Abstract
Hypoxia encountered at high altitude, blood loss and erythroleukemia instigate stress erythropoiesis, which involves glucocorticoid-induced proliferation of erythroid progenitors (ebls). The tumour suppressor p53 stimulates hematopoietic cell maturation and antagonizes glucocorticoid receptor (GR) activity in hypoxia, suggesting that it may inhibit stress erythropoiesis. We report that mouse fetal liver ebls that lack p53 proliferate better than wild-type cells in the presence of the GR agonist dexamethasone. An important mediator of GR-induced ebl self-renewal, the c-myb gene, is induced to higher levels in p53–/– ebls by dexamethasone. The stress response to anemia is faster in the spleens of p53–/– mice, as shown by the higher levels of colony forming units erythroids and the increase in the CD34/c-kit double positive population. Our results show that p53 antagonizes GR-mediated ebl expansion and demonstrate for the first time that p53–GR cross-talk is important in a physiological process in vivo: stress erythropoiesis.
Introduction
Physiological stresses to the erythroid system, such as blood loss, hemolysis, erythroleukemia and oxygen deprivation at high altitudes, induce rapid proliferation of erythroid progenitors that is driven by erythropoietin (Epo) and glucocorticoids. Glucocorticoids control the activity of the glucocorticoid receptor (GR), a member of the steroid receptor superfamily. They play an essential role in maintaining basal- and stress-related homeostasis and, in particular, stimulate erythropoiesis. They enhance the formation of murine erythroid colonies (Golde et al., 1976), increase the proliferation of erythroid cells in the presence of limiting amounts of Epo (Udupa et al., 1986) and restore normal erythropoiesis in humans afflicted by certain anemias (Zito and Lynch, 1977; Liang et al., 1994). Pathological changes of glucocorticoid levels affect erythropoiesis. Insufficient corticosteroid production, in Morbus Addison disease, is associated with anemia, whereas elevated glucocorticoid levels, in Cushing’s syndrome patients, is connected with increased red blood cell (RBC) count, hemoglobin and hematocrit values. Glucocorticoids cooperate with the activated receptors for Epo (EpoR) and stem cell factor (c-kit) to induce long-term proliferation and differentiation arrest of normal erythroblasts in vitro (Wessely et al., 1997a, 1999; Reichardt et al., 1998; von Lindern et al., 1999). This requires the DNA-binding and transactivation capacities of the GR (Wessely et al., 1997b; Reichardt et al., 1998).
The tumour suppressor p53 is frequently inactivated in human cancers, including leukemia and lymphoma (Hainaut and Hollstein, 2000). Amongst its different functions, p53 may be involved in hematopoietic re-population. Numerous in vitro studies indicate that p53 is involved in proliferation, differentiation and apoptosis of hematopoietic cells (Kastan et al., 1991; Lotem and Sachs, 1993; Prokocimer and Rotter, 1994). p53 and glucocorticoids are key mediators of stress responses with opposing activities that repress each other’s functions in normal cells, notably in responses to hypoxia (Sengupta and Wasylyk, 2001). These observations raised the possibility that p53 may antagonize the activity of glucocorticoids in stress erythropoiesis. Our results show that antagonism between p53 and GR helps to maintain the balance between proliferation and differentiation of erythroid cells in vivo. Specific inhibitors of p53–GR interactions may be useful to replenish the RBC pool, for example in anemic patients.
RESULTS AND DISCUSSION
Increased proliferation of p53–/– erythroblasts (ebls)
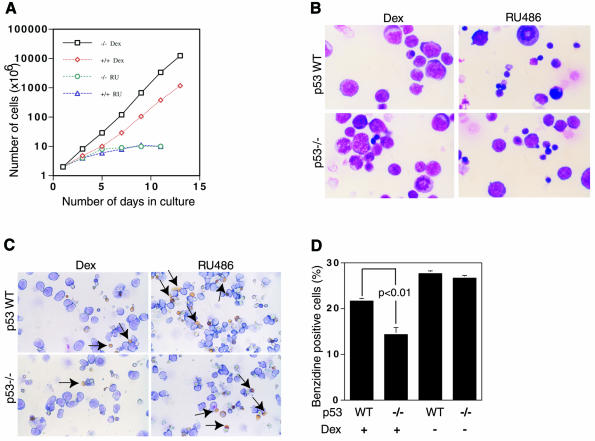
We compared the proliferative capacity of wild-type and p53–/– ebls from mouse fetal livers at E14.5 d.p.c. (after the onset of definitive erythropoiesis). Fetal liver cell suspensions were cultured in StemPro-34 medium supplemented with Epo, SCF and IGF. In the presence of Dex, the cells divided actively for at least 14 days, with a doubling time of about 24 h (Figure 1A). The p53–/– cells proliferated better than their wild-type counterparts from the earliest times in culture, and after 14 days the total number of cells was at least 10-fold higher (similar results were obtained in five independent experiments). In the presence of the GR antagonist RU486, p53–/– and wild-type cells grew much more slowly and at similar rates. These results show that p53 decreases the velocity (doubling time) of GR-dependent proliferation of ebls in fetal liver cultures.
Fig. 1. Proliferation of fetal liver cell erythroblasts in culture. (A) Cells were counted daily and cumulative cell numbers determined. (B and C) Aliquots on day 6 were centrifuged on slides and stained with May Grunwald (B) or neutral benzidine (C) and counter-stained with Giemsa. The number of benzidine positive cells was counted blindly. (D) The proportion of benzidine positive cells (yellow indicated with arrows) was significantly lower in the p53–/– than in the wild-type cultures (p <0.01).
Spontaneous differentiation during renewal was analysed after 12 days in culture by staining with May Grunwald for morphology (Figure 1B) and neutral benzidine for hemoglobin (Figure 1C). In the presence of Dex, p53–/– cultures contained a larger proportion of proliferating immature cells with morphological features of ebls (basophilic cytoplasm and large central nucleus) and significantly fewer (p <0.01) hemoglobin containing cells (Figure 1B–D). In the absence of Dex or in the presence of the antagonist RU486, p53–/– and wild-type cultures were very similar, with fewer ebls and more hemoglobinized reticulocytes/erythrocytes (Figure 1B–D; data not shown).
Spontaneous differentiation during renewal was also studied by FACS analysis of immature (c-kit, CD117) and late erythroid (Ter119) cell surface markers (Table I). In the presence of Dex at day 10, there was a larger proportion of immature erythroid cells in the p53–/– cultures (58 versus 43%) and fewer late erythroid cells (23 versus 29%). Similarly, at day 15, there were more immature erythroid cells in the p53–/– cultures (61 versus 34%) and fewer late erythroid cells (30 versus 40%). These cultures did not express the macrophage and granulocyte markers Mac-1 and Gr-1 (data not shown). In the absence of Dex, there were fewer immature erythroid and more Ter119 positive late erythroid cells (data not shown), as expected from the lack of Dex stimulation of ebl proliferation. These results show that the loss of p53 favours the expansion of immature erythroblasts at the expense of the more differentiated cells. Hence, p53 is important for the maturation of hematopoietic cells.
Table I. FACS analysis of fetal liver cell cultures (%) in the presence of Dex.
| 10 days |
15 days |
|||
|---|---|---|---|---|
| Cell surface markers | WT | KO | WT | KO |
| Early hematopoietic c-kit (CD117) | 43 | 58 | 34 | 61 |
| (±2.0) | (±3.5) | (±3.5) | (±3.6) | |
| Late erythroid Ter119 | 29 | 23 | 40 | 30 |
| (±2.0) | (±3.5) | (±2.4) | (±2.5) | |
Fetal liver cells from day 10 and 15 cultures were immunostained with fluorochrome-labelled antibodies against c-kit and Ter119. Three independent cultures were analysed by FACS. The results are expressed as the mean (±SD) of the three separate analyses.
Effect of p53 on expression of genes involved in erythroid cell proliferation and differentiation
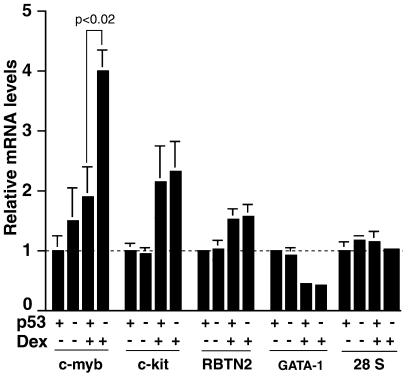
A number of genes have been identified that have roles in erythroid cell differentiation and proliferation, including c-myb, c-kit, RBTN2 and GATA-1. c-myb codes for a transcription factor that regulates the proliferation and differentiation of various hematopoietic cell lineages and is required for proliferation of erythroid progenitors (Introna et al., 1994; Wessely et al., 1997a; Weston, 1998; von Lindern et al., 1999). The c-kit receptor ligand SCF stimulates the proliferation of erythroid progenitors (Muta et al., 1995; Wessely et al., 1997b). c-myb and c-kit are downregulated upon differentiation of erythroid progenitors. The RBTN2 transcription factor is selectively expressed in immature erythroid cells and is required for progression past the proerythroblast stage (Warren et al., 1994). The GATA-1 transcription factor is required for terminal differentiation (Pevny et al., 1995; Fujiwara et al., 1996). Dex activation of GR in cultured erythroid progenitors enhances the expression of c-myb, c-kit and RBTN2 and inhibits the expression of GATA-1 (von Lindern et al., 1999). We tested whether p53 affects Dex induction of the expression of these genes, using real-time RT–PCR. Day 10 cultures of fetal liver cells were incubated for 48 h in medium containing growth factors but lacking steroid hormone to withdraw them from the inducer (Wessely et al., 1997a; von Lindern et al., 1999). Dex was then added and relative mRNA levels were measured after 24 h. As expected, in wild-type cultures (p53+/+), Dex induced the expression of c-myb, c-kit and, to a lesser extent, RBTN2 and decreased GATA-1 (Figure 2). In p53–/– cultures, Dex induced c-myb to significantly higher levels (p <0.02), whereas c-kit, RBTN2 and GATA-1 were not significantly different from the p53+/+ cultures. The control, 28S RNA, did not vary. Interestingly, c-myb expression has been shown previously to be repressed by p53, in one of three cell lines studied (Zhao et al., 2000). Our results show that c-myb, a key regulator of ebl proliferation, could be an important intermediary in a p53–GR-mediated decision between proliferation and differentiation.
Fig. 2. Relative mRNA levels. Day 10 cultures were withdrawn from Dex for 48 h, Dex was added and 24 h later total RNA was extracted. The levels of c-myb, c-kit, RBTN2, GATA-1 and 28S RNAs were measured by real-time RT–PCR and plotted relative to the wild-type samples (p53 +) in the absence of Dex (Dex –). Each column represents the average of three amplifications (error bars show the standard deviation). The relative levels of c-myb mRNA are significantly higher in the p53–/– compared to the wild-type cultures (p <0.02). Comparable results were obtained in a replicate experiment using a different set of mice.
Effect of p53 on ebl upregulation in spleen in response to anemia
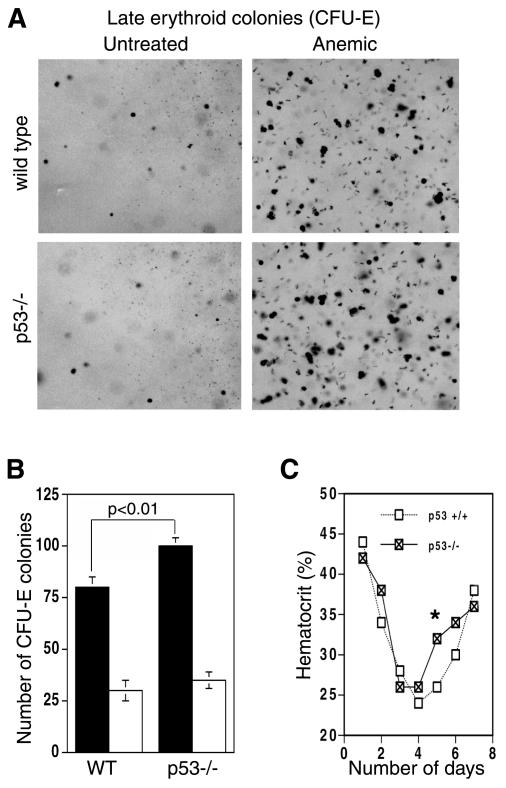
GR is required for the rapid expansion of ebls in the spleen in response to hemolytic anemia (Bauer et al., 1999). We studied whether p53 affects this response. Anemia was induced by injection of phenylhydrazine into wild-type and p53–/– mice, and spleen cell suspensions were seeded in methylcellulose medium. Three days after the injection, the number of early erythroid progenitors (colony forming units erythroids, CFU-Es) induced by hemolysis was higher in the p53–/– mice (Figure 3A and B; p < 0.01). We also studied a blood parameter, the hematocrit. Five days after the first phenyhydrazine injection, the hematocrit values of p53–/– mice were consistently but transiently higher than in the wild-type mice (Figure 3C; p <0.03). These results show that p53–/– mice recover faster from the anemic stress, with increased numbers of progenitors (CFU-Es) by day 3 and mature erythroid cells by day 5. Apparently, the p53 restraint on GR-mediated proliferation is lost, allowing faster recovery.
Fig. 3. Effect of p53 on the induction by anemia of spleen CFU-Es and circulating blood cells. (A) On day 4 (after the first phenylhydrazine injection), spleen cells were isolated and equal numbers of cells were seeded in duplicate into semi-solid media containing factors required for CFU-E formation. After 2–3 days, pictures were taken (A) and the numbers of CFU-Es were counted (B; solid bars, treated; empty bars, untreated). (C) Hematocrit values after phenylhydrazine injection. There was a reproducible and significant (*p <0.03) increase in the hematocrit values at day 5 in the p53–/– mice in four separate experiments.
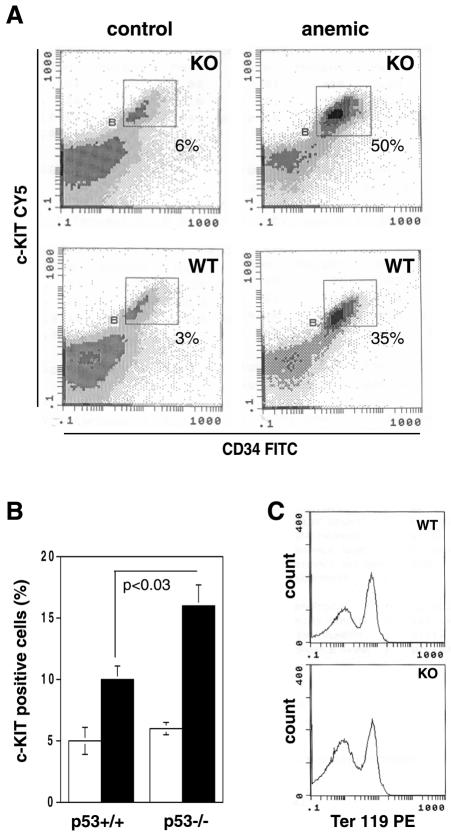
We examined the differentiation status of the spleen cells by FACS analysis of the early hemopoietic markers c-kit (CD117) and CD34 and the late erythroid marker Ter119 (Figure 4). The proportion of c-kit positive cells was found to be elevated to a significantly greater extent in the p53–/– anemic mice (Figure 4B; p <0.03). A distinct cell population, double stained by CD34 and c-kit, was upregulated to a greater extent in the p53–/– anemic mice (Figure 4A). As expected (Bauer et al., 1999), a fraction of these double positive cells also coexpressed Ter119 (Figure 4C), showing that they belong to the erythroid lineage. Thus, loss of p53 enhances the expansion of the particular type of spleen ebls (c-kit/CD34/Ter119 triple positive) that are responsible for the glucocorticoid- and GR-mediated response to erythrolytic stress (Axelrod and Reisine, 1984; Tronche et al., 1998; Bauer et al., 1999).
Fig. 4. Effect of p53 on the induction by anemia of erythroid progenitors in the spleen. Aliquots of spleen cell suspensions, prepared from mice treated with phenylhydrazine, were immunostained with fluorochrome-labelled antibodies against CD34, c-Kit (CD117) and Ter119. (A) Viable cells were gated and evaluated by FACS analysis. The CD34/c-kit double positive cells are outlined (squares), and the percentages of double positive cells are indicated. One representative result, from three independent experiments, is shown. (B) The fraction of c-kit positive cells from the untreated (empty bars) and the anemic (solid bars) animals are plotted. The percentages of c-kit positive cells are calculated from several independent experiments, and a p-value is indicated. (C) The CD34/CD117 double positive cells [the rectangle outlined in (A)] were gated and analysed for Ter119 expression. WT, wild type; KO, p53–/–.
In conclusion, our results show that stress erythropoiesis is a physiological process in which p53 antagonizes the GR. We showed previously that p53 antagonizes the activity of GR in normal cells in culture (Sengupta and Wasylyk, 2001), but the in vivo significance of these observations was unclear. Various studies have implicated p53 in differentiation (Almog and Rotter, 1997), in addition to cell-cycle arrest and apoptosis (Balint and Vousden, 2001). The levels of p53 protein increase with maturation in human hematopoietic cells, suggesting that p53 may play a role in hematopoietic cell maturation by contributing to the inhibition of proliferation that occurs during terminal differentiation (Kastan et al., 1991). GR is required in vivo for the rapid expansion of ebls under stress conditions (Bauer et al., 1999). Our observations fit with these studies and provide a molecular mechanism for the balance between precursor expansion and differentiation of erythroid cells. p53 and GR apparently function as opposing forces that help to maintain homeostasis and to make the important decision between proliferation and differentiation. GR regulates the expression of genes that are required for ebl proliferation, such as c-myb (von Lindern et al., 1999). p53 antagonizes the proliferative effects of GR and inhibits the expression of c-myb, thereby favouring differentiation.
METHODS
Isolation and cultures of mouse fetal liver cells. p53 heterozygote mice from mixed background (BL6/sv129) mice were crossed, and fetal livers were isolated from 14.5 d.p.c. embryos (Bauer et al., 1999). The livers were resuspended in 1 ml of serum free stem cell expansion medium (StemPro-34; Life Technologies Gibco-BRL), supplemented with 100 ng/ml mouse SCF, 2 U/ml Epo (R&D Systems), 40 ng/ml IGF-1 (Sigma) and 1 µM Dex (Sigma). Erythroid cultures were maintained at 2–4 × 106 cells/ml by daily medium changes plus re-addition of fresh factors with appropriate dilution. Cell numbers were determined manually on a daily basis.
Cell staining. Cytospins (1–20 × 104 cells in 200 µl of growth medium centrifuged on glass slides) were stained with May Grunwald and counter-stained with Giemsa, or fixed for 5 min in methanol, incubated in 1% 3′,3′-dimethoxybenzidine (Sigma) in methanol for 1.5 min, washed with bi-distilled H2O for 30 s and counter-stained with Giemsa (Beug et al., 1982). Dark yellow cytoplasms are benzidine (hemoglobin) positive, light blue negative.
FACS analysis. The cells were incubated with Fc block (Pharmingen) for 10 min, antibodies for 15 min at 4°C, followed by secondary antibodies coupled to CY5 (for c-kit) or FITC (for CD34) or with streptavidin linked to PE (for Ter119). Flow cytometry was performed with the EPICS profile analyser.
RNA extraction and RT–PCR. Ten-day fetal liver cell cultures were incubated in medium with growth factors but lacking Dex for 48 h, stimulated with Dex for 1 day and total RNA extracted with Trizol Reagent (Life Technologies Gibco-BRL). Real-time RT–PCR was performed with the LightCycler system (Roche Diagnostics) and the SYBR Green protocol. Primers for c-myb, c-kit, RBTN2, GATA-1 and 28S RNA were designed using the Oligo 4.0 programme. The reactions, containing 5 µl of RNA (100–500 ng) and 15 µl of reagents (including the primers, MgCl2, enzymes and SYBR green), were reverse transcribed for 60 s at 55°C, denatured for 30 s at 95°C and cycled 40 times for 2 s at 95°C, 10 s at 55°C and 10 s at 72°C (∼1 s per 25 bp depending on the size of the product). Amplification specificity was verified by melting curve analysis, and the data quantified with LightCycler software.
In vivo erythropoiesis. Anemia was induced with phenylhydrazine (60 mg/kg body weight; Sigma) injected intraperitoneally on two consecutive days (Broudy et al., 1996; Bauer et al., 1999). Cell suspensions were prepared by squeezing spleens collected on day 3 through 70 µm cell strainers and washing with cold PBS. For CFU-Es, the cells were resuspended in StemPro-34 medium and seeded (2 × 105 to 2 × 106 cells per well, four well dishes) into methocult 3236 supplemented with 5 U of human recombinant Epo, 100 ng/ml mouse SCF, 40 ng/ml IGF-1 and 40 mg/ml LDL (Sigma). Small compact colonies were counted on days 2–3. For the hematocrit experiments, blood samples were collected daily from four wild-type and four p53–/– mice, starting from the first phenylhydrazine injection. The blood samples were centrifuged in capillary tubes in a minicentrifuge (Bayers diagnostics).
Acknowledgments
ACKNOWLEDGEMENTS
We thank S. Chan and P. Kastner for critical reading of the manuscript; G. Buchwalter for advice; the IGBMC facilities; and Aventis, Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Hôpital Universitaire de Strasbourg, Association pour la Recherche sur le Cancer, Fondation pour la Recherche Médicale, and Ligue Nationale et Régionale (Haut et Bas Rhin) contre le Cancer (Equipe labellisée) for financial assistance.
REFERENCES
- Almog N. and Rotter, V. (1997) Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta, 1333, F1–F27. [DOI] [PubMed] [Google Scholar]
- Axelrod J. and Reisine, T.D. (1984) Stress hormones: their interaction and regulation. Science, 224, 452–459. [DOI] [PubMed] [Google Scholar]
- Balint E.E. and Vousden, K.H. (2001) Activation and activities of the p53 tumour suppressor protein. Br. J. Cancer, 85, 1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Tronche, F., Wessely, O., Kellendonk, C., Reichardt, H.M., Steinlein, P., Schutz, G. and Beug, H. (1999) The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev., 13, 2996–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Palmieri, S., Freudenstein, C., Zentgraf, H. and Graf, T. (1982) Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell, 28, 907–919. [DOI] [PubMed] [Google Scholar]
- Broudy V.C., Lin, N.L., Priestley, G.V., Nocka, K. and Wolf, N.S. (1996) Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood, 88, 75–81. [PubMed] [Google Scholar]
- Fujiwara Y., Browne, C.P., Cunniff, K., Goff, S.C. and Orkin, S.H. (1996) Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl Acad. Sci. USA, 93, 12355–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D.W., Bersch, N. and Cline, M.J. (1976) Potentiation of erythropoiesis in vitro by dexamethasone. J. Clin. Invest., 57, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut P. and Hollstein, M. (2000) p53 and human cancer: the first ten thousand mutations. Adv. Cancer Res., 77, 81–137. [DOI] [PubMed] [Google Scholar]
- Introna M., Luchetti, M., Castellano, M., Arsura, M. and Golay, J. (1994) The myb oncogene family of transcription factors: potent regulators of hematopoietic cell proliferation and differentiation. Semin. Cancer Biol., 5, 113–124. [PubMed] [Google Scholar]
- Kastan M.B. et al. (1991) Levels of p53 protein increase with maturation in human hematopoietic cells. Cancer Res., 51, 4279–4286. [PubMed] [Google Scholar]
- Liang R., Chan, T.K. and Todd, D. (1994) Childhood acute lymphoblastic leukaemia and aplastic anaemia. Leuk. Lymphoma, 13, 411–415. [DOI] [PubMed] [Google Scholar]
- Lotem J. and Sachs, L. (1993) Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood, 82, 1092–1096. [PubMed] [Google Scholar]
- Muta K., Krantz, S.B., Bondurant, M.C. and Dai, C.H. (1995) Stem cell factor retards differentiation of normal human erythroid progenitor cells while stimulating proliferation. Blood, 86, 572–580. [PubMed] [Google Scholar]
- Pevny L., Lin, C.S., D’Agati, V., Simon, M.C., Orkin, S.H. and Costantini, F. (1995) Development of hematopoietic cells lacking transcription factor GATA-1. Development, 121, 163–172. [DOI] [PubMed] [Google Scholar]
- Prokocimer M. and Rotter, V. (1994) Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood, 84, 2391–2411. [PubMed] [Google Scholar]
- Reichardt H.M. et al. (1998) DNA binding of the glucocorticoid receptor is not essential for survival. Cell, 93, 531–541. [DOI] [PubMed] [Google Scholar]
- Sengupta S. and Wasylyk, B. (2001) Ligand-dependent interaction of the glucocorticoid receptor with p53 enhances their degradation by Hdm2. Genes Dev., 15, 2367–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F., Kellendonk, C., Reichardt, H.M. and Schutz, G. (1998) Genetic dissection of glucocorticoid receptor function in mice. Curr. Opin. Genet. Dev., 8, 532–538. [DOI] [PubMed] [Google Scholar]
- Udupa K.B., Crabtree, H.M. and Lipschitz, D.A. (1986) In vitro culture of proerythroblasts: characterization of proliferative response to erythropoietin and steroids. Br. J. Haematol., 62, 705–714. [DOI] [PubMed] [Google Scholar]
- von Lindern M., Zauner, W., Mellitzer, G., Steinlein, P., Fritsch, G., Huber, K., Lowenberg, B. and Beug, H. (1999) The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood, 94, 550–559. [PubMed] [Google Scholar]
- Warren A.J., Colledge, W.H., Carlton, M.B., Evans, M.J., Smith, A.J. and Rabbitts, T.H. (1994) The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell, 78, 45–57. [DOI] [PubMed] [Google Scholar]
- Wessely O., Deiner, E.M., Beug, H. and von Lindern, M. (1997a) The glucocorticoid receptor is a key regulator of the decision between self-renewal and differentiation in erythroid progenitors. EMBO J., 16, 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely O., Mellitzer, G., von Lindern, M., Levitzki, A., Gazit, A., Ischenko, I., Hayman, M.J. and Beug, H. (1997b) Distinct roles of the receptor tyrosine kinases c-ErbB and c-Kit in regulating the balance between erythroid cell proliferation and differentiation. Cell Growth Differ., 8, 481–493. [PubMed] [Google Scholar]
- Wessely O., Bauer, A., Quang, C.T., Deiner, E.M., von Lindern, M., Mellitzer, G., Steinlein, P., Ghysdael, J. and Beug, H. (1999) A novel way to induce erythroid progenitor self renewal: cooperation of c-Kit with the erythropoietin receptor. Biol. Chem., 380, 187–202. [DOI] [PubMed] [Google Scholar]
- Weston K. (1998) Myb proteins in life, death and differentiation. Curr. Opin. Genet. Dev., 8, 76–81. [DOI] [PubMed] [Google Scholar]
- Zhao R., Gish, K., Murphy, M., Yin, Y., Notterman, D., Hoffman, W.H., Tom, E., Mack, D.H. and Levine, A.J. (2000) Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev., 14, 981–993. [PMC free article] [PubMed] [Google Scholar]
- Zito G.E. and Lynch, E.C. (1977) Prednisone-responsive congenital erythroid hypoplasia. J. Am. Med. Assoc., 237, 991–992. [PubMed] [Google Scholar]