Abstract
Cervical cancer is the fourth most common cancer among women globally. Historically, human papillomavirus (HPV) infection was considered necessary for the development of both precursor and invasive epithelial tumors of the cervix; however, studies in the last decade have shown that a significant proportion of cervical carcinomas are HPV-independent (HPVI). The 2020 World Health Organization (WHO) Classification of Female Genital Tumors separates both squamous cell carcinomas (SCCs) and endocervical adenocarcinomas (ECAs) by HPV status into HPV-associated (HPVA) and HPVI tumors. The classification further indicates that, in contrast to ECAs, HPVI and HPVA SCCs cannot be distinguished by morphological criteria alone and suggests that HPV testing or correlates thereof are required for correct classification. Moreover, while HPVA SCC precursor lesions (ie, high-grade squamous intraepithelial lesion [HSIL]) are well known and characterized, precursors to HPVI SCCs have only been described recently in a small number of cases. We studied 670 cases of SCCs from the International Squamous Cell Carcinoma Project (ISCCP) to analyze the reproducibility of recognition of invasive SCC growth patterns, presence of lymphovascular space invasion, tumor grade, and associations with patient outcomes. Consistent with previous studies, we found histologic growth patterns and tumor types had limited prognostic implications. In addition, we describe the wide morphologic spectrum of HPVA and HPVI SCCs and their precursor lesions, including tumor growth patterns, particular and peculiar morphologic features that can lead to differential diagnoses, as well as the role of ancillary studies in the diagnosis of these tumors.
Keywords: cervical cancer, human papillomavirus, squamous cell carcinoma, endocervical adenocarcinoma, precursor lesions
Introduction
Cervical cancer is the fourth most common cancer among women globally and one of the most commonly diagnosed cancers in women under the age of 45 years.1 Over 90% of cases occur in patients from less-developed countries where primary (vaccination) or secondary (screening) prophylactic programs are not widely available or do not exist.2
Historically, human papillomavirus (HPV) infection was believed to be a requisite for the development of most or all invasive cervical carcinomas and precursors.3–6 Recent studies, however, have reported a significant frequency of HPV-independent (HPVI) cervical carcinoma, particularly adenocarcinomas.7–11 To harmonize the classification across lower genital tract sites, the 2020 World Health Organization (WHO) Classification of Female Genital Tumors classifies squamous cell carcinomas (SCCs) and endocervical adenocarcinomas (ECAs) by HPV status – HPV-associated (HPVA) and HPVI tumors.1 Consistent data on differences in age, stage at diagnosis, precursor lesions, morphology, clinical behavior, and response to treatment between HPVA and HPVI ECAs are available.11–14 Similarities between HPVI SCCs and HPVI ECAs were noted in a few recent studies. In contrast to HPVA SCCs, HPVI SCCs occur in older age, are diagnosed at an advanced stage, are associated with lymph node metastases, have a worse prognosis, have a propensity to develop distant metastasis, and are typically resistant to conventional oncologic treatment.10,15–17
HPVA and HPVI ECAs can be distinguished microscopically on hematoxylin & eosin (H&E) stained slides by the presence of abundant apical mitotic figures and apoptotic bodies, as initially described in the International Endocervical Adenocarcinoma Criteria and Classification (IECC).11 In contrast, the 2020 WHO classification states that HPVI and HPVA SCCs cannot be distinguished by morphology alone, suggesting that additional staining and HPV testing may be required;1 however, when this classification was published, little was known about HPVI SCC morphology, and there was no evidence of HPVI precursor lesions. Only recently have HPVI precursor lesions been identified and described in a small number of cases.9
We studied 670 cases of cervical SCCs from the International Squamous Cell Carcinoma Project (ISCCP) and classified them by evaluating morphology, immunohistochemistry, HPV status, demographic data, and clinical outcomes. In this review, we describe the wide spectrum of morphologies observed in HPVA and HPVI SCCs and their precursor lesions. We note tumor growth patterns, particular and peculiar morphologic features that may lead to differential diagnoses, as well as the role of ancillary studies for diagnosis and classification. Distinctions between HPVA and HPVI SCCs may lead to discoveries in novel and targeted therapies.
I. HPVA SCCs
HPVA SCCs are squamous tumors with either stromal invasion or exophytic growth forming a mass.1 High-risk HPV genotypes (16, 18, 31, 35, 39, 45, 51, 52, 56, 58, 59) are most commonly found in HPVA SCCs, and 70% of all SCCs are related to the 16 and 18 genotypes.1,18,19 Associated low-risk HPV subtypes (ie, 6 and 11) have been reported in very rare cases.20 HPVA SCC is frequently associated with high-grade squamous intraepithelial lesion (HSIL) as a consequence of high levels of viral oncogenes (E6 and E7) as well as additional genetic and genomic alterations, immunosuppression, smoking, multiparity, and long-term use of oral contraceptives.1,21–24 HPVA HSIL precedes invasive SCC by 10 to 15 years and, in contrast to HPVI precursor lesions, can be detected by Papanicolaou smear and/or viral screening with high sensitivity.1,22–24
For HPVA SCCs, the mean age at diagnosis is 51 years, but importantly, 30% of patients are diagnosed before 30 years of age. The tumor develops at the transformation/junctional zone. Small HPVA SCCs are usually asymptomatic, while large tumors may present with vaginal bleeding, discharge, and pain. In advanced stages, patients can present with ureteric obstruction, uraemia, haematuria, tenesmus, and fistulas.1,25
When visible, the tumor is of variable size, white or gray in color, and of hard consistency. The tumor can be either exophytic (papillary or polypoid) or endophytic (stromal invasive), and tumor necrosis often can be appreciated on cut surfaces.25
Because it was not based on HPV association, the pattern-based 2014 WHO classification of cervical SCCs was replaced in the 2020 WHO classification;1,4 the prognostic significance of various morphologic patterns (eg, keratinizing, non-keratinizing, basaloid, warty/condylomatous, papillary, squamotransitional, and lymphoepithelial-like) was debated and determined to be not reproducible because of non-specific definitions.4 While the 2020 WHO classification does not recommend reporting histologic subtypes or growth patterns, we, along with the 2022 International Collaboration on Cancer Reporting (ICCR) guidelines, suggest this information remain in the surgical pathology report in a note or comment, given that primary tumor morphology is significant when looking for similarities to a recurrence.1,25,26 This information could also be used in research to better understand the interplay between HPV infection, growth patterns, and clinical outcomes (Table 1).
Table 1:
Comparison of clinical and pathologic features of HPV-associated and HPV-independent invasive squamous cell carcinoma.
| HPV-associated invasive squamous cell carcinoma | HPV-independent invasive squamous cell carcinoma | |
|---|---|---|
| Etiology/pathogenesis | HR-HPV and LR-HPV | No HR-HPV and LR-HPV |
| Clinical features | Small or large tumors | Large tumors |
| Mean age | 51 years | >60 years |
| Location | Transformation/junctional zone | Transformation/junctional zone |
| Microscopic features | Any growth pattern | Any growth pattern |
| p16 expression/HPV testing | Block-type positive for p16/ HR-HPV and LR-HPV ISH positive | Negative for p16/ HR-HPV and LR-HPV ISH negative |
| Treatment | Surgery and oncologic treatment | Surgery and oncologic treatment |
| Prognosis | Usually good | Worse |
HPV = human papillomavirus; HR = high risk; LR = low risk; ISH = in situ hybridization
Typical histologic appearances of HPVA SCC
Microscopically, stromal invasion is recognized by: (1) an irregular epithelial-stromal interface; (2) tumor growth in the form of angulated and irregular tumor nests and buds of multiple sizes and shapes; (3) paradoxical maturation of squamous tumor cells, with abundant and eosinophilic cytoplasm; (4) tumor cells with atypical and sometimes bizarre nuclei; (5) loss of polarity and basal palisading; and (6) presence of a desmoplastic stromal response (Figure 1). Architecturally, tumor cells may form nests, buds, cords, solid sheets, and papillae, or infiltrate as single cells. The stroma can be fibrous (pink in color and represented by a compact proliferation of fibroblasts) or myxoid (myxoedematous, lightly basophilic) and associated with inflammatory infiltrates, which can sometimes be abundant. There also may be a foreign body giant cell reaction in association with extracellular keratin and necrosis (Figure 2). Lymphovascular space invasion (LVSI), identified at the periphery of the tumor, is frequently present and is now increasingly quantitated by distinguishing between focal versus extensive (more than 3 or 5 intravascular tumor emboli) LVSI.26–28
Figure 1: Infiltrating human papillomavirus-associated (HPVA) squamous cell carcinoma (SCC):
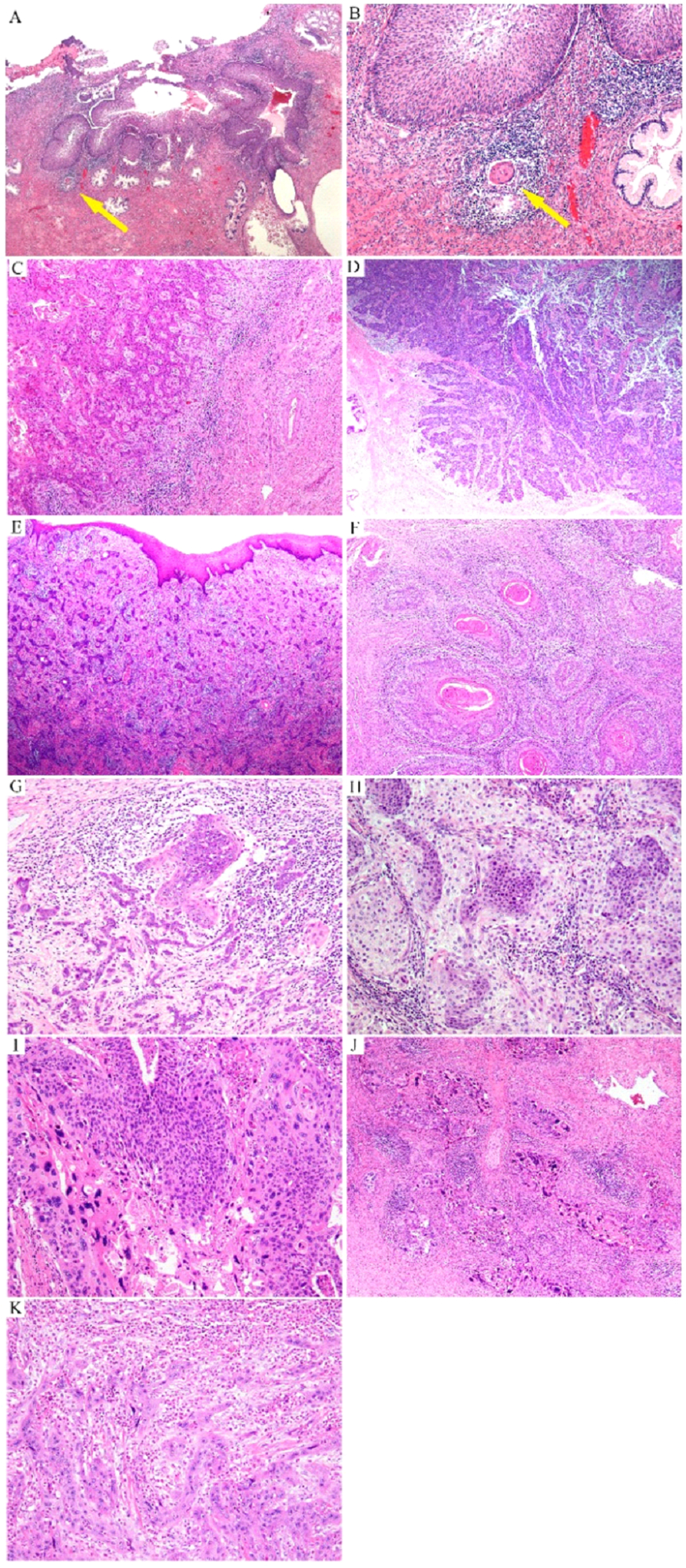
extensive high-grade squamous intraepithelial lesion (HSIL) with a tiny focus of invasion (arrow) (A) characterized by a small nest of tumor cells with paradoxical maturation (arrow) (B); invasive SCC with ragged epithelial-stromal interface (C); tumor cell buds invade the stroma with associated stromal desmoplasia (D, E); invasive buds of various sizes and shapes with central keratin pearls (F); buds and cords of tumor cells infiltrate the stroma in association with inflammatory infiltrates (G); infiltrating tumor cells display paradoxical maturation and uniform, small nuclei (H) or highly atypical nuclei (I, J) sometimes with a spindle shape, mimicking a sarcoma (K).
Figure 2: Infiltrating human papillomavirus-associated (HPVA) squamous cell carcinoma (SCC):
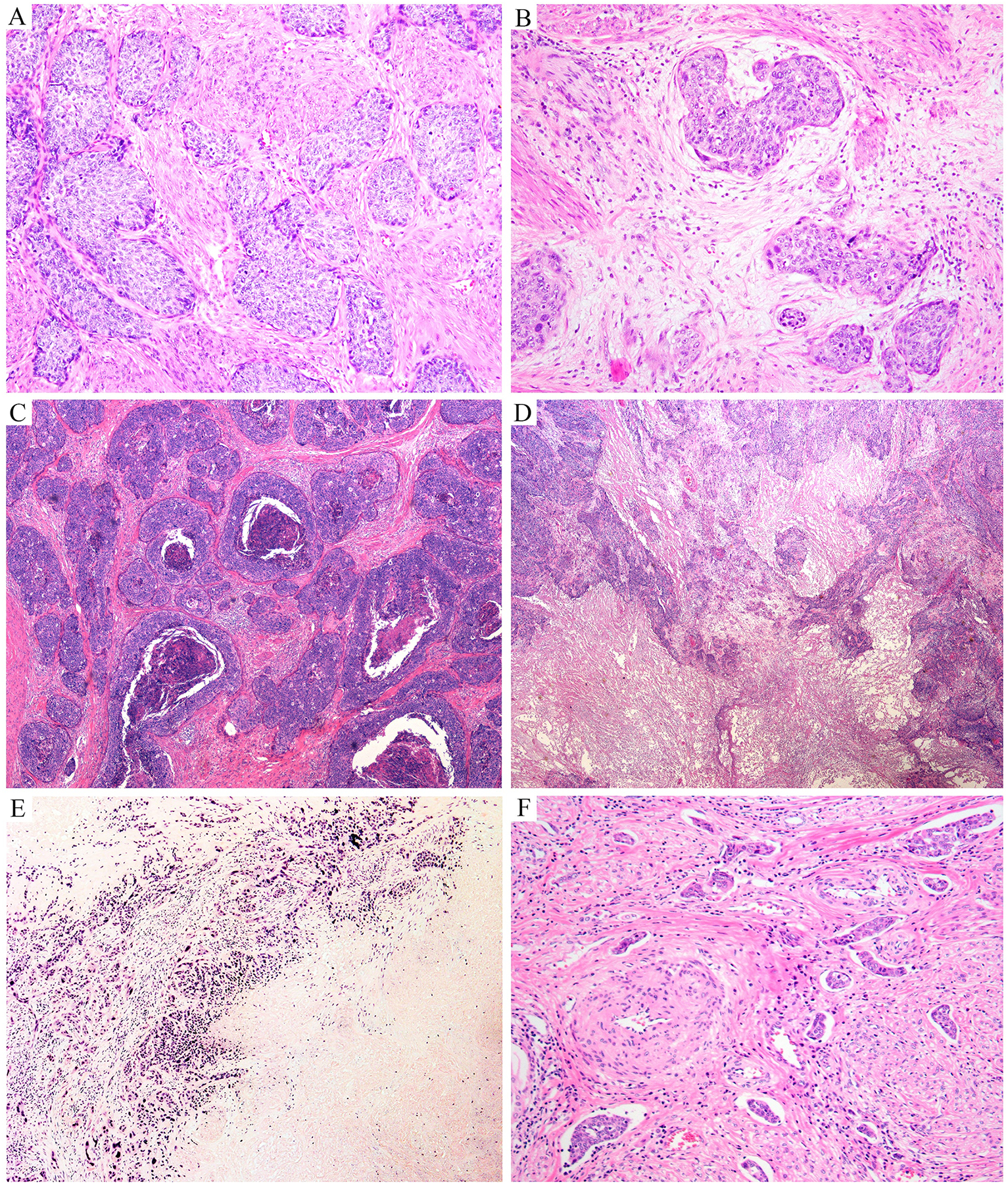
fibrous stroma (A); myxoid stroma (B); areas of comedo-type of necrosis (C) or massive necrosis (D, E); numerous foci of lymphovascular space invasion (LVSI) at the periphery of the tumor (F).
Spectrum of growth patterns in HPVA SCC
The most frequently encountered growth pattern is non-keratinizing, which is characterized by neoplastic tumor cells growing in nests or buds, in the absence of keratin pearls and keratohyalin granules.29 In contrast, the keratinizing growth pattern has ≥1 keratin pearls and usually large and polygonal tumor cells, with intercellular bridges, ample dense and eosinophilic cytoplasm, keratohyaline granules, and cytoplasmic keratinization.4,30,31 In the basaloid pattern, rounded nests of smaller, immature, and atypical basaloid squamous cells invade the stroma.4,32 The warty pattern is usually exophytic, superficially resembling a condyloma microscopically, and contains tumor cells with koilocytic features in both the deep infiltrative and superficial components.33 The papillary growth pattern is exophytic, with or without endophytic destructive stromal invasion, and presents with thin or broad papillae with fibrovascular cores lined by a multilayered epithelium with squamous differentiation, resembling HSIL.34–37 This pattern can be thought of as the squamous correlate to adenocarcinoma with a villoglandular pattern. Squamotransitional growth previously was used to describe papillary SCC with non-keratinizing immature squamous epithelium resembling urothelial mucosa; however, its immunohistochemical profile differs from transitional/urothelial carcinoma, so this terminology has been abandoned.34,38,39 The lymphoepithelioma-like growth pattern is represented by undifferentiated, non-keratinized tumor cells with large vesicular nuclei and prominent nucleoli that form invasive nests, and is associated with a massive inflammatory infiltrate.40,41 Some SCCs have a verrucous growth pattern, presenting with an exophytic mass in association with a pushing type of infiltrative component and with minimal cytologic atypia but abundant cytoplasm in the absence of koilocytes.19,42,43 Pure verrucous carcinomas, which are very rare cervical tumors, are HPVI and found almost exclusively in the vulva.25 While keratinizing, non-keratinizing, and basaloid growth patterns are frequently encountered in clinical practice, we rarely diagnose warty, papillary, and lymphoepithelioma-like growth patterns, and have never encountered a true verrucous carcinoma in the cervix (Figure 3; Table 1).
Figure 3: Microscopic growth patterns in human papillomavirus-associated (HPVA) squamous cell carcinomas (SCCs):
keratinizing (A); non-keratinizing (B); basaloid (C); papillary (D); inverted papillary (papillae invading the storm without an exophytic component) (E, F); warty (G); lymphoepithelioma-like (H); microscopic growth pattern, p16 is block-type positive (I).
In general, immunohistochemistry is not required to establish HPVA status. HPVA SCCs are block-type positive for p16, although there are situations when this marker is negative (patchy or no staining; Figure 3). p16-negative cases can be explained by methylation-induced inactivation of p16 and allelic loss of p16. In addition, sections from poorly fixed paraffin blocks or heterogeneous p16 positivity throughout the tumor may appear as p16 negative.11,15,44–46 In equivocal cases, repeat p16 staining is recommended, using another block or, if available, high-risk HPV in situ hybridization (HR-HPV ISH), which, if positive, establishes HPVA status. Almost all HPVA tumors have wild-type p53, in which case two staining patterns can be observed: (1) marked reduced staining or scattered (significantly attenuated with weak intensity in >70% of tumor cells), which can be confused with the true null mutational pattern; and (2) mid-epithelial (basal sparing).47 In the former, which is the most frequently encountered, the differential diagnosis includes the null staining pattern (no staining), indicating p53 mutation, a very rare event in HPVA SCC. In the ISCCP cohort, 1.57% of HPVA SCC cases were of p53 null type, which is similar to IECC ECAs cohort, in which 4% of cases were of p53 null type.11 In mid-epithelial (basal sparing), there may be staining of cells in the middle of invasive tumor cell nests, but not at the periphery. These patterns were described in vulvar SCCs and may be relevant in cervical SCCs, although our experience with interpreting p53 in cervical HPVA SCCs in association with HSIL is limited and more studies are needed.47
Spectrum of peculiar architectural and cytological features in HPVA SCC
a. Uncommon architectural features
We have encountered tumors with nests containing peripheral and internal palisades, adenoid basal-like and adenoid cystic-like growth patterns as well as inverted papillary, pseudoglandular, micropapillary, and mammary lobular-like growth patterns (single-cell infiltration), sarcomatoid, and HSIL-like features. Borrowing terminology from ECAs, some SCCs resemble Silva pattern A (usually pure adenoid basal carcinoma [ABC]), B (focally destructive invasion usually encountered in microscopically detected SCCs), or C (extensive destructive invasion, which is the most common pattern; Figures 4 and 5). The presence of these various morphologic patterns may impose diagnostic difficulties. Examination of multiple tumor sections usually uncovers unequivocal squamous differentiation with classic features. In scant samples, such as biopsies and curettings, the diagnosis may require special stains to detect squamous differentiation (ie, p63, p40) and association with HR-HPV infection.
Figure 4: Peculiar architectural patterns in human papillomavirus-associated (HPVA) squamous cell carcinomas (SCCs):

palisading (A, B); pseudoglandular pattern (C); papillary SCC with pseudoglandular areas (D, E); Silva pattern A (F); Silva pattern B (G, H); Silva pattern C (I); inverted papillary pattern (J); adenoid basal-like morphology (K); adenoid cystic-like morphology (L).
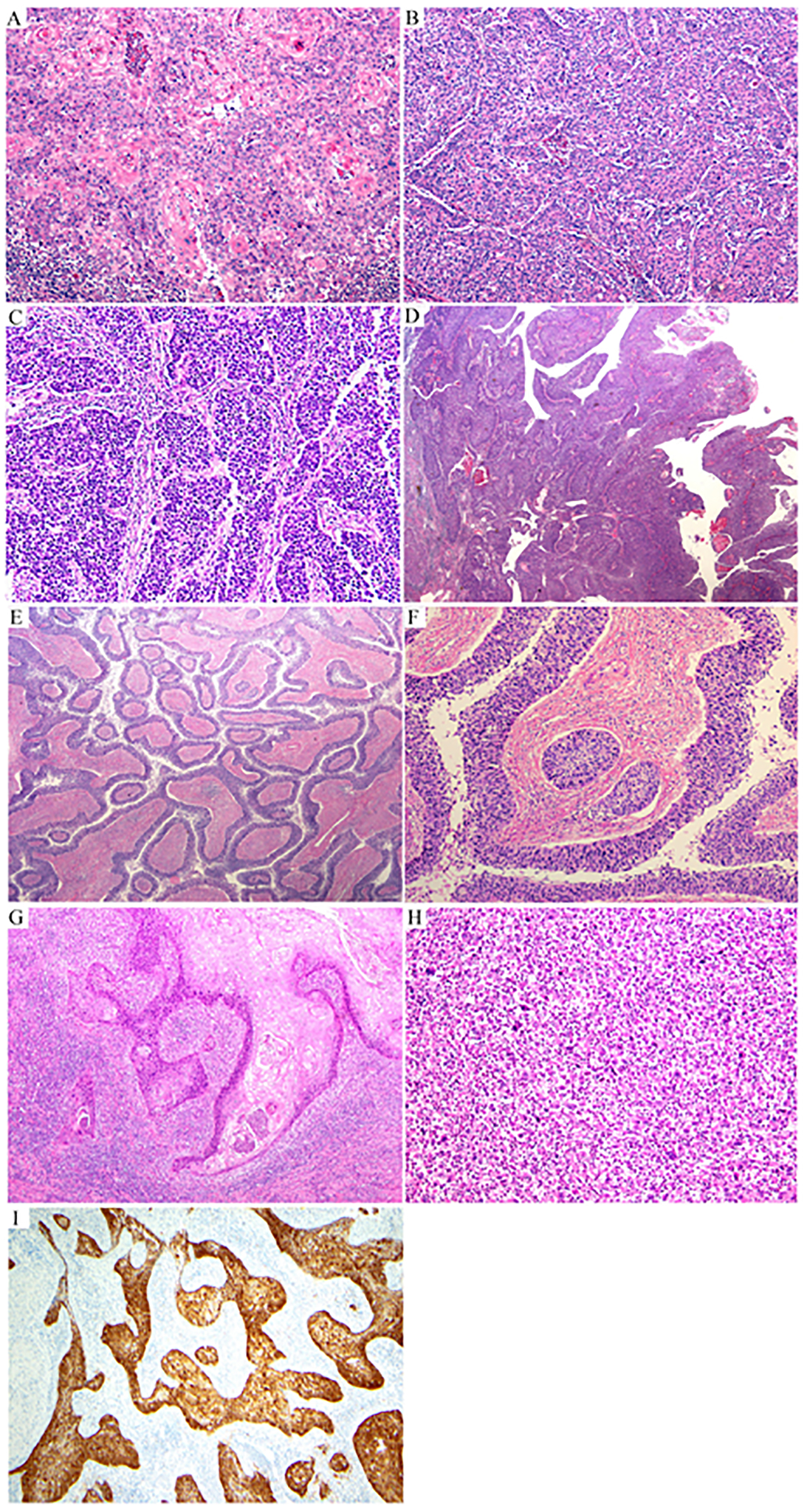
Figure 5: Peculiar architectural patterns in human papillomavirus-associated (HPVA) squamous cell carcinomas (SCCs):

lobular architecture (A); micropapillary architecture (B); massive squamous benign metaplasia at the top of the tumor (C) associated with numerous calcifications (D); giant multinucleate cells associated with nests of tumor cells (E); massive calcification (F); psammoma bodies (G).
Palisading is somewhat unusual in HPVA-invasive SCC, with approximately 2% of all SCCs presenting with this architectural feature; the primary differential diagnosis is invasive stratified mucinous carcinoma (ISMC) and ABC. Another unusual pattern is an internal palisade, in which the nuclearcluster is in the center of invasive tumor nests, with cytoplasm at the periphery. Although these patterns may suggest adenocarcinoma, these tumors are usually diffusely positive for squamous markers. In our review of ISCCP cases, we also observed a light and dark pattern in tumor cell nests – large tumor cells with eosinophilic or clear cytoplasm resembling koilocytes (light cells) and smaller cells with scanty and basophilic cytoplasm (dark cells). The basophilic cells are usually found at the periphery of cell nests.
HPVA SCCs may contain adenoid basal-like components in variable proportions. ABC, an uncommon cervical cancer subtype with low-grade histology, is thought to arise from pluripotential subcolumnar/reserve cells. ABCs feature small, rounded nests of basaloid squamous tumor cells, peripheral palisading and, frequently, glandular differentiation in the form of central lumina. The tumor cells must be small, bland, and never associated with a stromal reaction to invasion. They are always associated with HR-HPV, and most are associated with HSIL.48–51 Most cases coexist with conventional invasive HPVA SCC and, rarely, small-cell carcinoma, carcinosarcoma, or adenocarcinoma. Interestingly, NKX3.1 (a prostatic marker) expression in ABC has been reported recently.52
Among the 670 SCC cases in the ISCCP study, 27 (4%) contained an adenoid basal component, which was primarily identified at the periphery of invasive HPVA SCC. All cases were positive for p16 and HR-HPV. Appropriating terminology from ECAs, these cases demonstrated the Silva A non-destructive pattern of invasion, lacking stromal desmoplasia. Like pattern A ECAs, these are benign if excised with negative margins and if conventional invasive carcinomas and LVSI are not present.1,50
Although rare, HPVA SCCs may also contain adenoid cystic-like components. Adenoid cystic carcinoma was described alongside ABC in earlier publications;49,51 however, subsequent studies have demonstrated these are unrelated to adenoid cystic carcinomas of the salivary, mammary, and Bartholin glands, despite some histologic similarities.53 The characteristic t(6;9)(MYB-NFIB) gene fusion of true adenoid cystic carcinomas has not been consistently identified in tumors with cervical adenoid cystic-like differentiation, further confirming that although they may be morphologically similar, these cervical tumors are biologically distinct from their counterparts occurring in other parts of the body.53 The presence of an adenoid cystic-like component SCCs leads to difficulty differentiating such tumors from invasive ECAs.
Patterns of invasion similar to those described by Elvio Silva and colleagues for invasive adenocarcinomas have been observed in HPVA SCCs.54 Among the 584 cases in the ISCCP study in which Silva pattern was determined, 97% were Silva pattern B and C (destructive invasion ≥5 mm), and therefore, Silva pattern was not a predictor of overall survival or disease-free survival.
Other rare patterns have been reported in the literature. HPVA SCCs can have micropapillary architecture, which was recently described by Alvarado-Cabrero et al in HPVA and HPVI adenocarcinomas, characterized by an association with lymph node metastases and aggressive behavior.55 Mammary lobular carcinoma-like patterns can usually be distinguished from metastatic lobular carcinoma by the clinical and morphologic contexts, but presumably, this pattern could present problems in small samples. Immunohistochemistry may be helpful (p16 and p63-positive in the former; SOX10, mammaglobin, and estrogen receptor/progesterone receptor (ER/PR)-positive in the latter). HSIL-like SCC may be difficult to interpret. The invasive component is similar to HSIL that involve crypts and can have paradoxical maturation without peripheral palisading. These foci appear out of the plane of the endocervical crypts and do not conform to the usual undulations and invaginations of endocervical epithelium.56 Inverted papillary growth, especially when associated with a squamotransitional morphology, is rare but can mimic metastatic urothelial carcinoma. There is no individual marker that distinguishes these entities, but in general, urothelial carcinoma does not show block-like p16 staining or HR-HPV ISH positivity and is often GATA3 and CK20 positive, unlike most HPVA SCCs. Occasionally, HPVA SCCs contain calcifications resembling psammoma bodies, and in such cases, they should be differentiated from other tumor types in which psammoma bodies are characteristic. Massive inflammation can also occur and resemble lymphoepithelioma-like SCC or metastatic carcinoma. Giant cells are seen in some HPVA carcinomas, either in the form of tumor giant cells or foreign body giant cells, as a reaction to keratin (Figure 5).
b. Uncommon cytological features
Cytoplasmic clearing, usually due to glycogen, may be present. Theoretically, this phenomenon could suggest a clear cell carcinoma, but the cytoarchitectural features are discernibly different. Clear cell carcinomas typically have clear cytoplasm with hyperchromatic but uniform nuclei, sometimes with a prominent nucleolus. SCC may be readily diagnosed in the presence of other more typical features of this entity, including cytoplasmic eosinophilia and intercellular bridges, and in the absence of classic papillary and tubulocystic clear cell carcinoma architectures (Figure 6).
Figure 6: Peculiar cytologic features in human papillomavirus-associated (HPVA) squamous cell carcinomas (SCCs):

cytoplasmic clearing (A, B); rare cytoplasmic mucin (C, D); light and dark pattern (E); eosinophilic single cells (F); glassy-like morphology (G); multinucleated cells (H); dark and light pattern (I, J); psammoma bodies (K); sarcomatoid features (L).
Tumor cells can occasionally have intracytoplasmic mucin, which in our experience is usually a focal finding; although, some publications report between 20% and 35% of SCCs may contain intracellular mucin demonstrable with mucin stains.57 Scattered mucin-producing cells in SCC should not be interpreted as adenosquamous carcinoma, in which the two admixed squamous and glandular components are readily identified with H&E microscopy. Glassy cell carcinoma was previously thought to be related to adenosquamous carcinoma, although neither overt glandular nor squamous differentiation is obvious with standard stains. The tumor is composed of sheets of tumor cells with abundant ground glass eosinophilic cytoplasm, distinct cell borders, and enlarged nuclei with prominent nucleoli in association with necrosis and a prominent eosinophilic and/or neutrophilic infiltrate. Recent studies have shown that tumors with glassy cell features have glandular differentiation only and are in fact poorly differentiated ECAs.58 ISMC, a recently described histologic variant of mucin-producing HPVA ECA, may appear to have a mixed squamous and glandular appearance, but are in fact composed of nests of stratified glandular cells. Peripheral palisading is also typical.59 p63 and p40 stains are positive, but usually only in the palisade, which contrasts with more diffuse staining in SCC. Mucoepidermoid carcinoma, which is very rare and morphologically identical to those arising in salivary gland-type tissue, can occur in the cervix.60 As such, it exhibits three cell types: epidermoid, mucin-producing, and intermediate. These are unassociated with HPV infection and harbor genetic alterations in genes characteristically altered in mucoepidermoid carcinomas at other sites (CRTC1, MAML2).61
Sarcomatoid SCC features spindled tumor cells, sometimes arranged in fascicles, which as the name implies, resembles a cervical sarcoma (eg, a NTRK-rearranged fibroblastic sarcoma in a young patient, or a carcinosarcoma with sarcomatous stromal overgrowth).62 Sarcomatoid SCC has not be recognized as a histologic subtype or growth pattern by the WHO classification, but it has been described in the literature.63–65 It is controversial whether sarcomatoid SCC has a worse survival compared to epithelioid SCCs, but some studies report that survivals are comparable. Including this feature in the pathology report may help when diagnosing recurrences or metastases associated with this rare entity.63–65
Routine p16 staining in patients 60 years of age or older should be considered if therapeutic guidelines become dependent on HPV association. This would represent a much more limited expenditure, given the low frequency of cervical SCC in this age group.
c. Spectrum of precursor HPVA lesions
HPVA SCCs develop from HPVA precursor lesions (eg, HSIL) as a consequence of HPV infection, additional genetic alterations, and associated factors. HSIL develops at the transformation zone from HPV-infected reserve cells or primitive CK7-positive cuboidal cells in junctional epithelium.66,67 The lesion is clinically and macroscopically invisible, and is detected using HPV testing and Papanicolaou smears. According to the 2020 WHO classification and Lower Anogenital Squamous Terminology (LAST) criteria, both cervical intraepithelial neoplasia (CIN) grades 2 and 3 are considered HSIL.1,68 Microscopically, HSIL is characterized by loss of organization, polarity, and maturation, with immature squamous cells extending above the lower third of the epithelium. These cells have minimal cytoplasm and enlarged nuclei, with irregular nuclear membranes, dense chromatin and inconspicuous nucleoli, and mitotic figures above the basal third layer and in increased number.
Distinguishing between low-grade intraepithelial lesion (LSIL) and HSIL can be difficult. The LAST criteria suggest using p16 immunohistochemical staining to distinguish the two, particularly in younger patients to avoid unnecessary cone biopsies that could impair full-term pregnancies.68 Importantly, p16 staining should be used only in this context if there is significant concern that the lesion is HSIL and should be used only as confirmation that HSIL is present. Features that should raise significant concern for HSIL include atypical mitoses, mitoses in the superficial one-half of the lesion, and monster koilocytes that are more than five times larger than accompanying classical appearing koilocytes.69
Morphologic variants of HSIL have been described (eg, thin, keratinizing, pleomorphic, papillary, eosinophilic dysplasia). While the terms are not currently included in the pathology report, the significance of these variants deserves further investigation.70–76 Differential diagnoses include benign mimickers such as atrophy, atypical immature metaplasia, transitional cell metaplasia, reparative changes, and tangential sectioning. For these differential diagnoses, p16 band-like/block-positivity, HR-HPV ISH positivity, and a high Ki-67 index can assist. Neoplastic mimickers include invasive SCC, stratified mucin-producing intraepithelial lesion (SMILE), pagetoid spread of urothelial carcinoma, and HPVI precursor lesions.77,78 In SMILE, p63 and p40 are negative or positive only in the basal cell layer, while in pagetoid spread of urothelial carcinoma, HPV is negative, with CK20 and GATA3 positivity. HPVI precursor lesions rarely can be p16 positive but are HPV ISH negative (Figure 7; Table 2).
Figure 7: Human papillomavirus-associated (HPVA) precursor lesions:

high-grade squamous intraepithelial lesion (HSIL), cervical intraepithelial neoplasia (CIN) grade 2) (A, B); HSIL, CIN grade 3 (C).
Table 2:
Comparison of clinical and pathologic features of HPV-associated and HPV-independent squamous precursor lesions.
| HPV-associated squamous precursor lesion | HPV-independent squamous precursor lesion* | |
|---|---|---|
| Etiology/pathogenesis | HPV infection/additional genetic alterations | Gain of chromosome 3q TP53 mutation (possible) |
| Clinical/colposcopic features | Clinically invisible | Clinically invisible |
| Mean age | 35–40 years | 35–40 years |
| Location | Transformation/junctional zone | Transformation/junctional zone and upper endocervical canal |
| Microscopic features | Basaloid morphology | Basaloid or highly differentiated and keratinizing |
| p16 expression/HPV testing | Block-type positive for p16/ HR-HPV ISH positive | Block-type positive or negative for p16/ HR-HPV ISH negative |
| Treatment | Conization/loop excision with negative margins | Conization/loop excision with margins negative |
| Follow-up | Colposcopy, repeat smears and HPV testing | Colposcopy, repeat smears |
| Prognosis | Good | Not enough data in literature |
Extremely limited data
HPV = human papillomavirus; HR = high risk; ISH = in situ hybridization
II. Reproducibility of different growth patterns (original versus consensus tumor type)
Although the 2014 WHO classification was a useful guide, we believe it was insufficiently detailed to enable reproducible appraisal of patterns and tumor types. Finding that tumor type assignment (see Supplemental Material) is neither reproducible nor predictive of survival is consistent with previous studies that concluded this value has limited implications in SCCs. Consequently, removing these variables from the 2020 WHO classification and no longer recognizing them as core data in pathology reports are justified.1,26
As discussed above, we believe it may be useful to continue recording growth patterns and tumor types in the pathology report as a comment. Unfortunately, evidence suggests that HPVA and HPVI cannot be reliably distinguished by histologic review. Also, we evaluated grading in the ISCCP cases using different methods (Broders modified grading system and novel grade proposed by Jesinghaus), and established that similar to histological patterns, there were no prognostic implications, with low predictive accuracy for OS and progression-free survival (PFS).79,80
III. HPVI SCCs
According to the 2020 WHO definition, HPVI SCC are squamous tumors with stromal invasion and/or exophytic growth. This is a newly described entity, and its incidence is unknown. Negative HR-HPV results were previously interpreted as largely attributable to technical artifacts or to cases positive for low-risk HPV genotypes; however, recent studies with more sensitive techniques for HR-HPV detection reported 5% to 7% of cervical SCCs are HPVI.9,10,15 The etiology of HPVI SCCs is unknown, but there are reports these tumors show a high frequency of abnormal p53 immunostaining, suggestive of mutation.9,15 Mutations in genes such as KRAS, ARID1A, and PTEN have been described as well as inconsistent somatic gene mutations (PIK3CA, STK11, TP53, SMARC2B, and GNAS) in non-hotspot locations at low mutational frequency. Other genetic abnormalities include the pathogenic (angiogenic) germline polymorphism Q472H in KDR and chromosome 3q gains.9,21
Patients with HPVI SCCs are older than those with HPVA SCCs, according to previous studies, with a mean age at presentation of 60 years; however, in the ISCCP study, the mean age at diagnosis was older than that reported in the literature (mean age 72 for HPVIs vs 49 for HPVAs, P<.001). Clinical symptoms and macroscopic appearances are similar to HPVA SCCs. Patients with HPVI tumors are diagnosed at a higher International Federation of Gynecology and Obstetrics (FIGO) stage than patients with HPVA tumors, and present with larger tumors. Frequently, these tumors are ulcerated, making it impossible to detect a precursor lesion.
The 2020 WHO classification states that any histologic growth pattern can be seen in the HPVI category, suggesting morphology cannot reliably differentiate HPVA from HPVI SCCs. Ancillary testing is therefore required to make this distinction.1 Microscopically, HPVI tumors have been described as keratinizing and well-differentiated with numerous keratin pearls, as well as non-keratinizing.9,81 This is consistent with our review of ISCCP cases, in which most HPVI cases were keratinizing with keratin pearls and well-differentiated; however, we also observed non-keratinizing tumors and those with warty growth pattern. The presence of dense lymphocyte-dominated inflammatory infiltrates with plasma cells and occasional stromal eosinophils was a notable finding, and the presence of eosinophils in HPVI tumors has been linked to an unfavorable prognosis.82,83 The stroma is frequently highly vascularized with numerous small blood vessels, particularly around the basaloid invasive foci (Figure 8).9
Figure 8: Human papillomavirus-independent (HPVI) precursor and infiltrating squamous cell carcinoma (SCC):
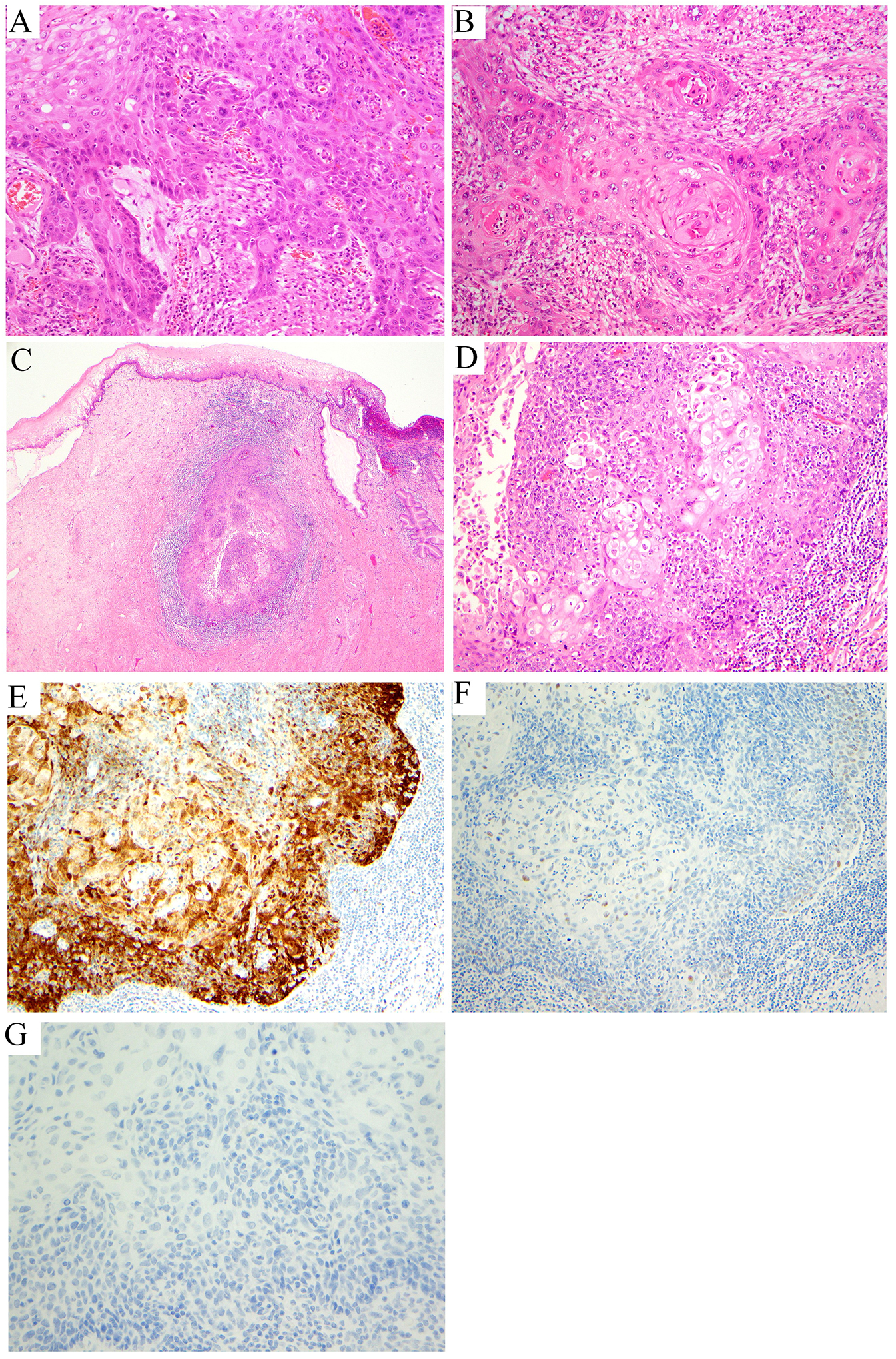
high-grade squamous intraepithelial lesion (HPVI) precursor lesion differentiated vulvar intraepithelial neoplasia (dVIN)-like (A); invasive HPVI keratinizing SCC with a dense inflammatory infiltrate (B); invasive HPVI SCC with a non-keratinizing growth pattern (C, D), p16 patchy (not overexpressed) (E), p53 wild type (patchy, low intensity nuclear staining) (F), HPV in situ hybridization (ISH) negative (G).
p16 and p53 immunohistochemistry, as well as HR-HPV ISH, should be important diagnostic adjuncts if clinical management becomes reliant upon HPV status. Molecular testing for HPV is negative in all HPVI SCCs, and p16 immunohistochemistry will show focal and patchy or negative staining (but not diffuse staining); both markers should be negative to indicate HPVI SCC. Rare equivocal cases of HPV-negative/p16-positive tumors can exist, and there are precedents in the literature for this phenotype in HPVI ECAs.11 p53 immunostaining is expected to be aberrant in a proportion of HPVI SCCs, whereas this phenomenon is very unusual in HPVA SCCs. In our analysis of ISCCP cases, only 20% of SCC cases p53 abnormal (null type) results that are similar to those of Nicolas et al (Nicolas, 2019).11 We recommend the interpretation of p53 staining be based on vulvar HPVI SCC criteria, with basal or parabasal/diffuse overexpression, as well as considering null (absent) and cytoplasmatic staining as aberrant. p63 and p40 were positive in nearly all cases (Figure 8).
To date, treatment of both HPVA and HPVI SCCs is similar across international guidelines, including the 2023 National Comprehensive Cancer Network (NCCN) and the European Society of Radiotherapy and Oncology/European Society of Gynaecological Oncology (ESTRO/ESGO) guidelines. However, evidence indicates HPVI tumors do not respond to conventional oncologic therapy (complete response to chemo- and radiotherapy is much lower in HPVI tumors compared to HPVA tumors), and thus additional studies will be beneficial to develop alternative management options.17 Turco et al suggested that HPV status should be assessed at initial diagnosis,17 but this would require extensive and mostly unnecessary testing.
a. Spectrum of precursor HPVI lesions
A recent characterization of HPVI precursor lesions demonstrated that all occurred at the transformation zone as well as the upper endocervical canal.9 Morphologically, the lesions were described as undifferentiated and basaloid or highly differentiated and keratinizing, similar to differentiated vulvar intraepithelial neoplasia (dVIN; Figure 8).9 In the study by Regauer, 2 of 3 HPVI precursor lesions and the in situ component of 1 of 6 invasive HPVI SCCs were p16 block-type positive, while most were wild-type p53; only 1 case was aberrant-type p53 (basal nuclei positivity).
Among the ISCCP cohort, there were several candidate HPVI precursor lesions in patients >60 years of age and in association with an invasive component of HPVI SCC. Of interest, some cases closely resembled dVIN morphology, while others were of basaloid morphology and resembled HSIL. All cases were HR HPV and low-risk HPV negative by ISH.
The spectrum of morphologies reported in HPVI precursor lesions makes it difficult to develop appropriate terminology. We propose either dCIN (differentiated cervical intraepithelial neoplasia) to provide continuity with vulvar terminology or, more accurately, HPVI CIN. It is premature to encourage p16 staining for most or selected cervical squamous precursor lesions because of their rarity and uncertain clinical relevance. The existence of the HPVI squamous precursor lesion raises questions related to the efficiency of screening programs in which the first step is HPV testing. Interestingly, while HPVI-invasive SCCs occur at an older age than HPVA SCCs, Regauer reported HPVI squamous precursor lesions in patients aged 36, 41, and 42 years. It is possible there is a long latency period between the identification of HPVI precursor lesions and the development of invasive HPVI SCC. Somatic gene mutations have not yet been identified, but some precursor lesions had a gain of chromosome 3q.9 Given the scarcity of data on these lesions, definitive conclusions would be premature.
The 2020 WHO classification distinguishes HPVA and HPVI tumors, and this distinction has prognostic and predictive implications. These tumor types cannot be distinguished on microscopic examination, necessitating additional immunohistochemical and molecular investigations. Both entities develop from precursor lesions, but while HPVA precursor lesions are well known, HPVI precursor lesions have been only recently described and their natural biology is still under investigation. Additional information on HPVI precursor lesions could have a major impact on clinical management, including screening programs and vaccinations against HPV infection.
Supplementary Material
Conflicts of Interest and Source of Funding:
Outside the current work, A. Iasonos reports consulting fees from Mylan. All other authors have no potential conflicts of interest to disclose. This research was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
References
- 1.WHO Classification of Tumours Female Genital Tumours. 5 ed. WHO Classification of Tumours. International Agency for Research on Cancer; 2020. [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. Jan 2022;72(1):7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. Jan 2003;16(1):1–17. doi: 10.1128/cmr.16.1.1-17.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurman RJ, Carcangiu ML, Herrington CS. World Health Organisation classification of tumours of the female reproductive organs. 4 ed. World Health Organization classification of tumours Lyon: International agency for research on cancer; 2014. [Google Scholar]
- 5.Park KJ, Soslow RA. Current concepts in cervical pathology. Arch Pathol Lab Med. May 2009;133(5):729–38. doi: 10.5858/133.5.729 [DOI] [PubMed] [Google Scholar]
- 6.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. Sep 1999;189(1):12–9. doi: [DOI] [PubMed] [Google Scholar]
- 7.Alexander C, White M, Maleki Z, Rodriguez EF. HPV-ISH-Negative Invasive Cervical Squamous Cell Carcinoma: Histologic and Pap Test Results. Acta Cytol. 2019;63(5):417–423. doi: 10.1159/000500595 [DOI] [PubMed] [Google Scholar]
- 8.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. May 2011;35(5):633–46. doi: 10.1097/PAS.0b013e31821534b9 [DOI] [PubMed] [Google Scholar]
- 9.Regauer S, Reich O, Kashofer K. HPV-negative Squamous Cell Carcinomas of the Cervix With Special Focus on Intraepithelial Precursor Lesions. Am J Surg Pathol. Feb 1 2022;46(2):147–158. doi: 10.1097/pas.0000000000001778 [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Carunchio L, Soveral I, Steenbergen RD, et al. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. Bjog. Jan 2015;122(1):119–27. doi: 10.1111/1471-0528.13071 [DOI] [PubMed] [Google Scholar]
- 11.Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am J Surg Pathol. Feb 2018;42(2):214–226. doi: 10.1097/pas.0000000000000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgson A, Park KJ, Djordjevic B, et al. International Endocervical Adenocarcinoma Criteria and Classification: Validation and Interobserver Reproducibility. Am J Surg Pathol. Jan 2019;43(1):75–83. doi: 10.1097/pas.0000000000001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren H, Almadani N, Pors J, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): An Independent Cohort With Clinical and Molecular Findings. Int J Gynecol Pathol. Nov 1 2021;40(6):533–540. doi: 10.1097/pgp.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 14.Stolnicu S, Hoang L, Chiu D, et al. Clinical Outcomes of HPV-associated and Unassociated Endocervical Adenocarcinomas Categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am J Surg Pathol. Apr 2019;43(4):466–474. doi: 10.1097/pas.0000000000001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolás I, Marimon L, Barnadas E, et al. HPV-negative tumors of the uterine cervix. Mod Pathol. Jul 2019;32(8):1189–1196. doi: 10.1038/s41379-019-0249-1 [DOI] [PubMed] [Google Scholar]
- 16.Pilch H, Günzel S, Schäffer U, et al. The presence of HPV DNA in cervical cancer: correlation with clinico-pathologic parameters and prognostic significance: 10 years experience at the Department of Obstetrics and Gynecology of the Mainz University. Int J Gynecol Cancer. Jan-Feb 2001;11(1):39–48. doi: 10.1046/j.1525-1438.2001.011001039.x [DOI] [PubMed] [Google Scholar]
- 17.Turco LC, Pedone Anchora L, Fedele C, et al. Human papillomavirus independent status on pathologic response and outcomes in locally advanced cervical cancer managed with chemoradiotherapy followed by surgery. Int J Gynecol Cancer. Apr 3 2023;33(4):489–497. doi: 10.1136/ijgc-2022-003940 [DOI] [PubMed] [Google Scholar]
- 18.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. Jan 13 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jesus M, Tang W, Sadjadi M, Belmonte AH, Poon TP. Carcinoma of the cervix with extensive endometrial and myometrial involvement. Gynecol Oncol. Feb 1990;36(2):263–70. doi: 10.1016/0090-8258(90)90185-n [DOI] [PubMed] [Google Scholar]
- 20.Guimerà N, Lloveras B, Lindeman J, et al. The occasional role of low-risk human papillomaviruses 6, 11, 42, 44, and 70 in anogenital carcinoma defined by laser capture microdissection/PCR methodology: results from a global study. Am J Surg Pathol. Sep 2013;37(9):1299–310. doi: 10.1097/PAS.0b013e31828b6be4 [DOI] [PubMed] [Google Scholar]
- 21.Integrated genomic and molecular characterization of cervical cancer. Nature. Mar 16 2017;543(7645):378–384. doi: 10.1038/nature21386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellsagué X, Muñoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;(31):20–8. [PubMed] [Google Scholar]
- 23.Deacon JM, Evans CD, Yule R, et al. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: a case-control study nested within the Manchester cohort. Br J Cancer. Dec 2000;83(11):1565–72. doi: 10.1054/bjoc.2000.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. Oct 29 1992;327(18):1272–8. doi: 10.1056/nejm199210293271804 [DOI] [PubMed] [Google Scholar]
- 25.Stolnicu S, Soslow RA. Squamous and Glandular Epithelial Tumors of the Cervix: A Pragmatical Review Emphasizing Emerging Issues in Classification, Diagnosis, and Staging. Surg Pathol Clin. Jun 2022;15(2):369–388. doi: 10.1016/j.path.2022.02.010 [DOI] [PubMed] [Google Scholar]
- 26.Park KJ, Selinger CI, Alvarado-Cabrero I, et al. Dataset for the Reporting of Carcinoma of the Cervix: Recommendations From the International Collaboration on Cancer Reporting (ICCR). Int J Gynecol Pathol. Nov 1 2022;41(Suppl 1):S64–s89. doi: 10.1097/pgp.0000000000000909 [DOI] [PubMed] [Google Scholar]
- 27.Alvarado-Cabrero I, Roma AA, Park KJ, Rutgers JKL, Silva EG. Factors Predicting Pelvic Lymph Node Metastasis, Relapse, and Disease Outcome in Pattern C Endocervical Adenocarcinomas. Int J Gynecol Pathol. Sep 2017;36(5):476–485. doi: 10.1097/pgp.0000000000000357 [DOI] [PubMed] [Google Scholar]
- 28.Bosse T, Peters EE, Creutzberg CL, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer--A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer. Sep 2015;51(13):1742–50. doi: 10.1016/j.ejca.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 29.Stoler MH. The pathology of cervical neoplasia. Cervical Cancer: From Etiology to Prevention. Kluwer Academic Publishers; 2004:3–59. [Google Scholar]
- 30.Kurman R, Ronnett B, Sherman M, Wilkinson E. Tumors of the cervix, vagina and vulva Armed Forces Institute of Pathlogy Atlas of Tumor Pathology. 2010;(4):114. [Google Scholar]
- 31.Reagan JW, Hamonic MJ, Wentz WB. Analytical study of the cells in cervical squamous-cell cancer. Lab Invest. May-Jun 1957;6(3):241–50. [PubMed] [Google Scholar]
- 32.Grayson W, Cooper K. A reappraisal of “basaloid carcinoma” of the cervix, and the differential diagnosis of basaloid cervical neoplasms. Adv Anat Pathol. Sep 2002;9(5):290–300. doi: 10.1097/00125480-200209000-00003 [DOI] [PubMed] [Google Scholar]
- 33.Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. Feb 1993;17(2):133–45. doi: 10.1097/00000478-199302000-00005 [DOI] [PubMed] [Google Scholar]
- 34.Al-Nafussi AI, Al-Yusif R. Papillary squamotransitional cell carcinoma of the uterine cervix: an advanced stage disease despite superficial location: report of two cases and review of the literature. Eur J Gynaecol Oncol. 1998;19(5):455–7. [PubMed] [Google Scholar]
- 35.Brinck U, Jakob C, Bau O, Füzesi L. Papillary squamous cell carcinoma of the uterine cervix: report of three cases and a review of its classification. Int J Gynecol Pathol. Jul 2000;19(3):231–5. doi: 10.1097/00004347-200007000-00006 [DOI] [PubMed] [Google Scholar]
- 36.Mirhashemi R, Ganjei-Azar P, Nadji M, Lambrou N, Atamdede F, Averette HE. Papillary squamous cell carcinoma of the uterine cervix: an immunophenotypic appraisal of 12 cases. Gynecologic oncology. 2003;90(3):657–661. [DOI] [PubMed] [Google Scholar]
- 37.Randall ME, Andersen WA, Mills SE, Kim J-AC. Papillary squamous cell carcinoma of the uterine cervix: a clinicopathologic study of nine cases. International journal of gynecological pathology. 1986;5(1):1–10. [DOI] [PubMed] [Google Scholar]
- 38.Albores-Saavedra J, Young RH. Transitional cell neoplasms (carcinomas and inverted papillomas) of the uterine cervix. A report of five cases. Am J Surg Pathol. Oct 1995;19(10):1138–45. [PubMed] [Google Scholar]
- 39.Koenig C, Turnicky RP, Kankam CF, Tavassoli FA. Papillary squamotransitional cell carcinoma of the cervix: a report of 32 cases. Am J Surg Pathol. Aug 1997;21(8):915–21. doi: 10.1097/00000478-199708000-00005 [DOI] [PubMed] [Google Scholar]
- 40.Hasumi K, Sugano H, Sakamoto G, Masubuchi K, Kubo H. Circumscribed carcinoma of the uterine cervix, with marked lymphocytic infiltration. Cancer. Jun 1977;39(6):2503–7. doi: [DOI] [PubMed] [Google Scholar]
- 41.Mills SE, Austin MB, Randall ME. Lymphoepithelioma-like carcinoma of the uterine cervix. A distinctive, undifferentiated carcinoma with inflammatory stroma. Am J Surg Pathol. Dec 1985;9(12):883–9. doi: 10.1097/00000478-198512000-00004 [DOI] [PubMed] [Google Scholar]
- 42.Degefu S, O’Quinn AG, Lacey CG, Merkel M, Barnard DE. Verrucous carcinoma of the cervix: a report of two cases and literature review. Gynecol Oncol. Sep 1986;25(1):37–47. doi: 10.1016/0090-8258(86)90062-4 [DOI] [PubMed] [Google Scholar]
- 43.Kraus FT, Perezmesa C. Verrucous carcinoma. Clinical and pathologic study of 105 cases involving oral cavity, larynx and genitalia. Cancer. Jan 1966;19(1):26–38. doi: [DOI] [PubMed] [Google Scholar]
- 44.Masoudi H, Van Niekerk DJ, Gilks CB, et al. Loss of p16 INK4 expression in invasive squamous cell carcinoma of the uterine cervix is an adverse prognostic marker. Histopathology. Nov 2006;49(5):542–5. doi: 10.1111/j.1365-2559.2006.02510.x [DOI] [PubMed] [Google Scholar]
- 45.Nuovo GJ, Plaia TW, Belinsky SA, Baylin SB, Herman JG. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc Natl Acad Sci U S A. Oct 26 1999;96(22):12754–9. doi: 10.1073/pnas.96.22.12754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poetsch M, Hemmerich M, Kakies C, et al. Alterations in the tumor suppressor gene p16(INK4A) are associated with aggressive behavior of penile carcinomas. Virchows Arch. Feb 2011;458(2):221–9. doi: 10.1007/s00428-010-1007-4 [DOI] [PubMed] [Google Scholar]
- 47.Tessier-Cloutier B, Kortekaas KE, Thompson E, et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod Pathol. Aug 2020;33(8):1595–1605. doi: 10.1038/s41379-020-0524-1 [DOI] [PubMed] [Google Scholar]
- 48.Baggish MS, Woodruff JD. Adenoid-basal carcinoma of the cervix. Obstet Gynecol. Aug 1966;28(2):213–8. doi: 10.1097/00003081-196608000-00013 [DOI] [PubMed] [Google Scholar]
- 49.Ferry JA, Scully RE. “Adenoid cystic” carcinoma and adenoid basal carcinoma of the uterine cervix. A study of 28 cases. Am J Surg Pathol. Feb 1988;12(2):134–44. doi: 10.1097/00000478-198802000-00007 [DOI] [PubMed] [Google Scholar]
- 50.Parwani AV, Smith Sehdev AE, Kurman RJ, Ronnett BM. Cervical adenoid basal tumors comprised of adenoid basal epithelioma associated with various types of invasive carcinoma: clinicopathologic features, human papillomavirus DNA detection, and P16 expression. Hum Pathol. Jan 2005;36(1):82–90. doi: 10.1016/j.humpath.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 51.van Dinh T, Woodruff JD. Adenoid cystic and adenoid basal carcinomas of the cervix. Obstet Gynecol. May 1985;65(5):705–9. [PubMed] [Google Scholar]
- 52.Stewart CJR, Moses J. NKX3.1 expression in cervical ‘adenoid basal cell carcinoma’: another gynaecological lesion with prostatic differentiation? Pathology. Feb 2021;53(2):193–198. doi: 10.1016/j.pathol.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 53.Xing D, Bakhsh S, Melnyk N, et al. Frequent NFIB-associated Gene Rearrangement in Adenoid Cystic Carcinoma of the Vulva. Int J Gynecol Pathol. May 2017;36(3):289–293. doi: 10.1097/pgp.0000000000000324 [DOI] [PubMed] [Google Scholar]
- 54.Diaz De Vivar A, Roma AA, Park KJ, et al. Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol. Nov 2013;32(6):592–601. doi: 10.1097/PGP.0b013e31829952c6 [DOI] [PubMed] [Google Scholar]
- 55.Alvarado-Cabrero I, McCluggage WG, Estevez-Castro R, et al. Micropapillary Cervical Adenocarcinoma: A Clinicopathologic Study of 44 Cases. Am J Surg Pathol. Jun 2019;43(6):802–809. doi: 10.1097/pas.0000000000001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart CJ, Crook ML. Cervical intraepithelial neoplasia (CIN) 3-like squamous cell carcinoma of the cervix: a review of 14 cases with comparison of E-cadherin and cyclin D1 expression in the CIN 3-like and infiltrative tumour elements. Histopathology. Feb 2017;70(3):367–374. doi: 10.1111/his.13094 [DOI] [PubMed] [Google Scholar]
- 57.PB C, RH Y. Atlas of Gynecologic Surgical Pathology. 2 ed. Elsevier; 2008. [Google Scholar]
- 58.Stolnicu S, Hoang L, Zhou Q, et al. Cervical Adenosquamous Carcinoma: Detailed Analysis of Morphology, Immunohistochemical Profile, and Outcome in 59 Cases. Int J Gynecol Pathol. Aug 31 2022;doi: 10.1097/pgp.0000000000000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lastra RR, Park KJ, Schoolmeester JK. Invasive Stratified Mucin-producing Carcinoma and Stratified Mucin-producing Intraepithelial Lesion (SMILE): 15 Cases Presenting a Spectrum of Cervical Neoplasia With Description of a Distinctive Variant of Invasive Adenocarcinoma. Am J Surg Pathol. Feb 2016;40(2):262–9. doi: 10.1097/pas.0000000000000543 [DOI] [PubMed] [Google Scholar]
- 60.Hellweg G [The muco-epidermoid carcinomas of the uterus]. Geburtshilfe Frauenheilkd. Oct 1957;17(10):963–71. Uber mukoepidermoide Karzinome des Uterus. [PubMed] [Google Scholar]
- 61.Lennerz JK, Perry A, Mills JC, Huettner PC, Pfeifer JD. Mucoepidermoid carcinoma of the cervix: another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion. Am J Surg Pathol. Jun 2009;33(6):835–43. doi: 10.1097/PAS.0b013e318190cf5b [DOI] [PubMed] [Google Scholar]
- 62.Chiang S, Cotzia P, Hyman DM, et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype With Features of Fibrosarcoma. Am J Surg Pathol. Jun 2018;42(6):791–798. doi: 10.1097/pas.0000000000001055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown J, Broaddus R, Koeller M, Burke TW, Gershenson DM, Bodurka DC. Sarcomatoid carcinoma of the cervix. Gynecol Oncol. Jul 2003;90(1):23–8. doi: 10.1016/s0090-8258(03)00200-2 [DOI] [PubMed] [Google Scholar]
- 64.Pang LC. Sarcomatoid squamous cell carcinoma of the uterine cervix with osteoclast-like giant cells: report of two cases. Int J Gynecol Pathol. Apr 1998;17(2):174–7. doi: 10.1097/00004347-199804000-00014 [DOI] [PubMed] [Google Scholar]
- 65.Steeper TA, Piscioli F, Rosai J. Squamous cell carcinoma with sarcoma-like stroma of the female genital tract. Clinicopathologic study of four cases. Cancer. Sep 1 1983;52(5):890–8. doi: [DOI] [PubMed] [Google Scholar]
- 66.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. Jun 26 2012;109(26):10516–21. doi: 10.1073/pnas.1202684109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang EJ, Quick MC, Hanamornroongruang S, et al. Microanatomy of the cervical and anorectal squamocolumnar junctions: a proposed model for anatomical differences in HPV-related cancer risk. Mod Pathol. Jul 2015;28(7):994–1000. doi: 10.1038/modpathol.2015.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. Oct 2012;136(10):1266–97. doi: 10.5858/arpa.LGT200570 [DOI] [PubMed] [Google Scholar]
- 69.Park K, Ellenson LH, Pirog EC. Low-grade squamous intraepithelial lesions of the cervix with marked cytological atypia-clinical follow-up and human papillomavirus genotyping. Int J Gynecol Pathol. Oct 2007;26(4):457–62. doi: 10.1097/pgp.0b013e31802f64ab [DOI] [PubMed] [Google Scholar]
- 70.Cho NH, Joo HJ, Ahn HJ, Jung WH, Lee KG. Detection of human papillomavirus in warty carcinoma of the uterine cervix: comparison of immunohistochemistry, in situ hybridization and in situ polymerase chain reaction methods. Pathol Res Pract. 1998;194(10):713–20. doi: 10.1016/s0344-0338(98)80131-3 [DOI] [PubMed] [Google Scholar]
- 71.Duggan MA, Akbari M, Magliocco AM. Atypical immature cervical metaplasia: immunoprofiling and longitudinal outcome. Hum Pathol. Nov 2006;37(11):1473–81. doi: 10.1016/j.humpath.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 72.Iaconis L, Hyjek E, Ellenson LH, Pirog EC. p16 and Ki-67 immunostaining in atypical immature squamous metaplasia of the uterine cervix: correlation with human papillomavirus detection. Arch Pathol Lab Med. Sep 2007;131(9):1343–9. doi: 10.5858/2007-131-1343-pakiia [DOI] [PubMed] [Google Scholar]
- 73.Ma L, Fisk JM, Zhang RR, Ulukus EC, Crum CP, Zheng W. Eosinophilic dysplasia of the cervix: a newly recognized variant of cervical squamous intraepithelial neoplasia. Am J Surg Pathol. Nov 2004;28(11):1474–84. doi: 10.1097/01.pas.0000141407.10204.c5 [DOI] [PubMed] [Google Scholar]
- 74.Miyatake T, Ueda Y, Yoshino K, et al. Clonality analysis and human papillomavirus infection in squamous metaplasia and atypical immature metaplasia of uterine cervix: is atypical immature metaplasia a precursor to cervical intraepithelial neoplasia 3? Int J Gynecol Pathol. Apr 2007;26(2):180–7. doi: 10.1097/01.pgp.0000235068.16054.39 [DOI] [PubMed] [Google Scholar]
- 75.Regauer S, Reich O, Kashofer K. Thin variant of high-grade squamous intraepithelial lesion - relationship with high-risk and possibly carcinogenic human papilloma virus subtypes and somatic cancer gene mutations. Histopathology. Sep 2019;75(3):405–412. doi: 10.1111/his.13869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reich O, Regauer S. Thin HSIL of the Cervix: Detecting a Variant of High-grade Squamous Intraepithelial Lesions With a p16INK4a Antibody. Int J Gynecol Pathol. Jan 2017;36(1):71–75. doi: 10.1097/pgp.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 77.Park JJ, Sun D, Quade BJ, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol. Oct 2000;24(10):1414–9. doi: 10.1097/00000478-200010000-00012 [DOI] [PubMed] [Google Scholar]
- 78.Reyes MC, Park KJ, Lin O, et al. Urothelial carcinoma involving the gynecologic tract: a morphologic and immunohistochemical study of 6 cases. Am J Surg Pathol. Jul 2012;36(7):1058–65. doi: 10.1097/PAS.0b013e318251eade [DOI] [PubMed] [Google Scholar]
- 79.Broders AC. Squamous-cell epithelioma of the lip: a study of five hundred and thirty-seven cases. Journal of the American Medical Association. 1920;74(10):656–664. [Google Scholar]
- 80.Jesinghaus M, Strehl J, Boxberg M, et al. Introducing a novel highly prognostic grading scheme based on tumour budding and cell nest size for squamous cell carcinoma of the uterine cervix. J Pathol Clin Res. Apr 2018;4(2):93–102. doi: 10.1002/cjp2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morrison C, Catania F, Wakely P Jr., Nuovo GJ. Highly differentiated keratinizing squamous cell cancer of the cervix: a rare, locally aggressive tumor not associated with human papillomavirus or squamous intraepithelial lesions. Am J Surg Pathol. Oct 2001;25(10):1310–5. doi: 10.1097/00000478-200110000-00013 [DOI] [PubMed] [Google Scholar]
- 82.van Driel WJ, Hogendoorn PC, Jansen FW, Zwinderman AH, Trimbos JB, Fleuren GJ. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol. Sep 1996;27(9):904–11. doi: 10.1016/s0046-8177(96)90216-6 [DOI] [PubMed] [Google Scholar]
- 83.Xie F, Liu LB, Shang WQ, et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. Aug 10 2015;364(2):106–17. doi: 10.1016/j.canlet.2015.04.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


