Abstract
On October 21, 2022, the FDA approved tremelimumab (Imjudo) in combination with durvalumab for adult patients with unresectable hepatocellular carcinoma (uHC). The approval was based on the results from the HIMALAYA study, in which patients with uHC who were naïve to previous systemic treatment were randomized to receive one of three study arms: tremelimumab in combination with durvalumab (n=393), durvalumab (n=389), or sorafenib (n=389). The primary objective of improvement in overall survival (OS) for tremelimumab in combination with durvalumab compared to sorafenib met statistical significance with a stratified hazard ratio (HR) of 0.78 (95% confidence interval [CI], 0.66, 0.92; P=0.0035). The median OS was 16.4 months (95% CI, 14.2 to 19.6) with tremelimumab in combination with durvalumab and 13.8 months (95% CI, 12.3 to 16.1) with sorafenib. Adverse reactions occurring in ≥20% of patients receiving tremelimumab in combination with durvalumab were rash, fatigue, diarrhea, pruritus, musculoskeletal pain, and abdominal pain. The recommended tremelimumab dose for patients weighing 30 kg or more is 300 mg intravenous (IV) as a single dose in combination with durvalumab 1500 mg at Cycle 1/Day 1, followed by durvalumab 1500 mg IV every 4 weeks. For those weighing less than 30 kg, the recommended tremelimumab dose is 4 mg/kg IV as a single dose in combination with durvalumab 20 mg/kg IV, followed by durvalumab 20 mg/kg IV every 4 weeks.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and the third leading cause of cancer deaths worldwide (1). In the United States, there are approximately 30,000 deaths a year due to liver cancer (2). At diagnosis, most patients have cancer spread to regional lymph nodes or distant metastasis, corresponding to a 5-year relative survival of 12% and 3%, respectively (3).
Tremelimumab (Imjudo, AstraZeneca) is human IgG2 monoclonal antibody (mAb) directed against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), leading to tumor-directed T-cell activation and expansion. Durvalumab (Imfinzi, AstraZeneca) is a human IgG1 mAb that interferes with the interaction of programmed death-ligand 1 (PD-L1) with its receptor, PD-1. Durvalumab is approved for various indications and settings in the treatment of lung cancer and biliary tract cancer.
Treatment with inhibition of angiogenesis with vascular endothelial growth factor (VEGF)-targeting tyrosine kinases such as sorafenib or lenvatinib and most recently the combination of atezolizumab (PD-L1 inhibitor) plus bevacizumab (mAb against VEGF) represents the standard of care in the first-line advanced or metastatic setting. CTLA-4 and PD-1 inhibit antitumor immunity through complementary and nonredundant mechanisms, suggesting dual blockade may have additive or synergistic antitumor responses (4).
Herein, we provide a summary of the FDA’s review of the marketing application that led to the approval of tremelimumab in combination with durvalumab for the treatment of unresectable or metastatic HCC.
Clinical Trial Design
AstraZeneca submitted the results of the HIMALAYA trial (NCT03298451) along with a safety database comprising patients who were exposed to tremelimumab plus durvalumab in various studies, to support the request for approval; the results of the HIMALAYA trial have been published (5).
HIMALAYA was a randomized, open-label, multicenter, global, phase 3 trial to evaluate the efficacy and safety of tremelimumab in combination with durvalumab compared to sorafenib in patients with uHCC who were not eligible for locoregional therapy and who had not previously received systemic therapy. Eligible patients had measurable disease, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, Child-Pugh A and Barcelona Clinic Liver Cancer (BCLC) stage B or C disease, and adequate hematologic and organ function. Patients with hepatitis B and C coinfection, history of hepatic encephalopathy, active or prior documented gastrointestinal bleeding within 12 months of study enrollment, main portal vein thrombosis on baseline imaging and ascites requiring non-pharmacologic intervention were ineligible. Randomization was stratified according to macrovascular invasion (yes or no), etiology of liver disease (hepatitis B or C or other/nonviral), and ECOG PS (0 or 1).
Patients were randomly assigned to receive one of three regimens: a single dose of tremelimumab 300 mg in combination with durvalumab 1500 mg followed by durvalumab 1500 mg every 4 weeks, durvalumab 1500 mg every 4 weeks monotherapy or sorafenib 400 mg twice daily. Only the results of the combination arm, however, were used to support the approval. Treatment continued until progression, unacceptable toxicity, consent withdrawal, or other discontinuation criteria were met. Patients could continue treatment after progression if the investigator assessed that they were benefiting from treatment and met prespecified criteria for continuation after progressive disease. Disease response and progression were measured at baseline and every 8 weeks until week 48, and every 12 weeks thereafter. The primary endpoint was OS (tremelimumab plus durvalumab vs sorafenib). Key secondary endpoints were OS for durvalumab monotherapy vs sorafenib for non-inferiority and OS for durvalumab monotherapy vs sorafenib for superiority. Additional secondary endpoints included OS rates (at 18, 24 and 36 months), progression-free survival (PFS), objective response rate (ORR) and duration of response (DOR) per investigator assessment using RECIST v1.1.
Efficacy Results
Efficacy data were analyzed in the intent-to-treat population (ITT), defined as all patients randomly assigned to tremelimumab plus durvalumab (n=393) and sorafenib (n=389). Baseline demographics and disease characteristics for patients were balanced (Table 1). The study enrolled 17 patients (2%) across both arms who identified as Black or African American.
Table 1.
Himalaya Trial: Baseline demographics and disease characteristics
| n (%) | Tremelimumab plus Durvalumab N=393 |
Sorafenib N=389 |
|---|---|---|
| Median age, years | 65.0 | 64.0 |
| Sex | ||
| Female | 327 (83.2) | 337 (86.6) |
| Male | 66 (16.8) | 52 (13.4) |
| Geographic region | ||
| Asia (excluding Japan) | 156 (39.7) | 156 (40.1) |
| Rest of World (plus Japan) | 237 (60.3) | 233 (59.9) |
| Race | ||
| White | 182 (46.3) | 179 (46.0) |
| Black or African American | 7 (1.8) | 10 (2.6) |
| Asian | 195 (49.6) | 189 (48.6) |
| Other | 8 (2.0) | 5 (1.3) |
| Ethnicity | ||
| Hispanic or Latino | 21 (5.3) | 21 (5.4) |
| Not Hispanic or Latino | 372 (94.7) | 362 (93.1) |
| ECOG | ||
| 0 | 246 (62.6) | 239 (61.4) |
| 1 | 147 (37.4) | 148 (38.0) |
| BCLC stage a | ||
| Early (A) | NA | NA |
| Intermediate (B) | 77 (19.6) | 66 (17.0) |
| Advanced (C) | 316 (80.4) | 323 (83.0) |
| Etiology of liver disease | ||
| HBV-positive | 122 (31.0) | 119 (30.6) |
| HCV-positive | 110 (28.0) | 104 (26.7) |
| Others | 161 (41.0) | 166 (42.7) |
| Child-Pugh Score | ||
| A/5 | 295 (75.1) | 277 (71.2) |
| A/6 | 92 (23.4) | 102 (26.2) |
| B/7 | 2 (0.5) | 10 (2.6) |
| ALBI score | ||
| 1 | 217 (55.2) | 203 (52.2) |
| 2 | 174 (44.3) | 185 (47.6) |
| 3 | 1 (0.3) | 1 (0.3) |
| Missing | 1 (0.3) | 0 |
In HIMALAYA, patients were enrolled only if they had BCLC Stage B (not eligible for locoregional therapy) or Stage C.
Source: U.S. Food and Drug Administration. BLA Multidisciplinary Review and Evaluation and Approval Package: IMJUDO (tremelimumab) (9).
At the final OS data cut off (27, August 2021), 555 deaths had occurred across the tremelimumab plus durvalumab and sorafenib arms. Treatment with tremelimumab in combination with durvalumab demonstrated a statistically significant, clinically meaningful, and sustained improvement in OS compared with sorafenib with the stratified HR of 0.78 (95% CI: 0.66, 0.92; stratified log-rank 2-sided p= 0.0035). The Kaplan-Meier (KM) estimates for median OS were 16.4 months (95% CI: 14.2, 19.6) in the T300+D arm and 13.8 months (95% CI: 12.3, 16.1) in the sorafenib arm, an estimated 2.7-month difference in median OS (Figure 1).
Figure 1.
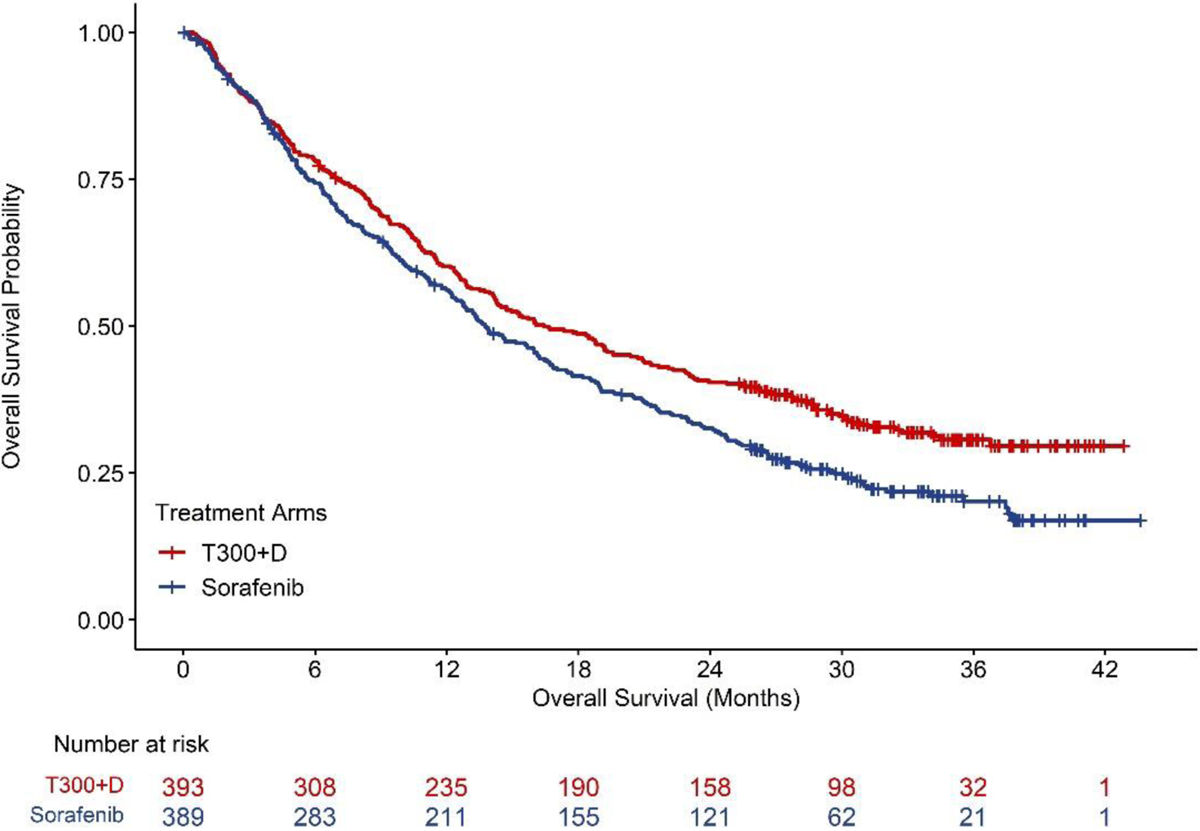
Himalaya Trial: K-M curves of overall survival. Table at the bottom represents the number of patients at risk by treatment arm. Source: U.S. Food and Drug Administration. BLA Multidisciplinary Review and Evaluation and Approval Package: IMJUDO (tremelimumab) (9).
Progression-free survival (PFS) was assessed by investigator assessment according to RECIST 1.1. The stratified HR for PFS was 0.90 (95% CI: 0.77, 1.05). The KM estimate for median PFS was 3.8 months (95% CI: 3.7, 5.3) in the tremelimumab plus durvalumab arm, and 4.1 months (95% CI: 3.7, 5.5) in the sorafenib arm. Efficacy analyses are summarized in Table 2.
Table 2.
Himalaya Trial: Efficacy results
| Endpoint | Tremelimumab plus Durvalumab N=393 |
Sorafenib N=389 |
|
|---|---|---|---|
| OS | |||
| Number of deaths (%) | 262 (66.7) | 293 (75.3) | |
| Median OS (months) (95% CI) | 16.4 (14.2, 19.6) | 13.8 (12.3, 16.1) | |
| HR (95% CI)1 | 0.78 (0.66, 0.92) | ||
| p-value2,3 | 0.0035 | ||
| PFS | |||
| Number of events (%) | 335 (85.2) | 327 (84.1) | |
| Median PFS (months) (95% CI) | 3.8 (3.7, 5.3) | 4.1 (3.7, 5.5) | |
| HR (95% CI)1 | 0.90 (0.77, 1.05) | ||
| ORR | |||
| ORR % (95% CI)4,5 | 20.1 (16.3, 24.4) | 5.1 (3.2, 7.8) | |
| Complete Response n (%) | 12 (3.1) | 0 | |
| Partial Response n (%) | 67 (17.0) | 20 (5.1) | |
| DoR | |||
| Median DOR (months) (95% CI) | 22.3 (13.7, NR) | 18.4 (6.5, 26.0) | |
| % with duration ≥ 6 months | 82.3 | 78.9 | |
| % with duration ≥ 12 months | 65.8 | 63.2 | |
HR (tremelimumab plus durvalumab vs. sorafenib) based on the stratified Cox proportional hazard model.
Based on a stratified log-rank test.
Based on a Lan-DeMets alpha spending function with O’Brien Fleming type boundary and the actual number of events observed, the boundary for declaring statistical significance for IMJUDO and durvalumab vs. sorafenib was 0.0398 (cite Lan and DeMets 1983).
Confirmed complete response or partial response.
Based on Clopper-Pearson method.
CI=Confidence Interval, HR=Hazard Ratio, NR=Not Reached
Source: U.S. product labeling, IMJUDO (tremelimumab) (10)
PFS, ORR, and DOR were not included in the multiple testing procedure (MTP) and no formal statistical comparisons were conducted for these endpoints.
The overall treatment effect was generally consistent across subgroups.
Safety Results
The safety analysis population consisted of patients who received at least one dose of tremelimumab plus durvalumab (n=388) or sorafenib (n=374). Table 3 lists all grade and grade 3–4 adverse events. The most common treatment-emergent adverse events (TEAE, >20%) in the combination arm were rash (32%) diarrhea (27%), fatigue (26%), pruritus (23%), musculoskeletal pain (22%), and abdominal pain (20%) and in the sorafenib arm, TEAEs >20% were rash (57%), diarrhea (45%), fatigue (30%), and abdominal pain (24%). Serious adverse reactions in >1% of patients receiving tremelimumab plus durvalumab included hemorrhage (6%), diarrhea (4%), sepsis (2.1%), pneumonia (2.1%), rash (1.5%), vomiting (1.3%), acute kidney injury (1.3%), and anemia (1.3%).
Table 3.
Himalaya Trial: Safety summary
| Tremelimumab plus Durvalumab N=388 |
Sorafenib N=374 |
|||
|---|---|---|---|---|
| Adverse Reaction | All Grades (%) | Grade 3–4 (%) | All Grades (%) | Grade 3–4 (%) |
| Gastrointestinal disorders | ||||
| Diarrhea | 27 | 6 | 45 | 4.3 |
| Abdominal pain | 20 | 1.8 | 24 | 4 |
| Nausea | 12 | 0 | 14 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Rash | 32 | 2.8 | 57 | 12 |
| Pruritus | 23 | 0 | 6 | 0.3 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 17 | 1.3 | 18 | 0.8 |
| General disorders and administration site conditions | ||||
| Fatigue | 26 | 3.9 | 30 | 6 |
| Pyrexia | 13 | 0.3 | 9 | 0.3 |
| Psychiatric disorders | ||||
| Insomnia | 10 | 0.3 | 4.3 | 0 |
| Endocrine disorders | ||||
| Hypothyroidism | 14 | 0 | 6 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Musculoskeletal pain | 22 | 2.6 | 17 | 0.8 |
Source: U.S. product labeling, IMJUDO (tremelimumab) (10)
Fatal adverse reactions occurred in 8% of patients who received tremelimumab plus durvalumab including death (1%), hemorrhage intracranial (0.5%), cardiac arrest (0.5%), pneumonitis (0.5%), hepatic failure (0.5%), and immune-mediated hepatitis (0.5%).
In the tremelimumab plus durvalumab arm, the most frequently reported immune-mediated adverse events (imAEs) reported by ≥ 2% patients were as follows: hypothyroidism (11%), colitis (6%), dermatitis (4.9%), hyperthyroidism (4.6%) and pancreatitis (2.3%). imAEs leading to discontinuation occurred in 22 (5.7%) patients in the tremelimumab plus durvalumab arm and 6 (1.6%) in the sorafenib arm. Six patients (1.5%) in the tremelimumab plus durvalumab arm died due to an imAE (pneumonitis, 3 hepatic events, myocarditis, and myasthenia gravis).
The median duration of exposure to tremelimumab plus durvalumab was 5.5 months (0.4 to 42.7) and to sorafenib was 4.1 months. Adverse events leading to discontinuation occurred in 53 (13.7%) patients in the tremelimumab plus durvalumab arm and 63 (16.8%) patients receiving sorafenib. The most frequently reported AEs leading to treatment discontinuation in the tremelimumab plus durvalumab arm (reported by ≥1% patients) were hemorrhage (1.8%), diarrhea (1.5%), aspartate aminotransferase increased (1.0%) and hepatitis (1.0%). The most frequently reported AEs leading to treatment discontinuation in the sorafenib arm (reported by ≥1% patients) were rash (3.5%), abdominal pain (1.3%), diarrhea (1.3%), fatigue (1.1%) and hemorrhage (1.1%).
Regulatory Considerations
This is the first FDA approval demonstrating an improvement in survival for combination immunotherapies in HCC and the first approved indication for tremelimumab. The preceding treatment landscape in the first line treatment setting consisted of sorafenib, which was approved in 2007 based on a survival benefit versus placebo followed by lenvatinib in 2018 based on noninferiority of survival and the assessment of PFS when compared with sorafenib. In 2021, atezolizumab plus bevacizumab was approved based on a survival benefit against sorafenib.
The clinical data supporting the FDA’s assessment of efficacy and safety were based primarily on the results of the HIMALAYA trial, which demonstrated a statistically significant and clinically meaningful improvement in OS in patients receiving tremelimumab in combination with durvalumab compared with patients receiving sorafenib.
The FDA review of the application considered that the study originally planned to randomize approximately 1310 patients in a 1:1:1:1 ratio to 4 treatment arms: durvalumab monotherapy, tremelimumab 75 mg for 4 doses plus durvalumab 1500 mg every 4 weeks (T75+D), tremelimumab 300 mg for one dose plus durvalumab 1500 mg Q4W and sorafenib. The applicant closed the T75+D arm after a total of 155 patients were enrolled following availability of the results of an interim analysis of Study 22 (NCT02519348; phase II randomized, open-label, comparative study of durvalumab or tremelimumab, or durvalumab in combination with tremelimumab or bevacizumab in advanced HCC) This analysis of the efficacy in the T75+D arm did not meaningfully differentiate from the durvalumab arm. Patients already randomized and receiving treatment with T75+D could continue assigned study treatment, provided the investigator and patient agreed it was in the best interest of the patient. After closure of the T75+D study arm, the study continued with patients randomized (1:1:1) to durvalumab monotherapy, tremelimumab 300 mg for one dose plus durvalumab 1500 mg Q4W, and sorafenib.
Study 22 served as an independent measure of contribution of components and provided supportive data to demonstrate the treatment effect of the proposed tremelimumab plus durvalumab regimen, in addition to the treatment effect of tremelimumab and durvalumab monotherapy, in the second line uHCC setting. Descriptive survival and response data directionally favored tremelimumab in combination with durvalumab and supported the primary efficacy results from the HIMALAYA trial. However, as Study 22 contained both randomized and non‐randomized patients with only descriptive efficacy analyses, and the study population differed from the proposed indication in this application (i.e., 1L uHCC), no definite conclusion can be made.
Key secondary endpoints included evaluating OS non‐inferiority and superiority for durvalumab monotherapy compared to sorafenib. Results showed that durvalumab monotherapy achieved statistical noninferiority (NI) relative to sorafenib based on the AstraZeneca’s pre‐specified NI margin of 1.08. FDA did not conduct a detailed assessment of the appropriateness of a non‐inferiority claim or study methodology with respect to the NI design and focused on the approvability of the combination regimen. Results also showed that durvalumab monotherapy did not achieve OS superiority relative to sorafenib. This result, along with data from Study 22, provided supportive evidence that both components of the tremelimumab plus durvalumab combination regimen are needed to achieve a statistically significant OS effect compared to sorafenib.
FDA notes that the HIMALAYA trial allowed enrollment of patients with esophageal varices unless there had been active or prior documented gastrointestinal bleeding within 12 months of study enrollment. Esophagogastroduodenoscopy was not mandated or bleeding risk assessment prior to enrollment but adequate endoscopic therapy according to institutional standards was required for patients with a history of esophageal variceal bleeding or those assessed at high risk for esophageal variceal bleeding as determined by the treating physician.
Although HIMALAYA allowed for the enrollment of patients with esophageal varices, it is not known whether the actual population of patients differed substantially from that in the trial that supported the approval of atezolizumab plus bevacizumab and overall, the HIMALAYA trial enrolled patients with relatively conserved liver function and lower bleeding risk compared with the general population of patients with advanced HCC. In addition, only 19 of 782 patients (2%) in HIMALAYA identified as Black or African American. Given that age-adjusted HCC incidence and mortality are higher in Black patients compared with White patients in the United States, increased efforts are needed to enroll a more representative patient population in future clinical trials (6–8).
Conclusions
In summary, the combination of tremelimumab plus durvalumab for the treatment of patients with unresectable HCC has a favorable benefit-risk profile, with a meaningful improvement in survival demonstrated in the HIMALAYA trial. Although there was an increased incidence of adverse events observed on the tremelimumab plus durvalumab arm compared to the sorafenib arm, the overall adverse event profile observed with the combination was consistent with that expected in patients receiving dual‐checkpoint inhibitor therapy with an anti‐PD‐L1 and anti‐CTLA‐4 therapy. FDA’s risk and benefit analysis is shown in Table 4.
Table 4.
FDA benefit: risk analysis.
| Dimension | Evidence and uncertainties | Conclusions and reasons |
|---|---|---|
| Analysis of Condition | HCC and intrahepatic bile duct cancer accounts for 2.2% of all new cancers in the United States and the 5-year relative survival is 12.8% for locoregional disease and 3.1% for metastatic disease. | Unresectable HCC is a serious and life-threatening condition with a poor prognosis. |
| Current treatment options | FDA approved therapies for the first line treatment of unresectable HCC include: atezolizumab plus bevacizumab, sorafenib, and lenvatinib. However, not all patients are eligible to receive bevacizumab and there are significant toxicities associated with tyrosine kinase inhibitors. | There is an unmet medical need for new effective treatments for patients with unresectable HCC |
| Benefit | In the HIMALAYA trial, tremelimumab in combination with durvalumab demonstrated a statistically significant and clinically meaningful improvement in OS compared to sorafenib (stratified HR of 0.78 [95% CI: 0.66, 0.92], stratified log-rank 2-sided p-value =0.0035). | The study met its primary objective with T300+D demonstrating superiority in OS over sorafenib and the statistically significant effect on OS is clinically meaningful. |
| Risk and risk management | The most common adverse reactions (≥20%) were rash, diarrhea, fatigue, pruritus, musculoskeletal pain, and abdominal pain. | The observed safety profile is acceptable when assessed in the context of the treatment of a life-threatening disease. Most of the adverse reactions were manageable with dosage modifications. The risks of severe and serious adverse reactions, such as immune mediated reactions, are adequately addressed in the Warnings and Precautions and Dosage Modifications sections of the product labeling. |
Source: U.S. Food and Drug Administration. BLA Multidisciplinary Review and Evaluation and Approval Package: IMJUDO (tremelimumab) (9).
The combination of tremelimumab plus durvalumab for the first-line treatment of unresectable HCC was approved on October 21, 2022. The applications were reviewed under various programs designed to expedite the review of applications for patients with cancer including the Real-Time Oncology program entailing early receipt of datasets prior to application submission and Assessment Aid (a voluntary review template submission from the applicant to facilitate FDA’s assessment).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors report no financial interests or relationships with the commercial sponsors of any products discussed in this report.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Ward EM, Sherman RL, Henley SJ, Jemal A, Siegel DA, Feuer EJ, et al. Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20–49 Years. J Natl Cancer Inst. 2019;111(12):1279–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer [Available from: https://seer.cancer.gov/statfacts/html/livibd.html.
- 4.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1(8):EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 6.Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and Geographic Disparities in Hepatocellular Carcinoma Outcomes. Am J Prev Med. 2018;55(5 Suppl 1):S40–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry - FDA Draft Guidance [Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations.
- 8.Fashoyin-Aje L, Beaver JA, Pazdur R. Promoting Inclusion of Members of Racial and Ethnic Minority Groups in Cancer Drug Development. JAMA Oncol. 2021;7(10):1445–6. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration, BLA Multidisciplinary Review and Evaluation and Approval Packadge: IMJUDO (tremelimumab), October 2022. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761289Orig1s000MultidisciplineR.pdf.
- 10.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Tremelimumab USPI. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761289Orig1s000lbl.pdf.


