Abstract
Objectives:
We examined within-individual changes in self-reported sleep health as community-dwelling older adults age as well as potential differences in these changes by self-reported sex and racial identity.
Methods:
Participants were from the United States and enrolled in the Rush Memory and Aging Project, Minority Aging Research Study, or Religious Orders Study (N=3,539, 20% Black, 75% female, mean 78 years [range 65–103]), and they received annual, in-person clinical evaluations (median 5 visits [range 1–27]). A sleep health composite score measured the number of poor sleep characteristics among satisfaction, daytime sleepiness, efficiency, and duration. Mixed effects models estimated associations of age, race, sex, and their interactions on the composite and individual sleep measures, accounting for key confounders.
Results:
As they aged, Black participants shifted from reporting two poor sleep characteristics to one poor sleep characteristic, while White participants shifted from one poor characteristic to two. Regardless of age, sex, and race, participants reported that they “often” felt satisfied with their sleep and “sometimes” had trouble staying asleep. Females over age 85 and males of all ages reported the most daytime sleepiness, and older White participants (> age 90) reported the most difficulty falling asleep.
Conclusions:
Although self-reported sleep characteristics were typically stable across age, identifying race and sex differences in self-reported sleep health can help guide future research to understand the mechanisms that underlie these differences.
Keywords: sleep duration, sleep quality, sleep latency, aging, healthy aging, health inequities
1. Introduction
Sleep characteristics including duration, timing, efficiency, and satisfaction are known to change across the lifespan as a result of both internal and external factors1–10. In older adults, sleep characteristics can be influenced by normal age-related changes in circadian and homeostatic processes,5 social stressors, and alterations in health and functioning7,8,11–14. However, many of these associations are considered bi-directional15–17, with sleep also predicting the onset and course of health outcomes in older adults 6,18–22. These associations – combined with the behavioral modifiability of many dimensions of sleep – make sleep a prime target for screening and interventions. Self-reported sleep is particularly beneficial because it is scalable for large-scale studies and uniquely captures subjective perceptions of sleep. However, to effectively utilize self-reported sleep as a gauge of overall health, it is critical to understand its normative patterns of change in older adults, including potential differences by sex and/or racial identity.
Prior examinations of sleep in older adults are primarily based on cross-sectional studies or meta-analyses 1,3,8–10,19, though some longitudinal studies exist 6,7. One point of relative consensus is that self-reported nighttime awakenings and wake after sleep onset increase with age in older adults 2,19,23, resulting in lower sleep efficiency 3,6,8. Napping and daytime sleepiness can increase in older age 2,19, although this may depend heavily on medical and/or psychiatric comorbidities including cardiovascular disease, depression, and pain as well as behavioral, environmental, and social factors.19 Findings are mixed regarding the extent to which self-report duration and timing change during older adulthood. While an early study reported increased sleep duration with age 8, more recent studies either indicate reductions in duration and a shift towards earlier timing 4,6 or suggest stable duration and timing in older age 3.
The study designs of these meta-analyses and cross-sectional studies may contribute to the mixed findings. Meta-analyses are based on a patchwork of samples across multiple individual studies with different sampling criteria. Thus, they typically cannot adequately account for key confounders, including co-morbidities and cognitive and physical functioning, which can alter findings. Cross-sectional studies cannot investigate within-individual sleep changes, and the few studies with longitudinal follow-up typically had shorter/limited follow-up 7,24, limited sleep measures 4,6,24, or small sample sizes,7 providing a constrained picture of sleep and aging.
Sleep health is known to be multidimensional25–27, and there is increased clinical interest in composite sleep health scores that capture the number of adverse sleep characteristics within an individual.25 Although composite sleep health scores are increasingly useful as a generalizable indicator of overall sleep health 18,21,28–31, few longitudinal studies have examined multiple domains (see 3,32 for exceptions), and prior studies have not examined changes in the number of poor sleep characteristics within aging older adults.
Sociodemographic factors including self-reported race/ethnicity and sex are central to understanding normative changes in sleep health in older adults. Cross-sectional race and sex differences have been widely observed in older adults.1,4,9,11,26,33–35 Specifically, women and people with minoritized identities self-report worse sleep outcomes (e.g., shorter duration, greater rates of insomnia, poorer sleep continuity and quality) relative to men and White non-Hispanic people. One relevant study examined differences in age-related self-reported sleep duration trajectories by race and sex from early adolescence to adulthood (11–44 years)36, but findings in this age range are not necessarily expected to extend to older adults. Thus, the extent to which observed cross-sectional sex and race inequities in older adults’ sleep health are maintained, accelerated, or diminished with age is uncertain. Older adults may be particularly informative because the adverse health correlates or consequences of poor sleep may be more proximal.
Two competing hypotheses – commonly known as “weathering” and “hardening” – provide a theoretical foundation on which we can interpret the extent to which sex and race differentiate sleep health trajectories in older adults.37 When applied to sleep health, the weathering hypothesis posits that the cumulative experience of stressors (e.g., structural and interpersonal discrimination) contributes to an earlier and more sharply declining sleep health trajectory for minoritized individuals or women. Conversely, the hardening hypothesis suggests that cumulative stressors (when constrained at moderate levels and coupled with resources to “reframe” the problem) buffer individuals from experiences of distress and ultimately result in better self-reported sleep health and slower declines over time. Beyond these hypotheses, there are established mental, physical, and cognitive health disparities by race and sex – many of which impact sleep – that could further alter the sleep health trajectory.
In this study, we aim to quantify longitudinal changes in sleep health in older adults while accounting for key health confounders. Given the clear cross-sectional differences in sleep health related to race and sex, we account for these potential differences and their possible interactions in our examination of age-related changes in multidimensional sleep health. We hypothesize that women and Black individuals will initially have worse sleep health but remain agnostic regarding whether these inequities will be maintained, accelerate, or diminish over time. We hope that our work generates hypotheses to promote future health equity research focused on understanding socio-environmental constructs which explain differential sleep health trajectories in older adults.
2. Participants and Methods
2.1. Parent Cohorts
Participants are from the Religious Orders Study (ROS, beginning in 1994), Rush Memory and Aging Project (MAP, beginning in 1997), and Minority Aging Research Study (MARS, beginning in 2004). Briefly, these are longitudinal, epidemiologic cohort studies from the Rush Alzheimer’s Disease Center (RADC) and include participants without known dementia at enrollment. Cohort harmonization is facilitated by shared study staff and common study designs and protocols, including highly overlapping measures harmonized within the RADC. Further details of RADC methods and cohort study designs are described elsewhere 38,39 and in Appendix A.
These RADC cohorts follow older adults (age 65+) for up to 27 years, providing annual data on self-reported sleep characteristics (e.g., satisfaction, daytime sleepiness, difficulty falling and staying asleep, and duration) and critical features of aging (e.g., cognitive and physical functioning, co-morbidities, and depressive symptoms). Annual visits also include a clinical evaluation of cognitive status (i.e., no cognitive impairment (NCI), mild cognitive impairment (MCI), and Alzheimer’s and other dementias). This clinical diagnosis is determined using a three-stage process including computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and diagnostic classification by a clinician (see details described elsewhere 40,41).
2.2. Analytic Samples
To enhance generalizability, we combined ROS, MAP, and MARS data into a single cohort with N=4,507 participants. We refined this cohort by selecting Black or White participants who were aged 65 or older with at least one self-reported sleep measure (N=4,242; Figure A.1). American Indian/Alaska Native, Native Hawaiian/Pacific Islander, Asian, Other, and Unknown racial categories comprised only 1% of the sample, representing sample sizes too small to extract interpretable results from the analysis. We developed a primary analytic sample and two sensitivity samples, differing by the strictness of inclusion criteria based on cognitive status. Although many mental and physical health characteristics affect (and are affected by) sleep, we focused on cognitive status because people with dementia and/or MCI may be unable to reliably self-report their sleep and other study measures 42,43.
In our primary analytic sample (“NCI at Visit”; N=3,539; median of 5 [range 1–27] follow-up visits) we removed potentially unreliable observations by excluding any time point with a diagnosis of MCI or dementia (N=703 excluded). In sensitivity analytic sample 1 (“Always NCI”; N=1,693; median of 5 [range 1–27] follow-up visits), we restricted the primary sample by excluding any participant who received a diagnosis of MCI or dementia during follow-up (N=1,846 excluded), thereby removing potential confounding related to current and future cognitive problems. In sensitivity analytic sample 2 (“Everyone”; N=4,242; median of 6 [range 1–27] follow-up visits), we included everyone, regardless of cognitive status. This sample is more reflective of a community sample where cognitive diagnosis is not necessarily known, and thus may be more generalizable to the larger population.
2.3. Outcomes
In MAP and ROS, self-reported items probing sleep health were measured annually from study onset. In MARS, they were included in 2012. Using the SATED (Satisfaction, Alertness, Timing, Efficiency, Duration) framework 25, we selected measures to represent available sleep health domains including Satisfaction, Daytime Alertness/Sleepiness, Efficiency, and Duration (Table 1). Timing measures were not available across all three cohorts nor during the full follow-up period and were not included in this analysis.
Table 1.
Self-reported sleep questions and cutoffs for poor sleep health characteristics
| Self-reported sleep question relative to the past montha | Poor | Sleep domain |
|---|---|---|
| (Q1) “how often do you have trouble falling asleep?” | ≥ 3 | Efficiencyb |
| (Q2) “how often are you troubled by waking up during the night?” | ≥ 3 | Efficiencyb |
| (Q3) “how often do you get so sleepy during the day or evening that you have to take a nap?” | ≥ 3 | Daytime Sleepiness |
| (Q4) “how often do you feel really rested when you wake up in the morning?” | ≤ 2 | Satisfaction |
| (Q5) “how many hours do you usually sleep at night?” | < 7 or > 8c | Duration |
Q1-Q3 responses: (0=never; 1=rarely; 2=sometimes; 3=often; 4=very often); Q4 responses are reversed.
If either Q1 or Q2 was “poor” then the Efficiency domain was coded as “poor”.
Based on recommendations by the National Sleep Foundation for older adults 62.
We used the SATED characteristics (Table 1) to operationalize a sleep health composite score representing the number of poor self-reported sleep characteristics. At the initial visit, Spearman correlations among the individual sleep characteristics ranged from −0.23 to 0.3, indicating generally weak to negligible correlations and relatively independent measures, consistent with the sleep health framework.25,44 Based on prior work 45,46, we selected cut-points indicating “poor” levels of each characteristic and summed these indicators as an aggregate score (range 0–4). The sleep health composite score is our primary outcome. Individual sleep health characteristics are secondary outcomes (sleep satisfaction, daytime sleepiness, trouble falling asleep, trouble staying asleep, and sleep duration).
2.4. Predictors and Covariates
Our main predictors were age at each visit (centered such that age 65 was coded as 0) and self-reported sex (male or female) and race at the initial visit (Black or White). We also selected covariates that were potentially sleep-related or may confound associations among sleep and our main predictors, including time-invariant (measured at the initial visit) and time-varying (measured at each follow-up visit) variables.
Time-invariant covariates included self-reported ethnic identity (Spanish/Hispanic/Latin origin), years of education, marital status (never married, married, widowed, or divorced/separated), grams of alcohol consumed per day, early life socioeconomic status (SES), a ROS cohort indicator, and a death during follow-up indicator. Grams of alcohol per day is a self-reported estimate of typical alcohol consumption per day in the past 12 months. Higher values [range 0–234.6g] indicate greater alcohol consumption. Early life SES is a previously-validated averaged Z-score composite index based on paternal and maternal years of education and number of children in the family 47. Values [range −2.76 to 2.31] above or below zero indicate above or below average SES within the cohorts, respectively. ROS participants are catholic nuns, priests, and brothers who may have unique lifestyles and sleep patterns compared to those in MAP or MARS.
Time-varying covariates included global cognitive function, number of self-reported medical conditions, use of insomnia medication, depressive symptoms, and instrumental activities of daily living (IADLs). Global cognitive function is a composite Z-score calculated from a battery of 19 cognitive tests assessing episodic memory, semantic memory, working memory, visuospatial ability, and perceptual speed 48. Positive Z-scores indicate an above average within-cohort overall score at the initial visit. The number of medical conditions considers clinician diagnosis of stroke or self-reported hypertension, diabetes, heart disease, cancer, or thyroid disease. Use of insomnia medications indicates taking an insomnia medication in the last 2 weeks based on direct visual inspection of medications. Depressive symptoms were assessed with a modified, 9-item version of the Center for Epidemiologic Studies Depression scale (CES-D) 49,50 with the sleep-specific item removed to avoid tautology. Higher scores [range 0–9] indicate greater depressive symptoms. IADL is a composite measure of disability summing 8 items adapted from the Duke Older Americans Resources and Services project, including measures of household management and self-care functions. Higher scores [range 0–8] indicate greater disability.
2.5. Statistical Analysis
In preliminary analyses, we summarized the sample by race and sex and assessed distributions of sociodemographic and baseline measures.
For primary aims, we fit generalized linear mixed effects models within our primary analytic sample (NCI at visit, N=3,539) and sensitivity samples (Always NCI [N=1,693], Everyone [N=4,242]). We used a linear model for continuous sleep duration. We used ordinal models for each of the other sleep measures, which were based on a Likert scale response or count. Each model included primary predictors of age, race, sex, and their two-way interactions, adjusting for covariates. We tested interactions among age, race, and sex, and excluded any not statistically significant (alpha=0.05) interactions one at a time to create a final model. To control for multiple comparisons, we also examined whether key covariates and their interactions met a Bonferroni-adjusted p-value threshold (p < 0.05/6 outcomes = 0.008). All continuous measures were standardized to facilitate comparison of model effect sizes across covariates. We included a random effect to account for within-subject correlations in all models. For the continuous duration model, we additionally considered age squared, a three-way age x race x sex interaction, and nested participants within each cohort as a random effect. We also considered these effects in ordinal models, but their inclusion resulted in non-convergence, so they were removed.
Using the final model from each sample, we estimated probabilities of each level of the outcome (if ordinal) or the predicted outcome (if continuous) by age, race, and sex, while holding other covariates constant at their mean to allow for model interpretation at “typical” covariate levels within the sample. Similarly, we estimated probabilities of each level of the sleep health composite score by cognitive status, number of medical conditions, IADLs, and depressive symptoms. We emphasized these features as key health domains for older adults.
In terms of evaluating mortality effect, we included a comprehensive list of covariates (physical, mental, and cognitive health) as proxies for mortality in all analyses (similar to prior longitudinal studies)51. Furthermore, in an additional sensitivity analysis using our primary analytic sample, we examined whether covarying for death during follow-up would alter our findings regarding the primary sleep health composite score outcome.
Analyses were performed in R; ordinal outcome mixed effects models used the clmm function from the ordinal package 52, and continuous outcome models used the lme function from the nlme package 53,54. We assumed missing outcome data to be missing completely at random or missing due to observed covariates in the model which is accommodated using mixed effects models.
3. Results
Descriptive statistics for the primary analytic sample are presented in Table 2 by sex and race (Tables A.1 and A.2 report descriptive statistics for sensitivity analytic samples). The primary analytic sample (N=3,539) had 20% Black and 75% female participants, with 49% from MAP, 16% from MARS, and 35% from ROS. In this sample, Black participants and female participants tended to be younger, had fewer years of education, differed from others by marital status, drank fewer grams of alcohol per day, and had a lower-than-average early life SES. Two-hundred and one (5.7%) participants in the primary analytic sample were excluded from fully-adjusted analytic models (Table 3) due to missing data, typically on marital status or early life SES (Table 2).
Table 2.
Descriptive characteristics of MAP, MARS, and ROS participants in the NCI at Visit sample at baseline by race and sex
| Overall | Black | White | Female | Male | |
|---|---|---|---|---|---|
| (N=3539) | (N=715) | (N=2824) | (N=2655) | (N=884) | |
| Visit age | |||||
| Mean (SD) | 77.6 (7.27) | 74.9 (6.47) | 78.2 (7.31) | 77.7 (7.29) | 77.2 (7.20) |
| Sex, n (%) | |||||
| Female | 2655 (75.0%) | 580 (81.1%) | 2075 (73.5%) | 2655 (100%) | 0 (0%) |
| Male | 884 (25.0%) | 135 (18.9%) | 749 (26.5%) | 0 (0%) | 884 (100%) |
| Race, n (%) | |||||
| Black | 715 (20.2%) | 715 (100%) | 0 (0%) | 580 (21.8%) | 135 (15.3%) |
| White | 2824 (79.8%) | 0 (0%) | 2824 (100%) | 2075 (78.2%) | 749 (84.7%) |
| Spanish/Hispanic/Latin origin, n (%) | |||||
| No | 3406 (96.2%) | 701 (98.0%) | 2705 (95.8%) | 2554 (96.2%) | 852 (96.4%) |
| Yes | 133 (3.8%) | 14 (2.0%) | 119 (4.2%) | 101 (3.8%) | 32 (3.6%) |
| Years of education | |||||
| Mean (SD) | 16.2 (3.72) | 15.2 (3.45) | 16.5 (3.74) | 16.0 (3.54) | 17.1 (4.10) |
| Missing, n (%) | 1 (0.0%) | 0 (0%) | 1 (0.0%) | 1 (0.0%) | 0 (0%) |
| Marital status, n (%) | |||||
| Divorced/Separated | 351 (9.9%) | 189 (26.4%) | 162 (5.7%) | 309 (11.6%) | 42 (4.8%) |
| Married | 817 (23.1%) | 199 (27.8%) | 618 (21.9%) | 478 (18.0%) | 339 (38.3%) |
| Never married | 1380 (39.0%) | 107 (15.0%) | 1273 (45.1%) | 1015 (38.2%) | 365 (41.3%) |
| Widowed | 830 (23.5%) | 220 (30.8%) | 610 (21.6%) | 739 (27.8%) | 91 (10.3%) |
| Missing | 161 (4.5%) | 0 (0%) | 161 (5.7%) | 114 (4.3%) | 47 (5.3%) |
| Grams alcohol per day | |||||
| Mean (SD) | 4.37 (11.7) | 3.04 (11.9) | 4.71 (11.6) | 2.96 (9.97) | 8.61 (15.0) |
| Missing, n (%) | 3 (0.1%) | 0 (0%) | 3 (0.1%) | 3 (0.1%) | 0 (0%) |
| Early life SES | |||||
| Mean (SD) | 0.019 (0.749) | −0.060 (0.777) | 0.038 (0.741) | −0.007 (0.758) | 0.096 (0.717) |
| Missing, n (%) | 34 (1.0%) | 10 (1.4%) | 24 (0.8%) | 29 (1.1%) | 5 (0.6%) |
| Study, n (%) | |||||
| MAP | 1735 (49.0%) | 84 (11.7%) | 1651 (58.5%) | 1315 (49.5%) | 420 (47.5%) |
| MARS | 559 (15.8%) | 559 (78.2%) | 0 (0%) | 442 (16.6%) | 117 (13.2%) |
| ROS | 1245 (35.2%) | 72 (10.1%) | 1173 (41.5%) | 898 (33.8%) | 347 (39.3%) |
Abbreviations: MAP=Memory and Aging Project; MARS=Minority Aging Research Study; ROS=Religious Orders Study; NCI=No cognitive impairment; SES=Socioeconomic status.
Table 3.
Associations of predictors with each sleep health outcome for MAP, MARS, and ROS participants in the NCI at Visit sample (N = 3,338)
| Sleep composite | Satisfaction | Sleepiness | Staying asleepa | Falling asleep | Duration | ||
|---|---|---|---|---|---|---|---|
| No. of | |||||||
| observations | 20,473 | 20,484 | 20,480 | 20,467 | 20,488 | 20,484 | |
| Predictors | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | Est. (SE) | |
| Age (years, centered at 65) | 0.98 (0.97, 1.00)b | 0.99 (0.98, 1.01) | 1.03 (1.01, 1.04)b | 0.98 (0.97, 1.00)c | 0.98 (0.96, 1.00)c | 0.02 (0.005)b | |
| White race (ref=Black) | 0.35 (0.26, 0.47)c | 0.48 (0.36, 0.65)b | 0.71 (0.52, 0.97)c | 0.88 (0.69, 1.13) | 0.46 (0.33, 0.64)b | 0.66 (0.094)b | |
| Male sex (ref=Female) | 1.32 (1.11, 1.56)b | 0.99 (0.66, 1.49) | 2.74 (2.08, 3.62)b | 0.83 (0.61, 1.12) | 0.36 (0.27, 0.49)b | 0.28 (0.194) | |
| Education (years) | 0.89 (0.82, 0.97) | 0.90 (0.82, 0.97) | 0.94 (0.86, 1.04) | 0.94 (0.88, 1.01) | 0.91 (0.82, 1.01) | 0.07 (0.023) | |
| Hispanic (ref=Not Hispanic) | 1.04 (0.71, 1.53) | 0.96 (0.66, 1.40) | 0.57 (0.37, 0.88) | 1.28 (0.95, 1.71) | 1.50 (0.97, 2.33) | −0.20 (0.102) | |
| Alcohol use (grams) | 0.93 (0.87, 1.00) | 1.00 (0.93, 1.06) | 0.90 (0.84, 0.97) | 1.02 (0.96, 1.07) | 0.97 (0.90, 1.05) | 0.05 (0.018) | |
| Early life SES | 0.93 (0.84, 1.02) | 1.03 (0.94, 1.14) | 0.89 (0.79, 0.99) | 0.94 (0.87, 1.01) | 0.89 (0.79, 1.00) | 0.08 (0.027) | |
| Marital status (ref=Never married) | |||||||
| Married | 0.90 (0.61, 1.31) | 0.97 (0.68, 1.40) | 0.81 (0.53, 1.23) | 1.18 (0.88, 1.57) | 1.17 (0.76, 1.80) | 0.18(0.102) | |
| Divorced/Separated | 1.42 (0.94, 2.15) | 1.56 (1.05, 2.31) | 0.92 (0.58, 1.45) | 1.44 (1.05, 1.97) | 1.82 (1.13, 2.92) | 0.05 (0.111) | |
| Widowed | 1.20 (0.82, 1.75) | 1.19 (0.83, 1.71) | 0.76 (0.50, 1.15) | 1.40 (1.05, 1.86) | 1.27 (0.82, 1.96) | −0.01 (0.102) | |
| ROS (ref=MAP/MARS) | 1.45 (1.00, 2.11) | 1.25 (0.87, 1.79) | 1.45 (0.96, 2.19) | 1.43 (1.08, 1.90) | 0.88 (0.58, 1.36) | 0.05 (0.101) | |
| Global cognition | 1.06 (0.95, 1.18) | 1.22 (1.10, 1.36) | 1.04 (0.93, 1.16) | 1.17 (1.06, 1.28) | 1.25 (1.12, 1.40) | −0.14 (0.023) | |
| No. of medical conditions | 1.14 (1.09, 1.20) | 1.11 (1.05, 1.17) | 1.14 (1.08, 1.20) | 1.04 (0.99, 1.08) | 1.05 (1.00, 1.11) | −0.01 (0.013) | |
| IADLs (composite disability) | 1.11 (1.07, 1.15) | 1.11 (1.07, 1.16) | 1.10 (1.06, 1.14) | 1.00 (0.97, 1.04) | 0.98 (0.94, 1.02) | 0.05 (0.008) | |
| Insomnia meds (ref=No meds) | 1.25 (1.09, 1.45) | 1.28 (1.11, 1.47) | 0.84 (0.73, 0.97) | 1.13 (0.99, 1.29) | 1.82 (1.56, 2.12) | −0.01(0.030) | |
| Depressive symptoms | 1.72 (1.66, 1.79) | 1.50 (1.44, 1.56) | 1.26 (1.22, 1.32) | 1.45 (1.40, 1.51) | 1.45 (1.39, 1.51) | −0.10 (0.008) | |
| Age x White race | 1.05 (1.03, 1.07)b | 1.03 (1.02, 1.05)b | 1.02 (1.00, 1.04)c | 1.02 (1.01, 1.04)b | 1.06 (1.04, 1.08)b | −0.01 (0.006) | |
| Age x Male sex | 1.02 (1.01, 1.03)b | 0.98 (0.97, 1.00)c | 1.02 (1.01, 1.04)b | −0.02 (0.013) | |||
| White race x Male sex | 0.62 (0.41, 0.93)c | 1.43 (1.04, 1.98)c | −0.60 (0.212)b | ||||
| Age x White race x Male sex | not tested | not tested | not tested | not tested | not tested | 0.04 (0.014)b | |
Abbreviations: ROS=Religious Orders Study; MAP=Memory and Aging Project; MARS=Minority Aging Research Study; NCI=No cognitive impairment; OR=Odds ratio; Est.=Coefficient estimate; SE=Standard error; SES=Socioeconomic status; IADLs=Instrumental activities of daily living.
One person was completely missing data for trouble staying asleep.
Key covariates (age, race, sex) and/or their interactions met a Bonferroni-adjusted p-value threshold < 0.008.
Key covariates (age, race, sex) and/or their interactions met an unadjusted p-value threshold < 0.05.
3.1. Self-reported Sleep in Primary Analytic Sample (NCI at Visit)
3.1.1. Sleep Health Composite
We observed a significant age by race interaction (OR=1.05, 95%CI=[1.03, 1.07]) (Table 3). For White participants, the probability of reporting zero or one poor characteristic decreased with age, while reporting two characteristics increased (Figure 1). Conversely, for Black participants, the probability of reporting zero or one poor characteristics increased with age, while reporting two characteristics decreased. We also observed a main effect of sex, indicating that males had 1.32 [1.11, 1.56] higher odds of reporting additional poor sleep characteristics compared to females, regardless of age or race. After covarying for death during follow-up, individuals who died during follow-up had higher odds of reporting additional poor sleep characteristics (1.48 [1.28, 1.72]) (Table A.3). However, patterns relating to key predictors (i.e., age, race, and sex) were very similar to those observed prior to adjusting for death (Figure A.2).
Figure 1.
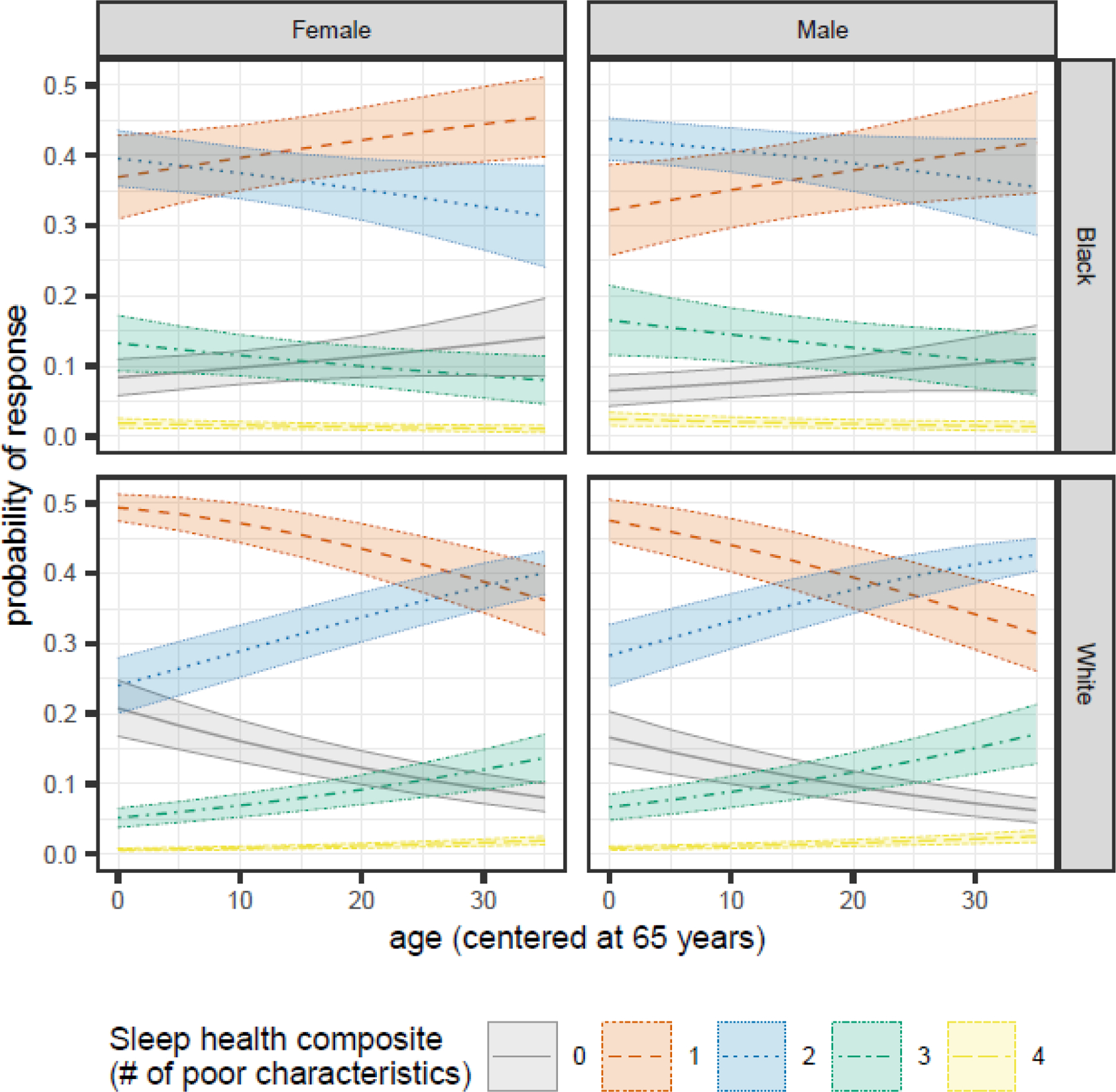
Sex- and race-specific changes in the predicted probabilities of each Sleep Health Composite Score as participants age for MAP, MARS, and ROS participants in the NCI at Visit sample.
3.1.2. Sleep Satisfaction
We observed a significant age by race interaction (1.03 [1.02, 1.05]), age by sex interaction (1.02 [1.01, 1.03]), and race by sex interaction (0.62 [0.41, 0.93]) (Table 3). All participants were most likely to report “often” feeling satisfied with their sleep (i.e., often feeling really rested when they wake in the morning) (Figure 2). For younger White participants (and especially males), “very often” feeling satisfied was the second most common response, but as they aged, they were more likely to report “sometimes” feeling satisfied. In contrast, Black participants consistently reported “sometimes” feeling satisfied as the second most likely response across age.
Figure 2.
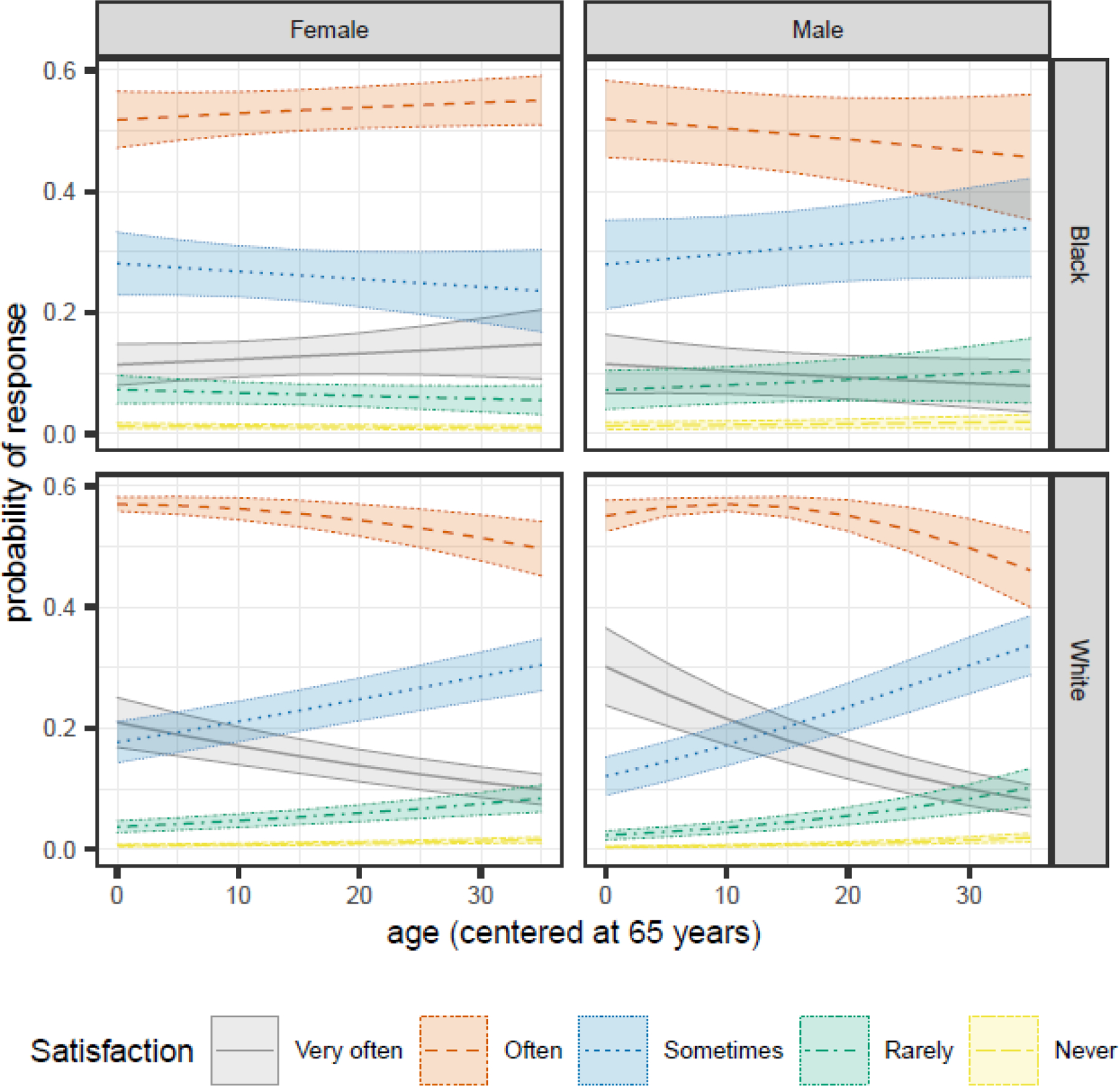
Sex- and race-specific changes in the predicted probabilities of each Satisfaction response as participants age for MAP, MARS, and ROS participants in the NCI at Visit sample.
3.1.3. Daytime Sleepiness
We observed a significant age by race interaction (1.02 [1.00, 1.04]) and age by sex interaction (0.98 [0.97, 1.00]) (Table 3). Participant response patterns over time differed by sex. Males were most likely to report “sometimes” feeling very sleepy, regardless of age (Figure 3). Younger females (age 65–75) were most likely to report “rarely” feeling very sleepy, but by 85 years old, both Black and White females were most likely to report “sometimes” feeling very sleepy. While significant at alpha=0.05, this interaction did not remain significant after adjusting for multiple comparisons (Table 3).
Figure 3.
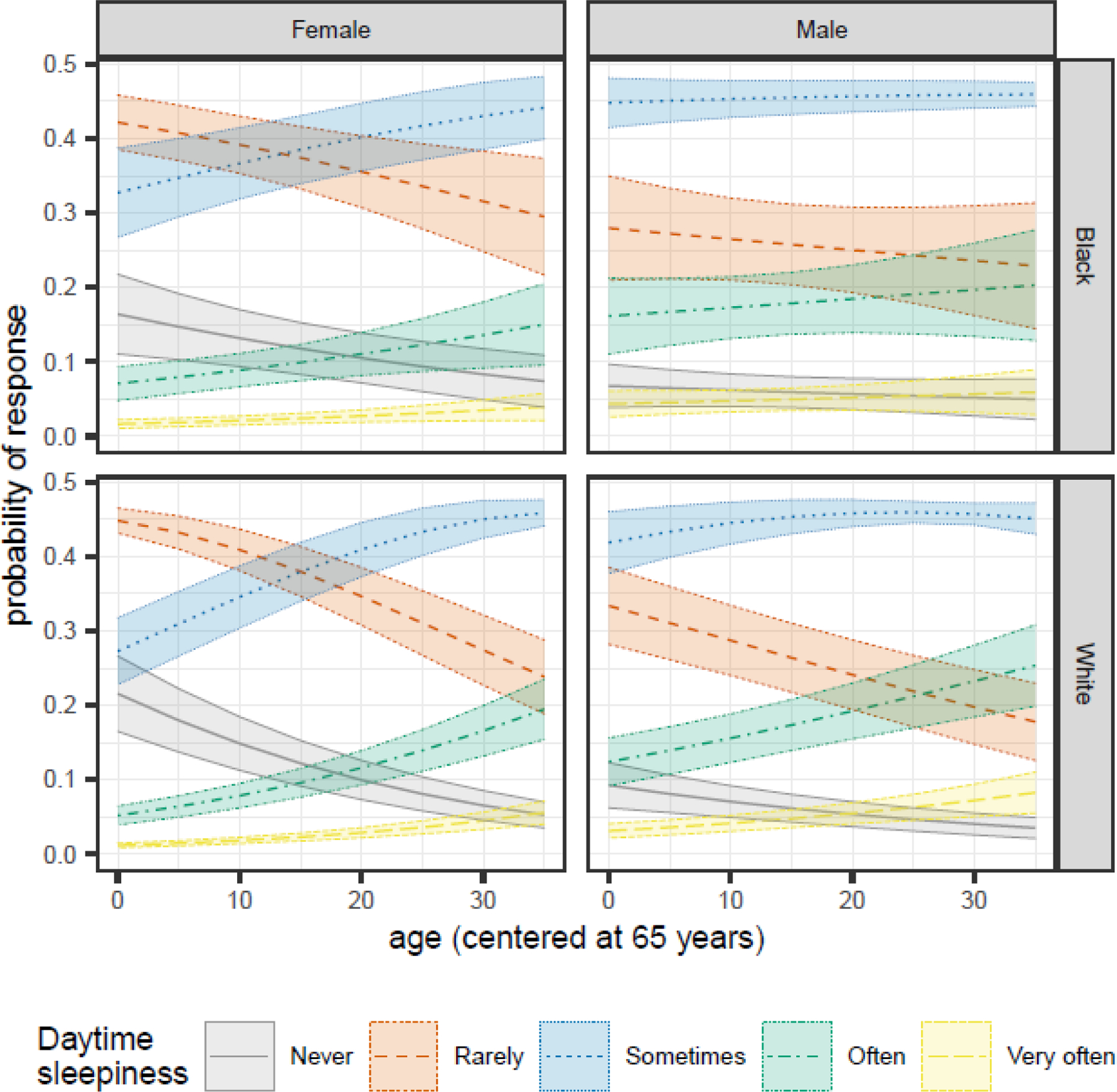
Sex- and race-specific changes in the predicted probabilities of each Daytime Sleepiness response as participants age for MAP, MARS, and ROS participants in the NCI at Visit sample.
3.1.4. Trouble Staying Asleep
We observed significant age by race (1.02 [1.01, 1.04]) and race by sex (1.43 [1.04, 1.98]) interactions (Table 3). All participants were most likely to report “sometimes” having trouble staying asleep (Figure 4). As Black participants aged, their likelihood of reporting “rarely” or “never” having trouble staying asleep increased, while their likelihood of reporting “often” or “very often” decreased. For White participants, the opposite tended to be true; reports of “often” or “very often” having trouble staying asleep increased with age, while reports of “rarely” or “never” decreased.
Figure 4.
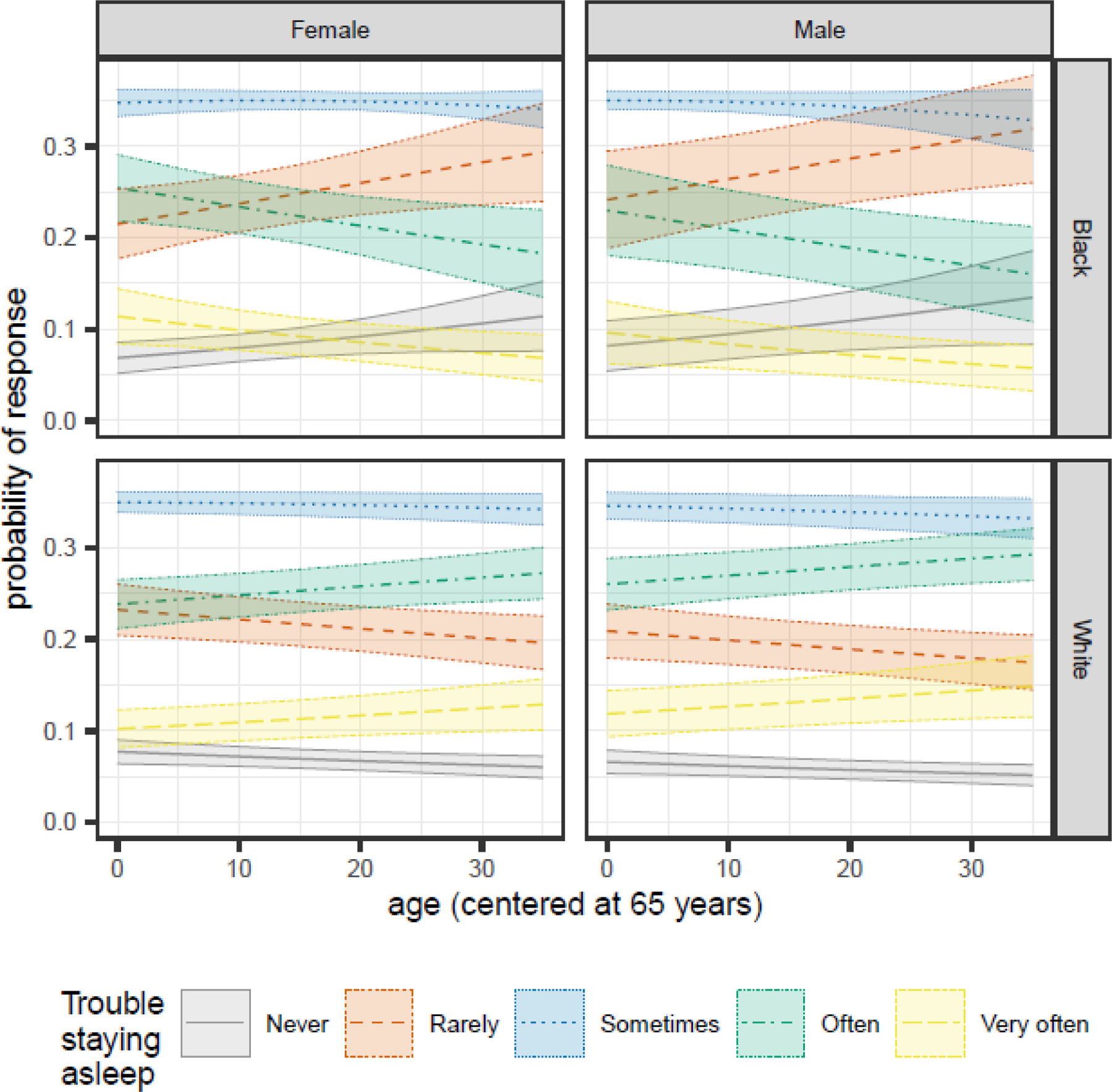
Sex- and race-specific changes in the predicted probabilities of each Trouble Staying Asleep response as participants age for MAP, MARS, and ROS participants in the NCI at Visit sample.
3.1.5. Trouble Falling Asleep
We observed significant age by race (1.06 [1.04, 1.08]) and age by sex (1.02 [1.01, 1.04]) interactions (Table 3). Black males were most likely to report “rarely” having trouble falling asleep, regardless of age (Figure 5). Younger Black females were about equally likely to report “rarely” or “sometimes” having trouble falling asleep. As they aged, they became more likely to report “rarely” having trouble falling asleep and less likely to report “sometimes”. For White participants, the probability of reporting “rarely” having trouble falling asleep decreased with age while reporting “sometimes” increased.
Figure 5.
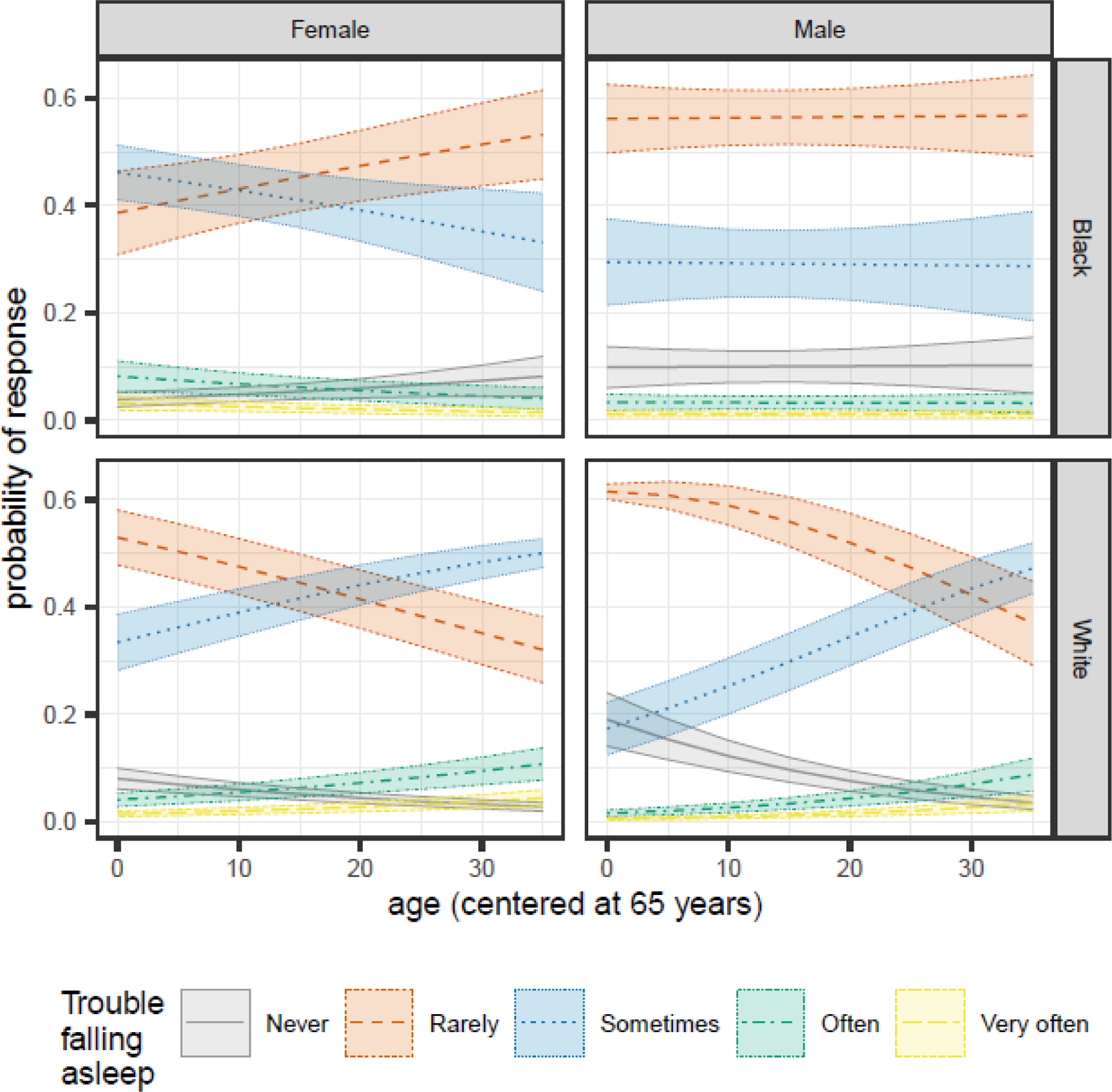
Sex- and race-specific changes in the predicted probabilities of each Trouble Falling Asleep response as participants age for MAP, MARS, and ROS participants in the NCI at Visit sample.
3.1.6. Sleep Duration
We observed a significant three-way interaction among age, race, and sex (estimate [standard error (SE)] = 0.04 [0.014]) (Table 3). White females generally slept longer than others, about 7.11 hours [SE=0.06] on average compared to White males (6.99 [0.07]), Black females (6.62 [0.08]), and Black males (6.52 [0.12]) (Figure 6). Duration increased slightly as participants aged at a rate of 0.017 [SE=0.005] hours per year for Black females, 0.006 [0.003] for White females, and 0.019 [0.004] for White males. Duration for Black males decreased 0.008 [0.012] hours per year. The slope for White females differed significantly from that of White males (P=0.03).
Figure 6.
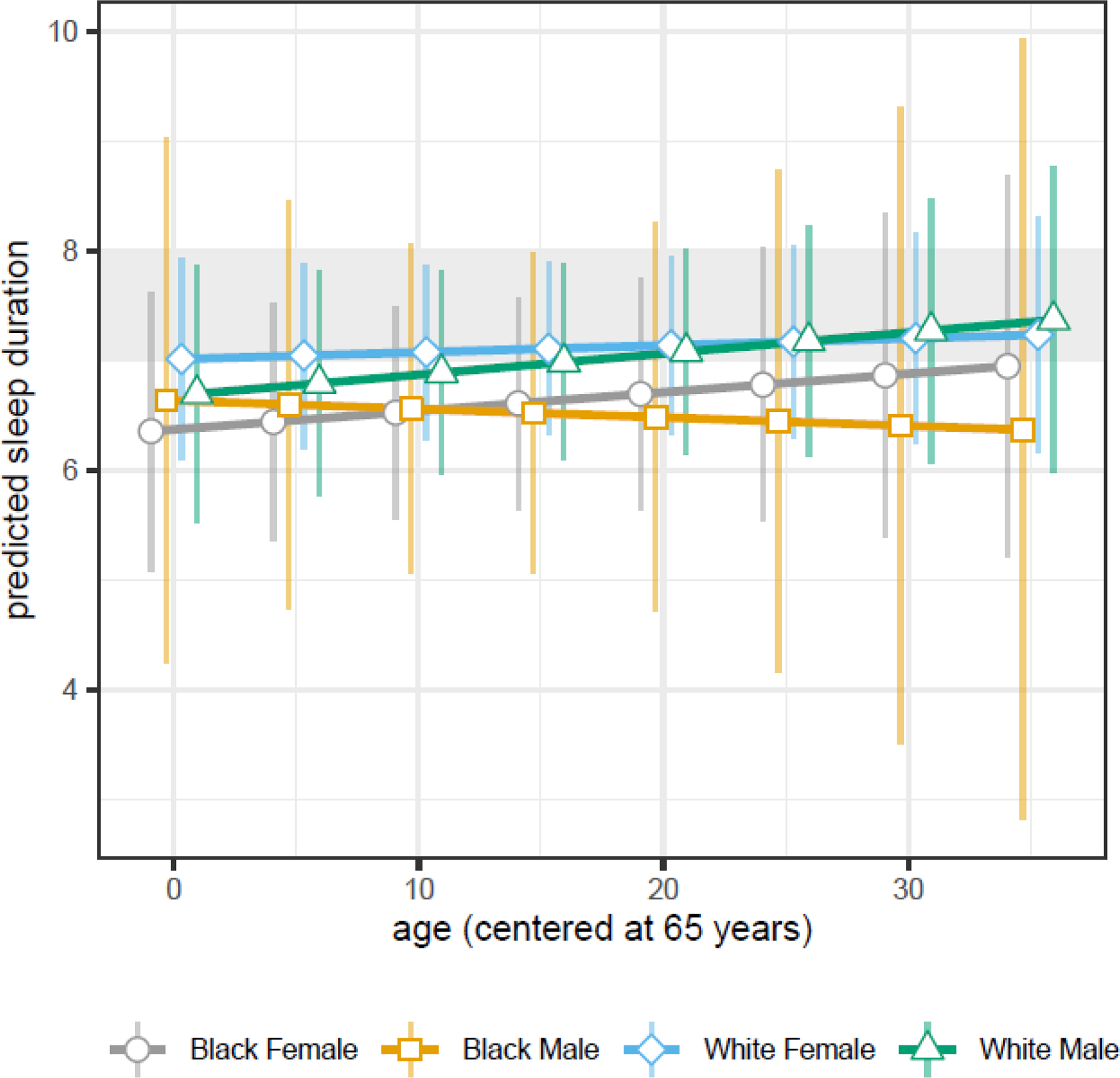
Sex- and race-specific changes in predicted sleep duration as participants age for MAP, MARS, and ROS participants in the NCI at Visit sample. Points denote the predicted average duration with lines connecting race- and sex-specific points at each age over time; vertical bars denote the 95% confidence interval around each predicted average; and the shaded gray area denotes the optimal sleep duration between 7–8 hours per night. Shown here is the extent to which race- and sex- specific changes in sleep duration overlap, the increasing duration for all females and White males, and the decreasing duration for Black males.
3.2. Self-reported Sleep in Sensitivity Analytic Samples
For each sleep outcome, effect sizes in the primary NCI at Visit sample were generally consistent with those in the Everyone sample. However, we observed some differences between the primary NCI at Visit sample and the Always NCI sample. Participants in the Always NCI sample compared to the primary NCI at Visit sample reported slightly higher probabilities of worse sleep satisfaction, especially as they aged; no age-related changes in trouble staying asleep; and small increases in sleep duration with age, regardless of race or sex. Tables A.4 and A.5 contain full results of sensitivity analytic samples.
3.3. Self-reported Sleep by Clinical Characteristics
Depressive symptoms accounted for the largest amount of variability in each sleep health outcome, with more depressive symptoms associated with a greater likelihood of reporting a higher (‘worse’) sleep health composite score (Table 3, Figure 7A). Participants with more medical conditions (Table 3, Figure 7B) and greater disability (Table 3, Figure 7D) were also more likely to report a higher sleep health composite score. These findings were primarily driven by associations with sleep satisfaction and daytime sleepiness. Finally, the association between global cognitive function and sleep health outcomes was most notable in the Everyone sample (Table A.5, Figure 7C), where higher cognitive function was associated with a greater likelihood of reporting worse sleep health.
Figure 7.
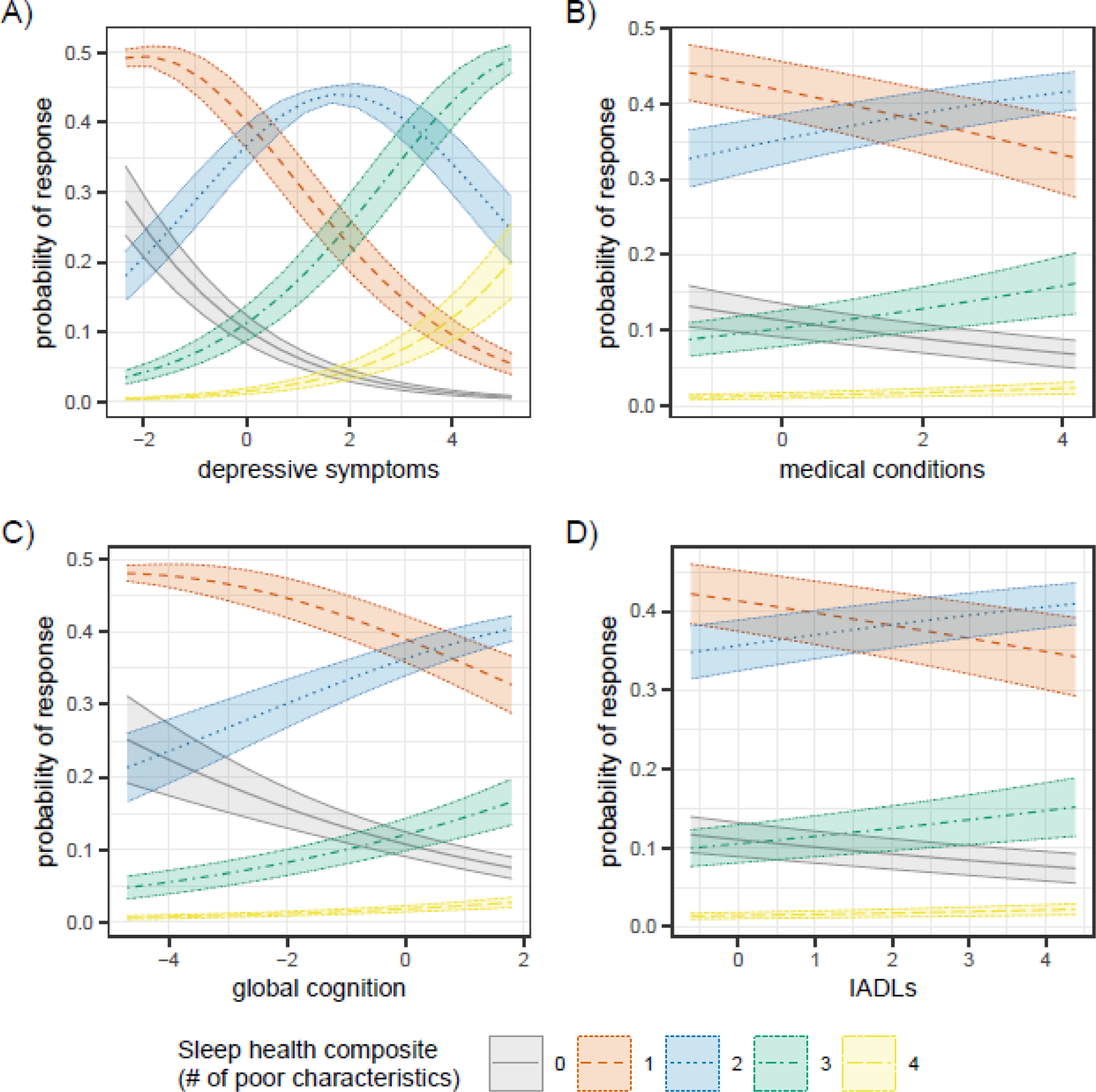
Changes in the predicted probabilities of each sleep health composite score by (A) depressive symptoms (NCI at Visit sample), B) medical conditions (NCI at Visit sample), C) global cognition (Everyone sample), and D) Instrumental activities of daily living (IADLs) (NCI at visit sample). All x-axis measures are standardized.
4. Discussion
In a large sample of community-dwelling diverse older adults with up to 27 years of annual follow-up, we quantified normative within-subject age trends in self-reported sleep health by sex and race. White participants (especially males) tended to report one additional poor sleep health characteristic as they aged, while Black participants tended to report one less poor sleep health characteristic. Upon investigating individual sleep characteristics contributing to this finding, we primarily observed race differences in difficulty falling asleep and sex differences in daytime sleepiness. Age, sex, and race interacted to set the trend for duration as individuals aged, with Black males showing a notable decrease in duration relative to Black females and White participants. Ratings of satisfaction and trouble staying asleep were relatively consistent regardless of age, sex, and race.
These novel findings contribute to our understanding of longitudinal changes in sleep health among older adults. Despite the previous consensus that sleep efficiency decreases and daytime sleepiness increases with age 2,3,6,8,19, our findings indicate that self-reports of these features differ by sex and/or race; this finding has important implications for future screening tools. Moreover, many self-reported sleep characteristics are relatively stable across older age, typically changing by only a single ‘category’, if at all. Thus, even seemingly minor alterations in self-reported sleep health (e.g., one additional poor health characteristics; shifting from ‘rarely’ to ‘sometimes’ experiencing difficulties) may be noteworthy. The extent to which a one-category change in sleep health predicts worsening health should be examined in future studies.
Worsening self-reported sleep health also likely reflects co-occurring changes in health conditions. For example, higher depressive symptoms were associated with additional poor sleep health characteristics. Strong associations between sleep health and depressive symptoms in older adults have been observed previously;55,56 this combination may indicate underlying common mechanisms including chronic stress or unmeasured medical disease. Higher medical conditions and functional limitations were also associated with additional poor sleep health characteristics, consistent with previous studies6,11,19. Chronic medical conditions, functional limitations, depressive symptoms, and sleep health likely interact with bi-directional associations, and interventions that address these comorbid problems in older adults are needed.57 Interestingly, worse cognitive function was associated with better self-reported sleep health. Poor cognitive ability may be associated with underreporting of poor sleep health characteristics; viewed another way, cognitively intact people are likely more aware of their sleep health. Future analyses should directly examine how changes in cognitive status relate to changes in self-reported sleep health.
Our analyses only partially support the extant literature suggesting that Black individuals report worse sleep health relative to White individuals. Prior to age 85, Black participants are indeed more likely to report worse sleep health; however, they are more likely to report improvements in sleep health with age. This pattern of apparent improvement with age, despite documented disparities cross-sectionally, has been observed in other studies examining racial/ethnic differences in self-reported measures such as functional limitations37 and serious medical conditions51. However, the life-course factors underlying racial/ethnic health disparities are likely condition specific and dependent on whether conditions are subjectively or objectively measured. Black participants are more likely to have experienced a lifetime of cumulative psychosocial and physiologic stressors as a result of systemic racism, and thus their self-reported improvements in sleep health are consistent with the hardening theory (i.e., cumulative stressors may lead to fewer subjective complaints of sleep health). Yet studies of objective health measures (e.g., inflammation, metabolic dysregulation) show that lifetime exposures to stressors among racial/ethnic minorities contribute to accelerated physiological deterioration58, consistent with the weathering hypothesis.
Our findings suggest further research is needed in at least two areas. First, we need to understand whether a similar pattern of findings is observed with objective sleep measures. Although self-reported measures are a key tool in large-scale screening, they do not correspond exactly with behavioral and physiological measures of sleep. While individuals may not self-report sleep complaints (supporting the hardening theory), we may very well observe deterioration in similar objective measures of sleep health (supporting the weathering theory). Second, research should examine components of systemic racism and socio-environmental factors59 to determine how the accumulation of racialized social stressors over a lifetime may impact both self-report and objective sleep measures in an aging population. Ultimately, future studies should collect and analyze data from other minoritized racial and ethnic identities to gain a fuller understanding of disparities in sleep health across the life course.
Our study uses harmonized measures across MAP, MARS, and ROS. However, ROS participants, as members of religious orders, may possess characteristics unique from MAP and MARS participants. In post-hoc analyses, we examined associations with the sleep health composite score, excluding ROS participants. We observed that ROS participants may be driving the observed main effect of sex in the primary NCI at Visit sample, although the overall patterns remained similar with and without ROS (Table A.6). Excluding ROS participants did not meaningfully impact associations with race, nor did it alter the sex- and race-specific sleep health composite score response patterns as participants aged (Figure A.3), suggesting that ROS participants’ trajectory of self-reported sleep health with age is not so fundamentally different from MAP/MARS participants. However, lifestyle factors, duties, and responsibilities (e.g., Matins) may impact the sleep of monks and nuns differently compared to the general population60,61 and should be further investigated in future studies.
This study poses some limitations. First, sleep regularity and timing are important domains of sleep health and increasingly related to adverse health and accelerated aging. However, these measures were not collected in all cohorts and could not be evaluated here. Second, although participants were community-dwelling older adults, they were healthy volunteers with relatively high levels of education; findings must be replicated in samples more comparable to the population. Third, we have not included occupation measures, and although this is an older-age cohort, occupational history and current employment status could impact sleep patterns in certain demographic sub-groups. Finally, mortality rates differed across analytic samples (primary NCI at Visit sample=50%, Always NCI=33%, and Everyone=55%), and we did not examine the competing risk of mortality. Adjusting for death during follow-up had little impact on our primary results, but higher group-specific mortality rates could explain some observed differences. Future studies should examine differences in sleep health trajectories by mortality, as well as by other physical, cognitive, and mental health characteristics pertinent to this aging population.
5. Conclusions
This study establishes normative changes in a sleep health composite score among older adults as a function of age, race, and sex. Given the overall stability of self-reported sleep characteristics in community-dwelling older adults, future studies should investigate whether a one-unit change in self-reported sleep health warrants further screening and/or intervention. Furthermore, race and sex differences in specific sleep health outcomes can help guide future research to improve screening tools and understand the mechanisms that underlie these differences.
Supplementary Material
Acknowledgements
We are grateful for the altruism of the thousands of participants in the Religious Orders Study, the Rush Memory and Aging Project, and the Minority Aging Research Study. We thank study coordinators, data and analytic programmers, and staff and faculty of the Rush Alzheimer’s Disease Center. All data are publicly available at https://www.radc.rush.edu/.
Funding Sources
This work was supported by the National Institute on Aging [RF1AG056331, R01AG052488, R01AG071638, RF1AG070436, P30AG010161, P3072975, R01AG17917, R01AG22018]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicts of Interest
Dr. Wallace reports grants from NIA RF1AG056331, during the conduct of the study; personal fees from Health Rhythms, personal fees from Noctem Health, personal fees from Sleep Number Bed, outside the submitted work. Dr. Buysse reports grants from NIA RF1AG05633, during the conduct of the study; personal fees from National Cancer Institute, personal fees from Pear Therapeutics, personal fees from Sleep Number, personal fees from Idorsia, personal fees from Eisai, personal fees from Weight Watchers International, outside the submitted work; and Dr. Buysse is an author of the Pittsburgh Sleep Quality Index, Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A), Brief Pittsburgh Sleep Quality Index (B-PSQI), Daytime Insomnia Symptoms Scale, Pittsburgh Sleep Diary, Insomnia Symptom Questionnaire, and RU_SATED (copyrights held by University of Pittsburgh). These instruments have been licensed to commercial entities for fees. He is also co-author of the Consensus Sleep Diary (copyright held by Ryerson University), which is licensed to commercial entities for a fee. He has received grant support from NIH, PCORI, AHRQ, VA and Sleep Number.
Figure A.2. Sex- and race-specific changes in the predicted probabilities of each Sleep Health Composite Score as participants age for MAP, MARS, and ROS participants in the NCI at Visit sample for the A) original model compared to the B) model with death included as a covariate.
Figure A.3. Sex- and race-specific changes in the predicted probabilities of each Sleep Health Composite Score as participants age for MAP and MARS (excluding ROS) participants in the NCI at Visit sample.
References
- 1.Kocevska D, Lysen TS, Dotinga A, et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis. Nat Hum Behav 2021;5(1):113–122. doi: 10.1038/s41562-020-00965-x [DOI] [PubMed] [Google Scholar]
- 2.Mander BA, Winer JR, Walker MP. Sleep and Human Aging. Neuron 2017;94(1):19–36. doi: 10.1016/j.neuron.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace ML, Kissel N, Hall MH, et al. Age Trends in Actigraphy and Self-Report Sleep Across the Life Span: Findings From the Pittsburgh Lifespan Sleep Databank. Psychosom Med 2022;84(4):410–420. doi: 10.1097/PSY.0000000000001060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonasdottir SS, Minor K, Lehmann S. Gender differences in nighttime sleep patterns and variability across the adult lifespan: a global-scale wearables study. Sleep 2021;44(2). doi: 10.1093/sleep/zsaa169 [DOI] [PubMed] [Google Scholar]
- 5.Taillard J, Gronfier C, Bioulac S, Philip P, Sagaspe P. Sleep in Normal Aging, Homeostatic and Circadian Regulation and Vulnerability to Sleep Deprivation. Brain Sci 2021;11(8). doi: 10.3390/brainsci11081003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Didikoglu A, Maharani A, Tampubolon G, Canal MM, Payton A, Pendleton N. Longitudinal sleep efficiency in the elderly and its association with health. J Sleep Res 2020;29(3):e12898. doi: 10.1111/jsr.12898 [DOI] [PubMed] [Google Scholar]
- 7.Bliwise DL, Ansari FP, Straight LB, Parker KP. Age Changes in Timing and 24-Hour Distribution of Self-Reported Sleep. Am J Geriatr Psychiatry 2005;13(12):1077–1082. doi: 10.1097/00019442-200512000-00007 [DOI] [PubMed] [Google Scholar]
- 8.Floyd JA, Medler SM, Ager JW, Janisse JJ. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health 2000;23(2):106–117. doi: [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Lemoine P. [Sleep and insomnia markers in the general population]. Encephale 2004;30(2):135–140. doi: 10.1016/s0013-7006(04)95423-1 [DOI] [PubMed] [Google Scholar]
- 10.Evans MA, Buysse DJ, Marsland AL, et al. Meta-analysis of age and actigraphy-assessed sleep characteristics across the lifespan. Sleep 2021;44(9). doi: 10.1093/sleep/zsab088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews KA, Hall MH, Lee L, et al. Racial/ethnic disparities in women’s sleep duration, continuity, and quality, and their statistical mediators: Study of Women’s Health Across the Nation. Sleep 2019;42(5). doi: 10.1093/sleep/zsz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulia KK, Kumar VM. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics 2018;18(3):155–165. doi: 10.1111/psyg.12319 [DOI] [PubMed] [Google Scholar]
- 13.Miner B, Kryger MH. Sleep in the Aging Population. Sleep Med Clin 2017;12(1):31–38. doi: 10.1016/j.jsmc.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean GE, Weiss C, Morris JL, Chasens ER. Impaired Sleep: A Multifaceted Geriatric Syndrome. Nurs Clin North Am 2017;52(3):387–404. doi: 10.1016/j.cnur.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 15.Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med 2019;23(4):2324–2332. doi: 10.1111/jcmm.14170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju YES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 2020;45(1):104–120. doi: 10.1038/s41386-019-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman MA, Kline CE, Buysse DJ, et al. Longitudinal Association Between Depressive Symptoms and Multidimensional Sleep Health: The SWAN Sleep Study. Ann Behav Med 2021;55(7):641–652. doi: 10.1093/abm/kaaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation “2003 Sleep in America” Poll. Am J Geriatr Psychiatry 2007;15(4):344–350. doi: 10.1097/01.JGP.0000249385.50101.67 [DOI] [PubMed] [Google Scholar]
- 20.Turner AD, Lim AS, Leurgans SE, Bennett DA, Buchman AS, Barnes LL. Self-Reported Sleep in Older African Americans and White Americans. Ethn Dis 2016;26(4):521–528. doi: 10.18865/ed.26.4.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Lawson KM. Beyond single sleep measures: A composite measure of sleep health and its associations with psychological and physical well-being in adulthood. Soc Sci Med 2021;274:113800. doi: 10.1016/j.socscimed.2021.113800 [DOI] [PubMed] [Google Scholar]
- 22.Alcántara C, Biggs ML, Davidson KW, et al. Sleep Disturbances and Depression in the Multi-Ethnic Study of Atherosclerosis. Sleep 2016;39(4):915–925. doi: 10.5665/sleep.5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep 2010;33(2):211–223. doi: 10.1093/sleep/33.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews KA, Kravitz HM, Lee L, et al. Does midlife aging impact women’s sleep duration, continuity, and timing?: A longitudinal analysis from the Study of Women’s Health Across the Nation. Sleep 2020;43(4). doi: 10.1093/sleep/zsz259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep 2014;37(1):9–17. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Langenberg SCN, Kocevska D, Luik AI. The multidimensionality of sleep in population-based samples: a narrative review. J Sleep Res 2022;31(4):e13608. doi: 10.1111/jsr.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace ML, Yu L, Buysse DJ, et al. Multidimensional sleep health domains in older men and women: an actigraphy factor analysis. Sleep 2021;44(2). doi: 10.1093/sleep/zsaa181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace ML, Buysse DJ, Redline S, et al. Multidimensional Sleep and Mortality in Older Adults: A Machine-Learning Comparison With Other Risk Factors. J Gerontol A Biol Sci Med Sci 2019;74(12):1903–1909. doi: 10.1093/gerona/glz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung J, Goodman M, Huang T, Bertisch S, Redline S. Multidimensional sleep health in a diverse, aging adult cohort: Concepts, advances, and implications for research and intervention. Sleep Health 2021;7(6):699–707. doi: 10.1016/j.sleh.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whibley D, Goldstein C, Kratz AL, Braley TJ. A multidimensional approach to sleep health in multiple sclerosis. Mult Scler Relat Disord 2021;56:103271. doi: 10.1016/j.msard.2021.103271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tighe CA, Brindle RC, Stahl ST, et al. Multidimensional Sleep Health and Physical Functioning in Older Adults. Gerontol Geriatr Med 2021;7:23337214211016224. doi: 10.1177/23337214211016222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schousboe JT, Kats AM, Stone KL, et al. Self-reported poor sleep on multiple dimensions is associated with higher total health care costs in older men. Sleep 2020;43(10). doi: 10.1093/sleep/zsaa073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandner MA. Sleep, Health, and Society. Sleep Med Clin 2017;12(1):1–22. doi: 10.1016/j.jsmc.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Wang R, Zee P, et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015;38(6):877–888. doi: 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson CL, Powell-Wiley TM, Gaston SA, Andrews MR, Tamura K, Ramos A. Racial/Ethnic Disparities in Sleep Health and Potential Interventions Among Women in the United States. J Womens Health . 2020;29(3):435–442. doi: 10.1089/jwh.2020.8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saelee R, Haardörfer R, Johnson DA, Gazmararian JA, Suglia SF. Racial/Ethnic and Sex/Gender Differences in Sleep Duration Trajectories From Adolescence to Adulthood in a US National Sample. Am J Epidemiol Published online August 25, 2022. doi: 10.1093/aje/kwac156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauerteig MR, Ferraro KF, Bauldry S. Life Course Stressors and Functional Limitations in Later Life Among White, Black, and Hispanic Adults: Deleterious, Hardening, or Benign? J Gerontol B Psychol Sci Soc Sci 2022;77(1):249–259. doi: 10.1093/geronb/gbab066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res 2012;9(6):734–745. doi: 10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 2018;64(s1):S161–S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006;27(3):169–176. doi: 10.1159/000096129 [DOI] [PubMed] [Google Scholar]
- 41.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198 [DOI] [PubMed] [Google Scholar]
- 42.Schneider S, Junghaenel DU, Zelinski EM, et al. Subtle mistakes in self-report surveys predict future transition to dementia. Alzheimers Dement 2021;13(1):e12252. doi: 10.1002/dad2.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider S, Junghaenel DU, Meijer E, et al. Quality of Survey Responses at Older Ages Predicts Cognitive Decline and Mortality Risk. Innov Aging 2022;6(3):igac027. doi: 10.1093/geroni/igac027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coelho J, Lopez R, Richaud A, et al. Toward a multi-lingual diagnostic tool for the worldwide problem of sleep health: The French RU-SATED validation. J Psychiatr Res 2021;143:341–349. doi: 10.1016/j.jpsychires.2021.09.008 [DOI] [PubMed] [Google Scholar]
- 45.Wallace ML, Lee S, Hall MH, et al. Heightened sleep propensity: a novel and high-risk sleep health phenotype in older adults. Sleep Health 2019;5(6):630–638. doi: 10.1016/j.sleh.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace ML, Stone K, Smagula SF, et al. Which Sleep Health Characteristics Predict All-Cause Mortality in Older Men? An Application of Flexible Multivariable Approaches. Sleep 2018;41(1). doi: 10.1093/sleep/zsx189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology 2005;25(1):8–14. doi: 10.1159/000085307 [DOI] [PubMed] [Google Scholar]
- 48.Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology 2015;85(11):984–991. doi: 10.1212/WNL.0000000000001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 50.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health 1993;5(2):179–193. doi: 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- 51.Brown TH, O’Rand AM, Adkins DE. Race-ethnicity and health trajectories: tests of three hypotheses across multiple groups and health outcomes. J Health Soc Behav 2012;53(3):359–377. doi: 10.1177/0022146512455333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen RHB. ordinal---Regression Models for Ordinal Data Published online 2019.
- 53.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS Published online 2000. doi: 10.1007/b98882 [DOI]
- 54.Pinheiro J, Bates D, R Core Team. nlme: Linear and Nonlinear Mixed Effects Models Published online 2022. https://CRAN.R-project.org/package=nlme [Google Scholar]
- 55.Hsu MF, Lee KY, Lin TC, Liu WT, Ho SC. Subjective sleep quality and association with depression syndrome, chronic diseases and health-related physical fitness in the middle-aged and elderly. BMC Public Health 2021;21(1):164. doi: 10.1186/s12889-021-10206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furihata R, Hall MH, Stone KL, et al. An Aggregate Measure of Sleep Health Is Associated With Prevalent and Incident Clinically Significant Depression Symptoms Among Community-Dwelling Older Women. Sleep 2017;40(3). doi: 10.1093/sleep/zsw075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Onen SH, Onen F. Chronic Medical Conditions and Sleep in the Older Adult. Sleep Med Clin 2018;13(1):71–79. doi: 10.1016/j.jsmc.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 58.Boen C Death by a Thousand Cuts: Stress Exposure and Black-White Disparities in Physiological Functioning in Late Life. J Gerontol B Psychol Sci Soc Sci 2020;75(9):1937–1950. doi: 10.1093/geronb/gbz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Billings ME, Cohen RT, Baldwin CM, et al. Disparities in Sleep Health and Potential Intervention Models: A Focused Review. Chest 2021;159(3):1232–1240. doi: 10.1016/j.chest.2020.09.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnulf I, Brion A, Pottier M, Golmard JL. Ring the bell for Matins: circadian adaptation to split sleep by cloistered monks and nuns. Chronobiol Int 2011;28(10):930–941. doi: 10.3109/07420528.2011.624436 [DOI] [PubMed] [Google Scholar]
- 61.Hoch CC, Reynolds CF 3rd, Kupfer DJ, Houck PR, Berman SR, Stack JA. The superior sleep of healthy elderly nuns. Int J Aging Hum Dev 1987;25(1):1–9. doi: 10.2190/P1A2-K0X5-K27M-TJ49 [DOI] [PubMed] [Google Scholar]
- 62.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1(1):40–43. doi: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


