Abstract
Objectives
This study explores racial and ethnic differences in 1) receiving tissue plasminogen activator (tPA) and endovascular thrombectomy (EVT) as treatment for ischemic stroke and 2) outcomes and quality of care after use of tPA or EVT in the US.
Materials and Methods
An observational analysis of 89,035 ischemic stroke patients from the 2019 National Inpatient Sample was conducted. We performed weighted logistic regressions between race and ethnicity and 1) tPA and EVT utilization and 2) in-hospital mortality. We also performed a weighted Poisson regression between race and ethnicity and length of stay (LOS) after tPA or EVT.
Results
Black patients had significantly lower odds of receiving tPA and EVT than White patients and minority populations (including but not limited to Black, Hispanic, Pacific Islander, Native American, and Asian) had significantly longer hospital LOS after treatment with tPA or EVT. We failed to find a significant difference between race/ethnicity and in-hospital mortality post-tPA or EVT.
Conclusions
Black ischemic stroke patients were less likely to receive tPA and EVT than White patients, and among patients who received tPA or EVT, minority patients had significantly longer hospital LOSs than White patients. While we failed to find a difference in in-hospital mortality, racial and ethnic disparities are still evident in the decreased usage of tPA and EVT and longer LOSs for minority patients. This study calls for interventions to expand the utilization of tPA and EVT and advance quality of care post-tPA or EVT in order to improve stroke care for minority patients.
Keywords: Ischemic stroke, Tissue Plasminogen Activator, Endovascular Thrombectomy, Racial and Ethnic Disparities, Ischemic Stroke Treatment
Introduction
Every year, more than 795,000 people in the United States have a stroke as reported by the American Heart Association (AHA) [1]. Strokes are the fifth leading cause of adult death in the United States and are a leading cause of long-term disability [1]. Strokes can occur in any population, but studies have shown that there are racial and ethnic discrepancies in stroke incidence and post-stroke outcomes nationally. Black and Hispanic patients experience a higher incidence of stroke and have strokes that occur earlier in life [2]. The AHA reports that Black patients have nearly a two-fold higher risk of first-time stroke when compared to Whites [1], and the Centers for Disease Control and Prevention reports that Black patients have the highest mortality rate due to strokes [3]. Black patients are also less likely to receive tissue plasminogen activator (tPA) [4], have higher door-to-imaging times [5], and are less likely to receive endovascular thrombectomy (EVT) than White patients [6]; all of which may contribute to the higher mortality rate post-stroke in Black patients.
TPA and EVT have been shown to improve functional outcomes after ischemic stroke [7,8,9] and are both widely accepted in guidelines for treating ischemic stroke [10,11]. While the national use of tPA and EVT have increased over the years, this increase in usage is not proportionate between races as tPA and EVT use is lower in Black populations [12]. In this study, we aim to continue the exploration of racial disparities in the usage of tPA and EVT nationally as well as to investigate outcomes in ischemic stroke patients who received either tPA or EVT. We hypothesize that tPA and EVT usage will be lower in Black patients as compared to White patients. Additionally, we hypothesize that Black patients will have higher in-hospital mortality and longer hospital length of stay (LOS) after being treated with tPA or EVT than White patients.
Methods
Data and sample
We used data from the 2019 National Inpatient Sample (NIS) collected by the Healthcare Cost Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ) for this observational analysis. The NIS data were de‐identified, and a data user agreement was signed before we analyzed the data. The HCUP is Health Insurance Portability and Accountability Act‐compliant, and therefore review by an institutional review board is not required. The 2019 NIS is the most recent dataset available for analyses. The NIS is the largest hospital discharge sample available and includes discharge records from all HCUP-participating hospitals covering more than 97% of the U.S. population. This NIS sample includes HCUP-participating community hospitals while excluding patients in prison hospitals, inpatient rehabilitation hospitals, and long-term acute care facilities. Sample weights were provided to generate nationally representative estimates.
The current study’s sample includes adults aged 18 or older with a primary diagnosis of ischemic stroke who received tPA or EVT. The International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) diagnosis and procedure codes were used for classification. Patients discharged with ischemic stroke were identified using principal diagnosis ICD-10 codes: 163.x [13].
Variable selection
Our initial analysis examined the usage of tPA and EVT in various racial and ethnic groups. Utilization of TPA and EVT served as the outcome variables and race and ethnicity was the primary predictor variable. Race and ethnicity were defined by NIS as Non-Hispanic (NH) White, NH Black, Hispanic, and Other which included but was not limited to Asian, Pacific Islanders, and Native Americans.
The procedural code ICD-10-PCS 3E03317 was used to define patients who received tPA [14]. The following procedural codes were used to define patients who underwent EVT: ICD-10-PCS 03CG3Z7, 03CG3ZZ, 03CG4Z6, 03CG4ZZ, 03CH3Z6, 03CH3Z7, 03CH3ZZ, 03CH4Z6, 03CH4ZZ, 03CJ3Z6, 03CJ3Z7, 03CJ3ZZ, 03CJ4Z6, 03CJ4ZZ, 03CK3Z6, 03CK3Z7, 03CK3ZZ, 03CK4Z6, 03CK4ZZ, 03CL3Z6, 03CL3Z7, 03CL3ZZ, 03CL4Z6, 03CL4ZZ, 03CP3Z6, 03CP3Z7, 03CP3ZZ, 03CP4Z6, 03CP4ZZ, 03CQ3Z6, 03CQ3Z7, 03CQ3ZZ, 03CQ4Z6, 03CQ4ZZ [12].
For our secondary analysis, we observed the outcomes after ischemic stroke in the patient subsample who received either tPA or EVT. We compared the differences in clinical outcomes, in-hospital mortality and length of stay (LOS), by using race and ethnicity as the primary predictor variable. In-hospital mortality is a binary variable with patients who died coded as 1 and patients who did not die coded as 0. LOS is a continuous variable that was defined by the NIS by subtracting the admission date from the discharge date.
Confounding variables included age, sex, neighborhood median household income [15], payers [16], calendar quarter of discharge [17], number of comorbidities [18], hospital’s location (rural or urban) [19], hospital teaching status [20.21], bed size [20,21, 22] and weekend discharge [23]. Age groups were divided into 18–44, 45–64, 65–74, and 75 and older. Neighborhood median household income quartiles were defined by the 2019 NIS income ranges: $1–24,999, $25,000–34,999, $35,000–44,999, $45,000 or more. NIS categorizes “payers” as Medicare, Medicaid, private insurance, self-pay, and no charge. The discharge quarters were categorized into four quarters: January-March, April-June, July-September, and October-December. We used the Charlson Comorbidity Index to categorize the number of comorbidities into two groups: 1 comorbidity or 2 or more comorbidities. Hospital location is a binary variable categorized as rural or urban. Teaching status is a binary variable categorized as teaching and non-teaching hospital. NIS categorizes hospital size as small, medium, or large based on number of beds depending on hospital location, region, and teaching status [24]. Weekend discharge describes whether the patient was discharged on a weekday (Monday-Friday) or a weekend (Saturday or Sunday). Existing literature that establishes differences in in-hospital mortality based on patient, neighborhood, and hospital characteristics guided our choice of these confounding variables [25].
Statistical Analysis
We first assessed the percentage of missing values in the study sample. There was a total of 3,807 (4.1%) observations that contained missing values. Previous studies have suggested that when an analytic sample has less than 5% missing values, performing imputations does not significantly reduce biases [26]. Therefore, with 4.1% missing values in our sample, we decided not to perform imputation but conduct the analysis in the complete sample.
In our first analysis, we examined whether there was a statistically significant difference in the rate of utilization of tPA and EVT in our sample (age ≥ 18 and primary diagnosis of ischemic stroke) for NH White, NH Black, Hispanic, and Other racial and ethnic groups (Asian, Pacific Islanders, Native Americans, and others), using Pearson Chi-Square (x2) test. We then performed weighted logistic regression to test the association between race and ethnicity and use of tPA and EVT, while controlling for sex, neighborhood median household income, payers, calendar quarter of discharge, number of comorbidities, hospital’s location, hospital teaching status, rural-urban status, bed size, and weekend discharge. The odds ratios (OR) and 95% confidence intervals (95% CI) are presented below.
For the second analysis, we examined the differences in-hospital mortality within our patient subsample who received tPA or EVT across racial and ethnic groups and tested their statistical significance using the x2 test. We then performed a weighted logistic regression to test the association between race and ethnicity and in-hospital mortality, while controlling for sex, neighborhood median household income, payers, calendar quarter of discharge, number of comorbidities, hospital’s location, hospital teaching status, rural-urban status, bed size, and weekend discharge. The OR and 95% CI are presented below.
For our third analysis, as LOS is a count measure, we performed a weighted Poisson regression to test the association between race and ethnicity and LOS, controlling for all the aforementioned confounding variables. The incidence rate ratios and 95% CI are presented below.
All analyses were performed using sampling weights, and Stata SE 17 was used for all statistical analyses (StataCorp, College Station, TX). One author (D.Z.) had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
The full sample included 89,035 ischemic stroke patients. Of ischemic stroke patients in our sample, 68.32% were NH White, 17.35% were NH Black, 8.11% were Hispanic, and 6.21% were Asian, Pacific Islanders, Native Americans, or others. Within our ischemic stroke sample, 10.23% received tPA, 6.57% received EVT, and 4.08% died in the hospital. Additional sample demographics including age, sex, number of comorbidities, household income quartiles, insurance type, time of discharge, location, teaching status, and bed size of hospital are shown in Table 1.
Table 1.
Sample characteristics by tPA and EVT for patients with ischemic stroke, National Inpatient Sample 2019 (n=89,035; N= 445,174).
| Variable | tPA | P-value | EVT | P-value |
|---|---|---|---|---|
| Race/Ethnicity | <0.001 | <0.001 | ||
| NH White | 6242 (10.25%) | 4007 (6.58%) | ||
| NH Black | 1425 (9.22%) | 890 (5.76%) | ||
| Hispanic | 811 (11.27%) | 494 (6.86%) | ||
| Other | 608 (11.05%) | 447 (8.12%) | ||
| Age | <0.001 | <0.001 | ||
| 18–44 | 587 (14.77%) | 342 (8.61%) | ||
| 45–64 | 2821 (11.04%) | 1719 (6.73%) | ||
| 65–74 | 2095 (9.58%) | 1377 (6.3%) | ||
| 75+ | 3583 (9.52%) | 2400 (6.37%) | ||
| Sex | 0.8457 | 0.029 | ||
| Male | 4571 (10.19%) | 2862 (6.38%) | ||
| Female | 4515 (10.22%) | 2976 (6.74%) | ||
| Household income quartiles | <0.001 | <0.001 | ||
| $1–24,999 | 2461 (8.88%) | 1688 (6.09%) | ||
| $25,000–34,999 | 2206 (9.73%) | 1483 (6.54%) | ||
| $35,000–44,999 | 2396 (11.08%) | 1475 (6.82%) | ||
| $45,000+ | 2023 (11.87%) | 1192 (6.99%) | ||
| Payers | <0.001 | <0.001 | ||
| Medicare | 5571 (9.59%) | 3602 (6.2%) | ||
| Medicaid | 813 (9.86%) | 572 (6.94%) | ||
| Private insurance | 2054 (12.27%) | 1292 (7.72%) | ||
| Other | 648 (10.88%) | 372 (6.25%) | ||
| Discharge Quarter | 0.6387 | 0.0956 | ||
| Jan-Mar | 2213 (10.06%) | 1385 (6.3%) | ||
| Apr-Jun | 2261 (10.09%) | 1437 (6.41%) | ||
| July-Sep | 2280 (10.29%) | 1484 (6.7%) | ||
| Oct-Dec | 2332 (10.37%) | 1532 (6.82%) | ||
| Discharge Day | <0.001 | 0.3832 | ||
| Weekday Discharge | 6601 (10%) | 4299 (6.51%) | ||
| Weekend Discharge | 2485 (10.79%) | 1539 (6.68%) | ||
| Location | <0.001 | <0.001 | ||
| Urban | 8811 (10.61%) | 5815 (7%) | ||
| Rural | 275 (4.57%) | 23 (0.38%) | ||
| Teaching Status | 0.2986 | <0.001 | ||
| No | 7591 (10.25%) | 5471 (7.39%) | ||
| Yes | 1495 (9.97%) | 367 (2.45%) | ||
| Hospital bed size | <0.001 | <0.001 | ||
| Small | 1375 (8.54%) | 392 (2.44%) | ||
| Medium | 2746 (10.54%) | 1274 (4.89%) | ||
| Large | 4965 (10.59%) | 4172 (8.9%) | ||
| Comorbidities | <0.001 | <0.001 | ||
| 1 | 937 (7.51%) | 356 (2.85%) | ||
| 2+ | 8149 (10.64%) | 5482 (7.16%) |
The “Other” category of race/ethnicity includes but is not limited to Asian, Pacific Islanders, and Native Americans
The proportion of ischemic stroke patients who received tPA and EVT as categorized by race and ethnicity is shown in Figure 1 and Figure 2, respectively. NH Black patients had the lowest proportion of tPA usage at 9.22% (P < 0.001) signifying that of those who received tPA, NH Black patients made up the lowest percentage. Similarly, NH Black patients also had the lowest proportion of EVT usage at 5.76% (P<0.001) as seen in Figure 2.
Figure 1.
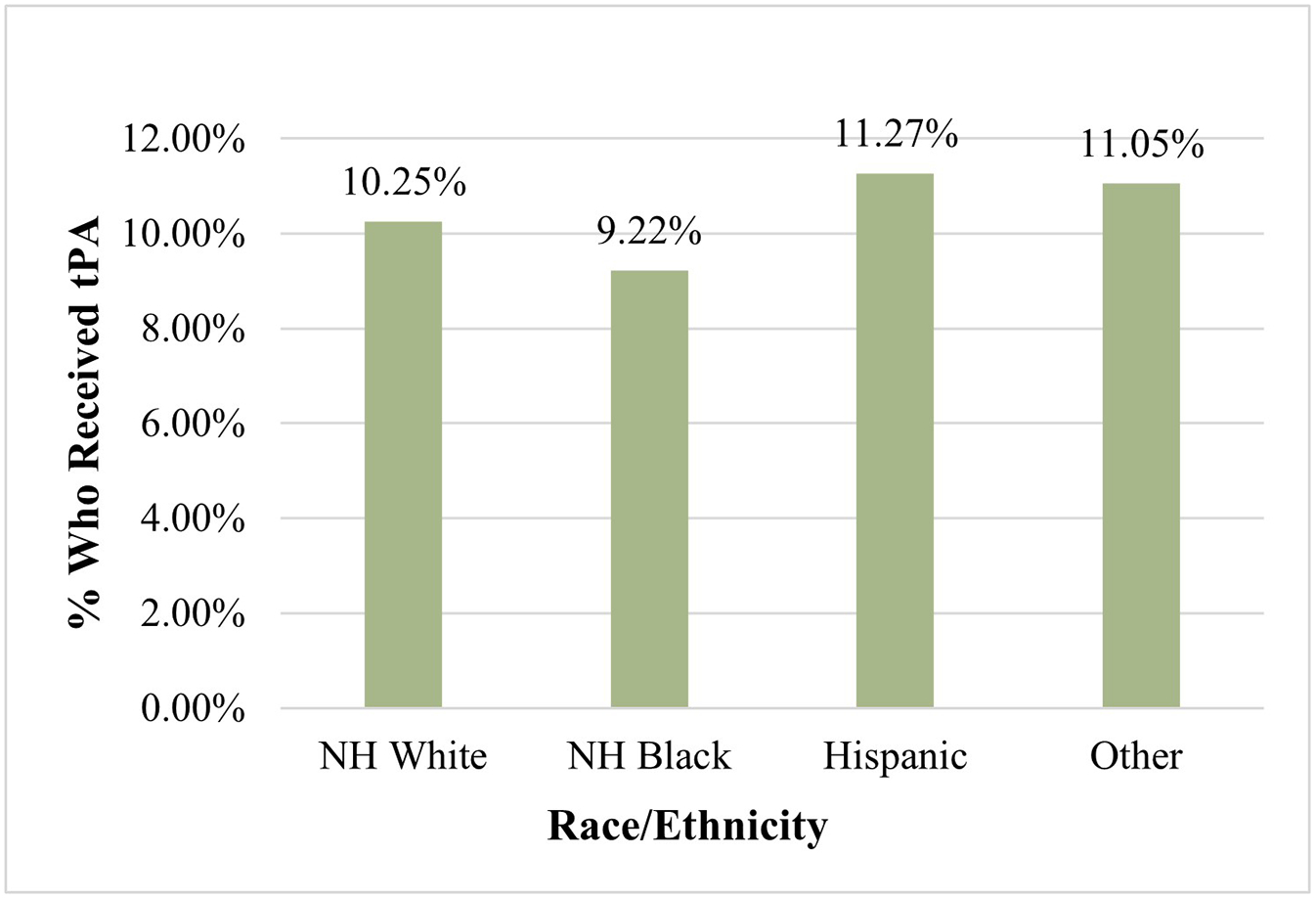
Proportion of ischemic stroke patients who received tPA (N=89,035)
*tPA: Tissue Plasminogen Activator
† The “Other” category of race/ethnicity includes Asian, Pacific Islanders, Native Americans, and “others” as classified by the NIS
Figure 2.
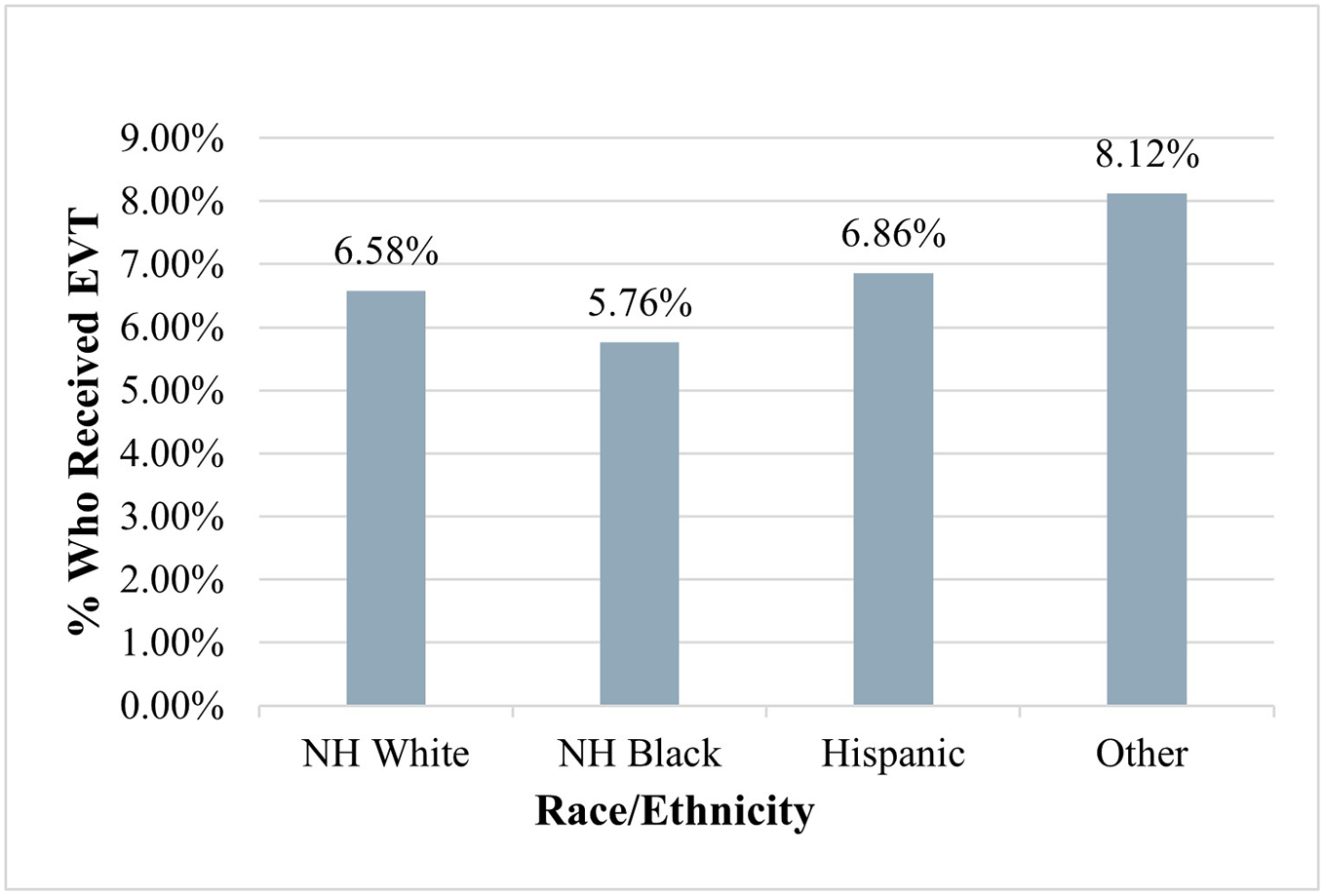
Proportion of Ischemic Stroke Patients Who Received EVT (N=89,035)
*EVT: Endovascular Thrombectomy
† The “Other” category of race/ethnicity includes Asian, Pacific Islanders, Native Americans, and “others” as classified by the NIS
The findings from the logistic regression assessing the association between race and ethnicity and the usage of tPA and EVT are described in Table 2. In the unadjusted analysis, NH Black patients were found to have significantly lower odds of receiving tPA than NH White patients (OR=0.88, 95% CI=0.83–0.94, P=<0.001) and that Hispanic patients had significantly higher odds of receiving tPA than NH White patients (OR=1.11, 95% CI=1.03–1.20, P=0.006). When adjusting for confounding variables, NH Black patients continued to have significantly lower odds of receiving tPA than NH White patients (AOR=0.85, 95% CI: 0.80–0.91, P=<0.001), but tPA usage in Hispanic patients was no longer significantly different than NH White patients. In the unadjusted analysis, NH Black patients were found to have significantly lower odds of receiving EVT than NH White patients (AOR=0.86, 95% CI=0.80–0.93, P=<0.001) and that Asian, Pacific Islander, and Native American patients had significantly higher odds of receiving EVT than NH Whites (AOR=1.24, 95% CI=1.12–1.37, P=<0.001). When adjusting for confounding variables, NH Black patients continued to have significantly lower odds of receiving EVT than NH White patients (AOR=0.75, 95% CI: 0.70–0.82, P=<0.001), and EVT usage in Asian, Pacific Islander, Native American patients was still significantly higher than NH White patients, though the strength of significance lessened (AOR=1.11, 95% CI=1.00–1.24, P=0.048). Overall, Table 2 demonstrates that NH Black patients were less likely to receive tPA and EVT than NH White patients after ischemic stroke.
Table 2.
Logit Regression Assessing the Association Between the Use of tPA and EVT and Race/Ethnicity in Ischemic Stroke Patients, National Inpatient Sample 2019 (n=89,035; N= 445,174).
| Ischemic stroke patients who received tPA | ||||
|---|---|---|---|---|
| Variable | Unadjusted Odds Ratio | Unadjusted 95% Confidence Interval | Adjusted Odds Ratio | Adjusted 95% Confidence Interval |
| Race/Ethnicity | ||||
| NH White | 1.00 | [1.00–1.00] | 1.00 | [1.00–1.00] |
| NH Black | 0.88*** | [0.83–0.94] | 0.85*** | [0.80–0.91] |
| Hispanic | 1.11** | [1.03–1.20] | 0.99 | [0.92–1.08] |
| Other | 1.08 | [0.99–1.18] | 0.95 | [0.87–1.04] |
| Ischemic stroke patients who received EVT | ||||
| Variable | Unadjusted Odds Ratio | Unadjusted 95% Confidence Interval | Adjusted Odds Ratio | Adjusted 95% Confidence Interval |
| Race/Ethnicity | ||||
| NH White | 1.00 | [1.00–1.00] | 1.00 | [1.00–1.00] |
| NH Black | 0.86*** | [0.80–0.93] | 0.75*** | [0.70–0.82] |
| Hispanic | 1.06 | [0.96–1.17] | 0.93 | [0.84–1.03] |
| Other | 1.24*** | [1.12–1.37] | 1.11* | [1.00–1.24] |
P < .05;
P < .01;
P < .001.
Results were presented as weighted N (%).
Models adjusted for patients’ age, sex, neighborhood median household income, payers, discharge quarter, number of comorbidities, hospital location, hospital teaching status, hospital bed size, and weekend discharge. Marginal probabilities were estimated from the logit regression model and converted from odds ratios. Note: P-values were calculated using Chi-square tests.
All statistics were adjusted using sampling weights
The “Other” category of race/ethnicity includes but is not limited to Asian, Pacific Islanders, and Native Americans
The results on the association between race and ethnicity and in-hospital mortality are shown in Table 3. We did not find a significant racial or ethnic difference for in-hospital mortality after receiving tPA or EVT in either the unadjusted or the adjusted analyses. This demonstrates that in-hospital mortality post-ischemic stroke did not significantly differ between racial and ethnic groups among those who received tPA or EVT.
Table 3.
Logit Regression Assessing the Association Between In-hospital Mortality and Race/Ethnicity in Ischemic Stroke Patients who Received tPA or EVT, National Inpatient Sample 2019.
| In-hospital mortality for tPA patients (n=89,035 ; N= 45,425) | ||||
|---|---|---|---|---|
| Variable | Unadjusted Odds Ratio | Unadjusted 95% Confidence Interval | Adjusted Odds Ratio | Adjusted 95% Confidence Interval |
| Race/Ethnicity | ||||
| NH White | 1.00 | [1.00–1.00] | 1.00 | [1.00–1.00] |
| NH Black | 0.94 | [0.70–1.26] | 1.19 | [0.86–1.64] |
| Hispanic | 0.98 | [0.68–1.42] | 1.05 | [0.70–1.58] |
| Other | 1.37 | [0.95–1.98] | 1.38 | [0.94–2.02] |
| In-hospital mortality for EVT patients (n=89,035 ; N=29,185) | ||||
| Variable | Unadjusted Odds Ratio | Unadjusted 95% Confidence Interval | Adjusted Odds Ratio | Adjusted 95% Confidence Interval |
| Race/Ethnicity | ||||
| NH White | 1.00 | [1.00–1.00] | 1.00 | [1.00–1.00] |
| NH Black | 0.77 | [0.60–0.98] | 0.85 | [0.65–1.10] |
| Hispanic | 1.07 | [0.81–1.41] | 1.08 | [0.81–1.46] |
| Other | 0.91 | [0.67–1.24] | 0.94 | [0.69–1.30] |
P < .05;
P < .01;
P < .001.
Results were presented as weighted N (%).
Models adjusted for patients’ age, sex, neighborhood median household income, payers, discharge quarter, number of comorbidities, hospital location, hospital teaching status, hospital bed size, and weekend discharge. Marginal probabilities were estimated from the logit regression model and converted from odds ratios. Note: P-values were calculated using Chi-square tests.
All statistics were adjusted using sampling weights
The “Other” category of race/ethnicity includes but is not limited to Asian, Pacific Islanders, and Native Americans
The results of the Poisson regression assessing the association between race and ethnicity and hospital LOS in patients who received tPA or EVT are shown in Table 4. Unadjusted and adjusted analyses showed that minority patients (NH Black, Hispanic, Asian, Native American, and Pacific Islander) who received tPA all had significantly longer LOSs than White patients (Black patients: Adjusted Incidence Rate Ratio [AIRR]=1.31, 95% CI: 1.21–1.41, P< 0.001, Hispanic patients: AIRR=1.15, 95% CI: 1.04–1.28, P=0.008, Other minority patients: AIRR=1.16, 95% CI: 1.07–1.27, P=0.001). Similarly, all minority patient groups who received EVT had significantly longer hospital LOSs than White patients within the unadjusted and adjusted analyses (Black patients: AIRR=1.30, 95% CI: 1.19–1.44, P< 0.001, Hispanic patients: AIRR=1.23, 95% CI: 1.10–1.38, P< 0.001, Other minority patients: AIRR=1.14, 95% CI: 1.14–1.44, P<0.001). These findings demonstrate that racial and ethnic minority patients who received either tPA or EVT stayed in the hospital significantly longer than White patients.
Table 4.
Poisson Regression Assessing the Association Between Hospital Length of Stay and Race/Ethnicity in Ischemic Stroke Patients Who Received tPA or EVT, National Inpatient Sample 2019.
| Length of Stay for tPA patients (n=89,035 ; N= 45,425) | ||||
|---|---|---|---|---|
| Variable | Unadjusted Incidence Rate Ratio | Unadjusted 5% Confidence Interval | Adjusted Incidence Rate Ratio | Adjusted 95% Confidence Interval |
| Race/Ethnicity | ||||
| NH White | 1.00 | [1.00–1.00] | 1.00 | [1.00–1.00] |
| NH Black | 1.37*** | [1.28.−1.48] | 1.31*** | [1.21–1.41] |
| Hispanic | 1.18** | [1.06–1.31] | 1.15** | [1.04–1.28] |
| Other | 1.19*** | [1.09–1.30] | 1.16*** | [1.07–1.27] |
| Length of Stay for EVT patients (n=89,035 ; N= 29,185) | ||||
| Variable | Unadjusted Incidence Rate Ratio | Unadjusted 95% Confidence Interval | Adjusted Incidence Rate Ratio | Adjusted 95% Confidence Interval |
| Race/Ethnicity | ||||
| NH White | 1.00 | [1.00–1.00] | 1.00 | [1.00–1.00] |
| NH Black | 1.45*** | [1.33–1.58] | 1.30*** | [1.19–1.43] |
| Hispanic | 1.33*** | [1.17–1.50] | 1.23*** | [1.10–1.38] |
| Other | 1.32*** | [1.17–1.49] | 1.28*** | [1.14–1.44] |
P < .05;
P < .01;
P < .001.
Results were presented as weighted N (%).
Models adjusted for patients’ age, sex, neighborhood median household income, payers, discharge quarter, number of comorbidities, hospital location, hospital teaching status, hospital bed size, and weekend discharge. Marginal probabilities were estimated from the logit regression model and converted from odds ratios. Note: P-values were calculated using Chi-square tests.
All statistics were adjusted using sampling weights
The “Other” category of race/ethnicity includes but is not limited to Asian, Pacific Islanders, and Native Americans
Discussion
The current study compares the usage of tPA and EVT between races and ethnicities and furthers this analysis by assessing the racial and ethnic differences for in-hospital mortality and hospital length of stay after receiving tPA or EVT. In accordance with previous literature, our study demonstrates that NH Black ischemic stroke patients are less likely to receive tPA and EVT than NH White patients. Both tPA and EVT have been shown to be effective in improving outcomes after ischemic stroke7,9, and the use of tPA and EVT has increased over the years [12,27]. However, even with the increase in usage of tPA and EVT, Black patients still receive these treatments at a lower proportion than White patients [6]. Additional research is needed to explore why the rate of use of EVT and tPA is increasing throughout America, yet the usage of EVT and tPA in Black patients is not equally increasing.
Of the patients who received tPA or EVT, we failed to find a racial or ethnic difference in in-hospital mortality. Despite our insignificant finding, we did find that patients from minority populations (NH Black, Hispanic, Asian, Pacific Islander, and Native American) all had a significantly longer hospital LOSs than NH White patients. This signifies that racial and ethnic minority patients who received tPA or EVT stayed in the hospital longer than White patients. This finding is consistent with previous research which demonstrated a longer LOS for minority ischemic stroke patients during 2011–2012 [28]. This increased LOS for minority patients may indicate suboptimal quality of care or poorer clinical and functional outcomes post tPA or EVT. LOS may be used as metric of quality of care as previous studies have demonstrated that shorter length of stay for stroke patients is associated with higher quality of care [29]. The current study reveals two important disparities in minority patient ischemic stroke care: 1. less utilization of tPA and EVT in NH Black patients and 2. longer length of stay for minority patients who received tPA and EVT when compared to NH White patients.
Delays in care could be a reason why Black patients are less likely to receive tPA and EVT than White patients. TPA is most effective when given within 4.5 hours of stroke onset and EVT is most effective when given within 6 hours of stroke onset [10]. The use of emergency medical services (EMS) for transportation to the hospital is associated with shorter transport time, however Black patients are less likely to use EMS at the onset of stroke thus often delaying their care [30, 31]. Black patients who use EMS experience prolonged time from calling 911 to arriving at the hospital as compared to White patients [32]. Once Black patients arrive to the hospital, they face longer wait times33 and experience longer door-to-imaging time which further delays care [5,34]. Furthermore, even for patients who meet the recommended time period, Black patients are still less likely to receive tPA than White patients [35]. Further research on why these delays occur could guide the development of interventions to improve the efficiency of care for Black ischemic stroke patients.
Limited access to hospitals that provide EVT and tPA may be another reason why Black patients are less likely to receive these treatments. There is significantly lower access to hospitals that provide tPA in rural areas [36], and we see a similar trend with EVT. EVT is performed primarily at large stroke centers that tend to be urban teaching hospitals [37]. Only 37% of stroke centers in the nation are able to perform EVT, and only 20% of Americans can be transported within 15 minutes to a hospital that provides EVT [38]. Black patients are less likely to arrive at hospitals that perform EVT procedures [37] which demonstrates limited access. Transfer to a hospital that provides EVT services can further delay care which may lessen the likelihood of Black patients receiving this treatment. Expansion of tPA and EVT use to more regional areas may improve access to stroke care for minority patients.
Lastly, implicit biases cannot go unrecognized when considering the racial and ethnic differences in usage of tPA and EVT and LOS after these procedures. Many healthcare providers have implicit or unconscious racial biases that can impact the relationship between the patient and physician [39]. One study showed that physician implicit bias against Black patients were correlated with a decreased likelihood of recommending thrombolysis treatment for myocardial infarction to Black patients and an increased likelihood of recommending thrombolysis treatment to White patients [40]. Studies have cited that medical provider racial biases may play a role in the racial disparities present in stroke care [30]. A study from the AHA Get With The Guidelines Stroke Program demonstrated that Black stroke patients are less likely to receive patient-centered evidence-based care than White stroke patients [4]. Evidence based care can contribute to both quality of care and length of stay. The AHA also suggests that a lack of minority physicians may play a role in the racial and ethnic disparities present in stroke care [30]. When discussing the disparities in the usage and outcomes of tPA and EVT in ischemic stroke patients, we must not overlook medical providers’ implicit racial and ethnic biases that impact stroke care in minority patients.
In addition to disparities in the utilization of tPA and EVT between non-Hispanic Black and non-Hispanic White patients, we did not observe significant differences in the receipt of these standard-of-care treatments for ischemic stroke among other racial and ethnic groups when compared to non-Hispanic White patients. Notably, the Other racial group that includes Asian, Pacific Islander, and Native American patients, demonstrated a higher likelihood of receiving EVT treatment, even after adjusting for potential confounding variables. Several factors may contribute to this phenomenon: First, the Other racial group is highly diverse, potentially encompassing individuals who maintain healthier lifestyles and better cardiovascular health (e.g., certain Asian groups) when compared to White patients [41]. However, it’s important to acknowledge that this categorization may obscure disparities faced by specific subgroups within the Other racial group, such as Native Americans, who may experience delayed access to health care, but they are less represented in our current grouping methodology [42,43]. Second, the Other racial group has a much smaller sample size compared to the White group, which could introduce potential biases in our estimates.
Limitations
Our study has several key limitations. First, we did not consider stroke severity in our analysis. While coding of the National Institutes of Health Stroke Scale (NIHSS) in the NIS dataset has been increasing over the past few years, the NIHSS is not yet coded for each patient12. Including only patients with coded NIHSS would have limited our sample size. However, we are hopeful that in future studies we can control for NIHSS as the coding in the NIS dataset increases. Additionally, the use of tPA and EVT is a patient specific medical decision. Within the NIS dataset, we cannot control for all reasons as to why a patient did or did not receive tPA or EVT such as time of arrival to hospital since onset of stroke, medical contraindications for treatment, or patient medical history. Also, a small number of patients received both tPA and EVT, and this was not controlled for in our analysis. Additionally, while we did not find a racial or ethnic difference in in-hospital mortality after EVT or tPA, this may be due to a lack of statistical power as only a small subset of our population died in the hospital. Because the dataset comes from medical records, there is a possibility of data coding errors regarding demographics, tPA and EVT use, mortality, and discharge status. Lastly, we cannot account for all comorbidities and confounding variables that may be underreported.
Conclusion
The current study demonstrates that Black ischemic stroke patients are less likely to receive tPA and EVT than White patients. Additionally, when assessing post-tPA or EVT outcomes, we failed to find a racial or ethnic difference for in-hospital mortality, but we did find that minority patients (including but not limited to Black, Hispanic, Asian, Pacific Islander, and Native American) had significantly longer LOS in the hospital than White patients. Even with an increasing trend in the usage of tPA and EVT in the US, we still see disparities when caring for minority patients with ischemic stroke. Black patients are less likely to receive both tPA and EVT, and minority populations have longer hospital stays than White patients which may be an indicator of suboptimal quality of care or poor clinical outcome. We hope that with additional research, we can gain a greater understanding of the racial and ethnic disparities in usage of the life-saving treatments of tPA and EVT in ischemic stroke patients to further encourage interventions to improve stroke care for minority patients.
Acknowledgments
Author contributions: Delaney Metcalf and Donglan Zhang conceptualized and designed the research. Delaney Metcalf and Donglan Zhang achieved and analyzed the data and wrote the article. All authors reviewed, discussed, and approved the final article.
Sources of Funding
The present study was supported by the National Institute on Minority Health and Health Disparities (1R01MD013886).
Footnotes
Disclosures
None.
Contributor Information
Delaney M. Metcalf, Medical College of Georgia and Augusta University/ University of Georgia Medical, Partnership, Athens, GA, 30605.
Donglan Zhang, Division of Health Services Research, Department of Foundations of Medicine, New York University Long Island School of Medicine, Mineola, NY, 11501.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Underlying Cause of Death, 1999–2018 In: Prevention CDCa, ed. CDC WONDER Online Database; 2018. [Google Scholar]
- 4.Schwamm LH, Reeves MJ, Pan W, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. doi: 10.1161/circulationaha.109.881490 [DOI] [PubMed] [Google Scholar]
- 5.Jacobs BS, Birbeck G, Mullard AJ, et al. Quality of hospital care in African American and white patients with ischemic stroke and TIA. Neurology. 2006;66:809–814. doi: 10.1212/01.wnl.0000203335.45804.72 [DOI] [PubMed] [Google Scholar]
- 6.Rinaldo L, Rabinstein AA, Cloft H, et al. Racial and Ethnic Disparities in the Utilization of Thrombectomy for Acute Stroke. Stroke. 2019;50:2428–2432. doi: doi: 10.1161/STROKEAHA.118.024651 [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. New England Journal of Medicine. 2018;378:708–718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. New England Journal of Medicine. 2017;378:11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke KET, Walsh CD, Kelly PJ. Safety and Efficacy of IV-TPA for Ischaemic Stroke in Clinical Practice – A Bayesian Analysis. Cerebrovascular Diseases. 2009;28:572–581. doi: 10.1159/000247601 [DOI] [PubMed] [Google Scholar]
- 10.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e99. doi: doi: 10.1161/STR.0000000000000158\ [DOI] [PubMed] [Google Scholar]
- 11.Qiu S, Xu Y. Guidelines for Acute Ischemic Stroke Treatment. Neurosci Bull. 2020;36:1229–1232. doi: 10.1007/s12264-020-00534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Havenon A, Sheth K, Johnston KC, et al. Acute Ischemic Stroke Interventions in the United States and Racial, Socioeconomic, and Geographic Disparities. Neurology. 2021;97:e2292–e2303. doi: 10.1212/wnl.0000000000012943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1 [DOI] [PubMed] [Google Scholar]
- 14.Saber H, Navi BB, Grotta JC, et al. Real-World Treatment Trends in Endovascular Stroke Therapy. Stroke. 2019;50:683–689. doi: 10.1161/strokeaha.118.023967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose KM, Suchindran CM, Foraker RE, et al. Neighborhood disparities in incident hospitalized myocardial infarction in four U.S. communities: the ARIC surveillance study. Ann Epidemiol. 2009;19:867–874. doi: 10.1016/j.annepidem.2009.07.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fargen KM, Neal D, Blackburn SL, et al. Health disparities and stroke: the influence of insurance status on the prevalence of patient safety indicators and hospital-acquired conditions. J Neurosurg. 2015;122:870–875. doi: 10.3171/2014.12.Jns14646 [DOI] [PubMed] [Google Scholar]
- 17.Bahonar A, Khosravi A, Khorvash F, M et al. Seasonal and Monthly variation in stroke and its subtypes-10 Year Hospital-Based Study. Mater Sociomed. 2017;29:119–123. doi: 10.5455/msm.2017.29.119-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ofori-Asenso R, Zomer E, Chin KL, et al. Effect of Comorbidity Assessed by the Charlson Comorbidity Index on the Length of Stay, Costs and Mortality among Older Adults Hospitalised for Acute Stroke. Int J Environ Res Public Health. 2018;15. doi: 10.3390/ijerph15112532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leira EC, Hess DC, Torner JC, et al. Rural-urban differences in acute stroke management practices: a modifiable disparity. Arch Neurol. 2008;65:887–891. doi: 10.1001/archneur.65.7.887 [DOI] [PubMed] [Google Scholar]
- 20.Alshekhlee A, Walbert T, DeGeorgia M, et al. The impact of Accreditation Council for Graduate Medical Education duty hours, the July phenomenon, and hospital teaching status on stroke outcomes. J Stroke Cerebrovasc Dis. 2009;18:232–238. doi: 10.1016/j.jstrokecerebrovasdis.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 21.Saposnik G, Baibergenova A, O’Donnell M, et al. Hospital volume and stroke outcome: does it matter? Neurology. 2007;69:1142–1151. doi: 10.1212/01.wnl.0000268485.93349.58 [DOI] [PubMed] [Google Scholar]
- 22.Witrick B, Zhang D, Switzer JA, et al. The Association Between Stroke Mortality and Time of Admission and Participation in a Telestroke Network. J Stroke Cerebrovasc Dis. 2020;29:104480. doi: 10.1016/j.jstrokecerebrovasdis.2019.104480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mekonnen B, Wang G, Rajbhandari-Thapa J, et al. Weekend Effect on in-Hospital Mortality for Ischemic and Hemorrhagic Stroke in US Rural and Urban Hospitals. J Stroke Cerebrovasc Dis. 2020;29:105106. doi: 10.1016/j.jstrokecerebrovasdis.2020.105106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson KM, Trochim WMK. Concept Mapping as an Alternative Approach for the Analysis of Open-Ended Survey Responses. Organizational Research Methods. 2005;5:307–336. doi: 10.1177/109442802237114 [DOI] [Google Scholar]
- 25.Skyrud KD, Vikum E, Hansen TM, et al. Hospital Variation in 30‐Day Mortality for Patients With Stroke; The Impact of Individual and Municipal Socio‐Demographic Status. Journal of the American Heart Association. 2019;8:e010148. doi: doi: 10.1161/JAHA.118.010148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Statistics in Medicine. 2010;29:2920–2931. doi: 10.1002/sim.3944 [DOI] [PubMed] [Google Scholar]
- 27.Faysel MA, Singer J, Cummings C, et al. Disparities in the Use of Intravenous t-PA among Ischemic Stroke Patients: Population-based Recent Temporal Trends. J Stroke Cerebrovasc Dis. 2019;28:1243–1251. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 28.Kumar N, Khera R, Pandey A, et al. Racial Differences in Outcomes after Acute Ischemic Stroke Hospitalization in the United States. J Stroke Cerebrovasc Dis. 2016; 25(8):1970–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.049 [DOI] [PubMed] [Google Scholar]
- 29.Svendsen ML, Ehlers LH, Andersen G, et al. Quality of care and length of hospital stay among patients with stroke. Med Care. 2009; 47(5):575–82. doi: 10.1097/MLR.0b013e318195f852 [DOI] [PubMed] [Google Scholar]
- 30.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24 [DOI] [PubMed] [Google Scholar]
- 31.Menon SC, Pandey DK, Morgenstern LB. Critical factors determining access to acute stroke care. Neurology. 1998;51:427–432. doi: 10.1212/wnl.51.2.427 [DOI] [PubMed] [Google Scholar]
- 32.Kleindorfer DO, Lindsell CJ, Broderick JP, et al. Community socioeconomic status and prehospital times in acute stroke and transient ischemic attack: do poorer patients have longer delays from 911 call to the emergency department? Stroke. 2006;37:1508–1513. doi: 10.1161/01.STR.0000222933.94460.dd [DOI] [PubMed] [Google Scholar]
- 33.Karve SJ, Balkrishnan R, Mohammad YM, et al. Racial/ethnic disparities in emergency department waiting time for stroke patients in the United States. J Stroke Cerebrovasc Dis. 2011;20:30–40. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 34.Polineni SP, Perez EJ, Wang K, et al. Sex and Race-Ethnic Disparities in Door-to-CT Time in Acute Ischemic Stroke: The Florida Stroke Registry. J Am Heart Assoc. 2021;10:e017543. doi: 10.1161/jaha.120.017543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messé SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87:1565–1574. doi: 10.1212/wnl.0000000000003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzales S, Mullen MT, Skolarus L, et al. Progressive rural-urban disparity in acute stroke care. Neurology. 2017;88:441–448. doi: 10.1212/wnl.0000000000003562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Attenello FJ, Adamczyk P, Wen G, et al. Racial and socioeconomic disparities in access to mechanical revascularization procedures for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:327–334. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarraj A, Savitz S, Pujara D, et al. Endovascular Thrombectomy for Acute Ischemic Strokes: Current US Access Paradigms and Optimization Methodology. Stroke. 2020;51:1207–1217. doi: 10.1161/strokeaha.120.028850 [DOI] [PubMed] [Google Scholar]
- 39.Blair IV, Steiner JF, Fairclough DL, et al. Clinicians’ implicit ethnic/racial bias and perceptions of care among Black and Latino patients. Ann Fam Med. 2013;11:43–52. doi: 10.1370/afm.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22:1231–1238. doi: 10.1007/s11606-007-0258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Summary Health Statistics: National Health Interview Survey:2018 Table A-1a. 2021.
- 42.Cromer KJ, Wofford L, Wyant DK. Barriers to Healthcare Access Facing American Indian and Alaska Natives in Rural America. J Community Health Nurs. 2019. Oct-Dec;36(4):165–187. doi: 10.1080/07370016.2019.1665320. [DOI] [PubMed] [Google Scholar]
- 43.Nesoff ED, Brownstein JN, Veazie M, O’Leary M, Brody EA. Time-to-Treatment for Myocardial Infarction: Barriers and Facilitators Perceived by American Indians in Three Regions. J Community Health. 2017. Feb;42(1):129–138. doi: 10.1007/s10900-016-0239-x. [DOI] [PubMed] [Google Scholar]


