Abstract
Objective:
To investigate in vitro fertilization (IVF) outcomes in an adolescent transmasculine mouse model mimicking gender-affirming-hormone therapy in prepubertal youth, both on testosterone (T) and after T washout.
Design:
Experimental laboratory study using a validated mouse model
Settings:
University-based basic science research laboratory
Animals:
80 prepubertal 26-day-old C57BL/6N female mice were used in this study
Intervention(s):
Animals (n=10/group) were subcutaneously implanted with gonadotropin releasing hormone agonist (GnRHa) at 3.6 mg or received sham surgery. 21 days after they were implanted with silastic tubing containing either testosterone 10mg or placebo for 6 weeks. After 6 weeks a group of animals were super ovulated for immediate IVF and other group had the implant removed and went through superovulation for IVF after 2 weeks (washout IVF). The total number of oocytes yielded, oocyte maturity rate, fertilization rate, and numbers of 2-cell embryos, 4–8 cell embryos, morula, blastocysts and hatching blastocysts were recorded.
Results:
Testosterone treatment negatively impacted IVF outcomes in animals stimulated while on T, but not after T washout. Pretreatment with GnRHa did not affect IVF outcomes.
Conclusion:
While current T had a negative impact on IVF outcomes compared to controls, animals were still able to produce viable oocytes for fertilization and developed to blastocysts. Future efforts to study the impact of long-term T exposure on oocyte quality, especially aneuploidy rates, pregnancy outcomes, and live birth rates are necessary.
Keywords: mouse model, GnRH agonist, testosterone, transmasculine youth, fertility outcomes
Capsule:
Prepubertal female mice pretreated with GnRHa and currently on T were able to produce viable oocytes, but at lower numbers than controls.
Introduction
Gender-affirming-hormone therapy (GAHT) in peripubertal transmasculine youth may comprise of gonadotropin-releasing hormone agonist (GnRHa) treatment to suppress puberty and block development of secondary sexual characteristics (1–4) followed later by testosterone (T) treatment to align physical characteristics with their gender identity (5–7).
The number of adolescents seeking GAHT has been increasing, and recent guidelines recommend the use of hormone therapy as early as 14 years old (7, 8). Given the possibility of fertility impairment, the World Professional Association for Transgender Health, Endocrine Society, and American Society of Reproduction recommend discussing fertility preservation and family planning before initiating GAHT (7, 9–12). Despite this, a recent study showed that only 2.8% of transgender youth chose fertility preservation before starting to transition (13). Barriers to fertility preservation include the desire to move forward with medical transition without delay, cost, invasiveness of procedures, discrimination, lack of awareness, and concern for worsening gender dysphoria (14–17).
Significant gaps exist in the literature regarding the fertility potential in transmasculine people who underwent treatment with GnRHa-T as adolescents (13, 14). Although there are case reports of successful oocyte cryopreservation for fertility preservation in transmasculine youth, these studies were performed prior to starting GAHT and did not evaluate later in vitro fertilization (IVF) outcomes (5, 18). Additional studies evaluating fertility preservation potential in adults varied in terms of whether it was before or after T cessation, the nature of T formulation, regimen of T treatment, the duration of pre-stimulation T cessation, and none of the cases received pretreatment with GnRHa as an adolescent (19, 20), thus precluding accurate assessment of fertility outcomes in this population.
We recently developed a translational mouse model mimicking GAHT in peripubertal transmasculine youth. We found that GnRHa halted puberty, and subsequent T treatment arrested cyclicity, sustained elevated testosterone levels, and lowered LH levels without affecting ovarian reserve (21) similar to reports in humans (10, 22). Using this model, we investigated reproductive potential and in vitro fertilization (IVF) outcomes both during and after cessation of testosterone treatment.
Materials and Methods
Animals
This study used 80 prepubertal 26-day-old C57BL/6N female mice (Envigo, Indianapolis, IN, USA). Animals were housed in groups of three to five in ventilated cages under standard housing conditions (ad libitum access to food and water, photoperiod 12h light and 12h dark) at the University of Michigan, Ann Arbor. All procedures involving mice were carried out according to the Guide for the Care and Use of Laboratory Animals approved by the University of Michigan Institutional Animal Care and Use Committee (PRO00009635).
Experimental design
GnRHa treatment
Forty peripubertal C57BL/6N females, 26 days old, were subcutaneously implanted with GnRH agonist (GnRHa, Goserelin acetate implant 3.6 mg (Zoladex ®, AstraZeneca, UK). Forty control animals received sham implants. To assess if the GnRH agonist treatment was able to suppress the HPG axis, we recorded the timing of vaginal opening, the first physical change in the external genitalia indicating the activation of the HPG axis in rats and mice that corresponds with the beginning of breast development in humans (23). Daily vaginal cytology was performed to monitor cyclicity for 3 weeks. To confirm that the HPG axis was suppressed, FSH and LH levels were measured in blood samples taken at weekly intervals (Figure 1A). We previously established that this model of GnRH agonist treatment halts puberty in C57BL/6N female mice for at least 21 days when the implant was left in situ. (21).
Figure 1). Experimental design and methodology.
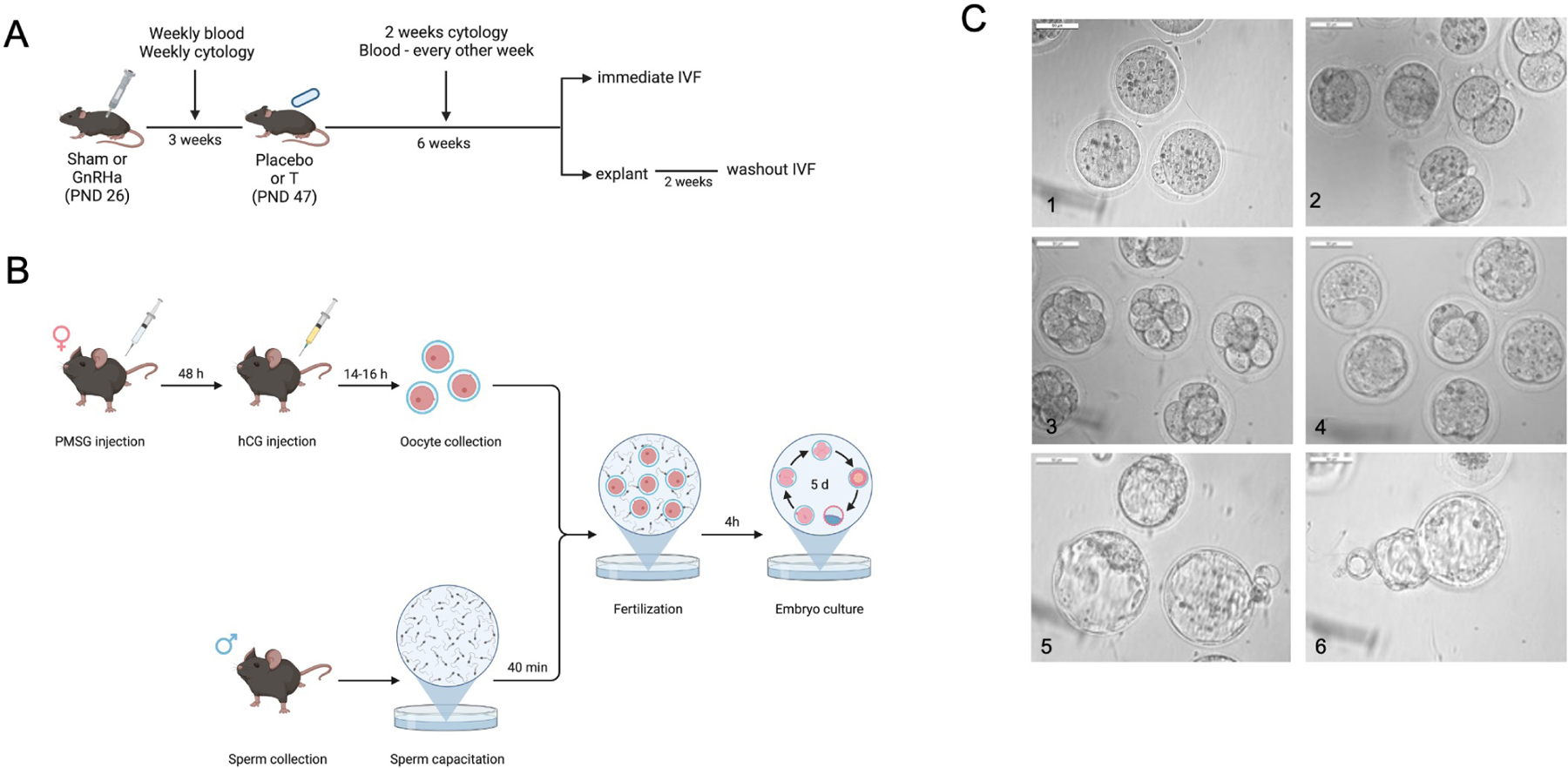
A) Experimental design showing the implantation day of gonadotropin-releasing-hormone agonist followed by testosterone, showing time of interventions. B) Scheme showing the in vitro fertilization protocol used in this study. C) Representative images of embryo development.
T treatment
On postnatal day (PND) 47 (21 days after sham or GnRHa implantation) animals were assigned to one of the following 4 treatment groups: 1) control, 2) GnRHa only, 3) T only, or 4) GnRHa plus T. Half of the animals from each of the above 4 groups underwent IVF immediately after 6 weeks of T treatment (Immediate IVF) and the other half underwent IVF 2 weeks after cessation of T treatment (washout IVF). Sham and T implantations were carried out under isoflurane anesthesia. All animals were subcutaneously implanted with a 16 mm silastic implant in the back (Dow Corning, USA; Inner Diameter: 1.98 mm, Outer Diameter: 3.17 mm). Control implants were filled with 25 μl (8 mm) of ethanol (200 Proof Ethanol, Decon Laboratories, King of Prussia, PA, USA) and sealed with 4 mm of adhesive (Factor II, Incorporated, Lakeside, AZ, USA) at both ends. T implants were filled with 10mg of crystalline testosterone dissolved in 25 μl of ethanol and similarly sealed. All implants were prepared one day before surgery to ensure the ethanol was evaporated entirely. On the day of surgical implantation, implants were soaked in ethanol for three minutes and allowed to air dry prior to being inserted in animals (24). Implants were left in place for six weeks. Vaginal cytology was performed for 2 weeks after T implantation to ensure T-only treated animals stopped cycling, and GnRHa+T-treated mice continued to remain acyclic during the T treatment period (Figure 1A).
At 6 weeks, immediate IVF animals (PND 89) were superovulated for IVF with the implants in place. Washout IVF animals underwent an implant removal to ensure well-defined timing of T washout (PND 103) and were superovulated two weeks later for IVF (Figure 1A). On the day of sacrifice, body weight and ovarian weight from one ovary were recorded, and the other ovary was saved for future transcriptome analysis. Terminal blood was collected to assess hormone levels.
Assessment of timing of vaginal opening and estrous cyclicity
Mice were assessed daily for vaginal opening, and starting one day after vaginal opening, vaginal cytology was performed to assess estrous cyclicity. The estrous cycle was staged using light microscopy analysis of the vaginal epithelial cellular distribution and characterized by cornified, nucleated epithelial cells and leukocytes (25).
Weekly blood collection and serum hormone analysis
Blood was collected weekly from the lateral tail vein, up to 0.5% of body weight. At the time of sacrifice, terminal blood was collected via decapitation. Samples were kept at 4°C overnight, centrifuged at 9200 × g for 10 minutes, and serum was stored at −20°C until analysis. Peptide hormone measurements of FSH and steroid hormones measurements of testosterone, estradiol, progesterone, and AMH were performed at the Ligand Assay and Analysis Core Facility, University of Virginia Center for Research in Reproduction.
The reportable dose range for FSH Mouse and Rat in-house protocol radioimmunoassay was 3–75 ng/ml, 10.0–1600.00ng/dL for testosterone mouse & rat IBL ELISA, 0.15–40.0 ng/mL and 0.30–80.0 ng/mL with a 2x dilution for progesterone mouse & rat IBL ELISA, and 5.0–3200.0 pg/mL and 10.0–6400.0 pg/mL with a 2x dilution for mouse & rat estradiol ALPCO ELISA. Reportable dose range for AMH was 4.0–260 ng/mL. The reportable ranges were established with a coefficient of variation of 1–10% for testosterone, 0.2–4.4% for estradiol, <20% for progesterone and FSH, and 0.8–3.7% for AMH. All immunoassays were performed in singlets.
Superovulation
Animals received 7.5 IU of PMSG (Bioworld, Dublin, OH, USA) intraperitoneally between 5 and 6 pm. Forty-eight hours post-PMSG, they were intraperitoneally injected with 7.5 IU of hCG (Sigma, CG5, St. Louis, MO, USA).
Preparing culture and IVF dishes
All culture dishes were prepared a day before the IVF procedure and placed in an incubator supplied with mixed gas (4.5% CO2, 5% O2) to maintain temperature (37°C) and pH (7.2–7.4) of the culture medium during the IVF procedure.
Each sperm dish was made by adding 500ul of HTF media (Millipore Sigma, Burlington, MA, USA) in a 4-well plate. Each COCs collection dish was made by adding 300ul of M2 media (Millipore Sigma, Burlington, MA, USA) in a 4-well plate. IVF dishes were made by adding 1mL of HTF media (Millipore Sigma, Burlington, MA, USA), and the washing and embryo culture dishes were made by adding four drops of 75ul of KSOM media (Millipore Sigma, Burlington, MA, USA) covered with OVOIL™ (Vitrolife, San Diego, CA, USA) in a 35mm petri dish.
In vitro fertilization (IVF)
In vitro fertilization was performed using a modified version of the method described by Sztein et al (2001) (26), Byers et al (2006) (27), and Takahashi et al. (2010) (28).
Male mice, 8–10 weeks old, were euthanized and sacrificed by decapitation. Bilateral cauda epididymis and distal vas deferens were removed and placed into a previously prepared sperm collection dish. Using forceps, the sperm of the vas deferens was expressed, and using a 26-gauge syringe, the epididymis was sliced ten times. The sperm dish was then returned to the incubator for 10 minutes to allow motile spermatozoa to exit the tissue. After 10 minutes, the dish was removed from the incubator, and 10ul of the sperm-containing media was applied to a Makler counting chamber (New York Microscopy Company, Hicksville, NY, USA) to calculate the total sperm concentration. A total of 1×106 sperm/ml were added to each IVF dish and then returned to the incubator to allow sperm capacitation for 40 minutes. In the meantime, experimental females were euthanized and sacrificed by decapitation fourteen to sixteen hours post-hCG, and the oviducts were immediately placed into COC’s collection dish The cumulus-oocyte complexes (COCs) were removed from the ampulla using micro dissecting forceps and combined with sperm in the IVF dish for fertilization (adapted from Byers et al (2006) (27) (Figure 1B).
Four hours later, the oocytes were removed from the IVF dish and transferred to the washing and embryo culture dish. During the washing procedure, the total number of oocytes yielded, as well as the number of immature and mature oocytes, were recorded. The maturity rate was calculated by dividing the total number of mature oocytes by the total number of oocytes yielded. Approximately 18 hours after the washing procedure, two cell embryos were counted, and the fertilization rate was calculated by dividing the total number of two-cells embryos by the total number of mature oocytes. Embryo development was followed for five days, and the number of 4–8 cell embryos, morula, blastocysts, and hatching blastocysts were recorded from each mouse (Figure 1C).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9. The results were analyzed by descriptive statistics to determine normality of data distribution. Two-way ANOVA followed by Tukey’s HSD post hoc test was performed. All data were presented as mean ± SD. The level of significance was defined as P < 0.05. For analysis purposes, hormone levels above and below the detection level were treated as the value set for the maximum or lower limit of quantification.
Results
GnRHa + T treatment suppressed the HPG axis, but the axis was reactivated after
T washout
GnRHa agonist treatment resulted in earlier vaginal opening (P<0.001) compared to controls (Figure 2A). Control animals cycled regularly throughout the study period (Figure 2D). GnRHa and GnRHa+T treated mice exhibited persistent diestrus that was maintained for at least 21 days after GnRHa implantation day, supportive of pubertal arrest. FSH levels were suppressed in GnRHa and GnRa+T animals when compared with controls and T-only treated animals (P<0.0001) (Figure 2B). Treatment with T increased weekly serum T levels in T-only and GnRHa+T animals compared to controls and GnRHa-only animals (P<0.0001) (Figure 2C). When the T implant was removed after six weeks of treatment, animals in the T-only and GnRHa+T groups resumed cycling 6.2 ± 1.13 days after the explantation day (Figure 2D). At this time T levels had returned to levels comparable to that of controls and GnRHa-only treated animals (control= 0.3 + 0.086; GnRHa= 0.4 + 0.19; T= 0.36 + 0.16; GnRHa+T= 0.33 + 0.10, P=0.5698) (Figure 2C).
Figure 2). Characterization of the transmasculine mouse model.
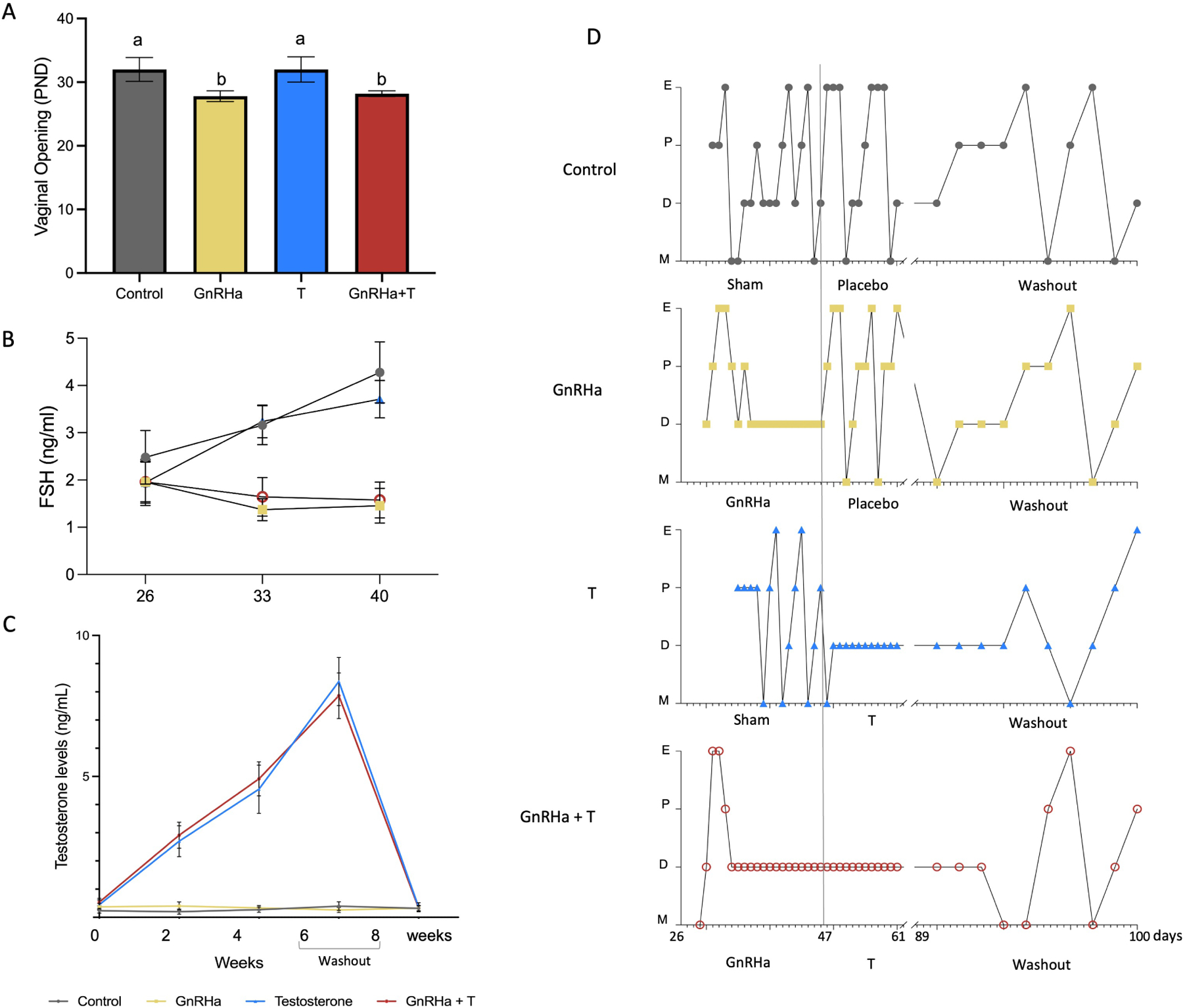
A) Vaginal opening. B) Weekly FSH levels on days 26, 33, 40. C) Testosterone levels over 6 weeks of treatment and after cessation of T. D) Estrous cyclicity. Control animals went through all estrous cycle phases. E, estrus; P, Proestrus; D, Diestrus; M, Metaestrus. GnRHa-treated mice showed a flare and subsequent persistent diestrus, and resume cyclicity after GnRHa clearance. T animals presented persistent diestrus after initiating T treatment and resumed cycling after T cessation. GnRHa+T animals showed persist diestrus during GnRHa and T treatment and resumed cyclicity after T washout.
T treatment reduced ovarian weight in T and GnRHa+T groups, which persisted even after T washout
T treatment caused a decrease in terminal ovarian weight in the T-only and GnRHa+T groups (immediate IVF group - control= 7.3 ± 1.2; GnRHa-only= 6.1±0.99, T-only=3.4±0.46; GnRHa±T=3.2+0.98, P<0.0001) (Figure 3B) in the absence of any change in body weight (Figure 3A). This difference persisted after T washout (washout IVF group - control= 7.2 ± 1.4; GnRHa-only= 7.2±1.2, T-only=5.0±1.2; GnRHa+T=5.1±0.98, P<0.0169) (Figure 3 B).
Figure 3). Body Measurements.
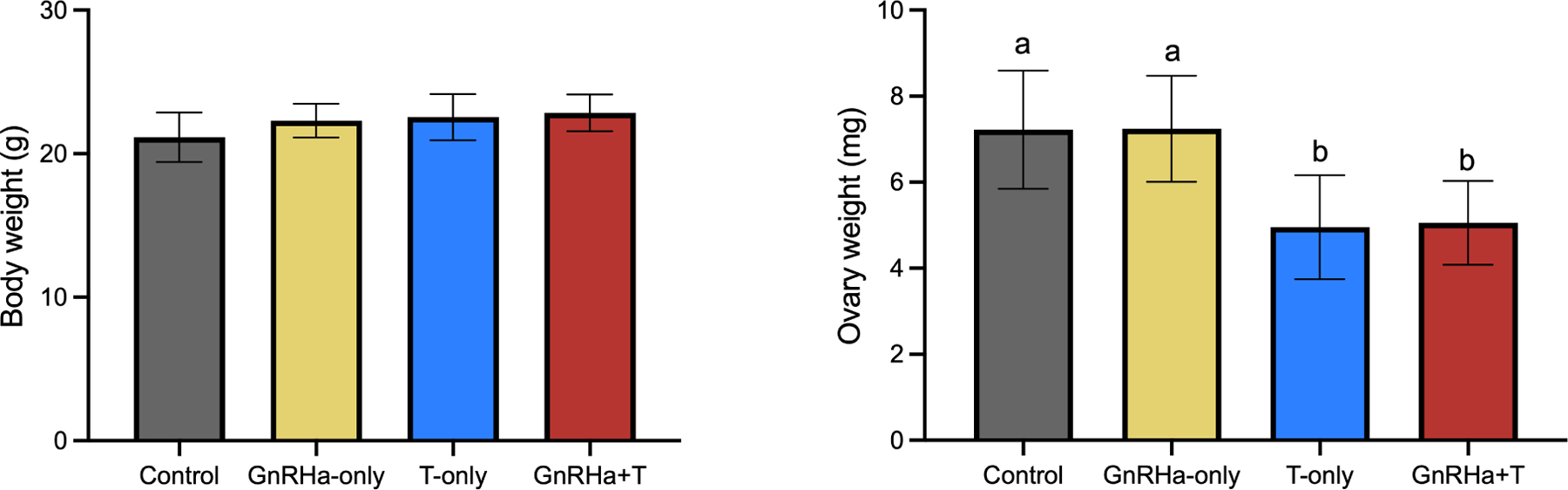
A) Body weight. B) Ovary weight. Data are expressed as mean ± SD, ANOVA followed by Tukey test; letters (a,b) denote significance.
Immediate IVF in T-treated animals had compromised IVF outcomes, but outcomes recovered after T washout.
T-only and GnRHa+T animals on T treatment (Immediate IVF group) (Figure 5) had reduced oocyte yield (Control=21±4.5; GnRHa=22±3.4; T=12±3.6; GnRHa+T=11±4.7, P<0.0001), fertilization rate (Control=90%±15; GnRHa=84%±17; T=49%±15; GnRHa±T=42%±17, P<0.0001), maturity rate (Control=23%±8.1; GnRHa=31%±6.9; T=11%±3.8; GnRHa±T=15%±6.7, P<0.0001), number of 2-cell embryos (Control=4.8±2.2; GnRHa=5.6±1.9; T=2.1±1.5; GnRHa+T=1.8±0.71, P=0.0004), number of the 4–8-cell embryos (Control=4.8±2.2; GnRHa=5.6±1.9; T=1.9±1.2; GnRHa+T=1.5±0.76, P<0.0013), number of morulae (Control=4.5±2.1; GnRHa=5.6±1.9; T=1.9±1.2; GnRHa+T=1.4±0.52, P<0.0044), and number of blastocysts (Control=4.4±1.8; GnRHa=4.8±2.0; T=1.4±0.53; GnRHa+T=1.4±0.52, P<0.005) when compared to controls and GnRHa-only animals. GnRHa+T on T treatment (immediate IVF) showed elevated levels of estradiol when compared to control group (p= 0.0302). Progesterone levels were decreased in T and GnRHa+T animals on T treatment (p= 0.0035 and p = 0.0094, respectively). No difference was found in AMH levels among groups (p>0.05) (Figure 4)
Figure 5). IVF outcomes.
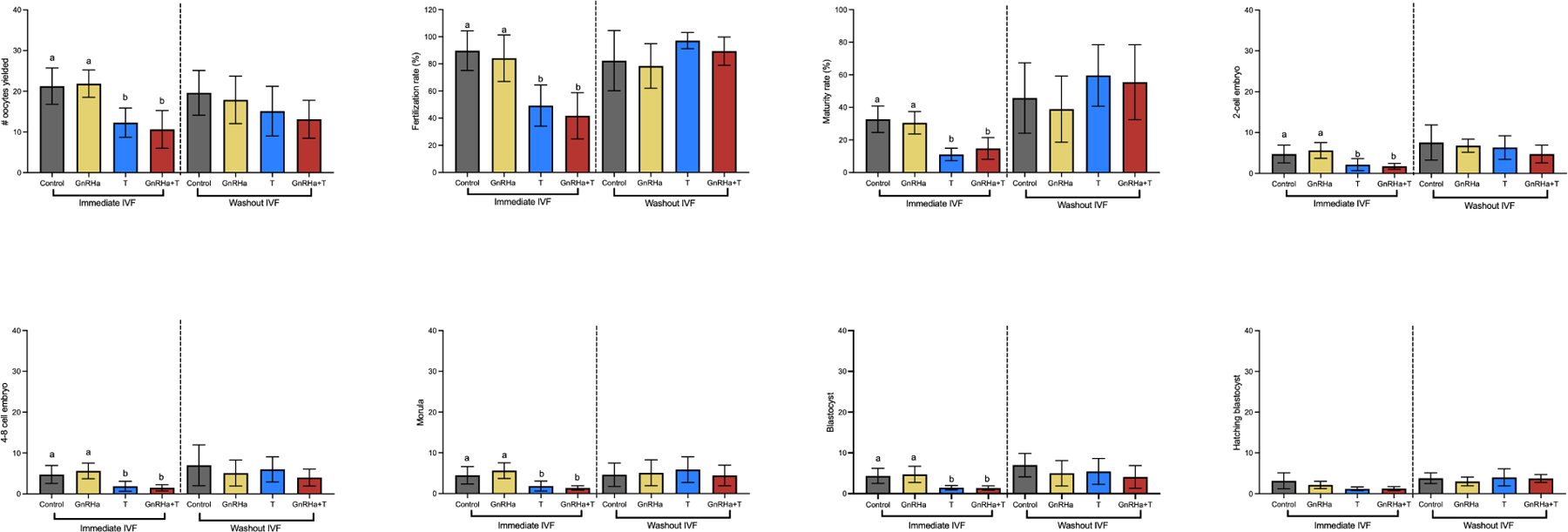
A) Numbers of oocytes yielded. B) Fertilization rate. C) Maturity rate. D) Numbers of 2-cell embryo. E) Numbers of 4–8-cell embryo. F) Numbers of morula. G) Numbers of blastocysts. H) Numbers of hatching blastocyst. Data are expressed as mean ± SD, ANOVA followed by Tukey test; letters (a,b) denote significance.
Figure 4). Terminal hormones levels.
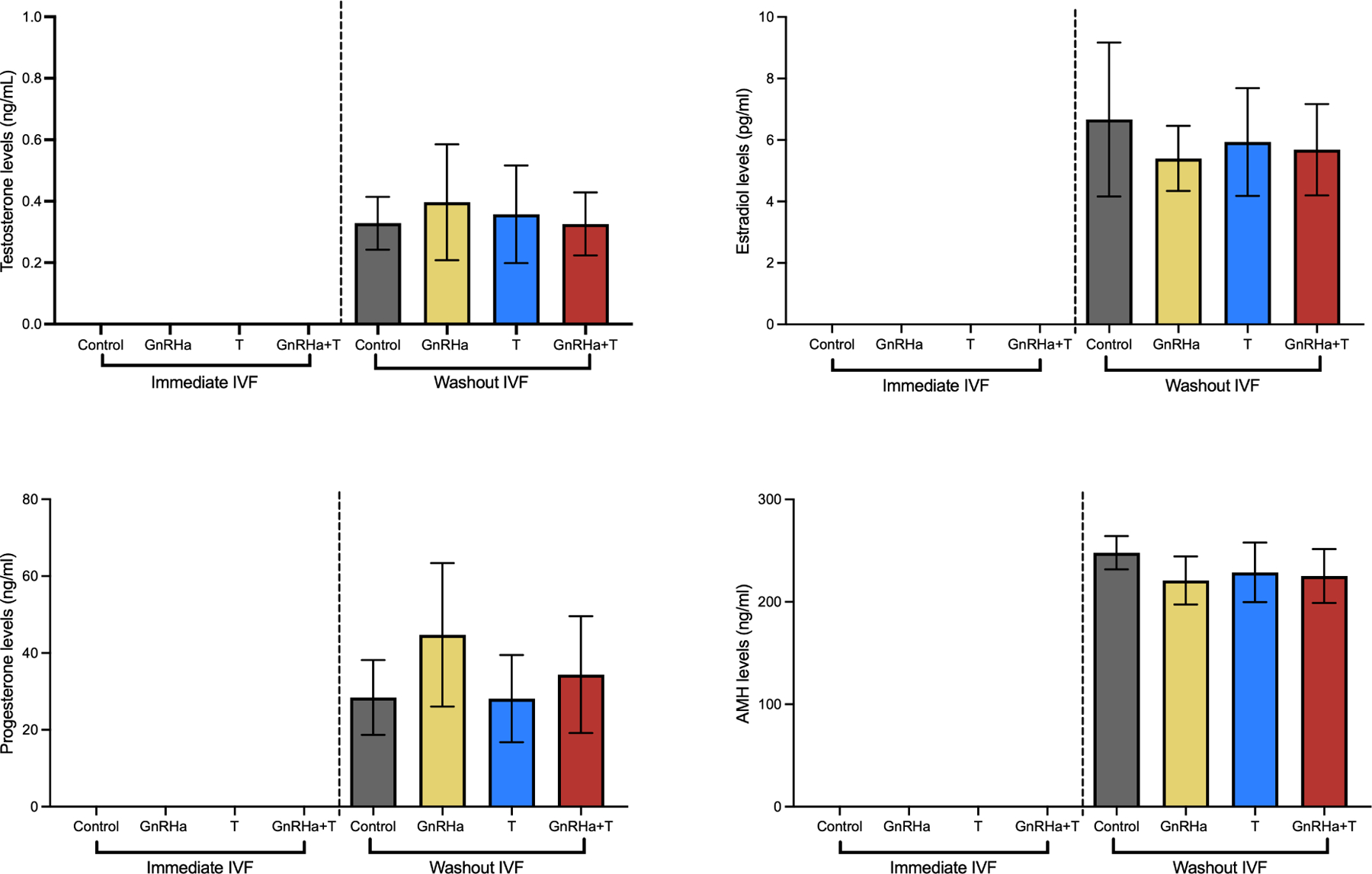
A) Testosterone levels; B) Estradiol levels; C) Progesterone levels; D) AMH levels. Data are expressed as mean ± SD, ANOVA followed by Tukey test.
For groups with IVF performed 2 weeks after cessation of T treatment (washout IVF) (Figure 5), T-only and GnRHa+T -treated mice had similar oocyte yields (P=0.0780), fertilization rate (P=0.1844), maturity rate (P=0.3277), number of 2-cell embryos (P=0.3326), number of 4–8-cell embryos (0.3820), number of morula (0.5192), and number of blastocysts (P=0.331), when compared to controls and GnRHa-only animals. Additionally, no alteration on estradiol, progesterone and AMH levels were found among groups after the cessation of T (Figure 4).
Discussion
Due to the currently limited available data about the effects of gender-affirming testosterone exposure on fertility outcomes, several medical organizations advise fertility preservation counseling before initiating T treatment. For patients already on T, many clinics discontinue T therapy for 1 to 6 months prior to stimulation or until the resumption of menses (29, 30). In this study, the total number of oocytes yielded were significantly reduced in animals currently on T treatment (immediate IVF: T and GnRHa+T groups) in relation to controls and GnRHa-only treated animals. These differences resolved if IVF was performed after a T washout period, and IVF outcomes did not appear to be affected by GnRHa pretreatment. The reduced ovarian weight in T and GnRHa+T treated animals in the immediate group is likely related to fewer corpora lutea, and it is consistent with the total number of oocytes retrieved after ovarian stimulation treatment. Fertilization and maturity rate were also impaired by T treatment as well as embryo development outcomes (2-cell to blastocyst) in the immediate IVF group. After T washout, these parameters also recovered and were comparable to controls and GnRHa animals. The persistently low ovarian weights in T and GnRHa+T animals after T cessation might be related to a morphological alteration of ovary. While we did not perform ovarian morphological analysis in this study, we recently showed that female mice treated with 0.9mg of T injected weekly for 6 weeks showed aberrant ovarian stroma alterations after T cessation (31). There were no significant differences in terminal estradiol among the washout IVF groups or AMH levels amongst the immediate IVF and washout IVF animals. The increase in estradiol levels in T-treated animals compared to the control group in the immediate IVF arm is likely because both the controls and T-only groups reached puberty in the absence of HPG axis suppression with GnRH agonist. As such, while controls continued to undergo cyclic ovarian changes, testosterone treatment in the T only group led to the arrest of follicle growth/atresia leading to persistence of a larger number of follicles. Indeed, physiological levels of testosterone in females are essential for normal follicular development, enhancing follicle recruitment and promoting follicular growth and development. Conversely, supraphysiological testosterone levels in females induce follicle development arrest and maintain premature follicles in the ovary (32–35). In this way, when the ovarian stimulation treatment was initiated, these arrested follicles resumed steroidogenesis and growth, resulting in higher estradiol levels. The decreased progesterone levels in T and GnRHa+T treated animals in the immediate IVF cohort may be related to the lower numbers of oocytes yielded. After ovulation, the rise in progesterone levels is maintained by corpora lutea. Since T and GnRHa+T animals in the immediate IVF cohort showed a lower number of oocytes retrieved, is also expected lower numbers of corpora lutea, which was reflected in progesterone levels (36, 37).
Concerns related to stopping or delaying hormonal treatment have been investigated in previous studies (17, 38, 39), and some transmasculine individuals may prefer to proceed with IVF without T cessation due to distress and worsening gender dysphoria (40, 41), while off of GAHT. The negative impact caused by testosterone treatment in animals from immediate IVF arm may relate to disruption of the follicular steroidal milieu (healthy follicles have a higher estrogen to androgen ratio) and consequent negative impact on oocyte/embryo quality. Treatment of in vitro-grown follicles with testosterone treatment were shown to have a negative impact on meiosis resumption reflected as a lower potential to reinitiate meiosis and presence of chromosomes outside the metaphase plate in MII oocytes, which could affect the fertilization rate and their ability to undergo normal embryonic development (42, 43). In the present study, we did not perform any molecular nor morphological analysis in the retrieved oocytes and embryos, which could better address the negative outcomes on embryo development (44). Other studies have shown that female mice treated with T responded to ovarian stimulation by ovulating similar numbers of oocytes as controls that were fertilized and cleaved in vitro (45). In this study, animals were injected with T suspended in oil as opposed to T implant in our study, and their weekly serum T levels were lower than in our study, which may have affected the fertility outcomes. Currently, no clinical studies address the fertility potential of transmasculine youth pretreated GnRHa followed immediately by T, and thus never completing natal puberty. However, there are currently two case reports in the literature demonstrating successful oocyte cryopreservation in transmasculine youth treated with GnRHa who had not yet started T (46, 47). Additionally, researchers showed for the first time a live birth from an oocyte retrieved from a transgender man on T during stimulation, indicating that this outcome is possible without T cessation (41). Despite a negative impact on oocyte and blastocyst quantity in animals currently on testosterone (immediate IVF: T and GnRHa+T groups), we showed these animals could produce mature oocytes, which were fertilized and cleaved from the two-cell stage until blastocyst. These findings are comparable with our previous study that showed the blockage of puberty and T treatment affected the pool of primary follicles, which can be used for in vitro maturation (21). Additionally, the results found in this present study regarding IVF outcomes after T cessation mirror the findings in humans, which demonstrated the number of retrieved oocytes, and maturity rate among transgender adolescents are comparable with those of adolescent cisgender females (48, 49).
Moreover, our data showed that pretreatment with GnRHa did not affect IVF outcomes, positively or negatively. Indeed, pubertal suppression via GnRHa is reversible, with the commencement of normal puberty upon cessation (50). However, it is important to consider the age of the animals and the experimental design used in this study when interpreting the results since the animals’ ages and different ovarian stimulation protocols/medications could interfere in the IVF outcomes. The limitations of this study include the non-optimization of the dose of gonadotropins utilized for ovarian stimulation, which could improve IVF outcomes particularly in the immediate IVF group. Additionally, we only worked with one time point of T exposure (6 weeks) and washout (2 weeks. These timepoints were chosen based on the timing when animals resumed cyclicity, an approach similar to that clinicians use to decide when the ovarian stimulation treatment should start in humans. Our findings are limited to a short-term T exposure and clearance paradigm of T.
Conclusion
In conclusion, this study demonstrated that besides the negative impact on IVF outcomes, female mice pretreated with GnRHa followed by testosterone for 6 weeks could produce viable oocytes, which reached the blastocyst stage after fertilization. We also showed that after T washout, reproductive outcomes were comparable to controls and GnRHa-treated animals. Significant gaps exist in the literature regarding the consequences of T-GAHT in transmasculine youth, and our study attempts to address some of these unknowns, although the findings we show here must be taken cautiously since a mouse model does not always translate directly to humans. Future efforts are needed to comprehensively understand long-term T’s impacts on oocyte quality, especially aneuploidy rates, pregnancy outcomes, and live birth rates.
Funding Statement:
This work was supported by the Michigan Institute for Clinical and Health Research grants KL2 TR 002241 and UL1 TR 002240 (C.D.C.); University of Michigan Office of Research funding U058227 (A.S.); American Society for Reproductive Medicine/Society for Reproductive Endocrinology and Infertility Grant (M.B.M.); and National Institutes of Health R01-HD098233 (M.B.M.). The University of Virginia Center for Research in Reproduction, Ligand Assay and Analysis Core Facility was supported by the Eunice Kennedy Shriver NICHD/NIH grants P50-HD028934 and R24-HD102061.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: CDC: none; AW: none; MB: none; VP: none; AS: none; MBM: none.
Data Sharing Statement:
The data underlying this article are available in the article.
References
- 1.Mejia-Otero JD, White P, Lopez X. Effectiveness of Puberty Suppression with Gonadotropin-Releasing Hormone Agonists in Transgender Youth. Transgend Health 2021;6:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP et al. Use of Gonadotropin-Releasing Hormone Analogs in Children: Update by an International Consortium. Horm Res Paediatr 2019;91:357–72. [DOI] [PubMed] [Google Scholar]
- 3.Clayton A Gender-Affirming Treatment of Gender Dysphoria in Youth: A Perfect Storm Environment for the Placebo Effect-The Implications for Research and Clinical Practice. Arch Sex Behav 2023;52:483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bungener SL, de Vries ALC, Popma A, Steensma TD. Sexual Experiences of Young Transgender Persons During and After Gender-Affirmative Treatment. Pediatrics 2020;146. [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Bernardi LA, Pavone ME, Feinberg EC, Moravek MB. Oocyte cryopreservation among transmasculine youth: a case series. J Assist Reprod Genet 2018;35:2057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safer JD, Tangpricha V. Care of the Transgender Patient. Ann Intern Med 2019;171:775–6. [DOI] [PubMed] [Google Scholar]
- 7.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017;102:3869–903. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Fuqua J, Eugster EA. Characteristics of Referrals for Gender Dysphoria Over a 13-Year Period. J Adolesc Health 2016;58:369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton E, Abhari S, Tangpricha V, Futral C, Mehta A. Family Planning and Fertility Counseling Perspectives of Gender Diverse Adults and Youth Pursuing or Receiving Gender Affirming Hormone Therapy. Urology 2023;171:244–50. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz AR, Moravek MB. Reproductive potential and fertility preservation in transgender and nonbinary individuals. Curr Opin Obstet Gynecol 2021;33:327–34. [DOI] [PubMed] [Google Scholar]
- 11.Borrás A, Manau MD, Fabregues F, Casals G, Saco A, Halperin I et al. Endocrinological and ovarian histological investigations in assigned female at birth transgender people undergoing testosterone therapy. Reprod Biomed Online 2021;43:289–97. [DOI] [PubMed] [Google Scholar]
- 12.De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online 2017;34:557–66. [DOI] [PubMed] [Google Scholar]
- 13.Nahata L, Chen D, Moravek MB, Quinn GP, Sutter ME, Taylor J et al. Understudied and Under-Reported: Fertility Issues in Transgender Youth-A Narrative Review. J Pediatr 2019;205:265–71. [DOI] [PubMed] [Google Scholar]
- 14.Kelley CE, Davidge-Pitts CJ. Breaking Down Barriers to Reproductive Care for Transgender People. AACE Clin Case Rep 2022;8:96–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murad MH, Elamin MB, Garcia MZ, Mullan RJ, Murad A, Erwin PJ et al. Hormonal therapy and sex reassignment: a systematic review and meta-analysis of quality of life and psychosocial outcomes. Clin Endocrinol (Oxf) 2010;72:214–31. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Simons L, Johnson EK, Lockart BA, Finlayson C. Fertility Preservation for Transgender Adolescents. J Adolesc Health 2017;61:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiniara LN, Viner C, Palmert M, Bonifacio H. Perspectives on fertility preservation and parenthood among transgender youth and their parents. Arch Dis Child 2019;104:739–44. [DOI] [PubMed] [Google Scholar]
- 18.Amir H, Oren A, Klochendler Frishman E, Sapir O, Shufaro Y, Segev Becker A et al. Oocyte retrieval outcomes among adolescent transgender males. J Assist Reprod Genet 2020;37:1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009;94:3132–54. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg ME, Gower AL, McMorris BJ, Rider GN, Shea G, Coleman E. Risk and Protective Factors in the Lives of Transgender/Gender Nonconforming Adolescents. J Adolesc Health 2017;61:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dela Cruz C, Kinnear HM, Hashim PH, Wandoff A, Nimmagadda L, Chang FL et al. A mouse model mimicking gender-affirming treatment with pubertal suppression followed by testosterone in transmasculine youth. Hum Reprod 2023;38:256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaish I, Tordjman K, Amir H, Malinger G, Salemnick Y, Shefer G et al. Functional ovarian reserve in transgender men receiving testosterone therapy: evidence for preserved anti-Müllerian hormone and antral follicle count under prolonged treatment. Hum Reprod 2021;36:2753–60. [DOI] [PubMed] [Google Scholar]
- 23.Laffan SB, Posobiec LM, Uhl JE, Vidal JD. Species Comparison of Postnatal Development of the Female Reproductive System. Birth Defects Res 2018;110:163–89. [DOI] [PubMed] [Google Scholar]
- 24.Hashim PH, Kinnear HM, Cruz CD, Padmanabhan V, Moravek MB, Shikanov A. Pharmacokinetic comparison of three delivery systems for subcutaneous testosterone administration in female mice. Gen Comp Endocrinol 2022;327:114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cora MC, Kooistra L, Travlos G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol Pathol 2015;43:776–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sztein JM, Noble K, Farley JS, Mobraaten LE. Comparison of permeating and nonpermeating cryoprotectants for mouse sperm cryopreservation. Cryobiology 2001;42:28–39. [DOI] [PubMed] [Google Scholar]
- 27.Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 2006;65:1716–26. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi H, Liu C. Archiving and distributing mouse lines by sperm cryopreservation, IVF, and embryo transfer. Methods Enzymol 2010;476:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deutsch MB, Feldman JL. Updated recommendations from the world professional association for transgender health standards of care. Am Fam Physician 2013;87:89–93. [PubMed] [Google Scholar]
- 30.asrm@asrm.org ECotASfRMEa. Access to fertility services by transgender and nonbinary persons: an Ethics Committee opinion. Fertil Steril 2021;115:874–8. [DOI] [PubMed] [Google Scholar]
- 31.Kinnear HM, Hashim PH, Dela Cruz C, Chang FL, Rubenstein G, Nimmagadda L et al. Presence of ovarian stromal aberrations after cessation of testosterone therapy in a transgender mouse model. Biol Reprod 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod 1999;14:2328–32. [DOI] [PubMed] [Google Scholar]
- 33.Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 1999;61:353–7. [DOI] [PubMed] [Google Scholar]
- 34.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 2005;146:3185–93. [DOI] [PubMed] [Google Scholar]
- 35.Yoo M, Tanaka T, Konishi H, Tanabe A, Taniguchi K, Komura K et al. The Protective Effect of Testosterone on the Ovarian Reserve During Cyclophosphamide Treatment. Onco Targets Ther 2020;13:2987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez EG, Giviziez CR, Sanchez HM, Agostinho PL, Barros PS, Approbato MS. Low progesterone levels and ovulation by ultrasound assessment in infertile patients. JBRA Assist Reprod 2016;20:13–6. [DOI] [PubMed] [Google Scholar]
- 37.Dozortsev DI, Diamond MP. Luteinizing hormone-independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil Steril 2020;114:191–9. [DOI] [PubMed] [Google Scholar]
- 38.Armuand G, Dhejne C, Olofsson JI, Stefenson M, Rodriguez-Wallberg KA. Attitudes and experiences of health care professionals when caring for transgender men undergoing fertility preservation by egg freezing: a qualitative study. Ther Adv Reprod Health 2020;14:2633494120911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahata L, Tishelman AC, Caltabellotta NM, Quinn GP. Low Fertility Preservation Utilization Among Transgender Youth. J Adolesc Health 2017;61:40–4. [DOI] [PubMed] [Google Scholar]
- 40.Stark BA, Mok-Lin E. Fertility preservation in transgender men without discontinuation of testosterone. F S Rep 2022;3:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenwald P, Dubois B, Lekovich J, Pang JH, Safer J. Successful In Vitro Fertilization in a Cisgender Female Carrier Using Oocytes Retrieved From a Transgender Man Maintained on Testosterone. AACE Clin Case Rep 2022;8:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderiesz C, Trounson AO. The effect of testosterone on the maturation and developmental capacity of murine oocytes in vitro. Hum Reprod 1995;10:2377–81. [DOI] [PubMed] [Google Scholar]
- 43.Romero S, Smitz J. Exposing cultured mouse ovarian follicles under increased gonadotropin tonus to aromatizable androgens influences the steroid balance and reduces oocyte meiotic capacity. Endocrine 2010;38:243–53. [DOI] [PubMed] [Google Scholar]
- 44.Anesetti G, Chávez-Genaro R. Neonatal androgenization in rats affects oocyte maturation. Reprod Sci 2021;28:2799–806. [DOI] [PubMed] [Google Scholar]
- 45.Bartels CB, Uliasz TF, Lestz L, Mehlmann LM. Short-term testosterone use in female mice does not impair fertilizability of eggs: implications for the fertility care of transgender males. Hum Reprod 2021;36:189–98. [DOI] [PubMed] [Google Scholar]
- 46.Martin CE, Lewis C, Omurtag K. Successful oocyte cryopreservation using letrozole as an adjunct to stimulation in a transgender adolescent after GnRH agonist suppression. Fertil Steril 2021;116:522–7. [DOI] [PubMed] [Google Scholar]
- 47.Rothenberg SS, Witchel SF, Menke MN. Oocyte Cryopreservation in a Transgender Male Adolescent. N Engl J Med 2019;380:886–7. [DOI] [PubMed] [Google Scholar]
- 48.Adeleye AJ, Cedars MI, Smith J, Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet 2019;36:2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leung A, Sakkas D, Pang S, Thornton K, Resetkova N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril 2019;112:858–65. [DOI] [PubMed] [Google Scholar]
- 50.Manasco PK, Pescovitz OH, Feuillan PP, Hench KD, Barnes KM, Jones J et al. Resumption of puberty after long term luteinizing hormone-releasing hormone agonist treatment of central precocious puberty. J Clin Endocrinol Metab 1988;67:368–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.


