Abstract
Objective:
Although animal models suggest a role for blood-brain barrier dysfunction in postoperative delirium-like behavior, its role in postoperative delirium and postoperative recovery in humans is unclear. Thus, we evaluated the role of blood-brain barrier dysfunction in postoperative delirium and hospital length of stay among older surgery patients.
Methods:
Cognitive testing, delirium assessment, and cerebrospinal fluid and blood sampling were prospectively performed before and after non-cardiac, non-neurologic surgery. Blood-brain barrier dysfunction was assessed using the cerebrospinal fluid-to-plasma albumin ratio (CPAR).
Results:
Of 207 patients (median age 68, 45% female) with complete CPAR and delirium data, 26 (12.6%) developed postoperative delirium. Overall, CPAR increased from before to 24-hours after surgery (median change 0.28, [IQR] [−0.48, 1.24]; Wilcoxon p=0.001). Preoperative to 24-hour postoperative change in CPAR was greater among patients who developed delirium vs those who did not (median [IQR] 1.31 [0.004, 2.34] vs 0.19 [−0.55, 1.08]; p=0.003). In a multivariable model adjusting for age, baseline cognition, and surgery type, preoperative to 24-hour postoperative change in CPAR was independently associated with delirium occurrence (per CPAR increase of 1, OR = 1.30, [95% CI 1.03-1.63]; p=0.026) and increased hospital length of stay (IRR = 1.15 [95% CI 1.09-1.22]; p<0.001)
Interpretation:
Postoperative increases in blood-brain barrier permeability are independently associated with increased delirium rates and postoperative hospital length of stay. Although these findings do not establish causality, studies are warranted to determine whether interventions to reduce postoperative blood-brain barrier dysfunction would reduce postoperative delirium rates and hospital length of stay.
Tweet:
“A Role for Blood-brain Barrier Dysfunction in Delirium Following Non-Cardiac Surgery. Link paper. @Mike_Devinney, @RealMilesBerger, and colleagues show that postoperative blood-brain barrier dysfunction is independently associated with increased postoperative delirium rates.
Introduction:
Postoperative delirium is a disorder of fluctuating changes in attention and level of consciousness that occurs in up to 40% of the >16 million older Americans who undergo surgery each year,1,2 and is associated with longer hospital stays,3 US health care costs over 25 billion dollars per year,4 and increased mortality and long-term dementia risk.1,5 Yet, there are no FDA-approved drugs to prevent delirium, largely because we understand so little of its underlying neuro-pathophysiology.
One potential mechanism underlying postoperative delirium is blood-brain barrier dysfunction, since animal models of postoperative delirium-like behavior exhibit significant blood-brain dysfunction.6-8 The blood-brain barrier contains non-fenestrated brain capillaries that restrict the free diffusion of solutes and cells into the central nervous system (CNS), which normally protects the CNS from peripheral toxins, pathogens, and inflammation. Although many neurologic diseases involve blood-brain barrier dysfunction, including multiple sclerosis,9 cerebrovascular injury,10 and Alzheimer’s disease,11 the role of blood-brain barrier dysfunction in postoperative delirium and overall postoperative recovery is unclear.12
Blood-brain barrier function in humans can be measured by the cerebrospinal fluid-to-plasma albumin ratio (CPAR), since albumin is an abundant plasma protein that does not normally diffuse through an intact blood-brain barrier.11 Indeed, increased CPAR has been observed in patients with Alzheimer’s disease.11,13 Further, non-cardiac surgery in animal models disrupts the blood-brain barrier,7 yet relatively few studies have examined postoperative blood-brain barrier dysfunction in humans.14,15 For example, significant blood-brain barrier dysfunction has been observed in 10 patients after cardiac surgery.16 Additionally, following thoracoabdominal aortic aneurysm repair in 11 patients, 24-hour postoperative CPAR increased to a greater extent in patients who developed delirium versus those who did not.12 These small cardiac surgery studies suggest that postoperative blood-brain barrier dysfunction can occur, though it is unknown to what extent it occurs following non-cardiac surgery (i.e., without exposure to cardiopulmonary bypass) and what role it might play in postoperative delirium and postoperative recovery.
One quantifiable surrogate of overall postoperative recovery is hospital length of stay, because hospital discharge usually requires that a patient has pain resolution, return of bowel function, and an ability to ambulate- all measures of overall postoperative recovery.17 Prolonged postoperative hospital length of stay occurs in patients with postoperative delirium,3,18 but the relationship of postoperative blood-brain barrier dysfunction with postoperative hospital length of stay is unknown. Blood brain barrier dysfunction could potentially lead to an increased postoperative hospital length of stay via increased delirium risk or via disruption of postoperative recovery processes, through exposure of relevant brain areas to peripheral inflammatory mediators. For example, blood-brain barrier dysfunction has been associated with impaired motor recovery in animal models of traumatic brain injury.19
Here, we sought to determine the extent that postoperative blood-brain barrier dysfunction occurs in older non-cardiac surgery patients, and its relationship with 1) postoperative delirium and 2) hospital length of stay. To investigate this, we performed preoperative and 24-hour postoperative lumbar punctures, postoperative delirium assessments, and measured pre- and 24-hour postoperative CPAR in 207 older adults that underwent a wide variety of major non-cardiac/non-neurosurgical procedures.
Methods:
Study Information
Samples and data were utilized from MADCO-PC (NCT01993836)20 and INTUIT (NCT03273335)21 studies. Markers of Alzheimer’s Disease and neuroCognitive Outcomes after Perioperative Care (MADCO-PC)20 was an observational cohort study that enrolled 140 older surgical patients (age ≥60) undergoing non-cardiac, non-neurologic surgery, and investigated the extent of correlations between postoperative changes in cognitive function and CSF biomarkers related to Alzheimer’s disease (AD). Investigating NeuroinflammaTion UnderlyIng postoperative cogniTive dysfunction (INTUIT)21 was an observational cohort study that enrolled 201 older surgical patients (age ≥60) undergoing non-cardiac, non-neurologic surgery, and was designed to determine the extent to which postoperative changes in cerebrospinal fluid (CSF) cytokines are associated with postoperative changes in cognition, resting state functional brain connectivity (measured by fMRI) and CSF AD-related biomarkers. MADCO-PC and INTUIT were both prospective observational studies; thus, no direction was given to anesthesia care providers about what fluids to administer (e.g., albumin versus crystalloids) in either study.
MADCO-PC and INTUIT were both approved by the Duke Institutional Review Board, and all participants in both studies provided written informed consent before enrollment. For both studies, patients were eligible if they were undergoing surgery scheduled for at least 2 hours at Duke University Hospital or Duke Regional Hospital. Patients were excluded if they were taking immunosuppressants, chemotherapy drugs with cognitive side effects, or anticoagulants that would preclude safe lumbar puncture. Subjects who received intravenous albumin during surgery were excluded from our analysis, since this could artificially reduce the CPAR ratio by increasing systemic albumin levels. No study patients received intraoperative fresh frozen plasma.
Lumbar Punctures, Blood draws, Sample Processing, and Albumin Assays
Using our protocol that reduces pain and adverse events,22 lumbar punctures were performed using standard sterile technique under local anesthesia before and 24 hours after the start of surgery, with the patient seated upright and leaning forward, or in the lateral decubitus position if the patient was unable to tolerate sitting. CSF was then gently aspirated into a 10 mL Luer-Lock polypropylene syringe,22 which was then emptied into a pre-chilled 15 ml conical tube (VWR; Radnor, PA) on ice. The CSF samples were then aliquoted with low binding pipette tips into Sarstedt 1.5-mL polypropylene microcentrifuge tubes (VWR; Radnor, PA), which were pre-chilled on ice. These aliquots were then frozen at −80°C within 1 hour of sample collection, and maintained at −80°C without any freeze/thaw cycles until they were thawed together for batched analysis.20
Up to 10 mL of whole blood was collected from patients before surgery and again 24 hours after surgery using standard sterile venipuncture technique, and were processed and aliquoted as described.20 In brief, blood was collected into pre-chilled K2 EDTA vacutainer tubes (Becton Dickinson; Franklin Lakes, NJ) and immediately placed on ice. The samples were then centrifuged at 3,000 RPM for 15 minutes, separating the plasma from the red cells and buffy coat layer. The plasma was divided into 0.5 ml aliquots and frozen at −80°C.
Plasma albumin levels were measured in duplicate with bromocresol purple dye-binding using a Beckman DxC 600 clinical analyzer. The coefficient of variation between duplicate measurements for plasma albumin was 0.73% (SD 1.02). CSF albumin levels were measured in 10 μl samples in duplicate, via immuno-turbidimetry with an anti-albumin antibody in a Beckman DxC 600 clinical analyzer. The coefficient of variation between duplicated measurements for CSF albumin was 1.24% (SD 0.98). The CSF albumin to plasma albumin ratio (CPAR) was calculated using the formula 1000 x [cerebrospinal fluid albumin (mg/dl)]/[serum albumin (mg/dl)].
Cognitive Testing
Preoperative cognitive function was measured with a 14 item test battery, which we have previously used in numerous studies of postoperative neurocognitive deficits.20,23,24 These tests included the Wechsler Test of Adult Reading, Revised Wechsler memory scale and Modified Visual Reproduction test, Hopkins verbal learning test, Randt Short Story memory test, Digit Span, Trail Making Test A, Trail Making Test B, Digit Symbol, and the Lafayette Grooved Pegboard Test. The tests generated a total of 14 different scores which were used for factor analysis. The Trails B was truncated at 300 seconds and the trails making tests were negatively log transformed, so that higher scores indicated better performance (similar to the other test variables) and could be used together with the other test variables for factor analysis. This factor analysis was performed with oblique rotation, and produced a 5-factor solution that explained 82% of the variability in test scores. These 5 factors reflected 5 cognitive domains: attention/concentration, structured verbal memory, unstructured verbal memory, visuospatial memory, and executive function. The average of these 5 cognitive domain scores produced the continuous cognitive index (CCI) score, a sensitive continuous measure of baseline cognition our group has used in numerous studies over the last 20+ years.20,23-25
Demographic and Baseline Clinical Data
Subject characteristics and clinical variables were extracted from the electronic medical record. Charlson comorbidity scores were determined as described.26 Subjects were administered the Duke Activity Status questionnaire preoperatively.27 Apolipoprotein E4 genotyping was performed as previously described.28 Surgery type was classified based on the operative surgical service. Length of stay was ascertained from the electronic medical record.
Delirium Assessment
Delirium was assessed daily with the Confusion Assessment Method (CAM) with a skip pattern (the assessment was stopped if inattention was not present) in MADCO-PC,20 and twice daily with the three minute diagnostic CAM (3D-CAM) in INTUIT.29 The CAM and 3-D CAM are both highly sensitive and specific for delirium in hospitalized patients.29,30 However, since the CAM and 3D-CAM require patients to be verbal, in both studies (MADCO-PC and INTUIT) the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) was used for delirium assessment whenever a patient was intubated or otherwise non-verbal at the time of assessment. The CAM-ICU is a well-validated method of delirium assessment in intubated/non-verbal patients.31 A validated method for chart review32 was additionally performed to detect any delirium cases that may have been missed by the research delirium assessments performed at discrete time points. Patients were defined as having postoperative delirium if they had at least one positive bedside CAM assessment (3D-CAM, CAM, or CAM-ICU) or if they had a positive delirium chart review.
Statistics
Because prior studies have found a continuous relationship between CPAR and blood-brain barrier leakiness,33 our analysis focused on CPAR as a continuous measure of blood-brain barrier disruption. Assuming a postoperative delirium rate of 10% and variability similar to that observed in our preliminary data (SD of 2.25), our sample size of ≥200 patients would provide 80% power with α = 0.05 level to detect a difference in pre- to 24-hour postoperative change in CPAR of ≥1.49 in patients with versus without delirium, which is in the range of CPAR differences found in other studies.11
Pre-to-post surgery CPAR change was examined with a Wilcoxon Signed Rank test. Wilcoxon Rank Sum tests and univariable logistic regression were used to evaluate the relationship between CPAR and delirium. A subsequent multivariable logistic regression model for delirium included the following baseline risk factors for delirium as adjustment terms: age, baseline cognitive function, and surgery type (since there are baseline differences in characteristics among patients scheduled for different surgery types). The association of CPAR (per CPAR increase of 1) and hospital length of stay (measured in days) was analyzed via multivariable negative binomial regression, adjusting for the same terms as the delirium model and for delirium status itself. We report odds ratios (OR) and 95% confidence intervals (CI) from the logistic regression models for delirium and incidence rate ratios (IRR) with 95% CI from the negative binomial regression models for hospital length of stay. The IRR is a multiplicative factor that corresponds to the average value of the outcome, analogous to percent change (i.e., IRR of 1.04 corresponds to a 4% increase). Normality was assessed for numeric variables via the Shapiro-Wilks test. If the data was significantly non-normal, nonparametric summary statistics and hypothesis testing were used. Model fit diagnostics were performed via the Hosmer-Lemeshow goodness of fit test and discrimination was evaluated with the area under the receiver operating curve.
Predicted Delirium Probability and Length of Stay Calculation:
We used results from the multivariable regression models for delirium and length of stay, to extract predicted values and standard error estimates using the predict function in R (v4.2, Project for Statistical Computing, Vienna Austria). Adjustment variables were fixed at either their average (age set to 68 years) or reference level (surgery type set to Urologic/gynecologic). For the delirium model we specified three values of interest for baseline cognitive performance, average (the cohort average baseline cognitive index value was 0.01), impaired (1 SD below the cohort average), and above average (1 SD above the cohort average) using CPAR change values across the observed range of −3 to 9. For the length of stay model we fixed baseline cognitive performance at the average and calculated the predicted values for subjects with versus without delirium. We then plotted the estimated probability (and confidence intervals) for each level of baseline cognitive performance across the range of CPAR change values. Bootstrapping of model estimates with 1000 replicates was utilized to estimate the empirical 95% confidence interval of difference between two predicted probabilities at fixed levels of CPAR change.
Results:
Subject characteristics
Subject enrollment is shown in Figure 1; baseline/preoperative characteristics are shown in Table 1. Those who developed postoperative delirium had lower years of education, lower baseline continuous cognitive index scores (a sensitive global measure of cognition), and lower mini-mental status exam scores, similar to findings in prior studies.12,34
Figure 1: Study flow diagram.
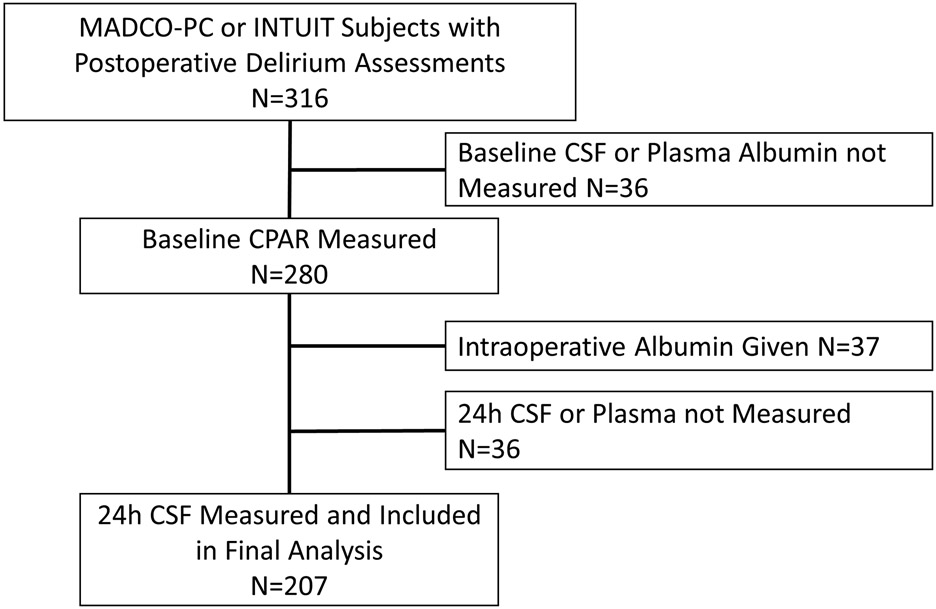
MADCO-PC, Markers of Alzheimers Disease and Cognitive Outcomes after Perioperative Care; INTUIT, Investigating Neuroinflammation UnderlyIng Postoperative CogniTive Dysfunction; CSF, cerebrospinal fluid; CPAR, Cerebrospinal fluid-to-Plasma Albumin Ratio.
Table 1:
Subject Characteristics
| Overall (N=207) |
Not Delirious (N=181) |
Delirious (N=26) |
Standardized Difference |
|
|---|---|---|---|---|
| Age | 68 [64, 73] | 68 [64, 72] | 70 [65, 75] | 0.256 |
| Sex (Male) | 114 (55.1%) | 99 (54.7%) | 15 (57.7%) | 0.635 |
| Race | 0.285 | |||
| Black or African American | 26 (12.6%) | 21 (11.6%) | 5 (19.2%) | |
| Caucasian/White | 178 (86.0%) | 158 (87.3%) | 20 (76.9%) | |
| Other | 3 (1.4%) | 2 (1.1%) | 1 (3.8%) | |
| Body Mass Index | 29.0 [25.1, 33.1] | 29.1 [25.5, 33.7] | 28.5 [23.9, 30.8] | 0.364 |
| APOE4 Carrier a | 54 (26.9%) | 48 (27.0%) | 6 (26.1%) | 0.020 |
| Years of Education | 16 [13, 18] | 16 [14, 18] | 14 [12, 16] | 0.483 |
| Baseline Cognitive Index b | 0.01 (0.72) | 0.11 (0.64) | −0.69 (0.86) | 1.051 |
| Mini-Mental Status Exam Score c | 29 [27, 29] | 29 [28, 29] | 26 [23, 29] | 1.103 |
| Duke Activity Status Index d | 21 [11, 39] | 21 [11, 39] | 21 [7, 42] | 0.065 |
| Charlson Comorbidity Index | 4 [3, 6] | 4 [3, 6] | 4 [3, 5] | 0.244 |
| ASA Physical Status Class | 3 [2, 3] | 3 [2, 3] | 3 [3, 3] | 0.290 |
| Surgery Type | 0.129 | |||
| Thoracic | 26 (12.6%) | 23 (12.7%) | 3 (11.5%) | |
| General/Abdominal/Plastic/ENT | 67 (32.4%) | 58 (32.0%) | 9 (34.6%) | |
| Urologic/Gynecologic | 58 (28.0%) | 50 (27.6%) | 8 (30.8%) | |
| Orthopedic | 56 (27.1%) | 50 (27.6%) | 6 (23.1%) |
Wilcoxon
Chi-square
APOE4 genotype missing for 6
baseline cognitive index missing for 4
MMSE missing for 1 subject
DASI missing for 2.
Perioperative blood-brain barrier dysfunction and postoperative delirium
Although there was no difference in preoperative versus 24-hour postoperative CPAR overall (Table 2), there was a modest yet significant within-subject change in preoperative to 24-hour postoperative CPAR (median change 0.28, interquartile range −0.48, 1.24; p = 0.001; Table 2).
Table 2:
Univariable Relationships Between CPAR and Postoperative Delirium
| CPAR | Overall Cohort (n = 207) Median [IQR] Mean (SD) |
Not Delirious (n= 181) Median [IQR] Mean (SD) |
Delirious (n = 26) Median [IQR] Mean (SD) |
Univariable Logistic Regression** |
|
|---|---|---|---|---|---|
| OR (95% CI) | p-value | ||||
| Baseline | 5.68 [4.44, 7.35] 6.4 (3.2) |
5.61 [4.33, 7.24] 6.4 (3.2) |
5.90 [4.50, 7.36] 6.4 (3.1) |
1.01 (0.87, 1.13) | 0.942 |
| 24 Hour | 5.92 [4.35, 8.58] 6.9 (3.8) |
5.79 [4.32, 8.42] 6.8 (3.7) |
6.84 [5.52, 9.08] 7.9 (3.9) |
1.07 (0.97, 1.17) | 0.169 |
| 24-Hour Change | 0.28 [−0.48, 1.24]* 0.6 (1.9) |
0.19 [−0.55, 1.08] 0.4 (1.9) |
1.31 [0.004, 2.34]# 1.5 (2.0) |
1.27 (1.05, 1.54) | 0.011 |
CPAR, cerebrospinal fluid-to-plasma albumin ratio, *Within-subject 24-hour change in CPAR, Wilcoxon Signed Rank test, p = 0.001; #Delirious vs. not delirous 24-Hour change in CPAR, Wilcoxon test, p = 0.003. **Univariable Logistic Regression compares the indicated CPAR variable among delirious vs not delirious patients.
Neither preoperative nor 24-hour postoperative CPAR levels differed between patients with vs without delirium (p > 0.05 for each, Table 2). However, preoperative to 24-hour postoperative CPAR change was greater in patients who did vs did not develop delirium (median [Q1, Q3] 1.31 [0.004, 2.34] vs 0.19 [−0.55, 1.08]; p = 0.003, respectively, Table 2, Figure 2). Increased preoperative to 24-hour postoperative CPAR change was associated with higher odds of postoperative delirium in both univariable analysis (OR = 1.27 per CPAR increase of 1, 95% CI 1.05, 1.54; p = 0.011; Table 2) and a multivariable logistic regression adjusted for age, baseline cognitive function (preoperative continuous cognitive index) and surgery type (OR 1.30 per CPAR increase of 1, 95% CI 1.03, 1.63; p = 0.026; Table 3). This model had no evidence of miss-fit (Hosmer-Lemeshow p=0.63) and had an area under the receiver operating curve of 0.81 (95% CI 0.71, 0.91). In a model that also included preoperative CPAR, there was no evidence of an interaction effect for baseline CPAR by preoperative to 24-hour postoperative CPAR change on postoperative delirium (interaction effect estimate −0.01; 95% CI, −0.07, 0.05; p = 0.798). Additionally, preoperative CPAR level was not correlated with preoperative to 24-hour postoperative CPAR change (Spearman ρ = −0.01; 95% CI, −0.15, 0.13; p = 0.878).
Figure 2: Postoperative cerebrospinal fluid-to-plasma albumin ratio change in patients with vs without postoperative delirium.
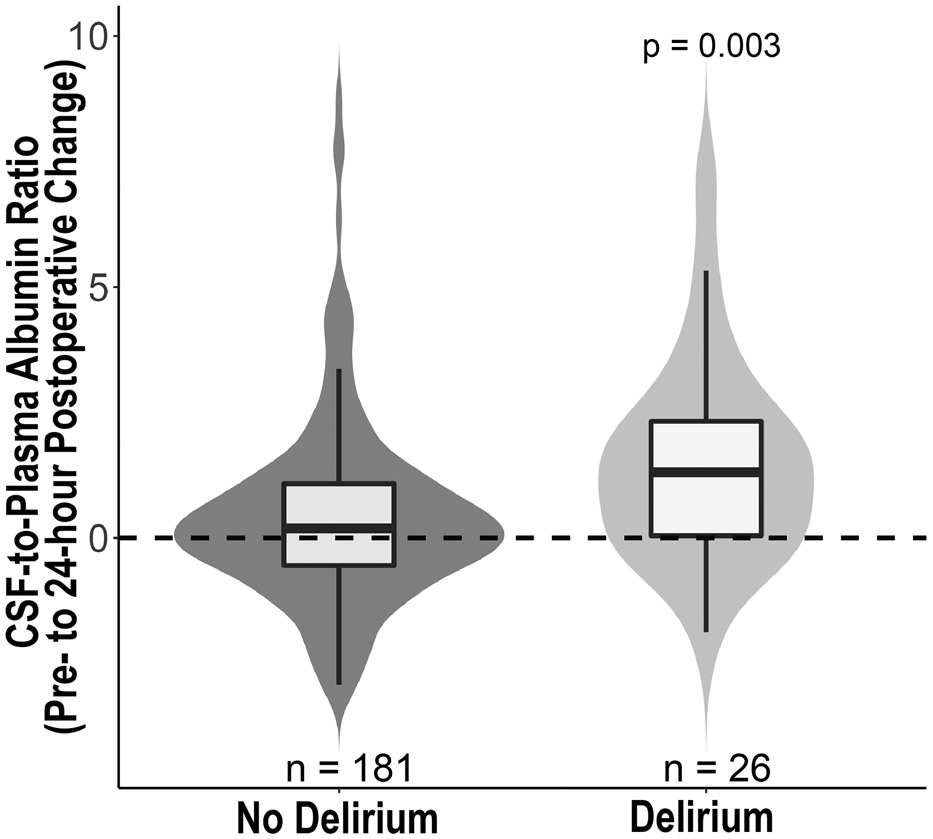
Comparison of preoperative to 24-hour postoperative change in cerebrospinal fluid-to-plasma albumin ratio (CPAR) change in patients who did vs did not develop postoperative delirium (p = 0.003, Wilcoxon).
Table 3:
Multivariable logistic regression examining effect of preoperative to 24-hour postoperative CPAR change on postoperative delirium
| Effect | OR (95% CI) | P- value |
|---|---|---|
| Pre- to 24-hour postoperative CPAR Change | 1.30 (1.03, 1.63) | 0.026 |
| Baseline cognition (per SD increase) | 0.29 (0.17, 0.49) | <0.001 |
| Age (per 5 years) | 0.74 (0.47, 1.16) | 0.192 |
| Surgery Type | ||
| General/Abdominal/Plastic/ENT v Urologic/Gynecologic | 0.77 (0.24, 2.51) | 0.661 |
| Orthopedic v Urologic/Gynecologic | 0.62 (0.16, 2.50) | 0.505 |
| Thoracic v Urologic/Gynecologic | 0.45 (0.08, 2.47) | 0.361 |
CPAR, cerebrospinal fluid-to-plasma albumin ratio
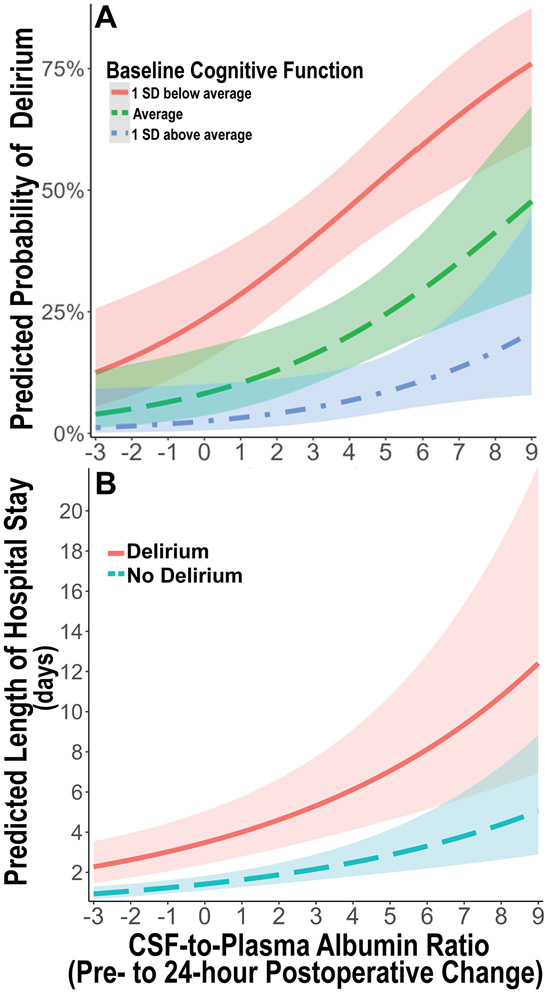
Independent of baseline cognitive impairment, there were dose-dependent additive effects of increased pre to 24-hour postoperative CPAR on postoperative delirium rates, as illustrated in Figure 3A. For a preoperative to 24-hour postoperative CPAR increase of 1 (versus no change), the delirium probability increase for a subject with 1 SD above average cognition was 0.7% (95% CI, 0.05%, 2.8%), versus a delirium probability increase of 5.0% for a subject with 1 SD below average cognition (95% CI, 0.8%, 11.7%, p = 0.026 for full model).
Figure 3: Predicted probability of postoperative delirium and hospital length of stay based on 24-hour change in cerebrospinal fluid-to-plasma ratio of albumin.
A. Predicted probability of postoperative delirium over the observed range of 24-hour postoperative cerebrospinal fluid-to-plasma ratio of albumin (CPAR) change according to baseline global cognitive function in our multivariable logistic regression model adjusted for age, baseline global cognitive function (continuous cognitive index), and surgery type. There is an additive dose-dependent association of increased 24-hour postoperative CPAR change with increased postoperative delirium rates, independent of baseline cognition. Baseline cognition is stratified as average (green ─ ─ ─), 1 standard deviation (SD) below average (red ───), and 1 SD above average (blue - ─ -). B. Predicted length of postoperative hospital length of stay over the observed range of 24 hour postoperative CPAR change according to postoperative delirium presence (red ───) or absence (blue ─ ─), in a multivariable negative binomial regression model adjusted for age, baseline global cognitive function, surgery type, and postoperative delirium status. Independent of postoperative delirium status, there is an additive dose-dependent association of increased 24-hour postoperative CPAR change with increased postoperative hospital length of stay. Shaded areas represent mean prediction error.
Blood-brain barrier dysfunction and postoperative length of stay
Next, we examined the relationship of postoperative blood-brain barrier dysfunction with hospital length of stay. Preoperative CPAR was not associated with increased hospital length of stay in a univariable (incidence rate ratio [IRR, i.e., percent increase] = 1.04 per CPAR increase of 1, 95% CI 0.99, 1.09; p = 0.129) or multivariable negative binomial regression controlling for surgery type, age, and baseline cognition (IRR = 1.02 per CPAR increase of 1, 95% CI 0.97, 1.06; p = 0.414). Yet, 24-hour postoperative CPAR was associated with increased postoperative hospital length of stay in univariable analysis (IRR = 1.08 per CPAR increase of 1, 95% CI 1.04, 1.13; p < 0.001), and in a multivariable negative binomial regression controlling for surgery type, age, baseline cognition (IRR = 1.06 per CPAR increase of 1, 95% CI 1.03, 1.10; p = 0.001). Further, increased pre to 24-hour postoperative CPAR was associated with a 20% and 18% increase in average hospital length of stay, respectively, in univariable analysis (IRR = 1.20 per CPAR increase of 1, 95% CI 1.12, 1.29; p < 0.001) and a multivariable negative binomial regression controlling for surgery type, age, and baseline cognition (IRR = 1.18 per CPAR increase of 1, 95% CI 1.11, 1.26; p < 0.001; Table 4).
Table 4:
Multivariable binomial regression examining effect of preoperative to 24-hour postoperative CPAR change on postoperative hospital length of stay
| Effect | Incidence Rate Ratio (95% CI) |
P- value |
|---|---|---|
| Pre- to 24-hour postoperative CPAR Change | 1.18 (1.11, 1.26) | <0.001 |
| Baseline cognition (per SD decrease) | 1.21 (1.06, 1.38) | 0.005 |
| Age (per 5 years) | 1.09 (0.97, 1.22) | 0.167 |
| Surgery Type | ||
| General/Abdominal/Plastic/ENT v Urologic/Gynecologic | 1.32 (0.95, 1.83) | 0.103 |
| Orthopedic v Urologic/Gynecologic | 0.86 (0.59, 1.24) | 0.422 |
| Thoracic v Urologic/Gynecologic | 2.48 (1.67, 3.68) | <0.001 |
CPAR, cerebrospinal fluid-to-plasma albumin ratio
To examine whether the relationship of blood-brain barrier dysfunction with postoperative hospital length of stay differed among patients with and without postoperative delirium, we performed a stratified analysis in patients with and without postoperative delirium using the same multivariable negative binomial regression (again controlling for surgery type, age, and baseline cognition). In patients without postoperative delirium, increased pre to 24-hour postoperative CPAR (per CPAR increase of 1) was associated with a 14% increased hospital length of stay (IRR 1.14, 95% CI 1.07, 1.20; p<0.001). In patients with postoperative delirium, increased pre to 24-hour postoperative CPAR (per CPAR increase of 1) was also associated with a 35% increased hospital length of stay (IRR 1.35, 95% CI 1.07, 1.69; p=0.011). Independent of postoperative delirium status, there were additive dose-dependent effects of increased pre to 24-hour postoperative CPAR on hospital length of stay, which is illustrated in Figure 3B. For example, the length of stay increase for a postoperative CPAR increase of 1 (versus no change) was 0.2 days (95% CI, 0.1, 0.3) for a subject without postoperative delirium compared to 0.5 days (95% CI, 0.2, 0.9) for a subject with postoperative delirium (p < 0.001 for full model).
Blood-brain barrier dysfunction and one-year postoperative mortality
We also examined the relationship of blood-brain barrier dysfunction with one-year postoperative mortality. Overall, one-year postoperative mortality was low as only 2.4% of subjects (5/207) died within one year after surgery. We found that preoperative CPAR was not significantly higher in patients that died with one year postoperatively (median 7.2, IQR 6.6, 8.7) versus those who did not (median 5.6, IQR 4.3, 7.2; Wilcoxon p = 0.14). Preoperative to 24-hour postoperative CPAR change was also not significantly higher in patients that died within one-year postoperatively (median 1.1, IQR 0.5, 1.3) versus those who did not (median 0.3, IQR −0.5, 1.2; Wilcoxon p = 0.35).
Discussion:
In this combined cohort of 207 older patients who underwent a variety of non-cardiac and non-neurologic surgeries, we found significant associations of postoperative blood-brain barrier dysfunction with postoperative delirium and increased length of hospital stay. Increased pre- to 24-hour postoperative cerebrospinal fluid-to-plasma albumin ratio was independently associated with increased odds of postoperative delirium and longer hospital stays even after accounting for surgery type and baseline cognitive status. We also demonstrate small but statistically significant surgery-induced increases in blood-brain barrier permeability (as measured by CPAR) across the entire cohort. These results provide key evidence that blood-brain barrier dysfunction occurs in older non-cardiac surgery patients, and that greater postoperative blood-brain barrier dysfunction is associated with increased postoperative delirium rates and increased hospital length of stay among older surgical patients.
Our findings are also consistent with previous work that demonstrated associations of postoperative blood-brain barrier dysfunction with cognitive dysfunction35 and delirium12 in small cohorts of cardiac/aortic surgery patients. Our work extends these findings by demonstrating that postoperative blood-brain barrier dysfunction is associated with delirium independent of baseline cognitive function, and in a much larger cohort of older non-cardiac surgery patients. This suggests that there may be a two-hit model for postoperative delirium that involves both a predisposing factor (i.e., impaired preoperative cognition) and a precipitating factor (i.e., postoperative blood-brain barrier dysfunction, as illustrated in Figure 3A).
While our data demonstrate an association between blood-brain barrier dysfunction and delirium, they do not prove causality. Indeed, our data are also compatible with the idea that blood-brain barrier dysfunction may simply be a marker of greater overall brain dysfunction after surgery, and that this greater overall brain dysfunction leads to delirium without a causal role for blood brain barrier dysfunction itself. Nonetheless, our results are similar to results from mouse models of perioperative neurocognitive disorders, in which postoperative blood-brain barrier dysfunction has been demonstrated following orthopedic surgery and has been associated with delirium-like behavioral changes.36-38 Moreover, in these animal studies, delirium-like behavioral changes are prevented when blood-brain barrier dysfunction is reduced by administration of netrin-1, a protein that upregulates endothelial tight junction proteins to increase blood-brain barrier integrity.39
Two other lines of evidence also suggest that it is biologically plausible for blood-brain barrier dysfunction to play an etiologic role in delirium. First, a leaky blood-brain barrier can allow both peripheral inflammatory molecules and leukocytes to enter the brain,12,40 both of which have been shown to result in cognitive dysfunction in animal models and other human disorders ranging from multiple sclerosis to major depression.41,42 Indeed, anti-inflammatory treatments have been shown to improve cognition in both depression41 and multiple sclerosis,43 which suggests that neuroinflammation plays a role in causing cognitive dysfunction in patients with these disorders. Second, it is well known that there is a significant peripheral inflammatory response after surgery,44 and blood-brain barrier dysfunction could allow these inflammatory mediators to enter the brain. If these inflammatory cytokines enter the brain, it is plausible that they could cause cognitive alterations seen in delirium, because cytokines have been shown to impair synaptic plasticity,45 a molecular mechanism that underlies human cognition and memory.
Taken in context of these prior findings, our results are consistent with the hypothesis that postoperative blood-brain barrier dysfunction allows peripheral and cellular inflammatory mediators into the brain after surgery, which then play an etiologic role in delirium. If this hypothesis is correct, four important questions for future studies arise. First, what factors contribute to postoperative blood-brain barrier dysfunction? A detailed analysis of intraoperative factors (such as hypotension) associated with postoperative blood-brain barrier dysfunction could help identify factors that potentially lead to postoperative blood-brain barrier dysfunction but is beyond the scope of the current study. Second, what are the molecular and cellular mechanisms that lead to blood-brain barrier dysfunction after surgery? Third, what specific mediators play a causal role in delirium after they enter the brain via a disrupted blood-brain barrier? Fourth, what interventions could block these mechanisms and/or prevent blood-brain barrier dysfunction after surgery?
Aside from the role of blood-brain barrier dysfunction in delirium, our data also show postoperative blood-brain barrier dysfunction was associated with increased postoperative hospital length of stay. Further, this association remained in stratified analyses among patients without delirium, which suggests that postoperative delirium does not fully account for the increased length of stay in patients with increased postoperative blood-brain barrier dysfunction. There are at least three potential explanations for these findings: First, increased postoperative blood-brain barrier dysfunction may play a role in other processes that limit postoperative recovery, such as (but not limited to) pain,46 depressed mood,47 and increased anxiety,47 that could affect willingness to participate in physical therapy and ambulation thus prolonging hospital discharge. Second, increased postoperative blood-brain barrier dysfunction may contribute to other unmeasured cognitive deficits that may slow postoperative recovery, such as sub-syndromal delirium (i.e., isolated attention deficits, unnoticed awareness changes, or subtle disorganized thinking). Because of the skip pattern in our delirium assessments in MADCO-PC, we do not have data on rates of sub-syndromal delirium or delirium severity scores. Third, given the fluctuating nature of delirium, it is possible that some cases of delirium were missed, despite our rigorous delirium assessments combined with chart reviews for delirium. Even though our data demonstrated an association of blood-brain barrier dysfunction on postoperative hospital length of stay independent of delirium, delirium could still be a mediator of this relationship if a substantial number of delirium cases were missed.
Further, similar to the relationship of blood-brain barrier dysfunction with delirium, the association of blood-brain barrier dysfunction with increased postoperative length of stay could reflect a causal relationship or simply an association without a causal relationship. Our data cannot distinguish between these possibilities, but evidence of causality (or its absence) could come from future studies to test the extent to which interventions that reduce postoperative blood-brain barrier dysfunction (such as the angiogenic growth factor netrin-139 or omega 3 fatty acids8) lead to reduced hospital length of stay.
We also examined the relationship of blood-brain barrier dysfunction with one-year postoperative mortality. Although blood-brain barrier dysfunction was not significantly higher in patients who died by one-year after surgery, our study is likely severely underpowered to determine the relationship between blood-brain barrier dysfunction and postoperative mortality given the low number of one-year postoperative deaths (n = 5). Thus, the relationship between blood-brain barrier dysfunction and one-year postoperative mortality should be investigated further in a larger study of a surgical cohort that exhibits greater one-year postoperative mortality, such as cardiac surgery patients.
This study has several strengths. First, because the overall size (n=207) of this study is significantly larger, this study significantly extends work in cardiac (n=10)16 and aortic surgery (n=11)12 patients that examined postoperative CPAR increases. Second, delirium assessments were carried out by trained staff and were supplemented with delirium chart reviews to minimize missed cases of delirium. Third, we studied a wide variety of non-cardiac surgeries, extending the findings from prior studies on blood-brain barrier dysfunction following cardiac surgery. Because postoperative blood-brain barrier dysfunction could result from the inflammatory response elicited by cardiopulmonary bypass, it was unclear whether blood-brain barrier dysfunction occurs with other types of surgery. Our findings provide new evidence that non-cardiac surgery also elicits blood-brain barrier dysfunction.
This study has several limitations. First, although these findings demonstrate 24-hour postoperative increases in blood-brain barrier permeability, the exact time course of when blood-brain barrier dysfunction develops within the first 24 hours after surgery remains unclear. Second, the MADCO-PC and INTUIT studies used different instruments for detecting delirium (i.e., CAM vs 3D-CAM), which may have increased variance in the relationship strength seen between blood-brain barrier dysfunction and postoperative delirium between these two cohorts. However, both instruments have high sensitivity and specificity for detecting delirium and both methods were supplemented by delirium chart reviews.48 Further, we found no significant effect of study cohort on the relationship of CPAR with postoperative delirium. Third, the delirium rate seen here was modest (12.6%), which while comparable to that reported in other studies of similarly aged non-cardiac surgical patients,49,50 reduces our ability to model additional covariates or to find interaction effects between postoperative blood-brain barrier dysfunction and baseline delirium risk factors (such as cognition). Fourth, the low delirium rate and high variance of CPAR within the delirium group (n=26) resulted in broad confidence intervals for our effect estimates. Thus a larger study could provide a more precise effect estimate for the strength of the association(s) between CPAR and postoperative delirium, and could help assess for potential interaction effects between additional baseline factors and CPAR on postoperative delirium risk. Fifth, our cohort was comprised of patients from a single center who were mostly Caucasian, and who spoke English. Thus, future studies are necessary to determine the extent to which these results generalize to other centers, patients of different races, and non-English speakers.
Conclusions:
In a large cohort of older patients undergoing elective non-cardiac surgery, we found that pre- to 24-hour postoperative blood-brain barrier permeability increases were associated with higher rates of postoperative delirium and increased postoperative hospital length of stay.
Summary for Social Media if Published:
1. Twitter Handles: @Mike_Devinney and @RealMilesBerger
2. What is the current knowledge on the topic? Findings in animal models suggest a role for blood-brain barrier dysfunction in delirium-like behavior following orthopedic surgery. Following cardiac surgery in humans, blood-brain barrier dysfunction occurs and may be associated with postoperative delirium.
3. What question did this study address? Does blood-brain barrier dysfunction occur in older adults following non-cardiac surgery? Is the extent of postoperative blood-brain barrier dysfunction associated with the occurrence of postoperative delirium and increased hospital length of stay?
4. What does this study add to our knowledge? This study provides key evidence that blood-brain barrier dysfunction occurs in older non-cardiac surgery patients, and that postoperative blood-brain barrier dysfunction is associated with increased postoperative delirium rates and increased hospital length of stay among older surgical patients.
5. How might this potentially impact on the practice of neurology? This study supports the idea that interventions to target blood-brain barrier dysfunction should be tested to prevent delirium. If successful, then these interventions could be used in clinical settings to reduce rates of postoperative delirium.
Acknowledgements:
We thank the patients who participated and the clinical staff who cared for them, for making this work possible. We thank Michael Muehlbauer, PhD and the Duke Molecular Physiology Institute for performing the albumin measurements. We also thank Kathy Gage for comments on this manuscript. Funding for this study was provided by NIH R03-AG067976 (MJD), the Duke Department of Anesthesiology, a Foundation for Anesthesia Education and Research (FAER) Research Fellowship Grant (MJD), a William L. Young Award from the Society of Neuroscience in Anesthesiology and Critical Care (MJD), an International Anesthesia Research Society Mentored Grant (MB), NIH R03-AG05918 (MB) and NIH K76-AG057022 (MB). MJD also acknowledges additional support from a FAER GEMSSTAR grant, a Merck Investigator-Initiated Studies Program grant, and a George Maddox Award from the Duke Center for the Study of Aging. ERM acknowledges support from NIH K24 AG035075. HEW acknowledges support from NIH 5-P30AG072958-02 and 5-P30AG028716-17. JPM acknowledges support from 5R01AG074185-02. MB acknowledges additional funding from P30 AG072958 (the Duke/UNC Alzheimer’s Disease Research Center). MB and JNB acknowledge additional support from 1R01AG076903-01 and 1R01AG073598-01A1. NT acknowledges support from R01AG057525 and RF1AG079138.
Collaborators:
MADCO-PC Study Team: Miles Berger, Brian E. Brigman, Jeffrey N. Browndyke, W. Michael Bullock, Jessica Carter, Joseph Chapman, Brian Colin, Mary Cooter, Thomas A. D’Amico, James K. DeOrio, Ramon M. Esclamado, Michael N. Ferrandino, Jeffrey Gadsden, Grant E. Garrigues, Stuart Grant, Jason Guercio, Dhanesh Gupta, Ashraf Habib, David H. Harpole, Mathew G. Hartwig, Ehimemen Iboaya, Brant A. Inman, Anver Khan, Sandhya Lagoo-Deenadayalan, Paula S. Lee, Walter T. Lee, John Lemm, Howard Levinson, Christopher Mantyh, Joseph P. Mathew, David L. McDonagh, John Migaly, Suhail K. Mithani, Eugene Moretti, Judd W. Moul, Mark F. Newman, Brian Ohlendorf, Alexander Perez, Andrew C. Peterson, Glenn M. Preminger, Quintin Quinones, Cary N. Robertson, Sanziana A. Roman, Scott Runyon, Aaron Sandler, Faris M. Sbahi, Randall P. Scheri, S. Kendall Smith, Leonard Talbot, Julie K. M. Thacker, Jake Thomas, Betty C. Tong, Steven N. Vaslef, Nathan Waldron, Xueyuan Wang, and Christopher Young.
INTUIT Study Team: Leah Acker, Cindy Louise Amundsen, Oke Anakwenze, Harel Anolick, David Attarian, Pallavi Avasarala, Chakib Ayoub, Matthew Barber, Rachel Beach, Andrew Berchuck, Dan G Blazer III, Michael Bolognesi, Rachele Brassard, Brian Brigman, William Michael Bullock, Thomas Bunning, Victor Cai, Yee Ching Vanessa Cheong, Soren Christensen, Brian Colin, Mitchell Wayne Cox, Thomas D’Amico, Brittany Anne Davidson, James Keith Deorio, Mark E. Easley, Sarada Eleswarpu, Detlev Erdmann, Mariana Feingold, Michael Nicolo Ferrandino, Jeffrey Gadsden, Mark Gage, Arun Ganesh, Grant Edward Garrigues, Rachel Adams Greenup, Ashraf Habib, Ashley Hall, Rhett K. Hallows, David Harpole Jr., Matthew Hartwig, Laura Havrilesky, Courtney Holland, Scott Thomas Hollenbeck, Thomas Hopkins, Edward Ross Houser ll, Samuel Huang, Ehimemen Iboaya, Brant Inman, William Jiranek, Russel Kahmke, Amie Kawasaki, Brendan Kelleher, Jay Han Kim, Jacob Klapper, Christopher Klifto, Rebecca Klinger, Stuart Knechtle, Sandhya A. Lagoo-Deenadayalan, Billy Lan, Walter Lee, Howard Levinson, Brian Lewis, Michael Lipkin, Christopher Mantyh, Hector Martinez-Wilson, John Migaly, Eugene Moretti, Judd Moul, Michael Muehlbauer, David Murdoch, Thomas L. Novick, Kathryn Odom, Brian Ohlendorf, Steven Olson, Shannon Page, Theodore Pappas, John Park, Andrew Peterson, Andreea Podgoreanu, Thomas J Polascik, Dana Portenier, Glenn M Preminger, Rebecca Ann Previs, Edward Nandlal Rampersaud Jr., Kenneth Roberts, Cary N Robertson, Sanziana Alina Roman, Jason Rothman, Aaron Sandler, Siddharth Sata, Charles Scales, Jr., Randall Scheri, Thorsten Seyler, Keri Anne Seymour, Nazema Y. Siddiqui, Shayan Smani, Michael Stang, Samuel David Stanley, Katherine Sweeney, Ayesha Syed, Martin V. Taormina, Julie Thacker, Jake Thomas, Betty Tong, Yanne Toulgoat-Dubois, Keith Vandusen, Nathan Waldron, Alison Weidner, Kent Weinhold, Samuel Wellman, David Williams, Marty Woldorff, Rosa Yang, Christopher Young, Sabino Zani, and Mimi Zhang.
Footnotes
Conflicts of Interest: Nothing to report.
References:
- 1.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;(11):1–25. [PubMed] [Google Scholar]
- 3.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gou RY, Hshieh TT, Marcantonio ER, et al. One-Year Medicare Costs Associated With Delirium in Older Patients Undergoing Major Elective Surgery. JAMA Surg. 2021;156(5):430–442. doi: 10.1001/jamasurg.2020.7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong TG, Inouye SK. The inter-relationship between delirium and dementia: the importance of delirium prevention. Nat Rev Neurol. 2022;18(10):579–596. doi: 10.1038/s41582-022-00698-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P, Velagapudi R, Kong C, et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement. 2020;16(5):734–749. doi: 10.1002/alz.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Gu C, Mandeville ET, et al. Anesthesia and Surgery Impair Blood-Brain Barrier and Cognitive Function in Mice. Front Immunol. 2017;8:902. doi: 10.3389/fimmu.2017.00902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T, Velagapudi R, Kong C, et al. Protective effects of omega-3 fatty acids in a blood-brain barrier-on-chip model and on postoperative delirium-like behaviour in mice. Br J Anaesth. 2023;130(2):e370–e380. doi: 10.1016/j.bja.2022.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cashion JM, Young KM, Sutherland BA. How does neurovascular unit dysfunction contribute to multiple sclerosis? Neurobiol Dis. 2023;178:106028. doi: 10.1016/j.nbd.2023.106028 [DOI] [PubMed] [Google Scholar]
- 10.Taheri S, Gasparovic C, Huisa BN, et al. Blood-Brain Barrier Permeability Abnormalities in Vascular Cognitive Impairment. Stroke. 2011;42(8):2158–2163. doi: 10.1161/STROKEAHA.110.611731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowman GL, Dayon L, Kirkland R, et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14(12):1640–1650. doi: 10.1016/j.jalz.2018.06.2857 [DOI] [PubMed] [Google Scholar]
- 12.Taylor J, Parker M, Casey CP, et al. Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth. Published online February 7, 2022:S0007-0912(22)00013-7. doi: 10.1016/j.bja.2022.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68(21):1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danielson M, Wiklund A, Granath F, et al. Neuroinflammatory markers associate with cognitive decline after major surgery: Findings of an explorative study. Ann Neurol. 2020;87(3):370–382. doi: 10.1002/ana.25678 [DOI] [PubMed] [Google Scholar]
- 15.Vasunilashorn SM, Ngo LH, Dillon ST, et al. Plasma and cerebrospinal fluid inflammation and the blood-brain barrier in older surgical patients: the Role of Inflammation after Surgery for Elders (RISE) study. J Neuroinflammation. 2021;18(1):103. doi: 10.1186/s12974-021-02145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinsfelt B, Ricksten SE, Zetterberg H, Blennow K, Fredén-Lindqvist J, Westerlind A. Cerebrospinal fluid markers of brain injury, inflammation, and blood-brain barrier dysfunction in cardiac surgery. Ann Thorac Surg. 2012;94(2):549–555. doi: 10.1016/j.athoracsur.2012.04.044 [DOI] [PubMed] [Google Scholar]
- 17.Poole L, Leigh E, Kidd T, Ronaldson A, Jahangiri M, Steptoe A. The combined association of depression and socioeconomic status with length of post-operative hospital stay following coronary artery bypass graft surgery: Data from a prospective cohort study. J Psychosom Res. 2014;76(1):34–40. doi: 10.1016/j.jpsychores.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PJ, Rivelli SK, Waters AM, et al. Delirium affects length of hospital stay after lung transplantation. J Crit Care. 2015;30(1):126–129. doi: 10.1016/j.jcrc.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ameliorate JL, Ghabriel MN, Vink R. Magnesium enhances the beneficial effects of NK1 antagonist administration on blood-brain barrier permeability and motor outcome after traumatic brain injury. Magnes Res. 2017;30(3):88–97. doi: 10.1684/mrh.2017.0427 [DOI] [PubMed] [Google Scholar]
- 20.Berger M, Browndyke JN, Cooter Wright M, et al. Postoperative changes in cognition and cerebrospinal fluid neurodegenerative disease biomarkers. Ann Clin Transl Neurol. Published online February 1, 2022. doi: 10.1002/acn3.51499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger M, Oyeyemi D, Olurinde MO, et al. The INTUIT Study: Investigating Neuroinflammation Underlying Postoperative Cognitive Dysfunction. J Am Geriatr Soc. Published online January 23, 2019. doi: 10.1111/jgs.15770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobuhara CK, Bullock WM, Bunning T, et al. A protocol to reduce self-reported pain scores and adverse events following lumbar punctures in older adults. J Neurol. 2020;267(7):2002–2006. doi: 10.1007/s00415-020-09797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601 [DOI] [PubMed] [Google Scholar]
- 24.Browndyke JN, Wright MC, Yang R, et al. Perioperative neurocognitive and functional neuroimaging trajectories in older APOE4 carriers compared with non-carriers: secondary analysis of a prospective cohort study. Br J Anaesth. 2021;127(6):917–928. doi: 10.1016/j.bja.2021.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman MF, Grocott HP, Mathew JP, et al. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32(12):2874–2881. doi: 10.1161/hs1201.099803 [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 27.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7 [DOI] [PubMed] [Google Scholar]
- 28.Browndyke JN, Wright MC, Yang R, et al. Perioperative neurocognitive and functional neuroimaging trajectories in older APOE4 carriers compared with non-carriers: secondary analysis of a prospective cohort study. Br J Anaesth. Published online September 14, 2021:S0007-0912(21)00519-5. doi: 10.1016/j.bja.2021.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554–561. doi: 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941 [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 32.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. doi: 10.1111/j.1532-5415.2005.53120.x [DOI] [PubMed] [Google Scholar]
- 33.Montagne A, Barnes SR, Sweeney MD, et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59 Suppl 2:S301–304. doi: 10.1111/j.1532-5415.2011.03679.x [DOI] [PubMed] [Google Scholar]
- 35.Abrahamov D, Levran O, Naparstek S, et al. Blood-Brain Barrier Disruption After Cardiopulmonary Bypass: Diagnosis and Correlation to Cognition. Ann Thorac Surg. 2017;104(1):161–169. doi: 10.1016/j.athoracsur.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 36.Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–995. doi: 10.1002/ana.22664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang T, Xu G, Newton PT, et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth. 2019;122(3):350–360. doi: 10.1016/j.bja.2018.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David-Bercholz J, Acker L, Caceres AI, et al. Conserved YKL-40 changes in mice and humans after postoperative delirium. Brain Behav Immun Health. 2022;26:100555. doi: 10.1016/j.bbih.2022.100555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li K, Wang J, Chen L, et al. Netrin-1 Ameliorates Postoperative Delirium-Like Behavior in Aged Mice by Suppressing Neuroinflammation and Restoring Impaired Blood-Brain Barrier Permeability. Front Mol Neurosci. 2021;14:751570. doi: 10.3389/fnmol.2021.751570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger M, Murdoch DM, Staats JS, et al. Flow Cytometry Characterization of Cerebrospinal Fluid Monocytes in Patients With Postoperative Cognitive Dysfunction: A Pilot Study. Anesth Analg. Published online March 2019. doi: 10.1213/ANE.0000000000004179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kermode AG, Thompson AJ, Tofts P, et al. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990;113 ( Pt 5):1477–1489. doi: 10.1093/brain/113.5.1477 [DOI] [PubMed] [Google Scholar]
- 43.Sprenger T, Kappos L, Sormani MP, et al. Effects of teriflunomide treatment on cognitive performance and brain volume in patients with relapsing multiple sclerosis: Post hoc analysis of the TEMSO core and extension studies. Mult Scler. 2022;28(11):1719–1728. doi: 10.1177/13524585221089534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verdonk F, Einhaus J, Tsai AS, et al. Measuring the human immune response to surgery: multiomics for the prediction of postoperative outcomes. Curr Opin Crit Care. 2021;27(6):717–725. doi: 10.1097/MCC.0000000000000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang S, Bhaskar K. Dynamics of the Complement, Cytokine, and Chemokine Systems in the Regulation of Synaptic Function and Dysfunction Relevant to Alzheimer’s Disease. J Alzheimers Dis. 2017;57(4):1123–1135. doi: 10.3233/JAD-161123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tajerian M, Clark JD. Novel cytogenic and neurovascular niches due to blood-brain barrier compromise in the chronic pain brain. Mol Pain. 2015;11:63. doi: 10.1186/s12990-015-0066-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Ma L, Luo Y, et al. Increasing of Blood Brain Barrier Permeability and the Association With Depression and Anxiety in Systemic Lupus Erythematosus Patients. Front Med (Lausanne). 2022;9:852835. doi: 10.3389/fmed.2022.852835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasunilashorn SM, Guess J, Ngo L, et al. Derivation and Validation of a Severity Scoring Method for the 3-Minute Diagnostic Interview for Confusion Assessment Method--Defined Delirium. J Am Geriatr Soc. 2016;64(8):1684–1689. doi: 10.1111/jgs.14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackenbom MF, Zyczynski HM, Butters MA, Lopa S, Orris SR, Davis EM. Postoperative delirium in older patients after undergoing pelvic organ prolapse surgery. Int Urogynecol J. 2023;34(1):201–209. doi: 10.1007/s00192-022-05170-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss Y, Zac L, Refaeli E, et al. Preoperative Cognitive Impairment and Postoperative Delirium in Elderly Surgical Patients - a Retrospective Large Cohort Study. Ann Surg. Published online August 1, 2022. doi: 10.1097/SLA.0000000000005657 [DOI] [PubMed] [Google Scholar]