Abstract
With limited evidence on the neurological impact of particulate matter (PM) exposure in China, particularly for PM1 which is smaller but more toxic, we conducted a large Chinese cohort study using causal inference approaches to comprehensively clarify such impact. A total of 36,271 participants in southern China were recruited in 2015 and followed up through 2020. We obtained the neurological hospitalizations records by linking the cohort data to the electronic reports from 418 medical institutions across the study area. By using high-resolution PM concentrations from satellite-based spatiotemporal models and the cohort data, we performed marginal structural Cox models under causal assumptions to assess the potential causal links between time-varying PM exposure and neurological hospitalizations. Our findings indicated that increasing PM1, PM2.5, and PM10 concentrations by 1 μg/m3 were associated with higher overall neurological hospitalization risks, with hazard ratios (HRs) of 1.10 (95% confidence interval (CI) 1.04–1.16), 1.09 (95% CI 1.04–1.14), and 1.03 (95% CI 1.00–1.06), respectively. PM1 appeared to have a stronger effect on neurological hospitalization, with a 1% and 7% higher impact compared to PM2.5 and PM10, respectively. Additionally, each 1-μg/m3 increase in the annual PM1 concentration was associated with an elevated risk of hospitalizations for ischemic stroke (HR: 1.15; 95% CI, 1.06–1.26), which tended to be larger than the estimates for PM2.5 (HR: 1.13, 95% CI, 1.04–1.23) and PM10 (HR: 1.05, 95% CI, 1.00–1.09). Furthermore, never-married or female individuals tended be at a greater risk compared with their counterparts. Our study provides important insights into the health impact of particles, particularly smaller particles, on neurological hospitalization risk and highlights the need for clean-air policies that specifically target these particles.
Keywords: Particulate matter, Neurological disorders
GRAPHICAL ABSTRACT
1. Introduction
Neurological disorders remain a significant public health issue worldwide, responsible for approximately 3.09 million deaths and 38 million disability-adjusted life-years (DALYs) in 2017 (Collaborators, 2018). In China, neurological disorders accounted for 2.6 million hospital admissions in 2022, accounting for 3.1% of all hospital admissions (Commission, 2022).
Exposure to particulate matter (PM) is approximated to result in 4.14 million deaths globally in 2019 (Collaborators, 2020). Previous epidemiologic research has shown an increased risk of neurological disorders following long-term PM exposure. For instance, a previous meta-analysis found that long-term exposure to each 10 μg/m3 increase in PM2.5 (ambient particles with aerodynamic diameter ≤2.5 μm) was associated with a 34% increase in the incidence of Parkinson’s disease and a 26% increase in Alzheimer’s disease (Fu et al., 2019). Mechanism studies suggested that PM exposure may affect biological pathways of oxidative stress, neuroinflammation, or neuronal damage, thereby accelerating the occurrence of neurological disorders (Xu et al., 2016). These findings suggest that PM exposure may be an important predictor underlying the increased risk of neurological disorders.
However, current studies on the neurological impact of PM exposure suffer from multiple research gaps. First, the majority of the current evidence is originated from western countries with relatively low PM concentrations. In contrast, China, one of the world’s most densely populated areas, has been suffering from the highest levels of air pollution globally, where the annual PM2.5 concentration was 7–8 times higher than the World Health Organization (WHO) interim target (5 μg/m3) (Organization, 2019). Therefore, high-quality studies from Chinese cohorts are urgently needed to clarify the neurological impact of PM exposure so as to inform policy and public health interventions to improve the wellbeing of residents in such heavily polluted settings. Furthermore, existing studies usually are focused on the impact of regular-sized particles such as PM2.5 and PM10, resulting in a significant gap in our knowledge regarding the detrimental neurological effects of PM1 (Wang et al., 2021). PM1 is a significant constituent of PM2.5 and PM10, and despite being smaller in size, it is more toxic than the regular-sized particles (Wang et al., 2015). The smaller size of PM1 particles allows them to reach deeper into the lung or brain, while the disproportionately large surface area increases the possibility of carrying large amounts of absorbable harmful components (Chen et al., 2017; Shih et al., 2018). In recent years, there has been a growing body of epidemiological research suggesting a potential association between PM1 exposure and health issues. For example, a large cross-sectional study of 33 communities in northeast China found that the risk of hypertension from PM1 tended to be 2% greater than that from PM2.5 (Yang et al., 2019). Another population-based study conducted in China found that a 10 μg/m3 increase in exposure to PM1, in comparison to PM2.5, was linked to a 3% higher risk of hospital admission for total respiratory diseases (Zhang et al., 2020). However, limited evidence is available on the neurological impact of PM1 exposure, and as of now, no air quality standards have been established for PM1. Last but not least, existing studies largely rely on traditional association assessment methods (e.g., Cox proportional hazards regression), which may suffer from residual confounding bias (Robins et al., 2000a). Over the last decade, novel modeling approaches have been developed under a set of causal assumptions. The basic idea underlying the causal inference approaches is to mimic a randomized controlled trial where both the measured confounders are well balanced by certain weighting procedures (Cole and Herán, 2008a). These methods have been applied to observational data in recent studies, although far too little attention has been paid to the causal association between PM exposure and neurological hospitalizations (Qiu et al., 2020).
This study aims to investigate the association between long-term exposure to PMs, with a particular focus on PM1, and neurological hospitalizations. We further examined the variation in effect estimates across the particle size, sociodemographic characteristics and health behaviors. The state-of-the-art causal inference approach for observational data were utilized to investigate the potential causal associations.
2. Methods
2.1. Cohort design and population
The present study includes individuals from over 35 communities randomly selected in Guangzhou area, as a part of the Major Projects of Science Research for the 11th and 12th Five-year Plans of China (Ruan et al., 2019). Based on the availability of outcome data, our study participants were all selected from 35 communities in Guangzhou, randomly selected based on sociodemographic characteristics. In this study, we included participants who were permanent residents, capable of undergoing a physical examination, and willing to sign an informed consent form. Meanwhile, the exclusion criteria involved individuals who were unable to undergo long-term follow-up or under the age of 18. More details about cohort have been described elsewhere (Zhang et al., 2023). More details about cohort have been described elsewhere. Beginning in January 2016, hospitalization reporting became mandatory in the study area. For this study, we focused on individuals who were hospitalized due to neurological disorders that occurred at least one year after enrollment. As a result, we included 36,271 participants recruited between January and December 2015, and followed them up until December 2020. Trained staff conducted in-person interviews, as well as clinical and laboratory examinations to obtain individual information on demographics (e.g., age, sex, ethnicity, body weight status, marital situation, educational attainment, medical insurance), lifestyle variables (e.g., exercise level, smoking habit status, alcohol consumption. The institutional review board (IRB) at Sun Yat-sen University approved this study, and all participants provided with a completed informed consent.
2.2. Outcome definition
We obtained information on the causes of hospitalization by linking records with electronic hospitalization reports from 418 medical institutions, including 71 tertiary medical institutions, 100 secondary medical institutions, 71 primary medical institutions, and 176 other medical institutions, covering the entire Guangzhou area. We identified the causes of hospitalization using the International Classification of Diseases, Tenth Revision (ICD-10) codes. The primary outcome of this study was the overall neurological hospitalizations (G00–99), as well as the major subtypes including ischemic stroke (G45–46), Parkinson disease (G20-G22) and Alzheimer disease (G30-G31). Each participant was followed until the occurrence of outcomes of interest or the end of the study period, whichever came first.
2.3. Data on PMs and environmental exposures
PMs data were obtained from the China High Air Pollutants (CHAP) database, which was estimated by satellite-based spatiotemporal models and space-time extremely randomized trees. The ground observations matched well with the anticipated levels of PM1, PM2.5, and PM10, with cross-validation coefficient of determination (CV-R2 value, a statistical measure used to assess the goodness of fit between a statistical model and the actual observed data) of 0.77, 0.89, and 0.86, and root mean square errors (RMSEs) of 14.60, 10.33, and 24.28 μg/m3, respectively (Wei et al., 2019, 2020, 2021a, 2021b). The relatively lower R-squared value for PM1 may be attributed to the sparse distribution of monitoring stations across mainland China, which might not adequately represent all possible surface types and atmospheric conditions, as well as the limited number of data samples, potentially affecting the model’s training and overall accuracy. However, it was still within an acceptable range. The database has been widely used in previous studies (Ao et al., 2022; Wu et al., 2022). We estimated the annual PM1, PM2.5, and PM10 concentrations during the study period (i.e., 2016–2020) for each participant by linking the pollution data with the cohort data via the residential address. To account for the potential confounding impacts of residential greenness, data on the normalized difference vegetation index (NDVI) were obtained from the Land Processing Distributed Active Archive Center (LPDAAC, https://lpdaac.usgs.gov), and the annual average NDVI within 500 m surrounding an address was computed for each participant (Twohig-Bennett and Jones, 2018).
2.4. Statistical analysis
We employed a marginal structural Cox proportional hazards model with time-dependent exposures to estimate the causal relationship between long-term PM exposure and neurological hospitalizations (Robins et al., 2000b; Bind, 2019). This approach imitates a randomized controlled trial by using inverse probability weights (IPWs) to balance confounding variables, thus, is believed to generate causal insights from a observational data (Cole and Hernán, 2008b). We developed models using distinct different methods to create IPWs including the linear model (LM), generalized estimating equation (GEE), and gradient boosting machine learning (ML) (van der Wal and Geskus, 2011; Chen and Guestrin, 2016). The average absolute correlation (AC) values were used to identify the optimal model with the best performance in the confounding balancing (Fig. S1). More methodological details have been described elsewhere (Wang et al., 2022, 2023). This study employs the LM-IPWS method to explore the impact of PMs on overall and neurological-specific hospitalization rates with each increase of 1-μg/m3. Additionally, we conducted a traditional Cox proportional hazards model with time-dependent exposures for comparison. Directed acyclic graph (DAG) was applied to identify potential covariates, including age, sex, ethnicity (Han or non-Han), marital status (single, married, widowed, or divorced), highest educational attainment (illiterate or semiliterate, elementary school, middle school, high school, or college and above), medical insurance (medical insurance for urban workers, for urban residents, the new rural cooperative medical insurance, or others), smoking status (non-smoker, former smoker, or current smoker), NDVI (500 m), physical activity (low, moderate, or high) (Fig. S2). Multivariate imputation by chained equations (MICE) was performed to impute missing data for confounding variables in our study (Table S1) (van Buuren and Groothuis-Oudshoorn, 2011).
The study employed four different models to analyze the data. Model 0 was a conventional Cox proportional hazards model that did not include any covariate adjustment. Model 1 added age as a covariate to Model 0. Model 2 included additional covariates such as sex, ethnicity, education level, marital status, smoking status, medical insurance, physical activity, and NDVI (500 m). Finally, Model 3 was a refitted version of Model 2, using the marginal structural Cox proportional hazards model.
We included the air pollution exposure as a penalized B-spline function with 3 degrees of freedom in the model to test nonlinearity of the association and visualize the exposure-response relationship between PMs and neurological hospitalizations. The lowest PM exposure level was used as the reference in estimating the association of exposure to PM with neurological hospitalizations. Using the Model 3, we also further stratified our results by sex (male or female), age (<65 years or ≥65 years), marital status (never married or ever married), education level (elementary school and below, middle and high school, college degree or above), and physical activity (low, moderate, or high) of the participants.
2.5. Sensitivity analyses
Sensitivity analyses were performed to evaluate the reliability of our results. First, we assessed the impact of missing data imputation by comparing the estimates based on the dataset before and after imputation. We also considered the potential confounding impact of NDVI by defining exposure using different buffer sizes (250 m, 500 m, and 1000 m) and performed sensitivity analyses. We utilized meta-regression models to assess whether there were differences in estimating between the main model and the sensitivity models. Additionally, to further evaluate the reliability of the findings, we applied three marginal structure Cox models and E-values (VanderWeele and Ding, 2017). All analyses were performed using R version 4.1.3.
3. Results
At baseline, the mean (SD) age was 51 (18) years, and 40.6% (n = 14,727) were men. During 209,676 person-years of follow-up, 723 (2.0%) participants were ever hospitalized due to neurological disorders, including 323 from ischemic stroke, 49 from Alzheimer disease and 41 from Parkinson disease (Table 1). Participants who had been hospitalized for neurological disorders were more likely to be older, ever married, less educated, or having medical insurance for urban workers or residents. Additionally, these participants tended to have no history of smoking and drinking, or engaging in more frequent exercise. The 5-year average concentration of PM1, PM2.5, PM10 was 17.41 μg/m3 (SD = 2.75), 33.73 μg/m3 (SD = 5.23), and 56.09 μg/m3 (SD = 6.52), respectively (Fig. 1).
Table 1.
Description of the study participants and annual average PM concentrations.
| Characteristics | Overall (N = 36,271) | Control group (N = 35,548) | Neurological hospitalization (N = 723) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean (SD)) | 50.93 (17.76) | 50.66 (17.74) | 64.20 (12.76) | <0.01 |
| Body Mass Index (mean (SD)) | 22.60 (2.95) | 22.59 (2.94) | 23.25 (3.15) | <0.01 |
| Gender (male %) | 14,727 (40.60) | 14,419 (40.56) | 308 (42.60) | 0.30 |
| Ethnicity (%) | 0.15 | |||
| Han | 67,147 (98.81) | 65,769 (98.79) | 1378 (99.42) | |
| Minority | 280 (0.77) | 278 (0.78) | 2 (0.28) | |
| Marital status (%) | <0.01 | |||
| Single | 9664 (14.22) | 9573 (14.38) | 91 (6.57) | |
| Married | 55,611 (81.83) | 54,423 (81.75) | 1188 (85.71) | |
| Widowed | 2107 (3.10) | 2019 (3.03) | 88 (6.35) | |
| Divorced | 576 (0.85) | 557 (0.84) | 19 (1.37) | |
| Education level (%) | <0.01 | |||
| Illiterate or semiliterate | 741 (2.04) | 711 (2.00) | 30 (4.15) | |
| Elementary school | 5052 (13.93) | 4893 (13.76) | 159 (21.99) | |
| Middle school | 7545 (20.80) | 7397 (20.81) | 148 (20.47) | |
| High school | 16,310 (44.97) | 15,989 (44.98) | 321 (44.40) | |
| College or above | 6623 (18.26) | 6558 (18.45) | 65 (8.99) | |
| Medical insurance (%) | ||||
| Medical insurance for urban workers | 24,922 (68.71) | 24,405 (68.65) | 517 (71.51) | <0.01 |
| Medical insurance for urban residents | 7308 (20.15) | 7144 (20.10) | 164 (22.68) | |
| The new rural cooperative medical insurance | 677 (1.87) | 673 (1.89) | 4 (0.55) | |
| Others | 3364 (9.27) | 3326 (9.36) | 38 (5.26) | |
| Lifestyle behaviors | ||||
| Physical activity (%) | <0.01 | |||
| Low | 17,357 (47.85) | 17,066 (48.01) | 291 (40.25) | |
| Moderate | 2574 (7.10) | 2510 (7.06) | 64 (8.85) | |
| High | 16,340 (45.05) | 15,972 (44.93) | 368 (50.90) | |
| Smoking status (%) | <0.01 | |||
| Non-smoker | 25,192 (69.45) | 24,698 (69.48) | 494 (68.33) | |
| Ever smoker | 529 (1.46) | 506 (1.42) | 23 (3.18) | |
| Current smoker | 10,550 (29.09) | 10,344 (29.10) | 206 (28.49) | |
| Alcohol consumption (%) | 0.01 | |||
| Never | 25,551 (70.44) | 25,050 (70.47) | 501 (69.29) | |
| Ever | 10,720 (29.56) | 10,498 (29.53) | 222 (30.71) | |
| Land-use variable | ||||
| NDVI (500), mean (SD) | 0.22 (0.04) | 0.22 (0.04) | 0.22 (0.04) | 0.20 |
Abbreviations: PM, particulate matter; SD, stand deviation; NDVI, normalized difference vegetation index.
Fig. 1.
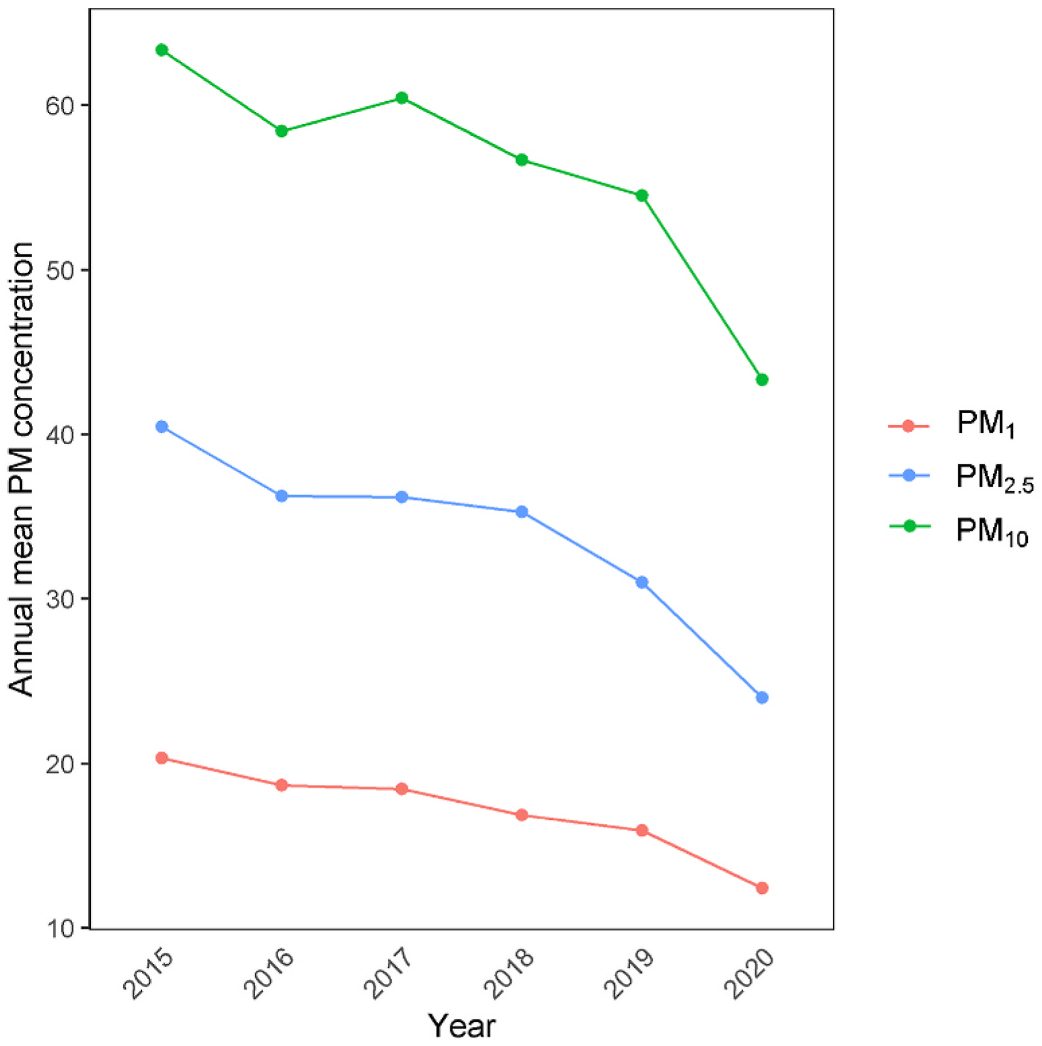
Annual mean concentrations of PM
Abbreviations: PM, particulate matter; PM1, particulate matter with an aerodynamic diameter ≤1 μm; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm.
The associations between long-term PM exposure and risk of hospital admissions due to overall neurological disorders and subtypes are presented in Table 2. The causal inference model revealed that PM1, PM2.5, and PM10 concentrations were associated with increased overall neurological hospitalization risk, with HRs of 1.10 (95% CI 1.04–1.16), 1.09 (95% CI 1.04–1.14) and 1.03 (95% CI 1.00–1.06) following each 1-μg/m3 increment in the PM concentrations (Fig. 2). Interestingly, the estimates tended to increase with the decreasing size of PM particles with the HR for PM1 exposure being 1–7% greater than the estimates for PM2.5, and PM10 exposures. These findings were consistent with the traditional Cox proportional hazards model. We observed a significant non-linear association between PM exposure and the risk of neurological hospitalization with reference to the lowest PMs exposure level (P for nonlinear trend <0.05, Fig. 3). Specifically, as PM1 exposure increased, there was a steady increase in the risk of neurological hospitalization, which then leveled off at higher concentrations. The risk of neurological hospitalization increased steadily up to 38 μg/m3 and 58 μg/m3 for PM2.5 or PM10 exposures but attenuated at higher exposure levels.
Table 2.
Associations between 1-μg/m3 increase in long-term PM exposure and hospital admission for neurological disorders.
| PM1 | PM2.5 | PM10 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Overall neurological disorders | 1.10 (1.04–1.16) | <0.01 | 1.09 (1.04–1.14) | <0.01 | 1.03 (1.00–1.06) | 0.04 |
| Parkinson disease | 1.12 (0.91–1.39) | 0.29 | 1.09 (0.94–1.27) | 0.26 | 1.01 (0.93–1.10) | 0.81 |
| Alzheimer disease | 1.20 (0.89–1.62) | 0.23 | 1.11 (0.88–1.40) | 0.39 | 1.07 (0.93–1.24) | 0.35 |
| Ischemic stroke | 1.15 (1.06–1.25) | <0.01 | 1.13 (1.04–1.23) | <0.01 | 1.05 (1.00–1.09) | 0.04 |
Abbreviations: PM, particulate matter; PM1, particulate matter with an aerodynamic diameter ≤1 μm; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm; HR, hazard ratios; CI, confidence interval.
Fig. 2.
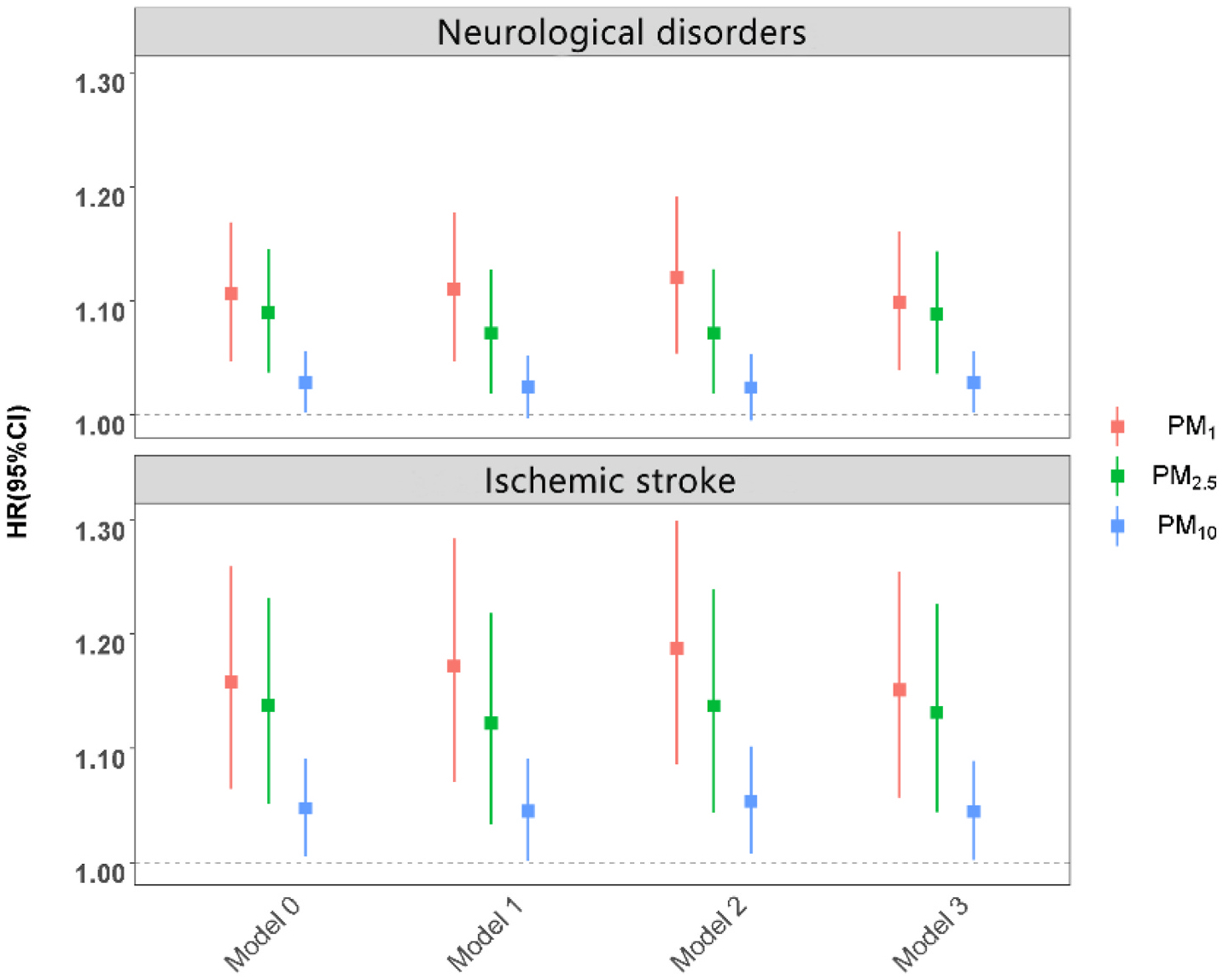
Association between 1-μg/m3 increase in long-term PM exposure and neurological disorders hospitalization under conventional and causal inference method Note: Model 0 as the conventional Cox proportional hazards model with no covariate adjustment. Model 1 as the model additionally adjusted for age based on Model 0. Model 2 as the model additionally adjusted for gender, ethnicity, education level, marital status, smoking status, medical insurance, physical activity, and NDVI (500 m) based on Model 1. Model 3 as the marginal structural Cox proportional hazards model based on model 2.
Abbreviations: HR, hazard ratio; CI, confidence interval; PM, particulate matter; PM1, particulate matter with an aerodynamic diameter ≤1 μm; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm.
Fig. 3.
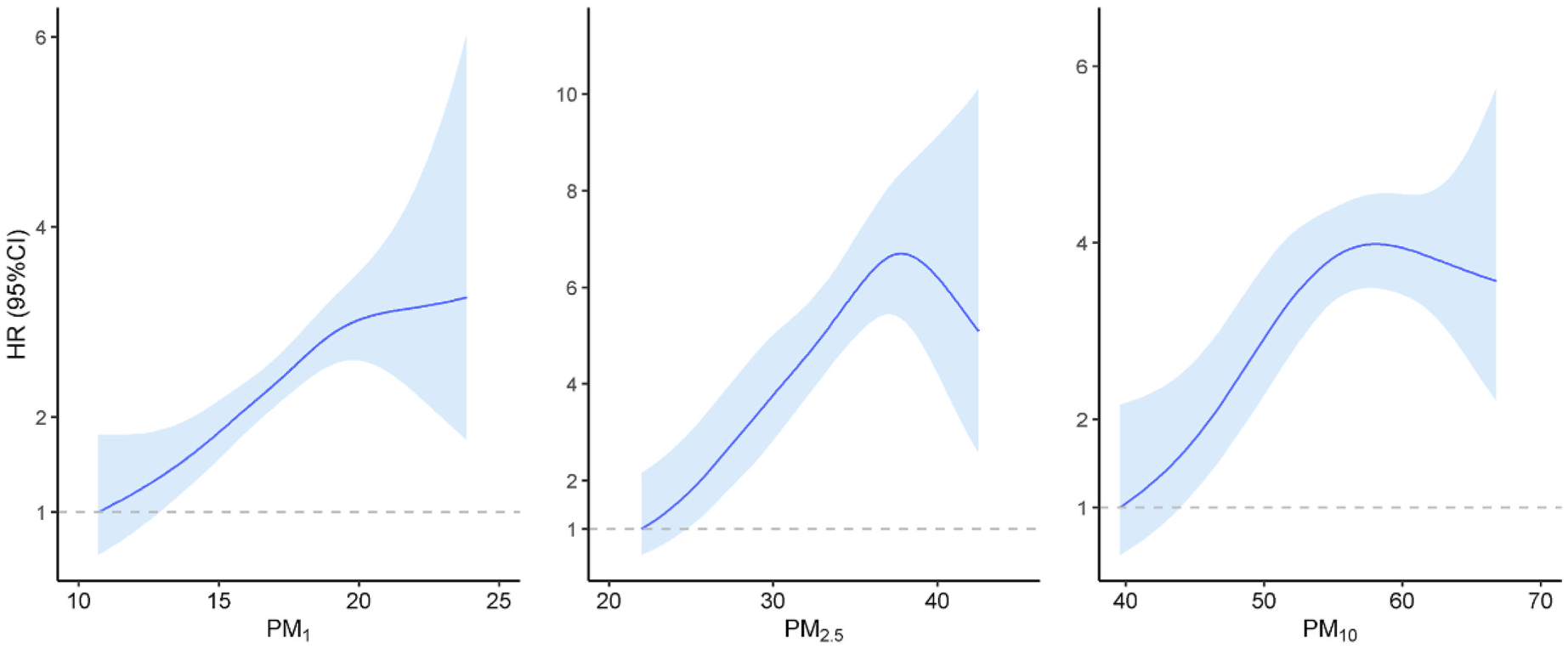
Exposure-Response Association of long-term exposure to PM with neurological disorders hospitalization
The solid blue lines with shaded regions indicate HRs of neurological disorders hospitalization and their 95% CIs, respectively. Abbreviations: HR, hazard ratio; CI, confidence interval; PM, particulate matter; PM1, particulate matter with an aerodynamic diameter ≤1 μm; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm.
In subgroup analysis by neurological subtype (Table 2), we also observed an increased risk of hospitalization following PM exposure, with HRs ranging from 1.05 to 1.15. However, the estimates generally were not significant, probably due to the sample size limit. Nevertheless, we observed that the risk of hospitalization for ischemic stroke increased with the average annual PM concentration, with PM1 being the most harmful (HR: 1.15, 95% CI 1.06–1.25), followed by PM2.5 (1.13, 95% CI 1.04–1.23), then, PM10 (1.05, 95% CI 1.00–1.09).
Table 3 presents the HRs of causal link between long-term PM exposure and neurological hospitalization stratified by demographics and lifestyle factors. We observed that results were generally consistent across different subgroups. However, we found that compared with the never-married participants (HRs ranging 1.03–1.25 across PMs), those who were ever married (i.e., married, widowed, divorced) generally had a reduced hazard for neurological hospitalization following long-term PM exposures (HR ranging 1.03–1.10), although the inter-group difference was only statistically significant for PM10. We also observed that female participants tended to be more vulnerable when exposed to PM (Table 3). The HR estimates were generally statistically significant among the females (HRs ranging 1.05–1.13 across different PMs, with P < 0.05), while not significant among the males (HRs ranging 1.00–1.07, with the 95% CIs overlapping with the null).
Table 3.
The modification effect of basic characteristics and lifestyle factors on the association between PM concentration and neurological disorders hospitalization.
| Effect modifiers | PM1 | PM2.5 | PM10 | |||
|---|---|---|---|---|---|---|
| Gender | HR (95% CI) | P interaction | HR (95% CI) | P interaction | HR (95% CI) | P interaction |
| Male | 1.06 (0.97–1.17) | 0.78 | 1.07 (0.99–1.16) | 0.39 | 1.00 (0.96–1.04) | 0.82 |
| Female | 1.13 (1.05–1.21) | 1.10 (1.03–1.17) | 1.05 (1.01 −1.09) | |||
| Age | ||||||
| < 65 years | 1.12 (1.04–1.21) | 0.56 | 1.12 (1.05–1.20) | 0.85 | 1.05 (1.02–1.09) | 0.81 |
| ≥65 years | 1.10 (1.01–1.20) | 1.05 (0.98–1.14) | 1.01 (0.97–1.05) | |||
| Marital status | ||||||
| Never married | 1.16 (0.96–1.40) | 0.22 | 1.25 (1.01 −1.56) | 0.09 | 1.03 (0.95–1.13) | 0.03 |
| Ever married | 1.10 (1.04–1.17) | 1.07 (1.02–1.13) | 1.03 (1.00–1.06) | |||
| Education level | ||||||
| Elementary school and below | 1.17 (1.03–1.34) | 0.71 | 1.08 (0.97–1.20) | 0.37 | 1.01 (0.96–1.07) | 0.42 |
| Middle and high school | 1.04 (0.97 −1.11) | 1.10 (1.03–1.17) | 1.03 (1.00–1.07) | |||
| College degree or above | 1.02 (0.88–1.19) | 0.97 (0.86–1.09) | 0.98 (0.93–1.05) | |||
| Physical activity | ||||||
| Low | 1.09 (0.99–1.21) | 0.70 | 1.04 (0.97–1.12) | 0.83 | 1.01 (0.97–1.05) | 0.81 |
| Moderate | 1.17 (1.03–1.32) | 1.14 (1.00–1.30) | 1.08 (1.00–1.16) | |||
| High | 1.12 (1.03–1.21) | 1.13 (1.04–1.22) | 1.04 (1.00–1.08) |
Abbreviations: PM, particulate matter; PM1, particulate matter with an aerodynamic diameter ≤1 μm; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm; HR, hazard ratios; CI, confidence interval.
We found that the associations did not significantly change across different buffer sizes for NDVI. The estimated HRs of neurological hospitalizations were also similar between the entire population dataset and the complete case dataset (all P-values >0.05) (Table S2). Furthermore, the association estimates also remained unchanged using three different weighting approaches in establishing the causal inference model, as described in Table S3. The E values shown in Table S4 suggested that the conclusions were less likely to be overturned by the potential unmeasured confounding bias.
4. Discussion
For this study, we identified an adverse association between PM exposure and neurological disorders in this cohort study from 2016 through 2020, which included over 36,000 adults. We observed that this association increased with decreasing size of particles, with the greatest risk observed for PM1 exposure. Our results further indicated that the risks were generally consistent across subgroups, however, participants that were never-married tended to be more vulnerable to the long-term neurological impact of PMs exposures.
According to our estimates, there was a 2.90%–9.90% increased rate of neurological hospitalizations following each 1-μg/m3 increment in the exposed concentrations of PMs. While studies on the prolonged impacts of PM exposure on neurological disorders, particularly based on large cohorts, remains quite limited in heavily polluted area, some existing findings on short-term PM exposure provide some clues. For example, a study utilizing time-series and aggregated data has revealed that an increase of 10 μg/m3 in daily exposure to PM2.5 is associated with a 2% rise in hospital admissions for neurological disorders (Gu et al., 2020). The long-term PM exposure may share similar biological mechanisms with the short-term exposure regarding the neurological toxicity. Mechanistic studies suggest that PM may affect the neurological system by direct absorption through olfactory tract or blood-brain barrier (Oberdörster et al., 2004; Wang et al., 2018). Animal and laboratory studies have suggested that the biological pathways might include mitochondrial damage, NLRP3 inflammasome activation, microglial activation, blood-brain barrier change, and neuronal damage (Block and Calderón-Garcidueñas, 2009; Wang et al., 2018). In addition, we assessed the exposure-response curve for PM-related hospitalizations due to neurological disorders. Our findings suggested that there was no identifiable safe threshold in the exposure range of 10–25 μg/m3 for PM1 and neurological hospitalization. The risk of neurological hospitalization increased steadily for PM2.5 or PM10 exposures but attenuated at higher exposure levels, which was also consistent with the existing evidence (Gu et al., 2020; Shim et al., 2023). Thus, protective measures are needed to mitigate the adverse neurological impact of PM exposures, particularly in highly-polluted areas.
Our results demonstrate a potential greater neurological toxicity as the particle diameter decrease. Specifically, the hospitalization risk for overall neurological disorders with PM1 was higher than PM2.5 and PM10 (7.40%, 7.10% and 3.30% for each 1-μg/m3). This is consistent with previous studies that have observed that smaller particles to be more hazardous to human health (Lin et al., 2016). For example, a 2-year cohort study on the admission risk for total respiratory diseases in China, wherein the estimate for PM1 was 3% larger than that for PM2.5. The size fraction of ambient PM is a significant component in determining its toxicity (Kelly and Fussell, 2012; Zhang et al., 2020). PM1 particles, with a disproportionally greater surface area than the regular particles, tend to penetrate more deeply into the lung and remain there longer, subsequently leading to a greater inflammatory response (Brown et al., 2001). Therefore, in addition to the health impacts of regular sized particles, we should also focus on the negative health impacts of PM1.
Furthermore, although the estimates for subtypes were generally not statistically significant, probably due to limited sample size, we still found a significant link between PM exposure and the hospital admission due to ischemic stroke. Similar findings were reported in a nationwide case-crossover study in China, which found that every 10 μg/m3 increase in PM1 exposure was linked to an increase of 0.6% in hospital admissions for transient ischemic attack (Liu et al., 2022). Previous studies also suggested that PM exposure may increase the risk of ischemic stroke through disturbance of the regulation of blood pressure and blood lipid dynamics, and subsequently decrease the blood vessel elasticity (Cascio et al., 2015; Yang et al., 2019).
Stratified analysis indicated that the never-married adults were likely to be more vulnerable than their ever-married counterparts when chronically exposed to PMs. This finding was understandable as marital status is considered to be a surrogate of social support, which has been linked to reducing the risk of neuropathological damage by increasing daily social interaction and elevating cognitive reserve (Perry et al., 2022). For instance, a meta-analysis reported lifelong single people had a higher vulnerability to dementia compared to those who were married (Sommerlad et al., 2018). Therefore, social engagement may be considered a modifiable risk factor among individuals never married.
Additionally, we observed that female participants generally had a greater vulnerability to the neurological impact of long-term PM exposure. Although previous studies have documented that males tended to have a higher prevalence of neurological disorders, emerging findings suggested that the severity of these conditions may be greater in females (Hanamsagar and Bilbo, 2016). For instance, a study conducted in South Korea found that women were more likely to experience cognitive decline with every 10 μg/m3 increase in PM10 compared to men (Kim et al., 2019). Findings documented that compared with males, females have been shown to have more microglia, which played a crucial role in modulation of inflammatory dysregulation (Schwarz et al., 2012). However, further research is needed to evaluate this hypothesis.
As far as we know, this study has been one of the few to estimate the potential causal relationship between long-term exposure to PM1 and regular-sized particulate exposure with neurological hospitalizations. In this study, we recruited participants from a variety of socioeconomic background in China, which enhances the generalizability of our results. Furthermore, we used advanced causal inference approaches combined with time-varying exposures, thereby providing more robust and reliable conclusions. Nevertheless, there are some limitations with our study. First, since participants with certain neurological symptoms are usually not required inpatient treatment, some patients with neurological disorders may not be captured in the hospital database. This could lead to an underestimation of the true impact of PM exposure on health in our results. Second, the study used grid-scale PM to simulate pollutant exposure, which means that residents from neighboring areas may be matched with the same PM concentration value, leading to exposure misclassification. However, this measurement error may be Berkson and classical error, causing bias toward a null (Zhang et al., 2018). Third, information on individual-level socioeconomic status was not collected in the baseline survey, however, similar variables (e.g., education, medical insurance) were included as surrogates. Furthermore, our findings were not significantly affected by residual confounding bias, as indicated by the E-values.
5. Conclusions
Our study, which followed a large group of participants over time, indicated that there may be a causal association between long-term exposure to PMs and hospital admissions for neurological disorders. PM1 appears to have a slightly stronger effect on neurological hospitalization, with a 1% and 7% higher impact compared to PM2.5 and PM10, respectively. In addition, the effect associated with PM exposure to ischemic stroke was stronger than other neurological subtypes. Females or unmarried individuals appeared to be more susceptible to neurological hospitalization due to PM-related factors compared to their counterparts, respectively, representing potential vulnerable subgroups. Therefore, it is crucial that environmental policies focus on reducing PM exposure levels, particularly PM1, and protecting vulnerable populations from the harmful impacts of pollution.
Supplementary Material
HIGHLIGHTS.
Long-term exposure to PMs increased the risk of neurological hospitalizations.
The hazard ratio for PM1 tends to be greater on neurological hospitalization.
Long-term exposure to PMs enhanced the risk of ischemic stroke hospitalization.
Unmarried residents might be more vulnerable than their counterparts.
Causal inference models with time-varying exposure minimized confounding bias.
Acknowledgment
This work was supported by National Natural Science Foundation of China (82204162, 81973150), Guangdong Provincial Pearl River Talents Program (0920220207), Basic and Applied Basic Research Foundation of Guangdong Province (2022A1515010823, 2022A1515110263), Guangzhou Municipal Science and Technology Bureau (2023A04J2072), and Fundamental Research Funds for the Central Universities, Sun Yat-sen University (23qnpy108). Professor Yuantao Hao gratefully acknowledges the support of KC Wong Education Foundation.
Footnotes
Declaration of competing interest
All authors declare that they have no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2023.140397.
Data availability
The data that has been used is confidential.
References
- Ao L, Zhou J, Han M, Li H, Li Y, Pan Y, Chen J, Xie X, Jiang Y, Wei J, Chen G, Li S, Guo Y, Hong F, Li Z, Xiao X, Zhao X, 2022. The joint effects of physical activity and air pollution on type 2 diabetes in older adults. BMC Geriatr 22, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, 2019. Causal modeling in environmental health. Annu. Rev. Publ. Health 40, 23–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L, 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K, 2001. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol 175, 191–199. [DOI] [PubMed] [Google Scholar]
- Cascio WE, Gilmour MI, Peden DB, 2015. Ambient air pollution and increases in blood pressure: role for biological constituents of particulate matter. Hypertension 66, 469–471. [DOI] [PubMed] [Google Scholar]
- Chen G, Li S, Zhang Y, Zhang W, Li D, Wei X, He Y, Bell ML, Williams G, Marks GB, Jalaludin B, Abramson MJ, Guo Y, 2017. Effects of ambient PM(1) air pollution on daily emergency hospital visits in China: an epidemiological study. Lancet Planet. Health 1, e221–e229. [DOI] [PubMed] [Google Scholar]
- Chen T, Guestrin C, 2016. XGBoost: A Scalable Tree Boosting System. Association for Computing Machinery, pp. 785–794. [Google Scholar]
- Cole SR, Hernán MA, 2008a. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol 168, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Hernán MA, 2008b. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol 168, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators, G.C.o.D., 2018. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators, G.R.F., 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission, N.H., 2022. China’s Health Statistical Yearbook 2022 China Union Medical College Press, Beijing. [Google Scholar]
- Fu P, Guo X, Cheung FMH, Yung KKL, 2019. The association between PM2.5 exposure and neurological disorders: a systematic review and meta-analysis. Sci. Total Environ 655, 1240–1248. [DOI] [PubMed] [Google Scholar]
- Gu J, Shi Y, Zhu Y, Chen N, Wang H, Zhang Z, Chen T, 2020. Ambient air pollution and cause-specific risk of hospital admission in China: a nationwide time-series study. PLoS Med 17, e1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Bilbo SD, 2016. Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol 160, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ, Fussell JC, 2012. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ 60, 504–526. [Google Scholar]
- Kim H, Noh J, Noh Y, Oh SS, Koh SB, Kim C, 2019. Gender difference in the effects of outdoor air pollution on cognitive function among elderly in Korea. Front. Public Health 7, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Tao J, Du Y, Liu T, Qian Z, Tian L, Di Q, Rutherford S, Guo L, Zeng W, Xiao J, Li X, He Z, Xu Y, Ma W, 2016. Particle size and chemical constituents of ambient particulate pollution associated with cardiovascular mortality in Guangzhou, China. Environ. Pollut 208, 758–766. [DOI] [PubMed] [Google Scholar]
- Liu T, Jiang Y, Hu J, Li Z, Guo Y, Li X, Xiao J, Yuan L, He G, Zeng W, Kan H, Rong Z, Chen G, Yang J, Wang Y, Ma W, 2022. Association of ambient PM1 with hospital admission and recurrence of stroke in China. Sci. Total Environ 828, 154131. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C, 2004. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol 16, 437–445. [DOI] [PubMed] [Google Scholar]
- Organization, W.H., 2019. Concentrations of fine particulate matter (PM2.5) Access January 17, 2023. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/concentrations-of-fine-particulate-matter-(pm2-5.
- Perry BL, McConnell WR, Coleman ME, Roth AR, Peng S, Apostolova LG, 2022. Why the cognitive “fountain of youth” may be upstream: pathways to dementia risk and resilience through social connectedness. Alzheimers Dement 18, 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Wei Y, Wang Y, Di Q, Sofer T, Awad YA, Schwartz J, 2020. Inverse probability weighted distributed lag effects of short-term exposure to PM2.5 and ozone on CVD hospitalizations in New England Medicare participants - exploring the causal effects. Environ. Res 182, 109095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Hernán MA, Brumback B, 2000a. Marginal structural models and causal inference in epidemiology. Epidemiology 11, 550–560. [DOI] [PubMed] [Google Scholar]
- Robins JM, Hernán MÁ, Brumback B, 2000b. Marginal structural models and causal inference in epidemiology. Epidemiology 11. [DOI] [PubMed] [Google Scholar]
- Ruan B, Yu Z, Yang S, Xu K, Ren J, Yao J, Wu N, Yu C, Deng M, Xie T, Chen P, Wang C, Li Y, Zhao Y, Sheng J, Hou Y, Wu Z, Jin S, Chen Y, Li M, Zhu F, Tang H, Hao Y, Pang X, Lu L, Yang W, Yuan Z, Xu A, Li Z, Ni M, Yan Y, Zhong Q, Zhou L, Li G, Meng Q, Hu J, Zhou H, Zhang G, Li D, Jiang W, Li Q, Wu P, Xing R, Gu J, Gao D, Li L, 2019. Establishment and development of national community-based collaborative innovation demonstration areas to achieve the control target of hepatitis B in China. BMC Infect. Dis 19, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD, 2012. Sex differences in microglial colonization of the developing rat brain. J. Neurochem 120, 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C-H, Chen J-K, Kuo L-W, Cho K-H, Hsiao T-C, Lin Z-W, Lin Y-S, Kang J-H, Lo Y-C, Chuang K-J, Cheng T-J, Chuang H-C, 2018. Chronic pulmonary exposure to traffic-related fine particulate matter causes brain impairment in adult rats. Part. Fibre Toxicol 15, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JI, Byun G, Lee JT, 2023. Long-term exposure to particulate matter and risk of Alzheimer’s disease and vascular dementia in Korea: a national population-based Cohort Study. Environ. Health 22, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerlad A, Ruegger J, Singh-Manoux A, Lewis G, Livingston G, 2018. Marriage and risk of dementia: systematic review and meta-analysis of observational studies. J. Neurol. Neurosurg. Psychiatry 89, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twohig-Bennett C, Jones A, 2018. The health benefits of the great outdoors: a systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res 166, 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, 2011. Mice: multivariate imputation by chained equations in R. J. Stat. Software 45, 1–67. [Google Scholar]
- van der Wal WM, Geskus RB, 2011. Ipw: an R package for inverse probability weighting. J. Stat. Software 43, 1–23. [Google Scholar]
- VanderWeele TJ, Ding P, 2017. Sensitivity analysis in observational research: introducing the E-value. Ann. Intern. Med 167, 268–274. [DOI] [PubMed] [Google Scholar]
- Wang BR, Shi JQ, Ge NN, Ou Z, Tian YY, Jiang T, Zhou JS, Xu J, Zhang YD, 2018. PM2.5 exposure aggravates oligomeric amyloid beta-induced neuronal injury and promotes NLRP3 inflammasome activation in an in vitro model of Alzheimer’s disease. J. Neuroinflammation 15, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Xu Y, Huang L, Wang K, Shen H, Li Z, 2021. Pollution characteristics and toxic effects of PM(1.0) and PM(2.5) in Harbin, China. Environ. Sci. Pollut. Res. Int 28, 13229–13242. [DOI] [PubMed] [Google Scholar]
- Wang Y, Du Z, Zhang Y, Chen S, Lin S, Hopke PK, Rich DQ, Zhang K, Romeiko XX, Deng X, Qu Y, Liu Y, Lin Z, Zhu S, Zhang W, Hao Y, 2022. Long-term exposure to particulate matter and COPD mortality: insights from causal inference methods based on a large population cohort in southern China. Sci. Total Environ 863, 160808. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wei J, Zhang Y, Guo T, Chen S, Wu W, Chen S, Li Z, Qu Y, Xiao J, Deng X, Liu Y, Du Z, Zhang W, Hao Y, 2023. Estimating causal links of long-term exposure to particulate matters with all-cause mortality in South China. Environ. Int 171, 107726. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Sun J, Zhang X, Che H, Li Y, 2015. Spatial and temporal variations of the concentrations of PM 10, PM 2.5 and PM 1 in China. Atmos. Chem. Phys 15, 13585–13598. [Google Scholar]
- Wei J, Li Z, Cribb M, Huang W, Xue W, Sun L, Guo J, Peng Y, Li J, Lyapustin A, Liu L, Wu H, Song Y, 2020. Improved 1 km resolution PM2.5 estimates across China using enhanced space-time extremely randomized trees. Atmos. Chem. Phys 20, 3273–3289. [Google Scholar]
- Wei J, Li Z, Guo J, Sun L, Huang W, Xue W, Fan T, Cribb M, 2019. Satellite-derived 1-km-Resolution PM1 concentrations from 2014 to 2018 across China. Environ. Sci. Technol 53, 13265–13274. [DOI] [PubMed] [Google Scholar]
- Wei J, Li Z, Lyapustin A, Sun L, Peng Y, Xue W, Su T, Cribb M, 2021a. Reconstructing 1-km-resolution high-quality PM2.5 data records from 2000 to 2018 in China: spatiotemporal variations and policy implications. Rem. Sens. Environ 252, 112136. [Google Scholar]
- Wei J, Li Z, Xue W, Sun L, Fan T, Liu L, Su T, Cribb M, 2021b. The ChinaHighPM10 dataset: generation, validation, and spatiotemporal variations from 2015 to 2019 across China. Environ. Int 146, 106290. [DOI] [PubMed] [Google Scholar]
- Wu H, Lu Z, Wei J, Zhang B, Liu X, Zhao M, Liu W, Guo X, Xi B, 2022. Effects of the COVID-19 lockdown on air pollutant levels and associated reductions in ischemic stroke incidence in shandong Province, China. Front. Public Health 10, 876615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MX, Zhu YF, Chang HF, Liang Y, 2016. Nanoceria restrains PM2.5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf 2 deficient mice. Free Radic. Biol. Med 99, 259–272. [DOI] [PubMed] [Google Scholar]
- Yang B-Y, Guo Y, Bloom MS, Xiao X, Qian Z, Liu E, Howard SW, Zhao T, Wang S-Q, Li S, Chen D-H, Ma H, Yim SH-L, Liu K-K, Zeng X-W, Hu L-W, Liu R-Q, Feng D, Yang M, Xu S-L, Dong G-H, 2019. Ambient PM1 air pollution, blood pressure, and hypertension: insights from the 33 Communities Chinese Health Study. Environ. Res 170, 252–259. [DOI] [PubMed] [Google Scholar]
- Zhang W, Lin S, Hopke PK, Thurston SW, van Wijngaarden E, Croft D, Squizzato S, Masiol M, Rich DQ, 2018. Triggering of cardiovascular hospital admissions by fine particle concentrations in New York state: before, during, and after implementation of multiple environmental policies and a recession. Environ. Pollut 242, 1404–1416. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ding Z, Xiang Q, Wang W, Huang L, Mao F, 2020. Short-term effects of ambient PM1 and PM2.5 air pollution on hospital admission for respiratory diseases: case-crossover evidence from Shenzhen, China. Int. J. Hyg Environ. Health 224, 113418. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Du Z, Chen S, Qu Y, Hao C, Ju X, Lin Z, Wu W, Xiao J, Chen X, Lin X, Chen S, Chen L, Jiang J, Zhang W, Hao Y, 2023. Potential causal links between long-term ambient particulate matter exposure and cardiovascular mortality: new evidence from a large community-based cohort in South China. Ecotoxicol. Environ. Saf 254, 114730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.


