The chemical industry does not enjoy a reputation for being an environmentally friendly undertaking. Incidents such as the release of toxic dioxines in Seveso, Italy, the dumping of hazardous wastes in the Love Canal near Niagara Falls, USA, or the poisoning of thousands of Indians due to a gas leak at a Union Carbide pesticide plant in Bhopal, India, are a few examples of the health and environmental dangers that come with this industry. Since then, increasingly stricter regulations have been applied to prevent such disasters, and, indeed, the environmental track record of the chemical industry has improved vastly. But these ordinances also put pressure on the industry to improve existing—or even to develop new—processes that are more environmentally friendly. This challenge often leads scientists to nature, as many chemicals used and produced by the pharmaceutical or chemical industries have natural origins or are modified natural compounds. And nature has also already provided the catalysts necessary to produce them: enzymes.
Clearly, due to their efficiency, specificity and reaction range, enzymes are far superior to most man-made catalysts for the synthesis of complex compounds and can be used to replace more energy-dependent and waste-producing reactions. ‘Within the next decade we will see large developments in chemistry’, Victor de Lorenzo, Vice Director of the National Centre for Biotechnology in Madrid, Spain, said about their potential, ‘There will be a new wave of biological products and applications of molecular biology in the area of the chemical industry’.
More than 3000 different enzymes from a wide variety of organisms have been identified and many have found uses in biotechnology as well as the pharmaceutical, chemical and food industries. But their usefulness can be limited by various factors, such as enzyme instability at high temperatures, extreme pH, the presence of organic solvents and the need for co-factors as well as their narrow substrate range. In the last decade, the discovery of organisms adapted to such extreme conditions has therefore sparked an interest in their enzymes, particularly thermophilic ones (Table I). ‘It is to be expected that these enzymes are even more extreme and that they have an enormous resistance [to extreme conditions]’, Karl Stetter, Director of the Department of Microbiology and the Centre for Archeae at the University of Regensburg, Germany, said. And as extremophile enzymes have great economic potential, various companies are now searching for new or modifying existing catalysts for use in industry and research laboratories.
Table I. Cloned thermophilic enzymes and their sources.
| Enzyme | Source | Optimal temperature (°C) |
|---|---|---|
| α-Amylases | Pyrococcus furiosus | 100 |
| Thermococcus profundus | 80 | |
| Pullulanases | Fervidobacterium pennavorans | 85 |
| Thermotaga maritima | 90 | |
| Pyrococcus wosei | 100 | |
| Pyrococcus furiosus | 105 | |
| Endoglucanase | Thermotaga neapolitana | 106 |
| Anaerocellum thermophilum | 95 | |
| Pyrococcus furiosus | 100 | |
| β-glucosidase | Pyrococcus furiosus | 102–105 |
| Thermotaga maritima | 75 | |
| Proteases | Pyrococcus furiosus | 85 |
| Thermococcus stetteri | 85 | |
| Sulfolobus acidocaldarius | 90 | |
| Bacterial DNA polymerases | Thermus aquaticus (Taq) | |
| Thermus thermophilus (Tth) | ||
| Thermotaga maritima (Tma) | ||
| Archaeal DNA polymerases | Pyrococcus wosei (Pwo) | |
| Pyrococcus furiosus (Pfu) | ||
| Thermococcus litoralis (Vent) | ||
| Glucose isomerase | Thermus flavus | 90 |
| Thermoanaerobacterium | 80 | |
| Thermotaga neapolitana | 95 | |
| Alcohol-dehydrogenase | Thermoanaerobacterium ethanolicus | 90 |
| Sulfolobus solfataricus | 95 | |
| Esterase | Pyrococcus furiosus | 100 |
Molecular biology and biotechnology are the most prominent examples of fields that have been truly revolutionised by thermostable enzymes. The DNA polymerase used in the polymerase chain reaction was originally isolated from the bacterium Thermus aquaticus in the hot springs of Yellowstone National Park, and, since then, other DNA polymerases with better proofreading abilities have been found and commercialised.
While biotechnology uses only tiny amounts of high-purity enzyme preparations, the food industry has been using vast amounts of enzymes in large-scale processes. The liquefaction, saccharification and isomerisation of starch and the degradation of cellulose are performed at high temperatures, and so the discovery of thermostable sugar-modifying enzymes from archaebacteria in the 1990s holds huge potential for increasing efficiency. The archaebacteria Pyrococcus wosei and Pyrococcus furioso, for example, were discovered in the truly hellish conditions near undersea volcanoes and can withstand temperatures above the boiling point of water. These organisms have already yielded α-amylases and pullulanases that function optimally at or above 100°C.
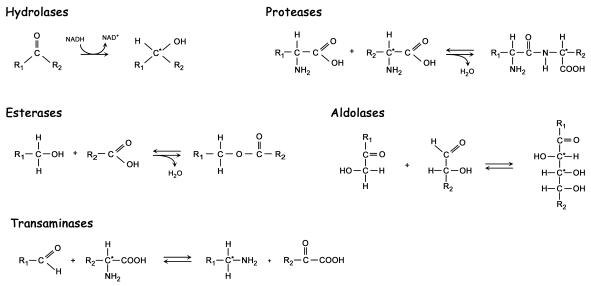
Thermophilic enzymes are also interesting due to their inherent stability in many of the organic solvents required for the synthesis of fine chemicals in cases where substrates are poorly water-soluble. This selectivity is particularly important when enantiomerically pure compounds are demanded, as in the production of many drugs—here enzymes clearly beat any man-made catalyst (Figure 1). Some of these processes can also be catalysed by whole organisms rather than isolated enzymes, such as in the production of various antibiotics. Another example is the field of bioremediation, where researchers combine various enzyme genes within micro-organisms to create new biochemical pathways capable of breaking down complex toxic molecules.
Fig. 1. Enzymes and reactions used in the synthesis of fine chemicals and pharmaceuticals with one or more optical active carbon atoms.
Mining and mineral processing firms are also using thermophilic and acidophilic microbes to release oxidised metals from mineral sulfides—mostly gold, copper and cobalt. Thiobacillus ferrooxidans, Leptospirillum ferrooxidans and other Thiobacillus species are the most important sulfur-oxidising bacteria in mineral-processing bioreactors. As tests have shown that the efficiency of metal extraction increases in proportion to temperature, the industry is now looking for more thermostable bacteria. Indeed, some archaebacteria, most notably Sulfolobus strains, are being tested in pilot projects at temperatures of ∼80°C.
As extremophile organisms hold a huge economic potential for a wide variety of applications, the search is on for novel bacteria and, more importantly, their enzymes. Of course, thermostable organisms generate the most interest, but researchers are also looking for bacteria that are able to survive at extreme pHs, high salt concentrations, drought or temperatures around freezing point. But the isolation of new organisms is not an easy undertaking and requires more than simply bringing a sample from the ocean floor or the Arctic into the laboratory. Microbiologists estimate that <10% of all organisms existing in a given environment are actually cultivatable. But here, genomics is also revolutionising this field, enabling researchers to now gather information on those organisms without having to cultivate them first. ‘They might not be culturable’, de Lorenzo said about them, ‘but if you have access to their DNA, who cares?’.
This is the approach adopted by Diversa, a company in San Diego, CA, specialised in extremophile enzymes. The company collects samples from diverse environments, such as deep-ocean vents, arctic tundra, contaminated industrial sites, deserts, endosymbionts of plants and bacteria, and prepares genomic DNA from them to create gene expression libraries representing more than 1 million micro-organisms. Diversa’s researchers then search these ‘metagenomic libraries’ for new variants of enzymes either in silico or by using high-throughput technology, which allows them to identify, for instance, up to 80 new lipase genes from 1 g of desert soil. Diversa is also looking for completely new enzyme activities by screening environmental samples with cell-sorters and labelled substrates. Karl Stetter, co-founder of Diversa and one of their scientific advisers, thinks that these new approaches will allow the company to find any enzyme that industry needs for a specific task. ‘My point is that nature has already solved these problems’, he said.
To search for extremophile organisms in the wild, one has to go to extreme places. Iceland is clearly one of them. Thousands of hot springs provide ample evidence that the Earth’s fiery interior is bubbling away just beneath the surface. This is where Prokaria, a company based in Reykjavik, looks for thermophilic enzymes. In contrast to Diversa, Prokaria carries out some degree of cultivation before they extract genomic DNA, using a technique they call ‘oligotrophic enrichment’. ‘The idea is that we imitate Nature’, Jakob Kristjansson, founder and CEO of Prokaria, explained. In 1999, the Icelandic government granted Prokaria an exclusive 5-year licence to carry out biospectoring in 28 geothermal areas, which provides the company with a wide range of various conditions—temperature, pH, salt, sulfour and other chemicals, many often even within one spring. ‘This is why hot springs are so good because you have an n-dimensional system’, Kristjansson said, ‘We go and find this spring and then we start’. The researchers simply put water from the spring into canisters and return them into the spring. Up to 3 weeks later, the organisms best adapted to the specific conditions have slowly accumulated, and their DNA is extracted from the sample. Whole bacterial genomes are sequenced at the facilities of deCODE Genetics—its CEO, Kári Stefánsson, provided the initial funding to establish Prokaria in 1998. To discover new variants of enzymes, the sequences are then screened in silico for specific gene families. ‘It doesn’t matter which gene family you take. Every time you find 50 to 100 new genes’, Kristjansson said about the efficiency of this approach. Prokaria is now marketing this technology to the biotech, chemical and pharmaceutical industries to provide tailor-made enzymes for particular applications and reaction conditions.
However, Maria-Regina Kula, Director of the Institute of Enzyme Technology at the University of Düsseldorf, Germany, thinks that this is just one solution. ‘Looking for new enzymes is always good, but right now there are many developments to improve existing ones’, she said, pointing out that today’s large-scale industrial processes already use modified, tailor-made enzymes. ‘Of course, enzymes have to be adapted to the process’, Stetter agreed, ‘They are just crude material that still has to be optimised’. Diversa therefore also use genomic technologies, such as site-specific mutagenesis, evolutionary mutagenesis or gene shuffling—coupled with high-throughput screening and the increasing knowledge of the structure of extremophile enzymes—to adapt them to industrial processes and conditions.
Indeed, industrial processes often rely on tailor-made enzymes that have undergone many rounds of modification. ‘And they will only be replaced if there is something that is much better’, Stetter said. In fact, although the interest in enzymes is certainly growing, their application in the chemical and pharmaceutical industry is often a matter of economics. ‘People who could improve the production of nylon by 0.1% would become rich’, said Kula. ‘The process just has to be large enough’. In contrast to these economies of scale, new enzymes for molecular biology can be financially viable even if they are produced in only tiny amounts, she pointed out, because their products are of great value.
But despite the genomics tools and technologies to circumvent cultivating micro-organisms, some problems remain. ‘They all have to screen’, Kula said about the companies, ‘the question is only how they do the screening’. And this is still the main bottleneck. Although high-throughput technologies allow companies to screen up to 1 billion clones a day, they can find only those enzymes that they are looking for and that are expressed as active enzymes. de Lorenzo thinks that ‘the expression systems that we have at the moment are very limited’, and will not necessarily produce active enzymes that are adapted to extreme conditions. Furthermore, most expression systems have been developed for bacterial genes, and are not suitable for expressing enzymes from other sources, such as archeae. Researchers in this field are therefore looking for what de Lorenzo calls ‘a unified expression system’ that eventually will produce active enzymes regardless of the organism from which the gene was isolated.
This can be bypassed through pure in silico screening for enzyme genes. But Kula is cautious about the application of these technologies. ‘Right now, there is still too much extrapolation’, she said, ‘We still don’t understand enough of this’. Indeed, even if a novel gene shares extensive homologies with an existing class of enzyme genes, it does not necessarily follow that its product has the same enzymatic activity. For Kula it is therefore still necessary to get the biochemistry right. ‘Without biochemical proof, I believe in only 10% of these claims’, she said.
Nevertheless, both Kula and de Lorenzo are confident that the use of genomic technologies and improved expression systems will make the search for new enzymes easier. And increasing what is known about the biochemistry of micro-organisms, their origins and the strategies they use to adapt to their environment will eventually improve screening and expression. Such developments will also allow researchers to access a largely untapped source of potentially useful enzymes. ‘Plants still have a large reservoir of useful enzymes but those are not so easily accessible’, Kula said. Indeed, they are true masters of synthesising complex molecules—many pharmaceutical compounds, such as aspirin, taxol and morphine, are plant secondary metabolites. Thus, elucidating the biochemical pathways of plants and gaining access to their enzymes would give the pharmaceutical and chemical industries completely new avenues to explore. ‘Plants will be the next frontier in the search for new enzymes’, de Lorenzo thinks.
Hot springs, such as the Grand Prismatic Hot Spring in Yellowstone National Park, Wyoming, USA, are ideal places to look for extremophile organisms
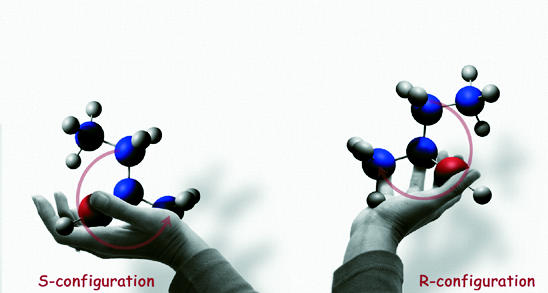
Enzymes are particularly interesting for the synthesis of enantiopure organic compounds with one or more optical active centers, as they are highly specific for either the R- or the S-isoform (2-butanol in this example)