Abstract
The actin-based motility of Listeria monocytogenes requires the addition of actin monomers to the barbed or plus ends of actin filaments. Immunofluorescence micrographs have demonstrated that gelsolin, a protein that both caps barbed ends and severs actin filaments, is concentrated directly behind motile bacteria at the junction between the actin filament rocket tail and the bacterium. In contrast, CapG, a protein that strictly caps actin filaments, fails to localize near intracellular Listeria. To explore the effect of increasing concentrations of gelsolin on bacterial motility, NIH 3T3 fibroblasts stably transfected with gelsolin cDNA were infected with Listeria. The C5 cell line containing 2.25 times control levels of gelsolin supported significantly higher velocities of bacterial movement than did control fibroblasts (mean ± standard error of the mean, 0.09 ± 0.003 μm/s [n = 176] versus 0.05 ± 0.003 μm/s [n = 65]). The rate of disassembly of the Listeria-induced actin filament rocket tail was found to be independent of gelsolin content. Therefore, if increases in gelsolin content result in increases in Listeria-induced rocket tail assembly rates, a positive correlation between gelsolin content and tail length would be expected. BODIPY-phalloidin staining of four different stably transfected NIH 3T3 fibroblast cell lines confirmed this expectation (r = 0.92). Rocket tails were significantly longer in cells with a high gelsolin content. Microinjection of gelsolin 1/2 (consisting of the amino-terminal half of native gelsolin) also increased bacterial velocity by more than 2.2 times. Microinjection of CapG had no effect on bacterial movement. Cultured skin fibroblasts derived from gelsolin-null mice were capable of supporting intracellular Listeria motility at velocities comparable to those supported by wild-type skin fibroblasts. These experiments demonstrated that the surface of Listeria contains a polymerization zone that can block the barbed-end-capping activity of both gelsolin and CapG. The ability of Listeria to uncap actin filaments combined with the severing activity of gelsolin can accelerate actin-based motility. However, gelsolin is not absolutely required for the actin-based intracellular movement of Listeria because its function can be replaced by other actin regulatory proteins in gelsolin-null cells, demonstrating the functional redundancy of the actin system.
The gram-positive rod Listeria monocytogenes is a food-borne pathogen that is capable of causing serious infections in pregnant women, neonates, elderly persons, and immunocompromised patients. The ability of Listeria to avoid the humoral immune system and cause disease in individuals with impaired cell-mediated immunity is explained by the unusual intracellular life cycle of this organism (26). Listeria is readily phagocytosed by host cells, including epithelial cells and hepatocytes (9, 11). By producing the exotoxin listeriolysin O, the organism is able to lyse the confining phagolysosomal membrane and escape into the cytoplasm of host cells (14, 19). Once within the cytoplasm, Listeria is able to stimulate the polar assembly of host cell actin. New actin monomers are added to the actin filament tails directly behind the bacterium, providing the force for the bacterium to migrate to the peripheral membrane of the cell (17, 21). Once the bacterium reaches the periphery, it pushes the cell membrane outward to form a projection, or filopodium. This filopodium is subsequently ingested by an adjacent cell, and the cycle begins again. In this way, Listeria is able to spread from cell to cell without ever coming in contact with the extracellular environment.
Exploration of the mechanisms by which Listeria usurps the host cell contractile system is leading to a better understanding of how this bacterium spreads from cell to cell and causes disease. As an added dividend, this new understanding promises to provide new insights into the regulation of actin-based motility in nonmuscle host cells. Although the roles of the host cell actin regulatory proteins vasodilator-stimulated phosphoprotein (4, 13), profilin (30), cofilin (3, 20), and ARPS 2/3 (33) have been explored, there has been little direct investigation of the potential role of actin filament capping proteins in Listeria-mediated actin assembly.
Actin-filament-capping proteins play a critical role in the regulation of actin filament growth in nonmuscle cells. Studies of the kinetics of actin filament growth have revealed that the two ends of an actin filament differ in their affinity and exchange rate for actin monomers. Electron micrographs have revealed that in the absence of ATP, myosin heads bind at a 45° angle, defining both barbed and pointed ends. The barbed end has a higher affinity for actin monomers as well as a higher exchange rate than the pointed end (2, 18). During normal cell motility, actin filament growth takes place primarily at the barbed end (28). Similarly, barbed-end growth is critical for the actin-based motility of Listeria. Cytochalasin D, a reagent that interferes with actin monomer addition to the barbed end, rapidly and reversibly blocks Listeria-induced actin rocket tail formation, bacterial movement, and cell-to-cell spread (6, 31). Nonmuscle host cells contain a number of actin regulatory proteins that bind to and block actin monomer exchange at the barbed end. Two of the most abundant barbed-end-capping proteins are CapG (also called macrophage capping protein and gCap39) (12, 24) and gelsolin (35). Both of these proteins are members of the gelsolin/villin family of proteins (7). Both proteins are activated by micromolar concentrations of ionized calcium. CapG caps the barbed ends but does not sever actin filaments. Gelsolin, in addition to capping actin filaments, binds to the sides of actin filaments and severs them. This action changes the consistency of the peripheral cytoplasm from a semisolid gel to a less viscous fluid.
We explored the potential roles of gelsolin and CapG in the actin-based motility of Listeria. We found that gelsolin localizes to the junction between the bacterium and the rocket tail and along the length of the rocket tail. CapG was not found in either of these regions. Using fibroblasts stably transfected with gelsolin cDNA, we found that increases in gelsolin levels to 2.25 times normal levels resulted in increased rates of actin rocket tail assembly and bacterial intracellular migration. Microinjection of a constitutively active form of gelsolin (gelsolin 1/2, containing the amino-terminal half of gelsolin) into infected cells also increased bacterial speeds by a factor of more than 2.2. Microinjection of CapG mixed with ionized calcium, on the other hand, had no effect on Listeria intracellular migration rates. These observations suggest that the assembly zone on the surface of Listeria is able to block barbed-end capping of actin filaments by gelsolin and CapG. Furthermore, the acceleration of bacterial motility associated with increased concentrations of gelsolin suggests that this severing protein can enhance actin-based motility, most likely by increasing the rate of recycling of actin monomers into Listeria-induced actin rocket tails or by decreasing the viscosity of the cytoplasm in host cells. Although increased levels of gelsolin can enhance actin-based motility, experiments with gelsolin-null cells revealed that these cells can support normal velocities of Listeria intracellular movement, indicating that gelsolin is not absolutely required for Listeria intracellular motility. This finding demonstrates that other actin regulatory proteins can replace the functional role of gelsolin and provides evidence for the functional redundancy of the contractile protein system in nonmuscle cells.
MATERIALS AND METHODS
Bacterial growth, tissue culture, and infection conditions.
L. monocytogenes 10403S, a virulent strain belonging to serotype 1 and having a 50% lethal dose for mice of 3 × 104, was used in this study as well as our previous actin studies (6, 21). Cells were infected as previously described (21), with one modification. After the addition of 1.6 × 107 bacteria in 2 ml of culture medium to each 35-mm dish containing adherent cultured cells, the dishes were centrifuged for 15 min at 400 × g and room temperature, followed by 45 min of incubation at 37°C. The dishes were then washed with phosphate-buffered saline, and the contents were resuspended in medium containing 10 μg of gentamicin sulfate per ml to prevent extracellular growth of bacteria, as previously described (6, 19, 21). The NIH 3T3 fibroblast cell lines C4, C5, C7, and C8 stably transfected with gelsolin and the LK cell line containing the expression plasmid without an insert were previously produced and characterized by Cunningham et al. (5). Fibroblasts from gelsolin-null mice and control littermates were also previously characterized (34). Ptk2 cells were cultured and infected as previously described (21).
Fixation and staining of cells.
Cells were fixed with 3.7% (vol/vol) formaldehyde in phosphate-buffered saline for 15 min at 25°C, followed by treatment with 0.4% Triton X-100 and 1.7 × 10−7 M BODIPY-phalloidin (Molecular Probes, Eugene, Oreg.) for 10 min at 37°C. Rabbit anti-recombinant murine gelsolin antibody (provided by D. Kwiatkowski) and rabbit anti-recombinant human CapG antibody (previously shown to specifically cross-react with gelsolin and CapG, respectively, on Western blots) (7, 34) were used for immunofluorescence microscopy, followed by a fluorescein-conjugated goat anti-rabbit immunoglobulin G secondary antibody. Controls with secondary antibody alone failed to reveal any immunolocalization in the intracellular bacteria (data not shown).
Microinjection of monomeric actin, CapG, and a constitutively active gelsolin mutant protein.
Three to 5 h after the initiation of infection, at a time when bacteria are moving within the cytoplasm, individual cells were microinjected with Lissamine rhodamine-conjugated actin, CapG, or the gelsolin 1/2 mutant protein by use of a micromanipulator and a microinjector (models 5171 and 5242, respectively; Eppendorf, Hamburg, Germany) as previously described (25). The temperature was maintained at 37°C during microinjection and observation by use of a warming block (MS-200D perfusion microincubation system; Narishige, Tokyo, Japan). Actin was purified from rabbit skeletal muscle by the method of Spudich and Watt (27) and labeled with Lissamine rhodamine-conjugated actin as previously described (21). Recombinant CapG was expressed and purified as previously described (23). The recombinant gelsolin 1/2 mutant protein cDNA was generated by PCR with human cytoplasmic gelsolin cDNA as the template. A premature stop codon (TGA) was introduced immediately after nucleic acid 1206 (corresponding to amino acid 370), and the truncated product was inserted into the pET12a vector expressed in Escherichia coli BL21 bacteria as previously described (23). The starting protein sample was loaded onto a hydroxylapatite chromatography column containing 10 mM potassium phosphate buffer (pH 7.0) and then eluted with a 10 to 120 mM potassium phosphate gradient. The protein eluted at the halfway point in the gradient as a single homogeneous peak. The recombinant truncated protein representing the first half of gelsolin was tested for severing activity by use of the pyrenyl actin-filament-severing assay (23) and found to sever actin filaments in the presence or absence of ionized calcium (32) (see Results).
Microscopy and image processing.
Cells were observed by phase-contrast microscopy with a Diaphot inverted microscope (Nikon, Tokyo, Japan) equipped with an image intensifier and a charge-coupled device camera (Dage-MTI, Inc., Michigan City, Ind.). Digital images were obtained and processed with an Image-1 video image analyzer (Universal Imaging Corp., West Chester, Pa.). Actin rocket tail lengths and bacterial speeds were determined by use of a length measurement analysis computer program (Image-1; Universal Imaging Corp.) calibrated with a Nikon micrometer. Differences between the rates of migration and tail lengths in the various cell lines were analyzed with the unpaired two-tailed Student t test or by Wilcoxon nonparametric analysis.
For measurements of the rates of disassembly of the actin rocket tails, Listeria-infected cells were microinjected with rhodamine-labeled actin monomers, followed by 15 min of incubation to allow the labeled actin to fully incorporate into the tails. Fluorescence images were captured digitally every 30 s. The relative fluorescence intensity of an area (4 × 4 pixels) directly behind the bacterium was analyzed in the initial frame, and the decrease in relative intensity was monitored over approximately 3 min by use of the area brightness function of the image analysis program. The relative intensity of a similar area (also 4 × 4 pixels) on either side of the tail was also measured, and the two values were averaged and subtracted as background from the relative intensity of the tail.
RESULTS
Immunofluorescence with antigelsolin and anti-CapG antibodies.
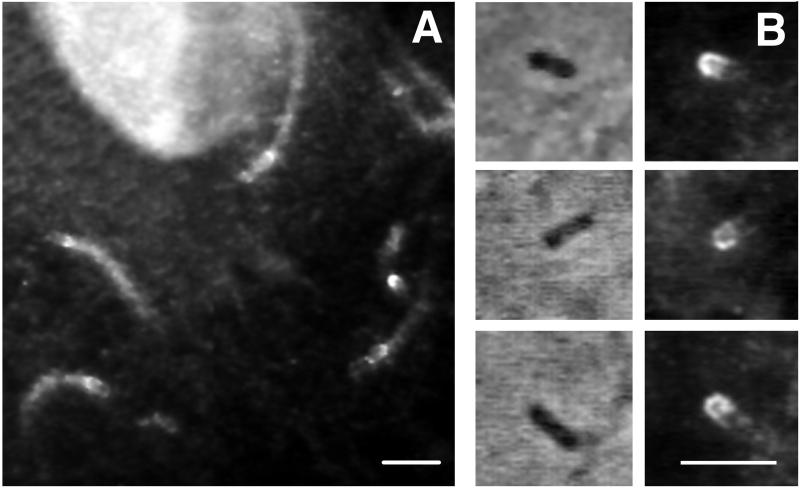
When PtK2 cells were infected with L. monocytogenes for 4 h, immunofluorescence staining with a highly specific antigelsolin antibody (see Materials and Methods) demonstrated strong cross-reactivity at the back of the bacterium, in the region of new actin filament assembly (Fig. 1A). Weaker immunofluorescence staining was also observed throughout the length of the rocket tail. Antigelsolin antibody staining was always apparent when actin filament structures were concentrated behind the moving bacteria. In bacteria that had not yet begun to form rocket tails, gelsolin was found to be localized at one end, suggesting that polar localization of gelsolin may precede the polarized assembly of actin filaments (Fig. 1B). Anti-CapG antibody failed to stain rocket tails or regions near the intracellular bacteria (data not shown).
FIG. 1.
Immunolocalization of gelsolin in PtK2 cells infected with Listeria. Four hours after the initiation of infection, PtK2 cells were fixed, permeabilized with Triton X-100, and stained with anti-murine gelsolin antibody as described in Materials and Methods. (A) Cross-reactive protein is noted throughout the actin filament rocket tails; higher-intensity staining is noted directly behind the bacterium. Antibody staining also faintly outlines each motile bacterium. Bar, 5 μm. (B) Phase-contrast micrographs of individual bacteria (left) and corresponding antigelsolin antibody immunofluorescence micrographs (right). These three bacteria have not yet begun to form phase-dense rocket tails; however, they do show gelsolin localized to one end. Bar, 5 μm.
Velocity of migration of Listeria in gelsolin-transfected and control fibroblasts.
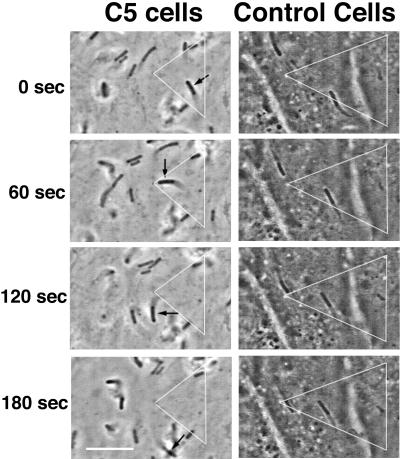
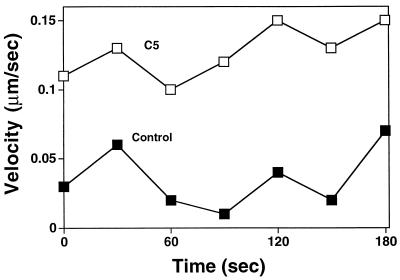
Because gelsolin and not CapG was shown to concentrate behind moving bacteria, we chose to explore the effects of various intracellular concentrations of gelsolin on Listeria intracellular movement. For this purpose, we used NIH 3T3 fibroblast cell lines stably transfected with gelsolin cDNA and expressing various concentrations of gelsolin, from 1.25 to 2.25 times control cell concentrations. The rate of Listeria intracellular migration within an individual cell varied considerably over time (Fig. 2, right), a condition which made detection of subtle differences in migration speed difficult. Therefore, we chose to compare only the migration speeds in cells with the highest gelsolin content (C5 cells, with a 2.25-fold increase) and cells with a normal gelsolin content. As shown in Fig. 2, bacteria tended to move faster in cells containing a high gelsolin content, with maximum speeds as high as 0.22 μm/s being observed, compared to maximum speeds of 0.11 μm/s in control cells. The mean rate of migration was nearly two times higher in cells with a high gelsolin content than in control cells (mean ± standard error of the mean [SEM], 0.09 ± 0.003 μm/s [n = 176] versus 0.05 ± 0.003 μm/s [n = 65]; P < 0.001).
FIG. 2.
(Left) Time-lapse video phase microscopy images of Listeria migrating in NIH 3T3 C5 fibroblasts expressing 2.25-fold-increased levels of gelsolin (left panels) and in NIH 3T3 control fibroblasts with a normal gelsolin level (right panels). Images were taken at 60-s intervals. The white triangles represent reference points drawn with fixed granules within the cytoplasm of each cell. A black arrow points to a rapidly moving bacterium within a C5 cell. The images also show other bacteria moving at rapid velocities within the cell. The images in the right panels show two bacteria moving slowly within a control cell. Bar, 10 μm. (Right) Graphic depiction of bacterial velocities over time. Open squares indicate the velocities of the bacterium designated by the black arrow in Fig. 2, left. Closed squares indicate the velocities of the bacterium seen in the upper-left-hand corner of the control cell panels in Fig. 2, left.
Rates of depolymerization of actin rocket tails in fibroblasts containing normal and increased levels of gelsolin.
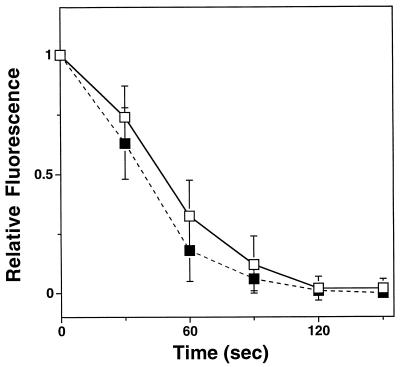
The increased velocity of Listeria motility in C5 cells suggested that gelsolin increased the rate of assembly of actin rocket tails. We were also interested in determining if the rates of disassembly of the rocket tails were affected by changes in gelsolin content. By microinjecting rhodamine-labeled actin monomers as previously described (21) and capturing images at 30-s intervals, we were able to measure the rates of disassembly of actin rocket tails in control cells and C5 cells, containing a 2.25-fold increase in gelsolin content. As shown in Fig. 3, the rates of rocket tail depolymerization were not statistically different in the two cell lines (mean ± SEM actin rocket tail half-life, 46.7 ± 3.5 s for C5 cells [n = 9] versus 40.5 ± 4.5 s for control cells [n = 4]; P = 0.4).
FIG. 3.
Mean rates of depolymerization of actin rocket tails induced by Listeria moving within C5 cells (open squares) and control cells (closed squares). Infected cells were microinjected with rhodamine-labeled actin monomers. Measurement were taken 30 min after microinjection. During this time, fluorescent actin equilibrated with native actin and became incorporated in the actin rocket tails. The fluorescence intensity of a single point within individual rocket tails was analyzed over time as previously described (21). Intensity is given in arbitrary units. Error bars represent the standard deviation. Each value represents the mean for four to nine determinations.
Actin rocket tail lengths in gelsolin-transfected and control fibroblasts.
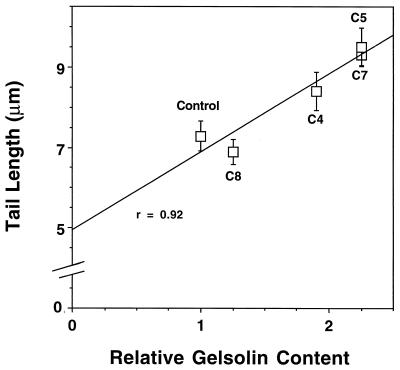
The rate of assembly of the actin rocket tail closely correlates with migration velocity (21) while the rate of disassembly remains constant in PtK2 cells (21, 29) and in gelsolin-transfected NIH 3T3 fibroblasts (see above). Therefore, the actin rocket tail length reflects the mean velocity of Listeria intracellular migration. The faster the migration speed, the longer the tail length. We used this characteristic to compare the average speeds within the different permanently transfected cell lines. Rocket tail lengths were significantly longer in Listeria-infected fibroblasts with a high gelsolin content (C5 and C7 cells) than in control fibroblasts, in fibroblasts with a slightly elevated gelsolin content (C8 cells), and in fibroblasts with a moderately elevated gelsolin content (C4 cells) (Fig. 4 and 5). A close positive correlation (r = 0.92) between mean rocket tail length and relative intracellular gelsolin content was observed (Fig. 5).
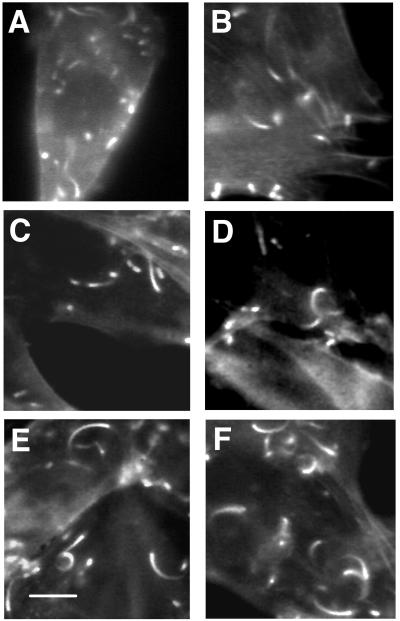
FIG. 4.
BODIPY-phalloidin-stained NIH 3T3 fibroblasts infected with Listeria. (A and B) Immunofluorescence micrographs of NIH 3T3 C8 fibroblast cells containing 1.25 times control gelsolin levels. Note the relatively short actin rocket tails. (C and D) Immunofluorescence micrographs of C4 cells containing 1.9 times control gelsolin levels. Note the intermediate lengths of the actin tails. (E and F) Immunofluorescence micrographs of C5 cells containing 2.25 times control gelsolin levels. Note the longer actin rocket tails. Bar, 10 μm.
FIG. 5.
Plot of rocket tail length versus gelsolin content. A linear relationship between gelsolin content and rocket tail length was observed, with a correlation coefficient of 0.92. Error bars represent the SEM. Mean tail lengths in control cells (7.3 ± 0.4 μm [n = 57]; P < 0.001), C8 cells (1.25 times control gelsolin content; 6.9 ± 0.3 μm [n = 52]; P < 0.001), and C4 cells (1.9 times control gelsolin content; 8.4 ± 0.5 μm [n = 58]; P = 0.02) were significantly shorter than that in C5 cells (2.25 times control gelsolin content; 9.5 ± 0.5 μm [n = 91]). C7 cells (2.25 times control gelsolin content) supported rocket tails with a length similar to that in C5 cells (9.3 ± 0.2 μm [n = 183]). At the x-axis value of 0 gelsolin content, the tail length was 4.95 μm.
Effects of microinjection of gelsolin 1/2 protein and CapG on Listeria intracellular motility.
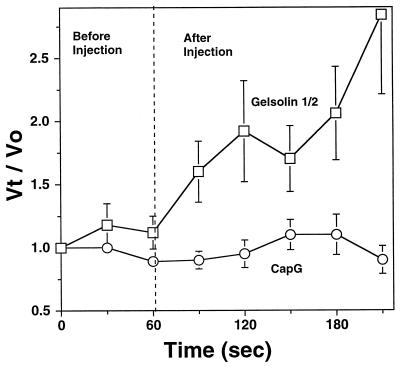
To further explore the functional consequences of increasing the intracellular concentrations of gelsolin in wild-type PtK2 cells, a constitutively active truncated form of gelsolin containing the amino-terminal half (gelsolin 1/2; see Materials and Methods) and capable of severing and capping actin filaments in the absence of micromolar calcium (32) was microinjected into Listeria-infected cells. We did not use full-length gelsolin because the wild-type molecule requires 10 to 100 μM ionized calcium to form an active complex capable of severing and capping actin filaments in vitro (1), calcium concentrations far in excess of those usually found in the cell. The kangaroo rat renal tubular epithelial cell line PtK2 was chosen for these experiments because of the ability of these cells to spread and flatten on glass coverslips, allowing the migration path of Listeria to be easily monitored in a single focal plane on phase-contrast microscopy. The in vitro activity of the recombinant protein was assessed by use of the pyrenyl actin-filament-severing assay. Depolymerization was induced by diluting 2 μM actin filaments to 100 nM in buffer alone (2 mM EGTA, 0.5 mM ATP, 10 mM imidizole, 0.1 M KCl, 1 mM MgCl2 [pH 7.5]) or in buffer plus a final concentration of 50 nM gelsolin 1/2. Gelsolin 1/2 markedly accelerated actin filament depolymerization in the absence of ionized Ca2+ (<0.5 μM Ca2+), indicative of actin filament severing (data not shown). The rate of actin filament disassembly was directly proportional to the number of free filament ends. Severing increased the number of ends and most readily explained the observed acceleration in actin filament disassembly. The ability of gelsolin 1/2 to sever actin filaments was comparable to that of full-length gelsolin, which, in the presence of ionized calcium, accelerates actin filament disassembly at nanomolar concentrations (23). Microinjection of an estimated intracellular gelsolin 1/2 concentration of 4 μM (needle concentration, 40 μM), well above the Kd for filament severing and capping (estimated to be in the nanomolar range based on our depolymerization experiments), failed to inhibit Listeria intracellular movement. Rather, introduction of the gelsolin 1/2 protein caused the velocity of Listeria intracellular movement to more than double (Fig. 6) (mean ± SEM preinjection velocity, 0.04 ± 0.01 μm/s [n = 30]; mean ± SEM postinjection velocity, 0.09 ± 0.01 μm/s [n = 30]; P < 0.001). Maximal increases in velocity were observed 150 s after microinjection, suggesting that some time is required for this protein to be appropriately incorporated into the actin cytoskeleton. The effects of increasing the levels of CapG in PtK2 cells were also examined by the same technique. An estimated intracellular ionized calcium concentration of 1 μM was introduced simultaneously to ensure maximal CapG activity (36). Introduction of an estimated intracellular CapG concentration of 4 μM (needle concentration, 40 μM), far exceeding the Kd for capping (0.5 to 1 nM) (23), had no significant effect on the velocity of Listeria intracellular movement (Fig. 6). Introduction of the same concentration of calcium alone also had no effect on Listeria motility.
FIG. 6.
Effects of microinjection of gelsolin 1/2 and CapG into PtK2 cells infected with Listeria. The vertical axis represents the velocity at each time point (Vt) divided by the velocity at time zero (V0). This determination allowed the comparison of multiple bacteria moving at different preinjection velocities. The broken vertical line represents the time at which an estimated intracellular concentration of 4 μM (needle concentration, 40 μM) constitutively active mutant gelsolin protein (gelsolin 1/2) or CapG was microinjected into the infected cells. An estimated intracellular concentration of 1 μM CaCl2 (10 μM needle concentration) was coinjected with CapG to activate the barbed-end-capping activity of this protein. Microinjection of gelsolin 1/2 caused a progressive increase in bacterial velocity over time, the mean velocity increasing to 2.5 times the preinjection value within 2 to 2.5 min. Introduction of CapG had no significant effect on Listeria motility. The error bars represent the SEM for 6 to 10 determinations.
Migration velocities and actin rocket tail lengths in gelsolin-null and wild-type mouse skin fibroblasts.
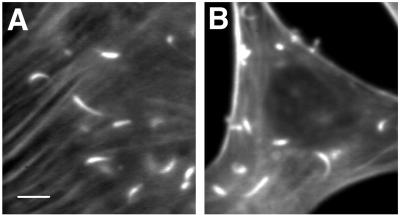
To determine whether or not gelsolin was absolutely required for the actin-based motility of Listeria, skin fibroblasts cultured from gelsolin-null and littermate control wild-type mice were infected with Listeria. After 4 to 5 h, Listeria began to move within both gelsolin-null and wild-type cells. The velocities of migration were found to be similar (0.21 ± 0.01 μm/s in gelsolin-null cells [n = 51] versus 0.20 ± 0.01 μm/s in wild-type cells [n = 18]; P = 0.4). Measurements of actin rocket tail lengths were also consistent with the velocity measurements, the mean tail lengths being nearly identical in null and wild-type cells (10.6 ± 0.6 μm [n = 38] versus 11.3 ± 0.6 μm [n = 66]; P = 0.4) (Fig. 7). It is of interest that both the mean velocities and the mean tail lengths of these mouse skin fibroblasts were considerably greater than those observed with both control and gelsolin-transfected NIH 3T3 fibroblasts (mean velocities, 0.05 to 0.09 μm/s; mean tail lengths, 6.9 to 9.5 μm/s), indicating that the relative concentrations of free actin monomers and actin regulatory proteins are likely to be considerably different in these two cell types.
FIG. 7.
BODIPY-phalloidin-stained skin fibroblasts from gelsolin-null and wild-type mice infected with Listeria. (A) Gelsolin-null cells. Note the prominent stress fibers commonly seen in these cells (34). (B) Wild-type cells. There was no statistical difference in actin rocket tail lengths between null and wild-type cells. Bar, 10 μm.
DISCUSSION
L. monocytogenes requires a force-generating motor to move within host cells and to form membrane projections called filopodia. These motile processes allow Listeria to spread from cell to cell without coming in contact with the humoral immune system. The ability to spread intracellularly explains many of the unique clinical characteristics of listeriosis, including the predisposition of the etiologic agent to cause disease in individuals with defects in cell-mediated immunity (26). Listeria generates the force for intracellular movement by assembling an actin-based motor. The bacterial surface protein ActA is capable of inducing the growth of new actin filaments directly behind the moving bacterium, causing the formation of an actin rocket tail that can be seen by phase-contrast microscopy in flat tissue culture cells, such as PtK2 cells, or by fluorescence microscopy (BODIPY-phalloidin stain) in thicker cells, such as fibroblasts and macrophages. New actin monomers are added at the junction between the bacterial cell wall and the actin rocket tail, defining a discrete polymerization zone (21). The elongation of filaments within this zone serves to drive the bacterium forward, the older, anchored regions of the tail providing the traction for directional movement. A number of actin regulatory proteins, including vasodilator-stimulated phosphoprotein (4), profilin (30), and ARPS 2/3 (33), likely to play roles in stimulating actin assembly have been found to concentrate in this polymerization zone. We have shown that gelsolin is also concentrated in this same region, suggesting that this barbed-end-capping and actin-filament-severing protein may also play an important role in regulating actin assembly in the actin-based motility of Listeria. Gelsolin has been demonstrated to be localized diffusely throughout actin rocket tails in XL177 cells (20); however, to our knowledge this is the first demonstration that gelsolin is focally concentrated at the bacterium-actin rocket tail junction. Furthermore, we have found that gelsolin is concentrated at one end of the bacterium prior to the formation of phase-dense actin filament rocket tails, suggesting that under normal conditions, this protein may participate in the early biochemical events required for the actin-based motility of Listeria.
To further explore the in vivo function of gelsolin in the generation of actin rocket tails, we examined the actin-based motility of Listeria in several NIH 3T3 fibroblast cell lines that were stably transfected with human cytoplasmic gelsolin cDNA. These NIH 3T3 clones have been shown to produce various concentrations of human gelsolin, ranging from 1.25 times to 2.25 times control cell levels (5). The rate of chemotaxis of these transfected cells was previously shown to directly correlate with gelsolin content; i.e., the higher the gelsolin content, the faster the rate of chemotaxis. As was observed in previous studies of normal amoeboid movement, cells with a higher gelsolin content supported higher mean velocities of the actin-based motility of Listeria. Because fibroblasts are relatively thick and the bacteria frequently moved out of the plane of focus, observation of Listeria by phase-contrast microscopy was difficult. Velocity measurements also tended to be inaccurate in these thick cells because distances of movement over time could be measured only in a two-dimensional plane. Furthermore, Listeria intracellular velocities vary considerably, making comparisons of mean velocities difficult over the short time frames of our experiments. Taking into consideration these limitations, comparisons between control cells and those with a 2.25-fold-higher gelsolin content did reveal statistically significant differences in velocity, cells with a higher gelsolin content moving twice as fast as those with a normal gelsolin content (Fig. 2).
To more accurately assess the mean velocities of Listeria in the different fibroblast clones, we took advantage of the kinetics of actin rocket tail assembly and disassembly. The rate of actin rocket tail assembly has been shown to vary with the velocity of intracellular migration, while the rate of disassembly of the tail is constant throughout the tail and is not dependent on the speed of movement (21, 29).
Experiments with rhodamine-labeled actin monomers confirmed that changes in gelsolin content did not detectably alter the rates of disassembly of rocket tails (Fig. 3). Therefore, tail length would be expected to directly correlate with bacterial speed, and this assumption has been confirmed experimentally (21, 29). Measurement of tail length can serve as a way to time average bacterial migration rates. During fixation, fibroblasts tend to flatten, allowing an accurate two-dimensional assessment of tail length following BODIPY-phalloidin actin filament staining. Using this assay, we found a close positive correlation between gelsolin content and actin rocket tail length; i.e., the higher the gelsolin content, the longer the actin rocket tail (Fig. 4 and 5). The relationship between tail length and gelsolin content was linear. Curiously, extrapolation of the data in Fig. 5 to 0 gelsolin content predicted that Listeria would be expected to continue to form rocket tails in the absence of gelsolin. Furthermore, recent experiments with Xenopus extracts demonstrated that depletion of gelsolin had no effect on tail length or migration rate. However, these extracts contained concentrations of EGTA that maintained ionized calcium in the submicromolar range, a condition that prevents gelsolin from binding actin (20) and that makes any conclusions concerning the role of gelsolin in rocket tail formation in these experiments suspect.
To explore whether or not gelsolin is absolutely required for the actin-based motility of Listeria, the velocities of intracellular migration and the actin rocket tail lengths were compared in skin fibroblasts derived from gelsolin-null and wild-type littermates. No statistical differences between the two cell lines were observed, indicating that gelsolin is not absolutely required and that knockout cells are capable of chemotaxis. These observations raise the possibility that gelsolin may not be absolutely necessary for the generation of an actin-based motor but may instead serve to modulate the motor by accelerating the rate of actin filament assembly. It is also possible that gelsolin-null mice compensate for the loss of gelsolin by upregulating the expression of other actin-filament-severing and -capping proteins. The actin regulatory system contains multiple proteins with redundant functions, and this condition provides an important survival advantage. A similar redundancy is observed when various T-cell subsets are knocked out. Mice lacking a T-cell subset demonstrate “normal” immune responses to infections with intracellular pathogens, indicating the compensatory potential of the incomplete immune system (15).
Although gelsolin is not absolutely required for Listeria intracellular movement, our experiments with NIH 3T3 fibroblast cell lines that overexpress this protein indicated that gelsolin serves a facilitatory role in the actin-based motility of Listeria. Further support for this conclusion is provided by our microinjection experiments with PtK2 cells. These kangaroo rat renal tubular epithelial cells spread extensively on tissue culture plates, providing a very flat substrate for Listeria motility studies. Intact gelsolin requires 10 to 100 μM ionized calcium to form an active complex capable of severing and capping actin filaments in vitro (1), concentrations far in excess of those found in the cell. Therefore, we took advantage of the gelsolin 1/2 mutant, which has been shown to be calcium insensitive; i.e., the molecule is active in the presence of submicromolar as well as micromolar ionized calcium (32). As evidenced by the in vitro pyrenyl actin-filament-severing assay, we were able to successfully generate calcium-independent truncated gelsolin using recombinant methods (data not shown). The gelsolin 1/2 mutant is constitutively active and, when introduced into infected PtK2 cells, increased Listeria velocity by more than 2.2-fold (Fig. 6). Thus, increasing the intracellular concentrations of gelsolin in intact cells by either overexpression or microinjection results in the acceleration of Listeria-induced actin filament assembly and intracellular motility.
The exact mechanism by which gelsolin participates in the assembly of actin filaments during Listeria motility remains to be clarified. The functional versatility of gelsolin makes this determination a particularly daunting task. Gelsolin is thought to play a critical role in recycling actin monomers and regulating the length of actin filaments as cells move (28). Our experiments comparing the effects of introducing CapG and gelsolin by microinjection provide some preliminary insights. CapG is structurally similar to gelsolin but has no actin-filament-severing function. This protein had no effect on Listeria motility at concentrations 1,000 times the apparent capping constant. Gelsolin, which both caps and severs actin filaments at similar concentrations, accelerated Listeria movement, indicating that severing is likely to play an important role in potentiating actin-based motility. The major effect of actin filament severing is to lower the viscosity of the cytoplasm, a condition that would be less resistant to bacterial movement. The severing action of gelsolin would not be expected to alter the architecture of the actin rocket tails because these actin filaments are bound with tropomyosin (6) and tropomyosin is known to protect actin filaments from gelsolin severing (8, 10). Unlike cofilin, which accelerates the rate of disassembly of actin rocket tails (3, 20), gelsolin was shown in both in vitro experiments with Xenopus extracts (20) and our own studies with Listeria-infected NIH 3T3 fibroblasts not to accelerate the overall rate of disassembly of actin rocket tails. The results of these experiments are consistent with the conclusion that gelsolin acts primarily on actin filaments not incorporated in the rocket tails. In order to accelerate Listeria intracellular movement, actin filament severing would be expected to take place primarily at the front of the moving bacterium; however, our immunofluorescence experiments and others (20) demonstrated that gelsolin is most highly concentrated at the back of the motile bacterium. Actin filaments that form behind the moving bacterium have their barbed ends oriented toward the back of the bacterium (22, 31); therefore, gelsolin is likely to play a role in regulating the growth of these actin filaments.
A most surprising finding was the inability of gelsolin and CapG to block Listeria-induced actin filament assembly at intracellular concentrations 1,000 times the capping constant for both proteins. If these proteins had capped the barbed ends of the actin filaments directly behind the bacterium, the actin-based movement of Listeria should have ceased. Our findings indicate that intracellular Listeria is able to prevent gelsolin and CapG from capping the barbed ends of actin filaments growing behind the motile bacterium. Previous quantitative analysis of the concentrations of monomeric sequestering proteins and monomeric actin in Xenopus extracts indicated that the majority of barbed ends are capped. This finding led to the suggestion that Listeria may induce actin assembly in such extracts by uncapping the high-affinity barbed ends (16), and our experiments support this model. The barbed ends have a higher affinity for actin monomers than for the host cell monomeric sequestering proteins, and uncapping of the high-affinity ends of the actin filaments would allow the addition of new monomers and elongation of the filaments.
The inability of microinjected actin-filament-capping proteins to block Listeria-induced actin filament assembly supports the existence of a polymerization zone on the surface of intracellular Listeria. This zone must block or minimize the capping of actin filaments. Recent electron micrographs (22) demonstrated that rocket tails induced by Listeria consist of long actin filaments lined up with their barbed ends facing toward the bacterium. This observation raises the possibility that preformed actin filaments present in the host cell cytoplasm may become uncapped on entering the polymerization zone, and these uncapped filaments can then serve as templates for the addition of new actin monomers. The existence of a discrete polymerization zone containing highly concentrated actin regulatory proteins at the surface of the motile bacterium has also been suggested by the recent biochemical studies of Kang et al. (13). The mechanisms that allow this zone to block barbed-end capping by gelsolin and CapG are likely to play a critical role not only in Listeria pathogenesis but also in filopodial and lamellipodial extension in normal host cell movements.
ACKNOWLEDGMENTS
We thank Daniel Purich, Paul Jamney, and Thomas Stossel for helpful discussions and Ping Shen for technical assistance.
This work was funded by National Institutes of Health grants RO1AI24276, RO1 AI23262, and RO1HL19429.
REFERENCES
- 1.Allen P G, Janmey P A. Gelsolin displaces phalloidin from actin filaments. A new fluorescence method shows that both Ca2+ and Mg2+ affect the rate at which gelsolin severs F-actin. J Biol Chem. 1994;269:32916–32923. [PubMed] [Google Scholar]
- 2.Bonder E M, Fishkind D J, Mooseker M S. Direct measurement of critical concentrations and assembly rate constants at the two ends of an actin filament. Cell. 1983;34:491–501. doi: 10.1016/0092-8674(83)90382-3. [DOI] [PubMed] [Google Scholar]
- 3.Carlier M-F, Laurent V, Santolini J, Melki R, Didry D, Xia G-X, Hong Y, Chua N-H, Pantaloni D. Actin depolymerization factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1323. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charkraborty T, Ebel F, Domann E, Niebuhr K, Gerstel B, Pistor S, Temm-Grove C J, Jockusch B M, Reinhard M, Walter U, Wehland J. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995;14:1314–1321. doi: 10.1002/j.1460-2075.1995.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham C C, Stossel T P, Kwiatkowski D J. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991;251:1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- 6.Dabiri G A, Sanger J M, Portnoy D A, Southwick F S. Listeria monocytogenes moves rapidly through the host cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci USA. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabiri G A, Young C L, Rosenbloom J, Southwick F S. Molecular cloning of human macrophage capping protein cDNA: a unique member of the gelsolin/villin family expressed primarily in macrophages. J Biol Chem. 1992;267:16545–16552. [PubMed] [Google Scholar]
- 8.Dabrowska R, Hinssen H, Galazkiewicz B, Nowak E. Modulation of gelsolin-induced actin-filament severing by caldesmon and tropomyosin and the effect of these proteins on the actin activation of myosin Mg(2+)-ATPase activity. Biochem J. 1996;315:753–759. doi: 10.1042/bj3150753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 10.Fattoum A, Hartwig J H, Stossel T P. Isolation and some structural and functional properties of macrophage tropomyosin. Biochemistry. 1983;22:1187–1193. doi: 10.1021/bi00274a031. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of the surface antigens from Gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 12.Johnston P A, Yu F-X, Reynolds G A, Yin H L, Moomaw C R, Slaughter C A, Sudhof T C. Purification and expression of gCap39. J Biol Chem. 1990;265:17946–17952. [PubMed] [Google Scholar]
- 13.Kang F, Laine R O, Bubb M R, Southwick F S, Purich D L. Profilin interacts with the Gly-Pro-Pro-Pro-Pro-Pro sequences of vasodilator-stimulated phosphoprotein (VASP): implications for actin-based Listeria motility. Biochemistry. 1997;36:8384–8392. doi: 10.1021/bi970065n. [DOI] [PubMed] [Google Scholar]
- 14.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutation in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann S H, Ladel C H. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 16.Marchand J B, Moreau P, Paoletti A, Cossart P, Carlier M F, Pantaloni D. Actin-based movement of Listeria monocytogenes: actin assembly results from the local maintenance of uncapped filament barbed ends at the bacterium surface. J Cell Biol. 1995;130:331–343. doi: 10.1083/jcb.130.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peskin C S, Odell G M, Oster G F. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys J. 1993;65:316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard T D, Mooseker M S. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J Cell Biol. 1981;88:654–659. doi: 10.1083/jcb.88.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenblatt J, Agnew B J, Abe H, Bamburg J R, Mitchison T J. Xenopus actin depolymerization factor/cofilin (XAC) is responsible for the turnover of actin filaments in Listeria monocytogenes tails. J Cell Biol. 1997;136:1323–1332. doi: 10.1083/jcb.136.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanger J M, Sanger J W, Southwick F S. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect Immun. 1992;60:3609–3619. doi: 10.1128/iai.60.9.3609-3619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sechi A S, Wehland J, Small J V. The isolated comet tail pseudopodium of Listeria monocytogenes: a tail of two actin filament populations, long and axial and short and random. J Cell Biol. 1997;137:155–167. doi: 10.1083/jcb.137.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Southwick F S. Gain-of-function mutations conferring actin-severing activity to human macrophage Cap G. J Biol Chem. 1995;270:45–48. doi: 10.1074/jbc.270.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Southwick F S, DiNubile M J. Rabbit alveolar macrophages contain a Ca2+-sensitive, 41,000 dalton protein which reversibly blocks the “barbed” ends of actin filaments but does not sever them. J Biol Chem. 1986;261:14191–14195. [PubMed] [Google Scholar]
- 25.Southwick F S, Purich D L. Arrest of Listeria movement in host cells by a bacterial ActA analogue: implications for actin-based motility. Proc Natl Acad Sci USA. 1994;91:5168–5172. doi: 10.1073/pnas.91.11.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Southwick F S, Purich D L. Mechanisms of disease: intracellular pathogenesis of listeriosis. N Engl J Med. 1996;334:770–776. doi: 10.1056/NEJM199603213341206. [DOI] [PubMed] [Google Scholar]
- 27.Spudich J A, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 28.Stossel T P. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 29.Theriot J A, Mitchison T J, Tilney L G, Portnoy D A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 30.Theriot J A, Rosenblatt J, Portnoy D A, Goldschmidt-Clermont P J, Mitchison T J. Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell. 1994;76:505–517. doi: 10.1016/0092-8674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 31.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Way M, Gooch J, Pope B, Weeds A G. Expression of human gelsolin in Escherichia coli and dissection of actin binding sites by segmental deletion mutagenesis. J Cell Biol. 1989;109:593–605. doi: 10.1083/jcb.109.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch M D, Iwamatsu A, Mitchison T J. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 34.Witke W, Sharpe A H, Hartwig J H, Azuma T, Stossel T P, Kwiatkowski D J. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- 35.Yin H L, Stossel T P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979;281:583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- 36.Young C L, Feierstein A, Southwick F S. Calcium regulation of actin filament capping and monomer binding by macrophage capping protein. J Biol Chem. 1994;269:13997–14002. [PubMed] [Google Scholar]