Summary
Primary cilia act as antenna receivers of environmental signals and enable effective neuronal or glial responses. Disruption of their function is associated with circuit disorders. To understand the signals these cilia receive, we comprehensively mapped cilia’s contacts within the human cortical connectome using serial-section EM reconstruction of a 1mm3 cortical volume, spanning the entire cortical thickness. We mapped the ‘contactome’ of cilia emerging from neurons and astrocytes in every cortical layer. Depending on the layer and cell type, cilia make distinct patterns of contact. Primary cilia display cell-type and layer-specific variations in size, shape, and microtubule axoneme core, which may affect their signaling competencies. Neuronal cilia are intrinsic components of a subset of cortical synapses and thus a part of the connectome. This diversity in the structure, contactome, and connectome of primary cilia endows each neuron or glial cell with a unique barcode of access to the surrounding neural circuitry.
Keywords: Primary cilia, cortical neurons, cortical circuits, ciliopathies, synapse, gap junction, connectome, human brain
eTOC Blurb
The unique pattern of primary cilia contacts with the surrounding cortical circuitry may enable cilia signaling to serve as a mechanism through which local environmental signals can shape and refine neuronal circuits. Disruptions in primary cilia connectome may thus contribute to circuit dysfunction in ciliopathies and other human brain disorders.
Graphical Abstract
Introduction
The primary cilium, a microtubule-based antennae-like organelle, is present in cerebral cortical neurons, and when defective, leads to clinically significant disorders known generally as ciliopathies. A distinguishing feature of ciliopathies is disruptions in neural circuit formation and function. These disruptions are associated with a range of disorders including autism, intellectual disabilities, mood disorders, obesity, and epilepsy1–30. Correspondingly, the expression of genes encoding cilia-associated proteins is significantly dysregulated in sets of patients with major psychiatric disorders including schizophrenia (SCZ), autism spectrum disorder (ASD), bipolar disorder (BP), and major depressive disorder (MDD)31. Further, copy number variation (CNV) of the complement component 4A (C4A) gene confers significant SCZ risk in humans, and disruption of primary cilia-related processes in excitatory neurons is a key contributor to this risk32. Likewise, disruption of ciliary function in interneurons and excitatory neurons can lead to altered synaptic connectivity and E/I balance5, 33, 34. Recent studies also reveal that novel axo-ciliary synapses can regulate the epigenetic state of post-synaptic neurons35. Collectively, these studies imply a role for primary cilia in neuronal function and circuit dynamics.
However, how primary cilia of diverse neurons and glia in the human cortex are organized, how they interact with adjacent cells, and how they are incorporated into the brain connectome are unknown. Primary cilia signaling may serve as a non-traditional synaptic signaling mechanism through which local environmental signals can shape and refine neuronal circuits in health and disease. Nonetheless, their signaling partners are poorly defined. We, therefore, sought to precisely map primary cilia interactions within the human cortical connectome.
We examined the distinct ultrastructural features of neuronal (both excitatory and inhibitory) and astroglial primary cilia in every layer of the human cerebral cortex and their entire interactions with their surrounding cellular milieu using the human brain serial-section EM connectome36. Our analysis reveals neuronal and glial-type and layer-specific diversity in primary cilia characteristics and their contactome. These characteristics may subserve their functions in cortical circuitry. This comprehensive, ultrastructural map of the nature and interactions of neuronal and astroglial primary cilia within the human cerebral cortex supports the functional significance of primary cilia in the ongoing maintenance and function of cortical neuronal circuitry in humans. Neuronal primary cilium, in addition to functioning as an antenna, sensing neuromodulators in the local environment, also influences neuronal functions via specialized contacts it makes with synapses and adjacent cells. We find that neuronal primary cilia are an intrinsic component of synaptic structure in a subset of cortical synapses. Primary cilia thus help create small, local modulatory signaling ensembles within larger neuronal circuits.
Results
Organization of neuronal and glial primary cilia in the cerebral cortex
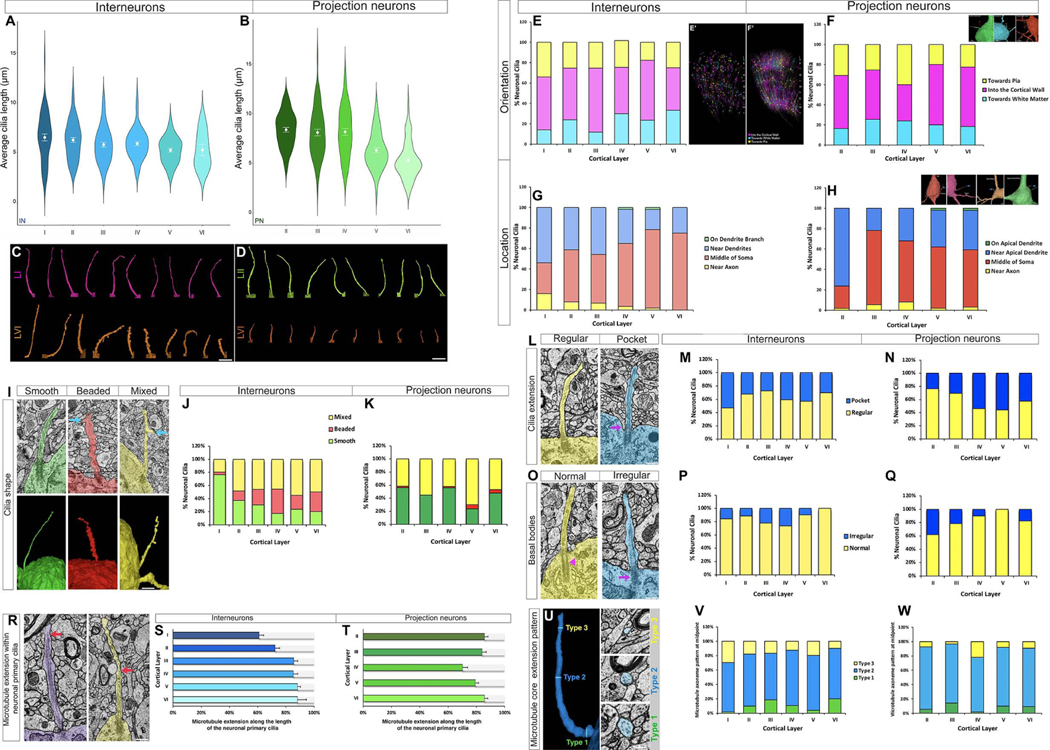
To evaluate the primary cilia contactome and connectome of the human cortical neurons, we first mapped the primary cilium of interneurons and projection neurons in all 6 layers of the cerebral cortex (Figures 1, 2, and 3; Movies S1 and S2a; Table S1). Primary cilia occupy 0.03% of cortical volume (Figure S1A). The total cellular density or volume of layers does not correlate with ciliary volume (Figure S1B–D). We then analyzed the length, orientation, morphology, and intra-ciliary structural features of neuronal primary cilia. In both the projection neurons and interneurons, upper layer primary cilia were significantly longer than the lower layer ones (layer 1 IN: 6.3±0.3μm, layer 6 IN: 4.9±0.4μm; layer 2 PN: 8.4±0.2μm, layer 6 PN: 5.5±0.2μm; Figure 4A–D). The average length of IN and PN cilia are 5.8±0.2μm and 7.4±0.6μm, respectively. The majority of primary cilia are oriented into the plane of the cortical wall (Figure 4E–F) and are located in closer proximity to the neuron’s dendrites than its axon (Figure 4G–H). Distinct neuron-specific differences are evident in cilia shape. Compared to the smoother, tubular primary cilia of projection neurons, interneuronal cilia tend to be more complex, with a convoluted or beaded ciliary membrane sheath (Figure 4I–K). These outpocketings of the ciliary membrane (arrow, Figure 4I) are reminiscent of budding ciliary ectosomes, the signaling vesicles released from cilia37.
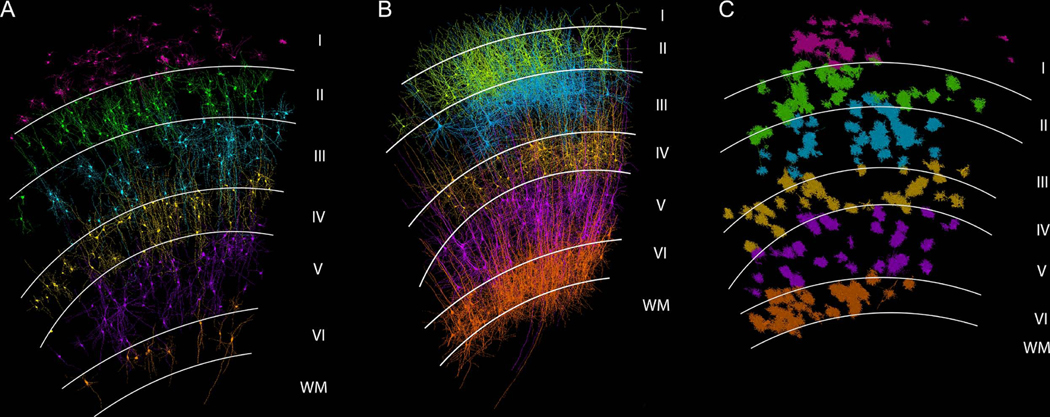
Figure 1. Examination of primary cilia in projection neurons, interneurons, and astrocytes of every cerebral cortical layer.
Primary cilium of interneurons (A), projection neurons (B), and astrocytes (C) from all different layers of the cerebral cortex were analyzed. Neurons and glia examined are shown. I-VI, different cortical layers; WM, white matter.
Figure 2. Cortical interneuronal primary cilia.
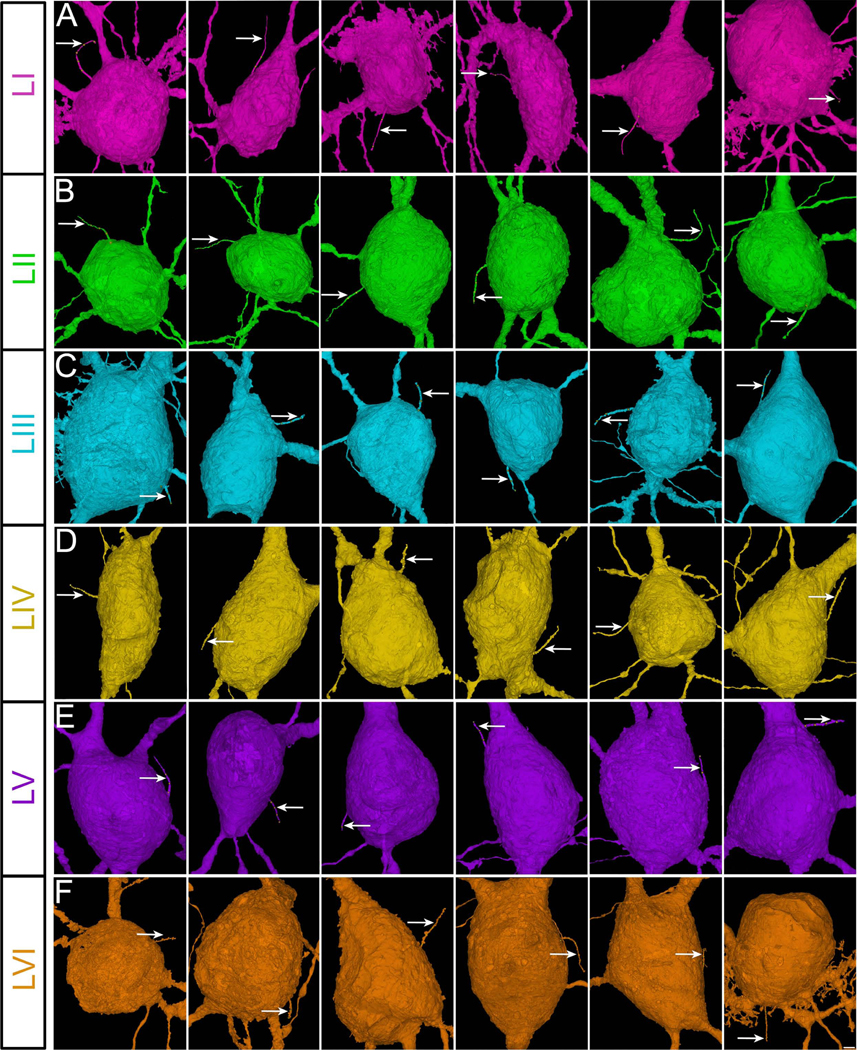
Sample images of the primary cilium (arrow) of human cortical interneurons from all six cortical layers (A-F). Cells are oriented so that axons are pointing downwards. Scale bar: 1.6μm. I-VI, cortical layers.
Figure 3. Cortical projection neuronal primary cilia.
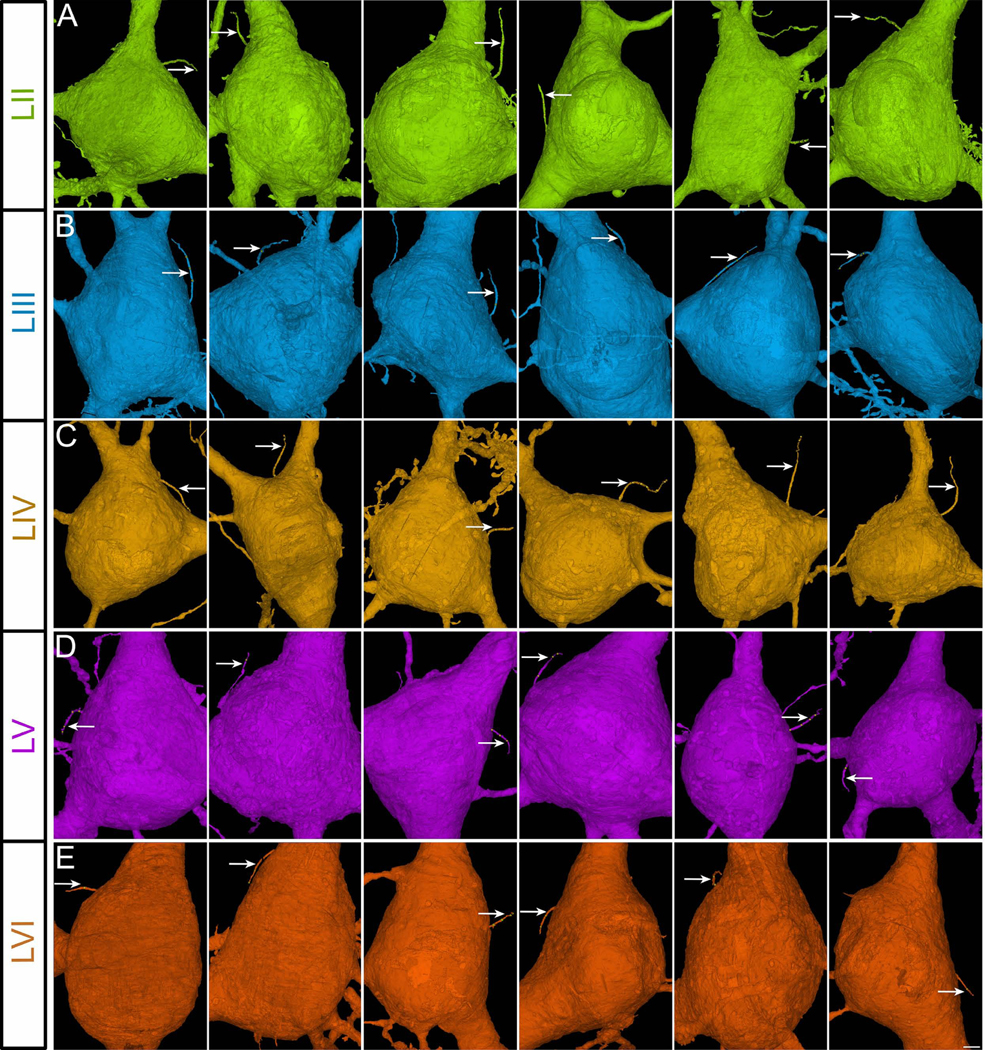
Sample images of the primary cilium (arrow) of human cortical projection neurons from cortical layers II-VI (A-E). There are no projection neurons in layer I. Cells are oriented so that apical dendrites are pointing upwards and axons are pointing downwards. Scale bar: 1.85μm. II-VI, cortical layers.
Figure 4. Diversity in the structure and organization of neuronal primary cilia.
(A-D) Diversity in the length of neuronal primary cilia. Quantification of the average length of interneuronal (A) and projection neuronal (B) primary cilia. (C) Sample images of interneuronal primary cilia from layers I and VI. (D) Sample images of projection neuronal primary cilia from layers II and VI. Data shown are mean ± SEM. One-way ANOVA: IN-F5, 286[cilia length] = 3.33, p = 0.0061; PN- F4, 303[cilia length] = 29.85, p<1−16. Two-way ANOVA: F1,540[cilia lengthIN versus PN] = 86.24, p<1E−16. Scale bar: 2μm (C-D). IN, interneuron; PN, projection neuron; I-VI, cortical layers.
(E-F) Quantification of neuronal primary cilia orientation. Orientation of neuronal primary cilia towards the pial surface, white matter, or into the cortical wall was quantified (E-F). Sample images of cilia orientation are shown in inset (top left, F). Panels E’ and F’ show the layer location of interneurons (E’) and projection neurons (F’) with different cilia orientations. Cell soma of these neurons are highlighted in colors corresponding to different cilia orientation. One-way ANOVA (orientation): IN- F2,15= 7.26, p= 0.0062; PN- F2,12= 5.72, p= 0.018.
(G-H) The position of neuronal primary cilia relative to the position of the dendrites, axon, or cell soma was examined in interneurons and projection neurons. For projection neurons, the apical dendrite was used as the landmark, whereas any dendrite was used for interneurons. Sample images of cilia (arrow) position are shown in inset (top left, H). I-VI, cortical layers. One-way ANOVA (position): IN- F3,20= 14.67, p= 0.0000278; PN- F3,16= 12.66, p= 0.00017.
(I-K) Diversity of cortical neuronal primary cilia shape. (I) Sample cilia images (EM and 3D reconstruction) of each category of shape (smooth, beaded, mixed). Arrows point to ciliary membrane outpocketings. (J-K) Quantification of neuronal cilia shape. Shape of interneuronal cilia and projection neuronal cilia from different layers were quantified. IN, interneuron; PN, projection neuron; I-VI, cortical layers. Two-way ANOVA: F2,27[cilia type] = 20.22, p = 4.3E−06. Post-hoc p[beaded cilia, IN versus PN]= 0.0064. Scale bar: 1.5μm.
(L-Q) Organization of neuronal primary cilia. Quantification of cilia extending from a ciliary pocket at the base (L-N) and basal body organization (normal or irregular) of cilia (O-Q). Sample cilia images of each category are shown in panels L (normal or ciliary pocket [arrow]) and O (normal [arrowhead] or irregular-shaped basal body [arrow]). Interneuronal cilia and projection neuronal cilia from different layers were quantified (M-N, P-Q). IN, interneuron; PN, projection neuron; I-VI, cortical layers. One-way ANOVA (cilia pocket organization): IN- F1,5 = 16.68, p= 0.002; PN- F1,4 = 25.52, p= 0.00098. One-way ANOVA (basal body organization): IN- F1,5 = 19.28, p = 0.0014; PN- F1,4= 29.04, p = 0.00065. Scale bar: 1μm (L, O).
(R-W) Changing dynamics of axoneme MT core of neuronal cilia. Sample images of a cilium with MT filaments extending through the majority of its length (arrow, R[left panel]) and a cilium with MT filaments extending only midway through its length (arrow, R[right panel]). Quantification of the extension of MT filaments within interneuronal (S) or projection neuronal (T) cilia from different layers. (U) Sample images of cross sections from the base, middle, and tip of a neuronal cilium. Cross sections containing 9–8, 7–4, and 3 or fewer MT doublet filaments are classified as type 1, 2, and 3, respectively. Quantification of the MT type at the midpoint of interneuronal (V) or projection neuronal (W) cilia from different layers. IN, interneuron; PN, projection neuron; I-VI, cortical layers. Data shown are mean ± SEM. Two-way ANOVA: F2,27[MT organization-midpoint] =254.9, p<1E−16, post-hoc p[IN versus PN]= 0.03. Scale bar: 1.4μm (R); 0.7μm (U).
We next analyzed, if the cilia of interneurons and projection neurons in different layers have distinguishing membrane specializations and cytoskeletal organization. Ciliary pockets are indicative of active vesicle transport and docking at the base of the cilium38, 39. In general, depending on the cortical layer and neuronal subtype, 24 to 56% of neuronal primary cilia have a ciliary pocket membrane specialization at their base (arrow, Figure 4L; Figure 4L–N). Furthermore, basal body organization is thought to be a key modulator of ciliary membrane extension and MT axoneme orientation and stability40, 41. A subset of cortical neurons in all layers display an irregular basal body structure (arrow, Figure 4O–Q), instead of the well-organized mother centriole-based, microtubule-organizing basal body at the base (arrowhead, Figure 4O). Layer 4 interneurons and layer 2 projection neurons have the highest percentage of irregular basal bodies (26% and 38%, respectively).
The microtubule (MT) core of neuronal primary cilia dynamically varies across neuronal types. In cortical projection neurons, microtubule filaments extend up to 70–86% of the ciliary length, with the layer 4 projection neuron cilia displaying the shortest microtubule core extension (70±3.58% of the ciliary length; Figure 4R–T). Similarly, microtubule filaments extend up to 61–88% of interneuronal cilia length, with layer 1 interneuron cilia exhibiting the shortest microtubule core extension (61±3.12% of the ciliary length; Figure 4R–T). The neuronal primary cilia MT architecture is highly diverse in contrast to the commonly observed 9+0 MT arrangement in primary cilia elsewhere28. The MT axoneme of neuronal cilia gradually changes from base to tip with devolving numbers of MT core units. We measured MT core units at the base, middle, and tip of neuronal primary cilia. A rich variety in MT axoneme architecture across different interneuron and projection neuron populations is evident (Figure 4U–W). Importantly, in the majority of cortical neurons of all subtypes, the 9+0 MT core organization is not present at the midpoint of the ciliary length; instead, 7 or fewer MT core filaments are present at the midpoint (Figure 4U–W, Movies S3 and S4).
Occasionally, we noticed oddities in neuronal ciliary organization, such as a cilium emanating from a major dendrite at a significant distance away from the soma (Figure S2A–B) or an interneuron with two basal body structures and two cilia (Figure S2C–D). We did not detect a primary cilium only in 0.48% (9/1890) of neurons, all of which were interneurons.
We then similarly mapped primary cilia of astrocytes in all 6 layers of the cerebral cortex (Table S2). Astrocyte cilia have features that are rather distinct from neuronal cilia. In contrast to neurons, astrocyte cilia are often embedded in deep ciliary pockets (greater than 50% of the ciliary axoneme length is inside a membrane pocket in 78±3% of cortical astrocytes. The average length of the astrocyte cilium is 3.97±0.09 μm, shorter than neuronal cilia (INcilia: 5.8±0.2μm, PNcilia: 7.4±0.6μm; Figure S3A). The majority of them are oriented into the cortical wall (Figure S3B–C) and are of smooth tubular shape (Figure S3D). ~40% or more of astrocytes in every cortical layer display an irregular basal body structure (Figure S3E). Unlike neurons, in 10.58±2 % of cortical astrocytes, the ciliary axoneme was found to be still within the ciliary sheath embedded inside the astrocyte cell soma (Figure S3F–H; Movie S5). These astrocytes do not have externally visible cilia. We did not detect such cilia in any neurons. A 9+0 microtubule structure is mostly absent at the midpoint of astrocyte cilium (Figure S3I–K). The majority of them contain fewer than 4 microtubule core units at the midpoint (Figure S3I–K). No ciliary structures were detected in mature myelinating oligodendrocytes.
Neuronal and glial primary cilia contactome in the cerebral cortex
To explore how neuronal primary cilia interact with the surrounding cells in the human cerebral cortex, we mapped every cell that came in membrane contact with the primary cilium of interneurons and projection neurons in all 6 layers of the cerebral cortex (Figures 5 and 6; Movies S2b, S6–9; Table S3). We first analyzed the cell type, cell domain, and the layer position of these ciliary contacts. On average, an individual interneuronal primary cilium is contacted by 41±0.4 other cells, whereas 47±0.5 cells contact a projection neuronal cilium (P<0.0001, t-test). Only rarely does a neuronal cilium contact the same cell twice or more. Interneuronal cilia, across layers, make a comparably similar number of contacts, whereas upper layer projection neuronal cilia make a significantly higher number of contacts than lower layer projection neuronal cilia (layer 2 PN cilia: 57±1.6, layer 6 PN cilia: 39±0.7, P<0.0001 [t-test]; Figures 5, 6, Figure S4A–B). The length of a neuronal cilium positively correlates with the number of contacts it makes with other neural cells in the cortex (r, 0.5974, p=1E−14).
Figure 5. Cortical interneuronal primary cilia contactome.
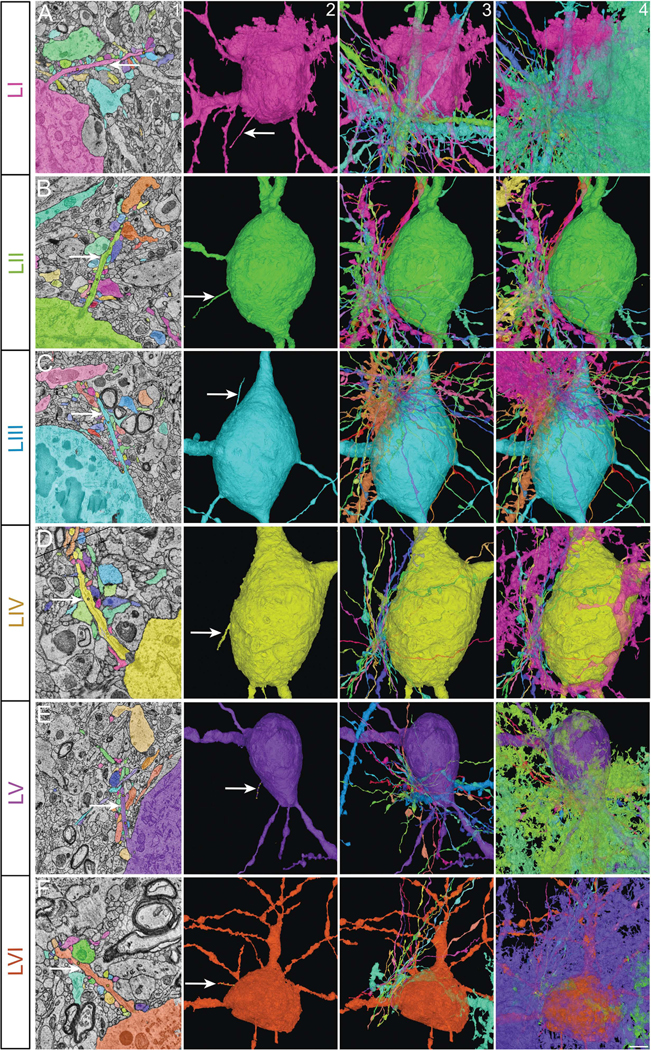
Primary cilia of cortical interneurons from all six cortical layers (arrow, columns 1 and 2; A-F) and all the axons and dendrites of other neurons (column 3) as well as the non-neural cells contacting them (column 4). Column 1 shows electron micrographs of the relevant cilium (arrow) and its contacting cells. I-VI, cortical layers. Scale bar: 2μm (column 1); 5μm (columns 2–4).
Figure 6. Cortical projection neuronal primary cilia contactome.
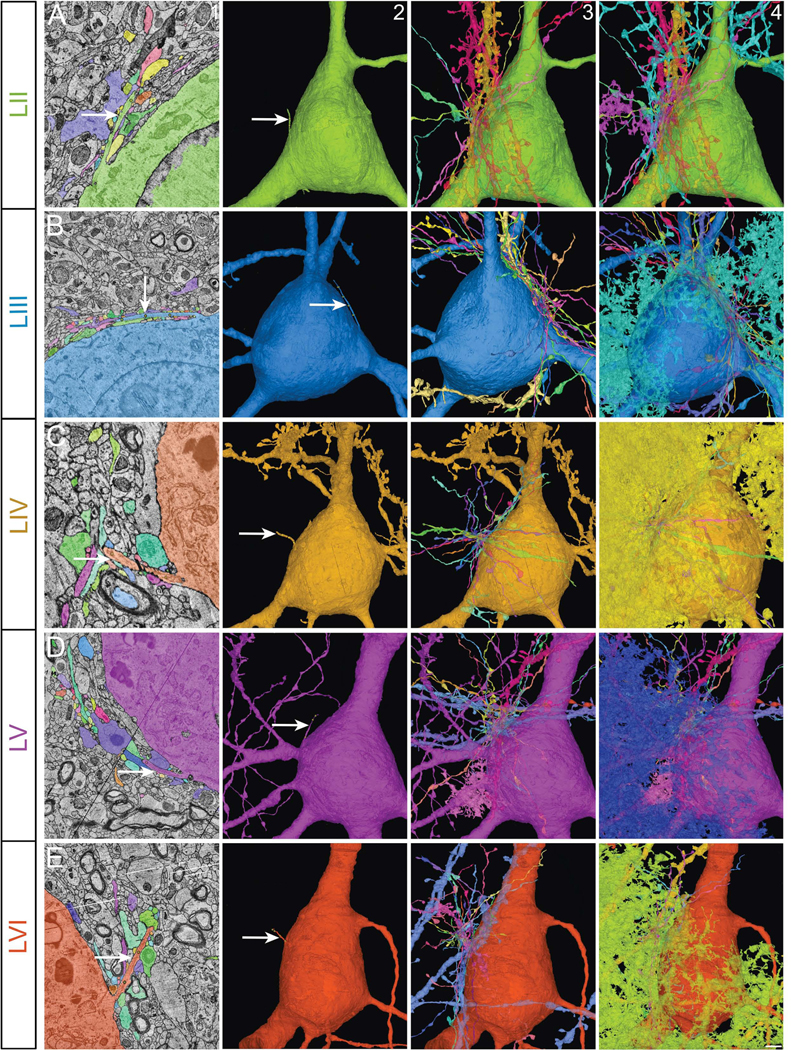
Primary cilia of cortical projection neurons from layers II-VI (arrow, columns 1 and 2; A-E) and all the axons and dendrites of other neurons (column 3) as well as the non-neural cells contacting them (column 4). Column 1 shows electron micrographs of the relevant cilium (arrow) and its contacting cells. II-VI, cortical layers. Scale bar: 2μm (column 1); 5μm (columns 2–4).
In general, projection neuronal cilia make significantly more contact with other projection neurons, whereas interneuronal cilia make contact with both interneurons and projection neurons (PN cilia contacts [# projection neurons/ # interneurons]: 6.86±0.37, IN cilia contacts [# projection neurons/ # interneurons]: 1.97±0.08, P<0.0001 [t-test]; Figure S4A–D; Table S4). This pattern is seen in all cortical layers even though the ratio of projection neuron to interneuron numbers varies layer by layer (0.0068[L1], 1.83 [L2], 1.73[L3], 3.80[L4], 3.46[L5], 6.29[L6])36.
Cortical neuronal cilia make substantially more membrane contacts with axons than dendrites (~10 fold higher). Compared to interneuronal cilia, projection neuronal cilia predominantly contact other excitatory axons than inhibitory axons (PN cilia contacts [excitatory/inhibitory axons]: 6.81±0.37 vs IN cilia contacts [excitatory/inhibitory axons]: 1.84±0.09, P<0.0001 [t-test]; Figure S4G–J; Table S5). In contrast, both interneuronal and projection neuronal cilia make significantly more contact with excitatory, spiny dendrites than with inhibitory dendrites (IN cilia: 0.27±0.03 [inhibitory] vs 2.04±0.10 [spiny], PN cilia: 0.26±0.03 [inhibitory] vs 1.93±0.10 [spiny], P<0.0001 [t-test]; Figure S4K–L).
Projection neurons within the six-layered human cortex are hierarchically organized based on their projection patterns42. The intratelencephalic, upper layer neurons (2–3) project to other domains of the cerebral hemisphere and striatum, whereas the extratelencephalic, deeper layer neurons (5–6) project to thalamus and subcortical targets. The ratio of inhibitory neurons contacting the subcortically projecting, deeper layer (L5–6) projection neurons’ cilia is significantly higher than the intracortically projecting, upper layer (L2–3) projection neurons’ cilia (excitatory/inhibitory: 4.04±0.46L5–6 vs 7.53±1.71L2–3, P<0.0001 [t-test]). Similar to projection neurons, cortical interneuronal diversity can be further distinguished based on molecular, morphological, connectivity, and activity patterns43. Morphologically, cortical interneurons can be broadly divided into basket cells, bipolar cells, neurogliaform cells, Martinotti cells, and chandelier cells. Primary cilia of chandelier cells, which make unique axo-axonic synapses on the AIS of cortical projection neurons44, contact 1.6 fold more cells and almost twice as many projection neurons as compared to primary cilia of other cortical interneurons (P<0.01, Tukey’s multiple comparison test; Figure S5A–D).
Compared to neuronal cilia-neuron contacts, neuronal cilia-glial contacts are ~15-fold less frequent (Figure S4A–B). Both interneuronal and projection neuronal cilia are contacted primarily by astrocytes than other glial cell types (i.e., microglia and oligodendroglia). However, projection neuronal cilia make significantly more glial contacts (+22.3±2.7%) than interneuronal cilia (P<0.0001, t-test; Figure S4A–B, E–F).
To clarify the distinctiveness of neuronal ciliary contacts with the surrounding cortical cellular milieu, we mapped the cellular contacts of adjacent axons of the same phenotype (i.e., excitatory or inhibitory) and length as the respective neuronal cilia (Figure S6A–B, D–E; Table S6). The patterns of cellular contacts of these axonal domains are significantly different and did not form the same pattern, based on layers or neuronal types, noticed for neuronal cilia (Figure S6A–B, D–E; Tables S4, and S5), thus indicating the specificity of the neuronal ciliary contactome. The distinctiveness of the cellular contacts made by a cilia-sized domain of the respective neuronal AIS further supports this specificity (Figure S6C, F; Tables S7, S8A, and S8B).
To further explore the distinct nature of the neuronal cilia contactome, we mapped the primary cilia contactome of the astrocytes in every cortical layer (Figure 1C; Figure 7; Movies S10 and S11). Astrocytes intimately associate with neurons and modulate their function45. In contrast to neurons, majority of astrocyte cilia are embedded in deep ciliary pockets. Astrocyte cilia make a significantly fewer number of contacts compared to neurons (7.6±0.12 [vs INcilia: 41±0.4 or PNcilia: 47±0.5, P<0.0001, Tukey’s multiple comparison test]); the majority of them are with projection neurons and their axons (Figure 7; Tables S9A and SB). The distinctly different pattern of the astrocyte cilia contactome as compared to the neuronal cilia contactome indicates the uniqueness and diversity of neuronal and glial ciliary interactions with the surrounding cortical circuitry.
Figure 7. Cortical astrocyte primary cilia contactome.
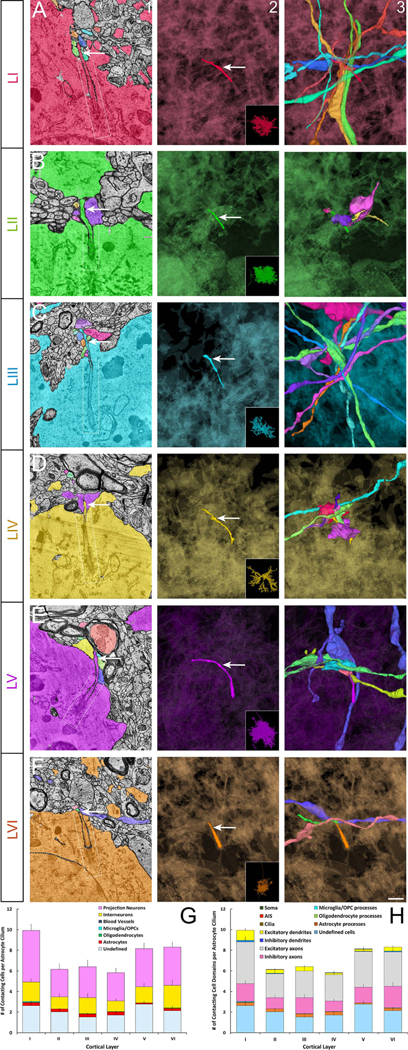
Primary cilia (arrow) of astrocytes from layers I-VI (arrow, columns 1 and 2; A-F) and all the axons and dendrites of other neurons as well as the non-neural cell processes contacting them (column 3). Column 1 shows electron micrographs of the relevant cilium (arrow) and its contacting cells. Ciliary pockets are outlined in column 1 (white box). In columns 2 and 3 astrocyte cell body is shown in a translucent background so as not to obstruct the cilium. Insets in column 2 show the whole astrocyte. (G-H) Quantification of astrocyte primary cilia contactome. Cell types (G) and different cell domains (H) contacting the primary cilium of different cortical layer astrocytes were quantified. Data shown are mean ± SEM. Two-way ANOVA (cell types): F6, 30= 85.93, p= 1.1E−15. Two-way ANOVA (cell domains): F10, 50= 102, p=1E−15. I-VI, cortical layers. Scale bar: 0.45μm (column1); 0.8μm (columns 2–3).
The Diversity of neuronal and glial ciliary interactions with the cortical connectome
We then examined the defining features of neuronal ciliary membrane contacts with other cells of the cortical connectome. In particular, we examined if there are membrane specializations (i.e., synapse, gap junction, tight junction, adherens junction, etc.) at the sites of contact between a neuronal cilium and nearby cells, and if any preferential distribution of organelles (vesicles, lysosomes, mitochondria, ER, Golgi, etc.) are evident at these sites of contact.
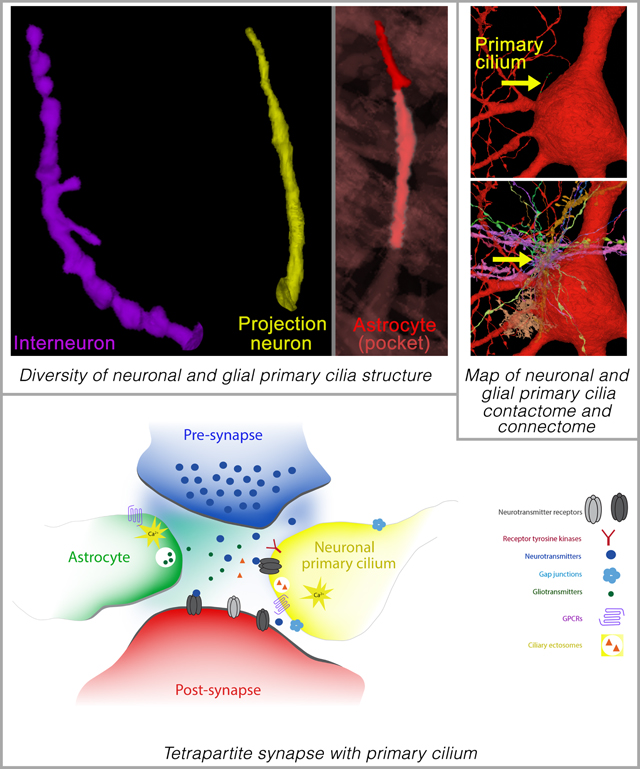
Three types of neuronal cilia-axon contacts are evident. First, some of the contacts between axons and cilia are gap junction-like. These contacts are distinguished by a narrow membrane separation of 2–4nm with electron-dense intercellular bridges in the middle (arrow, Figure S7A–D), characteristics similar to known GAP junctions49–52. Consistent with the presence of gap junctions in cilia, expression of a neuronal gap junction hemichannel, connexin-3653, is detected in subsets of (9.6±0.7%) human cortical neuronal primary cilia (Figure S7E). The second type of axon-ciliary contact is characterized by a dense accumulation of vesicles (arrow [F, H]) on the axonal side of cilia-axon contacts (Figure S7F–L). Reminiscent of presynaptic axon compartments, mitochondria are also present in axons at these sites (asterisk, Figure S7F). Whether these axon-cilia contacts are the cortical analogs of the recently described axo-ciliary synapses between serotonergic brainstem neurons and hippocampal CA1 pyramidal neurons35 remains to be established. Intriguing are the third kind of neuronal cilia-axon contacts. These ciliary contacts are immediately adjacent to the synapses (Figure 8). Primary cilia of both interneurons and projection neurons of every cortical layer can be found intimately associated with synapses (Figure 8; Movie S12), often forming a triangular partnership in which the primary ciliary compartment is intercalated next to the synaptic cleft. Primary ciliary tips are also found in tripartite (axon-dendrite-astrocyte) synaptic clefts (Figure 8). 51% of neuronal primary cilia engage in synaptic contacts. The majority of these synapses are excitatory (67%) as is the case for synapses generally in this sample. These observations indicate that primary cilia are intrinsic components of synaptic structure in subsets of cortical synapses. In contrast, control axonal segments adjacent to the respective neuronal cilia rarely make such contacts (neuronal cilia: 0.6±0.09 vs axon control: 0.05±0.03, P<0.0001 [t-test]). Compared to simple, tubular neuronal cilia, complex, beaded neuronal cilia make a significantly higher number of axo-ciliary associations, synaptic partnerships, and gap junction-like contacts (smooth cilia: 1.53±0.19, beaded cilia: 4.16±0.42, P<0.0001 [t-test]). Furthermore, 10% of neuronal cilia made only synaptic contacts, 14% made only axo-ciliary type contacts, and 7% formed only gap junction-like contacts. The majority of cilia made two or all three types of contacts.
Figure 8. Neuronal primary cilia as an intrinsic component of synaptic structure.
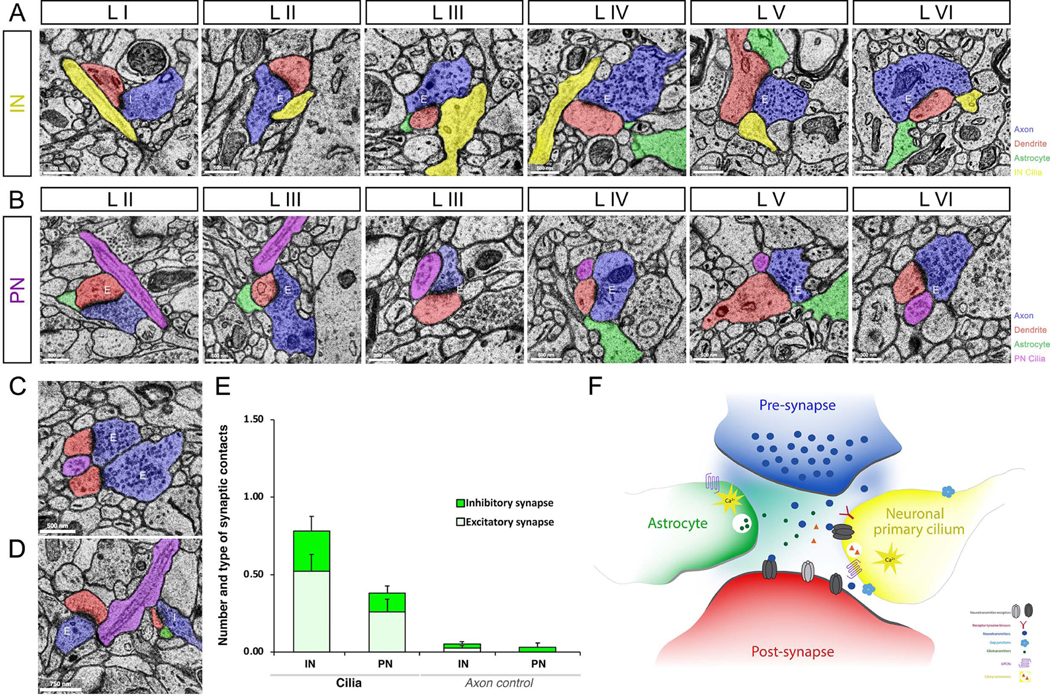
Neuronal primary cilia tips are located right next to synapses. Primary cilia highlighted in yellow and purple indicate primary cilia from inhibitory (A) and excitatory neurons (B), respectively. Astrocytes, axons, and dendrites are colored in green, blue, and red, respectively. Cortical layers (I-VI) and synapse type (excitatory [E] and inhibitory [I]) are indicated in each panel. IN, interneuron; PN, projection neuron. (C) Electron micrograph of two synapses with a contacting projection neuronal primary cilium in the middle. (D) Electron micrograph of two synapses (excitatory [E] and inhibitory [I]) contacted by a projection neuronal primary cilium. (E) Quantification of synaptic association of neuronal cilia. For each cilium, the number of ciliary contacts with bonafide chemical synapses was quantified. Synapse type (excitatory [E] and inhibitory [I]) was also noted. Similar quantifications were made for adjacent control axonal segments of the same length and phenotype as the respective cilium. IN, interneuron; PN, projection neuron. Data shown are mean±SEM. INcilia vs INaxon control, P<0.05 (t- test). PNcilia vs PNaxon control, P<0.05 (t-test). (F) Using the cilia localized signaling machinery, synaptic primary cilium may sense and respond to synaptic activity and environment. Unlike the previously known tripartite synapse (axon, dendrite, astrocyte), the integration of neuronal primary cilium leads to the formation of a tetrapartite (pre, post, astrocyte, cilium) synapse. Neuroglancer links to images in A-D are as follows- A: LI, LII, LIII, LIV, LV, LVI; B: LII, LIII, LIII, LIV, LV, LVI; C, D.
Neuronal primary cilia also make numerous contacts with dendritic spines (Figure S8A–D). Large mitochondria are seen in 32.5% of cilia-contacting dendritic spines (asterisk, Figure S8A–C)54. In contrast to neurons, both astrocyte (Figure S8E–G; Movie S13) and microglial (Figure S8H–I) processes encircle the neuronal cilium at the sites of contact, often fully embedding them into their network of processes.
Akin to neuronal cilia, a subset of astrocyte cilia associates with synaptic clefts (Figure S9A–B, E), and can form axo-ciliary contacts (Figure S9C–D, E). Astrocyte cilia makes significantly fewer such contacts than neuronal cilia (neuronal cilia: 1.78±0.16 vs astrocyte cilia: 0.0297±0.016, P<0.001 [t-test]). Further, astrocyte primary cilia also form contacts with dendritic spines (Figure S9F). As like neuronal cilia, astrocyte cilia are often engulfed by astrocyte processes (Figure S9G).
The majority of neuronal ciliary contacts emerge from other neurons or glia in the same or adjacent layer (99.9% of IN or PN cilia contacts with other neurons; 99.8% of IN or PN cilia contacts with glia) (Figure S10A–B). However, occasional long-distance contacts spanning multiple layers, between a neuronal cilium and dendritic projections from neurons a few layers away, were also noticed (Figure S2E–F). Overall, this predominantly local nature of the neuronal ciliary connectome is in contrast to the extensive, multi-layer, and region-spanning nature of the respective neuron’s synaptic connectome (Figure S11). As with neurons, the majority of astrocyte ciliary contacts (99.9% of contacts with neurons; 100% of contacts with other glia) originate from other neurons and glia in the same or an adjacent layer (Figure S10C).
Relationship between cilia and neuronal structural and functional features
Since neuronal primary cilia are thought to modulate the growth, connectivity, and function of neurons, we analyzed whether neuronal ciliary characteristics and the corresponding functional (e.g., synaptic input or output) or structural features of neurons (e.g., spininess, volume, etc.) are correlated. During image segmentation, automated predictions of the neuronal subcompartment composition (axon, dendrite, dendritic spine, AIS, etc.) were generated at points along each segment36. We used these predictions to quantify neuronal characteristics, for example, the spininess of a neuron is defined by the ratio of spine predictions to dendrite predictions along the segment. The number and type of synaptic connections and volume (voxels) of each neuron were also quantified during image segmentation and annotation36. We found a moderate but significant positive correlation between the cilia length and the spininess of a neuron (r, 0.41, p<1E−16; Figure S12A). The spininess of a neuron positively correlates with the total number of neuronal ciliary contacts (r, 0.352, p<1E−16; Figure S12B), in particular with the number of excitatory axonal contacts (r, 0.614, p<1E−16; Figure S12C). In addition, the number of incoming synapses to neurons positively correlates with the number of excitatory axonal contacts of cilia (r, 0.43, p<1E−16; Figure S12D). In contrast, both the spininess and the number of incoming synapses to neurons negatively correlate with the number of neuronal ciliary inhibitory axonal contacts (r, −0.39spininess [p<1E−16], −0.34number of incoming synapses [p=1E−16]; Figure S12E–F). These observations provide a rationale to further examine the intentional or incidental nature of the correlation between the patterns within the ciliary connectome and the functional characteristics of cortical neurons.
Discussion
Disrupted primary cilia signaling and the resultant changes in neuronal circuit formation and function lead to brain malformations and neurodevelopmental disorders, including developmental delay, autism, intellectual disabilities, mood disorders, schizophrenia, obesity, ataxia, apraxia, and epilepsy. Whilst these observations implicate primary cilia signaling as a potential non-synaptic mechanism through which environmental signals may shape and refine neuronal circuits, how neuronal primary cilia are organized within the cortical connectome to impact neural circuit function in the human cerebral cortex remain unresolved. Here, using serial EM reconstructed human cortex, we precisely and comprehensively delineated primary cilia organization and the connectome that animates ciliary signaling mechanisms in a diverse array of neurons and astrocytes in all layers of the human temporal cortex.
The length of a neuronal cilium positively correlates with the number of contacts it makes. Cortical neuronal cilia can make several types of functional associations with axons, dendrites, and synapses to effect changes in neuronal function. First, neuronal cilia can form intimate contacts with the synaptic cleft, thus exposing neuronal ciliary membrane receptors to synaptically released neurotransmitters, neuromodulators, second messengers, and other neuroactive components. A perisynaptic cilium may bind synaptically released neurotransmitters (NTs). NTs such as norepinephrine, serotonin, dopamine, and somatostatin that can activate different ciliary GPCRs are widely expressed in the mature cerebral cortex. Furthermore, the ion channels in cilium (e.g., PKD1L1, PKD2L1, PKD2, TRPC1 and 4, TRPM2 and 3, and TRPV4)55–61 may help maintain the appropriate ionic milieu of the synaptic cleft necessary to facilitate proper synaptic function. Single cell RNAseq-based expression analysis of these cilia-associated receptors, ion channels, and second messengers in diverse human cortical neurons indicate distinct patterns of neuronal expression (Figure S13)21, 23, 62–65, thus suggesting a neuron-type specific diversity in the signaling competence of cortical neuronal primary cilia. Compellingly, 75% of the known cilia-associated GPCRs are differentially expressed in the frontal cortices of a major neural circuit disorder, schizophrenia31.
Neuronal cilia also make numerous contacts with axonal segments that are full of synaptic vesicles. These contacts are distinct from classical chemical synapses. A few of these cilia-axonal contacts have mitochondria (Figure S7) in addition to vesicles, suggestive of a presynaptic style architecture. However, post synaptic density-type membrane contrast enhancement is not consistently seen on the ciliary side of these contacts, though this should be further explored with different EM fixation methods designed to enhance contrast. Neuronal cilia make comparatively more axo-ciliary-type associations than contacts with chemical synapses (1.18±0.12 vs 0.6±0.09, P<0.001, t-test). This intriguing pattern of ciliary interactions with different synaptic compartments may provide an avenue of functional flexibility to ciliary signaling.
Aside from the interactions with chemical synapses and axo-ciliary contacts, gap junction-like, electrical synaptic contacts between cilia and axons are evident as well. These gap junctional contacts may enable direct diffusion of second messengers and small metabolites, such as Ca2+, cAMP, ATP, glucose, and inositol triphosphate, between a neuronal primary cilium and the axonal terminal46–48. Due to their ability to electrically couple cells, these gap junctions may also directly facilitate the spreading of presynaptic electrical currents to the postsynaptic neuron via its primary cilium. However, it is important to note that due to the current resolution (4X4nm2) of the EM dataset, our observations might underestimate the electrical synapses associated with cilia due to their small size (1–20 nm). Enhanced electron-dense labeling methods during the EM procedure coupled with molecular tagging of gap junction proteins will help resolve this deficit in the future. Further, human cortical neurons from both sexes were used in our cilia-gap junction co-localization studies, but the influence of sex on the pattern of co-localization is unknown.
Of these associations of neuronal primary cilia with the activity-related compartments of other neurons, the primary cilium as an intrinsic component of subsets of cortical synapses is compelling (Figure 8). A single neuronal primary cilium can contact up to ~5 synapses, both excitatory and inhibitory. Synaptically released ligands can act on the ciliary membrane receptors, generate Ca2+ transients5, 66, and thus provide neurons an additional ability to monitor and modulate their functional homeostasis in response to local activity. However, several questions remain: How do cilia-derived signals or ciliary invagination of synaptic structures affect the function, maturation, maintenance, and sculpting of synaptic circuits? Contact by primary cilia may regulate critical aspects of synapse formation, function, maintenance, or elimination, as the other cellular partner of synapses, i.e., astrocytes, do45. Nonetheless, primary cilia do not intercalate with all synapses; thus it will be important to establish if the primary cilia-synapse association is a dynamic process that depends on neuronal or astroglial activity. Other related questions, including if the primary cilia’s association with synapses is part of key epochs of cortical circuit development and plasticity, such as the critical period, LTP, LTD, and homeostatic scaling, remain completely open.
Astrocyte-derived signals may not only directly regulate the synapses45, 67, 68, but may also fine-tune neuronal activity via the adjacent primary cilia. For example, astrocyte-released signals may regulate neurotransmitter or neuromodulator receptor localization on the ciliary membrane at synaptic sites. Furthermore, synaptic activity can trigger astrocytic Ca2+ rise and the secretion of neuroactive gliotransmitter molecules, such as glutamate, D-serine, taurine, TNF-a, and ATP45, 69–71. Similarly, microglia can also release modulators such as ATP in response to circuit activity72–74. The ciliary membrane is known to be decorated with receptors for these gliotransmitter ligands (e.g., P2YR2 for ATP), and thus may enable glial modulation of neuronal or glial homeostasis via primary cilia.
Primary cilia can form contacts with axons, dendrites, astrocytes, and microglia, but rarely associate with neuronal AIS or blood vessels. Neuronal primary cilia contact 10 fold or more axons than dendrites, even though the numerical ratio of axonal to dendritic processes in the cortical volume is ~1.8:136. Similarly, despite the presence of twice as many glia than neurons, only ~6% of neuronal ciliary contacts are with glia. Further, there are nearly 3.7 times as many oligodendrocytes than astrocytes in the cortical volume36, but neuronal cilia associate with ~14 fold or more astrocytes than with oligodendrocytes (Table S5). However, other than cell numbers, the volume occupied by the different cell types and cellular domains in each layer may also be an important contributing factor to the pattern of ciliary contactome we observed. Further evaluation of the functional relevance of these unique features of ciliary association with the surrounding cells in the cortical connectome and examination of the intentional vs incidental nature of these associations will help determine how neuronal and glial cells form and organize their contacts with neuronal primary cilia to subserve a circuit modulating function.
In addition to rapidly modulating neural circuits through its rich and diverse interactions with the cortical circuit, neuronal cilia may also generate a direct link to the nucleus to modulate neuronal activity via transcriptional changes. Ciliary receptor activation can trigger changes in the accessibility of gene regulatory elements and chromatin landscape in neurons35, 75, 76. The positive correlation between the cilia length and the spininess or the number of incoming synapses in a neuron raises the intriguing possibility that neuronal ciliary signaling may impact the synaptic competence of a neuron via such cilia-mediated gene regulation.
Structurally, it is remarkable that almost all cortical neuronal cilia do not extend a 9+0 microtubule axoneme all the way to the tip. The majority of cortical neuronal cilia do not display a 9+0 MT doublet-based axoneme core at the ciliary midpoint. This may help create neuronal ciliary domains unencumbered by a rigid MT axoneme core to facilitate efficient ciliary signaling vesicle (i.e, ectosome)37 release. Importantly, the changing microtubule core along the neuronal ciliary length may also modulate the anterograde and retrograde intraflagellar transport of signaling molecules within a cilium and thus its signaling competence77–80. The layer and neuronal type-specific variations in the MT axoneme in cortical neurons may thus underly the functional diversity in neuronal primary cilia. A similar variation in 9+0 MT axoneme core is also evident in astrocyte cilia. However, unlike neurons, the majority of astrocyte cilia (~75%) are in deep membrane pockets, without access to surrounding circuitry. Further, the presence of a ciliary axoneme embedded within the ciliary sheath inside an astrocyte soma in subsets (~10%) of adult human cortical astrocytes suggests an active intracellular pathway-mediated ciliogenesis in these cells81, 82. Such diversity between neuronal and glial cilia may help modulate the distinct roles primary cilia play in neurons versus glia in the human cortex.
Collectively, these observations suggest that there exists a neuronal cell type-specific ciliary signaling avenue that can exert a dynamic influence on the functional state of neurons. By coincidence or design, each cortical neuronal or astroglial primary cilium interacts with a unique subset of other cortical neurons and glia. This remarkable diversity in the individual neuronal and glial ciliary connectome and their fine structure may underlie how primary cilia activity is transformed into changes in neural circuit function. The primary cilia connectome endows a neuron or astrocyte with a unique barcode of association with the surrounding neural circuitry. It raises the intriguing possibility that this diversity enables primary cilia of different cortical neurons and glia to sample and fine-tune local neuronal circuit activity and neuronal homeostasis in a unique manner. When perturbed in ciliopathies or other human brain disorders, alterations in this unique neuromodulation may trigger cortical neural circuit dysfunction.
STAR Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to, and will be fulfilled by, the lead contact, E. S. Anton (anton@med.unc.edu).
Materials availability
This study did not generate unique reagents.
Data and code availability
Serial EM dataset used in this study is available at https://h01-release.storage.googleapis.com/landing.html. Microscopy data reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental Model and Study Participant Details
Serial EM reconstruction of human cortical volume
A cortical thickness slab (2.5cm X 0.8cm) of histologically normal, unaffected left anterior temporal lobe from a 45-year-old female with a history of simple and complex partial seizures, resistant to drug treatment, was removed during surgical ablation of an epileptic focus in her left medial temporal lobe. This normal tissue was used for the generation of serial EM dataset36. The human brain tissue sample was collected and processed according to the Massachusetts General Hospital, Harvard University, and NIH guidelines on the use of human tissue.
Human cortical neuronal cells
The fetal tissue (GW15–18) harvesting and neurosphere collection were done as described previously (Konopka et al., 2012; Stein et al., 2014)84–85 and cells were derived from frozen pHNPC stocks. Neuronal cells from both males and females were used. Cells were maintained at 37°C/ 5% CO2 in Neurobasal A (Invitrogen) supplemented with 10% BIT (Stem Cell Technologies), Primocin (Invivogen), GlutaMAX (Gibco), and heparin (1 μg/mL; Sigma)] with freshly added EGF, FGF2, PDGF (each at 20 ng/mL; Invitrogen), and LIF (2 ng/mL; Invitrogen).
Method Details
Serial EM reconstruction of primary cilia in human cortical volume
Serial EM reconstruction of human cortical volume is described in Shapson-Coe et al., 2021 (and submitted)36. Briefly, a full cortical thickness slab (2.5cm X 0.8cm) of histologically normal human temporal lobe from a 45-year-old female was harvested during hippocampal resection surgery to treat epilepsy. The sample was stained with osmium, trimmed down to a rectangular area of 4584 X 1975 micrometers spanning all cortical layers from layer 1 to superficial white matter, sectioned at 30nm, and imaged at 4X4 nm resolution in multibeam (61 electron beams) scanning electron microscope mSEM (Zeiss). Images from more than 5292 slices were aligned, stitched, rendered, and segmented36. The total data set has a volume of ~ 1 mm3 volume and contains ~1.4 PB of image data, containing the three-dimensional structure of 56,000 cells, hundreds of millions of neurites, and >100 million synaptic connections. Training data based on human tracing of >700 cells were used to obtain a machine-learning algorithm to locate each cilium in the tissue. Primary cilia in different cortical neurons and astrocytes in all 6 layers of the cerebral cortex were reconstructed from this data set. These cilia annotations and the rest of this human connectomic data are available at https://h01-release.storage.googleapis.com/landing.html.
Analysis of primary cilia in neurons and glia of all cerebral cortical layers
We randomly identified 50 or more non-spiny local interneurons (INs) and pyramidal-shaped spiny projection neurons (PNs) with primary cilia in each layer (Table S1). The primary cilium of each neuron was confirmed by its characteristic microtubule-based axoneme and basal body anchor. We then analyzed (1) if there are neuron type-specific differences in the structure, shape, content, and cell domain origin of the primary cilium, (2) a complete census of all the cellular contacts [‘contactome’] made by individual neuronal cilium, (3) if these neuronal cilia form specific, distinguishable, functional contacts [‘connectome’] with other neurons (e.g., with axons, dendritic spines, synapses, and AIS [axon initial segments]), glia (i.e., astrocytes, oligodendroglia, and microglia), or blood vessels in the environment, (4) the distinguishing features and diversity of primary cilium-neuron or glial contact sites, and (5) the neuronal layer and subtype-specific characteristics of primary cilia. Further, we examined the contactome and connectome of astroglial primary cilia in every cortical layer. Due to the complex morphology of astrocytes, astrocyte cilia were identified by first locating the basal bodies in these cells and then tracing the microtubule (MT) based cilia emanating from them. The following number of neurons and glia were analyzed: Interneurons (Layer I-50, Layer II-63, Layer III-59, Layer IV-57, Layer V-51, Layer VI-12); Projection neurons (Layer II-55, Layer III-55, Layer IV-50, Layer V-50, Layer VI-98); Astrocytes (Layer I-38, Layer II-32, Layer III-32, Layer IV-33, Layer V-31, Layer VI-36).
If >75 % of the length of ciliary surface was smooth or beaded, it was binned as smooth or beaded cilium, respectively. If a cilium had both membrane characteristics and neither (smooth or beaded) covered at least 75% of its length, it was binned as a mixed type. A cilium was considered to be near an axon or a dendrite if the ciliary base is less than 5μm from the base of the axon/dendrite. For cilia orientation, a cilium is classified as “towards pia” or “towards white matter” if it is angled at 45 degrees or greater away from the cell soma in the direction of pia or white matter, respectively. Otherwise, its direction is noted as ‘into the cortical wall’. Cell fragments that were less than 10μm in size could not be classified (for example, as axon, dendrite, soma, or glia, etc.) and were binned as undefined cells. Organelles such as lysosomes were defined as described in Barral et al., 202283. Lysosomes range in size from 200 to 600 nm, are often electron-dense, and have membrane whorls.
As a control for the specificity of neuronal primary ciliary interactions with the surrounding milieu, we identified a segment of the axon of the same phenotype (excitatory or inhibitory) that is immediately adjacent to the neuron on the other side of the cilium and of the same length as the respective neuronal cilium. We then mapped all the cells and cellular sub-compartments that are in contact with that segment of the axon the same way we did for the neuronal cilium. Patterns of control axonal contacts were then compared to the patterns of neuronal ciliary contacts. Similar quantification was also done for the axon initial segments of a subset of interneurons and projection neurons in layer I and VI, respectively.
Immunohistochemistry
Human cortical neuronal cells (4 weeks in vitro) were generated from human neural precursors (GW15–18)84,86. The following primary antibodies were used to immunohistochemically label these cells: Arl13B (mouse, 857602, Biolegend), Connexin 36 (rabbit, 710663, ThermoFisher), and neuron-specific class III β-tubulin [Tuj1] (chicken, ab9354, Millipore). Appropriate Cy2, Cy3, or Alexa dye-conjugated secondary antibodies (Jackson ImmunoResearch, Molecular Probes) were used to detect primary antibody binding. DAPI (Sigma-Aldrich, D9542) was used as a nuclear counterstain. 2914 neurons from 8 independent experiments were analyzed. Images were obtained using a Zeiss LSM780 confocal microscope.
Quantification and Statistical Analysis
General statistical analysis
Excel, SAS, GraphPad, or R language were used for data analysis. Statistical significance was determined by two-tailed Student’s t-test for comparisons between two groups and by ANOVA with Tukey-Kramer’s test for comparisons among multiple groups. Correlation between groups was tested by Pearson’s or Point Biserial correlation4–6, 8, 86. No a priori power analyses were performed. All data are expressed as mean ± standard error of the mean (SEM). Statistical details, including p values, are indicated in the text or figure legends. The number of cells analyzed (n) is indicated in the relevant methods sections (Analysis of primary cilia in neurons and glia of all cerebral cortical layers, Immunohistochemistry) or the figure legends.
Supplementary Material
Supplemental movie 1 (Related to Figures 2 and 3). A comprehensive EM stack of a cortical neuronal primary cilium. EM sections of a layer 4 interneuron cilium (highlighted in teal; sagittal view) illustrating the entire length of the microtubule-based ciliary axoneme and the cilia anchoring basal bodies.
Supplemental movie 2a (Related to Figures 2 and 3). A comprehensive EM stack of a cortical neuronal primary cilium and its contacts. EM sections of a layer 4 interneuron cilium (highlighted in teal; same cilium as in Movie S1) and all the surrounding cells making contact with it. Contacting cells are colorized.
Supplemental movie 2b (Related to Figures 2 and 3). A comprehensive EM stack of a cortical neuronal primary cilium and its contacts in cross-sectional view. EM sections of a layer 6 interneuron cilium (highlighted in yellow; cross-sectional view) and all the surrounding cells making contact with it as it extends into cortical parenchyma. Contacting cells are colorized and are labeled accordingly (astrocytes, inhibitory axons, excitatory axons, and excitatory dendrites).
Supplemental movie 3 (Related to Figure 4). Changing 9+0 MT axoneme core of an interneuron. Cross section of a layer 3 cortical interneuronal neuronal primary cilium (yellow) was tracked in EM stacks as it extends into the surrounding cortical connectome. The 9+0 MT axoneme core devolves into fewer and fewer MT filaments as the cilium extends away from the neuronal soma.
Supplemental movie 4 (Related to Figure 4). Changing 9+0 MT axoneme core of a projection neuron. Cross section of a layer 5 cortical projection neuronal primary cilium (blue) was tracked in EM stacks as it extends into the surrounding cortical connectome. 9+0 MT axoneme core devolves into fewer and fewer MT filaments as the cilium extends away from the neuronal soma.
Supplemental movie 5 (Related to Figure S3). An astrocyte primary cilium embedded inside the soma. EM sections of a layer 4 astrocyte primary cilium (yellow arrow) as it remains within a ciliary sheath inside the soma.
Supplemental movie 6 (Related to Figure 5). Layer 2 interneuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 2 interneuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 7 (Related to Figure 5). Layer 5 interneuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 5 interneuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 8 (Related to Figure 6). Layer 2 projection neuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 2 projection neuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 9 (Related to Figure 6). Layer 5 projection neuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 5 projection neuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 10 (Related to Figure 7). A comprehensive EM stack of an astrocyte primary cilium and its contacts. EM sections of a layer 1 astrocyte cilium (teal arrow) as it emerges from a deep ciliary pocket and all the surrounding cells making contact with it. Contacting cells are colorized and labeled accordingly (inhibitory axons, excitatory axons, and excitatory dendrites).
Supplemental movie 11 (Related to Figure 7). Astrocyte primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 5 astrocyte and all the surrounding cells coming into membrane contact with it. Right panel shows the astrocyte cilium and its contacts in 3D. Note that the cilium is for the most part inside a membrane pocket.
Supplemental movie 12 (Related to Figure 8). Neuronal primary cilia association with synapses. A cortical projection neuronal primary cilium (blue; cross-sectional view) was tracked in EM stacks as it extends into the surrounding cortical connectome. Spotlight illustrates its position between two excitatory synapses and then contact with another excitatory synapse. E, excitatory synapse. Frames pause when cilium contacts synapses.
Supplemental movie 13 (Related to Figures 5, 6, Figure S4, and Figure S8). Neuronal primary ciliary association with an astrocyte. A cortical projection neuronal primary cilium (purple) was tracked in EM stacks as it extends into the surrounding cortical connectome. An astrocyte (green) surrounds and eventually fully encircles the primary cilium.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-connexin 36 | ThermoFisher | Cat#710663, RRID: AB_2576616 |
| Mouse anti-arl13b | Biolegend | Cat# 857602, RRID: AB_2801216 |
| Chicken anti-β tubulin | Millipore | Cat# 9354, RRID: AB_570918 |
| AlexaFluor goat anti-mouse 647 | Invitrogen | Cat# A21236 |
| AlexaFluor goat anti-rabbit Cy3 | Invitrogen | Cat# A10520 |
| AlexaFluor goat anti-chicken 488 | Invitrogen | Cat# A11039 |
| Experimental Models: Organisms/ Strains | ||
| Adults human cerebral cortex | Shapson-Coe et al., 202136 | N/A |
| Human neural progenitor cells | Stein et al., 201484 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DAPI | Invitrogen | Cat# D21490 |
| Osmium tetroxide-thiocarbohydrazide | EMS, Sigma | Cat# 19150, Cat# T2137 |
| Uranyl acetate | EMS | Cat# 22400 |
| Software and Algorithms | ||
| Excel | Microsoft | https://products.office.com/en-us/exce |
| SAS | SAS Institute | https://oda.sas.com |
| GraphPad | GraphPad Software Inc. |
https://graphpad.com |
| Neuroglancer | Shapson-Coe et al., 202136 | https://github.com/google/neuroglancer |
Highlights.
Cortical neuronal and astroglial cilia are structurally and connectomically diverse.
Unlike neuronal cilia, astrocyte cilia are mostly in pockets or embedded inside soma.
The contactome of each cilium enables unique access to surrounding neural circuitry.
Human neuronal primary cilia are components of tetrapartite synapses.
Acknowledgments
This research was supported by NIH grants MH132710 (R.Y., J.L, and E.A.), NS116859 (E.A.), and U24 NS109102(J.L.). We thank Yukako Yokota and Troy Ghashghaei for insightful discussions.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez Retuerto AI, Cantor RM, Gleeson JG, et al. (2008). Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet 17, 3887–3896. doi: 10.1093/hmg/ddn291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arellano JI, Guadiana SM, Breunig JJ, Rakic P, and Sarkisian MR (2012). Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol 520, 848–873. doi: 10.1002/cne.22793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guemez-Gamboa A, Coufal NG, and Gleeson JG (2014). Primary Cilia in the Developing and Mature Brain. Neuron 82, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J, Higginbotham H, Li J, et al. (2015). Developmental disruptions underlying brain abnormalities in ciliopathies. Nat Commun 6, 7857. doi: 10.1038/ncomms8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J, Otis JM, Higginbotham H, Monckton C, Cheng J, Asokan A, Mykytyn K, Caspary T, Stuber GD, & Anton ES (2017). Primary Cilia Signaling Shapes the Development of Interneuronal Connectivity. Developmental cell, 42(3), 286–300.e4. 10.1016/j.devcel.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo J, Otis JM, Suciu SK, Catalano C, Xing L, Constable S, Wachten D, Gupton S, Lee J, Lee A, Blackley KH, Ptacek T, Simon JM, Schurmans S, Stuber GD, Caspary T, & Anton ES (2019). Primary Cilia Signaling Promotes Axonal Tract Development and Is Disrupted in Joubert Syndrome-Related Disorders Models. Developmental cell, 51(6), 759–774.e5. 10.1016/j.devcel.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green JA, and Mykytyn K (2014). Neuronal primary cilia: an underappreciated signaling and sensory organelle in the brain. Neuropsychopharmacology 39, 244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higginbotham H. et al. (2012). Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell 23, 925–938. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildebrandt F, Benzing T, and Katsanis N. (2011). Ciliopathies. N Engl J Med. 364, 1533–1543. doi: 10.1056/NEJMra1010172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilgendorf KI, Johnson CT, and Jackson PK (2016). The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr Opin Cell Biol 39, 84–92. doi: 10.1016/j.ceb.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, et al. (2009). INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41, 1027–1031. [DOI] [PubMed] [Google Scholar]
- 12.Jovasevic V, Zhang H, Sananbenesi F, Guedea AL, Soman KV, Wiktorowicz JE, Fischer A, and Radulovic J. (2021). Primary cilia are required for the persistence of memory and stabilization of perineuronal nets. iScience 24, 102617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirschen GW, Liu H, Lang T, Liang X, Ge S, and Xiong Q. (2017). The radial organization of neuronal primary cilia is acutely disrupted by 5 seizure and ischemic brain injury. Frontiers in Biology 12, 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster MA and Gleeson JG (2009). The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev 19, 220–229. doi: 10.1016/J.GDE.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CH, Kang GM, & Kim MS (2022). Mechanisms of Weight Control by Primary Cilia. Molecules and cells, 45(4), 169–176. 10.14348/molcells.2022.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marley A. and von Zastrow M. (2012). A Simple Cell-Based Assay Reveals That Diverse Neuropsychiatric Risk Genes Converge on Primary Cilia. PLoS One 7, doi: 10.1371/journal.pone.0046647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meffre J, Chaumont‐Dubel S, Cour C. la, Loiseau F, Watson DJ, Dekeyne A, S veno M, Rivet J, Gaven F, D l ris P, et al. (2012). 5 ‐HT6 receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Molecular Medicine 4, 1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchison HM and Valente EM (2017). Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 241, 294–309. doi: 10.1002/path.4843 [DOI] [PubMed] [Google Scholar]
- 19.Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, Als TD, Bigdeli TB, B rte S, Bryois J, et al. (2021). Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet 53, 817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novarino G, Akizu N, and Gleeson JG (2011). Modeling Human Disease in Humans: The Ciliopathies. Cell 147, 70–79. doi: 10.1016/J.CELL.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Trupiano MX, Simon J, Guo J, & Anton ES (2021). The essential role of primary cilia in cerebral cortical development and disorders. Current topics in developmental biology, 142, 99–146. 10.1016/bs.ctdb.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SM, Jang HJ, & Lee JH (2019). Roles of Primary Cilia in the Developing Brain. Frontiers of Cellular Neuroscience 13(218). doi: 10.3389/fncel.2019.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter JF and Leroux MR (2017). Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 18, 533–547. doi: 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkisian MR and Guadiana SM (2015). Influences of Primary Cilia on Cortical Morphogenesis and Neuronal Subtype Maturation. Neurosci 21, 136–151. doi: 10.1177/1073858414531074 [DOI] [PubMed] [Google Scholar]
- 25.Sattar S. and Gleeson JG (2011). The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Dev Med Child Neurol 53, 793–798. doi: 10.1111/j.14698749.2011.04021.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stubbs T, Koemeter-Cox A, Bingman JI, Zhao F, Kalyanasundaram A, Rowland LA, Periasamy M, Carter CS, Sheffield VC, Askwith CC, & Mykytyn K. (2022). Disruption of dopamine receptor 1 localization to primary cilia impairs signaling in striatal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience, JN-RM-0497–22. 10.1523/JNEUROSCI.0497-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas S, Boutaud L, Reilly ML, & Benmerah A. (2019). Cilia in hereditary cerebral anomalies. Biology of the Cell 111, 217–231. doi: 10.1111/boc.201900012 [DOI] [PubMed] [Google Scholar]
- 28.Sengupta P. (2017) Cilia and sensory signaling: The journey from “animalcules” to human disease. PLoS Biol 15(4): e2002240. doi: 10.1371/journal.pbio.2002240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaisse C, Reiter JF, & Berbari NF (2017). Cilia and Obesity. Cold Spring Harbor perspectives in biology, 9(7), a028217. 10.1101/cshperspect.a028217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valente EM, Rosti RO, Gibbs E, and Gleeson JG (2014). Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 10, 27–36. doi: 10.1038/nrneurol.2013.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alhassen W, Chen S, Vawter M, Robbins BK, Nguyen H, Myint TN, Saito Y, Schulmann A, Nauli SM, Civelli O, Baldi P, & Alachkar A. (2021). Patterns of cilia gene dysregulations in major psychiatric disorders. Progress in neuro-psychopharmacology & biological psychiatry, 109, 110255. 10.1016/j.pnpbp.2021.110255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M, Haney JR, Zhang P, Hernandez LM, Wang LK, Perez-Cano L, Loohuis L, de la Torre-Ubieta L, & Gandal MJ (2021). Brain gene co-expression networks link complement signaling with convergent synaptic pathology in schizophrenia. Nature neuroscience, 24(6), 799–809. 10.1038/s41593-021-00847-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, & Ge S. (2012). A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nature neuroscience, 15(3), 399–S1. 10.1038/nn.3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tereshko L, Gao Y, Cary BA, Turrigiano GG, & Sengupta P. (2021). Ciliary neuropeptidergic signaling dynamically regulates excitatory synapses in postnatal neocortical pyramidal neurons. eLife, 10, e65427. 10.7554/eLife.65427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheu SH, Upadhyayula S, Dupuy V, Pang S, Deng F, Wan J, Walpita D, Pasolli HA, Houser J, Sanchez-Martinez S, Brauchi SE, Banala S, Freeman M, Xu CS, Kirchhausen T, Hess HF, Lavis L, Li Y, Chaumont-Dubel S, & Clapham DE (2022). A serotonergic axon-cilium synapse drives nuclear signaling to alter chromatin accessibility. Cell, 185(18), 3390–3407.e18. 10.1016/j.cell.2022.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapson-Coe A. et al. (2021). A connectomic study of a petascale fragment of human cerebral cortex. bioRxiv, 2021.05.29.446289; doi: 10.1101/2021.05.29.446289 [DOI] [Google Scholar]
- 37.Nager AR, Goldstein JS, Herranz-Pérez V, Portran D, Ye F, Garcia-Verdugo JM, & Nachury MV (2017). An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell, 168(1–2), 252–263.e14. 10.1016/j.cell.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, Saunier S, Spassky N, Bastin P, & Benmerah A. (2010). The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. Journal of cell science, 123(Pt 10), 1785–1795. 10.1242/jcs.059519 [DOI] [PubMed] [Google Scholar]
- 39.Benmerah A. (2013). The ciliary pocket. Current opinion in cell biology, 25(1), 78–84. 10.1016/j.ceb.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa T, Ueno H, Omori T, & Kikuchi K. (2021). Cilia and centrosomes: Ultrastructural and mechanical perspectives. Seminars in cell & developmental biology, 110, 61–69. 10.1016/j.semcdb.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 41.Vertii A, Hung HF, Hehnly H, & Doxsey S. (2016). Human basal body basics. Cilia, 5, 13. 10.1186/s13630-016-0030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris KD, & Shepherd GM (2015). The neocortical circuit: themes and variations. Nature neuroscience, 18(2), 170–181. 10.1038/nn.3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim L, Mi D, Llorca A, & Marín O. (2018). Development and Functional Diversification of Cortical Interneurons. Neuron, 100(2), 294–313. 10.1016/j.neuron.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallo NB, Paul A, & Van Aelst L. (2020). Shedding Light on Chandelier Cell Development, Connectivity, and Contribution to Neural Disorders. Trends in neurosciences, 43(8), 565–580. 10.1016/j.tins.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen NJ, & Eroglu C. (2017). Cell Biology of Astrocyte-Synapse Interactions. Neuron, 96(3), 697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong A, Liu S, & Li Y. (2018). Gap Junctions in the Nervous System: Probing Functional Connections Using New Imaging Approaches. Frontiers in cellular neuroscience, 12, 320. 10.3389/fncel.2018.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans WH, & Martin PE (2002). Gap junctions: structure and function (Review). Molecular membrane biology, 19(2), 121–136. 10.1080/09687680210139839 [DOI] [PubMed] [Google Scholar]
- 48.Miller AC, & Pereda AE (2017). The electrical synapse: Molecular complexities at the gap and beyond. Developmental neurobiology, 77(5), 562–574. 10.1002/dneu.22484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rash JE, Yasumura T, & Dudek FE (1998). Ultrastructure, histological distribution, and freeze-fracture immunocytochemistry of gap junctions in rat brain and spinal cord. Cell biology international, 22(11–12), 731–749. 10.1006/cbir.1998.0392 [DOI] [PubMed] [Google Scholar]
- 50.Shivers RR, & McVicar LK (1995). Gap junctions revealed by freeze-fracture electron microscopy. Microscopy research and technique, 31(5), 437–445. 10.1002/jemt.1070310512 [DOI] [PubMed] [Google Scholar]
- 51.Wu L, Dong A, Dong L, Wang SQ, & Li Y. (2019). PARIS, an optogenetic method for functionally mapping gap junctions. eLife, 8, e43366. 10.7554/eLife.43366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zihni C, Mills C, Matter K, & Balda MS (2016). Tight junctions: from simple barriers to multifunctional molecular gates. Nature reviews. Molecular cell biology, 17(9), 564–580. 10.1038/nrm.2016.80 [DOI] [PubMed] [Google Scholar]
- 53.Söhl G, Maxeiner S. & Willecke K. (2005). Expression and functions of neuronal gap junctions. Nat. Rev. Neurosci. 6, 191–200. [DOI] [PubMed] [Google Scholar]
- 54.Hirabayashi Y, Kwon SK, Paek H, Pernice WM, Paul MA, Lee J, Erfani P, Raczkowski A, Petrey DS, Pon LA, & Polleux F. (2017). ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science (New York, N.Y.), 358(6363), 623–630. 10.1126/science.aan6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delling M, DeCaen PG, Doerner JF, Febvay S, and Clapham DE (2013). Primary cilia are specialized calcium signalling organelles. Nature 504, 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeCaen PG, Delling M, Vien TN, and Clapham DE (2013). Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doerner JF, Delling M, & Clapham DE (2015). Ion channels and calcium signaling in motile cilia. eLife, 4, e11066. 10.7554/eLife.11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lishko P, & Kirichok Y. (2015). Signaling the differences between cilia. eLife, 4, e12760. 10.7554/eLife.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, & Nachury MV (2015). Proteomics of Primary Cilia by Proximity Labeling. Developmental cell 35(4), 497–512. 10.1016/j.devcel.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pablo JL, DeCaen PG, & Clapham DE (2017). Progress in ciliary ion channel physiology. The Journal of general physiology, 149(1), 37–47. 10.1085/jgp.201611696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tajhya R, & Delling M. (2020). New insights into ion channel-dependent signalling during left-right patterning. The Journal of physiology, 598(9), 1741–1752. 10.1113/JP277835 [DOI] [PubMed] [Google Scholar]
- 62.van Dam TJ, Wheway G, Slaats GG, SYSCILIA Study Group, Huynen MA, & Giles RH (2013). The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2(1), 7. 10.1186/2046-2530-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dam T, Kennedy J, van der Lee R, de Vrieze E, Wunderlich KA, Rix S, Dougherty GW, Lambacher NJ, Li C, Jensen VL, Leroux MR, Hjeij R, Horn N, Texier Y, Wissinger Y, van Reeuwijk J, Wheway G, Knapp B, Scheel JF, Franco B, … Huynen MA (2019). CiliaCarta: An integrated and validated compendium of ciliary genes. PloS one 14(5), e0216705. 10.1371/journal.pone.0216705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishikawa H, Thompson J, Yates JR 3rd, Marshall WF (2012). Proteomic analysis of mammalian primary cilia. Curr Biol. 22(5):414–419. doi: 10.1016/j.cub.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodge RD, Bakken TE, et al. (2019) Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, & Inoue T. (2013). Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nature methods 10(11), 1105–1107. 10.1038/nmeth.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bushong EA, Martone ME, Jones YZ, & Ellisman MH (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of neuroscience : the official journal of the Society for Neuroscience, 22(1), 183–192. 10.1523/JNEUROSCI.22-0100183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, & Nedergaard M. (2009). Uniquely hominid features of adult human astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29(10), 3276–3287. 10.1523/JNEUROSCI.4707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harada K, Kamiya T, & Tsuboi T. (2016). Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Frontiers in neuroscience, 9, 499. 10.3389/fnins.2015.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koizumi S. (2022). Glial Purinergic Signals and Psychiatric Disorders. Frontiers in cellular neuroscience, 15, 822614. 10.3389/fncel.2021.822614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savtchouk I, & Volterra A. (2018). Gliotransmission: Beyond Black-and-White. The Journal of neuroscience : the official journal of the Society for Neuroscience, 38(1), 14–25. 10.1523/JNEUROSCI.0017-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ginhoux F, & Garel S. (2018) The mysterious origins of microglia. Nat Neurosci 21, 897–899. [DOI] [PubMed] [Google Scholar]
- 73.Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, and Garel S. (2014) Microglia modulate wiring of the embryonic forebrain. Cell Rep 8, 1271–1279. [DOI] [PubMed] [Google Scholar]
- 74.Thion MS, & Garel S. (2017) On place and time: microglia in embryonic and perinatal brain development. Curr Opin Neurobiol 47, 121–130. [DOI] [PubMed] [Google Scholar]
- 75.Hilgendorf KI, Johnson CT, Mezger A, Rice SL, Norris AM, Demeter J, Greenleaf WJ, Reiter JF, Kopinke D, and Jackson PK (2019). Omega-3 fatty acids activate ciliary FFAR4 to control adipogenesis. Cell 179, 12891305.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kong JH, Siebold C, & Rohatgi R. (2019). Biochemical mechanisms of vertebrate hedgehog signaling. Development (Cambridge, England), 146(10), dev166892. 10.1242/dev.166892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klena N, & Pigino G. (2022). Structural Biology of Cilia and Intraflagellar Transport. Annual review of cell and developmental biology, 38, 103–123. 10.1146/annurev-cellbio-120219-034238 [DOI] [PubMed] [Google Scholar]
- 78.Mallet A, & Bastin P. (2022). Restriction of intraflagellar transport to some microtubule doublets: An opportunity for cilia diversification?. BioEssays : news and reviews in molecular, cellular and developmental biology, 44(7), e2200031. 10.1002/bies.202200031 [DOI] [PubMed] [Google Scholar]
- 79.Jordan MA, & Pigino G. (2021). The structural basis of intraflagellar transport at a glance. Journal of cell science, 134(12), jcs247163. 10.1242/jcs.247163 [DOI] [PubMed] [Google Scholar]
- 80.Sun S, Fisher RL, Bowser SS, Pentecost BT, & Sui H. (2019). Three-dimensional architecture of epithelial primary cilia. Proceedings of the National Academy of Sciences of the United States of America, 116(19), 9370–9379. 10.1073/pnas.1821064116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sorokin SP (1968). Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3, 207–230. [DOI] [PubMed] [Google Scholar]
- 82.Ganga AK, Kennedy MC, Oguchi ME, Gray S, Oliver KE, Knight TA, De La Cruz EM, Homma Y, Fukuda M, & Breslow DK (2021). Rab34 GTPase mediates ciliary membrane formation in the intracellular ciliogenesis pathway. Current biology : CB, 31(13), 2895–2905.e7. 10.1016/j.cub.2021.04.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barral DC, Staiano L, Guimas Almeida C, Cutler DF, Eden ER, Futter CE, Galione A, Marques ARA, Medina DL, Napolitano G, Settembre C, Vieira OV, Aerts JMFG, Atakpa-Adaji P, Bruno G, Capuozzo A, De Leonibus E, Di Malta C, Escrevente C, Esposito A, … Seabra MC (2022). Current methods to analyze lysosome morphology, positioning, motility and function. Traffic (Copenhagen, Denmark), 23(5), 238–269. 10.1111/tra.12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stein JL, de la Torre-Ubieta L, Tian Y, Parikshak NN, Hernández IA, Marchetto MC, Baker DK, Lu D, Hinman CR, Lowe JK, Wexler EM, Muotri AR, Gage FH, Kosik KS, & Geschwind DH (2014). A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron, 83(1), 69–86. 10.1016/j.neuron.2014.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Konopka G, Wexler E, Rosen E, Mukamel Z, Osborn GE, Chen L, Lu D, Gao F, Gao K, Lowe JK, & Geschwind DH (2012). Modeling the functional genomics of autism using human neurons. Molecular psychiatry, 17(2), 202–214. 10.1038/mp.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakagawa N, Plestant C, Yabuno-Nakagawa K, Li J, Lee J, Huang CW, Lee A, Krupa O, Adhikari A, Thompson S, Rhynes T, Arevalo V, Stein JL, Molnár Z, Badache A, & Anton ES (2019). Memo1-Mediated Tiling of Radial Glial Cells Facilitates Cerebral Cortical Development. Neuron, 103(5), 836–852.e5. 10.1016/j.neuron.2019.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental movie 1 (Related to Figures 2 and 3). A comprehensive EM stack of a cortical neuronal primary cilium. EM sections of a layer 4 interneuron cilium (highlighted in teal; sagittal view) illustrating the entire length of the microtubule-based ciliary axoneme and the cilia anchoring basal bodies.
Supplemental movie 2a (Related to Figures 2 and 3). A comprehensive EM stack of a cortical neuronal primary cilium and its contacts. EM sections of a layer 4 interneuron cilium (highlighted in teal; same cilium as in Movie S1) and all the surrounding cells making contact with it. Contacting cells are colorized.
Supplemental movie 2b (Related to Figures 2 and 3). A comprehensive EM stack of a cortical neuronal primary cilium and its contacts in cross-sectional view. EM sections of a layer 6 interneuron cilium (highlighted in yellow; cross-sectional view) and all the surrounding cells making contact with it as it extends into cortical parenchyma. Contacting cells are colorized and are labeled accordingly (astrocytes, inhibitory axons, excitatory axons, and excitatory dendrites).
Supplemental movie 3 (Related to Figure 4). Changing 9+0 MT axoneme core of an interneuron. Cross section of a layer 3 cortical interneuronal neuronal primary cilium (yellow) was tracked in EM stacks as it extends into the surrounding cortical connectome. The 9+0 MT axoneme core devolves into fewer and fewer MT filaments as the cilium extends away from the neuronal soma.
Supplemental movie 4 (Related to Figure 4). Changing 9+0 MT axoneme core of a projection neuron. Cross section of a layer 5 cortical projection neuronal primary cilium (blue) was tracked in EM stacks as it extends into the surrounding cortical connectome. 9+0 MT axoneme core devolves into fewer and fewer MT filaments as the cilium extends away from the neuronal soma.
Supplemental movie 5 (Related to Figure S3). An astrocyte primary cilium embedded inside the soma. EM sections of a layer 4 astrocyte primary cilium (yellow arrow) as it remains within a ciliary sheath inside the soma.
Supplemental movie 6 (Related to Figure 5). Layer 2 interneuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 2 interneuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 7 (Related to Figure 5). Layer 5 interneuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 5 interneuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 8 (Related to Figure 6). Layer 2 projection neuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 2 projection neuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 9 (Related to Figure 6). Layer 5 projection neuronal primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 5 projection neuron and all the surrounding cells coming into membrane contact with it. Right panel shows the neuronal cilium and its contacts in 3D.
Supplemental movie 10 (Related to Figure 7). A comprehensive EM stack of an astrocyte primary cilium and its contacts. EM sections of a layer 1 astrocyte cilium (teal arrow) as it emerges from a deep ciliary pocket and all the surrounding cells making contact with it. Contacting cells are colorized and labeled accordingly (inhibitory axons, excitatory axons, and excitatory dendrites).
Supplemental movie 11 (Related to Figure 7). Astrocyte primary cilia contactome. Left panel shows EM image of the primary cilium extending from a layer 5 astrocyte and all the surrounding cells coming into membrane contact with it. Right panel shows the astrocyte cilium and its contacts in 3D. Note that the cilium is for the most part inside a membrane pocket.
Supplemental movie 12 (Related to Figure 8). Neuronal primary cilia association with synapses. A cortical projection neuronal primary cilium (blue; cross-sectional view) was tracked in EM stacks as it extends into the surrounding cortical connectome. Spotlight illustrates its position between two excitatory synapses and then contact with another excitatory synapse. E, excitatory synapse. Frames pause when cilium contacts synapses.
Supplemental movie 13 (Related to Figures 5, 6, Figure S4, and Figure S8). Neuronal primary ciliary association with an astrocyte. A cortical projection neuronal primary cilium (purple) was tracked in EM stacks as it extends into the surrounding cortical connectome. An astrocyte (green) surrounds and eventually fully encircles the primary cilium.
Data Availability Statement
Serial EM dataset used in this study is available at https://h01-release.storage.googleapis.com/landing.html. Microscopy data reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.