Abstract
(Oligodendro)glial cytoplasmic inclusions composed of α-synuclein (αSYN) characterize multiple system atrophy (MSA). Mature oligodendrocytes (OLs) do not normally express αSYN, so MSA pathology may arise from aberrant expression of αSYN in OLs. To study pathological deposition of αSYN in OLs, transgenic mice were generated in which human wild-type αSYN was driven by a proteolipid protein promoter. Transgenic αSYN was detected in OLs but no other brain cell type. At the light microscopic level, the transgenic αSYN profiles resembled glial cytoplasmic inclusions. Strikingly, the diagnostic hyperphosphorylation at S129 of αSYN was reproduced in the transgenic mice. A significant proportion of the transgenic αSYN was detergent insoluble, as in MSA patients. The histological and biochemical abnormalities were specific for the disease-relevant αSYN because control green fluorescent protein was fully soluble and evenly distributed throughout OL cell bodies and processes. Thus, ectopic expression αSYN in OLs might initiate salient features of MSA pathology.
INTRODUCTION
Multiple system atrophy (MSA) is an age-related syndrome that includes striatonigral degeneration causing Parkinsonism and olivopontocerebellar atrophy leading to ataxia, as well as autonomic failure and urinary dysfunction (Gilman et al., 1999). White matter from MSA brain is characterized by the presence of glial cytoplasmic inclusions (GCIs) that are composed of α-synuclein (αSYN) fibrils (Arima et al., 1998; Spillantini et al., 1998; Tu et al., 1998). αSYN belongs to a family of normally synaptic proteins, which can be phosphorylated at their acidic C-terminus (Okochi et al., 2000; Pronin et al., 2000). S129 was identified as the major phosphoacceptor in αSYN (Okochi et al., 2000). Hyperphosphorylation at S129 was recently found to be a diagnostic modification of αSYN in pathological lesions, including GCIs (Fujiwara et al., 2002).
The source of oligodendroglial αSYN, which aggregates into GCIs, is unknown. Mature oligodendrocytes (OLs) do not express αSYN mRNA and protein under physiological conditions (Iwai et al., 1995; Solano et al., 2000). It is possible that pathological expression of αSYN in OLs causes GCI formation. To validate this hypothesis, we generated a transgenic mouse model in which human wild-type αSYN was expressed under the control of an OL-specific proteolipid protein (PLP) promoter (Fuss et al., 2000). Robust expression of transgenic αSYN was achieved in mouse brain white matter. The transgenic αSYN in OL cell bodies was typically arranged in a triangular or half-moon-shaped manner, as in human MSA patients (Lantos, 1998). These profiles were immunopositive for a phospho-specific anti-αSYN (Fujiwara et al., 2002), corroborating the pathological nature of αSYN deposited in transgenic mouse OLs. Moreover, a substantial proportion of the transgenic αSYN was detergent insoluble, as in MSA brain samples.
RESULTS AND DISCUSSION
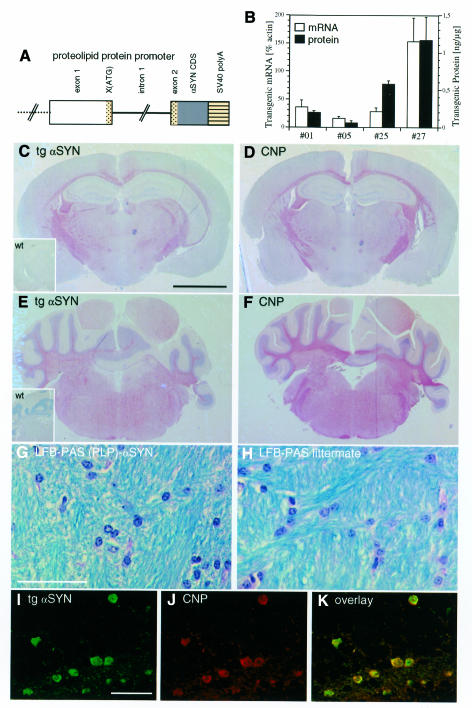
Four founder mice stably transmitted the transgene (Figure 1A). The expression of transgenic human αSYN mRNA (0.8–0.9 kb) and protein was low in line 05, intermediate in lines 01 and 25, and high in line 27 (Figure 1B). In all four lines, transgenic human αSYN immunoreactivity was detected in white matter and OL cell bodies giving a staining pattern similar to that of the OL marker enzyme 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNP) (Figure 1C–F). Myelin was stained with Luxol fast blue periodic acid Schiff (LFB-PAS). No obvious demyelination was observed in the transgenic mice up to 18 months (mo), compared to non-transgenic littermates (Figure 1G and H). The number of OLs was determined in the cerebellar white matter in five different visual fields from three transgenic mice (line 27) and three non-transgenic littermates. OL counts were similar for both groups: 21.6 ± 2.6 per 0.02 mm2 and 22.8 ± 2.8 per 0.02 mm2, respectively.
Fig. 1. OL-specific expression of PLP-driven αSYN in transgenic mice. (A) Schematic representation of (PLP)-αSYN construct (not drawn to scale). The 5′ region of the PLP gene is shown as an open box, and exon 1 and the initial segment of exon 2 is shown as a dotted region with the mutated ATG→GAG and the first 13 codons of PLP in exon 2. Intron 1 is shown as a solid line, human wild-type αSYN coding sequence as a dark box and the SV40 polyadenylation signals as a striped box. (B) Transgenic αSYN mRNA expression levels relative to β-actin (open bars) were determined in duplicate (error bars: range) by quantitative northern blotting. The amount of transgenic αSYN protein (closed bars) was determined in three individual mice of each line (error bars: standard deviation) by quantitative western blotting of whole-brain cytosol samples. (C–F) Coronal sections from a representative (PLP)-αSYN mouse (line 27) at the level of the hippocampus (C and D) and cerebellum (E and F) stained with 15G7 anti-αSYN (C and E) and anti-CNP (D and F). Inserts in C and E show no staining with the 15G7 antibody in wild-type mice. Scale bar in (C) corresponds to 2.5 mm. (G and H) Cerebellar white matter of a transgenic mouse (G) and a non-transgenic littermate (H) stained with LFB-PAS shows no demyelinization. Scale bar in (G) corresponds to 50 µm. (I–K) Confocal laser scanning images of a section from (PLP)-αSYN mouse cerebellum double-labeled with 15G7 anti-αSYN (green, I) and anti-CNP (red, J); overlay (K). Scale bar in (I) corresponds to 50 µm.
Laser confocal microscopy of sections double-labeled with 15G7 anti-αSYN and anti-CNP revealed that the transgenic αSYN was strictly localized in cells immunopositive for CNP (Figure 1I–K). Large-diameter neurons were spared of transgenic αSYN immunoreactivity (Figure 2A and C). Likewise, no transgenic αSYN was found in the neuropil (Figure 1C and E), where synucleins are normally enriched.
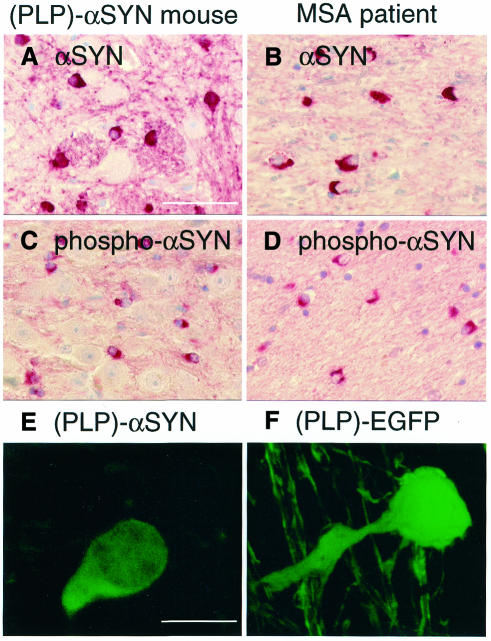
Fig. 2. Pathological subcellular localization and hyperphosphorylation of αSYN expressed in transgenic mouse OLs. (A–D) Cerebellar white matter sections from (PLP)-αSYN mice (A and C) and MSA patients (B and D) were stained with human-specific 15G7 anti-αSYN (A and B) and phospho-specific anti-αSYN (C and D). Scale bar in (A) corresponds to 50 µm. (E and F) Individual OL from a (PLP)-αSYN mouse stained with 15G7 anti-αSYN (E) and green fluorescence of a (PLP)-EGFP mouse OL (F). Scale bar in (E) corresponds to 10 µm.
The subcellular distribution of αSYN was predominantly cytosolic in transgenic mouse OLs, with a minor proportion of nuclear staining. The transgenic human αSYN was often arranged in a triangular or half-moon-shaped localization around the nucleus (Figure 2A). This profile was reminiscent of αSYN staining in human MSA patients, who also showed triangular or half-moon-shaped cytosolic immunoreactivity of αSYN in OL cell bodies (Figure 2B). Remarkably, the GCI-resembling profiles in (PLP)-αSYN mice and human MSA patients contained hyperphosphorylated αSYN (Figure 2C and D), as evidenced by a phospho-specific anti-αSYN. In contrast, white matter from non-transgenic mice and control human brain was entirely devoid of phospho-αSYN immunoreactivity (data not shown). Asymmetrical somal accumulation of hyperphosphorylated αSYN was a common feature of OLs in all brain regions that expressed the transgenic protein.
The striking similarity of transgenic mouse αSYN staining with human MSA was specific for the disease-relevant αSYN. When a control green fluorescent protein (EGFP) was expressed under control of the PLP promoter, a different localization was found. In contrast to the triangular accumulation of transgenic αSYN in OL cell bodies (Figure 2E), the control protein EGFP was also prominently visualized in the proximal processes (Figure 2F). The half-moon-shaped arrangement of transgenic αSYN was a common finding in all four (PLP)-αSYN lines but was not observed in six different GFP and EGFP lines, indicating that asymmetrical somal accumulation of transgenic αSYN was not due to unequal expression levels or transgene integration artifacts.
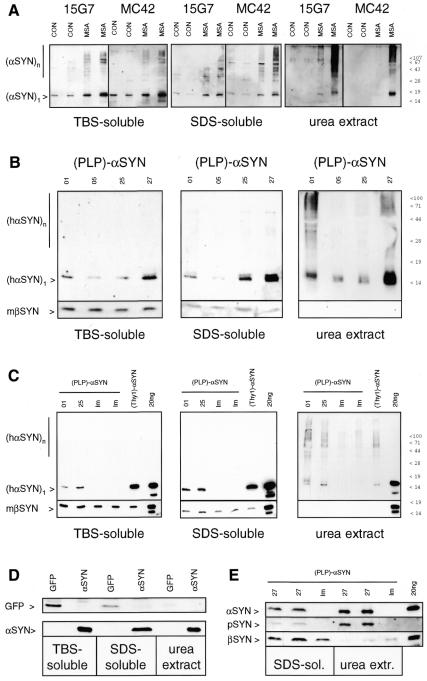
Detergent-insolubility is a characteristic biochemical feature of α-synucleinopathies, including MSA (Tu et al., 1998; Dickson et al., 1999; Duda et al., 2000; Campbell et al., 2001). We optimized a differential detergent extraction protocol for the analysis of human and transgenic mouse brain (Kahle et al., 2001). Briefly, whole brain was homogenized in buffer, and the 100 000 g pellet was extracted with 5% SDS. The remaining detergent-insoluble pellet was solubilized with 8 M urea/ 5% SDS. First, cerebellar white matter from human MSA patients and controls was analyzed. In control samples, much of the αSYN was extractable with buffer and the remainder was solubilized entirely with 5% SDS (Figure 3A). Monomeric αSYN was the predominant species in control brain. Interestingly, high-molecular-mass aggregates of αSYN were also visible in the buffer- and detergent-soluble fractions of MSA brains. Moreover, some αSYN remained insoluble after repeated extraction with 5% SDS. Strong denaturing conditions (8 M urea) were necessary to recover the detergent-insoluble αSYN from MSA samples (Figure 3A). On western blots of urea extracts from MSA patients, but not controls, the monomeric αSYN band as well as higher-molecular-mass oligomers and aggregates that remained in the stacking gel were seen using two different monoclonal antibodies against αSYN. A similar αSYN band pattern was also observed upon GCI immunoisolation from MSA white matter (Gai et al., 1999).
Fig. 3. Detergent-insoluble αSYN in MSA patients and (PLP)-αSYN mice. (A) Extracts from cerebellar cortex of controls (CON) and MSA patients were prepared. TBS-soluble and SDS-soluble material (20 µg per lane) and TCA precipitates from urea extracts were western blotted and sequentially probed with two monoclonal antibodies against αSYN (15G7, MC42). αSYN aggregates are labeled by a vertical bar. (B) Two whole brains of 3 mo (PLP)-αSYN mice (lines indicated on top) were pooled. Buffer- and detergent-soluble proteins (upper panels: 10 µg per lane; lower panels: 50 µg per lane), and TCA precipitates of urea extracts were western blotted and probed with human-specific anti-αSYN 15G7 and anti-βSYN 6485, as indicated to the left. (C) Whole brains of 6 mo (PLP)-αSYN mice (lines indicated on top) and age-matched non-transgenic littermates (lm) were differentially extracted. Brains from age-matched, neuronally expressing (Thy1)-[wt]αSYN mice were extracted in parallel. Buffer- and detergent-soluble proteins (10 µg per lane) and TCA precipitates of urea extracts were western blotted and sequentially probed with human-specific anti-αSYN 15G7 and anti-βSYN 6485, as indicated to the left. Control lanes were loaded with mixtures of 20 ng αSYN and βSYN. (D) Whole brains of a (PLP)-GFP and a (PLP)-αSYN mouse (as indicated on top) were extracted in parallel. Buffer-soluble (25 µg) and detergent-soluble (50 µg) fractions and TCA precipitates of urea extracts were western blotted and probed with anti-GFP (left panel) or 15G7 anti-αSYN (right panel). (E) Two whole brains of 8 mo (PLP)-αSYN heterozygotes (line 27) or littermates (lm) were pooled. Detergent-soluble proteins (50 µg) and TCA precipitates of urea extracts were western blotted and sequentially probed with human-specific anti-αSYN (upper panel), phospho-specific anti-αSYN (middle panel) and anti-βSYN 6485 (lower panel). The control lane to the far right was loaded with a mixture of unphosphorylated αSYN and βSYN (20 ng each).
This method, which proved diagnostic for α-synucleinopathy in MSA (see above), was applied to transgenic mice. Most of the synuclein was extracted with buffer and the remainder was solubilized with 5% SDS (Figure 3B). The non-amyloidogenic βSYN (Kahle et al., 2001) was not detected in urea extracts, both in transgenic mice and non-transgenic littermates. In sharp contrast, a portion of the transgenic αSYN remained detergent insoluble and required the harsh urea extraction step for recovery (Figure 3B). All four transgenic (PLP)-αSYN mouse lines contained some detergent-insoluble αSYN (Figure 3B). Interestingly, although the overall expression levels of human αSYN in the PLP-driven transgenic mice were lower than in (Thy1)-αSYN mice, the amount of detergent-insoluble transgenic αSYN was similar (Figure 3C). This result suggests that the relative insolubility of transgenic αSYN in OLs is higher than in neurons.
As a control, transgenic mouse brain expressing GFP under control of the same PLP promoter was extracted. The 27 kDa GFP band was found in the buffer- and detergent-soluble fractions but was not present in the urea extracts (Figure 3D). Thus, detergent-insolubility was a specific feature of transgenic αSYN and not non-specifically due to overexpression of an ectopic protein in OLs.
Finally, the specific accumulation of pathologically phosphorylated αSYN observed in histological sections (Figure 2C) was confirmed in biochemical experiments. Phosphorylated αSYN was detected on western blots of urea extracts from (PLP)-αSYN mice but not from littermates (Figure 3E). Phospho-αSYN immunoreactivity was enriched in the detergent-insoluble fractions, suggesting that phosphorylation of αSYN is a modification strictly associated with pathological lesions.
Although αSYN is the major component of GCIs, it is not understood how this predominantly neuronal protein appears in MSA OLs. In a recent study, no difference was found between controls and MSA patients comparing the αSYN expression levels in cerebral cortex (Ozawa et al., 2001), a brain region not obviously affected in MSA. Higher-resolution methods (such as single-cell RT–PCR) are necessary to determine the αSYN expression levels in white matter from the affected brain regions in MSA. OLs do have the potential to induce αSYN expression. Rat brain OLs transiently expressed αSYN during in vitro differentiation (Richter-Landsberg et al., 2000). Some pathological signal could therefore trigger oligodendroglial expression of αSYN in MSA. An interesting candidate for such a pathological MSA mediator is the pro-inflammatory cytokine interleukin-1β, which stimulated the expression of αSYN in U251 glioma cells (Tanji et al., 2001).
Upon ectopic expression in transgenic mouse OLs, αSYN arranged in profiles reminiscent of GCIs, whereas the disease-irrelevant control protein GFP freely distributed throughout the OL cytosol including processes. In this aberrant subcellular site, αSYN was pathologically phosphorylated. Because S129 phosphorylation enhanced αSYN fibrillization, the accumulation of hyperphosphorylated αSYN in OL cell bodies might be a first event during MSA pathology (Fujiwara et al., 2002). However, the transgenic αSYN did not give rise to argyrophilic inclusions for up to 18 mo, and no obvious changes were found in motor behavior of transgenic mice at the age of 10 mo (rotarod performance, spontaneous locomotor activity; data not shown). Thus, there may be factors that exacerbate α-synucleinopathy in OLs. It is likely that these putative risk factors enhance oxidative stress, because oxidative alterations of αSYN have been detected in MSA brains (Giasson et al., 2000). Aging may play an important role in the pathogenesis of MSA, which has a relatively high incidence in the >65-years-old population (Trenkwalder et al., 1995). Environmental toxins have been implicated with MSA (Hanna et al., 1999). Genetic factors might be involved as well, although the lack of known MSA families indicates that MSA is unlikely to be inherited as a monogenic trait. Nevertheless, an influence of (multiple) risk genes with low penetrance cannot be ruled out. Genomic studies on MSA patients and the mouse model presented here will clarify this point.
Speculation
If MSA was caused by ectopic expression of αSYN in OLs, the earliest manifestations of disease may include somal accumulation of detergent-insoluble hyperphosphorylated αSYN in OLs. These features were reproduced in transgenic (PLP)-αSYN mice. It will be interesting to follow these mice into highest age to observe additional traits of human MSA, including demyelination, neurodegeneration and behavioral abnormalities. But already, at this stage, our mice may serve as a model to study the deposition and pathological phosphorylation of αSYN in GCI-like profiles.
METHODS
Generation and characterization of transgenic mice. The coding sequence of human wild-type αSYN was first subcloned into the XhoI site of a modified intermediate vector (Fuss et al., 2000) derived from pNEB193 (New England Biolabs, Beverly, MA). This construct was digested with AscI and PacI, and the insert was subcloned into a PLP cassette (Fuss et al., 2000) that was modified to contain a multiple cloning site after the 3′ boundary of intron 1 (Figure 1A). A linear ApaI–SacII fragment of 11 kb comprising the transgene without plasmid sequences was isolated and injected into C57Bl/6 × DBA/2 fertilized oocytes according to standard techniques (Hogan et al., 1995). Founder mice were identified by tail clip PCR using PLP and human αSYN specific primers, amplifying a 650 bp fragment.
Transgenic mice were backcrossed into the C57Bl/6 background. At each generation the mouse with the highest C57Bl/6 background content as determined by the genotyping of microsatellite markers (36 markers analyzed covering all the chromosomes but sex chromosomes) was used for further backcrossing. Markers were all of C57Bl/6 haplotype from N3 for lines 01 and 27 and from N4 for line 25. The integration site of the transgene in line 05 is most probably nearby D10Mit95, since this marker has not recombined after N7 backcrosses. The mice used in this analysis were from N1 to N5.
Whole-brain mRNA was extracted and quantitative northern blots were probed with human-specific αSYN probes and a β-actin probe as internal standard (Kahle et al., 2000). Cytosolic protein from whole mouse brain was analyzed by quantitative western blotting using the human-specific monoclonal anti-αSYN 15G7 (Kahle et al., 2000).
Histological and immunohistochemical analysis of brains from transgenic mice and MSA patients. Brains from transgenic mice and MSA patients were fixed in 4% formalin in phosphate-buffered saline and embedded in paraffin. Sections (4 mm) were stained with hematoxylin and eosin, Bielschowsky, Gallyas and LFB-PAS, or used for immunohistochemistry using human-specific rat monoclonal 15G7 anti-αSYN (Kahle et al., 2000), phospho-specific rabbit polyclonal anti-αSYN (Fujiwara et al., 2002) and mouse monoclonal SMI 91 anti-CNP (Sternberger Monoclonals, Lutherville, MD). To enhance immunoreactivity, sections were boiled in 0.01 M citrate buffer (pH 6.0) five times for 3 min. Antibody binding was detected using the alkaline phosphatase/anti-alkaline phosphatase system (Dako, Glostrup, Denmark). Double-immunolabeling of anti-αSYN and anti-CNP was performed with fluorescein-conjugated goat anti-rat IgG and tetramethyl rhodamine isothiocyanate-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR), respectively. Confocal analysis was performed with the Leica TCS-NT confocal laser scanning microscope. (PLP)-EGFP transgenic mice (Mallon et al., 2002) were analyzed following perfusion with 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde overnight, and vibratome sections (30 mm) were imaged by confocal microscopy for EGFP epifluorescence.
Detergent extraction of transgenic mouse and MSA brain. Whole brains (∼0.4 g wet weight) from (PLP)-αSYN (the present study) or (PLP)-GFP (Fuss et al., 2000) transgenic mice or ∼0.5g cerebellar cortex from controls and MSA patients were homogenized and extracted with buffer 5% SDS and 8 M urea/ 5% SDS. A detailed description of the method was published previously (Kahle et al., 2001). Western blots were probed with anti-αSYN monoclonals 15G7 and MC42 (Transduction Laboratories, Lexington, KY), anti-βSYN antiserum 6485 (Kahle et al., 2000), a polyclonal antiserum specifically recognizing phosphorylated αSYN (Fujiwara et al., 2002) and Living Colors A.v. peptide antibody against GFP (BD Clontech, Heidelberg, Germany). Mixtures of purified human recombinant αSYN and βSYN (Kahle et al., 2001) were used as standards.
Acknowledgments
ACKNOWLEDGEMENTS
We thank I. Pigur, A. Albientz, M. Hänggi, B. Morand and S. Odoy for technical assistance and H. Schubert and N. Stonka for animal care. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 596, project A1).
REFERENCES
- Arima K., Uéda, K., Sunohara, N., Arakawa, K., Hirai, S., Nakamura, M., Tonozuka-Uehara, H. and Kawai, M. (1998) NACP/α-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol. (Berl.), 96, 439–444. [DOI] [PubMed] [Google Scholar]
- Campbell B.C.V., McLean, C.A., Culvenor, J.G., Gai, W.P., Blumbergs, P.C., Jäkälä, P., Beyreuther, K., Masters, C.L. and Li, Q.-X. (2001) The solubility of α-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J. Neurochem., 76, 87–96. [DOI] [PubMed] [Google Scholar]
- Dickson D.W. et al. (1999) Widespread alterations of α-synuclein in multiple system atrophy. Am. J. Pathol., 155, 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda J.E. et al. (2000) Immunohistochemical and biochemical studies demonstrate a distinct profile of α-synuclein permutations in multiple system atrophy. J. Neuropathol. Exp. Neurol., 59, 830–841. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Hasegawa, M., Dohmae, N., Kawashima, A., Masliah, E., Goldberg, M.S., Shen, J., Takio, K. and Iwatsubo, T. (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biol., 4, 160–164. [DOI] [PubMed] [Google Scholar]
- Fuss B., Mallon, B., Phan, T., Ohlemeyer, C., Kirchhoff, F., Nishiyama, A. and Macklin, W.B. (2000) Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev. Biol., 218, 259–274. [DOI] [PubMed] [Google Scholar]
- Gai W.P., Power, J.H.T., Blumbergs, P.C., Culvenor, J.G. and Jensen, P.H. (1999) α-Synuclein immunoisolation of glial inclusions from multiple system atrophy brain tissue reveals multiprotein components. J. Neurochem., 73, 2093–2100. [PubMed] [Google Scholar]
- Giasson B.I., Duda, J.E., Murray, I.V.J., Chen, Q., Souza, J.M., Hurtig, H.I., Ischiropoulos, H., Trojanowski, J.Q. and Lee, V.M.-Y. (2000) Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science, 290, 985–989. [DOI] [PubMed] [Google Scholar]
- Gilman S. et al. (1999) Consensus statement on the diagnosis of multiple system atrophy. J. Neurol. Sci., 163, 94–98. [DOI] [PubMed] [Google Scholar]
- Hanna P.A., Jankovic, J. and Kirkpatrick, J.B. (1999) Multiple system atrophy: the putative causative role of environmental toxins. Arch. Neurol., 56, 90–94. [DOI] [PubMed] [Google Scholar]
- Hogan B., Constantini, F. and Lacy, E. (1995) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY, pp. 89–204.
- Iwai A., Masliah, E., Yoshimoto, M., Ge, N., Flanagan, L., Rohan de Silva, H.A., Kittel, A. and Saitoh, T. (1995) The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron, 14, 467–475. [DOI] [PubMed] [Google Scholar]
- Kahle P.J. et al. (2000) Subcellular localization of wild-type and Parkinson’s disease-associated mutant α-synuclein in human and transgenic mouse brain. J. Neurosci., 20, 6365–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle P.J. et al. (2001) Selective insolubility of α-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am. J. Pathol., 159, 2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos P.L. (1998) The definition of multiple system atrophy: a review of recent developments. J. Neuropathol. Exp. Neurol., 57, 1099–1111. [DOI] [PubMed] [Google Scholar]
- Mallon B.S., Shick, H.E., Kidd, G.J. and Macklin, W.B. (2002) Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci., 22, 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M., Walter, J., Koyama, A., Nakajo, S., Baba, M., Iwatsubo, T., Meijer, L., Kahle, P.J. and Haass, C. (2000) Constitutive phosphorylation of the Parkinson’s disease associated α-synuclein. J. Biol. Chem., 275, 390–397. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Okuizumi, K., Ikeuchi, T., Wakabayashi, K., Takahashi, H. and Tsuji, S. (2001) Analysis of the expression level of α-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol. (Berl.), 102, 188–190. [DOI] [PubMed] [Google Scholar]
- Pronin A.N., Morris, A.J., Surguchov, A. and Benovic, J.L. (2000) Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J. Biol. Chem., 275, 26515–26522. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C., Gorath, M., Trojanowski, J.Q. and Lee, V.M.-Y. (2000) α-Synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J. Neurosci. Res., 62, 9–14. [DOI] [PubMed] [Google Scholar]
- Solano S.M., Miller, D.W., Augood, S.J., Young, A.B. and Penney, J.B., Jr (2000) Expression of α-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: genes associated with familial Parkinson’s disease. Ann. Neurol., 47, 201–210. [PubMed] [Google Scholar]
- Spillantini M.G., Crowther, R.A., Jakes, R., Cairns, N.J., Lantos, P.L. and Goedert, M. (1998) Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett., 251, 205–208. [DOI] [PubMed] [Google Scholar]
- Tanji K., Imaizumi, T., Yoshida, H., Mori, F., Yoshimoto, M., Satoh, K. and Wakabayashi, K. (2001) Expression of α-synuclein in a human glioma cell line and its up-regulation by interleukin-1β. Neuroreport, 12, 1909–1912. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C., Schwarz, J., Gebhard, J., Ruland, D., Trenkwalder, P., Hense, H.-W. and Oertel, W.H. (1995) Starnberg trial on epidemiology of Parkinsonism and hypertension in the elderly. Prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch. Neurol., 52, 1017–1022. [DOI] [PubMed] [Google Scholar]
- Tu P. et al. (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann. Neurol., 44, 415–422. [DOI] [PubMed] [Google Scholar]