Abstract
At the G2/M transition of the cell cycle, the cdc25c phosphatase dephosphorylates inhibitory residues of cdc2, and cyclin-B–cdc2 kinase (MPF) is activated. Phosphorylation of cyclin B1 induces its nuclear accumulation, and, since cdc25c is also believed to accumulate and activate shortly before G2/M in the nucleus, it has been proposed that this induces cyclin-B1–cdc2 kinase activation. We demonstrate that cyclin B1 phosphorylation has another essential function in vivo: it is required for cdc25c and MPF activation, which does not require nuclear accumulation of cyclin B1, and occurs in the cytoplasm.
Introduction
Soon after maturation-promoting factor (MPF) was established to be an heterodimer comprising one molecule of cdc2 and one molecule of cyclin B (Labbé et al., 1989; Dorée and Hunt, 2002), it became obvious that phosphorylation of cyclin B on multiple conserved N-terminal residues is universally associated with MPF activation at the G2/M transition (Meijer et al., 1989; Pines and Hunter, 1989; Gautier and Maller, 1991; Izumi and Maller, 1991; Li et al., 1995; Borgne et al., 1999). Besides promoting nuclear accumulation of the cyclin-B–cdc2 complex, no other function of cyclin B phosphorylation has been documented (Yang and Kornbluth, 1999; Takizawa and Morgan, 2000). Oocytes are particularly well suited for studying the role of cyclin B phosphorylation, because nuclear translocation is not required for cdc2 kinase activation in response to hormonal stimulation, which readily occurs in enucleated oocytes (Masui and Markert, 1971; Dabauvalle et al., 1988; Fisher et al., 1998). In Xenopus, in response to progesterone, cyclin B1 has been shown to undergo phosphorylation on five sites, identified as Ser2, Ser94, Ser96, Ser101 and Ser113 (Izumi and Maller, 1991; Li et al., 1995), the last four of which are conserved throughout evolution. In contrast to wild-type cyclin B1 (WT-B1), the quintuple alanine mutant for each of these phosphorylation sites (Ala5-B1) neither translocates into the germinal vesicle, the large nucleus of oocytes, nor induces germinal vesicle breakdown (GVBD) efficiently, unless an artificial nuclear localization signal is appended (Li et al., 1995, 1997). Thus, it was assumed that Ala5-B1 fails to induce GVBD because it does not translocate into the nucleus and hence cannot phosphorylate nuclear substrates, including lamins, whose phosphorylation by cyclin-B–cdc2 kinase is a landmark of the G2/M transition (Peter et al., 1990). In the present study, we addressed the question of whether cyclin B phosphorylation plays a more fundamental role in the process of MPF activation, independently of nuclear translocation. We focused on cyclin B1, because genetic studies in mouse showed it is essential in early embryogenesis, at variance with the other major cyclin B variant, cyclin B2, the loss of which has no effect on viability and fertility (Brandeis et al., 1998).
RESULTS
Initial cyclin B1 phosphorylation is required for cdc25c-dependent activation of MPF in Xenopus oocytes
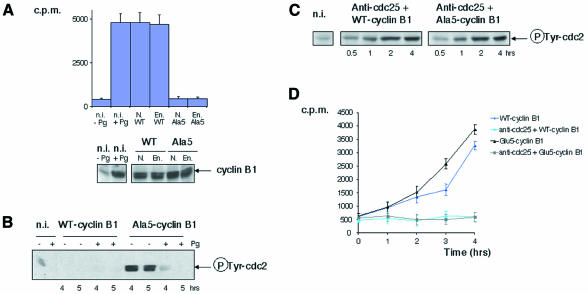
If the only function of cyclin B phosphorylation was to allow nuclear accumulation of cyclin-B–cdc2 complexes, those assembled from endogenous cdc2 and the quintuple alanine mutant should undergo activation in the cytoplasm. However, this was not the case: whereas ectopic WT-B1 readily supported the formation of active cyclin-B1–cdc2 complexes in both nucleated and enucleated oocytes when expressed in fully grown immature oocytes to levels similar to those of endogenous cyclin B1 in mature oocytes, no significant H1 kinase activity was generated in non-hormone-stimulated oocytes from Ala5-B1 (Figure 1A) and GVBD never occurred (354 oocytes scored from seven females), although both WT-B1 and Ala5-B1 assembled as efficiently with cdc2 (data not shown; Li et al., 1995). Consistent with previous results (Li et al., 1995, 1997), Ala5-B1–cdc2 complexes readily underwent activation when oocytes were stimulated with progesterone, and, as expected, activation was associated with tyrosine dephosphorylation of cdc2 (Figure 1B). Thus, endogenous WT-B1 is sufficient to trigger MPF activation, which can then act in a feedback loop on mutant cyclin-B1–cdc2 kinase incapable of activating MPF. In other words, cyclin B1 phosphorylation is required to trigger, but not maintain, the MPF activation feedback loop. When either WT-B1 or Ala5-B1 was expressed in oocytes injected with an antibody directed against cdc25c that suppressed tyrosine dephosphorylation of cdc2, they generated inactive complexes with similar kinetics and to equivalent levels (Figure 1C). This suggests that, although not required for cyclin-B–cdc2 kinase activity per se, phosphorylation of cyclin B is required to trigger the positive feedback mechanism that activates cdc25c.
Fig. 1. Cyclin B1 phosphorylation is required for MPF activation in Xenopus oocytes. (A) Intact nucleated oocytes (N.) or enucleated oocytes (En.) were microinjected with either WT-B1 (WT) or Ala5-B1 (Ala5) mRNA (constructs containing a C-terminal VSVG-tag). Five hours later, groups of three oocytes were collected, lysed and immunoprecipitated with anti-P5D4 monoclonal antibodies. The beads were then analysed by H1 kinase assay (upper panel) or western blotting for cyclin B1 content (lower panel). Non-injected oocytes (n.i.), stimulated (+Pg) or not (–Pg) with progesterone, were used directly for H1 kinase assay or western blotting. The equivalent of one oocyte was used for each assay. Relative levels of cyclin B1 and B2 were shown recently to be a variable feature of different batches of mature oocytes, cyclin B1 often exceeding cyclin B2 by far (see figure 6 in Hochegger et al., 2001). Assuming that cyclin-B1–cdc2 and cyclin-B2–cdc2 complexes contribute to equivalent levels to H1 kinase activity in such oocytes, as reported in an earlier study (Kobayashi et al., 1991), exogenous cyclin B1 should not have been expressed to levels exceeding twice the endogenous cyclin B1 level in this experiment. (B) Oocytes were microinjected as in (A) (with higher levels of cyclin B1 expression) and treated or not with progesterone. At the indicated times, groups of three oocytes were homogenized and submitted to an immunoprecipitation with anti-P5D4 antibodies, followed by a western blot for the tyrosine-phosphorylated form of cdc2. Non-injected oocytes were used directly for western blotting. The equivalent of one oocyte was loaded per lane. (C) Oocytes were microinjected with affinity-purified anti-cdc25 antibodies and either WT-B1 or Ala5-B1 mRNA. Groups of three oocytes were lysed and analysed by western blotting for tyrosine-phosphorylated cdc2 content. The equivalent of one oocyte was loaded per lane. (D) Oocytes were microinjected or not with anti-cdc25 antibodies and with either WT-B1 or Glu5-B1 mRNA. Extracts from individual oocytes selected at the indicated times after microinjection were assayed for H1 kinase. The error bars represent standard deviation.
To confirm this view, we used a quintuple glutamic acid mutant (Glu5-B1) with serine residues 2, 94, 96, 101 and 113 mutated to glutamic acid to mimic phosphoserine residues. Not only did this mutant readily generate active H1 kinase when expressed in non-hormone-stimulated oocytes, but it did so more rapidly (Figure 1D), and, as a consequence, oocytes underwent GVBD with more rapid kinetics than WT-B1, as reported previously (Li et al., 1995). Moreover, no H1 kinase activity was generated and Glu5-B1–cdc2 complexes accumulated with tyrosine-phosphorylated cdc2 in oocytes injected with anti-cdc25c antibodies, ruling out the possibility that Glu5-B1–cdc2 complexes could bypass inactivation through phosphorylation of cdc2 on inhibitory residues Thr14 and Tyr15. Similar results were obtained in enucleated oocytes (data not shown). Taken together, the above results strongly suggest that cyclin B1 phosphorylation is required in intact oocytes, independently of its role in promoting nuclear accumulation of cyclin B1, for cdc25c to be converted into an active form able to dephosphorylate inhibitory residues of cdc2.
Cyclin B1 phosphorylation is required for initial tyrosine dephosphorylation of cdc2 in Xenopus egg extracts
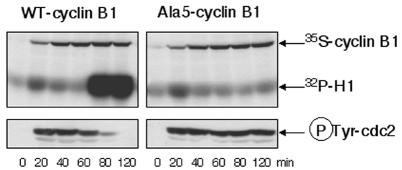
Next, we expressed Ala5-B1 in cytosolic extracts prepared from unfertilized eggs in the absence of a Ca2+ chelator; such extracts do not contain any nucleus nor endogenous cyclin. The mutant cyclin targeted endogenous cdc2 for tyrosine phosphorylation, and the Ala5-B1–cdc2 complexes failed to undergo efficient activation in such extracts, as observed in oocytes, and in contrast to complexes formed from WT-B1, which readily produced H1 histone kinase activity after a lag phase (Figure 2). No difference was detected in the kinetics of tyrosine phosphorylation during the lag phase between cdc2 complexes comprising wild-type or unphosphorylatable cyclin. This is important, because unfertilized eggs mainly use the cytosolic wee1 tyrosine kinase to inactivate cdc2, in contrast to oocytes in the first meiotic cell cycle, which exclusively use the membrane-associated myt1 tyrosine kinase (Mueller et al., 1995; Murakami and Vande Woude, 1998; Nakajo et al., 2000). Thus, in both cases, cyclin B1 phosphorylation is necessary to trigger the MPF activation loop.
Fig. 2. Cyclin B1 phosphorylation is required for MPF activation in Xenopus interphase egg extracts. Extracts were prepared from unfertilized eggs in the absence of any Ca2+ chelator. After centrifugation, extracts were treated with RNase and, subsequently, with RNase inhibitor and supplemented with tRNA and [35S]methionine. Then, mRNA encoding either WT-B1 or Ala5-B1 wase added. One microlitre of extract was taken per point and analysed by H1 kinase assay or western blotting for the tyrosine-phosphorylated form of cdc2. Phosphorylated histones and [35S]cyclin-B1 synthesis were revealed by autoradiography.
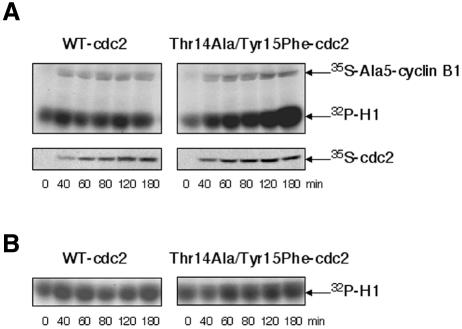
Activation of cyclin-B–cdc2 complexes is believed to be a two-step process (Murray and Hunt, 1993; King et al., 1994): a limited activation above a threshold level occurs first through a poorly understood process (initiation), followed by a positive feedback process that activates cdc25c and inactivates myt1/wee1, resulting in maximal dephosphorylation of cdc2 on inhibitory residues and kinase activation (amplification). In addition, a non-covalent inhibitor has been shown to cooperate with inhibitory phosphorylations to maintain cyclin-B–cdc2 complexes inactive in Xenopus oocytes and eggs, at least during the initiation step (Lee and Kirschner, 1996). As shown in Figure 3, Ala5-B1 readily formed active kinase complexes when coexpressed with a Thr14Ala/Tyr15Phe-cdc2 mutant. This rules out the possibility that cyclin phosphorylation could be required independently of cdc2 dephosphorylation.
Fig. 3. Ala5-B1 forms active kinase complexes when coexpressed with a Thr14Ala/Tyr15Phe-cdc2 mutant. (A) Extracts prepared as in Figure 2 were supplemented with both Ala5-B1 mRNA and either WT-cdc2 mRNA or Thr14Ala/Tyr15Phe-cdc2 mRNA. One microlitre of extract per point was analysed by H1 kinase assay. Phosphorylated histones and synthesis of [35S]Ala5-B1 and [35S]cdc2 were revealed by autoradiography. (B) Extracts were supplemented with only either WT-cdc2 or Thr14Ala/Tyr15Phe-cdc2 mRNA. One microlitre of extract per point was analysed by H1 kinase assay.
Dual role of xPlk1 in cdc25c activation
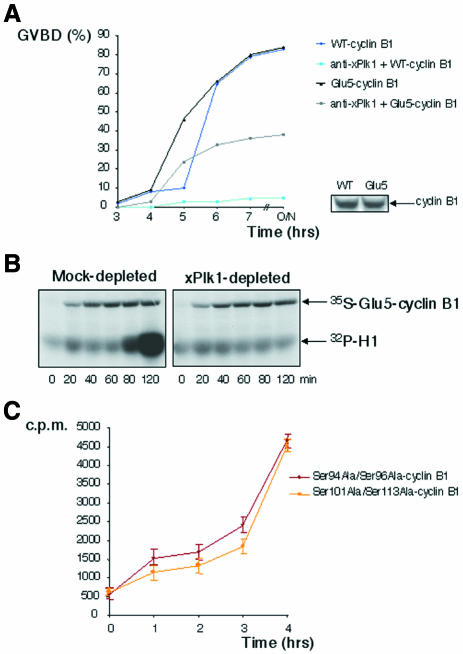
Whereas MPF itself can phosphorylate Ser94 and Ser96 in Xenopus cyclin B1 (Izumi and Maller, 1991; Borgne et al., 1999; Hagting et al., 1999), polo-like kinase 1 phosphorylates at least one residue homologous to Ser113 in mammalian cyclin B1 (Toyoshima-Morimoto et al., 2001). Xenopus oocytes prevented from activating this kinase (xPlk1) are strongly delayed in activating MPF in response to progesterone, although they ultimately undergo GVBD (data not shown; Qian et al., 2001). In the same way, suppression of xPlk1 activation through microinjection of specific antibodies severely inhibited H1 kinase activation and GVBD in non-hormone-stimulated oocytes expressing WT-B1 (Figure 4A). Inhibition still occurred but was considerably reduced in oocytes expressing Glu5-B1, suggesting that cyclin B phosphorylation partially fulfils the requirement for xPlk1 kinase activity. Using Xenopus egg extracts, we demonstrated previously that xPlk1 is required for cdc25c and cyclin-B–cdc2 kinase activation in the first mitotic cell cycle, although not for cyclin-A–cdc2 kinase, which neither requires (Abrieu et al., 1998) nor induces cdc25c activation (Hoffmann et al., 1993; Abrieu et al., 1998). As shown in Figure 4B, Glu5-B1 failed to generate active kinase complexes with cdc2 in the absence of xPlk1 activity in similar conditions. This confirms that xPlk1 serves purposes other than cyclin B phosphorylation in the process of cyclin-B–cdc2 activation, consistent with the previous report that it directly phosphorylates and activates cdc25c (Kumagai and Dunphy, 1996). Finally, either Ser94Ala/Ser96Ala-cyclin B1 or Ser101Ala/Ser113Ala-cyclin B1 double mutants assembled active kinase complexes with cdc2 when expressed in Xenopus oocytes (Figure 4C), suggesting that phosphorylation of cyclin B1 by either xPlk1 or MPF itself may be sufficient to fire the MPF amplification loop and that both kinases cooperate in this process.
Fig. 4. Besides cyclin B1 phosphorylation, xPlk1 is still required for MPF activation. (A) Left panel: time-course of oocyte maturation, following microinjection (or not) of affinity-purified anti-xPlk1 antibodies and microinjection of either WT-B1 mRNA or Glu5-B1 mRNA. Lower right panel: western blot showing the levels of expression of WT-B1 and Glu5-B1. (B) Extracts were prepared as in Figure 2 and either Mock-depleted or xPlk1-depleted. Then Glu5-B1 mRNA was added, and one microlitre of extract per point was analysed by H1 kinase assay, revealed by autoradiography, as well as [35S]Glu5-B1 synthesis. (C) Oocytes were microinjected with Ser94Ala/Ser96Ala-cyclin B1 mRNA or Ser101Ala/Ser113Ala-cyclin B1 mRNA. Extracts from individual oocytes selected at the indicated times after microinjection were assayed for H1 kinase. The error bars represent standard deviation.
DISCUSSION
Nuclear import of cyclin B1, itself dependent on cyclin B phosphorylation, is not essential for cyclin-B–cdc2 kinase activation, which first occurs in the cytoplasm, as also suggested by reports that certain cyclin-B–cdc2-dependent mitotic events in the cytoplasm, such as centrosome separation, occur before cyclin B1 nuclear import (Blangy et al., 1995; Sluder and Hinchcliffe, 2000). When Ala5-B1–cdc2 complexes are artificially targeted to the nucleus, due to leptomycin treatment or appending a nuclear localization signal, they become directly activated without the need for prior cdc25c activation above its basal activity (Li et al., 1997; Hagting et al., 1998), probably because nuclear targeting protects them from inhibitory phosphorylation in oocytes by cytoplasmic myt1. As demonstrated here, however, cyclin B phosphorylation is required for efficient dephosphorylation of cdc2 inhibitory residues and thus possibly for cdc25c activation in physiological conditions. Indeed, we provided evidence that unphosphorylatable cyclin B assembles with and targets cdc2 for inhibitory phosphorylation, including phosphorylation of Tyr15, with kinetics similar to wild-type cyclin in both intact oocytes (which do not express wee1 and use myt1 exclusively to phosphorylate cdc2 on inhibitory residues) and cell-free extracts prepared after completion of meiotic maturation (which predominantly use the wee1 kinase). Yet complexes including ectopic wild-type cyclin B readily activate the MPF amplification loop and cdc25c after a lag phase in both intact non-hormone-treated oocytes and cell-free extracts, as expected from previous work (Solomon et al., 1990). In contrast, complexes containing the unphosphorylatable cyclin B accumulate but do not activate the cdc25-dependent pathway required to dephosphorylate cdc2. Moreover, unphosphorylatable cyclin B readily forms active complexes if associated with a mutant of cdc2 lacking the phosphorylation sites for wee1/myt1 or if expressed in oocytes containing fully activated cdc25c. How cyclin B phosphorylation promotes cdc25c activation remains to be investigated.
Speculation
Cyclin B1 has been reported to interact through its P-box with cdc25c (Zheng and Ruderman, 1993), and this interaction, detected in vivo only at the time when cyclin B is phosphorylated (Jessus and Beach, 1992), was claimed to activate cdc25c, at least in vitro (Galaktionov and Beach, 1991). Possibly, phosphorylation of cyclin B1 by kinases including Plk1 facilitates cyclin B1 binding to cdc25c and subsequent activation of the mitotic inducer. According to this view, dissociation of 14-3-3 by interaction of the cdc25c–14-3-3 complex with phosphorylated cyclin B1 in the cytoplasm would also expose the nuclear localization signal of cdc25c adjacent to Ser287 (Xenopus) or Ser216 (human), allowing its nuclear import. Coordinated import of cyclin B1 and cdc25c may then be used as an accessory mechanism to speed up cdc2 kinase activation (Takizawa and Morgan, 2000) and elicit sudden phosphorylation of its nuclear targets. Yet the triggering mechanism occurs in the cytoplasm and requires cyclin B phosphorylation, at least in oocytes and early embryos. Thus, it may be a previously unidentified component of the universal control mechanism regulating onset of M phase in eukaryotic cells (Nurse, 1990).
METHODS
Xenopus oocytes and egg extracts. Oocyte manipulations in MMR buffer (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.1 mM EGTA, 5 mM HEPES pH 7.3) and homogenates in oocyte buffer (50 mM β-glycerophosphate, 10 mM MgCl2, 7.5 mM EGTA, 1 mM DTT, pH 7.3) were performed as described previously (Peter et al., 2001). Progesterone was used at a final concentration of 1 µM.
Plasmids and mRNA. Xenopus WT-B1, Ala5-B1 and Glu5-B1 constructs (containing a C-terminal epitope tag derived from the glycoprotein G of vesicular stomatitis virus) as well as Ser94Ala/Ser96Ala-cyclin B1 and Ser101Ala/Ser113Ala- cyclin B1 constructs have been described previously (Li et al., 1995). Xenopus WT-cdc2 and Thr14Ala/Tyr15Phe-cdc2 constructs have already been described (Gautier et al., 1991).
mRNA were prepared with mMESSAGEmMACHINE kit (Ambion).
Immunological procedures. Monoclonal anti-P5D4 antibodies were obtained from Sigma. Polyclonal antibodies against the tyrosine-phosphorylated form of cdc2 were obtained from New England Biolabs (9111S). Other antibodies used were rabbit polyclonal antisera against Xenopus full-length recombinant cyclin B1 (His6 tag), cdc25c and xPlk1 C-terminus, and they were used affinity-purified. The second anti-rabbit immunoglobulin-horseradish peroxydase conjugate was diluted according to the supplier’s recommendations (Amersham).
Immunoprecipitations were performed according to standard procedures. Briefly, anti-P5D4 antibodies were preincubated with protein G–Sepharose beads (Pharmacia) for 1 h. After oocyte homogenate centrifugation, the clear supernatant was incubated with the beads for 1 h, and the beads were washed twice in RIPA buffer and twice in Tris 50 mM pH 7.5.
Immunodepletions were performed as follows. Anti-xPlk1 antibodies or mock immunoglobulins were preincubated with Dynabeads Protein A (Dynal). Interphase egg extracts were incubated with the beads for 30 min. The depleted extracts were then used for in vitro translation.
Kinase activities of MPF. Total histone H1 kinase activities were assayed as described previously (Labbe et al., 1991).
Acknowledgments
ACKNOWLEDGEMENTS
Grateful acknowledgement is due to Dr Daniel Fisher for critical reading of the manuscript. This work was supported by grants from the Association pour la Recherche sur le Cancer and the Ligue Nationale Contre le Cancer. M.P. was a recipient of funding from the Ligue Nationale Contre le Cancer.
REFERENCES
- Abrieu A., Brassac, T., Galas, S., Fisher, D., Labbé, J.C. and Dorée, M. (1998) The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci., 111, 1751–1757. [DOI] [PubMed] [Google Scholar]
- Blangy A., Lane, H.A., d’Herin, P., Harper, M., Kress, M. and Nigg, E.A. (1995) Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell, 83, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Borgne A., Ostvold, A.C., Flament, S. and Meijer, L. (1999) Intra-M phase-promoting factor phosphorylation of cyclin B at the prophase/metaphase transition. J. Biol. Chem., 274, 11977–11986. [DOI] [PubMed] [Google Scholar]
- Brandeis M., Rosewell, I., Carrington, M., Crompton, T., Jacobs, M.A., Kirk, J., Gannon, J. and Hunt, T. (1998) Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl Acad. Sci. USA, 95, 4344–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabauvalle M.C., Dorée, M., Bravo, R. and Karsenti, E. (1988) Role of nuclear material in the early cell cycle of Xenopus embryos. Cell, 52, 525–533. [DOI] [PubMed] [Google Scholar]
- Dorée M. and Hunt, T. (2002) From Cdc2 to Cdk1: when did the cell cycle kinase join its partner. J. Cell Sci., in press. [DOI] [PubMed] [Google Scholar]
- Fisher D., Coux, O., Bompard-Marechal, G. and Dorée, M. (1998) Germinal vesicle material is dispensable for oscillations in cdc2 and MAP kinase activities, cyclin B degradation and synthesis during meiosis in Xenopus oocytes. Biol. Cell, 90, 497–508.9923074 [Google Scholar]
- Galaktionov K. and Beach, D. (1991) Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell, 67, 1181–1194. [DOI] [PubMed] [Google Scholar]
- Gautier J. and Maller, J.L. (1991) Cyclin B in Xenopus oocytes: implications for the mechanism of pre-MPF activation. EMBO J., 10, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Solomon, M.J., Booher, R.N., Bazan, J.F. and Kirschner, M.W. (1991) cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell, 67, 197–211. [DOI] [PubMed] [Google Scholar]
- Hagting A., Karlsson, C., Clute, P., Jackman, M. and Pines, J. (1998) MPF localization is controlled by nuclear export. EMBO J., 17, 4127–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A., Jackman, M., Simpson, K. and Pines, J. (1999) Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol., 9, 680–689. [DOI] [PubMed] [Google Scholar]
- Hochegger H., Klotzbücher, A., Kirk, J., Howell, M., Le Guellec, K., Fletcher, K., Duncan, T., Sohail, M. and Hunt, T. (2001) New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development, 128, 3795–3807. [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Clarke, P.R., Marcote, M.J., Karsenti, E. and Draetta, G. (1993) Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J., 12, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T. and Maller, J.L. (1991) Phosphorylation of Xenopus cyclins B1 and B2 is not required for cell cycle transitions. Mol. Cell. Biol., 11, 3860–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessus C. and Beach, D. (1992) Oscillation of MPF is accompanied by periodic association between cdc25 and cdc2-cyclin B. Cell, 68, 323–332. [DOI] [PubMed] [Google Scholar]
- King R.W., Jackson, P.K. and Kirschner, M.W. (1994) Mitosis in transition. Cell, 79, 563–571. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Minshull, J., Ford, C., Golsteyn, R., Poon, R. and Hunt, T. (1991) On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J. Cell Biol., 114, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy, W.G. (1996) Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science, 273, 1377–1380. [DOI] [PubMed] [Google Scholar]
- Labbé J.C., Capony, J.P., Caput, D., Cavadore, J.C., Derancourt, J., Kaghad, M., Lelias, J.M., Picard, A. and Dorée, M. (1989) MPF from starfish oocytes at first meiotic metaphase is an heterodimer comprising one molecule of cdc2 and one molecule of cyclin B. EMBO J., 8, 3053–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J.C., Cavadore, J.C. and Dorée, M. (1991) M phase-specific cdc2 kinase: preparation from starfish oocytes and properties. Methods Enzymol., 200, 291–301. [DOI] [PubMed] [Google Scholar]
- Lee T.H. and Kirschner, M.W. (1996) An inhibitor of p34cdc2/cyclin B that regulates the G2/M transition in Xenopus extracts. Proc. Natl Acad. Sci. USA, 93, 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Meyer, A.N. and Donoghue, D.J. (1995) Requirement for phos-phorylation of cyclin B1 for Xenopus oocyte maturation. Mol. Biol. Cell, 6, 1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Meyer, A.N. and Donoghue, D.J. (1997) Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc. Natl Acad. Sci. USA, 94, 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y. and Markert, C.L. (1971) Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool., 177, 129–145. [DOI] [PubMed] [Google Scholar]
- Meijer L., Arion, D., Golsteyn, R., Pines, J., Brizuela, L., Hunt, T. and Beach, D. (1989) Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J., 8, 2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P.R., Coleman, T.R., Kumagai, A. and Dunphy, W.G. (1995) Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science, 270, 86–90. [DOI] [PubMed] [Google Scholar]
- Murakami M.S. and Van de Woude, G.F. (1998) Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development, 125, 237–248. [DOI] [PubMed] [Google Scholar]
- Murray A. and Hunt, T. (1993) The Cell Cycle: An Introduction. W.H. Freeman & Co., New York.
- Nakajo N., Yoshitome, S., Iwashita, J., Iida, M., Uto, K., Ueno, S., Okamoto, K. and Sagata, N. (2000) Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev., 14, 328–338. [PMC free article] [PubMed] [Google Scholar]
- Nurse P. (1990) Universal control mechanism regulating onset of M-phase. Nature, 344, 503–508. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa, J., Dorée, M., Labbé, J.C. and Nigg, E.A. (1990) In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell, 61, 591–602. [DOI] [PubMed] [Google Scholar]
- Peter M., Castro, A., Lorca, T., Le Peuch, C., Magnaghi-Jaulin, L., Dorée, M. and Labbé, J.C. (2001) The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nature Cell Biol., 3, 83–87. [DOI] [PubMed] [Google Scholar]
- Pines J. and Hunter, T. (1989) Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell, 58, 833–846. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Erikson, E., Taieb, F.E. and Maller, J.L. (2001) The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes. Mol. Biol. Cell, 12, 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G. and Hinchcliffe, E.H. (2000) The coordination of centrosome reproduction with nuclear events during the cell cycle. Curr. Top. Dev. Biol., 49, 267–289. [DOI] [PubMed] [Google Scholar]
- Solomon M.J., Glotzer, M., Lee, T.H., Philippe, M. and Kirschner, M.W. (1990) Cyclin activation of p34cdc2. Cell, 63, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Takizawa C.G. and Morgan, D.O. (2000) Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol., 12, 658–665. [DOI] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi, E., Shinya, N., Iwamatsu, A. and Nishida, E. (2001) Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature, 410, 215–220. [DOI] [PubMed] [Google Scholar]
- Yang J. and Kornbluth, S. (1999) All aboard the cyclin train: subcellular trafficking of cyclins and their CDK partners. Trends Cell Biol., 9, 207–210. [DOI] [PubMed] [Google Scholar]
- Zheng X.F. and Ruderman, J.V. (1993) Functional analysis of the P box, a domain in cyclin B required for the activation of Cdc25. Cell, 75, 155–164. [PubMed] [Google Scholar]