Graphical Abstract
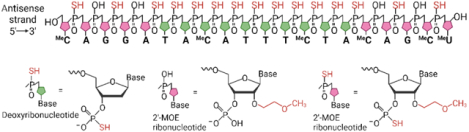
STRUCTURE: Tofersen, an antisense oligonucleotide (ASO) drug, is a 20-base residue with an RNA-DNA-RNA (5-10-5) gapmer mixed backbone oligonucleotide. Within the molecule, there are nineteen inter-nucleotide linkages, with fifteen of them being 3’-O to 5’-O phosphorothioate diesters and the remaining four being 3’-O to 5’-O phosphate diesters. In terms of sugar residues, ten out of the twenty are 2-deoxy-D-ribose, while the others consist of 2’-O-(2-methoxyethyl)-D-ribose (2’-MOE). The arrangement of residues involves five MOE nucleosides located at both the 5’ and 3’-ends, surrounding a central gap containing ten 2’-MOE. Me indicates that the cytosine and uridine bases are methylated at the 5-position. Tofersen’s molecular formula is C230H317N72O123P19S15, and it possesses a molecular weight of 7127.86 atomic mass units.
Graphical Abstract
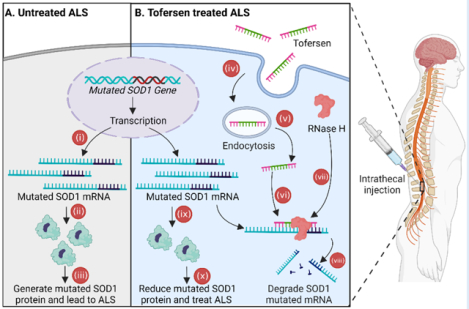
MECHANISM OF ACTION: (A) Approximately 2% of cases of amyotrophic lateral sclerosis (ALS) in adults is caused by a genetic mutation in the superoxide dismutase 1 (SOD1) gene. This mutation results in (i) the production of mutated SOD1 mRNA, which is then (ii) translated into dysfunctional SOD1 protein, (iii) contributing to the development of ALS. (B) Tofersen is indicated for the treatment of ALS in adults who have the mutation in the SOD1 gene. Tofersen molecules enter motor neurons and astrocytes through (iv) endocytosis, with some molecules (v) escaping the endosomal pathway. Once inside the cytoplasm, (vi) tofersen binds to the mutated SOD1 mRNA, forming a DNA:RNA heteroduplex structure. (vii) The enzyme RNase H recognizes this hybrid chain and (viii) subsequently cleaves the mRNA, (ix) leading to a reduction in the production of mutated SOD1 protein. By inhibiting the synthesis of dysfunctional SOD1 protein, (x) tofersen offers a therapeutic approach for the treatment of ALS.
NAME:
The drug name is tofersen and the brand name is Qalsody.
DRUG CLASS:
Tofersen belongs to a large molecule class of drugs known as antisense oligonucleotides (ASO) therapeutics. It is an orphan drug with an accelerated approval.
CLINICAL USE:
Tofersen is an intrathecally administered therapeutic indicated for the treatment of amyotrophic lateral sclerosis (ALS) in adult patients with a confirmed mutation in the superoxide dismutase 1 (SOD1) gene. The recommended dose for tofersen is 100 mg (15 mL). The treatment protocol involves an initial phase consisting of three loading doses administered at 14-day intervals. Following this phase, a maintenance dose should be administered once every 28 days thereafter.
DEVELOPED BY:
Tofersen was developed by Ionis Pharmaceuticals. Biogen licensed tofersen from Ionis Pharmaceuticals under a collaborative development and license agreement.
ADVERSE EFFECTS:
The most common adverse reactions are pain, fatigue, arthralgia, cerebrospinal fluid white blood cell counts increase, and myalgia. It is important to highlight other certain clinically adverse reactions associated with the treatment, including myelitis and/or radiculitis, papilledema, elevated intracranial pressure, and aseptic meningitis. These adverse reactions require attention due to their potential impact on patient health and well-being.
TIMELINE:
2015–2021: Phase 1/2/3 trial (NCT03764488 VALOR, NCT02623699 VALOR)
2017–present: Phase 3 trials (still recruiting) (NCT03070119 OLE, NCT04856982 ATLAS)
4/25/2023: FDA approval for Qalsody (tofersen), application number: 215887.
Acknowledgments
X-b.Z. is funded by the National Institutes of Health/National Institute of General Medical Sciences (NIGMS) R35GM140862.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no conflict of interest.
Literature
- 1.Miller T et al. (2020) Phase 1–2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med 383:109–119 [DOI] [PubMed] [Google Scholar]
- 2.Miller TM et al. (2022) Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med 387:1099–1110 [DOI] [PubMed] [Google Scholar]
- 3.Benatar M et al. (2022) Design of a randomized, placebo-controlled, phase 3 trial of tofersen initiated in clinically presympotomatic SOD1 variant carriers: the ATLAS study. Neurotherapeutics. 19(4):1248–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monine M et al. (2021) A physiologically-based pharmacokinetic model to describe antisense oligonucleotide distribution after intrathecal administration. J Pharmacokinet Pharmacodyn. 48(5):639–654 [DOI] [PubMed] [Google Scholar]
- 5.Meyer T et al. (2023) Neurofilament light chain response during therapy with antisense oligonucleotide Tofersen in SOD1 - related ALS – treatment experience in clinical practice. Muscle Nerve. 67(6):515–521 [DOI] [PubMed] [Google Scholar]
- 6.Dhuri K et al. (2020) Antisense oligonucleotides: An emerging area in drug discovery and development. J. Clin. Med 9(6):2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migliorati JM et al. (2022) Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs. Drug Metab Dispos. 50, 888–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. (2023) Labelling guidelines for Qalsody. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215887s000lbl.pdf


