Abstract
Exogenous glucocorticoids are known to inhibit wound repair, but the roles and mechanisms of action of endogenous glucocorticoids during the healing process are as yet unknown. Therefore, we wounded mice expressing a DNA-binding-defective mutant version of the glucocorticoid receptor (GRdim mice) and also analysed fibroblasts from these animals in vitro. We found a remarkably enlarged granulation tissue with a high fibroblast density in GRdim mice. This difference is likely to result from an increased migratory and proliferative capacity of GRdim fibroblasts and from elevated expression levels of soluble factors involved in granulation tissue formation in wounds of GRdim mice. In spite of the larger granulation tissue seen in early wounds, late wounds appeared normal, most likely due to an enhanced ability of GRdim fibroblasts to contract collagen. These results demonstrate an as yet unidentified role of endogenous glucocorticoids in the regulation of wound repair.
INTRODUCTION
It is clear from a large number of experimental and clinical studies that treatment with exogenous glucocorticoids (GCs) is detrimental to tissue repair. This inhibitory effect on wound healing is due largely to the anti-inflammatory action of GCs (Wong and Wahl, 1989). Furthermore, GCs at pharmacological doses regulate the expression of several key proteins during the repair process, such as pro-inflammatory cytokines, growth factors and extracellular matrix proteins (Beer et al., 2000). However, the potential roles of endogenous GCs in tissue repair have been largely ignored, and no data are available concerning the molecular mechanism by which GCs control wound healing.
Having diffused from the bloodstream into the cell cytoplasm, GCs bind glucocorticoid receptors (GRs) and translocate to the nucleus as monomers. These monomers may then dimerise and bind to glucocorticoid response elements (GREs) or negative-acting GREs in the upstream region of target genes. Alternatively, they may act in a DNA-binding-independent fashion by interfering directly with other transcription factors such as AP-1 and NFκB (Tuckermann et al., 1999). To delineate the role of endogenous GCs and their molecular mechanism of action during tissue repair, we wounded adult mice expressing a DNA-binding-defective mutant version of the GR (GRdim mice). These animals are deficient in the GC signalling pathway that acts via GREs but retain the regulation that occurs in the absence of direct DNA binding. Consequently, they lack inducibility of GRE-dependent genes and show an impairment of several physiological functions of the GR, including regulation of the hypothalamic–pituitary–adrenal (HPA) axis, thymocyte apoptosis and proliferation of erythroid progenitors (Reichardt et al., 1998). In this study we provide evidence for a role of endogenous GCs in wound repair.
RESULTS AND DISCUSSION
An important side effect of high-dose GC treatment is the potent inhibitory effect of GCs on wound repair, which limits their therapeutic application considerably. Endogenous GCs have also been reported as being detrimental to wound healing in experiments where circulating GC levels were raised artificially by restraint stress conditions (Padgett et al., 1998), but it has been unclear whether normal levels of endogenous GCs affect tissue repair. To address this question, we wounded mice in which the GC signalling pathway dependent on dimerisation and DNA binding of the GR is disrupted.
We observed no histological abnormalities in unwounded skin (data not shown), suggesting that any difference in repair is not due to skin alterations present prior to injury. Furthermore, no significant difference in serum corticosterone levels was detected either between GRdim mice and controls, or between mice killed 1 or 5 days post-wounding (data not shown).
Enhanced granulation tissue formation in GRdim mice
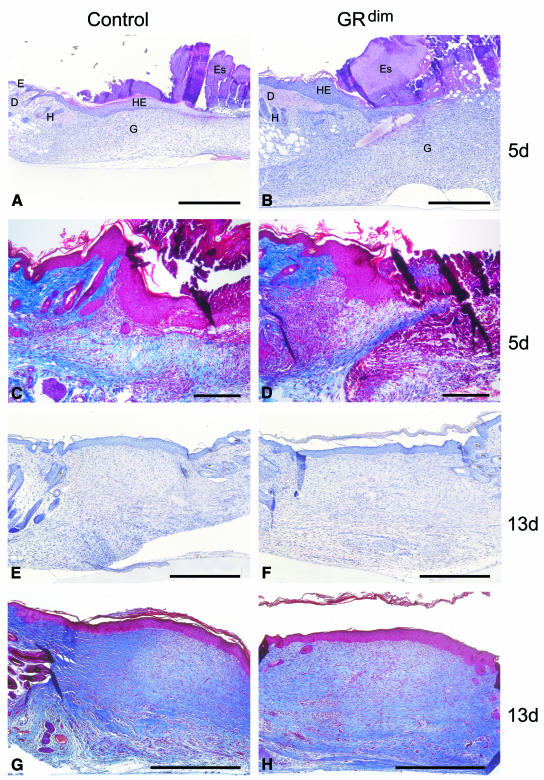
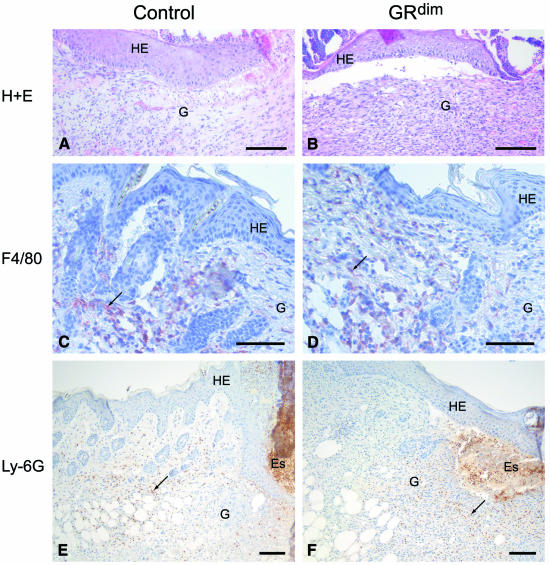
After skin injury, no obvious abnormalities in wound closure or appearance were observed (data not shown). However, when histological sections from the middle of the wounds were processed for either haematoxylin/eosin (H/E) (Figure 1A, B, E and F) or Masson trichrome staining (Figure 1C, D, G and H), 5-day wounds from GRdim mice consistently showed a strongly extended area of granulation tissue. A morphometric analysis of 10 wounds each from five control and five GRdim mice revealed a 64% increase in the area of granulation tissue in the latter (SEM ±10.6% for wild-type and ±9.7% for GRdim animals). Furthermore, a higher density of the cellular infiltrate was seen in wounds of GRdim mice when compared with controls (results from two wounds each of 15 control and 15 GRdim mice) (Figure 1A–D). In particular, the total number and density of fibroblasts in the GRdim wounds were increased strongly (Figure 2A and B), with quantitation of BrdU-positive fibroblasts suggesting a slightly higher number of proliferating cells in the granulation tissue of 5-day wounds of GRdim mice (30% higher, SEM ±18%). In contrast, the density of macrophages was identical in wild-type and GRdim mice, as shown by immunostaining with an antibody against monocytes/macrophages (Figure 2E and F) and cell counting (data not shown). In addition, immunohistochemistry with antibodies against neutrophils (Figure 2C and D) and endothelial cells (data not shown) revealed no differences in the density of these cells. Nevertheless, their total number was higher in GRdim mice due to the more extended granulation tissue. There was no visible difference in re-epithelialisation at any time during repair.
Fig. 1. Enhanced granulation tissue formation in GRdim mice. Sections from the middle of excisional wounds of GRdim and wild-type mice were stained with H/E (A, B, E and F) or Masson trichrome (C, D, G and H). (A, C) 5-day and (E, G) 13-day wounds of a control mouse; (B, D) 5-day and (F, H) 13-day wounds of a GRdim mouse. D, dermis; E, epidermis; Es, eschar; G, granulation tissue; H, hair follicle; HE, hyperproliferative epithelium. Scale bars: A, B and E–H, 200 µm; C and D, 40 µm.
Fig. 2. Granulation tissue composition in 5-day wounds of GRdim mice. (A and B) H/E stainings of the granulation tissue of control (A) and GRdim mice (B) are shown at high magnification. (C–F) Wax sections from control (C, E) and GRdim (D, F) mice were stained with the F4/80 antibody that detects monocytes/macrophages (C, D) or with the Ly-6G antibody that recognizes neutrophils (E, F). See Figure 1 for abbreviations. Scale bars: A–D, 20 µm; E and F, 40 µm.
By 13 days post-wounding, the histological difference between control and GRdim mice was no longer apparent (results from two wounds each of five control and five GRdim mice) (Figure 1E–H).
GRdim mice show enhanced expression of IL-1β
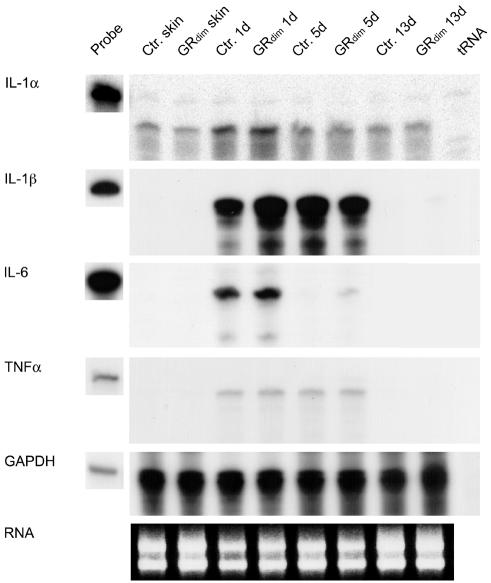
To determine a possible anti-inflammatory action of endogenous GCs, we analysed the expression of pro-inflammatory cytokines in the wounds of GRdim mice. For this purpose we performed RNase protection assays on at least two independent pools of total RNA, each isolated from two wounds from each of five control and five GRdim mice. Levels of IL-1β mRNA were 2.2-fold higher in 1-day wounds of GRdim mice compared with controls (Figure 3), and there was a minor upregulation of IL-6 mRNA in GRdim mice at this time point (Figure 3). The levels of TNFα and IL-1α mRNAs were unaltered (Figure 3). Since neutrophils and macrophages are the major producers of IL-1β in early wounds (Hübner et al., 1996), the increase in IL-1β expression is either the result of (i) an indirect effect due to enhanced recruitment of inflammatory cells to the wounds of GRdim mice or (ii) direct suppression of cytokine expression by GCs in inflammatory cells of control mice. The first possibility is supported by the inhibitory effect of exogenous GCs on inflammatory cell infiltration into wounds (Leibovich and Ross, 1975; Wong and Wahl, 1989), and the latter possibility is supported by the direct inhibitory effect of GCs on IL-1β gene transcription and mRNA stability in a human promonocytic cell line (Lee et al., 1988). However, since the DNA-binding-defective GRdim can still repress both IL-1β and IL-6 gene expression in isolated peritoneal macrophages (Reichardt et al., 2001), it appears likely that differences in cell recruitment to the wound site account for the observed effects. Previous studies from our laboratory point towards neutrophils being the most likely source of these pro-inflammatory cytokines in 1-day wounds (Hübner et al., 1996). However, exact quantitation of the neutrophil presence at this time point was hampered by their massive infiltration into the clot, where their numbers are not readily discernible.
Fig. 3. Increased expression of IL-1β in early wounds of GRdim mice. mRNA levels of IL-1α, -1β, -6, TNFα or GAPDH (loading control) were determined by RNase protection assay on 20 µg RNA samples from non-wounded back skin plus 1-, 5- and 13-day wounds from control and GRdim mice. 1000 c.p.m. of the hybridization probes were loaded in the lanes labeled ‘probe’ and used as a size marker. The results were reproduced in at least two independent RNase protection assays using RNAs from independent wound healing experiments.
Expression of growth factors and extracellular matrix proteins after wounding
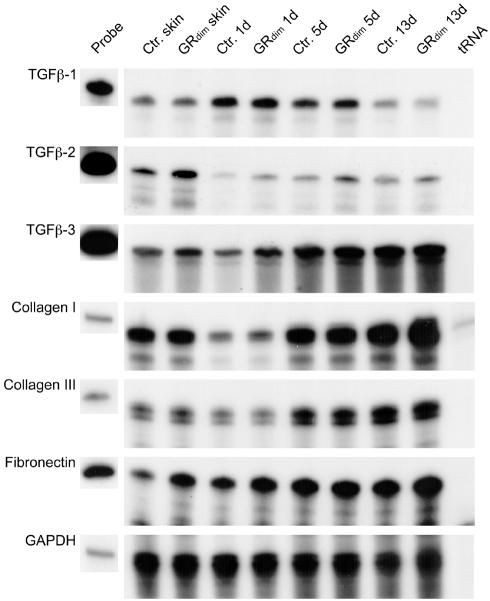
To determine a possible reason for the increase in the area of granulation tissue, we investigated the expression of growth factors that regulate granulation tissue formation (Figure 4; data not shown). We found no difference in transforming growth factor (TGF)β-1, platelet-derived growth factor (β-chain), or connective tissue growth factor expression between GRdim and control mice. However, GRdim mice showed a 1.5- or 3-fold increase in TGFβ-2 mRNA in unwounded skin or 1-day wounds, respectively. TGFβ-3 mRNA was 1.2-fold higher in unwounded skin of GRdim mice, and reached a maximum difference of ∼2-fold in 1-day wounds. In 13-day wounds, TGFβ-2 mRNA was still elevated in GRdim mice, but TGFβ-3 showed indistinguishable expression levels compared with wild-type animals.
Fig. 4. Expression of growth factors and extracellular matrix proteins after wounding. Expression levels of TGFβ-1, -2 and -3, collagens α1(I) and α1(III), fibronectin and GAPDH were determined by RNase protection assay on 20 µg RNA samples from non-wounded back skin plus 1-, 5- and 13-day wounds from control and GRdim mice. See Figure 3 for probe details.
The mRNA levels of the major wound matrix proteins collagen α1(I), collagen α1(III) and tenascin-C were unaltered in GRdim mice. However, the levels of fibronectin mRNA were increased in unwounded skin and 1-day wounds of GRdim mice (Figure 4; data not shown).
Enhanced migration and proliferation of GRdim fibroblasts
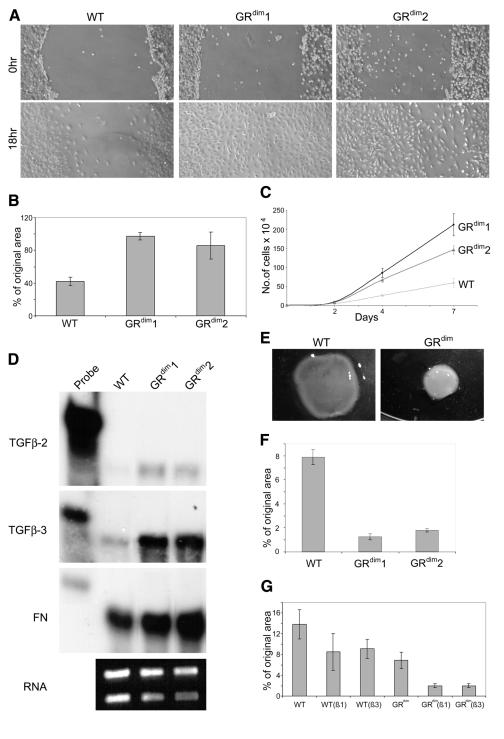
In addition to increased growth factor levels, the more extended granulation tissue in the wounds of GRdim animals could also result from a direct inhibitory effect of GCs on cells present in the wound mesenchyme. This hypothesis is supported by results obtained with pharmacological doses of exogenous GC, where inhibition of migration and/or proliferation of inflammatory cells, endothelial cells and fibroblasts was observed (reviewed in Beer et al., 2000). Since the density of fibroblasts was particularly increased in the wounds of GRdim mice, we determined whether GRdim fibroblasts have an increased migratory and/or proliferative capacity in vitro. Since primary fibroblasts become senescent after four or five passages, we used immortalized fibroblasts for most experiments to avoid artifacts associated with the onset of senescence. Using a scratch-wounding assay, we followed the movement of cells into the injured area. As shown in Figure 5A and B, significantly fewer wild-type cells had migrated into the wounded area compared with GRdim cells within 18 h. This result was observed with two different cell lines in four independent experiments. Furthermore, fibroblasts from GRdim mice also showed an enhanced proliferation rate compared with control fibroblasts, as shown by cell counting (Figure 5C) and BrdU incorporation assays (data not shown).
Fig. 5. Enhanced migration, proliferation, collagen gel contraction and altered gene expression of GRdim fibroblasts. Immortalized fibroblasts from wild-type (wt) and GRdim mice were allowed to attach on the dish. A clear space was produced in the monolayer, and the wounded fibroblast layer was photographed immediately and 18 h after wounding (A). The distance of migration from the original borders was determined in seven independent experiments and is indicated as a percentage of the original distance from the middle of the cleared space (B). (C) Control and GRdim fibroblasts were seeded into culture dishes and counted in triplicate dishes at different time points. (D) Samples of 10 µg of cellular RNA from wild-type and GRdim fibroblasts were analysed for the expression of TGFβ-2, TGFβ-3 and fibronectin. As a loading control, 1 µg of all RNA samples was loaded on a 1% agarose gel before hybridization and stained with ethidium bromide. Immortalized fibroblasts from wild-type and GRdim animals were seeded into collagen gels. They were photographed 21 h after seeding (E) and the area of the gel was measured in triplicate dishes [indicated as a percentage of the original area in (F)]. (G) Contraction assays were performed in 5% FCS in the absence or presence of 1 ng/ml TGFβ-1 or TGFβ-3. The area of the gels was measured in triplicate dishes 20.5 h after seeding and is indicated as a percentage of the original area.
Increased expression of TGFβs and fibronectin in fibroblasts from GRdim mice
Finally, we determined whether fibroblasts could be responsible for the increased expression of TGFβ-2, TGFβ-3 and fibronectin seen in the wounds of GRdim mice. Indeed, expression of TGFβ-2 and particularly of TGFβ-3 was strongly enhanced (7- and 5-fold, respectively) in GRdim cells compared with control fibroblasts. This result was obtained with cells grown on plastic (Figure 5D) or in collagen lattices (data not shown). In addition, a 1.5-fold higher expression of fibronectin was seen in GRdim fibroblasts compared with control cells. This finding as well as the strong expression of TGFβs and fibronectin in fibroblasts of healing skin wounds (Brown et al., 1993; Frank et al., 1996) suggests that these cells contribute strongly to the enhanced mRNA levels of TGFβs in wounds of GRdim mice. The less pronounced difference in the expression of these genes in vivo is due most likely to the fact that changes in a single cell type are dwarfed by large numbers of other cells present in wound tissue.
Enhanced collagen gel contraction of GRdim fibroblasts
Since the extensive granulation tissue of GRdim wounds is so efficiently contracted, we determined the ability of fibroblasts to freely contract three-dimensional collagen lattices, in which collagen fibrils are reorganized by the fibroblasts to build a structure resembling connective tissue (Bell et al., 1979). A remarkable difference was observed between fibroblasts from control and GRdim mice in six independent experiments. Although we often observed a delayed onset of contraction (data not shown), the rate of contraction of GRdim fibroblasts was enhanced strongly in all experiments (Figure 5E–G). This result was reproduced with primary fibroblasts (data not shown). These findings suggest that the increased capacity of GRdim fibroblasts to contract collagen is at least partially responsible for the normal appearance of late wounds in these mice. Finally, we determined whether the difference in collagen gel contraction between wild-type and GRdim fibroblasts could be due to the higher expression of TGFβs in the latter. Addition of TGFβ-1 or TGFβ-3 to wild-type fibroblasts grown in 5% fetal calf serum (FCS) increased their collagen gel contraction capacity, although the contraction rate of GRdim fibroblasts in the absence of TGFβs was still higher (Figure 5G). TGFβs further increased the gel contraction capacity of the latter, indicating that the difference in TGFβ expression is not the only reason for the increased gel contraction capacity of GRdim fibroblasts.
CONCLUSIONS
In summary, our results show that, in a toned down version of the dramatic effects resulting from exogenous GC treatment, endogenous GCs appear to exert a suppressive effect on early wound healing. Although this may seem disadvantageous, it is a small price to pay for the benefits derived from endogenous GC signalling, particularly in the response to stress, and ultimately has no deleterious effect on repair. Indeed, endogenous GC signalling could play a useful regulatory role in circumstances where the inflammatory response is excessively strong, for example in an infected wound.
The inhibitory effect of endogenous GCs on wound healing is obviously mediated by the DNA-binding activity of the GR, suggesting that some of the adverse effects of exogenous GCs on wound healing are also mediated via GRE binding. Since the side effects of treatment with exogenous GCs pose such a severe clinical problem, this work suggests that the use of more-specific GCs, which act only via the DNA-binding-independent GC signalling pathway, could be beneficial for the therapy of disorders currently being treated with GCs.
METHODS
Identification of GRdim mice. GRdim mice and their genotyping by PCR have been described previously (Reichardt et al., 1998). Wild-type and GRdim mice had been intercrossed for more than 20 generations into a C57Bl/6–129SvEv mixed background. GRdim mice and age- and sex-matched wild-type controls from this background were used for all experiments.
Wounding and preparation of wound tissues. Four full-thickness excisional wounds (5 mm diameter) were generated on the back of female GRdim and control mice (10–12 weeks old) as described previously (Werner et al., 1994). Mice were killed 1, 5 or 13 days after injury. The complete wounds, including 2 mm of the epithelial margins, were excised and used for RNA isolation. Non-wounded back skin served as a control. For histological analysis, the complete wounds were isolated, bisected, fixed in 4% paraformaldehyde in PBS and embedded in paraffin. Sections (7 µm) from the middle of the wound were analysed by H/E and Masson trichrome stainings. Wounding experiments were carried out with the permission of the local veterinary authorities.
Determination of GC levels in serum. Mice were housed individually prior to and after wounding, killed by decapitation and 500 µl of blood collected in a tube containing 50 µl 0.5 M EDTA pH 8.0. Samples were centrifuged in a cooled benchtop centrifuge, supernatants collected and snap-frozen in liquid nitrogen. Hormone concentrations in the serum were measured using commercially available radio-immunoassay kits according to the supplier’s instructions (ICN Biomedicals, Meckenheim, Germany).
RNA isolation and RNase protection assay. RNA isolation and RNase protection assays were performed as described previously (Werner et al., 1992). All protection assays were carried out at least in duplicate with different sets of RNA from independent experiments. Template cDNAs are described in Grose et al. (2002) and references therein. Differences in the intensity of bands were quantified using ImageQuant (Molecular Dynamics).
Immunohistochemistry. Bisected wounds were fixed overnight in 1% acetic acid/95% ethanol in PBS at 4°C. Sections (7 µm) were incubated with a biotinylated rat antibody targeted to Ly-6G (BD Biosciences, Heidelberg, Germany), or with a rat antibody targeted to F4/80 (Serotec, Oxford, UK), washed in PBS containing 0.1% Tween-20 and, for anti-F4/80, incubated with biotinylated donkey anti-rat IgG (Dianova GmbH, Hamburg, Germany). The Vectastain avidin-biotin-peroxidase complex kit was then used according to the manufacturer’s instructions, prior to peroxidase detection with a diaminobenzidine-peroxidase substrate kit (both from Vector Laboratories, Burlingame, CA). Sections were counterstained with Mayer’s Haemalum.
Fibroblast in vitro studies. Primary mouse embryonic fibroblasts were isolated and immortalized as described previously (Todaro and Green, 1963; Brusselbach et al., 1995). Heterozygous GRdim mice were mated and E14.5 embryos of homozygous GRdim and wild-type genotype were obtained from one litter. Each primary fibroblast culture was isolated from a single embryo, which was homogenized to single cells after removal of the head and the liver and subsequently immortalized. Cells were cultured in DMEM supplemented with 10% FCS without additional GCs. Some gel contraction experiments were performed in 5% FCS and in the absence or presence of 1 ng/ml TGFβ-1 (Roche, Rotkreuz, Switzerland) or TGFβ-3 (R&D Systems, Abingdon, UK). Contraction of collagen lattices was performed as described previously (Kessler et al., 2001). 9 × 104 cells/ml gel were seeded into 6-cm Petri dishes, and collagen gel areas were measured at different time points. For in vitro wound closure assays, cells were seeded at high density (1 × 106 cells/3.5-cm dish). Two hours after seeding, when the cells were completely attached, a scratch was made within the cell layer with a sterile pipette tip. Eighteen hours later, the same spot was photographed under phase-contrast microscopy (Eckes et al., 1998) and the distance of migration from the original border was determined. For proliferation studies, 1.4 × 104 cells were seeded into 3.5-cm cell culture dishes. Cells were counted in triplicate dishes after 2, 4 and 7 days.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr G. Schütz for support, Drs C. Mauch and B. Eckes for help with the fibroblast experiments and for helpful suggestions, C. Born-Berclaz for excellent technical assistance and Dr P. Martin for critical reading of the manuscript. This work was supported by grants from the German Ministry for Education and Research (BMBF) (to S.W.) and the Swiss National Science Foundation (No. 31-61358 to S.W.).
REFERENCES
- Beer H.D., Fässler, R. and Werner, S. (2000) Glucocorticoid-regulated gene expression during cutaneous wound repair. Vitam. Horm., 59, 217–239. [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson, B. and Merill, C. (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl Acad. Sci. USA, 76, 1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.F., Dubin, D., Lavigne, L., Logan, B., Dvorak, H.F. and van de Water, L. (1993) Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am. J. Pathol., 142, 793–801. [PMC free article] [PubMed] [Google Scholar]
- Brusselbach S.U., Mohle-Steinlein, Z.Q., Wang, M., Schreiber, F.C., Lucibello, R., Muller, R. and Wagner, E.F. (1995) Cell proliferation and cell cycle progression are not impaired in fibroblasts and ES cells lacking c-Fos. Oncogene, 10, 79–86. [PubMed] [Google Scholar]
- Eckes B. et al. (1998) Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci., 111, 1897–1907. [DOI] [PubMed] [Google Scholar]
- Frank S., Madlener, M. and Werner, S. (1996) Transforming growth factors b1, b2 and b3 and their receptors are differentially regulated during normal and impaired wound healing. J. Biol. Chem., 271, 10188–10193. [DOI] [PubMed] [Google Scholar]
- Grose R., Hutter, C., Bloch, W., Thorey, I., Watt, F., Fässler, R., Brakebusch, C. and Werner, S. (2002) A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development, 129, 2303–2315. [DOI] [PubMed] [Google Scholar]
- Hübner G., Brauchle, M., Smola, H., Madlener, M., Fassler, R. and Werner, S. (1996) Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine, 8, 548–556. [DOI] [PubMed] [Google Scholar]
- Kessler D., Dethlefsen, S., Haase, I., Plomann, M., Hirche, F., Krieg, T. and Eckes, B. (2001) Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J. Biol. Chem., 276, 36575–36585. [DOI] [PubMed] [Google Scholar]
- Lee S.W., Tsou, A.-P., Chan, H., Thomas, J., Petrie, K., Eugui, E.M. and Allison, A.C. (1988) Glucocorticoids selectively inhibit the transcription of the interleukin 1β gene and decrease the stability of interleukin 1β mRNA. Proc. Natl Acad. Sci. USA, 85, 1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S.J. and Ross, R. (1975) The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol., 78, 71–100. [PMC free article] [PubMed] [Google Scholar]
- Padgett D.A., Marucha, P.T. and Sheridan, J.F. (1998) Restraint stress slows cutaneous wound healing in mice. Brain Behav. Immun., 12, 64–73. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M. et al. (1998) DNA binding of the glucocorticoid receptor is not essential for survival. Cell, 15, 531–541. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Tuckermann, J.P., Gottlicher, M., Vujic, M., Weih, F., Angel, P., Herrlich, P. and Schutz, G. (2001) Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J., 20, 7168–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G.J. and Green, H. (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol., 17, 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckermann J.P., Reichardt, H.M., Arribas, R., Richter, K.H., Schutz, G. and Angel, P. (1999) The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J. Cell Biol., 147, 1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Peters, K.G., Longaker, M.T., Fuller-Pace, F., Banda, M. and Williams, L.T. (1992) Large induction of keratinocyte growth factor in the dermis during wound healing. Proc. Natl Acad. Sci. USA, 89, 6896–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Smola, H., Liao, X., Longaker, M.T., Krieg, T., Hofschneider, P.H. and Williams, L.T. (1994) The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science, 266, 819–822. [DOI] [PubMed] [Google Scholar]
- Wong H.L. and Wahl, S.M. (1989) Tissue repair and fibrosis. In Zembale, M. and Asherson, G.L. (eds), Human Monocytes. Academic Press, San Diego, CA, pp. 383–394.