Unstructured Abstract
Acute kidney injury (AKI) is a common complication in cardiac surgery patients, with a reported incidence of 20–30%. The development of AKI is associated with worse short- and long-term mortality, and longer hospital length of stay. The pathogenesis of cardiac surgery-associated AKI is poorly understood but likely involves an interplay between pre-operative comorbidities and peri-operative stressors. AKI is commonly diagnosed by using increases in serum creatinine or decreased urine output and staged using standardized definition such as the Kidney Disease Improving Global Outcomes (KDIGO) classification. Novel biomarkers under investigation may provide earlier detection and better prediction of AKI, enabling mitigating therapies early in the perioperative period. Recent clinical trials of cardiac surgery patients have demonstrated the benefit of goal-directed oxygen delivery, avoidance of hyperthermic perfusion and specific fluid and medication strategies. This review article highlights both advances and limitations regarding the prevention, prediction, and treatment of cardiac surgery-associated AKI.
Summary Statement:
Cardiac surgery-associated AKI occurs in ~20% of patients. Clinical trials identified intraoperative and early postoperative interventions that significantly reduce AKI. Improved patient risk stratification using clinical and biomarker parameters should allow better AKI prevention trials.
Introduction
Approximately 2 million patients undergo cardiac surgery each year,1 with cardiac surgery-associated acute kidney injury (AKI) occurring in an estimated 20–30% of them.1–3 Acute kidney injury is an abrupt decline in kidney function that occurs over hours and sometimes days and is characterized by a rapid increase in serum creatinine, decrease in urine output, or both. Only a small subset (i.e., 2–3%) of patients who develop AKI after cardiac surgery require renal replacement therapy. While cardiac surgeries are done to increase quality of life and survival, development of cardiac surgery-associated AKI is significantly associated with higher hospitalization costs4 and with increased short and long-term postoperative mortality.3,5–7 Several registry and retrospective cohort studies report significant associations between cardiac surgery-associated AKI and later development of chronic kidney disease,8 end-stage renal disease,9 heart failure,10 and major adverse cardiovascular events.11–14 Given the high incidence of cardiac surgery-associated AKI and its potentially devastating sequelae, there is an urgent clinical need to learn how best to prevent and mitigate cardiac surgery-associated AKI.
This review article highlights both advances and limitations in present medical knowledge regarding effective preventions, risk predictions and sub-phenotypes, and treatments of cardiac surgery-associated AKI. This review also touches on some new and exploratory preventive or therapeutic approaches to cardiac surgery-associated AKI that could be assessed in future randomized clinical trials. Finally, we will summarize recommendations outlined in recent guidelines and clinical practice updates15,16 regarding areas of perioperative practice that may prevent or mitigate cardiac-surgery AKI and its sequelae.
Defining Cardiac Surgery-Associated AKI
Accurate prediction of cardiac surgery-associated AKI requires a consistent definition of the AKI outcome that can be compared between studies. Historical definitions used for cardiac surgery-associated AKI have ranged from subtle increases in serum creatinine to the need for new post-operative renal replacement therapy. The Society of Thoracic Surgeons (STS) defines AKI as a 3-fold increase in serum creatinine, creatinine > 4 mg/dL or initiation of dialysis after cardiac surgery. Eventually, clinicians and researchers began to adopt more uniform definitions of AKI, with the creation of the Risk, Injury, Failure, Loss, and End-stage renal failure (RIFLE) and Acute Kidney Injury Network (AKIN) classifications. Both classifications use elevations in serum creatinine over different periods of time (7 days in RIFLE vs. 48 hours in AKIN) and decreases in urine output to categorize AKI into stages. In 2012, the Kidney Disease: Improving Global Outcomes (KDIGO)17 became the newest classification for AKI, integrating components of both RIFLE and AKIN criteria, and including rises in serum creatinine over both 48 hours and 7 days after cardiac surgery. Table 1 reviews the elements of the RIFLE, AKIN and KDIGO criteria. Over the past 10 plus years the KDIGO criteria have become an established standard for defining cardiac surgery-associated AKI both in clinical practice and in clinical research. However, there are some discrepancies in how the KDIGO criteria are used in cardiac surgery studies. Some of these studies diagnosed AKI using serum creatinine increases alone, while others used both the serum creatinine criteria and the KDIGO criteria for oliguria18. These discrepancies exist because some investigators find urine output to be unreliable in the setting of diuretic use.
Table 1.
Diagnostic criteria for RIFLE, AKIN and KDIGO. RIFLE, Risk, Injury, Failure, Loss, and End-stage renal failure. AKIN, Acute Kidney Injury Network. KDIGO, Kidney Disease: Improving Global Outcomes. SCr, Serum creatinine. GFR, Glomerular Filtration Rate. RRT, Renal Replacement Therapy.
| RIFLE | AKIN | KDIGO | ||||||
|---|---|---|---|---|---|---|---|---|
| Class | Creatinine Criteria | Urine Output Criteria | Stage | Creatinine Criteria | Urine Output Criteria | Stage | Creatinine Criteria | Urine Output Criteria |
| Risk | SCr ≥ 1.5 times baseline OR GFR decline ≥ 25% | <0.5 mL/kg/h for ≥6 h | Stage 1 | SCr ≥ 1.5 times baseline or increase by 0.3 mg/dL within 48 hours | <0.5 mL/kg/h for ≥6 h | Stage 1 | SCr increase by 0.3 mg/dL within 48 hours OR SCr ≥ 1.5–1.9 times baseline within 7 days | <0.5 mL/kg/h for ≥6 h |
| Injury | SCr ≥ 2 times baseline OR GFR decline ≥ 50% | <0.5 mL/kg/h for ≥12 h | Stage 2 | SCr ≥ 2 times baseline | <0.5 mL/kg/h for ≥12 h | Stage 2 | SCr ≥ 2.0–2.9 times baseline within 7 days | <0.5 mL/kg/h for ≥12 h |
| Failure | SCr ≥ 3 times baseline or increase by 0.5 mg/dL to ≥ 4 mg/dL OR GFR decline ≥ 75% OR RRT Initiation | <0.3 mL/kg/h for 24 h OR Anuria for 12 h | Stage 3 | SCr ≥ 3 times baseline or increase by 0.5 mg/dL to ≥ 4 mg/dL OR RRT Initiation | <0.3 mL/kg/h for 24 h OR Anuria for 12 h | Stage 3 | SCr ≥ 3 times baseline or increase by 0.5 mg/dL to ≥ 4 mg/dL OR RRT Initiation | <0.3 mL/kg/h for 24 h OR Anuria for 12 h |
| Loss | Loss of renal function for ≥ 4 weeks | |||||||
| End-Stage | Loss of renal function for ≥ 3 months | |||||||
Epidemiology of Cardiac Surgery-Associated AKI
A meta-analysis which included 32 observational studies of cardiac surgery patients published between 2006 and 2014 reported a median pooled incidence for AKI of 22.1%.19 The incidence of Stage 1, 2 and 3 AKI defined by either RIFLE, AKIN or KDIGO criteria among 19 studies which reported these data was 17.9%, 4.4% and 3.5% respectively. Another meta-analysis of 91 observational studies of cardiac surgery patients published between 2004 and 2014 reported a similar pooled incidence rate of AKI of 22.3%3. The pooled AKI rate in this study was lower in patients with coronary artery bypass surgery (CABG) (19.0%) compared to those who underwent valve surgery (27.5%) or aortic surgery (29.0%). A large epidemiological study was used to create the Mehta score reported that type of surgery an independent predictor of dialysis-dependent AKI after cardiac surgery with combined CABG-mitral valve surgery (Odds Ratio (OR) 2.57) and mitral valve surgery (OR 2.01) having the highest risk.20 AKI was also associated with significantly higher length of stay in the intensive care unit (ICU) (5.4 days vs. 2.2 days) and hospital (15.0 days vs. 10.5 days) when compared to patients without AKI.21
AKI-associated in-hospital and long-term (1–5 year) mortality in the latter meta-analysis was 10.7% and 30.0%, respectively.3 Duration of cardiac surgery-associated AKI has been found to predict mortality in a number of published studies. One meta-analysis of 9 observational studies of cardiac surgery patients reported that patients who recovered renal function prior to hospital discharge had a significantly lower long-term mortality risk compared to patients discharged with persistent abnormal renal function.21 However, even patients who recovered their renal function after developing AKI in this study had a significantly higher mortality than those who did not develop AKI. A 90-day follow-up of participants of the RENAL study, which randomized critically ill patients (including cardiac surgery patients) between receiving higher and lower levels of renal replacement therapy dose intensity, reported an overall mortality of 62.3% among all patients with AKI requiring renal replacement.22 This was confirmed in a retrospective study of cardiac surgery patients which reported a mortality of 58.6% in patients requiring new post-operative renal replacement therapy.23
Pathogenesis of Cardiac Surgery-Associated AKI
The pathogenesis of cardiac surgery-associated AKI is complex, resulting from a multitude of potential perioperative insults to the kidney. The commonality of these insults is lower oxygen delivery to the kidneys relative to the kidneys’ oxygen demand, resulting in renal tubular injury. The hypothesized mechanisms that contribute to cardiac surgery-associated AKI include hypoperfusion, atheroembolic events, exposure to nephrotoxins, inflammation, and oxidative stress.24
The kidneys receive 20% of the total cardiac output; however, intrarenal oxygen availability requires an orchestrated balance between supply (i.e. blood flow) and demand, which is determined by basal metabolism and tubule-glomerular functions. For example, the renal glomeruli extract only 10–20% of the oxygen delivered to them and maintain a low oxygen tension at baseline,25 which makes them particularly vulnerable to hypoxia during periods of hypoperfusion. Renal hypoperfusion can occur throughout the perioperative period due to hypotension, decreased cardiac output, sympathetic stimulation, the administration of vasoconstrictive medications, and activation of the renin-angiotensin-aldosterone system. Each of these events can interfere with renal autoregulation and reduce glomerular filtration rate.26
Cardiopulmonary bypass is associated with non-pulsatile flow, altered hemodynamics, decreased oxygen delivery, inflammation, and oxidative stress, each of which can contribute to AKI26. Renal perfusion while on cardiopulmonary bypass is directly proportional to mean arterial pressure (MAP) and pump flow rate, suggesting that normal autoregulatory mechanisms may be impaired during this period.27 Lannemyer et al. found that cardiopulmonary bypass induces renal vasoconstriction and redistribution of blood from the kidneys in the setting of unchanged glomerular filtration rate and renal oxygen consumption.28 This oxygen supply-demand mismatch on may explain why the duration of time29, degree of hemodilution30,31 and hypotension32 on cardiopulmonary bypass have all been linked to post-operative AKI. Atherosclerotic disease is also prevalent in patients undergoing cardiac surgery and elements of the surgical procedure are associated with atheroembolic events, including placement of intra-aortic balloon pumps, manipulation of the left atrium, and both clamping and de-clamping of the aorta. One autopsy-based study found that among patients who died in the hospital after cardiac surgery, 10.4% had atheroembolic findings in the kidneys.33 Rewarming from cardiopulmonary bypass provides a period of time when the kidney is susceptible to hypoxic injury. During this time, oxygen consumption increases with temperature in the renal medulla, and can exceed available supply.34
Perioperative medications associated with nephrotoxicity include antibiotics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, non-steroidal anti-inflammatory drugs (NSAIDs), diuretics and intravascular contrast agents (Table 2). Commonly used antibiotics, including aminoglycosides, beta-lactams and vancomycin, mediate AKI through immune and non-immune mechanisms. Aminoglycosides accumulate in proximal tubular cells and cause apoptosis and acute tubular necrosis.35 Beta-lactam antibiotics can cause a hypersensitivity reaction resulting in acute interstitial nephritis and can also mediate crystal development and obstructive injury to the tubules.35 Vancomycin is thought to cause AKI by promoting free radical formation and oxidative stress resulting in acute tubular necrosis, causing a hypersensitivity reaction resulting in acute interstitial nephritis, and through intratubular crystal obstruction. Among these antibiotics, KDIGO guidelines recommend avoiding aminoglycosides unless a suitable alternative is unavailable.36 Intravascular contrast agents are associated with acute tubular necrosis due to their cytotoxic effect on tubular cells and by mediating renal vasoconstriction. Since this association is based on observational studies, there is controversy regarding the true incidence of contrast-associated AKI and effective interventions in high-risk patients. Even studies utilizing propensity matching to control for non-contrast associated etiologies of AKI reported disparate results with one study finding a higher incidence of contrast-associated AKI in patients with impaired baseline kidney function37 and one study finding no association between contrast exposure and AKI regardless of baseline kidney function.38 Observational studies of cardiac surgical patients suggest that exposure to intravascular iodinated contrast agents less than 7 days before surgery may result a higher incidence of post-operative AKI.39–41 Until data from randomized clinical trials is available, the decision to postpone cardiac surgery after contrast exposure should be based on an individual evaluation of the patient and other risk factors for post-operative AKI.42
Table 2.
Medication Classes associated with Nephrotoxicity. ACEi, Angiotensin Converting Enzyme Inhibitors. ARBs, Angiotensin Receptor Blockers. NSAIDs, Non-steroidal anti-inflammatory drugs.
| Medication | Mechanism for Nephrotoxicity |
|---|---|
| ACEi and ARBs | Functional renal insufficiency (Hypotension) |
| NSAIDS | Reduction in prostaglandin synthesis |
| Antibiotics (Vancomycin, Aminoglycosides and Beta-lactams) | Acute tubular necrosis, acute interstitial nephritis, Crystal-induced acute kidney injury |
| Intravenous contrast agents | Acute tubular necrosis |
| Diuretics | Hypovolemia and hypotension |
Though angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with improved renal blood flow, their pre-operative administration is associated with an increased risk of AKI in patients undergoing cardiac surgery. The mechanism for this injury may be functional renal insufficiency, or hypotension resulting in decreased renal perfusion pressure.43 For this reason, the KDIGO “bundle of care” recommends discontinuing these medications for 48 hours after cardiac surgery.36 Diuretics, including the loop diuretic furosemide and mannitol, have not been found to reduce cardiac surgery-associated AKI and furosemide may actually increase the risk of AKI by inducing hypovolemia in some patients.15
Cardiac surgery patients are also susceptible to pre-renal and post-renal causes of AKI throughout the perioperative period. Aggressive pre-operative diuresis, hemofiltration during cardiopulmonary bypass, hemorrhage and vasoplegia can contribute to an absolute or relative hypovolemia and impaired renal perfusion. Post-renal AKI can result from urinary catheter obstruction (e.g., from blood clots or kinking of the catheter tubing), or due to urinary retention after the catheter is removed in the post-operative period. A thorough review of historical details (e.g., diuretic administration, prostatic hyperplasia), physical examination (e.g., flushing the urinary catheter, bladder ultrasonography) and laboratory tests (e.g., fractional excretion of sodium, fractional excretion of urea) are necessary to distinguish between pre-renal, post-renal and intrinsically renal etiologies of AKI after cardiac surgery.
Predicting Cardiac Surgery-Associated AKI
Clinical and Perioperative Risk Factors
Identifying patients with modifiable risk factors for cardiac surgery-associated AKI may facilitate interventions to mitigate their risk throughout the perioperative period. Numerous papers have assessed clinical and surgical risk factors for cardiac surgery-associated AKI, but, to date, the variables identified for risk prediction are most accurate for predicting that small group of patients who develop Stage 3 cardiac surgery-associated AKI, which is renal failure associated with a three-fold increase in serum creatinine or requiring renal replacement therapy. These models include the Cleveland Clinic score44, the Mehta score20, and the Simplified Renal Index.45 Each of these uses pre-operative risk factors to predict the risk of severe cardiac surgery-associated AKI, which are listed in Table 3. The Cleveland Clinic score has been validated in a cohort of 12,096 to have the best discriminatory value (area under the receiver operating characteristic (AUROC): 0.86) to predict the need for renal replacement therapy after cardiac surgery compared to the Mehta score and the Simplified Renal Index (AUROC: 0.81 and 0.79, respectively).46 Despite this, it has not been widely implemented in clinical practice. Research is now being done to improve prediction of cardiac surgery-associated AKI using biomarkers of renal function and renal injury, as predictive models have integrated clinical variables with these biomarkers47 and incorporated machine learning algorithms48 to improve clinical risk prediction for cardiac surgery-associated AKI. The greatest limitation of these newer machine learning models is their use of data obtained at static intervals to predict an outcome that is impacted by the multimodal dynamic data during the perioperative period. Future models that incorporate these multimodal dynamic data (e.g., perioperative hemodynamics and oxygenation variables, among others) and key biomarkers of kidney health obtained at various time points may be able to better predict all stages of AKI with greater accuracy and within actionable time points to intervene.
Table 3.
Summary of features used in clinical prediction models. US, United States. STS, Society of Thoracic Surgeons. CHF, Congestive Heart Failure. LV, Left Ventricle. EF, Ejection Fraction. IABP, Intra-aortic balloon pump. COPD, Chronic obstructive pulmonary disease. DM, GFR, Glomerular Filtration Rate. NYHA, New York Heart Association.
| Cleveland Clinic Score | Mehta Score | Simplified Renal Index | |||
|---|---|---|---|---|---|
| Derivation Cohort: 15,838 cardiac surgery patients, Single US Center, 1993–2002 | Derivation Cohort: 449,524 cardiac surgery patients, Database of > 600 centers, 2002–2004 | Derivation Cohort Details: 10,751 cardiac surgery patients, Single Canadian Center, 1999–2004 | |||
| Validation Cohort: 17,379 cardiac surgery patients from the same center and time period | Validation Cohort: 86,009 cardiac surgery patients from the same database, 2005 | Validation Cohort: 9,380 cardiac surgery patients, Two Canadian Centers, 1999–2003 | |||
| Variable | Points | Variable | Points | Variable | Points |
| Female Gender | 1 | Age ≥ 55 | 0–10 | Pre-operative GFR | 1–2 |
| CHF | 1 | Nonwhite race | 2 | Diabetes requiring medications | 1 |
| LV EF < 35% | 1 | Pre-operative Serum Creatinine | 5–40 | LV EF ≤ 40% | 1 |
| Pre-operative IABP | 2 | NYHA Class IV Heart Failure | 3 | Previous cardiac surgery | 1 |
| COPD | 1 | Diabetes treated with oral medications | 2 | Pre-operative IABP | 1 |
| Insulin-dependent Diabetes | 1 | Insulin-dependent Diabetes | 5 | Non-elective surgery | 1 |
| Previous cardiac surgery | 1 | COPD | 3 | Type of Surgery | 0–1 |
| Emergency surgery | 2 | Recent Myocardial Infarction | 3 | ||
| Type of Surgery | 0–2 | Previous cardiac surgery | 3 | ||
| Pre-operative Serum Creatinine | 0–5 | Cardiogenic shock | 7 | ||
| Type of Surgery | 0–7 | ||||
| Score Range | 0–17 | Score Range | 0–85 | Score Range | 0–8 |
In terms of identifying demographic and preoperative clinical characteristics that predict overall AKI after cardiac surgery, multiple parameters have been identified from observational studies, although not always consistently. Preoperative risk factors frequently identified include advanced age49,50, female sex49, higher body mass index,49,51, proteinuria52 and the presence of systemic comorbidities including hypertension49, diabetes49,50,53, chronic kidney disease42,49,50,53, chronic obstructive pulmonary disease49, left ventricular dysfunction49,53 and perioperative anemia49. Patients with chronic kidney disease have lower kidney reserve, hence, during cardiac surgery and cardiopulmonary bypass the kidney can be overwhelmed by surgical stressors, inflammation, and oxidative stress, resulting in further decline in glomerular filtration rate. Husain-Syed and colleagues studied patients who underwent CABG surgery at a single center and calculated their pre-operative renal functional reserve using a high oral protein load. The authors compared patients who had an in-hospital postoperative increase in serum creatinine and returned to baseline to a group who did not experience a rise in postoperative creatinine. The patients who experienced a transient increase in postoperative serum creatinine that returned to preoperative baseline concentration were subsequently found to have significantly lower renal functional reserve when compared to patients who did not have any rise in serum creatinine after surgery.54 This illustrates that AKI leaves patients with reduced long-term function and less renal functional margin in the setting of additional subsequent AKI events. Hypertension and diabetes are highly prevalent in patients undergoing cardiac surgery. Chronic hypertension can lead to autoregulatory vasoconstriction of the preglomerular vasculature, which can impair autoregulatory mechanisms and make the kidneys vulnerable to injury when the perfusion pressure is inadequate.55 Diabetes is associated with vasculopathy and nephropathy resulting from tissue hypoxia, inflammation, endothelial damage, and other mechanisms. Proteinuria is a marker of tubulointerstitial damage and the resorption of filtered protein in the proximal tubules is associated with upregulation of inflammatory mediators. One study found that pre-operative proteinuria was an independent predictor of cardiac surgery-associated AKI requiring renal replacement therapy by AKIN criteria.52
Urgent surgery, re-operative surgery and the pre-operative placement of an intra-aortic balloon pump are also associated with cardiac surgery-associated AKI diagnosed using RIFLE criteria. Compared to patients undergoing CABG, those undergoing isolated valve or aortic surgery have a higher incidence of cardiac surgery-associated AKI.49,53
Intra-operative and early postoperative risk factors include cardiopulmonary bypass duration42,49, anemia while on cardiopulmonary bypass, blood transfusion,49 and development of early postoperative proteinuria.56 While prolonged cardiopulmonary bypass time has been associated with cardiac surgery-associated AKI, large trials comparing outcomes after on-pump and off-pump CABG have failed to show a reduction in cardiac surgery-associated AKI among patients who underwent off-pump procedures57,58. Post-operative tissue hypoperfusion defined by elevated serum lactate levels59 and the use of inotropes59,60 have been associated with cardiac surgery-associated AKI. Additionally, re-exploration after surgery is a strong, independent predictor of cardiac surgery-associated AKI49,61.
Blood and Urine Biomarkers
Current diagnostic criteria for cardiac surgery-associated AKI utilize increases in serum creatinine and decreases in urine output. While creatinine provides a good approximation of glomerular filtration rate when kidney function is normal, its accuracy is diminished in non-steady state conditions such as the perioperative period. The increased volume of distribution in post-operative cardiac surgery patients or in other critically ill populations who are exposed to aggressive fluid resuscitation62 can lead to dilution of serum creatinine, delaying the diagnosis and underestimating the degree of kidney injury.63 The inclusion of other AKI-related biomarkers may enable earlier detection and better prediction of cardiac surgery-associated AKI (greater sensitivity and/or specificity) than with clinical and surgical risk factors alone (Figure 2).47 This may allow discovery of mitigating therapies to prevent the incidence and further progression of AKI. While a number of blood and urine biomarkers that have been assessed for prediction of cardiac surgery-associated AKI, many of these perform inconsistently between sub-populations of cardiac surgery patients and only one is approved for clinical use by the U.S. Food and Drug Administration. Additionally, evaluating biomarkers of kidney injury using functional biomarkers such as serum creatinine as clinical endpoints poses a challenge. Biomarker discovery and development is a vast area of research that is beyond the scope of this review, but we include a brief summary of some of the most relevant AKI biomarkers to date. These biomarkers are briefly reviewed in Table 4.
Figure 2.
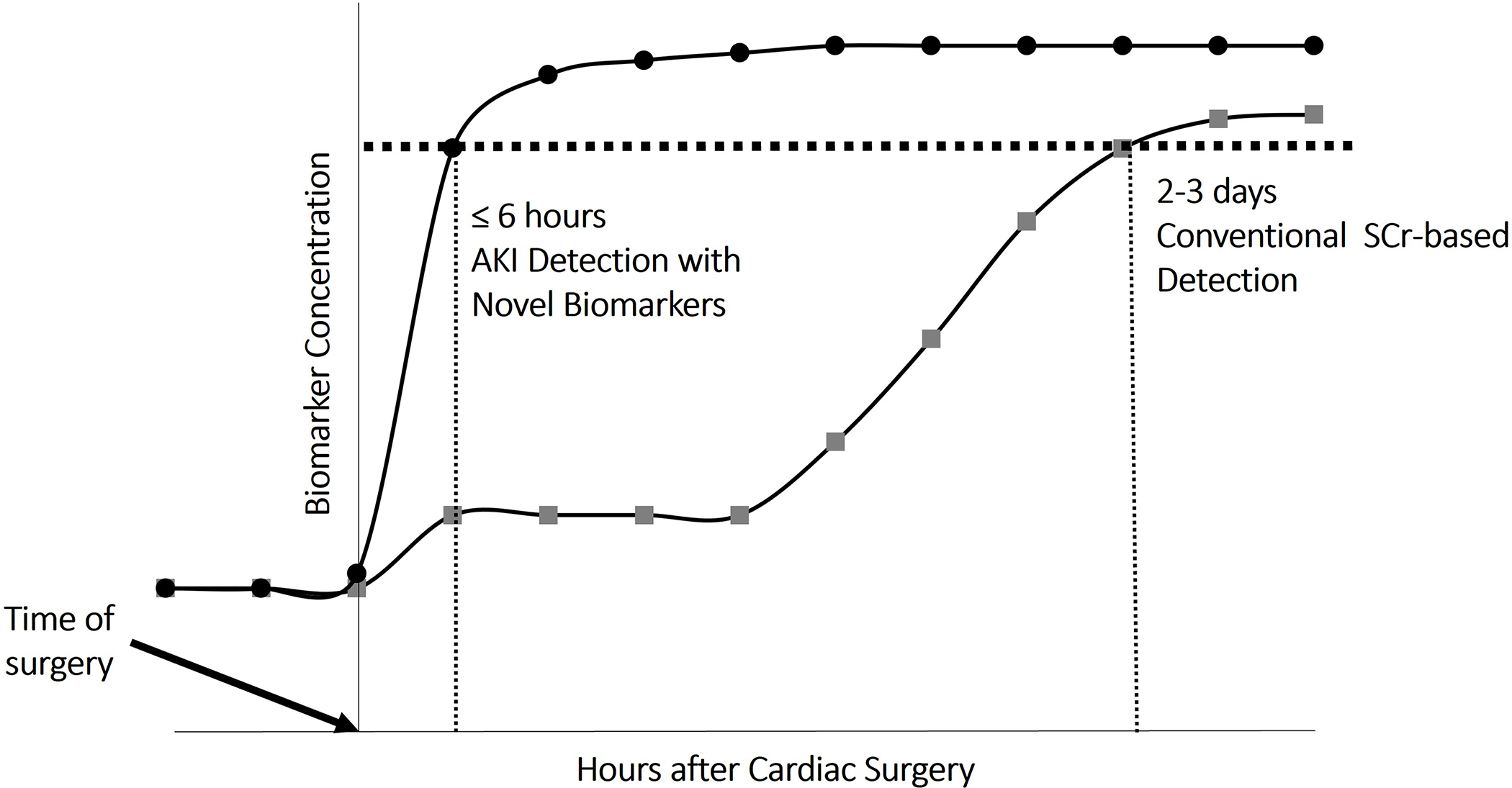
Timeline highlighting the potential for novel biomarkers to identify AKI as compared to serum creatinine-based criteria. AKI, acute kidney injury. SCr, Serum creatinine.
Table 4.
Novel blood and urine biomarkers being investigated for detection of cardiac surgery-associated AKI. NGAL, Neutrophil gelatinase-associated lipocalin. CysC, Cystatin C. IL-18, Interleukin-18. KIM-1, Kidney Injury Molecule-1. TIMP-2, tissue inhibitor of metalloproteinase. IGFBP7, insulin-like growth factor-binding protein. CCL-14, C-C motif chemokine ligand 14. AKI, acute kidney injury. CKD, chronic kidney disease.
| Biomarker | Source | Pathophysiology | Utility in Cardiac Surgery | Limitations |
|---|---|---|---|---|
| NGAL | Blood, Urine | Upregulated in the proximal tubules after ischemic or nephrotoxic injury to the kidneys | Early detection of AKI | More specific in children and adults without CKD. |
| CysC | Blood | Functional biomarker with decreased clearance in AKI | Early detection of AKI Unaffected by differences in muscle mass. | Some studies have indicated that CysC has lower predictive value. |
| IL-18 | Urine | Mediates ischemic and inflammatory kidney injury in the proximal tubules | Early detection of AKI | Some studies have indicated that IL-18 has lower predictive value. |
| KIM-1 | Urine | Rapidly expressed in proximal tubular cells after ischemic kidney injury | Early detection of AKI | Some studies have indicated that it peaks up to 2–3 days after kidney injury. |
| [TIMP-2]*[IGFBP7] | Urine | Induces cell cycle arrest in renal tubular cells | Early detection of AKI Better sensitivity and specificity in predicting AKI. | Some studies have indicated that these biomarkers have lower specificity. |
| CCL-14 | Urine | Mediates inflammatory kidney injury in the proximal tubules | Predicts persistent AKI and the need for RRT and can be used as a marker for progression of AKI to CKD | Does not provide early detection of AKI |
Neutrophil gelatinase-associated lipocalin is part of the innate immune system and functions by binding to bacterial iron-binding proteins. It is sensitive and specific marker of injury to the kidneys and can be detected in the blood and urine after both nephrotoxic and ischemic injuries. While increases in blood and urine neutrophil gelatinase-associated lipocalin concentration have been associated with cardiac surgery-associated AKI in children64, a meta-analysis of observational studies found that it had a much lower predictive value in adults undergoing cardiac surgery (AUC 0.83 in adults vs. 0.89 in children) and those with chronic kidney disease (AUC 0.81 in patients with chronic kidney disease vs. 0.87 in patients without chronic kidney disease).65
Cystatin C is a low molecular weight protein that undergoes complete reabsorption by the renal tubules. Unlike creatinine which is produced by muscle cells and undergoes tubular filtration, cystatin C is produced by all nucleated cells and undergoes glomerular filtration. It has therefore been investigated as a functional biomarker for kidney clearance. A post-hoc analysis of a randomized controlled trial of cardiac surgery patients found that an early post-operative rise in serum cystatin C was associated with AKI requiring renal replacement therapy.66 A meta-analysis evaluating the diagnostic accuracy of cystatin C in predicting AKI in adults in a variety of settings also found that it demonstrated high sensitivity (0.82) and specificity (0.82), with an AUC of 0.89.67
Urinary interleukin-18 is specific to the renal tubules and is thought to mediate ischemic and inflammatory injury to the kidneys. Urinary levels of interleukin-18 were found to be significantly elevated in patients with cardiac surgery-associated AKI, as early as 4–6 hours after cardiopulmonary bypass66. In cardiac surgery patients, one study found that patients with the highest concentration of urinary interleukin-18 (5th quintile) had a nearly 7-fold incidence of post-operative AKI. The addition of interleukin-18 to a clinical prediction model that included perioperative variables improved its receiver-operating characteristic curve (AUC) from 0.69 to 0.76.47
Kidney Injury Molecule-1 is a transmembrane protein with two extracellular domains, which separate from the cell surface and enter the urine after kidney injury.68 The urinary concentration of kidney injury molecule-1 peaks as early as 3 hours after kidney injury, making it an earlier marker of cardiac surgery-associated AKI compared to serum creatinine.69 One study evaluating biomarkers in post-cardiac surgery patients found that the combination of kidney injury molecule-1 and interleukin-18 had excellent predictive value (AUC 0.93) for Stage 3 AKIN and mortality.70
Tissue inhibitor of metalloproteinase and insulin-like growth factor-binding protein are inducers of cell cycle arrest in renal tubular cells, which occurs in the very early phases of cellular injury. Urinary concentrations of these biomarkers were found to have a superior sensitivity and specificity in predicting KDIGO stage 2–3 AKI when compared to other novel biomarkers.71 The product of the urinary concentrations of these biomarkers has been approved by the U.S. Food and Drug Administration for early clinical AKI prediction.
C-C motif chemokine ligand 14 is a chemokine that is released from tubular epithelial cells in response to injury. It mediates the renal inflammatory response that results from AKI by binding to receptors on monocytes and T-cells and promoting chemotaxis in these cells.72 An observational study in cardiac surgery patients found that elevated serum C-C motif chemokine ligand 14 was predictive of KDIGO Stage 3 AKI (AUC 0.93). Since C-C motif chemokine ligand 14 is a biomarker of kidney disease progression, it has also been investigated as a predictor of chronic kidney disease. One study of ICU patients found that urinary C-C motif chemokine ligand 14 levels were associated with renal non-recovery.73
Key Clinical Research for Prevention and Mitigation of Cardiac Surgery-Associated AKI
This section highlights results from some key areas that clinical trials and studies have focused on to date for the purposes of preventing or mitigating cardiac surgery-associated AKI.
Rewarming Temperature on cardiopulmonary bypass:
A study which included two separate randomized controlled trials assigned patients undergoing CABG to rewarming from 32°C to 34°C vs. 32°C to 37°C in one trial (n = 233) and a strategy of mild sustained hypothermia (34°C) vs. sustained normothermia (37°C) without rewarming in the second trial (n=267).74 These two randomized trials found that rewarming from 32°C to 37°C in a 10–15-minute period resulted in an increased incidence of AKI compared to rewarming to 34°C, however, that sustained mild hypothermia did not have a nephroprotective effect. Newland and colleagues analyzed a large multi-center registry of cardiac surgery patients using propensity scoring to adjust for confounders and found that the duration of hyperthermic perfusion (> 37°C) was an independent predictor of increased cardiac surgery-associated AKI.75
Goal Directed Oxygen Delivery (DO2) on Cardiopulmonary Bypass:
Clinical trials have recently sought to determine the impact of a goal directed oxygen delivery while on cardiopulmonary bypass on cardiac surgery-associated AKI. The Goal-Directed Perfusion Trial (GIFT) evaluated maintaining DO2 > 280 ml/min/m2 versus conventional perfusion on the rate of AKI in cardiac surgery patients.76 In this multi-center randomized controlled trial which enrolled 350 patients from 9 European institutions, patients in the intervention group had a lower incidence of AKIN stage 1 AKI while no difference in the incidence of AKIN stage 2 or 3 AKI. A similar study which randomized 300 patients undergoing cardiac surgery between targeting a DO2 > 300 ml/min/m2 versus conventional perfusion while on cardiopulmonary bypass found a higher rate of AKI (defined by KDIGO) in the conventional perfusion group.77 A subgroup analysis of the study found that the goal directed oxygen delivery strategy was superior in patients with lower hematocrits and lower body surface area (BSA). The results of these two trials suggest the need to individualize DO2 while on cardiopulmonary bypass rather than use conventional BSA-derived perfusion targets.
Vasopressors:
Vasoplegia is common in cardiac surgery patients.78 Since endogenous vasopressin is thought to be reduced after cardiac surgery and because vasopressin preferentially binds receptors in the efferent arterioles to potentially increase glomerular filtration rate, vasopressin use should be considered before using norepinephrine to treat post-cardiopulmonary bypass vasoplegia.15 The VANCS trial randomized 300 patients who experienced post-cardiopulmonary bypass vasoplegia to receive vasopressin versus norepinephrine as a first line agent.79 Patients who received vasopressin as a first-line agent had a significantly lower incidence of moderate-severe AKI and a lower mortality.
Perioperative Hypotension:
Cardiac surgery is associated with potentially turbulent hemodynamics related to anesthetics, surgical manipulation of the heart and great vessels, comorbidities including cardiovascular and coronary artery disease, non-pulsatile flow on cardiopulmonary bypass, anemia, vasoplegia and pump failure. Systemic hypotension due to any of these etiologies for a prolonged period can lead to impaired renal perfusion, renal tubular ischemia, and a consequent decrease in glomerular filtration rate. While evidence of an association between intra-operative hypotension and AKI has been established in the non-cardiac surgery population80, there is controversy regarding this association in cardiac surgery patients. Several studies have attempted to evaluate the impact of intraoperative hypotension on cardiac surgery-associated AKI. One observational study of 6523 patients reported an association between a MAP < 65 for 10 minutes or more after cardiopulmonary bypass and the need for new post-operative renal replacement therapy.81 Another study of 4984 patients found an association between each 10 minute period of hypotension throughout the cardiac surgical period and a composite outcome of stroke, AKI or death after cardiac surgery.82 Two randomized controlled trials83,84 found that targeting higher mean arterial pressures on cardiopulmonary bypass did not reduce the incidence of cardiac surgery-associated AKI, while another clinical trial85 showed that in the higher MAP group, there was a significantly larger number of patients who doubled their post-operative creatinine. These findings suggest the need for further investigation on the role of intra-operative blood pressure optimization as a modality to avoid cardiac surgery-associated AKI.
Anemia and Transfusion:
Both anemia86,87 and blood transfusion88 have been linked to cardiac surgery-associated AKI in multiple cohort studies. However, a large retrospective study found that combined exposure to anemia and blood transfusion increased the risk of post-cardiac surgery AKI more than either exposure alone.89 The Transfusion Requirements in Cardiac Surgery (TRICS) III study, a multicenter randomized controlled trial, found no difference in the incidence of new-onset AKI requiring renal replacement therapy in patients who were transfused to a restrictive (7.5 g/dL) versus a liberal transfusion (9.5 g/dL) threshold.90 A prespecified sub-study of this trial also found no difference in the incidence of milder forms of AKI between patients randomized to restrictive vs. liberal transfusion thresholds.91 Avoiding anemia and transfusion during the cardiac surgery perioperative period may necessitate multi-modal pre-operative anemia management with oral iron therapy, erythropoietin administration in patients with iron deficiency anemia and supplementation with vitamin B12 and folate for B12 and folate deficiency anemia, respectively.92
Hemolysis:
Hemolysis occurs frequently in cardiac surgery because of shear stress applied on the red blood cells, which is invoked by blood flowing through the cardiopulmonary bypass circuit, use of venting, and suction devices. Erythrocytes washing and the transfusion of cell-salvaged blood are additional sources of hemolysis products during cardiac surgery.93,94 Injured erythrocytes release cell-free hemoglobin and other heme-derived products, which normally bind to the hemoglobin and heme scavengers, haptoglobin and hemopexin, respectively, and then cleared by splenic and liver macrophages.95 However, if these scavenging systems are overwhelmed during prolonged or excessive hemolysis, plasma levels of cell-free hemoglobin and heme increase, and their clearance relies on renal filtration. Renal cell exposure to cell-free hemoglobin and other hemolysis products results in increased oxidative stress, inflammation, and reduced bioavailability of nitric oxide (NO) in the kidneys, all of which may exacerbate renal ischemia-reperfusion injury and endothelial dysfunction, thus promoting tubular renal cell death.96–100
Observational studies in patients undergoing cardiac surgery with cardiopulmonary bypass report that elevated levels of cell-free hemoglobin, as well as low levels of its scavenger, haptoglobin, are associated with increased cardiac surgery-associated AKI and increased mortality.101–106 Furthermore, it has been demonstrated that elevated levels of catalytic iron, another by-product of hemolysis, is associated with increased AKI and mortality, both after cardiac surgery and in other critically ill patient groups.107,108
Removal of hemolysis products:
Extracorporeal cytokine removal to decrease the cardiopulmonary bypass-related inflammatory response via hemoadsorption, has been attempted for some time. The technology is based on highly porous, biocompatible nonpolar polymer beads that bind and remove inflammatory mediators through size exclusion and non-specific surface adsorption. The technology of polymer bead-based cytokine hemoadsorption has shown to rapidly eliminate cytokines in vitro and in vivo.109,110 The Cytosorb® hemoadsorption filter device (Cytosorbents Corporation, USA) has been designed to directly capture and reduce mid-molecular weight inflammatory mediators (~10–60 kDa). The substances are adsorbed due to physiochemical binding depending on their concentration. Several studies have examined the ability of the Cytosorb® filter to remove cell-free hemoglobin and other hemolysis products during cardiopulmonary bypass, but the results were not promising, showing a very limited ability to remove cell-free hemoglobin.111,112 Furthermore, even if effective, it is important to remember that the use of hemoadsorption requires patients to be on extracorporeal circulation such as during cardiopulmonary bypass, ECMO or renal replacement therapy.113
KDIGO “Bundle of Care”:
The KDIGO Clinical Practice Guidelines includes recommendations for the prevention and treatment of AKI.114 This section discusses the implementation of these recommendations in cardiac surgery as a “Bundle of Care”.
Functional Hemodynamic Monitoring:
Cardiac output and stroke volume monitoring throughout the intraoperative and postoperative period is necessary to evaluate and optimize kidney perfusion. The PrevAKI single-center trial evaluated the impact of adherence to a bundle of interventions recommended by KDIGO to reduce AKI in high-risk patients undergoing cardiac surgery and reported a significant reduction in all stages of AKI in the KDIGO bundled care group as compared with the control group of routine care delivered in the post cardiac surgery ICU115. Patients in the intervention group underwent monitoring with a Pulse index Continuous Cardiac Output (PiCCO) monitor that provided stroke volume variation and cardiac index to guide the administration of fluids and inotropes, respectively. The multicenter PrevAKI trial, conducted by the same group of investigators, also utilized functional hemodynamic monitoring and optimized MAP and cardiac index in their intervention group.116
Hemodynamic optimization:
Perioperative strategies for preventing cardiac surgery-associated AKI aim to optimize kidney perfusion – primarily by maintaining blood pressure and cardiac output within physiologic limits. The multi-center PrevAKI study reported that cardiac surgery patients at high risk for AKI who were randomized to receive the KDIGO bundle of interventions had a significantly lower incidence of post cardiac surgery Stage 2 or Stage 3 AKI (14% vs. 24%). However, there were no significant between group differences in the multi-center study when Stage 1 AKI was also assessed. This bundle included an algorithmic approach to hemodynamic optimization to maintain MAP > 65 and cardiac index > 2.5 L/min/m2, resulting in intervention group patients receiving significantly more fluids and being more likely to receive dobutamine.116
Perioperative Medications:
Changes in autoregulation due to comorbidities and medications such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may potentiate renal hypoperfusion even when the systemic blood pressure is within the normal range. Patients with chronic hypertension, for example, have their autoregulatory curves shifted rightward resulting in higher MAP requirement to ensure adequate renal perfusion. Both the single-center and multi-center PrevAKI trials discontinued angiotensin-converting enzyme inhibitors and angiotensin receptor blockers during the first 48 hours after surgery as part of the KDIGO “bundle of care.”115,116 However, a systematic review of six studies (3 randomized controlled trials and 3 prospective cohort studies) evaluating the discontinuation of these agents before coronary angiography and cardiac surgery found no evidence that drug cessation impacted the incidence of AKI, citing a low quality of Grading of Recommendations, Assessment, Development and Evaluation (GRADE) evidence.117
Dobutamine has been shown to reduce cardiac surgery-associated AKI when implemented as part of the KDIGO “bundle of care” in both the single center and the multi-study Prev-AKI studies, suggesting that it should be used as part of a goal-directed strategy to maintain kidney perfusion in the postoperative ICU. Significantly more patients in the goal-directed management groups received dobutamine in both the single-center (31% vs. 9%) and multi-center (32.6% vs 18.8%) trials, resulting in higher MAPs at various time points.115,116
Intravenous fluids:
Compared to 0.9% saline, balanced crystalloid solutions such as Ringer’s lactate have been associated with a lower risk of AKI in critically ill patients, though the effect size was low in one meta-analysis of 6 studies (relative risk of 0.96).118 This may be due to the effect of chloride-mediated vasoconstrictions of the kidneys as well as the detrimental effect of chloride-associated metabolic acidosis.119 The Acute Disease Quality Initiative international consensus guidelines recommend resuscitation with a balanced crystalloid solution rather than 0.9% saline.116 A meta-analysis evaluating the role of urinary alkalinization with sodium bicarbonate to prevent cardiac surgery-associated AKI found no benefit in the general cardiac surgery population.117 However, there was a significantly lower incidence of renal replacement therapy in patients undergoing elective CABG. Further studies are needed before the routine use of sodium bicarbonate can be recommended. The Albumin in Cardiac Surgery trial randomized cardiac surgery patients to receive Ringer’s acetate solution or 4% albumin both in the cardiopulmonary bypass prime and used for intraoperative fluid resuscitation and found no significant difference in the incidence of AKI between the two groups.120 However, the higher incidence of bleeding and infection seen in patients who received albumin may limit its use in cardiac surgery. Hydroxyethyl starch should be avoided throughout the perioperative period because of their association with AKI in critically ill patients121, including cardiac surgery patients122.
Renal Replacement Therapy:
The indications, clinical timing of initiation, and different modalities of renal replacement therapy have not been specifically studied in the context of cardiac surgery-associated AKI. In the general critically ill patient population, indications of renal replacement therapy are focused on solute control (e.g., electrolyte or acid-base imbalances) and volume control to restore euvolemia when patients are no longer diuretic-responsive.123,124 For timing, data from recently completed clinical trials suggest that “early” initiation of renal replacement therapy in the absence of emergent indications of solute/volume control does not significantly improve mortality outcomes and is associated with more episodes of hypotension and hypophosphatemia.125 Further, in secondary outcome analysis of the STARRT-AKI trial, survivors of AKI-renal replacement therapy had higher risk of being renal replacement therapy-dependent at 90 days when compared to patients randomized to the “delayed” renal replacement therapy initiation strategy.126 Therefore, current consensus recommendation is that renal replacement therapy should be provided for specific solute/volume control indications that are refractory to medical management, which applies to patients with cardiac surgery-associated AKI. In this context, it is critical to carefully monitor the perioperative clinical trajectory of patients at risk for or with cardiac surgery-associated AKI to evaluate the need of renal replacement therapy.127 When renal replacement therapy is indicated, both intermittent and continuous renal replacement therapy are viable options for patients with cardiac surgery-associated AKI. Typically, renal replacement therapy is reserved for hemodynamically unstable patients given its lower net ultrafiltration rate, lower rate of osmotic shift, and slower change in extracellular fluid electrolyte concentrations with less impact on resting membrane potentials when compared to intermittent hemodialysis.123,124
Recent Novel Exploratory Studies of Cardiac Surgery AKI Prevention
Urine Oximetry:
Non-invasive urine oximetry is a novel concept for intraoperative and postoperative ICU use in cardiac surgery patients to assess adequacy of kidney perfusion and oxygen delivery. This can be potentially done by continuously measuring urine oxygen partial pressure. When measured from urine as it is excreted, urine oxygen partial pressure is thought to approximate the partial pressure of oxygen in the renal medulla.128 For this reason, urine oxygen partial pressure measurement is being investigated in cardiac surgery patients as a real time monitor of oxygen delivery to the kidneys. Zhu and colleagues used fiberoptic probes attached to the tip of urinary catheters to measure the partial pressure of urine in the bladders of patients undergoing cardiac surgery. The authors reported that low urine oxygen partial pressure predicted post-operative AKI and both the duration of low partial pressure as well as the nadir value increased the odds of AKI. 129 Another study which measured urine oxygen partial pressure using a prototype device placed between the urinary catheter and collection bag in cardiac surgery found that mean oxygen partial pressure in the post-cardiopulmonary bypass period was associated with post-operative AKI.130 These studies suggest that future studies and technical modifications may ultimately result in clinically reliable real-time monitoring of kidney oxygen delivery, allowing early interventions to mitigate risk of cardiac surgery-associated AKI.
Haptoglobin Administration:
Animal studies in models of hemolysis-associated AKI have shown that administration of haptoglobin prevented circulating free hemoglobin-induced kidney injury.131–133 In humans, a propensity-matched retrospective study reported that intraoperative administration of human haptoglobin (to bind and remove excessive cell-free hemoglobin) to patients exhibiting macro-hemoglobinuria during cardiopulmonary bypass, was associated with a reduced incidence of cardiac surgery-associated AKI.134 As haptoglobin is the natural scavenger of hemoglobin in the body, this approach seems clinically promising, however, human haptoglobin is only available for use in Japan and is not available for clinical use in other countries.
Nitric Oxide (NO):
One of the consequences of intravascular hemolysis is depletion in plasma NO135,136, which may result in vasoconstriction and impaired tissue perfusion106 Cell-free hemoglobin is released in the form of ferrous oxyhemoglobin and quickly reacts with plasma NO to form ferric methemoglobin, hence reducing the level of plasma NO.137 A second process contributing to reduced NO bioavailability during hemolysis, is the increase in the level of the enzyme arginase-1, which is released from hemolyzed erythrocytes.138 This enzyme metabolizes L-arginine, the main precursor of NO, therefore decreasing the available substrate for NO production137. Finally, injured erythrocytes also release asymmetric dimethylarginine, which directly inhibits endothelial NO synthase.139 Several studies have explored the effects of intraoperative administration of NO on postoperative outcomes in cardiac surgery patients. In a prospective study of 244 adult patients undergoing multiple valve surgeries, Lei and colleagues demonstrated that administration of NO (intraoperatively during cardiopulmonary bypass [80 ppm via the cardiopulmonary bypass circuit] and postoperatively for 24 hours) compared to controls who received nitrogen, was associated with a significant reduction in cardiac surgery-associated AKI and major adverse kidney events up to 1 year after surgery.100 Similarly, Kamenshchikov et al, reported that intraoperative administration of NO (40 ppm via the cardiopulmonary bypass circuit) was associated with a significant decrease in the incidence of cardiac surgery-associated AKI.140 Interestingly, there was no difference in the concentration of cell-free hemoglobin between treated patients and the controls in the latter study. Please note that using NO to prevent AKI is still investigational and not an approved indication.
Acetaminophen:
Acetaminophen has anti-inflammatory and anti-oxidative properties, specifically reducing the oxidation of cell-free hemoglobin by preventing the conversion of iron from the ferric ion to its more inflammatory and nephrotoxic ferryl ion form.141,142 In an animal model of rhabdomyolysis-induced kidney injury, acetaminophen significantly decreased markers of lipid peroxidation and preserved kidney function.142 Several observational retrospective studies have reported that exposure to acetaminophen was associated with decreased incidence of cardiac surgery-associated AKI in both adults and children undergoing cardiac surgery with cardiopulmonary bypass143,144. Furthermore, Janz and colleagues reported a phase IIa randomized placebo-controlled trial of acetaminophen in patients with severe sepsis and elevated circulating cell-free hemoglobin. Patients who received acetaminophen had lower levels of markers of lipid peroxidation as well as lower serum creatinine.145 In addition, two small clinical trials in adults and children undergoing cardiac surgery demonstrated that acetaminophen decreased markers of lipid peroxidation in the plasma after cardiopulmonary bypass. Although no differences in the incidence of AKI between the acetaminophen and placebo groups were reported, those studies were limited by small sample sizes and low event rates.146,147 Taken together, it seems that acetaminophen may have a kidney- protective effect in cardiac surgery, however, further studies, including appropriately powered prospective trials are needed to fully understand the role of acetaminophen as a kidney-protective medication.
Guidelines and Practice Updates
Published guidelines by the STS/Society of Cardiovascular Anesthesiologists (SCA)/American Society for Extracorporeal Technology (AmSECT)15 and a practice update by the SCA’s AKI Working group16 summarize the available literature and provide recommendations for the management of cardiac surgery-associated AKI. The STS/SCA/AmSECT guidelines graded the available evidence using the American College of Cardiology/American Heart Association Recommendation System. The SCA practice update assessed published randomized controlled trials using the GRADE methodology.
The STS/SCA/AmSECT guidelines recommended avoidance of hyperthermic perfusion on cardiopulmonary bypass and recommended goal directed DO2 delivery on cardiopulmonary bypass as Class I recommendations -evidence of benefit15 to prevent cardiac surgery-associated AKI. Adopting the KDIGO “bundle of care” for high-risk postoperative patients is also recommended to reduce the risk of cardiac surgery-associated AKI (Class IIa recommendation – should be considered). The use of minimally invasive extracorporeal circulation techniques was recommended as a Class IIb recommendation - may be considered. The guidelines recommend the use of fenoldopam in patients who tolerate the drug without hypotension (Class IIb recommendation – may be considered) but use of this medication has not been widely accepted in clinical settings. The guidelines recommend against the perioperative use of dopamine and mannitol, citing evidence that neither drug has shown to protect against the development of cardiac surgery-associated AKI (Class III recommendation – not recommended).
The SCA Practice Update endorses the use of goal-directed oxygen delivery and the KDIGO “bundle of care” in high-risk patients, citing moderate level of GRADE evidence16. This bundle, described in greater detail previously, includes volume and hemodynamic optimization, and avoidance of nephrotoxins and hypoglycemia. They also recommend the use of vasopressin over norepinephrine for treatment of vasoplegic shock after cardiac surgery, citing a low level of GRADE evidence. The practice update also reported that targeting higher MAP goals during cardiopulmonary bypass, and the perioperative use of dopamine and dexmedetomidine did not reduce cardiac surgery-associated AKI, citing a low level of GRADE evidence.
Summary
AKI is common after cardiac surgery and is associated with worse outcomes than patients who do not develop post-cardiac surgery AKI. While the pathogenesis of cardiac surgery-associated AKI is complex and multifactorial, the common consequence is renal tubular injury with a decline in glomerular filtration rate. The risk factors for cardiac surgery-associated AKI have been well characterized and used to develop predictive models. These models have performed well in predicting the need for renal replacement therapy but have less discriminatory ability to predict milder forms of AKI which are also associated with worse outcomes. Novel biomarkers of kidney function, inflammation and injury may allow earlier detection of cardiac surgery-associated AKI, better prediction models for developing cardiac surgery-associated AKI and will facilitate clinical trialists to study potential preventive and treatment strategies in the patients that are truly at risk for developing cardiac surgery-associated AKI. Preventing AKI requires the identification of patients with modifiable risk factors and mitigating this risk using evidence-based interventions throughout the perioperative period. Discontinuing nephrotoxic medications, optimizing chronic medication conditions, and treating anemia are recommended prior to elective cardiac surgery. During the intra-operative and post-operative phases, utilizing balanced crystalloids for resuscitation and employing a goal-directed hemodynamic strategy to optimize cardiac output and kidney perfusion pressure have been shown to improve kidney outcomes. Research driven advances in monitoring, real-time data capture and sub-phenotyping analyses, as well as discovery of novel detection and prediction biomarkers of kidney function and injury may ultimately allow real-time detection of kidney injury in the operating room or in the early post-operative period.
Figure 1.
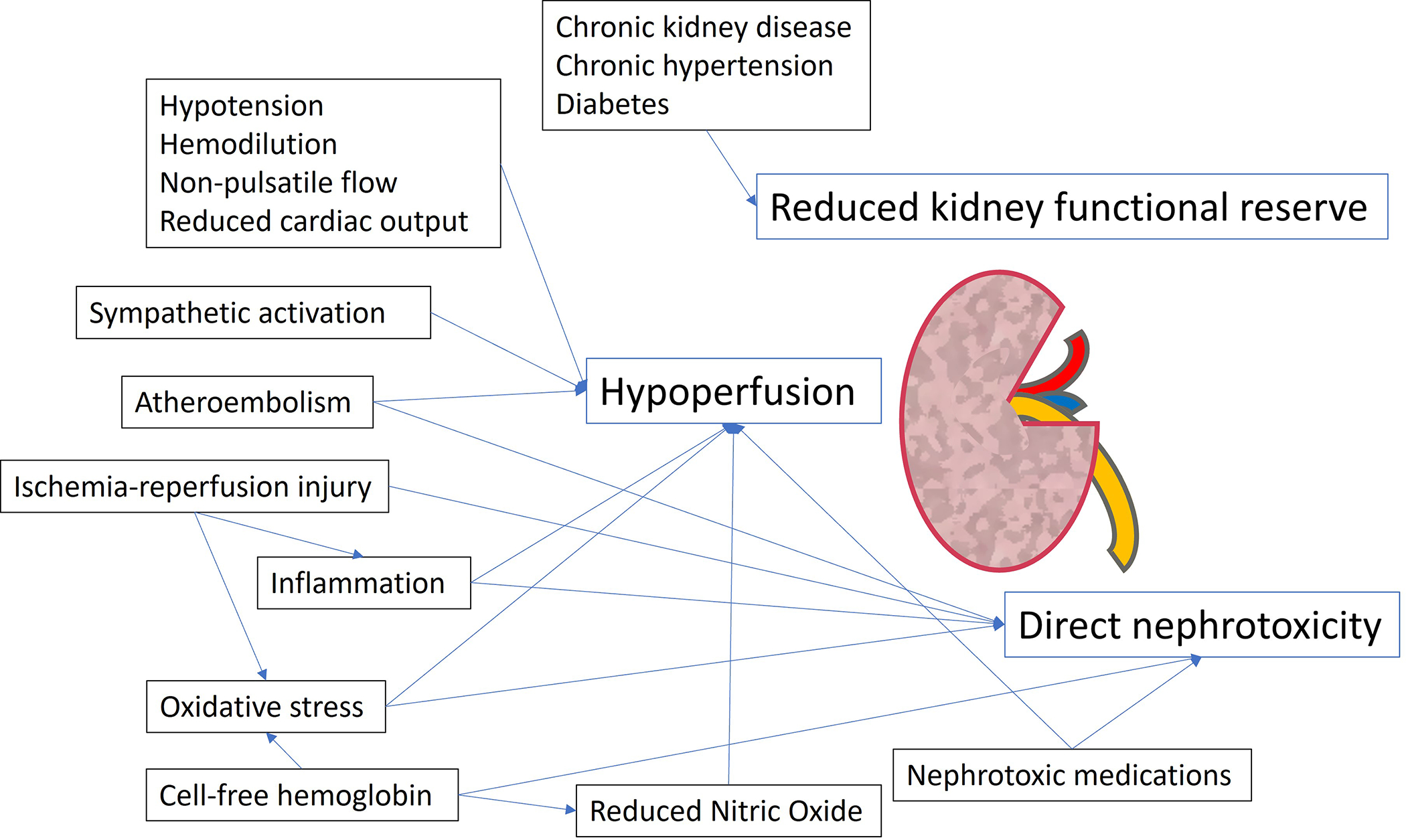
Pathophysiology of Cardiac Surgery-Associated Acute Kidney Injury.
Funding Statement:
This paper was supported by National Institutes of Health (NIH) NHLBI grant R01HL148448 (AF) and grants from NIDDK R01DK128208 (JN), R01DK133539 (JN), U01DK129989 (JN), and P30 DK079337 (JN).
Footnotes
Conflicts of Interest: JR has consulted for Octapharma USA, Inc. JAN is supported by JAN has received consulting honoraria from Baxter, Outset, Vifor, and AcelRx.
Clinical trial number: Not applicable
Prior presentations: Not applicable
Contributor Information
Sreekanth R. Cheruku, Department of Anesthesiology and Pain Management, University of Texas Southwestern Medical Center, Dallas, Texas.
Jacob Raphael, Department of Anesthesiology and Perioperative Medicine, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Javier A. Neyra, Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas; Department of Medicine, Division of Nephrology, University of Alabama at Birmingham, Birmingham, Alabama.
Amanda A. Fox, Department of Anesthesiology and Pain Management, University of Texas Southwestern Medical Center, Dallas, Texas; McDermott Center for Human Growth and Development, University of Texas Southwestern Medical Center, Dallas, Texas.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R: Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137: e67–e492 [DOI] [PubMed] [Google Scholar]
- 2.Moore EM, Simpson JA, Tobin A, Santamaria J: Preoperative estimated glomerular filtration rate and RIFLE-classified postoperative acute kidney injury predict length of stay post-coronary bypass surgery in an Australian setting. Anaesthesia and intensive care 2010; 38: 113–121 [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Chen R, Liu S, Yu X, Zou J, Ding X: Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. Journal of cardiothoracic and vascular anesthesia 2016; 30: 82–89 [DOI] [PubMed] [Google Scholar]
- 4.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA: Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrology Dialysis Transplantation 2008; 23: 1970–1974 [DOI] [PubMed] [Google Scholar]
- 5.Engoren M, Habib RH, Arslanian-Engoren C, Kheterpal S, Schwann TA: The effect of acute kidney injury and discharge creatinine level on mortality following cardiac surgery. Critical care medicine 2014; 42: 2069–2074 [DOI] [PubMed] [Google Scholar]
- 6.Pickering JW, James MT, Palmer SC: Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. American Journal of Kidney Diseases 2015; 65: 283–293 [DOI] [PubMed] [Google Scholar]
- 7.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A, Danesi TH, Kim JC, Nayak A, Neri M: Cardiac surgery-associated acute kidney injury. Cardiorenal medicine 2013; 3: 178–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J-R, Zhu J-M, Jiang J, Ding X-Q, Fang Y, Shen B, Liu Z-H, Zou J-Z, Liu L, Wang C-S: Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine 2015; 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rydén L, Sartipy U, Evans M, Holzmann MJ: Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130: 2005–2011 [DOI] [PubMed] [Google Scholar]
- 10.Olsson D, Sartipy U, Braunschweig F, Holzmann MJ: Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circulation: Heart Failure 2013; 6: 83–90 [DOI] [PubMed] [Google Scholar]
- 11.Hansen MK, Gammelager H, Jacobsen C-J, Hjortdal VE, Layton JB, Rasmussen BS, Andreasen JJ, Johnsen SP, Christiansen CF: Acute kidney injury and long-term risk of cardiovascular events after cardiac surgery: a population-based cohort study. Journal of cardiothoracic and vascular anesthesia 2015; 29: 617–625 [DOI] [PubMed] [Google Scholar]
- 12.Rydén L, Ahnve S, Bell M, Hammar N, Ivert T, Sartipy U, Holzmann MJ: Acute kidney injury after coronary artery bypass grafting and long-term risk of myocardial infarction and death. International journal of cardiology 2014; 172: 190–195 [DOI] [PubMed] [Google Scholar]
- 13.Liotta M, Olsson D, Sartipy U, Holzmann MJ: Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. The American journal of cardiology 2014; 113: 70–75 [DOI] [PubMed] [Google Scholar]
- 14.Parikh CR, Puthumana J, Shlipak MG, Koyner JL, Thiessen-Philbrook H, McArthur E, Kerr K, Kavsak P, Whitlock RP, Garg AX: Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. Journal of the American Society of Nephrology: JASN 2017; 28: 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JR, Shore-Lesserson L, Fox AA, Mongero LB, Lobdell KW, LeMaire SA, De Somer FM, von Ballmoos MW, Barodka V, Arora RC: The Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists/American Society of Extracorporeal Technology Clinical Practice Guidelines for the Prevention of Adult Cardiac Surgery-Associated Acute Kidney Injury. The Journal of ExtraCorporeal Technology 2022; 54: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng K, McIlroy DR, Bollen BA, Billings FT, Zarbock A, Popescu WM, Fox AA, Shore-Lesserson L, Zhou S, Geube MA: Society of Cardiovascular Anesthesiologists clinical practice update for management of acute kidney injury associated with cardiac surgery. Anesthesia & Analgesia 2022; 135: 744–756 [DOI] [PubMed] [Google Scholar]
- 17.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120: c179–c184 [DOI] [PubMed] [Google Scholar]
- 18.McIlroy D, Bellomo R, Billings IV F, Karkouti K, Prowle J, Shaw A, Myles P: Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: renal endpoints. British journal of anaesthesia 2018; 121: 1013–1024 [DOI] [PubMed] [Google Scholar]
- 19.Vandenberghe W, Gevaert S, Kellum JA, Bagshaw SM, Peperstraete H, Herck I, Decruyenaere J, Hoste EA: Acute kidney injury in cardiorenal syndrome type 1 patients: a systematic review and meta-analysis. Cardiorenal medicine 2016; 6: 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED: Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 2006; 114: 2208–2216 [DOI] [PubMed] [Google Scholar]
- 21.Corredor C, Thomson R, Al-Subaie N: Long-term consequences of acute kidney injury after cardiac surgery: a systematic review and meta-analysis. Journal of cardiothoracic and vascular anesthesia 2016; 30: 69–75 [DOI] [PubMed] [Google Scholar]
- 22.Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, Lo S, McGuinness S, Myburgh J, Parke R: Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS medicine 2014; 11: e1001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu B, Sun J, Liu S, Yu X, Zhu Y, Mao H, Xing C: Relationship among mortality of patients with acute kidney injury after cardiac surgery, fluid balance and ultrafiltration of renal replacement therapy: an observational study. Blood Purification 2017; 44: 32–39 [DOI] [PubMed] [Google Scholar]
- 24.Ronco C, Bellomo R, Kellum JA: Acute kidney injury. The Lancet 2019; 394: 1949–1964 [DOI] [PubMed] [Google Scholar]
- 25.Evans RG, Smith DW, Lee CJ, Ngo JP, Gardiner BS: What makes the kidney susceptible to hypoxia? The Anatomical Record 2020; 303: 2544–2552 [DOI] [PubMed] [Google Scholar]
- 26.Massoth C, Zarbock A, Meersch M: Acute kidney injury in cardiac surgery. Critical Care Clinics 2021; 37: 267–278 [DOI] [PubMed] [Google Scholar]
- 27.Andersson L, Bratteby L, Ekroth R, Hallhagen S, Joachimsson P, Van der Linden J, Wesslén O: Renal function during cardiopulmonary bypass: influence of pump flow and systemic blood pressure. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery 1994; 8: 597–602 [DOI] [PubMed] [Google Scholar]
- 28.Lannemyr L, Bragadottir G, Krumbholz V, Redfors B, Sellgren J, Ricksten S-E: Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology 2017; 126: 205–213 [DOI] [PubMed] [Google Scholar]
- 29.Karim HMR, Yunus M, Saikia MK, Kalita JP, Mandal M: Incidence and progression of cardiac surgery-associated acute kidney injury and its relationship with bypass and cross clamp time. Annals of cardiac anaesthesia 2017; 20: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranucci M, Romitti F, Isgrò G, Cotza M, Brozzi S, Boncilli A, Ditta A: Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. The Annals of thoracic surgery 2005; 80: 2213–2220 [DOI] [PubMed] [Google Scholar]
- 31.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M: The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. The Annals of thoracic surgery 2003; 76: 784–791 [DOI] [PubMed] [Google Scholar]
- 32.Ranucci M, Pavesi M, Mazza E, Bertucci C, Frigiola A, Menicanti L, Ditta A, Boncilli A, Conti D: Risk factors for renal dysfunction after coronary surgery: the role of cardiopulmonary bypass technique. Perfusion 1994; 9: 319–326 [DOI] [PubMed] [Google Scholar]
- 33.Blauth CI, Cosgrove DM, Webb BW, Ratliff NB, Boylan M, Piedmonte MR, Lytle BW, Loop FD: Atheroembolism from the ascending aorta: an emerging problem in cardiac surgery. The Journal of thoracic and cardiovascular surgery 1992; 103: 1104–1112 [PubMed] [Google Scholar]
- 34.Sgouralis I, Evans RG, Gardiner BS, Smith JA, Fry BC, Layton AT: Renal hemodynamics, function, and oxygenation during cardiac surgery performed on cardiopulmonary bypass: a modeling study. Physiological reports 2015; 3: e12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clifford KM, Selby AR, Reveles KR, Teng C, Hall RG 2nd, McCarrell J, Alvarez CA: The Risk and Clinical Implications of Antibiotic-Associated Acute Kidney Injury: A Review of the Clinical Data for Agents with Signals from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) Database. Antibiotics 2022; 11: 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kdigo A: Kidney Disease Improving Global Outcomes. AKI Work Group: Clinical practice guideline for acute kidney injury. Kidney Int. Suppl 2012; 2: 1–138 [Google Scholar]
- 37.Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH: Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013; 268: 719–728 [DOI] [PubMed] [Google Scholar]
- 38.McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE: Risk of intravenous contrast material–mediated acute kidney injury: a propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology 2014; 271: 65–73 [DOI] [PubMed] [Google Scholar]
- 39.Kim K, Joung K-W, Ji S-M, Kim J-Y, Lee E-H, Chung C-H, Choi I-C: The effect of coronary angiography timing and use of cardiopulmonary bypass on acute kidney injury after coronary artery bypass graft surgery. The Journal of thoracic and cardiovascular surgery 2016; 152: 254–261. e3 [DOI] [PubMed] [Google Scholar]
- 40.Jiang W, Yu J, Xu J, Shen B, Wang Y, Luo Z, Wang C, Ding X, Teng J: Impact of cardiac catheterization timing and contrast media dose on acute kidney injury after cardiac surgery. BMC cardiovascular disorders 2018; 18: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dayan V, Stanham R, Soca G, Genta F, Mariño J, Lorenzo A: Early surgery after angiography in patients scheduled for valve replacement. Asian Cardiovascular and Thoracic Annals 2017; 25: 18–23 [DOI] [PubMed] [Google Scholar]
- 42.Del Duca D, Iqbal S, Rahme E, Goldberg P, de Varennes B: Renal failure after cardiac surgery: timing of cardiac catheterization and other perioperative risk factors. The Annals of thoracic surgery 2007; 84: 1264–1271 [DOI] [PubMed] [Google Scholar]
- 43.Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, Lohr J: Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clinical journal of the American Society of Nephrology: CJASN 2008; 3: 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakar CV, Arrigain S, Worley S, Yared J-P, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. Journal of the American Society of Nephrology 2005; 16: 162–168 [DOI] [PubMed] [Google Scholar]
- 45.Wijeysundera DN, Karkouti K, Dupuis J-Y, Rao V, Chan CT, Granton JT, Beattie WS: Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. Jama 2007; 297: 1801–1809 [DOI] [PubMed] [Google Scholar]
- 46.Englberger L, Suri RM, Li Z, Dearani JA, Park SJ, Sundt III TM, Schaff HV: Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. American journal of kidney diseases 2010; 56: 623–631 [DOI] [PubMed] [Google Scholar]
- 47.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL: Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. Journal of the American Society of Nephrology 2011; 22: 1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penny-Dimri JC, Bergmeir C, Reid CM, Williams-Spence J, Cochrane AD, Smith JA: Machine learning algorithms for predicting and risk profiling of cardiac surgery-associated acute kidney injury, Seminars in thoracic and cardiovascular surgery, Elsevier, 2021, pp 735–745 [DOI] [PubMed] [Google Scholar]
- 49.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis J-Y, Fremes SE, Kent B, Laflamme C: Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119: 495–502 [DOI] [PubMed] [Google Scholar]
- 50.Parolari A, Pesce LL, Pacini D, Mazzanti V, Salis S, Sciacovelli C, Rossi F, Alamanni F, Outcomes MRGoCS: Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. The Annals of thoracic surgery 2012; 93: 584–591 [DOI] [PubMed] [Google Scholar]
- 51.Zou Z, Zhuang Y, Liu L, Shen B, Xu J, Luo Z, Teng J, Wang C, Ding X: Role of body mass index in acute kidney injury patients after cardiac surgery. Cardiorenal Medicine 2017; 8: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang T-M, Wu V-C, Young G-H, Lin Y-F, Shiao C-C, Wu P-C, Li W-Y, Yu H-Y, Hu F-C, Lin J-W: Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. Journal of the American Society of Nephrology: JASN 2011; 22: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kristovic D, Horvatic I, Husedzinovic I, Sutlic Z, Rudez I, Baric D, Unic D, Blazekovic R, Crnogorac M: Cardiac surgery-associated acute kidney injury: risk factors analysis and comparison of prediction models. Interactive cardiovascular and thoracic surgery 2015; 21: 366–373 [DOI] [PubMed] [Google Scholar]
- 54.Husain-Syed F, Ferrari F, Sharma A, Hinna Danesi T, Bezerra P, Lopez-Giacoman S, Samoni S, De Cal M, Corradi V, Virzì GM: Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrology Dialysis Transplantation 2019; 34: 308–317 [DOI] [PubMed] [Google Scholar]
- 55.Bidani AK, Griffin KA: Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 2004; 44: 595–601 [DOI] [PubMed] [Google Scholar]
- 56.Molnar AO, Parikh CR, Sint K, Coca SG, Koyner J, Patel UD, Butrymowicz I, Shlipak M, Garg AX: Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clinical journal of the American Society of Nephrology: CJASN 2012; 7: 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamy A, Devereaux P, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR: Off-pump or on-pump coronary-artery bypass grafting at 30 days. New England Journal of Medicine 2012; 366: 1489–1497 [DOI] [PubMed] [Google Scholar]
- 58.Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D: On-pump versus off-pump coronary-artery bypass surgery. New England Journal of Medicine 2009; 361: 1827–1837 [DOI] [PubMed] [Google Scholar]
- 59.Hernández-Leiva E, Hernández-Huertas F: The use of inotropes and not hyperchloremia is an independent risk factor for acute kidney injury during the postoperative period of cardiac surgery. A prospective cohort-study. International Journal of Surgery Open 2022; 45: 100507 [Google Scholar]
- 60.Nielsen DV, Hansen MK, Johnsen SP, Hansen M, Hindsholm K, Jakobsen C-J: Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score–matched analysis. Anesthesiology 2014; 120: 1098–1108 [DOI] [PubMed] [Google Scholar]
- 61.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M: Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. The Journal of thoracic and cardiovascular surgery 1996; 111: 1037–1046 [DOI] [PubMed] [Google Scholar]
- 62.Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, Wright P, Peterson MW, Rock P, Hyzy RC: Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Critical care medicine 2011; 39: 2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellomo R, Kellum JA, Ronco C: Defining acute renal failure: physiological principles. Intensive care medicine 2004; 30: 33–37 [DOI] [PubMed] [Google Scholar]
- 64.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. The Lancet 2005; 365: 1231–1238 [DOI] [PubMed] [Google Scholar]
- 65.Zhou F, Luo Q, Wang L, Han L: Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. European Journal of Cardio-Thoracic Surgery 2016; 49: 746–755 [DOI] [PubMed] [Google Scholar]
- 66.Kiessling A-H, Dietz J, Reyher C, Stock UA, Beiras-Fernandez A, Moritz A: Early postoperative serum cystatin C predicts severe acute kidney injury following cardiac surgery: a post-hoc analysis of a randomized controlled trial. Journal of Cardiothoracic Surgery 2014; 9: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yong Z, Pei X, Zhu B, Yuan H, Zhao W: Predictive value of serum cystatin C for acute kidney injury in adults: a meta-analysis of prospective cohort trials. Scientific reports 2017; 7: 41012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charlton JR, Portilla D, Okusa MD: A basic science view of acute kidney injury biomarkers. Nephrology Dialysis Transplantation 2014; 29: 1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khreba NA, Abdelsalam M, Wahab A, Sanad M, Elhelaly R, Adel M, El-Kannishy G: Kidney injury molecule 1 (KIM-1) as an early predictor for acute kidney injury in post-cardiopulmonary bypass (CPB) in open heart surgery patients. International journal of nephrology 2019; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD, Investigators S: Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney international 2014; 85: 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS: Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Critical care 2013; 17: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massoth C, Küllmar M, Enders D, Kellum JA, Forni LG, Meersch M, Zarbock A, Weiss R, Saadat-Gilani K, Roy-Ali T: Comparison of CC motif chemokine ligand 14 with other biomarkers for adverse kidney events after cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery 2021 [DOI] [PubMed] [Google Scholar]
- 73.Qian B-S, Jia H-M, Weng Y-B, Li X-C, Chen C-D, Guo F-X, Han Y-Z, Huang L-F, Zheng Y, Li W-X: Analysis of urinary C–C motif chemokine ligand 14 (CCL14) and first-generation urinary biomarkers for predicting renal recovery from acute kidney injury: a prospective exploratory study. Journal of Intensive Care 2023; 11: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boodhwani M, Rubens FD, Wozny D, Nathan HJ: Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. The Annals of thoracic surgery 2009; 87: 489–495 [DOI] [PubMed] [Google Scholar]
- 75.Newland RF, Baker RA, Mazzone AL, Quinn SS, Chew DP, Collaboration PD: Rewarming temperature during cardiopulmonary bypass and acute kidney injury: a multicenter analysis. The Annals of Thoracic Surgery 2016; 101: 1655–1662 [DOI] [PubMed] [Google Scholar]
- 76.Ranucci M, Johnson I, Willcox T, Baker RA, Boer C, Baumann A, Justison GA, De Somer F, Exton P, Agarwal S: Goal-directed perfusion to reduce acute kidney injury: a randomized trial. The Journal of thoracic and cardiovascular surgery 2018; 156: 1918–1927. e2 [DOI] [PubMed] [Google Scholar]
- 77.Mukaida H, Matsushita S, Yamamoto T, Minami Y, Sato G, Asai T, Amano A: Oxygen delivery-guided perfusion for the prevention of acute kidney injury: A randomized controlled trial. The Journal of Thoracic and Cardiovascular Surgery 2021 [DOI] [PubMed] [Google Scholar]
- 78.Lambden S, Creagh-Brown BC, Hunt J, Summers C, Forni LG: Definitions and pathophysiology of vasoplegic shock. Critical Care 2018; 22: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, Rhodes A, Landoni G, Osawa EA, Melo RR, Sundin MR, Grande SM, Gaiotto FA: Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology 2017; 126: 85–93 [DOI] [PubMed] [Google Scholar]
- 80.Sun LY, Wijeysundera DN, Tait GA, Beattie WS: Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015; 123: 515–523 [DOI] [PubMed] [Google Scholar]
- 81.Ngu JM, Jabagi H, Chung AM, Boodhwani M, Ruel M, Bourke M, Sun LY: Defining an intraoperative hypotension threshold in association with de novo renal replacement therapy after cardiac surgery. Anesthesiology 2020; 132: 1447–1457 [DOI] [PubMed] [Google Scholar]
- 82.De La Hoz MA, Rangasamy V, Bastos AB, Xu X, Novack V, Saugel B, Subramaniam B: Intraoperative hypotension and acute kidney injury, stroke, and mortality during and outside cardiopulmonary bypass: a retrospective observational cohort study. Anesthesiology 2022; 136: 927–939 [DOI] [PubMed] [Google Scholar]
- 83.Azau A, Markowicz P, Corbeau J, Cottineau C, Moreau X, Baufreton C, Beydon L: Increasing mean arterial pressure during cardiac surgery does not reduce the rate of postoperative acute kidney injury. Perfusion 2014; 29: 496–504 [DOI] [PubMed] [Google Scholar]
- 84.Kandler K, Nilsson JC, Oturai P, Jensen ME, Møller CH, Clemmesen JO, Arendrup HC, Steinbrüchel DA: Higher arterial pressure during cardiopulmonary bypass may not reduce the risk of acute kidney injury. Journal of Cardiothoracic Surgery 2019; 14: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vedel AG, Holmgaard F, Rasmussen LS, Langkilde A, Paulson OB, Lange T, Thomsen C, Olsen PS, Ravn HB, Nilsson JC: High-target versus low-target blood pressure management during cardiopulmonary bypass to prevent cerebral injury in cardiac surgery patients: a randomized controlled trial. Circulation 2018; 137: 1770–1780 [DOI] [PubMed] [Google Scholar]
- 86.Ranucci M, Conti D, Castelvecchio S, Menicanti L, Frigiola A, Ballotta A, Pelissero G: Hematocrit on cardiopulmonary bypass and outcome after coronary surgery in nontransfused patients. The Annals of thoracic surgery 2010; 89: 11–17 [DOI] [PubMed] [Google Scholar]
- 87.Loor G, Li L, Sabik III JF, Rajeswaran J, Blackstone EH, Koch CG: Nadir hematocrit during cardiopulmonary bypass: end-organ dysfunction and mortality. The Journal of thoracic and cardiovascular surgery 2012; 144: 654–662. e4 [DOI] [PubMed] [Google Scholar]
- 88.Koch CG, Li L, Duncan AI, Mihaljevic T, Loop FD, Starr NJ, Blackstone EH: Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. The Annals of thoracic surgery 2006; 81: 1650–1657 [DOI] [PubMed] [Google Scholar]
- 89.Loor G, Rajeswaran J, Li L, Sabik III JF, Blackstone EH, McCrae KR, Koch CG: The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? The Journal of thoracic and cardiovascular surgery 2013; 146: 1480–1487. e6 [DOI] [PubMed] [Google Scholar]
- 90.Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, Khanykin B, Gregory AJ, de Médicis É, McGuinness S: Restrictive or liberal red-cell transfusion for cardiac surgery. New England Journal of Medicine 2017; 377: 2133–2144 [DOI] [PubMed] [Google Scholar]
- 91.Garg AX, Badner N, Bagshaw SM, Cuerden MS, Fergusson DA, Gregory AJ, Hall J, Hare GM, Khanykin B, McGuinness S: Safety of a restrictive versus liberal approach to red blood cell transfusion on the outcome of AKI in patients undergoing cardiac surgery: a randomized clinical trial. Journal of the American Society of Nephrology 2019; 30: 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guinn NR, Schwartz J, Arora RC, Morton-Bailey V, Aronson S, Brudney CS, Bennett-Guerrero E: Perioperative Quality Initiative and Enhanced Recovery After Surgery-Cardiac Society Consensus Statement on the Management of Preoperative Anemia and Iron Deficiency in Adult Cardiac Surgery Patients. Anesth Analg 2022; 135: 532–544 [DOI] [PubMed] [Google Scholar]
- 93.Goksedef D, Omeroglu SN, Balkanay OO, Denli Yalvac ES, Talas Z, Albayrak A, Ipek G: Hemolysis at different vacuum levels during vacuum-assisted venous drainage: a prospective randomized clinical trial. Thorac Cardiovasc Surg 2012; 60: 262–8 [DOI] [PubMed] [Google Scholar]
- 94.Tan Z, Besser M, Anderson S, Newey C, Iles R, Dunning J, Falter F: Pulsatile Versus Nonpulsatile Flow During Cardiopulmonary Bypass: Extent of Hemolysis and Clinical Significance. Asaio j 2020; 66: 1025–1030 [DOI] [PubMed] [Google Scholar]
- 95.Vallelian F, Buehler PW, Schaer DJ: Hemolysis, free hemoglobin toxicity, and scavenger protein therapeutics. Blood 2022; 140: 1837–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deuel JW, Schaer CA, Boretti FS, Opitz L, Garcia-Rubio I, Baek JH, Spahn DR, Buehler PW, Schaer DJ: Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis 2016; 7: e2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haase M, Haase-Fielitz A, Bagshaw SM, Ronco C, Bellomo R: Cardiopulmonary bypass-associated acute kidney injury: a pigment nephropathy? Contrib Nephrol 2007; 156: 340–53 [DOI] [PubMed] [Google Scholar]
- 98.Hu J, Rezoagli E, Zadek F, Bittner EA, Lei C, Berra L: Free Hemoglobin Ratio as a Novel Biomarker of Acute Kidney Injury After On-Pump Cardiac Surgery: Secondary Analysis of a Randomized Controlled Trial. Anesth Analg 2021; 132: 1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Avondt K, Nur E, Zeerleder S: Mechanisms of haemolysis-induced kidney injury. Nat Rev Nephrol 2019; 15: 671–692 [DOI] [PubMed] [Google Scholar]
- 100.Lei C, Berra L, Rezoagli E, Yu B, Dong H, Yu S, Hou L, Chen M, Chen W, Wang H, Zheng Q, Shen J, Jin Z, Chen T, Zhao R, Christie E, Sabbisetti VS, Nordio F, Bonventre JV, Xiong L, Zapol WM: Nitric Oxide Decreases Acute Kidney Injury and Stage 3 Chronic Kidney Disease after Cardiac Surgery. Am J Respir Crit Care Med 2018; 198: 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ: Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg 2011; 142: 1–11 [DOI] [PubMed] [Google Scholar]
- 102.Billings FTt, Ball SK, Roberts LJ 2nd, Pretorius M: Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med 2011; 50: 1480–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cholette JM, Pietropaoli AP, Henrichs KF, Alfieris GM, Powers KS, Gensini F, Rubenstein JS, Sweeney D, Phipps R, Spinelli SL, Refaai MA, Eaton MP, Blumberg N: Elevated free hemoglobin and decreased haptoglobin levels are associated with adverse clinical outcomes, unfavorable physiologic measures, and altered inflammatory markers in pediatric cardiac surgery patients. Transfusion 2018; 58: 1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim-Campbell N, Gretchen C, Callaway C, Felmet K, Kochanek PM, Maul T, Wearden P, Sharma M, Viegas M, Munoz R, Gladwin MT, Bayir H: Cell-Free Plasma Hemoglobin and Male Gender Are Risk Factors for Acute Kidney Injury in Low Risk Children Undergoing Cardiopulmonary Bypass. Crit Care Med 2017; 45: e1123–e1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mamikonian LS, Mamo LB, Smith PB, Koo J, Lodge AJ, Turi JL: Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children*. Pediatr Crit Care Med 2014; 15: e111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vermeulen Windsant IC, de Wit NC, Sertorio JT, van Bijnen AA, Ganushchak YM, Heijmans JH, Tanus-Santos JE, Jacobs MJ, Maessen JG, Buurman WA: Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol 2014; 5: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Rawn JD, Frendl G, Waikar SS: Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int 2015; 87: 1046–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Waikar SS: Plasma catalytic iron, AKI, and death among critically ill patients. Clin J Am Soc Nephrol 2014; 9: 1849–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kellum JA, Song M, Venkataraman R: Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit Care Med 2004; 32: 801–5 [DOI] [PubMed] [Google Scholar]
- 110.Rimmelé T, Kellum JA: Clinical review: blood purification for sepsis. Crit Care 2011; 15: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gleason TG, Argenziano M, Bavaria JE, Kane LC, Coselli JS, Engelman RM, Tanaka KA, Awad A, Sekela ME, Zwischenberger JB: Hemoadsorption to Reduce Plasma-Free Hemoglobin During Cardiac Surgery: Results of REFRESH I Pilot Study. Semin Thorac Cardiovasc Surg 2019; 31: 783–793 [DOI] [PubMed] [Google Scholar]
- 112.Bernardi MH, Rinoesl H, Ristl R, Weber U, Wiedemann D, Hiesmayr MJ: Hemoadsorption does not Have Influence on Hemolysis During Cardiopulmonary Bypass. Asaio j 2019; 65: 738–743 [DOI] [PubMed] [Google Scholar]
- 113.Datzmann T, Träger K: Extracorporeal membrane oxygenation and cytokine adsorption. J Thorac Dis 2018; 10: S653–s660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS: Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney international supplements 2012; 2: 1–138 [Google Scholar]
- 115.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive care medicine 2017; 43: 1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarbock A, Küllmar M, Ostermann M, Lucchese G, Baig K, Cennamo A, Rajani R, McCorkell S, Arndt C, Wulf H: Prevention of cardiac surgery–associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: The PrevAKI-multicenter randomized controlled trial. Anesthesia & Analgesia 2021; 133: 292–302 [DOI] [PubMed] [Google Scholar]
- 117.Whiting P, Morden A, Tomlinson LA, Caskey F, Blakeman T, Tomson C, Stone T, Richards A, Savović J, Horwood J: What are the risks and benefits of temporarily discontinuing medications to prevent acute kidney injury? A systematic review and meta-analysis. BMJ open 2017; 7: e012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hammond DA, Lam SW, Rech MA, Smith MN, Westrick J, Trivedi AP, Balk RA: Balanced crystalloids versus saline in critically ill adults: a systematic review and meta-analysis. Annals of Pharmacotherapy 2020; 54: 5–13 [DOI] [PubMed] [Google Scholar]
- 119.Bailey M, McGuinness S, Haase M, Haase-Fielitz A, Parke R, Hodgson CL, Forbes A, Bagshaw SM, Bellomo R: Sodium bicarbonate and renal function after cardiac surgery: a prospectively planned individual patient meta-analysis. Anesthesiology 2015; 122: 294–306 [DOI] [PubMed] [Google Scholar]
- 120.Vlasov H, Juvonen T, Hiippala S, Suojaranta R, Peltonen M, Schramko A, Arvonen K, Salminen U-S, Kleine Budde I, Eränen T: Effect and safety of 4% albumin in the treatment of cardiac surgery patients: study protocol for the randomized, double-blind, clinical ALBICS (ALBumin In Cardiac Surgery) trial. Trials 2020; 21: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perel P, Roberts I: Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database of Systematic Reviews 2012 [DOI] [PubMed] [Google Scholar]
- 122.Lagny M-G, Roediger L, Koch J-N, Dubois F, Senard M, Donneau A-F, Hubert MB, Hans GA: Hydroxyethyl starch 130/0.4 and the risk of acute kidney injury after cardiopulmonary bypass: a single-center retrospective study. Journal of cardiothoracic and vascular anesthesia 2016; 30: 869–875 [DOI] [PubMed] [Google Scholar]
- 123.Wald R, Beaubien-Souligny W, Chanchlani R, Clark EG, Neyra JA, Ostermann M, Silver SA, Vaara S, Zarbock A, Bagshaw SM: Delivering optimal renal replacement therapy to critically ill patients with acute kidney injury. Intensive Care Med 2022; 48: 1368–1381 [DOI] [PubMed] [Google Scholar]
- 124.Teixeira JP, Neyra JA, Tolwani A: Continuous KRT: A Contemporary Review. Clin J Am Soc Nephrol 2023; 18: 256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gaudry S, Hajage D, Benichou N, Chaïbi K, Barbar S, Zarbock A, Lumlertgul N, Wald R, Bagshaw SM, Srisawat N, Combes A, Geri G, Jamale T, Dechartres A, Quenot JP, Dreyfuss D: Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2020; 395: 1506–1515 [DOI] [PubMed] [Google Scholar]
- 126.Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, Du B, Gallagher MP, Gaudry S, Hoste EA, Lamontagne F, Joannidis M, Landoni G, Liu KD, McAuley DF, McGuinness SP, Neyra JA, Nichol AD, Ostermann M, Palevsky PM, Pettilä V, Quenot JP, Qiu H, Rochwerg B, Schneider AG, Smith OM, Thomé F, Thorpe KE, Vaara S, Weir M, Wang AY, Young P, Zarbock A: Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med 2020; 383: 240–251 [DOI] [PubMed] [Google Scholar]
- 127.Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, Kane-Gill SL, Liu KD, Prowle JR, Shaw AD, Srisawat N, Cheung M, Jadoul M, Winkelmayer WC, Kellum JA: Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int 2020; 98: 294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Evans RG, Smith JA, Wright C, Gardiner BS, Smith DW, Cochrane AD: Urinary oxygen tension: a clinical window on the health of the renal medulla? American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2014; 306: R45–R50 [DOI] [PubMed] [Google Scholar]
- 129.Zhu MZ, Martin A, Cochrane AD, Smith JA, Thrift AG, Harrop GK, Ngo JP, Evans RG: Urinary hypoxia: an intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrology Dialysis Transplantation 2018; 33: 2191–2201 [DOI] [PubMed] [Google Scholar]
- 130.Silverton NA, Lofgren LR, Hall IE, Stoddard GJ, Melendez NP, Van Tienderen M, Shumway S, Stringer BJ, Kang W-s, Lybbert C: Noninvasive urine oxygen monitoring and the risk of acute kidney injury in cardiac surgery. Anesthesiology 2021; 135: 406–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schaer CA, Deuel JW, Schildknecht D, Mahmoudi L, Garcia-Rubio I, Owczarek C, Schauer S, Kissner R, Banerjee U, Palmer AF, Spahn DR, Irwin DC, Vallelian F, Buehler PW, Schaer DJ: Haptoglobin Preserves Vascular Nitric Oxide Signaling during Hemolysis. Am J Respir Crit Care Med 2016; 193: 1111–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW: Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 2012; 122: 1444–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boretti FS, Buehler PW, D’Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ: Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 2009; 119: 2271–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kubota K, Egi M, Mizobuchi S: Haptoglobin Administration in Cardiovascular Surgery Patients: Its Association With the Risk of Postoperative Acute Kidney Injury. Anesth Analg 2017; 124: 1771–1776 [DOI] [PubMed] [Google Scholar]
- 135.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT: Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 2002; 8: 1383–9 [DOI] [PubMed] [Google Scholar]
- 136.Rother RP, Bell L, Hillmen P, Gladwin MT: The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. Jama 2005; 293: 1653–62 [DOI] [PubMed] [Google Scholar]
- 137.Doyle MP, Hoekstra JW: Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem 1981; 14: 351–8 [DOI] [PubMed] [Google Scholar]
- 138.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM Jr., Gladwin MT: Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama 2005; 294: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Landburg PP, Teerlink T, van Beers EJ, Muskiet FA, Kappers-Klunne MC, van Esser JW, Mac Gillavry MR, Biemond BJ, Brandjes DP, Duits AJ, Schnog JJ: Association of asymmetric dimethylarginine with sickle cell disease-related pulmonary hypertension. Haematologica 2008; 93: 1410–2 [DOI] [PubMed] [Google Scholar]
- 140.Kamenshchikov NO, Anfinogenova YJ, Kozlov BN, Svirko YS, Pekarskiy SE, Evtushenko VV, Lugovsky VA, Shipulin VM, Lomivorotov VV, Podoksenov YK: Nitric oxide delivery during cardiopulmonary bypass reduces acute kidney injury: A randomized trial. J Thorac Cardiovasc Surg 2022; 163: 1393–1403.e9 [DOI] [PubMed] [Google Scholar]
- 141.Aronoff DM, Oates JA, Boutaud O: New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther 2006; 79: 9–19 [DOI] [PubMed] [Google Scholar]
- 142.Boutaud O, Moore KP, Reeder BJ, Harry D, Howie AJ, Wang S, Carney CK, Masterson TS, Amin T, Wright DW, Wilson MT, Oates JA, Roberts LJ 2nd: Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci U S A 2010; 107: 2699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Driest SL, Jooste EH, Shi Y, Choi L, Darghosian L, Hill KD, Smith AH, Kannankeril PJ, Roden DM, Ware LB: Association Between Early Postoperative Acetaminophen Exposure and Acute Kidney Injury in Pediatric Patients Undergoing Cardiac Surgery. JAMA Pediatr 2018; 172: 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Young AM, Strobel RJ, Rotar EP, Kleiman A, McNeil JS, Teman NR, Hawkins RB, Raphael J, Mehaffey JH: Perioperative acetaminophen is associated with reduced acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2022 [DOI] [PubMed] [Google Scholar]
- 145.Janz DR, Bastarache JA, Rice TW, Bernard GR, Warren MA, Wickersham N, Sills G, Oates JA, Roberts LJ 2nd, Ware LB: Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis trial. Crit Care Med 2015; 43: 534–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Billings FTt Petracek MR, Roberts LJ 2nd, Pretorius M: Perioperative intravenous acetaminophen attenuates lipid peroxidation in adults undergoing cardiopulmonary bypass: a randomized clinical trial. PLoS One 2015; 10: e0117625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simpson SA, Zaccagni H, Bichell DP, Christian KG, Mettler BA, Donahue BS, Roberts LJ 2nd, Pretorius M: Acetaminophen attenuates lipid peroxidation in children undergoing cardiopulmonary bypass. Pediatr Crit Care Med 2014; 15: 503–10 [DOI] [PMC free article] [PubMed] [Google Scholar]


