Abstract
This work reviews the growing body of interdisciplinary research on music cognition, using biomechanical, kinesiological, clinical, psychosocial, and sociological methods. The review primarily examines the relationship between temporal elements in music and motor responses under varying contexts, with considerable relevance for clinical rehabilitation. After providing an overview of the terminology and approaches pertinent to theories of rhythm and meter from the musical-theoretical and cognitive fields, this review focuses on studies on the effects of rhythmic sensory stimulation on gait, rhythmic cues’ effect on the motor system, reactions to rhythmic stimuli attempting to synchronize mobility (i.e., musical embodiment), and the application of rhythm for motor rehabilitation for individuals with Parkinson’s disease, stroke, mild cognitive impairment, Alzheimer’s disease, and other neurodegenerative or neurotraumatic diseases. This work ultimately bridges the gap between the musical-theoretical and cognitive science fields to facilitate innovative research in which each discipline informs the other.
Keywords: music cognition, music embodiment, psychology of music, rhythm and meter, rhythmic entrainment, rhythmic stimuli and gait, sensorimotor neurocognitive studies
Graphical Abstract
This review of recent research on the growing interdisciplinary area of music cognition examines the relationship between the temporal element in music and the way it affects motor response under varying contexts, ultimately providing relevance for clinical rehabilitation perspectives. One such study illustrates innovative rhythmic movement sequences for the gait modifications that are spatial biomechanical targets for evaluating and treating deficits in cognitive-motor integration.
INTRODUCTION
Music cognition, an interdisciplinary growing and dynamic field, explains how humans experience, understand, embody, and perceive music in our lives. In a harmonious blend of music theory and cognitive science. music cognition merges behavioral, neurophysiological, and computational perspectives.1 The study of music cognition integrates ideas from cognitive and experimental psychology, neuroscience, neuropsychology, philosophy, anthropology, linguistics, and computational modeling.2 The field of music theory describes different structures and organizational levels in the musical stimulus. Cognitive science in relation to music enables us to determine which aspects of musical structure (e.g., rhythm, harmony, melody, texture, and timbre, among others) are relevant for cognition. Music cognition identifies what knowledge is acquired by listeners and how detailed the structural discrimination can be in the perception of complex, nonverbal, musical stimuli.1
One subfield within music cognition, specifically the temporal organization of sound patterns, (e.g., rhythm, meter, and pulse), is steadily being used in clinical research to capitalize on the impact of auditory cues on the human motor and cognitive systems. The application of the temporal element in the form of musical cues, such as rhythm, also informs approaches on how clinicians, healthcare providers, and therapists can enhance and restore mobility and cognitive function through sensorimotor neurocognitive rehabilitation.
The objective of this review is to survey recent and not so recent studies from the growing interdisciplinary area of music cognition, the psychology of rhythm, and clinical neurorehabilitation to examine the relationship between the temporal element of music and the way it affects motor response under varying contexts. This review focuses on studies pertaining to rhythmic sensory stimulation on gait, effects of rhythmic cues on the motor system, reactions to rhythmic stimuli in attempts to synchronize mobility (i.e., musical embodiment), and the application of rhythm to enhance health. It situates the work within theories of music cognition, human auditory processing, and rhythm as a potent neural stimulus. Importantly the goal of this work is to bridge the gap between music cognition and clinical neurorehabilitation to facilitate innovative research where each discipline informs the other.
We begin by enumerating the definitions of fundamental concepts that define the theories of rhythm and meter (beat, grouping, meter, rhythm, resonance, and entrainment, among others). We then present hypotheses of well-formedness, precise timing, active sensing, and dynamic attending in the first section (Psychology of Music). In the second section (Rhythm, Meter, Embodiment, and Synchronization in the Human Brain), we focus on literature that examines brain functioning related to rhythm, meter, and synchronization through musical embodiment. In the third part of this review (Rhythmic Auditory Cueing and Music-Supported Therapy for Motor Rehabilitation in Diverse Neurological Populations), we present studies that link meter and rhythm to pathology and impairment, as well as explore motor rehabilitation through the application of musical rhythm.
THE PSYCHOLOGY OF MUSIC
Theories of rhythm and meter
By drawing on techniques of behavioral research and cognitive science, music psychology allows us to interpret and understand musical sounds, musical behaviors, and effects. As Elizabeth Hellmuth Margulis notes, music psychology “views music as a product of the human mind.”2 In the study led by Dr. Margulis, the authors focus on cognitive explanations of the temporal sphere in music, namely its principal elements of rhythm and meter.3, 4, 5 In kind, we will explain the theories of rhythm and meter from a music theory approach, followed by a review of theoretical propositions to further understand temporal processing, auditory–motor coupling, and its relevance for clinical rehabilitation perspectives.
To start, it is important to keenly define rhythm as we conceive it for the purpose of this review. Rhythm involves “patterns of duration,” referred to as “rhythmic groups” that are phenomenally present in the music.6 Rhythm in music typically refers to “the sound pattern” or to the “perception of the sound pattern.”7 As a sound pattern, these durations are marked by a series of musical events, called sounds and silences (Figure 1; See Supporting Information for definitions of musical terminology. Regarding “perception,” rhythm is the perceived temporal organization of the physical sound pattern—in essence, a series of notes and rests.7 This series of notes and rests gives a sense of movement in time.7, 8, 9
FIGURE 1.
An example of durational levels of notes (sounds) and their rhythmic grouping.
This perceived temporal organization of music is characterized by three fundamental concepts: beat, meter, and grouping. Grouping refers to the way in which a series of notes are perceived to be grouped together.7 Beats (or pulses), and more specifically perceived beats, are a series of periodic and accented time points in music.7 We clap to music in a periodic manner by recognizing the perceived beat. The meter is the temporal organization of beats on multiple time scales (i.e., levels of temporal organization or the hypermeter). Thus, the meter involves our initial perception as the subsequent anticipation of a series of beats that we abstract from the rhythmic surface of the music as it unfolds in time.
The music scholar, William Benjamin, sets up a conceptual framework in which meter, group structure, and accent (timepoints of marked change in any of the perceived properties of sound)9 are related to one another. Benjamin explores the practical and aesthetic functions of metric perception, an essential framework for metric perception. Benjamin describes accents and groupings as “the basic […] modes of partitioning musical time.”10 Importantly, the placement and duration of rhythmic patterns determine the level of metric complexity. Further, Benjamin notes that meter is a “secondary construct,” our perceptional response to aesthetic needs (i.e., our grouping and metric preferences). Meter is an attentional behavior, i.e., “a particular form of entrainment or attunement,” which is, “a synchronization of some aspect of our biological activity with regularly recurring events in the environment.”6 As the scholar of music theory and cognitive sciences, Justin London, summarizes succinctly in psychological terms: rhythm involves the structure of the temporal stimulus, while meter involves our perception and cognition of such stimuli.
The interdisciplinary approach to examining musical rhythm and meter from the perspective of psychology started with Fred Lerdahl and Ray Jackendoff’s landmark book, Generative Theory of Tonal Music (1983).9 Lerdahl, a composer and music theorist, and Jackendoff, a linguist, examined meter through a rule-based approach—perceptual and cognitive constraints that are governed by well-formedness rules, Metrical Well-Formedness Rules (MWFR) and Grouping Well-Formedness Rules (GWFR)11 These rules inform how we hear the well-formedness patterns using preference rules—Metrical Preference Rules (MPR) and Grouping Preference Rules (GPR).9, 11 Lerdahl and Jackendoff visually presented these rules via a metrical grid (as seen in Supporting Information) and defined the principles as follows:
Grouping Well-Formedness Rules: Establish the formal structure of grouping sequences that can constitute a piece; a group may contain smaller groups.12
Metrical Well-Formedness Rules: The placement and prominence of strong beats within each metrical layer.12
Grouping Preference Rules: Establish the possible formal structures that can be assigned to a musical piece corresponding to the listener’s actual intuitions (e.g., proximity, symmetry, parallelism). These rules explain many listeners’ preference for symmetry in music (i.e., division of units into equal parts, or more technically a preference for grouping that most closely approaches the subdivision of groups into two parts of equal length) and parallelism (preference for musical units that are the same as or similar to previously heard units).12
Metrical Preference Rules: Preference rules for a metrical structure that model the criteria by which the listener chooses the most stable metrical structure for a given musical structure.12
Stability within meter and rhythm refers to a clearly defined/delineated timing without changes or disruptions to how the listener understands the grouping to work. Adopting a viewpoint from linguistics, namely, the school of generative-transformational grammar,13, 14, 15 Lerdahl and Jackendoff extend the theory of generative linguistics—the modeling of unconscious knowledge of a formal system of principles of rules, or grammar, which generates the possible sentences of the language—to the principles of musical grammar. Thus, our reaction to musical events is based on our understanding of musical grammar, akin to how we understand grammar in language. Well-Formedness Rules define the metrical structure, whereas Metrical Preference Rules model the criteria by which the listener chooses the most stable metrical structure (i.e., the most clearly perceived grouping) for a given musical structure. These preference rules reveal partiality in the learned inclination towards isochronous meters—i.e., “temporally equidistant beats and/or beat divisions”16 in Western music, which is manifested as a preference for simple and compound meters, with a stronger affinity for duple meter, as the listener’s musical knowledge is acculturated, while non-isochronous meters—”unequal patterns of time”16 (e.g., complex, asymmetrical, or additive) are evident in many non-Western music styles. This principle is demonstrated by Polak et al.’s study on the non-isochronous meter in Malian djembe percussion music, which concludes that entrainment and precision of rhythm do not depend on isochrony (i.e., rhythmic and metric regularity) but rather on culturally learned behavior.
The scholar, Diana Deutsch, notes that when presented with a complex pattern, “the auditory system groups elements according to some rule based on frequency, amplitude, timing, spatial location, or some multidimensional attribute such as timbre”—attributes that can be used as the basis for grouping according to various rules.17, 18 Deutsch further notes that grouping according to these various, simple principles enables us to interpret our environment most effectively and to understand how patterns/grouping work for anything we perceive with our auditory system.17 As such, grouping is the most basic component of musical rhythmic understanding, and its most fundamental characteristic is that it is heard in a hierarchical fashion.
To elaborate, Deutsch explains that the guiding principles of musical grouping are: (1) proximity—where closer elements are grouped together in preference to those that are further apart; (2) similarity—elements are grouped by how similar they are; (3) continuation—elements that follow each other in a given direction are perceptually linked together; (4) common fate—elements that change in the same way they are perceptually linked together; and (5) we tend to form groupings to perceive configurations that are familiar to us.17 Thus, as Margulis posits, we not only recognize patterns, which are necessary for comprehending music, but prefer hearing their repetition in music.19, 20, 21
The repetition of music and the recognition of patterns leads the listener to project future repetitions of such patterns. Christopher Hasty defines projection as a process in which a mensurally determinate duration (i.e., rhythms that can be grouped into musical meter/measures) provides a definite durational potential for the beginning of an immediately successive event. Thus, the projective and the projected are aspects of a single process: a projection.22
In music theory studies, scholars have addressed how metrical dissonance,23 the juxtaposition or superimposition of noncoincident pulses and rhythmic patterns, the misalignment of heard metrical layers—and a deviation from projected expectations affects our comprehensibility of music (i.e., our perceived grouping). Pieter van den Toorn closely scrutinizes metrical displacements—ways in which repeated themes and its fragments, motives, or chords are repositioned relative to a steady metrical framework—in the music of Igor Stravinsky.24, 25, 26, 27, 28, 29, 30, 31, 32, 33 This kind of displaced repetition associated with Stravinsky’s music upsets the listener’s expectations of metrical parallelism, namely, the expectations that a theme, motive, or chord will be repeated at a metrically parallel location.31 Borrowing the terminology of “metrical parallelism” from Lerdahl and Jackendoff and David Temperley and Christopher Bartlette,34 and the notion of metric readjustment in listeners,35 parallelism plays a role in the establishment of a meter in the listener’s mind because of the listener’s strong sense of expectation of where the repetition should occur. Our strong sense of using parallelism to establish a meter is evident in popular Western music, like those used in “dance” music, where the rhythmic structure within phrases and verses tends to remain the same throughout.
Theories on temporal processing and auditory–motor coupling
In addition to the music theory regarding interpreting and understanding musical rhythm and meter, other theories enable us to further understand temporal processing and its relevance for clinical rehabilitation practice. These theories include the Dynamic Attending Theory, Precise Auditory Timing Hypothesis (PATH), Active Sensing, and Action Simulation for Auditory Prediction (ASAP) theories, and they bridge the disciplines of music and health, music cognition (and temporal processing), and its application to clinical therapy.
The foundation of the Dynamic Attending Theory is the relationship between time and synchrony (e.g., how the rhythms we experience interact with our surroundings.)11 Attending is defined as a dynamic activity that involves "the interaction between an attender’s internal cortical rhythms with the external rhythms that make up an event in the world around us.”11 An “event” refers to things in our environment that unfold over time, such as a song that we hear and attend to. The Dynamic Attending Theory focuses on the “time structure of biological rhythms inherent to an individual attender and on the rhythmic patterning of an external (to-be-attended) event” that we perceive.11 Synchrony between an individual’s internal and external rhythms of an event is the basic and crucial interaction that defines attending, enabled through the principles of entrainment and resonance.11 Mari Riess Jones defines resonance as the amplitude heightening of an attender’s neural oscillation by frequency matching with the frequency of its driving rhythm, as well as entrainment as phase and frequency adjustments of a driven oscillation in response to properties of a driving rhythm.11, 36 These theories can explain how listeners respond to systematic changes in everyday events while retaining a general sense of their rhythmic structure. This approach may illustrate applications of the dynamic attending concepts to musical and speech events, such as understanding musical rhythm and meter or the precepts of phonemes and words in speech learning.
Illustrating how sensory processing is a dynamic process is the theory of Active Sensing. This theory suggests that (1) most sensory processing is active and largely determined by motor and attentional sampling routines of the central nervous system, (2) sensory flow tends to be rhythmic due to the rhythmicity in the motor routine and its entrainment of ambient rhythms in sensory regions, and (3) attentional manipulation of rhythms in sensory pathways is instrumental to perceptual selection.37 A practical application of Active Sensing can be used for understanding how hierarchically organized neuronal oscillations, which present potential physiological substrates for brain operations that require temporal prediction, can be used to parse and encode complex input streams in speech. Thus, the motor system is considered a major source of rhythms in auditory processing.38
Further studies show that dorsal auditory pathway connections between auditory regions and motor planning regions via the parietal cortex may be stronger in humans than in non-human primates due to the evolution of vocal learning.39 In the ASAP theory, the authors treat beat perception as a complex brain function involving temporally-precise communication between auditory regions and motor planning regions of the cortex. Moreover, this approach allows us to understand the simulation of periodic movement in motor-planning regions that provide a neural signal that helps the auditory system predict the timing of upcoming beats.a
PATH is another approach that aims to explain the transfer between phonological skills and musical training. PATH proposes that entrainment practice is the core mechanism underlying enhanced phonological abilities in musicians.40 Rhythmic synchronization and language skills (such as consonant discrimination, detection of word and phrase boundaries, and conversational turn-taking) rely on the perception of extremely fine-grained timing details in sound. Auditory–motor timing is an acoustic feature that meets all five of the pre-conditions necessary for cross-domain enhancement to occur.40, 41, 42, 43 While neural networks that process timing in the context of both music and language overlap, musical entrainment demands more precise timing sensitivity than language processing.40
RHYTHM, METER, EMBODIMENT, AND SYNCHRONIZATION IN THE HUMAN BRAIN
In studies of motions in music, Robert Gjerdingen observed that meter in music is a mode of attending while we attend to rhythm.44 He remarked that while rhythm signifies movement, musical tones themselves do not move. Instead, we hear a virtual motion in a virtual, acousmatic space.b This perceived movement of rhythm is what led Levitin et al. to remark that “the urge to move to music is universal among humans.”45 The authors further elaborate that the temporal qualities of music—its pulse, tempo, and rhythmic patterns—evoke this impulse for the listener to move to music. Thus, patterned sequences in music (both in sound and rhythm/movement) induce a tendency for us to move in response, due to the coupling between perception and action. This phenomenon is illustrated in behavioral and neuroimaging studies that pertain to neural mechanisms that underlie musical experience.46
Studies have confirmed that musical groove, “the pleasurable urge to move to a rhythm”, relies on the interplay between predictability (formed by repetitive rhythmic patterns) and surprise arising from rhythmic deviations.47, 48, 49, 50, 51 Groove is found in rhythmic patterns with a moderate level of rhythmic complexity, whereas rhythms with low or high complexity are associated with a weaker experience of groove.52, 53, 54 When a listener switches from hearing simple to more complex meter, i.e., when transitioning between duple meter (as in marches) to triple (as in waltzes), there is modulation in the auditory evoked responses in the temporal lobe and motor cortex, basal ganglia, and cerebellum. Thus, evidence shows neural activity changes distinctly between different meters.55
Musical embodiment, choreography, and socially moving to music
The studies of musical embodiment address how we respond to and move to metrical irregularities and complexities and shape our interpretation of musical texts, and our engagement with music practices including composing, improvising, listening, and performing. These studies consider “how theory and analysis address music’s literal and figurative bodies.”56 Some scholars have focused on the embodiment of metrical dissonance in the ballet music of Pyotr Tchaikovsky. Researchers posit that the choreographers who set dances to Tchaikovsky’s music responded to and participated in these metrical dissonances by analyzing the music and choosing movements that matched the dissonance.57 Similarly, Kara Yoo Leaman examines the relationship between musical events and dance movements for metrical alignment or dissonance.58, 59 She raises the example of George Balanchine, a choreographer and a musician, known for choreographing dances to follow musical patterns closely, a prime example of musical embodiment. Balanchine created the neoclassical style and has inspired multiple choreographers to follow his music-guided approach to choreography. Considering social, and non-choreographed dance, Burger and Toiviainen studied relationships between metrical structures and electronic dance music (EDM)—a genre produced with the primary aim of making people move.60 They found that participants moved with significantly higher acceleration of torso, head, hands, and feet and more overall movement to the EDM stimuli than to other genres (e.g., Latin music, funk, and jazz). Further, with the increase in clarity and percussiveness of rhythm, there was an increased acceleration of body parts moving to the beat. The authors postulate this finding was due to increased predictability of the rhythm. This observation is consistent with studies of human motor control and neuromechanics. Humans are thought to use internal models—models unifying the human nervous system and environment, possibly originating from the cerebellum61—to predict the consequences of motor actions. In the presence of uncertainty, such as a destabilizing environment with ambiguous musical tempo, humans may rely less on these predictive internal models and more on error-based feedback relative to their movement goals.62 Motor commands must be generated to move synchronously with the music’s rhythm, which requires accurately perceiving the music’s temporal characteristics. Relying on feedback can reduce movement velocity. Easy-to-identify rhythms, like in EDM, may enable individuals to rely less on feedback and more on their predictive internal model of the music, potentially resulting in faster movements.62, 63
However, in more rhythmically complex passages of music, such as music that features syncopations and metrical displacements or music with non-isochronous meters (such as tango, salsa, and music within the region of Southeast Europe, South and Southeast Asia, Africa, and the African diaspora, among other non-Western musics), the choreographic accent pattern seems to derive from a metrically dissonant rhythmic motive in the musical score. Rebecca Simpson-Litke draws similar conclusions about the complex interactions between salsa music and dance by focusing on physical interpretations of specific types of metric ambiguities and disruptions.64 By choosing a genre of music (e.g., salsa music) that features frequent displacement dissonances that arise when the established clave pattern is flipped, paused, or broken and whose grouping dissonances rarely occur, the author shows that dancers tend to respond to these metric disruptions. The Norwegian telespringar—a music and dance tradition that has developed in tandem over time and features a non-isochronous meter (i.e., a temporal organization where the durations between adjacent beats are unequal—may have derived from embodied sensations related to the dance.65 Adjustment to complexities in the meter may be explained by a historical analysis of choreography in eighteenth-century dances. These dances promoted the development of hypermetrical hearing (i.e., a high-level rhythmic structure).66, 67 Dance movements accompanying and responding to music impacted the way people of this time heard and embodied music, which still continues today.
Central to the concept of music embodiment, inherent to the relationship between metrical structures and dance, is the notion that humans tend to synchronize movements to the beat (both in individual and joint response).68 Thus, consistent with the ASAP and other theories, motor areas may be involved in detecting or generating a beat.69, 70 Research on rhythmicity provides insights into the understanding of the temporal coding of music and temporal information processing in the human brain.71 Studying the neural dynamics of entrainment (i.e., synchronization) and brain imaging, Thaut identified regions including the primary sensorimotor and cingulate areas, bilateral opercular premotor areas, bilateral SII, ventral prefrontal cortex, as well as subcortical areas including the anterior insula, putamen, and thalamus.71 However, the brain’s activity in response to rhythmic stimuli is directly linked to the complexity of rhythm sequences. For instance, the use of functional magnetic resonance imaging (fMRI) to identify brain areas involved in auditory rhythm perception with rhythmic sequences that varied in temporal predictability confirms that the most predictable sequence is an isochronous rhythm sequence of a single interval. Further, it shows that the complexity of rhythm sequences is an important factor in modulating activity in the prefrontal cortex, given that it has been demonstrated that the superior prefrontal cortex is more active when listening to metric and non-metric rhythmic stimuli compared to being at rest.72
Auditory–motor coupling and sensorimotor synchronization
We embody rhythm (i.e., move to the beat or tap along) in response to the processing of the beat by motor areas.73 While studies have shown that several motor areas respond when attending to rhythms,72 Grahn and Brett investigated whether specific motor regions respond to the beat in rhythm, correctly predicting that the basal ganglia and supplementary motor area would respond in the presence of a regular beat and mediate beat perception. Chen et al.74 similarly found that the supplementary motor area, mid-premotor cortex, and cerebellum were observed to be activated in participants listening with anticipation of rhythm perception, suggesting an inherent link between auditory and motor systems in the context of rhythm.
The scholar, Pressing, makes an anthropological argument that because musical rhythm arises from the evolved cognitive capacity to predict and anticipate future events, rhythmic and metrical elements must be relevant for human action and predictions.75 Studies on the phenomenology of pulse and meter use music and rhythms to explore the theoretical predictions of neural resonance, the complexity of human musical rhythm, and its implications for the coordination of perception, attention, and behavior.76
When we listen to music, we often move spontaneously with the rhythm, synchronizing our body movements by tapping our feet, clapping our hands, or swaying to the beat. Although some individuals may not be able to synchronize their movements to the musical beat due to inaccurate motor synchronization to the beat or poor beat perception,77 tapping can be extremely precise as the earliest tapping studies illustrate humans tapping to a metronome can reproduce a temporal interval with high consistency.78
Sensorimotor synchronization (SMS), in which an action is temporally coordinated with a predictable external event,75 is the rhythmic coordination of perception and action.79, 80 SMS is a fundamental human skill that contributes to sensorymotor control in daily life. Research related to SMS has focused on adaptive error correction mechanisms that support the synchronization of periodic movements with events in regular pacing sequences. A thorough review of tapping literature81 highlights theories and empirical findings derived from laboratory experiments that sequence auditory stimuli consisting of variations of tones or clicks.79 For instance, after introducing unexpected changes to a rhythmic sequence, Thaut observed that synchronization deviates from the stimulus onset as people tend to tap 20–60 ms before the metronome click.78, 80 Timing deviations in individual synchronization response, among both musicians and non-specialists showed that the error margin for accurately tapping along with the beat was smaller among musicians, and both groups showed a fast phase correction following a tempo change in the pacing sequence.80
Synchronization tapping studies date back to the late nineteenth and early twentieth century,78, 82, 83, 84 and were advanced by notable pioneers Paul Fraisse,8, 85, 86 John Michon,87 and Thaut in the 21st century.79 New studies on rhythmic synchronization examine how more complex rhythmic structures affect our motor response. Paul Fraisse has examined the synchronization accuracy of musical syncopation—a rhythmical structure where a weak-beat note is played as the strongest note.88 Their experiment confirms that syncopation becomes easier to play when the tempo is slower and that a successful execution is possible when the succession of sounds reaches a rate of about one per second. More recent research, such as “ADAM” (an ADaptation and Anticipation Model), investigates the role of adaptation and anticipatory mechanisms in SMS.89 ADAM combines reactive error correction processes (adaptation) with predictive temporal extrapolation processes (anticipation) inspired by the computational neuroscience concept of internal models.
Physiological research has shown that auditory rhythm profoundly affects the motor system.90 Chen et al.91 demonstrated that rhythm’s metric structure can facilitate motor responses using fMRI. In their experiment, the participant tapped in synchrony to five variants of isochronous rhythm while employing intensity accentuation (i.e., increasing the contrast in sound amplitude between accented and unaccented tones) progressively highlighting the rhythm's metric structure. As such, metric organization, as manipulated via intensity accentuation, modulates motor behavior and neural responses in the auditory and dorsal premotor cortex. Further, auditory–motor interactions may occur at these regions with the dorsal premotor cortex interfacing sensory cues with temporally organized movement. Another study that synchronizes movements with auditory beats compared to visual effects shows divergent activation in timing-related brain areas as well as more stable tapping synchronization.92 These differences in timing-related brain activation could reflect differences in tapping synchronization stability, rather than differences between modality (that is, audio-motor vs. visuo-motor integration), as illustrated through fMRI imaging. Participants in that study were asked to synchronize their finger taps with four types of visual and auditory pacing sequences—flashes, a moving bar, beeps, and a frequency-modulated siren. Visuo-motor synchronization improved with moving targets whereas audio-motor synchronization degraded with frequency-modulated sirens. Imaging showed that activation in the putamen, a key timing area of the subcortical region of the brain, paralleled the behavioral results such that putamen activation was highest for beeps, intermediate for the siren and moving bar, and lowest for the visually produced flashes.
In summary, studies on music cognition grounded in theories of rhythm and meter support the notion of a strong tendency to embody musical beats by moving to them. Musical embodiment research examines the ways we respond to rhythm, which is informed by the studies of grouping and metrical preferences and well-formedness. This tendency to move to music arises from the interplay between predictability (i.e., our projections that we form based on the established grouping patterns) and surprise (a deviation from the established patterns/our projections). The greater the imbalance between the two elements (predictability and surprise), the greater the metrical irregularities and complexities. Studies on neural dynamics of entrainment show how the brain responds to rhythmic stimuli and which motor processes are modulating the responses. Although most of these neural studies use the metronome as the basis of their rhythmic stimuli, more recent research is incorporating more complex musical rhythmic stimuli to show the positive effect of using musical rhythm for improving movement and gait, as discussed below, in individuals with motor and cognitive impairment.
RHYTHMIC AUDITORY CUEING AND MUSIC-SUPPORTED THERAPY FOR MOTOR REHABILITATION IN DIVERSE NEUROLOGICAL POPULATIONS
The recent rich, diverse, and creative clinical research on the effectiveness of music, particularly rhythmic auditory stimulation, and motor therapy for stroke, Parkinson’s Disease (PD), Alzheimer’s disease (AD), and individuals with mild cognitive impairment (MCI) have shown promising results in rehabilitation. The topics range greatly and have included the development of robotic technology in partner dance for use in rehabilitation scenarios, the scientific investigations of whole-body motor coordination to improve gait in people with PD and in older adults,93, 94 rhythmic auditory cueing using a metronome,95 commercial music to improve gait,96 and even rhythm used in speech and language rehabilitation.97 A synthesized analysis of rhythmic auditory cueing shows improved rehabilitative practice and clinical effectiveness for individuals with cognitive impairment.98 Other analyses exist showing the benefits of music-informed and dance-based therapies for populations coping with neurodegenerative conditions. Schiavio and Altenmüller99 proposed that by framing music-supported therapy (MST) within a paradigm that “music cognition depends on the kinds of experiences that come from having a body with...perceptual and motor capacities that are inseparably linked and...form the matrix within which memory, emotion, language, and all other aspects of life are meshed.”100 Their work supports implementing insights from embodied cognitive science in research on the brain’s anatomical adaptation and for music-based motor rehabilitation. Further research on the connection between rhythmicity and brain function,101 and tracking how temporal modulations additionally activate predominantly right prefrontal, anterior cingulate, intraparietal regions, and posterior cerebellar hemispheres71 show strong evidence for the substantial benefits of rhythmic stimuli in rehabilitation training for motor disorders. Based on these neuropsychological connections the auditory system is clearly a fast and precise processor of temporal information and projects into motor structures in the brain, creating entrainment between the rhythmic signal and the motor response. Clinical studies over the past two decades90 have demonstrated the effectiveness of rhythm and music in facilitating motor responses and improving sequential movement in older individuals with neurodegenerative diseases, including stroke, PD, AD, and MCI. Much research has been conducted on PD, so this review places emphasis on this neurodegenerative disease affecting mostly older adults. This novel work in PD should be considered a model for music mechanism research in other neurodegenerative and neuroinsult conditions.
In a healthy population, Lorenzoni et al.102 studied the effect of (a)synchronous music on runners’ ground reaction force exerted on the foot when it impacts the ground. Increased impact was observed for running sessions with music compared to running in silence. Music-induced gait changes may be retained following training. For example, Brake et al.103 found that music-induced increases in running cadence were retained following an intervention. These findings are consistent with prior studies of metronome-based retraining of running gait.104, 105, 106 In the context of gait rehabilitation in individuals with motor or cognitive deficits, gait mechanics could be desirably altered (e.g., increasing paretic-limb loading in stroke survivors107) by precisely entraining cadence to music. Optimizing music to elicit desirable gait adaptations is a relatively new area of research but has potential for systematically and positively altering gait with music.108
In an early study, rhythmic auditory stimulation (RAS) was used as a pacemaker in a gait-training program for individuals with PD. Those who used rhythmic cues significantly improved their gait velocity by 25%, stride length by 12%, and step cadence by 10% over participants who did not use RAS (whose velocity decreased by 7%) and participants who did not train (whose velocity decreased by 7%).109 Enzensberger et al.95 confirmed that metronome simulation significantly reduced the time and number of steps needed to complete the test course and diminished the number of freezing episodes in patients with PD. The results of the McIntosh et al. study110 are consistent with prior studies showing that the use of rhythmic auditory facilitation in gait rehabilitation for patients with mild to moderate impairment resulting from PD (including those participating in an at-home program111) can be effective for gait rehabilitation in PD. Another study showed that 14 patients could walk faster and exhibit greater stride length in non-cued gait after four weeks of RAS. The authors postulated that sensorimotor timing skills underpinning the synchronization of steps to an auditory cue may predict the success of RAS in PD.112
Coupling gait to rhythmic auditory cues in people with PD relies on a neuronal network engaged in both perceptual and motor timing. This strategy can be used successfully in the rehabilitation of motor function in patients with motor disorders. If synchronization of movement to an auditory cue relies on a “supramodal timing system” involved in perceptual, motor, and sensorimotor integration, then auditory (musical) cueing can affect both motor and perceptual timing. One study tested the hypothesis by assessing perceptual and motor timing in 15 PD patients before and after a four-week music training program with rhythmic auditory cueing. Training improved patients’ performance in tasks requiring synchronization with isochronous sequences and enhanced their ability to adapt to durational changes in a sequence of hand-tapping tasks.113
Auditory cueing leads to benefits beyond gait. Coupling steps to external rhythmic cues (the beat of music or the sounds of a metronome) leads to long-term motor improvements, such as increased walking speed and greater stride length in people with PD. Because these effects may be due to compensatory brain mechanisms involving cerebellar–thalamocortical networks, areas that are also involved in perceptual and motor timing, parallel improvement in timing tasks is expected in PD beyond purely motor benefits.
As Ebersbach et al.114 point out, individuals in even early stages of PD tend to walk slower with smaller steps, resembling the gait of normal older adults. Similarly, Blin et al.115 found that the variability of stride length was more marked in individuals with parkinsonism. Hallett116 showed that freezing of gait in PD appears to result from impaired automaticity and internal generation of movement. As such, many of the rehabilitation studies focus particularly on the effects of rhythmic sensory stimulation on the kinematic parameters of gait (i.e., cadence, step amplitude, velocity, coefficient of variation of stride time, and the coefficient of variation of the step amplitude) in individuals with PD.117 Using auditory stimulation at a frequency matching preferred walking cadence decreased stride time and increased step amplitude in patients with more advanced PD.118
Del Olmo, et al.119 studied spatiotemporal gait parameters and temporal gait variability in patients with PD and control subjects under different walking conditions to investigate whether rhythmic auditory cues could reduce temporal variability. The study yielded consistent results that rhythmic auditory facilitation in PD rehabilitation studies is a valuable method of improving gait timing in these patients. Similarly, using the auditory stimulation of a metronome to examine gait performance in persons with PD confirmed prior findings that auditory stimulation by a metronome can benefit gait of persons with PD.120 Further investigation shows that patients with early-stage PD can modulate cadence and thus gait velocity when adjusting the rate of auditory cues.121
Musical cues may work better for those with PD and other conditions than a simple metronome. Bella et al.122 reported behavioral data showing beneficial effects of musically-cued gait training on gait performance (i.e., increased stride length and speed), perceptual timing (e.g., discriminating stimulus durations), and sensorimotor timing abilities (i.e., in paced tapping tasks) in PD patients.
The use of rhythm-based auditory cueing is not without challenges. Previous research has shown that PD patients are impaired in the timing of isochronous intervals.123, 124, 125 Testing PD patients and controls on a rhythm discrimination task to determine if basal ganglia dysfunction results in an impairment of processing rhythms that have a beat showed (as predicted) significantly impaired discrimination of beat-based rhythms in PD patients compared to controls, whereas discrimination of non-beat-based rhythms did not differ significantly.123 In conclusion, the authors suggested that the basal ganglia is part of a system involved in detecting or generating an internal beat and that this system is compromised in individuals with PD, who, as a result of their neurodegeneration, have often lost automaticity, or the ability to perform movements and tasks smoothly with little cognitive engagement.
The observed neural/cognitive benefits of music in PD are not always perceived by the patients. This presents a motivational challenge for music-based therapies. One study126 was more skeptical about the effects of using non-drug therapies for treatments of PD patients. Referring to the benefits of using music and rhythm stimulation on the movement of PD patients as “anecdotal,” those findings were based not on a clinical study but rather on a written music questionnaire that asked the participants how commonly they perceived an improvement in their motor symptoms with music and rhythm. The results showed a discrepancy between the evolving literature on music, rhythm, and PD, and the frequency of spontaneously reported benefits of music from patients in the clinic.126 However, in a review of neuroimaging studies on the influence of rhythm on gait in several pathological conditions, such as PD, Huntington's disease, and stroke, the authors noted that these studies provide notable insights for developing potential movement therapies that capitalize on music and rhythm in a targeted fashion.127 Specifically, neuroimaging studies show that rhythm perception activates structures within key motor networks, such as premotor and supplementary motor areas, basal ganglia, and the cerebellum. Again, the automatic engagement of motor areas during rhythm perception may be the connecting link between music and motor improvements in PD.
Rochester et al. focused on the feasibility and effectiveness of auditory cues to improve gait in PD and MCI. A small study involving only nine patients used the metronome to cue the participants at a preferred stepping frequency while on medication during single and dual task gait. Their results found that the cue that focused on both temporal and spatial parameters of gait significantly improved single- and dual-task walking speed and stride amplitude, thus further affirming the evidence that these types of studies provide the potential of cueing to improve gait in PD and cognitively impaired individuals.128 Brown et al.129 also investigated the effect of concurrent music on Parkinsonian gait in single- and dual-task contexts, focusing on the outcome measures of mean gait velocity, stride length, and the percentage of the gait cycle spent in double-limb support. Their study also showed that gait among the PD patients was adversely affected by concurrent music and that the added requirement of a cognitive task differentially influenced gait performance in PD patients and control subjects, with PD patients displaying a further decrease in spatiotemporal parameters of gait and control subjects displaying a marginal improvement.
Rather than using metronome cueing, de Bruin et al.96 examined whether gait modulation can be achieved using commercially available music. They concluded that listening to music while walking was an enjoyable activity that influenced gait. Specifically, they noted that salient music selections increased measures of cadence, velocity, and stride length; in contrast, gait was unaltered by the presence of non-salient music. They also observed that music tempo did not differentially affect gait performance (gait velocity, stride length, cadence, stride time variability) in these participants.
Novel research using paradigms with more complex rhythmic structure
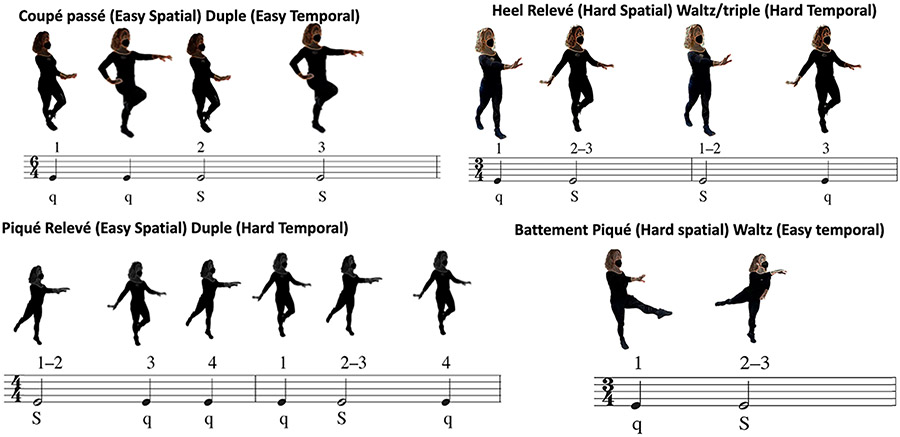
The aforementioned studies addressed very simple auditory cues in terms of rhythmic complexity. To further this field of music used in medicine, we propose that new research should integrate our understanding of music cognition and metric structure with our knowledge of neuropathology and impairment, as others have recommended. We posit that despite the advances in music neuroscience and the documented value and benefits of music-based neurological rehabilitation,106 quality research on this type of rehabilitation still remains low to moderate for most populations and outcomes. An opportunity lies with conditions like MCI, which afflicts one in ten Americans over the age of 65 years. In one of the most recent studies that innovatively applied music cognition, specifically theories of rhythm and meter, the authors devised musical arrangements according to the musical grouping and preference rules of music with more complex metrical and rhythmic structure, such as syncopations in tango and compound meter of waltzes, to develop dance-based therapy aiming at improving motor and cognitive function in older adults with MCI130 (see Figure 2). The authors evaluated the effects of age and MCI on the ability to accurately modulate spatial (i.e., joint kinematics), temporal (i.e., step timing), and spatiotemporal features of gait to achieve spatial and temporal targets during walking. Deficits associated with increasing age (healthy young adults versus healthy older adults) primarily reduced the ability to perform spatial modifications to gait, whereas MCI (healthy older adults versus those with MCI) primarily reduced the ability to perform temporal modifications in duple meter, particularly for longer step sequences. These findings suggest that age-related declines in strength and balance reduce the ability to accurately modulate spatial gait features, while declines in working memory in individuals with MCI may reduce the ability to perform longer temporal gait modification sequences. Differences in performance on rhythmic movement sequences highlight the impacts of motor and cognitive deficits on gait modulation capacity, which may assist when personalizing therapies based on these deficits (e.g., identifying optimally challenging rhythm–meter pairings for therapy96, 99, 131) and provide more sensitive indices to track intervention efficacy.130 Another recent study132 on people with chronic post-stroke hemiparesis leveraged auditory–motor entrainment principles to combine real-time gait sensing with adaptive music algorithms to fully automate gait training that can be personalized to the immediate and evolving needs of each patient. This training can be delivered autonomously without direct input from a clinician. Similar music-based gait retraining interventions can enhance cognitive and motor function, while also being feasible for use in community and home settings.133 Such research will lead the way to a more sophisticated understanding of music’s power and potential use in therapeutic applications across ages and conditions.
FIGURE 2.
Schematic showing innovative rhythmic movement sequences (RMS) developed and tested by Rosenberg et al.130
Although clinical studies are limited to date, existing experimental evidence demonstrates overlapping brain networks that are crucial in the design of rehabilitation approaches through the use of music and rhythm. While more recent studies on the effectiveness of auditory cueing in improving gait in individuals with MCI use musical rhythmic stimuli rather than just metronome cueing, the hybrid studies of music and health still are not fully integrated. To do so, it would be necessary to engage on a more significant level with the musical element. That is, understanding what constitutes a metrical complexity or irregularity from a music theory perspective (i.e., understanding music theory and music cognition) to how our body responds to different metrical structures (i.e., musical embodiment). Such understanding would facilitate more innovative, exciting, and effective music-based motor rehabilitation applications.
LIMITATIONS
This review does not discuss polyrhythmic music (a research topic with currently substantially less research) and the potential role it could play in health and well-being contexts. This review also restricts the discussion of clinical applications for aging-associated diseases and neurodegeneration. There are many other conditions (e.g., cancer, autism, and orthopedic conditions) that likely also could benefit from an application of musically-driven therapies.
CONCLUSIONS
Research on RAS and rehabilitative motor therapy for patients with cognitive impairment, stroke, PD, and other neurodegenerative diseases has been showing promising results in rehabilitation therapies. This article highlights how music cognition (i.e., music theory and psychology of music) together with theories of musical embodiment (i.e., entrainment and synchronization) contribute to our understanding of the ways we respond and move to rhythmic cueing and metrical irregularities and complexities. The considerations developed in this review should be considered when devising musically-driven motor and cognitive rehabilitation programs for therapeutic application.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this manuscript was supported by the National Institute of Child Health and Human Development and the National Institute on Aging of the National Institutes of Health under award numbers F32HD108927 and R01AG062691, respectively. This research was also supported by Emory University through a Goizueta Alzheimer’s Disease Research Center CEP Innovation Accelerator Seed Grant, an award from the Senior Vice President of Research at the Intersection Fund, and a Synergy II/Nexus Award.
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
This type of cross-species research allows us to determine which species are capable of human-like beat perception, that is, beat perception that involves the accurate temporal prediction of beat times across a fairly broad range of tempi.
Gjerdingen posits that the motion in music is perceived as apparent rather than real. Thus, Gjerdingen likens the perceived motion in music to acousmatic listening, defined as the sound that is “heard without the causes from which it originates being seen.”44 A concrete example of an acousmatic sound would be listening to a recorded music over loudspeakers.
REFERENCES
- 1.Tillmann B 2005. “Music Cognition.” In Encyclopedia of Social Measurement. Kempf-Leonard K, Ed.: 795–801. Amsterdam: Elsevier Inc. https://www.sciencedirect.com/topics/neuroscience/music-cognition. [Google Scholar]
- 2.Margulis EH 2019. The psychology of music: A very short introduction. Oxford: Oxford University Press. [Google Scholar]
- 3.Fraisse P 1982. “Rhythm and tempo.” In Psychology of music. Deutsch D, Ed.: 149–180. Academic Press. [Google Scholar]
- 4.Clarke EF 1999. “Rhythm and timing in music.” In Psychology of music, 2nd ed. Deutsch D, Ed.: 473–500. Academic Press. [Google Scholar]
- 5.Honing H. 2013. “Structure and interpretation of rhythm in music.” In The psychology of music, 3rd ed. Deutsch D, Ed.: 369–404. Academic Press. [Google Scholar]
- 6.London J. 2012. Hearing in time: Psychological aspects of musical meter, 2nd ed. Oxford University Press. [Google Scholar]
- 7.McAuley JD. 2010. “Tempo and Rhythm.” In Music Perception. Jones MR, Fay RJ, Popper AN, Eds.:165–199. New York: Springer. [Google Scholar]
- 8.Fraisse P. (1966). L’anticipation de stimulus rythmiques: Vitesse d’établissement et précision de la synchronisation [Anticipation of rhythmic stimuli: Speed of establishment and precision of synchronization]. L’Année Psychologique, 66: 15–36. [PubMed] [Google Scholar]
- 9.Lerdahl F & Jackendoff R. 1983. A generative theory of tonal music. Cambridge, MA: MIT Press. [Google Scholar]
- 10.Benjamin WE 1984. A theory of musical meter. Music Perception, 1(4), 355–413. 10.2307/40285269. [DOI] [Google Scholar]
- 11.Jones MR 2019. Time Will Tell: A Theory of Dynamic Attending. Oxford: Oxford University Press. [Google Scholar]
- 12.Lerdahl F & Jackendoff R. 1996. A generative theory of tonal music, 2nd paperback printing. Cambridge, MA: MIT Press. [Google Scholar]
- 13.Chomsky N. 1965. Aspects of the theory of syntax. Cambridge, MA: MIT Press. [Google Scholar]
- 14.Chomsky N. 1975. Reflections on language. Pantheon. [Google Scholar]
- 15.Chomsky N, & Halle M. 1968. The sound patterns of English. Harper & Row. [Google Scholar]
- 16.Polak R, London J, & Jacoby N. 2016. Both isochronous and non-isochronous metrical subdivision afford precise and stable ensemble entrainment: A corpus study of Malian jembe drumming. Frontiers in Neuroscience, 28. 10.3389/fnins.2016.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deutsch D. 2013. Grouping mechanisms in music. In The psychology of music, 3rd ed. Deutsch D, Ed.: 183–248. Academic Press. [Google Scholar]
- 18.Wertheimer M. 1923. Untersuchung zur Lehre von der Gestalt II. Psychologische Forschung, 4: 301–350. [Google Scholar]
- 19.Margulis EH 2013. Aesthetic responses to repetition in unfamiliar music. Empirical Studies of the Arts, 31(1): 45–57. [Google Scholar]
- 20.Margulis EH 2014. On repeat: How music plays the mind. Oxford University Press. [Google Scholar]
- 21.Margulis EH 2015. Repetition and musicality. SMT-V, 1.1. http://www.smt-v.org/archives/volume1.html#repetition-and-musicality. [Google Scholar]
- 22.Hasty C. 1997. Meter as Rhythm. Oxford University Press. [Google Scholar]
- 23.Krebs H. 1999. Fantasy Pieces: Metrical dissonance in the music of Robert Schumann. Oxford University Press. [Google Scholar]
- 24.van den Toorn P. 1987a. The music of Igor Stravinsky. Yale University Press. [Google Scholar]
- 25.van den Toorn P. 1987b. Stravinsky and The Rite of Spring: The beginnings of a musical language. University of California Press. [Google Scholar]
- 26.van den Toorn P. 1988. Stravinsky re-barred. Music Analysis, 7(2): 165–195. [Google Scholar]
- 27.van den Toorn P. 2003. Stravinsky, Les Noces, and the prohibition against expressive timing. Journal of Musicology, 20(2): 285–304. 10.1525/jm.2003.20.2.285. [DOI] [Google Scholar]
- 28.van den Toorn P 2004. Stravinsky, Adorno, and the art of displacement. Musical Quarterly, 87(3): 468–509. 10.1093/musqtl/gdh017. [DOI] [Google Scholar]
- 29.van den Toorn P. 2017a. “The physicality of The Rite: Remarks on the forces of meter and their disruption.” In The Rite of Spring at 100. Neff S & Carr MA, Eds.: pp. 285–303. Indiana University Press. [Google Scholar]
- 30.van den Toorn P. 2017b. The Rite of Spring briefly revisited: Thoughts on Stravinsky’s stratifications, the psychology of meter, and African polyrhythm. Music Theory Spectrum, 29(2): 158–181. [Google Scholar]
- 31.van den Toorn P, & McGinness J. 2012. Stravinsky and the Russian period. Cambridge University Press. [Google Scholar]
- 32.Horlacher G. 2017. Rethinking blocks and superimposition: Form in the “Ritual of the Two Rival Tribes.” In The Rite of Spring at 100. Neff S & Carr MA, Eds.: 331–338. Indiana University Press. [Google Scholar]
- 33.Emmery L. Forthcoming. The Bad Plus Stravinsky: Metrical displacement, segmentation, and stratification in the jazz trio’s original works. Jazzforschung/Jazz Studies, 50–51. [Google Scholar]
- 34.Temperley D, & Bartlette C. 2002. Parallelism as a factor in metrical analysis. Music Perception, 20(1): 117–149. [Google Scholar]
- 35.Imbrie A. 1973. “Extra” measures and metrical ambiguity in Beethoven. In Beethoven Studies. Tyson A, Ed.: 45–66. Norton. [Google Scholar]
- 36.Large EW, & Jones MR. 1999. The dynamics of attending: How people track time-varying events. Psychological Review 106(1): 119–159. doi: 10.1037/0033-295x.106.1.119. [DOI] [Google Scholar]
- 37.Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. 2010. Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol 20(2):172–6. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morillon B, Hackett TA, Kajikawa Y, Schroeder CE. 2015. Predictive motor control of sensory dynamics in auditory active sensing. Curr Opin Neurobiol 230–8. doi: 10.1016/j.conb.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel A, & Iversen J. 2014. The evolutionary neuroscience of musical beat perception: the Action Simulation for Auditory Prediction (ASAP) hypothesis. Front Syst Neurosci 8:57. doi: 10.3389/fnsys.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tierney A, & Kraus N. 2014. Auditory-motor entrainment and phonological skills: precise auditory timing hypothesis (PATH). Frontiers in Human Neuroscience 27. 10.3389/fnhum.2014.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel A. 2011. Why would musical training benefit the neural encoding of speech? The OPERA hypothesis. Front. Psychol 2:142. doi: 10.3389/fpsyg.2011.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel AD 2012. The OPERA hypothesis: assumptions and clarifications. Ann N Y Acad Sci 1252:124–8. doi: 10.1111/j.1749-6632.2011.06426.x. [DOI] [PubMed] [Google Scholar]
- 43.Patel A. 2014. Can nonlinguistic musical training change the way the brain processes speech? The expanded OPERA hypothesis. Hear Res. 308, 98–108. doi: 10.1016/j.heares.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 44.Gjerdingen R. 1994. Apparent motion in music? Music Perception, 11(4): 335–370. 10.2307/40285631. [DOI] [Google Scholar]
- 45.Levitin DJ, Grahn JA, & London J, J. 2018. The psychology of music: Rhythm and movement. Annual Review of Psychology, 69: 51–75. [DOI] [PubMed] [Google Scholar]
- 46.Janata P, & Grafton S. 2003. Swinging in the brain: shared neural substrates for behaviors related to sequencing and music. Nat Neurosci 6: 682–687. 10.1038/nn1081. [DOI] [PubMed] [Google Scholar]
- 47.Madison G. 2006. Experiencing groove induced by music: Consistency and phenomenology. Music Perception, 24(2): 201–208. doi: 10.1525/mp.2006.24.2.201. [DOI] [Google Scholar]
- 48.Janata P, Tomic ST, & Haberman JM. 2012. Sensorimotor coupling in music and the psychology of the groove. Journal of Experimental Psychology: General, 141(1): 54–75. 10.1037/a0024208. [DOI] [PubMed] [Google Scholar]
- 49.Stupacher J, Hove MJ, Novembre G, Schütz-Bosbach S, & Keller PE. 2013. Musical groove modulates motor cortex excitability: A TMS investigation. Brain and Cognition, 82(2): 127–136. doi: 10.1016/j.bandc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Câmara GS, & Danielsen A, A. 2020. “Groove.” In The Oxford Handbook of Critical Concepts in Music Theory. Rehding A & Rings S, Eds.: 271–294. Oxford University Press. [Google Scholar]
- 51.Senn O, Bechtold T, Rose D, Câmara GS, Düvel N, Jerjen R, Kilchenmann L, Hoesl F, Baldassarre A, & Alessandri E. 2020. Experience of groove questionnaire: Instrument development and initial validation. Music Perception, 38(1): 46–65. doi: 10.1525/mp.2020.38.1.46 [DOI] [Google Scholar]
- 52.Stupacher J, Matthews TE, Pando-Naude V, Elst OFV, & Vuust P, P. 2022. The sweet spot between predictability and surprise: Musical groove in brain, body, and social interactions. Frontiers in Psychology (perspective article). 10.3389/fpsyg.2022.906190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuust P, & Witek MAG. 2014. Rhythmic complexity and predictive coding: A novel approach to modeling rhythm and meter perception in music. Frontiers in Psychology, 5: 1111. doi: 10.3389/fpsyg.2014.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witek MAG, Clarke EF, Wallentin M, Kringelbach ML, & Vuust P. 2014. Syncopation, body-movement and pleasure in groove music. PLoS ONE, 9: Article e94446. 10.1371/journal.pone.0094446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujioka T, Fidali BC, & Ross B. 2014. Neural correlates of intentional switching from ternary to binary meter in a musical hemiola pattern. Frontiers in Psychology, 5(1257): 1–15. doi: 10.3389/fpsyg.2014.01257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reyland N, & Thumpston R Eds. 2018. Music, analysis, and the body: Experiments, explorations, and embodiments. Peeters Publishers. [Google Scholar]
- 57.Bell M. 2021. Danses Fantasiques: Metrical dissonance in the ballet music of P. I. Tchaikovsky. Journal of Music Theory, 65(1): 107–137. 10.1215/00222909-9124750. [DOI] [Google Scholar]
- 58.Leaman KY 2021. Dance as music in George Balanchine’s Concerto Barocco. SMT-V, Volume 7.2. http://www.smt-v.org/archives/volume7.html#dance-as-music-in-george-balanchines-concerto-barocco. [Google Scholar]
- 59.Leaman KY 2022. George Balanchine’s art of choreographic musicality in Tschaikovsky's Pas de Deux. Music Theory Spectrum, 44(2): 430–469. 10.1093/mts/mtac007. [DOI] [Google Scholar]
- 60.Burger B & Toiviainen P. 2018. Embodiment in electronic dance music: Effects of musical content and structure on body movement. Musicae Scientiae, 24(2): 186–205. 10.1177/1029864918792594. [DOI] [Google Scholar]
- 61.Wolpert DM, Miall RC, & Kawato M. 1998. Internal models in the cerebellum. Trends in Cognitive Sciences, 1;2(9): 338–347. [DOI] [PubMed] [Google Scholar]
- 62.Crevecoeur F, McIntyre J, Thonnard JL, & Lefevre P. 2010. Movement stability under uncertain internal models of dynamics. Journal of Neurophysiology, 104(3): 1301–1313. DOI: 10.1152/jn.00315.2010. [DOI] [PubMed] [Google Scholar]
- 63.Bazzi S, Ebert J, Hogan N, & Sternad D. 2018. Stability and predictability in human control of complex objects. Chaos: An Interdisciplinary Journal of Nonlinear Science, 28(10). 10.1063/1.5042090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpson-Litke R. 2021. Flipped, broken, and paused clave: Dancing through metric ambiguities in salsa music. Journal of Music Theory, 65(1): 39–80. 10.1215/00222909-9124726. [DOI] [Google Scholar]
- 65.Haugen MR 2021. Investigating music-dance relationships: A case study of Norwegian telespringar. Journal of Music Theory, 65(1): 17–38. 10.1215/00222909-9124714. [DOI] [Google Scholar]
- 66.Stevens A. 2021. Music in the body: The eighteenth-century contredanse and hypermetrical hearing. Journal of Music Theory, 65(1): 81–106. 10.1215/00222909-9124738. [DOI] [Google Scholar]
- 67.Yust J. 2018. Organized time: Rhythm, tonality, and form. Oxford University Press. [Google Scholar]
- 68.Keller PE, Novembre G, & Hove MJ. 2014. Rhythm in joint action: psychological and neurophysiological mechanisms for real-time interpersonal coordination. Royal Society: Biological Sciences 369(1658). 10.1098/rstb.2013.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merchant H, Grahn J, Trainor L, Rohrmeier M, & Fitch WT. 2015. Finding the beat: A neural perspective across humans and non-human primates. Philosophical Transactions of the Royal Society, 370(1664): 20140093. doi: 10.1098/rstb.2014.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Proksch S, Comstock DC, Médé B, Pabst A, & Balasubramaniam R. 2020. Motor and predictive processes in auditory beat and rhythm perception. Frontiers in Human Neuroscience, 14: 578546. doi: 10.3389/fnhum.2020.578546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thaut MH 2003. Neural basis of rhythmic timing networks in the human brain. Annals of the New York Academy of Sciences, 999: 364–373. 10.1196/annals.1284.044. [DOI] [PubMed] [Google Scholar]
- 72.Bengtsson SL, Ullén F, Ehrsson H, Hashimoto T, Kito T, Naito E, Forssberg H, & Sadato N. 2008. Listening to rhythms activates motor and premotor cortices. Cortex, 45(1): 62–71. 10.1016/j.cortex.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Grahn JA, & Brett M. 2007. Rhythm and beat perception in motor areas of the brain. Journal of Cognitive Neuroscience, 19(5): 893–906. doi: 10.1162/jocn.2007.19.5.893. [DOI] [PubMed] [Google Scholar]
- 74.Chen JL, Penhune VB, & Zatorre RJ. 2008. Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex, 18(12): 2844–2854. doi: 10.1093/cercor/bhn042. [DOI] [PubMed] [Google Scholar]
- 75.Pressing JL 2002. Black Atlantic rhythm: Its computational and transcultural foundation. Music Perception, 19(3): 285–310. 10.1525/mp.2002.19.3.285. [DOI] [Google Scholar]
- 76.Large EW 2008. “Resonating to musical rhythm: Theory and experiment.” In The psychology of time. Grondin S, Ed.: 189–231. Emerald Publishing. [Google Scholar]
- 77.Sowiński J, & Bella S. 2013. Poor synchronization to the beat may result from deficient auditory-motor mapping. Neuropsychologia 51(10): 1952–1963. 10.1016/j.neuropsychologia.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 78.Dunlap K. 1910. Reaction to rhythmic stimuli with attempt to synchronize. Psychological Review, 17(6): 399–416. 10.1037/h0074736. [DOI] [Google Scholar]
- 79.Thaut MH, Miller RA, & Schauer LM. 1998. Multiple synchronization strategies in rhythmic sensorimotor tasks: Phase vs period correction. Biological Cybernetics, 79: 241–250. 10.1007/s004220050474. [DOI] [PubMed] [Google Scholar]
- 80.Repp BH 2010. Sensorimotor synchronization and perception of timing: Effects of music training and task experience. Human Movement Science, 29(2): 200–213. 10.1016/j.humov.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Repp BH 2005. Sensorimotor synchronization: A review of the tapping literature. Psychonomic Bulletin and Review, 12(6): 969–992. https://link.springer.com/content/pdf/10.3758/BF03206433.pdf. [DOI] [PubMed] [Google Scholar]
- 82.Miyake I. 1902. Researches on rhythmic activity. Studies From the Yale Psychological Laboratory, 10: 1–48. [Google Scholar]
- 83.Stevens LT 1886. On the time-sense. Mind, 11: 393–404. [Google Scholar]
- 84.Woodrow H. 1932. The effect of rate of sequence upon the accuracy of synchronization. Journal of Experimental Psychology, 15: 357–379. [Google Scholar]
- 85.Fraisse P. 1948. Rythmes auditifs et rythmes visuels [Visual and auditory rhythms]. L’Année Psychologique, 49: 21–41. [Google Scholar]
- 86.Fraisse P. 1974. “Cues in sensori-motor synchronization.” In Chronobiology. Scheving LE, Halberg F, & Pauly JE, Eds.: 517–522. Igaku Shoin. [Google Scholar]
- 87.Michon JA (1967). Timing in temporal tracking. Van Gorcum. [Google Scholar]
- 88.Fraisse P, & Ehrlich S. 2009. Note on the possibility of syncopating as a function of the tempo of a sequence, trans. B. Repp. Psychomusicology: Music, Mind and Brain, 20(1–2): 167–169. 10.1037/h0094214. [DOI] [Google Scholar]
- 89.van der Steen MC, & Keller PE. 2013. The ADaptation and Anticipation Model (ADAM) of sensorimotor synchronization. Frontiers in Human Neuroscience 7. 10.3389/fnhum.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thaut MH, & Abiru M. 2010. Rhythmic auditory stimulation in rehabilitation of movement disorders: A review of current research. Music Perception, 27(4): 263–269. 10.1525/mp.2010.27.4.263. [DOI] [Google Scholar]
- 91.Chen JL, Penhune VB, & Zatorre RJ. 2006. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage, 32(4): 1771–1781. doi: 10.1016/j.neuroimage.2006.04.207. [DOI] [PubMed] [Google Scholar]
- 92.Hove MJ, Fairhurst MT, Kotz SA, & Keller PE. 2013. Synchronizing with auditory and visual rhythms: An fMRI assessment of modality differences and modality appropriateness. NeuroImage 67: 313–321. 10.1016/j.neuroimage.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 93.Chen TL, Bhattacharjee T, McKay JL, Borinski JE, Hackney ME, Ting LH, & Kemp CC. 2015. Evaluation by expert dancers of a robot that performs partnered stepping via haptic interaction. PLoS ONE, 10(5): Article e0125179. 10.1371/journal.pone.0125179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen TL, Bhattacharjee T, Beer JM, Ting LH, Hackney ME, Rogers WA, & Kemp CC. 2017. Older adults’ acceptance of a robot for partner dance-based exercise. PLoS ONE, 12(10): Article e0182736. 10.1371/journal.pone.0182736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Enzensberger W, Oberlander U, & Stecker K. 1997. Metronome therapy in patients with Parkinson disease. Der Nervenarzt, 68(12): 972–977. doi: 10.1007/s001150050225. [DOI] [PubMed] [Google Scholar]
- 96.de Bruin N, Kempster C, Doucette A, Doan JB, Hu B, & Brown LA. 2015. The effects of music salience on the gait performance of young adults. Journal of Music Therapy, 52(3): 394–419. 10.1093/jmt/thv009. [DOI] [PubMed] [Google Scholar]
- 97.Fujii S, & Wan CY. 2014. The role of rhythm in speech and language rehabilitation: the SEP hypothesis. Frontiers in Human Neuroscience 8. 10.3389/fnhum.2014.00777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoo GEY, & Kim SJ. 2016. Rhythmic auditory cueing in motor rehabilitation for stroke patients: Systematic review and meta-analysis. Journal of Music Therapy, 53(2): 149–177. 10.1093/jmt/thw003. [DOI] [PubMed] [Google Scholar]
- 99.Schiavio A, & Altenmüller E. 2015. Exploring music-based rehabilitation for Parkinsonism through embodied cognitive science. Frontiers in Neurology, 6. 10.3389/fneur.2015.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thelen E, Schöner G, Scheier C, & Smith LB. 2001. The dynamics of embodiment: A field theory of infant preservative reaching. The Behavioral and Brain Society, 24(1): 1–86. doi: 10.1017/S0140525X01003910. [DOI] [PubMed] [Google Scholar]
- 101.Thaut MH, Kenyon GP, Schauer ML, & McIntosh GC. 1999. The connection between rhythmicity and brain function. IEEE Engineering in Medicine and Biology Magazine, 18(2): 101–108. 10.1109/51.752991. [DOI] [PubMed] [Google Scholar]
- 102.Lorenzoni V, de Bie T, Marchant T., Van Dyck E, & Leman M/. 2019. The effect of (a)synchronous music on runners’ lower leg impact loading. Musicae Scientiae, 23(3): 332–347. 10.1177/1029864919847496. [DOI] [Google Scholar]
- 103.Brake MT, Stolwijk N, Staal B, Van Hooren B. 2022. Using beat frequency in music to adjust running cadence in recreational runners: A randomized multiple baseline design. European Journal of Sport Science, Mar 1:1–0 (online ahead print). doi: 10.1080/17461391.2022.2042398. [DOI] [PubMed] [Google Scholar]
- 104.Bood RJ, Nijssen M, Van Der Kamp J, & Roerdink M. 2013. The power of auditory-motor synchronization in sports: Enhancing running performance by coupling cadence with the right beats. PloS ONE, 8(8): Article e70758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bramah C, Preece SJ, Gill N, & Herrington L. 2019. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. The American Journal of Sports Medicine, 47 (14): 3406–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grau-Sánchez J, Jamey K, Paraskevopoulos E, Bella SD, Gold C, Schlaug G, Belleville S, Rodríguez-Fornells A, Hackney ME, & Särkämö T. 2022. Putting music to trial: Consensus on key methodological challenges investigating music-based rehabilitation. Annals of the New York Academy of Sciences, September 30, 2022. doi: 10.1111/nyas.14892. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Awad LN, Lewek MD, Kesar TM, Franz JR, & Bowden MG. 2020. These legs were made for propulsion: Advancing the diagnosis and treatment of post-stroke propulsion deficits. Journal of NeuroEngineering and Rehabilitation, 17(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buhmann J, Moens B, Van Dyck E, Dotov D, & Leman M. 2018. Optimizing beat synchronized running to music. PloS ONE, 6;13(12): Article e0208702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, & Brault JM. 1996. Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Movement Disorders: Official Journal of the Movement Disorder Society, 11(2): 193–200. 10.1002/mds.870110213. [DOI] [PubMed] [Google Scholar]
- 110.McIntosh GC, Brown SH, Rice RR, & Thaut MH. 1997. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry, 62(1): 22–26. 10.1136/jnnp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McIntosh GC 1998. Long-term training effects of rhythmic auditory stimulation on gait in patients with Parkinson's disease. Movement Disorders, 13, Supple 2: 212. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03316365.9539332 [Google Scholar]
- 112.Bella S, Benoit CE, Farrugia NN. et al. 2017. Gait improvement via rhythmic stimulation in Parkinson’s disease is linked to rhythmic skills. Sci Rep 7: 42005. 10.1038/srep42005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Benoit CE, Bella SD, Farrugia N, Obrig H, Manika S, & Kotz SA. 2014. Musically cued gait-training improves both perceptual and motor timing in Parkinson’s disease. Hum. Neurosci 8. 10.3389/fnhum.2014.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ebersbach G, Heijmenberg M, Kindermann L, Trottenberg R, Wissel J, & Poewe W. 1999. Interference of rhythmic constraint on gait in healthy subjects and patients with early Parkinson's disease: Evidence for impaired locomotor pattern generation in early Parkinson's disease. Movement Disorders, 14(4): 619–625. doi: . [DOI] [PubMed] [Google Scholar]
- 115.Blin O, Ferrandez AM, & Serratrice G. 1990. Quantitative analysis of gait in Parkinson patients: Increased variability of stride length. Journal of The Neurological Sciences, 98(1): 91–97. doi: 10.1016/0022-510x(90)90184-o. [DOI] [PubMed] [Google Scholar]
- 116.Hallett M. 2008. The intrinsic and extrinsic aspects of freezing of gait. Movement Disorders, 23, Suppl 2(02): S439–S443. 10.1002/mds.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arias P, & Cudeiro J. 2010. Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PloS ONE, 5(3): Article e9675. doi: 10.1371/journal.pone.0009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arias P, & Cudeiro J. 2008. Effects of rhythmic sensory stimulation (auditory, visual) on gait in Parkinson's disease patients. Experimental Brain Research, 186(4): 589–601. DOI: 10.1007/s00221-007-1263-y. [DOI] [PubMed] [Google Scholar]
- 119.del Olmo MF, & Cudeiro J. 2005. Temporal variability of gait in Parkinson disease: Effects of a rehabilitation programme based on rhythmic sound cues. Parkinsonism & Related Disorders, 11(1): 25–33. doi: 10.1016/j.parkreldis.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 120.Freedland RL, Festa C, Sealy M, McBean A, Elghazaly P, Capan A, Brozycki L, Nelson AJ, & Rothman J. 2002. The effects of pulsed auditory stimulation on various gait measurements in persons with Parkinson's disease. NeuroRehabilitation, 17(1): 81–87. https://pubmed.ncbi.nlm.nih.gov/12016350/ [PubMed] [Google Scholar]
- 121.Howe TE, Lövgreen B, Cody FWJ, Ashton VJ, & Oldham JA. 2003. Auditory cues can modify the gait of persons with early-stage Parkinson's disease: A method for enhancing parkinsonian walking performance? Clinical Rehabilitation, 17(4): 363–367. 10.1191/0269215503cr621oa. [DOI] [PubMed] [Google Scholar]
- 122.Bella S, Benoit CE, Farrugia N, Schwartze M, & Kotz SA. 2015. Effects of musically cued gait training in Parkinson's disease: beyond a motor benefit. Annals of the New York Academy of Sciences 1337(1): 77–85. 10.1111/nyas.12651 [DOI] [PubMed] [Google Scholar]
- 123.Grahn JA, & Brett M. 2009. Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex, 45(1): 54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 124.Harrington DL, Haaland KY, & Hermanowitz N. 1998. Temporal processing in the basal ganglia. Neuropsychology, 12: 3–12. [DOI] [PubMed] [Google Scholar]
- 125.O’Boyle DJ, Freeman JS, & Cody FWJ. 1996. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson's disease. Brain, 119: 51–70. [DOI] [PubMed] [Google Scholar]
- 126.Nombela C, Rae CL, Grahn JA, Barker RA, Owen AM, & Rowe JB. 2013. How often does music and rhythm improve patients’ perception of motor symptoms in Parkinson's disease? Journal of Neurology, 260(5): 1404–1405. 10.1007/s00415-013-6860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nombela C, Hughes LE, Owen AM, & Grahn JA. 2013. Into the groove: Can rhythm influence Parkinson’s disease? Neuroscience & Biobehavioral Reviews, 37( 10), Part 2: 2564–2570. 10.1016/j.neubiorev.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Rochester L, Burn DJ, Woods G, Goodwin J, & Nieuwboer A. 2009. Does auditory rhythmical cueing improve gait in people with Parkinson's disease and cognitive impairment? A feasibility study. Movement Disorders, 24(6): 839–845. 10.1002/mds.22400. [DOI] [PubMed] [Google Scholar]
- 129.Brown LA, de Bruin N, Dean JB, Suchowersky O, & Hu B. 2009. Novel challenges to gait in Parkinson's disease: The effect of concurrent music in single- and dual-task contexts. Archives of Physical Medicine and Rehabilitation, 90(9): 1578–1583. https://pubmed.ncbi.nlm.nih.gov/19735787/. [DOI] [PubMed] [Google Scholar]
- 130.Rosenberg M, Slusarenko A, Cao K, Mckay JL, Emmery L, Kesar T, & Hackney ME. 2023. Motor and cognitive deficits limit the ability to flexibly modulate spatiotemporal gait features in older adults with mild cognitive impairment. Frontiers in Human Neuroscience: Motor Neuroscience 17. 10.3389/fnhum.2023.1040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guadagnoli MA, & Lee TD. 2004. Challenge point: A framework for conceptualizing the effects of various practice conditions in motor learning. Journal of Motor Behavior, 1;36(2): 212–224. doi: 10.3200/JMBR.36.2.212-224. [DOI] [PubMed] [Google Scholar]
- 132.Collimore AN, Roto Cataldo AV, Aiello AJ, et al. 2003. Autonomous Control of Music to Retrain Walking After Stroke. Neurorehabilitation and Neural Repair, 37(5): 255–265. doi: 10.1177/15459683231174223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grau-Sánchez J, Segura E, Sanchez-Pinsach D et al. 2021. Enriched Music-supported Therapy for chronic stroke patients: a study protocol of a randomised controlled trial. BMC Neurol 21, 19. 10.1186/s12883-020-02019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.