Abstract
Noise and light levels during hospitalizations can disrupt sleep and circadian health, resulting in worsened health outcomes. This study describes patterns of noise and light for inpatient children undergoing stem cell transplants. Objective meters tracked noise and light levels every minute for 6 months. Median overnight sound was 55dB (equivalent to conversational speech). There were 3.4 loud noises (>80dB) per night on average. Children spent 62% of the 24-hour cycle in non-optimal lighting, with daytime light dimmer than recommended 98% of the time. Over the 6-month period, the lowest overnight noise level recorded exceeded World Health Organization recommendations for sleep, with frequent spikes into ranges known to cause wakings. During the day, children were rarely exposed to light sufficient to preserve healthy circadian rhythms. Hospitals should address systematic environmental and workflow disruptors to improve the sleep and circadian health of patients, particularly those already at elevated risk for health morbidities.
Author-input Keywords: inpatient sleep, circadian rhythms, pediatric cancer patient
Manuscript Keywords: sleep, pediatric hospital medicine, observational study designs, complications of cancer
Introduction
Noise and light are known disruptors of sleep and circadian health for hospitalized patients in pediatric hospitals.1 For example, noise often spikes at night on inpatient units as a result of alarms, medication administrations, and room entries, which can average 7–12 entries per night.1,2 Irregular and “unnatural” light-dark cycles that are not aligned with natural light patterns can cause circadian disruptions.3 As a consequence, both noise and light disruptions are independently associated with less sleep among hospitalized patients, including children.2 Poor sleep can lead to adverse health effects, including metabolic and immune system dysregulation. Therefore, it is essential to ensure the best possible inpatient environment for the sleep and circadian health of hospitalized patients.
Pediatric patients undergoing stem cell transplant (SCT) are hospitalized for extended periods (averaging multiple weeks and even ≥100 days)4 and are at high risk for sleep disturbances. While the negative impacts of noise and light for adult inpatients are widely recognized, our understanding of the impact on pediatric SCT patients is limited. In the pediatric setting, noise and light levels have only been tracked for brief periods (e.g., three days), primarily focusing on the nighttime.5 As there is variability in noise/light that not only occurs over a 24-hour day, but also seasonally over the weeks and months that pediatric SCT patients remain hospitalized, this study aimed to better characterize the 24-hour noise/light patterns in pediatric SCT rooms over a prolonged data collection period.
Methods
Over 6 months, we recorded sound and light levels at one-minute intervals within an inpatient room on the SCT unit at a children’s hospital in the northeast U.S. using the Extech SDL600 (noise) and SDL400 (light). Meters were wall-mounted near the head of the bed to approximate the patients’ most common light/sound experience (see Figure 1). We recorded admission/discharge dates and patient demographics. In this unit, patient doors are kept closed, three visitors are allowed at a time, and patients are restricted to the unit. The study was deemed IRB exempt.
Figure 1:
Room layout and meter placement.
We examined patterns of sound and light across three periods based on the hospital’s schedule: overnight (8:00pm-7:59am), daytime (8:00am-3:59pm), and evening (4:00pm-7:59pm). For noise, we evaluated whether median levels at night exceeded 40 dB, the World Health Organization’s (WHO) recommended maximum for nighttime noise spikes in inpatient hospital rooms.6 For light, we determined the percent of time that patients were exposed to inappropriate light levels. Recent consensus-based recommendations for optimal light conditions include exposure to a melanopic equivalent daylight illuminance (EDI) of ≥250 lux during daytime hours, ≤10 lux during the evening, and ≤1 lux overnight.7 Because our meter was wall mounted, we converted melanopic EDI to vertically measured illuminance by multiplying the recommended melanopic EDI by a correction factor of 1.79 based on a melanopic daylight efficiency ratio of 0.56 for 4000K fluorescent lights (Kenneth Wright Jr., PhD & Timothy Brown, PhD., email communication, http://cie.co.at/news/launch-cie-s-026-toolbox-and-user-guide, November 2022). Therefore, the vertically measured illuminance thresholds are 448 lux during the daytime, 18 lux during the evening, and 2 lux overnight. For the nighttime hours, we also measured the frequency of noise spikes >80 dB, sound level changes (SLCs, the difference between two consecutive sound measurements) >17.5 dB, and light spikes >150 lux – all of which are associated with night wakings.5,8 Our analyses were restricted to the dates that rooms were occupied, excluding admission/discharge days. Due to device malfunction, light data were not captured from 03/03/2022 to 05/22/2022, sound data were not captured from 07/07/2022 to 08/13/2022, and 1% of sound data were corrupted. We manually scanned for and agreed via consensus to remove corrupted data along with the five minutes immediately preceding and following the affected ones to ensure removal of any potential artifacts. Data were analyzed using RStudio.
Results
Six SCT patients aged 1–17 years (Mage=8.4 ± 6.6; 67% male) occupied the observed room from February to August 2022. Their average length of stay was 31.5 days (range=13–61).
Across all overnight data points in our study, the minimum noise level was 51.4 dB, with a median of 55.0 dB. Overnight noise peaked at 98.4 dB. See Figures 2a–b. On an average night, children in our study were exposed to exceedingly disruptive noise: 3.4 noise spikes >80 dB (roughly equivalent to a gas-powered lawnmower) and 5.6 SLCs >17.5 dB. Either a noise spike or a SLC occurred on 96.5% of nights (139/141 nights).
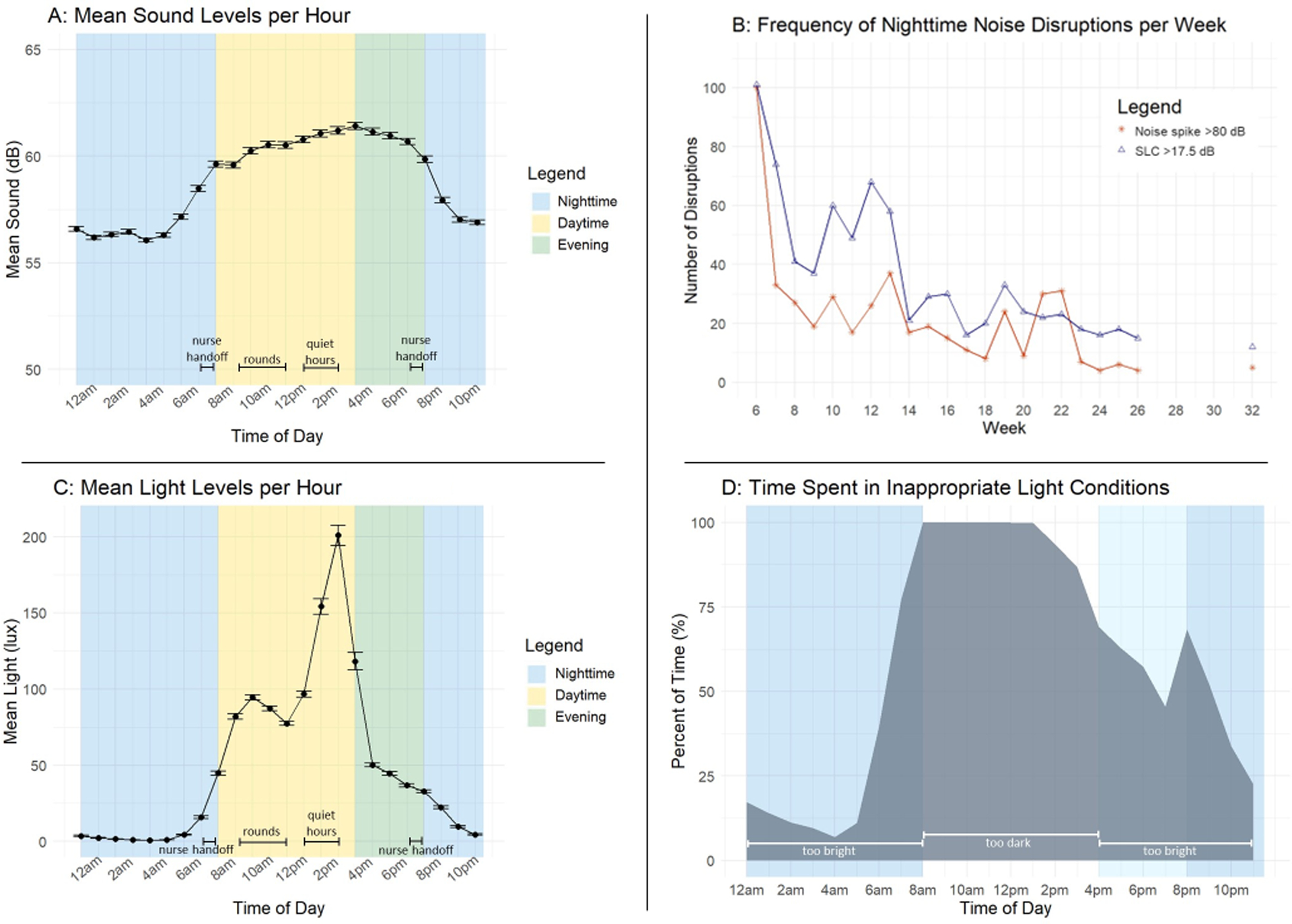
Figure 2a: Mean noise levels per hour (with 95% confidence intervals) in a pediatric hospital room.
Figure 2b: Frequency of noise spikes in a pediatric hospital room that are above thresholds previously associated with night wakings (>80 dB and SLCs >17.5 dB) per week.
Figure 2c: Mean light levels per hour (with 95% confidence intervals) in a pediatric hospital room.
Figure 2d: Average percent of time hospitalized patients spent in inappropriate light conditions across the 24-hour day.
Across a 24-hour day, patients were exposed to non-optimal light conditions 61.9% of the time. See Figures 2c–d. During the daytime, light levels were too dim (<448 lux) 97.5% of the time, with a median of 73.0 lux (range=0–1454 lux). In the evening, light was too bright (>18 lux) for 58.6% of the time, with a median of 29.0 lux (range=0–2370 lux). Overnight, children spent 30.2% of their time in light >2 lux, with a median of 1 lux and minimum of 0 lux. Light spiked to a maximum of 513 lux at night, with children being exposed to light spikes >150 lux on 4.9% of nights (4/82 nights).
Discussion
Hospitalized pediatric SCT patients in this study were consistently exposed to disruptive noise and light. Nighttime noise levels always exceeded WHO recommendations for sleep,6 with patients regularly exposed to multiple noise spikes associated with night wakings.5,8 Our data are consistent with a recent review, which found average noise varied from 44.1–62.5 dB on individual units.1 Humans can become accustomed to some persistent sounds; however, noise level variability is known to drive night wakings.5,8,9 It is therefore important to focus intervention efforts on minimizing the brief, but problematic, bursts of sound these patients were exposed to.
While light levels were frequently dim enough to be conducive to sleep at night, patients were rarely exposed to light bright enough to preserve healthy circadian rhythms during the day, which has the potential to dysregulate sleep at night.7 These levels of insufficient daytime light are consistent with prior research among adults on both ICU and non-ICU wards.7,8 As a child’s circadian rhythms may be affected by evening light to a greater extent than adults, such light spikes may be particularly disruptive to circadian rhythms in pediatric patients.7 The relatively low and afternoon-skewed daytime light, combined with the brighter than recommended light during the evening and early night, is thought to lead to longer hospitalizations and higher morbidity among adults.10
Limitations
We did not measure light/sound directly at eye/ear level or assess patient outcomes. Next, it was not feasible to blind hospital staff to monitor placement, so it is possible that they altered their behavior while in the room. Furthermore, these results may not be generalizable to all hospitals, child inpatient populations, and rooms. For example, room layout, window coverings/orientation, and location impact light exposure.10 Although this project was limited by equipment malfunction, we believe these data provide important information for pediatric SCT patients.
Recommendations
Our findings dovetail with those from other settings to suggest there is considerable room for improving the inpatient pediatric SCT environment to better support sleep and circadian health. Environmental factors are known to be the most common sleep disruptors; however, the majority of hospital sleep-promoting interventions target individuals’ behaviors, not their environments.1 We briefly outline several recommendations that hospitals can implement to improve sleep outcomes.
1). Intervene at the Institutional Level
Due to the known challenges with sustaining interventions that target individual patients or providers, institutions should make systematic changes that prioritize sleep and circadian function. For instance, reducing nighttime noise on the unit not only creates a more sleep friendly environment for patients, but also for their caregivers.10 Given that alarms are frequently implicated as a source of disruptive inpatient noise and can lead to staff alarm fatigue, better setting and control of alarm thresholds is warranted.11 Electronic health record systems can also be leveraged to promote sleep-friendly night care practices. For example, prompts to forgo overnight blood pressure monitoring for clinically stable patients were associated with fewer night wakings, increased sleep duration for patients >24 months, and an annual cost savings of >$15,000 USD.12 Additionally, limiting nighttime disruptions reduced sedative use among hospitalized adults.13
2). Establish Sleep/Circadian Friendly Standards
Almost all section chiefs of hospital medicine rate patient sleep as important, but fewer than half of their hospitals have adopted sleep friendly practices.14 This speaks to the need for the establishment of sleep/circadian health standards that institutions are incentivized to implement.15 For example, the development of a set of “Sleep/Circadian Friendly” room guidelines would standardize what administrators would want to consider when planning the design of a pediatric SCT unit.
3). Encourage Sleep/Circadian Health Research
Improving sleep/circadian health should be an area of growing interest among pediatric researchers and clinicians. Future studies could involve: a) qualitative assessment of key stakeholders to determine what would be important in an appropriate sleep/circadian patient environment; b) objective assessments of patients, including sleep disruptions (e.g. actigraphy or wearables), and circadian rhythm timing (e.g., urinary melatonin metabolite timing, dim light melatonin onset timing from saliva samples, or single timepoint circadian phase markers); and c) studying patient outcomes (e.g., length of stay).
Conflict of Interest Disclosures:
The authors have no conflicts of interest relevant to this article to disclose. Eric S. Zhou has received grant funding from Jazz Pharmaceuticals and Harmony Biosciences, and he has received consulting fees from Samsung and MindUP. Brian D. Gonzalez reports fees unrelated to this work from Sure Med Compliance and Elly Health. Jo M. Solet owns stock and is an advisor to Kunason, Inc, DreamZZZ, Inc, Lark Health, Inc, and Ascento Medical. The remaining authors have no known conflicts of interest to disclose.
Funding Support:
This research was generously supported by grant funding from Pedals for Pediatrics (PI: Zhou), a Clinician Scientist Development Grant, CSDG-21-080-01 - CTPS, from the American Cancer Society (PI: Zhou), and an institutional research training grant (T32DK063929).
Role of Funders:
The funder/sponsor did not participate in the work.
Footnotes
Clinical trial registry name and registration number: N/A
References
- 1.Fidler AL, Voorhees S, Zhou ES, Stacciarini JM, Fedele DA. A systematic review and proposed conceptual model of sleep disturbances during pediatric hospitalizations. Sleep. 2022;2022:1–17. doi: 10.1093/SLEEP/ZSAC038 [DOI] [PubMed] [Google Scholar]
- 2.Linder LA, Christian BJ. Nighttime sleep disruptions, the hospital care environment, and symptoms in elementary school-age children with cancer. Oncol Nurs Forum. 2012;39(6):553–561. doi: 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cain SW, Phillips AJK. Do no harm: the beginning of the age of healthy hospital lighting. Sleep. 2021;44(3):zsab016. doi: 10.1093/sleep/zsab016 [DOI] [PubMed] [Google Scholar]
- 4.Davis L, Yao Y, Jin Z, et al. Length of Stay and Health Care Utilization Among Pediatric Autologous Hematopoietic Cell Transplantation Recipients. Transplantation and Cellular Therapy. 2021;27(7):613.e1–613.e7. doi: 10.1016/j.jtct.2021.03.025 [DOI] [PubMed] [Google Scholar]
- 5.Stremler R, Micsinszki S, Adams S, Parshuram C, Pullenayegum E, Weiss SK. Objective sleep characteristics and factors associated with sleep duration and waking during pediatric hospitalization. JAMA Network Open. 2021;4(4):e213924–e213924. doi: 10.1001/JAMANETWORKOPEN.2021.3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berglund Birgitta, Lindvall T, Schwela Dietrich H, World Health Organization Occupational and Environmental Health Team. Guidelines for Community Noise. World Health Organization; 1999. [Google Scholar]
- 7.Brown TM, Brainard GC, Cajochen C, et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 2022;20(3):e3001571. doi: 10.1371/journal.pbio.3001571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal S, Garcia S, Owens R. Sound and Light Levels Are Similarly Disruptive in ICU and non-ICU Wards. J Hosp Med. 2017;12(10):798–804. doi: 10.12788/jhm.2826 [DOI] [PubMed] [Google Scholar]
- 9.Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med. 2012;157(3):170–179. doi: 10.7326/0003-4819-157-3-201208070-00472 [DOI] [PubMed] [Google Scholar]
- 10.Tan X, van Egmond L, Partinen M, Lange T, Benedict C. A narrative review of interventions for improving sleep and reducing circadian disruption in medical inpatients. Sleep Medicine. 2019;59:42–50. doi: 10.1016/j.sleep.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 11.Solet JM, Barach PR. Managing alarm fatigue in cardiac care. Progress in Pediatric Cardiology. 2012;33(1):85–90. doi: 10.1016/j.ppedcard.2011.12.014 [DOI] [Google Scholar]
- 12.Cook DJ, Arora VM, Chamberlain M, et al. Improving hospitalized children’s sleep by reducing excessive overnight blood pressure monitoring. Pediatrics. 2020;146(3). doi: 10.1542/peds.2019-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartick MC, Thai X, Schmidt T, Altaye A, Solet JM. Decrease in as-needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–24. doi: 10.1002/jhm.549 [DOI] [PubMed] [Google Scholar]
- 14.Affini MI, Arora VM, Gulati J, et al. Defining existing practices to support the sleep of hospitalized patients: A mixed-methods study of top-ranked hospitals. J Hosp Med. 2022;17(8):633–638. doi: 10.1002/jhm.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlov NM, Arora VM. A call for a “Sleep-Friendly” designation in hospitals. Sleep. 2022;45(5). doi: 10.1093/SLEEP/ZSAC066 [DOI] [PubMed] [Google Scholar]


