Abstract
Introduction:
Previous Alzheimer’s disease and related dementia (ADRD) research studies have illustrated the significance of studying alterations in white matter (WM). Fewer studies have examined how changes in WM integrity, measured with diffusion tensor imaging (DTI), are associated with changes in volume of gray matter regions and measures of cognitive function in aged participants spanning the dementia continuum.
Methods:
Magnetic resonance imaging and cognitive data were collected from 241 Boston University Alzheimer’s Disease Research Center participants who spanned from cognitively normal controls to amnestic mild cognitive impairment to having dementia. Primary diffusion tracts of interest were the cingulum ventral (CV) and cingulum dorsal (CD) pathways. Gray matter (GM) regions of interest (ROIs) were in the medial temporal lobe (MTL), prefrontal cortex, and retrosplenial cortex. Analyses of covariance models were used to assess differences in WM integrity across groups (control, aMCI, and dementia). Multiple linear regression models were used to assess associations between WM integrity and GM volume with measures of memory and executive function.
Results:
Changes in WM integrity were shown in both cingulum pathways in participants across the dementia continuum. Associations between WM integrity of both cingulum pathways and volume of selected GM ROIs were widespread. Functionally significant associations were found between WM of the CV pathway and memory, independent of MTL GM volume.
Discussion:
Changes in WM integrity of the cingulum bundle and surrounding GM ROI are likely related to the progression of ADRD. Such changes should continue to be studied, particularly in association with memory performance.
Keywords: aging, cingulum, white matter integrity, dementia, cognition, MRI
1. Introduction
Imaging research has historically focused on the atrophy of gray matter, yet recent work has shown that changes to microscopic white matter (WM), such as myelin loss, axonal injury, and edema, also occur in early phases of Alzheimer’s disease and related dementias (ADRD) (Brun and England, 1986; Gold et al., 2012; Shao et al., 2019). This pathology is heavily studied because decreased WM integrity is thought to lead to less efficient conduction of neural signals across the brain (Fan et al., 2019). Likewise, many studies have shown reduced WM integrity is associated with age-related cognitive decline (Mayo et al., 2019; O’Sullivan et al., 2001). In recent decades, measuring such changes in WM in-vivo has become possible due to improvements in magnetic resonance imaging (MRI) techniques and the development of diffusion tensor imaging (DTI) (Gold et al., 2012; Lee et al., 2015). Through DTI, researchers can calculate quantitative measures of extracellular water diffusion that are believed to reflect WM integrity. Such measures include mean diffusivity (MD) and fractional anisotropy (FA) (Esrael et al., 2021; Fan et al., 2019).
Previous ADRD studies have shown strong evidence that decreased WM integrity is demonstrated by a decrease in FA and an increase in MD (Fan et al., 2019; Gold et al., 2012). Yet what remains less clear is how early in the progression of ADRD do these changes become detectable and which WM pathways show the greatest vulnerability (Araque Caballero et al., 2018; Lee et al., 2015; Liu et al., 2017). A pathway of interest in studies of dementia is the cingulum bundle (Fan et al., 2019; Gold et al., 2012; Jacobs et al., 2018; Mito et al., 2018; Shao et al., 2019). The cingulum bundle has dense projections to the hippocampus and other regions in the medial temporal lobe (MTL), in addition to projections which extend through the retrosplenial cortex (RSC), anterior cingulate and into the frontal lobe (Bubb, Metzler-Baddeley, & Aggelton, 2018; Shao et al., 2019). Due to the complexity of this pathway, tractography imaging toolboxes used in conjunction with DTI further divide the cingulum into “ventral” and “dorsal” pathways with the goal of being able to better localize where and when changes within this pathway occur (Bubb et al., 2018).
Following the growth of DTI and tractography, research studies have examined the association between WM and cognitive functions, such as executive function and memory (Mayo et al., 2019; Shao et al., 2019). Fewer studies have assessed whether associations between diffusion measures and cognition are further related to volume of neighboring gray matter (GM) regions, and whether associations of WM integrity and cognition persist after accounting for GM atrophy (Bubb et al., 2018). Through use of participants enrolled in in a study led by the Boston University Alzheimer’s Disease Research Center (BU-ADRC), the goals of this study were, first, to assess relationships between measures of WM integrity spanning from participants who were cognitively normal to amnestic mild cognitive impairment (aMCI) to those with dementia. Next, we examined associations between WM integrity with regional GM volume. Lastly, we explored associations between WM integrity and volume of selected GM regions of interest (ROIs) with measures of cognition.
We tested three primary hypotheses. (1) Participants with dementia would show lower FA and higher MD than participants with aMCI or controls in both the cingulum ventral (CV) and cingulum dorsal (CD) pathways. Between cognitive groups, greater differences in WM integrity would be shown between control participants and participants with aMCI in the CV pathway because the volume and connectivity between MTL regions are often affected in earlier stages of dementia than frontal regions (Tucholka et al., 2018). Likewise, we hypothesized that greater differences between participants with aMCI and dementia would be shown in the CD pathway, To assess whether these differences in WM integrity are ubiquitous or specific to these tracts of interest, diffusion measures in a control region, the corticospinal tract (CST), were examined. (2) Based upon its projections to the frontal lobe, we hypothesized that WM integrity of the CD would show associations with volume of regions in the prefrontal cortex (PFC) and WM integrity of the CV would show associations with volume of regions in the MTL. We expected both pathways would show associations with volume of the RSC. (3) Lastly, we hypothesized that WM integrity of the CD pathway and volume of PFC regions would predict executive function and that WM integrity of the CV pathway and volume of MTL regions would predict memory performance.
2. Materials and Methods
2.1. Participants
We had access to MRI, demographic, and cognitive data from 286 participants within the Health Outreach Program for the Elderly (HOPE), a cohort study led by the BU-ADRC. The BU-ADRC is a center of >40) funded by the National Institute on Aging that contributes standardized data to the National Alzheimer’s Coordinating Center (NACC) each year. The BU-ADRC HOPE registry, including subject recruitment and inclusion/exclusion criteria, has previously been described (Ashendorf et al., 2017; Galetta et al., 2017; Sugarman et al., 2020). All participants provided the BU-ADRC with written consent to participate in all aspects of this study. Procedures conducted by the BU-ADRC were approved by our local IRB. The BU-ADRC Executive Committee approved of the sharing of the data for this study.
All participants had a NACC diagnosis that was made at BU-ADRC consensus conferences following presentation and discussion of all medical history and evaluation results, such as clinical interview, informant input, neuropsychological test scores, and MRI scans viewed for hippocampal atrophy, WM signal abnormalities, and evidence of microbleeds after the consensus diagnosis was made for the clinical syndrome. For the purposes of this study, only participants with the diagnosis of “control” (indicating no detectable cognitive impairment), “aMCI” (with either single or multiple cognitive domains affected), or “AD/dementia” (hereafter referred to as “dementia”) were included (n = 241). Participants with diagnoses such as “non-amnestic MCI” (in either single or multiple domains, n = 10) and “cognitively impaired, not MCI” (n = 35) were excluded.
2.2. Clinical Evaluation
All the participants in this study had a MRI scan and neuropsychological data collected either at the time of the MRI visit (n = 107) or at a visit shortly thereafter (subsequent visit closest to MRI was used). We used performance on the Craft Delayed Recall (verbatim) as our measure of memory (n = 124) and (time to complete) Part B of the Trailmaking Test as our measure of executive function (n = 197). Raw scores for both measures were converted to z-scores for statistical analysis. Z-scores for both measures were calculated so that positive numbers indicate above the mean and negative numbers indicate below the mean.
2.3. MRI
All participants were scanned at the Center for Biomedical Imaging at Boston University Chobanian & Avedisian School of Medicine between 2015–2020. T1 and high angular resolution diffusion images (HARDI) sequences were acquired for all participants on a 3T Philips Acheiva scanner. For T1 scans, a 32-channel head coil and sense factor of 2 was used with the following imaging parameters: TR = 6.8 ms, TE = 3.1 ms, flip angle = 9°, reconstructed and acquisition voxel size = .98 mm × .98 mm × 1.2 mm , FOV = 250 mm × 250 mm × 180 mm, 150 sagittal slices. For HARDI scans, a 32-channel headcoil and sense factor of 2 was used with the following imaging parameters: TR = 10,153 ms, TE = 97 ms, flip angle = 90°, reconstructed and acquisition voxel size = 2 mm × 2 mm × 2 mm, FOV = 224 mm × 224 mm × 128 mm, 64 transverse slices, B = 3000
All T1 scans were processed with Freesurfer version 7.2 on a Mac Pro 2013 running OS version 11.6 to obtain cortical parcellations and subcortical segmentations of anatomical regions (Desikan et al., 2006; Iglesias et al., 2015). The three PFC ROIs that were used in our study were the rostral middle frontal gyrus, caudal middle frontal gyrus, and superior frontal gyrus. The two MTL ROIs were the hippocampus and entorhinal cortex. Additionally, we examined volume of the isthmus of the cingulate gyrus as our measure of the retrosplenial cortex (RSC) (Desikan et al., 2006; Vann et al., 2009). This region was included in analyses due to its neuroanatomical location and proximity to both cingulum pathways as well as its role in navigation, spatial and episodic memory (Vann, Aggleton & Maguire, 2009). Total volume of all GM ROI was used in statistical analyses by adding the right and left volume of each region. Estimated total intracranial volume (eTIV) was also generated by Freesurfer. GM volume measures were adjusted for eTIV and adjusted-GM volumes were converted to z-scores for statistical analyses.
HARDI scans were processed with TRActs Constrained by UnderLying Anatomy (TRACULA), an updated toolbox available as part of FreeSurfer version 7.2 (Maffei et al., 2021; Yendiki et al, 2011). This processing included eddy-current compensation via FMRIB Software Library (FSL), intra- and inter-subject registration, ball-and-stick modeling, and tensor fitting (Vipin et al., 2019). Intra-subject registration was completed using boundary-based cost (bbregister) and inter-subject registration was completed using systemic normalization from Advanced Normalization Tools. TRACULA is a novel method for automated global probabilities reconstruction of 42 major WM pathways (https://dmri.mgh.harvard.edu/tract-atlas/).To reconstruct these WM tracts, TRACULA utilizes prior information on anatomy from a set of training participants where the tracts of interest were labeled manually. This prior information is the likelihood of each tract to travel through or next to each of the cortical and subcortical segmentation labels previously generated from processing T1 scans through Freesurfer. Our tracts of interest were the CD and CV pathways (Figure 1), and our control tract was the CST. Following reconstruction of all three tracts, FA and MD, were extracted and averaged from both right and left pathways. Averages of both measures were z-scored and used in subsequent analyses.
Figure 1. Freesurfer Reconstruction of Cingulum Pathways.
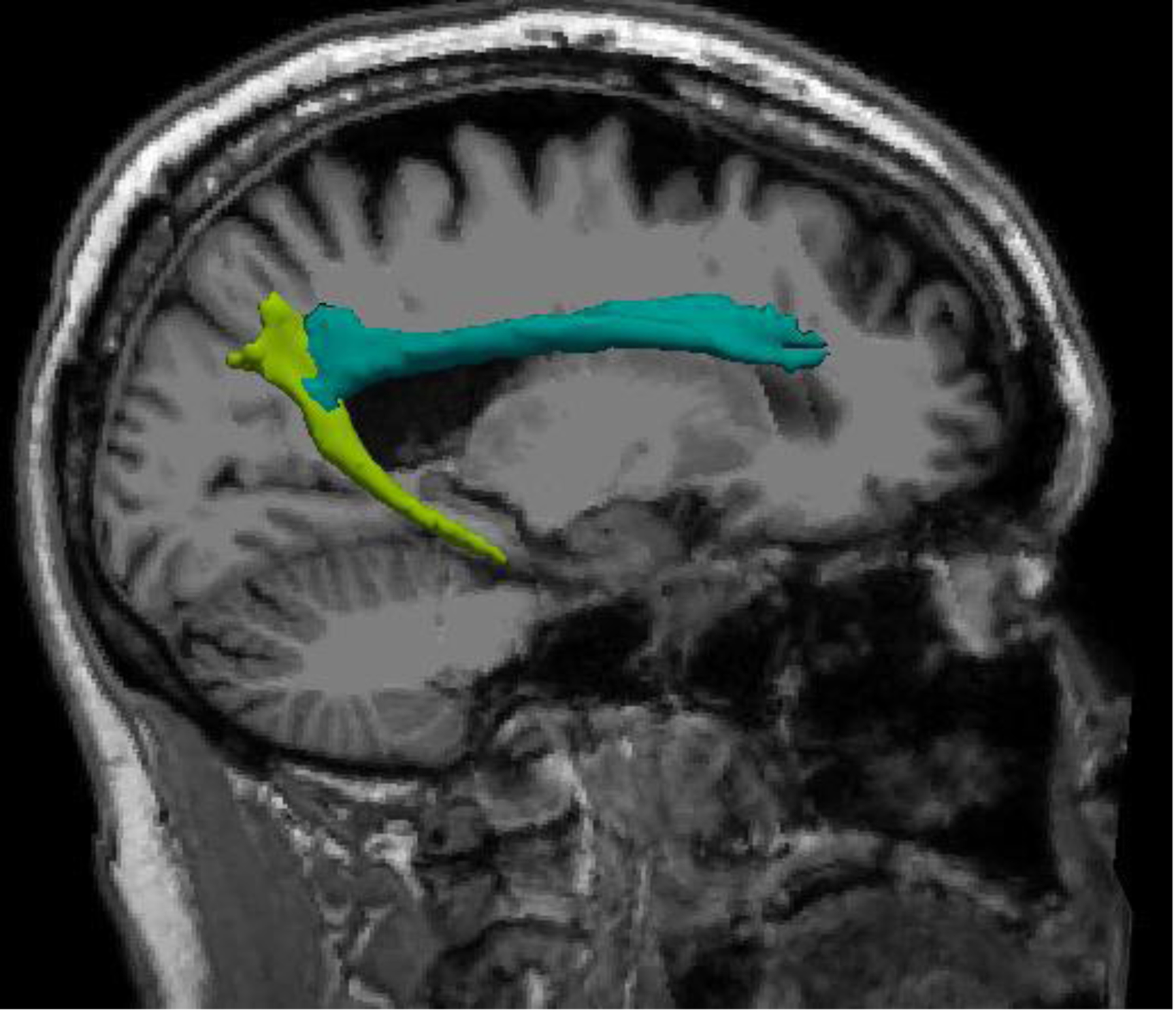
Left cingulum dorsal pathway shown in aqua, left cingulum ventral pathway shown in light green
2.4. Statistical Analysis
Analyses of variance (ANOVAs) were used to assess differences between the control, aMCI, and dementia groups in continuous measures such as age and years of education. Chi-square tests were used to assess differences in the distribution of categorical variables such as sex, race, and apolipoprotein E (APOE) ε4 status. Statistical significance for demographic comparisons was set at p < 0.05.
All raw continuous values (diffusion measures, adjusted-GM volumes and cognitive scores) were converted to z-scores for statistical analyses. All statistical analyses were adjusted for age (model 1) with further adjustment for sex in secondary models (model 2). Because GM volume was previously adjusted for eTIV, primary analyses and inferences are based on findings from model 1. Other demographic factors, such as education and APOE ε4 status, had no effect on outcome measures and were excluded as covariates. Analysis of covariance (ANCOVA) models were created assessing the effect of cognitive group on FA and MD for CV, CD, and CST. Post hoc Tukey’s Honestly Significant Difference (HSD) test was performed for significant model 1 results.
Multiple linear regression models were used to assess associations between WM integrity (FA/MD of CD and CV) and volume of selected ROIs (PFC, MTL, and RSC), as well as to assess the predictive power of WM integrity and volume of GM ROI in relation to cognitive performance. Beta (β) coefficients were calculated for every standard deviation (SD) increase in volume; thus, the positive β coefficients associated with FA illustrate that larger ROI volumes are expected with higher FA. Likewise, negative β coefficients reflect that larger ROI volumes are expected with lower MD. This same relationship applies between measures of cognitive function and measures of WM integrity. ANOVAs, ANCOVAs, and chi-squared testing were performed in JMP Pro V15.2 on a MacBook Pro 2015 running OS version 10.15.7. Stata SE, version 17 for Macintosh (Stata Corp) was used for multiple linear regression analyses and data visualization. Multiple comparisons were corrected for by use of the Benjamini-Hochberg method (Benjamini & Hochberg, 1995).
3. Results
3.1. Demographic and cognitive data
Sex significantly differed between cognitive groups (p < 0.001; Table 1). Follow-up pairwise chi-square testing revealed differences within the control (p < 0.001, significantly more females) and dementia groups (p = 0.008, significantly more males). Chi-square testing showed that the proportion of APOE ε4 carriers was greatest in participants with dementia (p = 0.04). No differences were found between cognitive groups in age, years of education, or race. As expected, cognitive performance significantly differed between groups (p < 0.001;Table 1).
Table 1.
Demographic and Cognitive Data
| Control (n=141) | aMCI (n=59) | Dementia (n=41) | p value | |
|---|---|---|---|---|
| Age, mean (SD) | 70.88 (8.63) | 72 (9.33) | 74.12 (8.55) | 0.11 |
| Education, mean (SD) | 16.42 (2.25) | 16.85 (2.54) | 16.17 (2.64) | 0.34 |
| Female sex, n (%) | 97 (68.79) | 32 (54.24) | 12 (29.27) | <0.001 |
| Race | 124 White | 53 White | 34 White | 0.46 |
| 3 Asian | 1 Asian | 0 Asian | ||
| APOE ε4 carrier, n (%) | 39 (29.77) | 23 (41.82) | 20 (50.0) | 0.04 |
| Craft Delayed Verbatim (n = 124) | 19.49 (5.51) (n = 68) | 14.98 (6.82) (n = 36) | 3.8 (6.26) (n = 20) | < 0.001 |
| Part B of Trailmaking Test (in seconds) (n = 197) | 70.61 (25.82) (n = 125) | 93.78 (46.38) (n = 49) | 223.91 (86.89) (n = 23) | < 0.001 |
One-way ANOVAs used for continuous data; chi-square testing used for categorical data. Race is self-reported by participant. No participants identified as “Pacific”, “More than one race”, or “Unknown”.
Abbreviations: aMCI, amnestic mild cognitive impairment; APOE, apolipoprotein E; SD, standard deviation
3.2. WM integrity across cognitive groups
ANCOVA models assessing the main effect of cognitive group with age as a covariate (model 1) showed a significant effect of cognitive group on the FA and MD of both cingulum pathways following correction for multiple comparisons (Supplemental Table 1). ANCOVA models assessing the main effect of cognitive group with age as a covariate for the CST did not show differences in either FA (p = 0.36) or MD (p = 0.97). Age had a significant effect on all four measures (p < 0.001) and sex had a significant effect on FA of the CV and MD of the CST (Supplemental Table 1). The distribution of MD and FA of each pathway as well as significant pairwise cognitive group differences following Tukey’s HSD test are shown in Figure 2 (Supplemental Table 2). Differences were shown in all four measures between control and dementia groups with additional differences between control-aMCI in FA of the CD and between aMCI-dementia in MD of the CV.
Figure 2. Boxplot of MD and FA of White Matter Pathways.
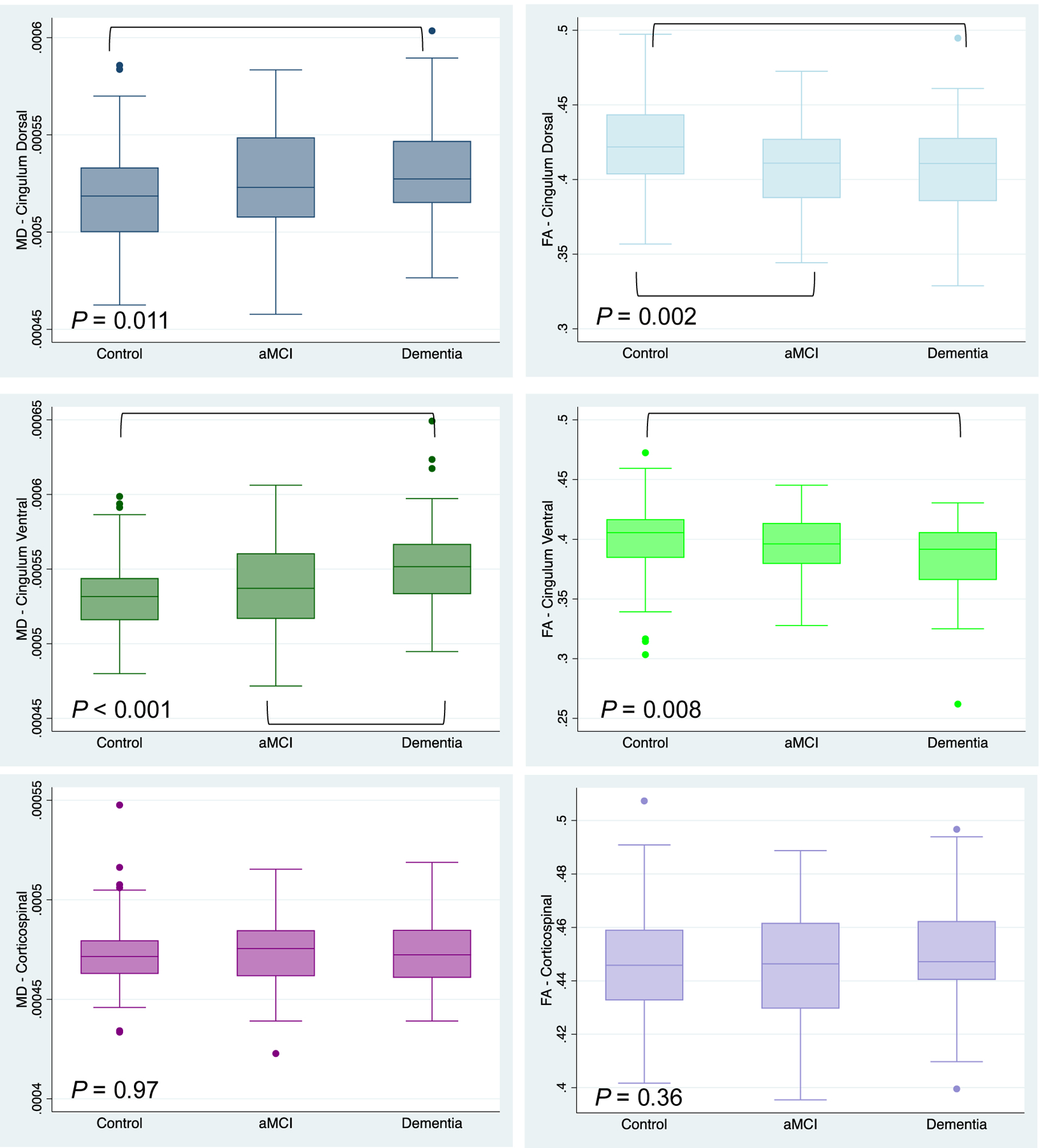
ANCOVA Model 1 p values shown. Cingulum dorsal (blue) in top panel, cingulum ventral (green) in middle panel, corticospinal (purple) bottom panel. MD on left (darker shade), FA (lighter shade) on right. Post hoc Tukey’s HSD between group differences indicated with brackets (full statistics shown in Supplemental Table 2).
Abbreviations: aMCI, amnestic mild cognitive impairment; FA, fractional anisotropy; MD, mean diffusivity
3.3. Associations between integrity of WM integrity and volume of GM ROIs
Multiple linear regression models showed significant associations between WM integrity of both cingulum pathways with volume of GM ROIs following corrections for multiple comparisons. The examined associations are outlined in Table 2 with significant statistical models shown in Table 3. A scatterplot displaying the association between WM integrity of the CD and hippocampal volume is visualized in Figure 3. Of the 15 significant associations between WM integrity and volume of GM ROIs, 12 remained significant when sex was added to the models (Supplemental Tables 3–4).
Table 2.
Associations between White Matter Integrity of Cingulum Pathways and Volume Gray Matter ROIs
| Cingulum Dorsal | Cingulum Ventral | ||||
|---|---|---|---|---|---|
| Region of Interest | FA | MD | FA | MD | |
| Medial Temporal Lobe | Hippocampus | X | X | X | X |
| Entorhinal Cortex | |||||
| Prefrontal Cortex | Superior frontal | X | X | X | X |
| Caudal middle frontal | X | X | X | X | |
| Rostral middle frontal | X | X | X | ||
| Other | Retrosplenial cortex | ||||
Significant associations (model 1; after correction for multiple comparisons using the Benjamini-Hochberg method) indicated by “X.”
Abbreviations: FA, fractional anisotropy; MD, mean diffusivity; ROIs; regions of interest
Table 3.
Associations between White Matter Integrity of Cingulum Pathways and Volume Gray Matter ROIs
| FA - Cingulum Dorsal Model 1 | MD – Cingulum Dorsal Model 1 | FA - Cingulum Ventral Model 1 | MD – Cingulum Ventral Model 1 | |||||
|---|---|---|---|---|---|---|---|---|
| β coefficient (95% CI) | SE | β coefficient (95% CI) | SE | β coefficient (95% CI) | SE | β coefficient (95% CI) | SE | |
| Volume Hippocampus (per SD) | 0.23 (0.11 – 0.35)** | 0.06 | −0.19 (−0.30 – −0.07)** | 0.06 | 0.21 (0.08 – 0.34)** | 0.07 | −0.23 (−0.35 – −0.11)** | 0.06 |
| Age (per 1 year) | −0.04 (−0.05 –0.03)†† | 0.01 | 0.05 (0.04 – 0.07)†† | 0.01 | −0.02 (−0.04 – −0.01)†† | 0.01 | 0.04 (0.03 – 0.06)†† | 0.01 |
| Volume Superior Frontal (per SD) | 0.17 (0.05 – 0.30)** | 0.06 | −0.16 (−0.27 – −0.04)* | 0.06 | 0.19 (0.05 – 0.32)** | 0.07 | −0.17 (−0.30 – −0.05)** | 0.06 |
| Age (per 1 year) | −0.04 (−0.06 – −0.03)†† | 0.01 | 0.05 (0.04 – 0.07)†† | 0.01 | −0.02 (−0.04– −0.01)†† | 0.01 | 0.05 (0.03 – 0.06)†† | 0.01 |
| Volume Caudal Middle Frontal (per SD) | 0.14 (0.02 – 0.26)* | 0.06 | −0.18 (−0.29 – −0.07)** | 0.06 | 0.23 (0.10 – 0.36)** | 0.06 | −0.19 (−0.31 – −0.07)** | 0.06 |
| Age (per 1 year) | −0.05 (−0.06 – −0.03)†† | 0.01 | 0.05 (0.04 – 0.07)†† | 0.01 | −0.02 (−0.04 – −0.01)†† | 0.01 | 0.05 (0.03 – 0.06)†† | 0.01 |
| Volume Rostral Middle Frontal (per SD) | 0.12 (0 – 0.25) | 0.06 | −0.12 (−0.24 – −0.01)* | 0.06 | 0.14 (0.01 – 0.28)* | 0.07 | −0.19 (−0.31 – −0.07)** | 0.06 |
| Age (per 1 year) | −0.04 (−0.06 – −0.03)†† | 0.01 | 0.06 (0.04 – 0.07)†† | 0.01 | −0.02 (−0.04 – −0.01)†† | 0.01 | 0.04 (0.03 – 0.06)†† | 0.01 |
Benjamini-Hochberg Adjusted p values <0.01**, <0.05*.
Unadjusted p values <0.01††, < 0.05†.
Only volume of GM ROIs significantly associated (after Benjamini-Hochberg correction for multiple comparisons) with WM integrity shown. Notably, the association between the volume rostral middle frontal gyrus and FA-Cingulum Dorsal was non-significant but statistics are included since other associations between this ROI and WM measures were significant.
Abbreviations: β, beta; CI, confidence interval; FA, fractional anisotropy; MD, mean diffusivity, ROIs, regions of interest, SD, standard deviation; SE, standard error
Figure 3. Associations between White Matter Integrity of Cingulum Dorsal and Volume of Hippocampus.
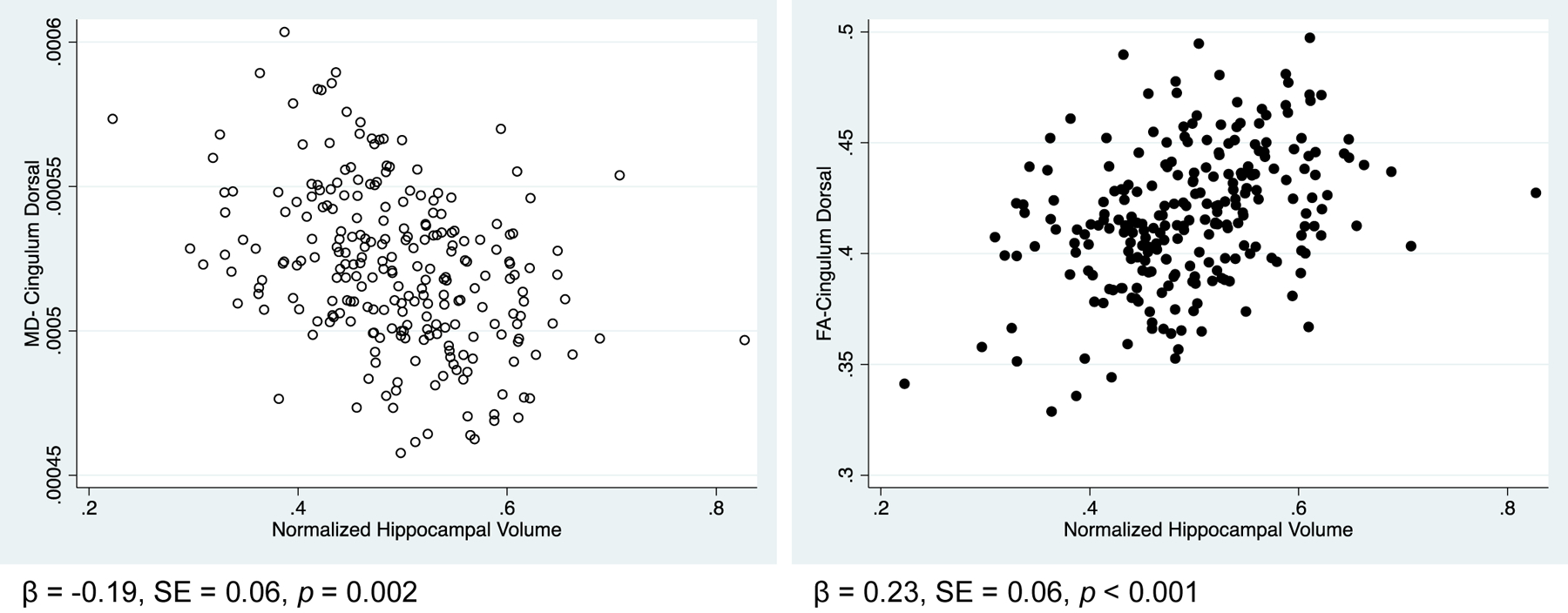
Regression model 1 p values shown. Hippocampal volume adjusted for eTIV.
Abbreviations: β, beta; eTIV, estimated total intracranial volume; FA, fractional anisotropy; MD, mean diffusivity; SE, standard error
3.4. Associations between WM integrity, volume of GM ROIs, and cognition
Due to our interest in examining the predictive value of the cingulum pathways, both in addition to and after accounting for GM atrophy, only findings from models where both WM integrity and GM ROI had significant predictive value (after correction for multiple comparisons) of cognitive function are reported.
3.4.1. Cingulum Dorsal – PFC – Executive Function
Of the six multiple linear regression models which examined whether WM integrity of the CD and volume of three PFC ROIs added explanatory power of executive function, none of these models showed significant predictive value of both factors.
3.4.2. Cingulum Ventral – MTL – Memory
Of the four multiple linear regression models which examined whether WM integrity of the CV and volume of two MTL ROIs added explanatory power of memory performance, three models were significant (Table 4). Lower MD (β: −0.35, p < 0.001) and larger entorhinal cortex volume (β: 0.23, p = 0.01) were independently associated with better memory performance. Likewise, lower MD (β: −0.27, p = 0.005) and larger hippocampal volume (β: 0.42, p <0.001) were also independently associated with memory performance. When sex was added to the models, the associations between MD of the CV, volume of both MTL ROI, and memory persisted although being female was further indicative of better memory (Supplemental Table 5). Higher FA (β: 0.25, p = 0.008) and larger entorhinal cortex volume (β: 0.27, p = 0.004) were further associated with better memory performance but the predictive association between FA of CV and memory performance was mitigated when sex was added to the model (Supplemental Table 5).
Table 4.
Associations between White Matter Integrity of Cingulum Ventral Pathway, Volume MTL ROIs, and Memory
| Memory Model 1 | ||
|---|---|---|
| β coefficient (95% CI) | SE | |
| MD – Cingulum Ventral (per SD) | −0.35 (−0.55 – −0.16)** | 0.10 |
| Volume Entorhinal Cortex (per SD) | 0.23 (0.05 – 0.41)† | 0.09 |
| Age (per 1 year) | 0.02 (−0.01 – 0.04) | 0.01 |
| MD – Cingulum Ventral (per SD) | −0.27 (−0.45 – −0.08)** | 0.09 |
| Volume Hippocampus (per SD) | 0.42 (0.25 – 0.59)†† | 0.09 |
| Age (per 1 year) | 0.02 (0 – 0.05)† | 0.01 |
| FA – Cingulum Ventral (per SD) | 0.25 (0.07 – 0.43)** | 0.09 |
| Volume Entorhinal Cortex (per SD) | 0.27 (0.09 – 0.45)†† | 0.09 |
| Age (per 1 year) | 0.01 (−0.01 – 0.03) | 0.01 |
Benjamini-Hochberg Adjusted p values <0.01**, <0.05*.
Unadjusted p values <0.01††, < 0.05†.
Abbreviations: β, beta; CI, confidence interval; FA, fractional anisotropy; MD, mean diffusivity; MTL, medial temporal lobe; ROIs, regions of interest; SD, standard error; SE, standard error
3.4.3. Retrosplenial Cortex
Of the eight multiple linear regression models assessing the predictive power of WM integrity of both cingulum pathways and volume of the RSC with either measure of cognition, both models assessing WM integrity of the CV with executive function were significant. The direction of these associations was opposite that expected (Table 5; Supplemental Table 6). Furthermore, higher FA of the CV (β: 0.23, p = 0.01) and larger RSC volume (β: 0.21, p = 0.02) were associated with better memory performance but the association between FA of the CV and memory was mitigated when sex was added to the model (Table 6; Supplemental Table 7).
Table 5.
Associations between White Matter Integrity of Cingulum Ventral Pathway, Volume RSC, and Executive Function
| Executive Function Model 1 | ||
|---|---|---|
| β coefficient (95% CI) | SE | |
| MD – Cingulum Ventral (per SD) | 0.23 (0.07 – 0.39)* | 0.08 |
| Volume RSC (per SD) | −0.18 (−0.33 – −0.04)† | 0.07 |
| Age (per 1 year) | 0.01 (−0.01 – 0.03) | 0.01 |
| FA – Cingulum Ventral (per SD) | −0.17 (−0.31 – −0.03)* | 0.07 |
| Volume RSC (per SD) | −0.21 (−0.36 – −0.06)†† | 0.07 |
| Age (per 1 year) | 0.02 (0 – 0.03)†. | 0.01 |
Benjamini-Hochberg Adjusted p values <0.01**, <0.05*.
Unadjusted p values <0.01††, < 0.05†.
Abbreviations: β, beta; CI, confidence interval; FA, fractional anisotropy; MD, mean diffusivity; RSC, retrosplenial cortex; SD, standard deviation; SE, standard error
Table 6.
Associations between White Matter Integrity of Cingulum Ventral Pathway, Volume RSC, and Memory
| Memory Model 1 | ||
|---|---|---|
| β coefficient (95% CI) | SE | |
| FA – Cingulum Ventral (per SD) | 0.23 (0.05 – 0.42)* | 0.09 |
| Volume RSC (per SD) | 0.21 (0.03 – 0.38)† | 0.09 |
| Age (per 1 year) | 0 (−0.02 – 0.03) | 0.01 |
Benjamini-Hochberg Adjusted p values <0.01**, <0.05*.
Unadjusted p values <0.01††, < 0.05†.
Abbreviations: β, beta; CI, confidence interval; FA, fractional anisotropy; RSC, retrosplenial cortex; SD, standard deviation; SE, standard error
4. Discussion
Our findings demonstrate that between group differences in participants ranging from cognitively normal controls to aMCI to dementia are specific to the CD and CV pathways, but not to the CST. We sought to build upon these findings by further exploring associations between WM integrity and less explored neighboring regions outside of the hippocampus, such as the entorhinal cortex, RSC, and PFC. Our robust findings highlight the widespread number of ROI that show atrophy in association with decreases in WM integrity of the cingulum. Furthermore, we report functionally significant associations exist between WM integrity of the CV pathway, volume of MTL ROIs, and memory. Such findings persisted, even after accounting for GM atrophy, which adds to existing literature that has highlighted how integrity of the cingulum relates to cognition (Fan et al., 2019; Kantarci et al., 2011; Mayo et al., 2019; Metzler-Baddeley et al., 2012; Shao et al., 2019). Altogether, a primary interpretation of these findings may be that the cingulum, although segmented as having distinct “dorsal” and “ventral” pathways, is truly a circuitous pathway connecting the PFC to the hippocampus through the parietal and temporal lobes. Our findings suggest that weakening of any of these structural components may be related to the progression of ADRD.
As predicted, group differences in diffusion measures of WM integrity were shown in both cingulum pathways in the expected directions: lower FA and higher MD between clinical groups (dementia > aMCI > control). These changes did not extend to the CST thereby affirming that these are not ubiquitous WM changes in the brain. These findings extend those of previous studies which have shown between cognitive group differences in FA and MD of the cingulum (Lee et al., 2015; Mito et al., 2018; Shao et al., 2019; Tucholka et al., 2018). When sex was added to the models, cognitive group differences in FA of the CV were mitigated. Greater FA of the CV was shown in females relative to males. We are not aware of other studies that have reported this sex difference in WM integrity of the cingulum, thus it is possible this finding was a characteristic of the study sample.
Post hoc testing revealed that the mean value of all four measures (FA of CD, MD of CD, FA of CV, and MD of CV) were significantly different between control-dementia groups. However, the differences between control-aMCI and aMCI-dementia groups were contrary to our hypothesis which suggested that changes in the CV and MTL ROI would occur in earlier stages of dementia. Meanwhile our results showed differences in FA of the CD between control-aMCI groups and differences in MD of the CV between aMCI-dementia groups. Interestingly, an Alzheimer’s Disease Neuroimaging Initiative (ADNI) study using a previous release of TRACULA showed changes in FA and MD of the cingulum-angular bundle (analogous to the CV pathway) existed between all three groups (e.g. control-AD, control-MCI, and MCI-AD). Unlike the present study, no differences were reported between the three groups in the cingulum-cingulate gyrus bundle (analogous to the CD pathway) (Lee et al., 2015). This may reflect group differences between the cohorts sampled (ADNI vs. BU-ADRC) among other reasons. Nonetheless, these findings are important as we continue to build upon our understanding of the sequence of changes in a region with strong functional and anatomical implications in the progression of ADRD.
Our findings showing associations between WM tracts and volume of GM ROI widely supported the predictions of our second hypothesis. Further, these findings revealed that associations between WM integrity and GM volume were more widespread than originally predicted. In addition to hypothesized associations, MD of the CV showed negative relationships with volume of all three PFC ROIs and both FA and MD of the CD showed significant associations with hippocampal volume. Our findings that show associations between hippocampal volume with diffusion measures of both cingulum pathways are similar to those reported in the ADNI-TRACULA study by Lee and colleagues (Lee et al., 2015). Unexpectedly, our results did not show any significant associations between volume of the entorhinal cortex and/or RSC with WM integrity of either cingulum pathway.
Lastly, we sought to incorporate how findings pertaining to structure, as represented by WM integrity and GM volume, predict cognitive function. First, we hypothosized that WM integrity of the CD and volume of PFC ROIs would predict executive function. This prediction was based upon the historical view that declines in executive function are the direct result of neurodegeneration in the PFC (West, 1996). Interestingly, we saw no relationship between diffusion measures of the CD, GM volume of PFC ROIs, and executive function. The second part of our prediction, stating that WM integrity of the CV and volume of MTL ROIs would predict memory performance, was supported by our findings. Furthermore, associations between WM integrity and memory existed even when volume of either the hippocampus or entorhinal cortex was accounted for in the models. The positive association between WM integrity of the CV, GM volume of both MTL ROIs, and memory performance is consistent with previous studies that have studied relationships between these measures (Ezzati et al., 2016; Jacobs et al, 2018; Kantarci et al., 2011; Wang et al., 2017). These findings highlight potential areas of interest to monitor in aging adults in the progression of ADRD. The basis of the counterintuitive association between WM integrity of the CV and volume of RSC with executive function remains unclear, although our recurring null findings concerning the RSC may suggest that volume of this region is not as sensitive to WM changes in the cingulum as we hypothesized.
A conclusion from our findings, specific to our final two hypotheses, is the observation that that MD may be a more sensitive measure than FA based upon the number of relationships shown between either pathway and volume of GM ROIs. The associations observed between MD of CV with memory function were further unique to measures of diffusivity (as opposed to anisotropy) and the ventral pathway. This is interesting because while FA is a more forthright measure for assessing integrity of the WM, others in the field have argued that MD is more sensitive to detecting fiber tract alterations (Araque Caballero et al., 2018; Mayo et al., 2019).
Limitations
Although we based our primary inferences on model 1, we acknowledge the additional influence sex may have on structural measures such as WM integrity, GM volume, and cognitive performance. We initially saw a sex difference in FA of the CV indicating females had higher FA than males. It appears this difference was further illustrated in subsequent multiple linear regression models adjusted for sex (e.g. there were instances when the associations between structural measures with one another or with cognition were mitigated in model 2). Since there does not seem to be consensus as to sex differences in WM integrity of the cingulum (Lawrence et al., 2021; Cox et al., 2016), we are hopeful future work can build upon our findings and continue to examine this important area of research as it pertains to ADRD risk.
Next, whereas the sample size (n = 241) is a strength of the present study, this precluded us from being able to manually check all Freesurfer and TRACULA segmentations for accuracy. Furthermore, not all 241 participants had measures of memory and executive function and thus models examining cognition had smaller sample sizes than the imaging sample. Lastly, while we had access to structural MRI, HARDI, and cognitive data, we did not have access to other AD biomarkers such as cerebrospinal fluid (CSF) or positron emission tomography (PET) measures of amyloid-beta (Aβ) and tau. The addition of this data could provide valuable insight as to how the presence of Aβ and tau are potentially impacting WM integrity, volume in GM ROIs, and cognitive function.
Conclusion
The results of this study confirmed our hypothesis that diffusion measures in the cingulum bundle are sensitive to the progression of ADRD. Furthermore, our findings provide compelling evidence that GM volume of regions ranging from the PFC to the MTL are associated with WM integrity of the cingulum, and that these changes have functional significance, particularly in relation to memory.
Supplementary Material
Key Points:
Differences exist in the white matter integrity of the cingulum bundle between participants who are on the dementia continuum.
White matter integrity of the cingulum is associated with gray matter volume of regions spanning from the medial temporal lobe to the prefrontal cortex.
White matter integrity of the cingulum ventral, independent of gray matter atrophy, may be predictive of changes in memory performance
Key Findings:
Differences exist in the white matter integrity of the cingulum bundle between participants who are on the dementia continuum.
White matter integrity of the cingulum is associated with gray matter volume.
White matter integrity of the cingulum ventral, independent of gray matter changes, may be predictive of memory performance.
Acknowledgements
We would like to thank Andy Ellison for acquiring the MR images used in this study. Data collection and sharing for this project was funded by the by the Boston University-Alzheimer’s Disease Research Center (NIH- NIA P30-AG072978).
Footnotes
Conflict: None of the authors have a conflict of interest with this work.
Ethics and consent statements: All procedures in this study were approved by the Boston University Medical School Institutional Review Board. All participants provided informed consent to participate in the procedures described in this work. Data sharing from the Boston University Alzheimer’s Disease Research Center was approved by the executive committee.
Disclaimer: This article was prepared while first author, Dr. Renee C. Groechel, was at Boston University Chobanian & Avedisian School of Medicine. Dr. Groechel is now employed at the National Institute of Neurological Disorders & Stroke (NINDS) Intramural Research Program, National Institutes of Health (NIH). The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
Data Sharing:
The data that support the findings of this study are available upon reasonable request from the Boston University Alzheimer’s Disease Research Center (https://www.bu.edu/alzresearch/information-for-investigators/)
References
- Araque Caballero MÁ, Suárez-Calvet M, Duering M, et al. White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer’s disease. Brain. 2018; 141(10), 3065–3080. 10.1093/brain/awy229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashendorf L, Alosco ML, Bing-Canar H, et al. Clinical utility of select neuropsychological assessment battery tests in predicting functional abilities in dementia. Archives Clinical Neuropsychology, 2017; 33: 530–540. doi: 10.1093/arclin/acx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y & Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995; 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bettcher BM, Mungas D, Patel N, et al. Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia. 2016; 85, 100–109. 10.1016/j.neuropsychologia.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, & Englund E A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Annals of neurology, 1986; 19(3), 253–262. [DOI] [PubMed] [Google Scholar]
- Bubb EJ, Metzler-Baddeley C, & Aggleton JP The cingulum bundle: anatomy, function, and dysfunction. Neuroscience & Biobehavioral Reviews, 2018; 92: 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, et al. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nature communications, 2016. 7(1), 13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for Subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006; 31(3): 968–980. 10.1016/j.NeuroImage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Esrael SMAM, Hamed AMM, Khedr EM, & Soliman RK Application of diffusion tensor imaging in Alzheimer’s disease: quantification of white matter microstructural changes. Egyptian Journal of Radiology and Nuclear Medicine. 2021; 52(1): 1–8. [Google Scholar]
- Ezzati A, Katz MJ, Lipton ML, Zimmerman ME, & Lipton RB Hippocampal volume and cingulum bundle fractional anisotropy are independently associated with verbal memory in older adults. Brain Imaging and Behavior. 2016; 10(3), 652–659. 10.1007/s11682-015-9452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YT, Fang YW, Chen YP, et al. Aging, cognition, and the brain: effects of age-related variation in white matter integrity on neuropsychological function. Aging & mental health. 2019; 23(7): 831–839. [DOI] [PubMed] [Google Scholar]
- Galetta KM, Chapman KR, Essis MD, et al. Screening utility of the King–Devick test in mild cognitive impairment and Alzheimer’s disease dementia. Alzheimer’s Disease & Associated Disorders. 2017; 31(2): 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD White matter integrity and vulnerability to Alzheimer’s disease: Preliminary findings and future directions. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012; 1822(3), 416–422. 10.1016/j.bbadis.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the Hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage. 2015;115:117–137. 10.1016/j.NeuroImage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Hedden T, Schultz AP, et al. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nature Neuroscience. 2018; 21(3): 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, Przybelski SA, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Petersen RC, Jack CR Jr Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011. 5;77:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KE, Nabulsi L, Santhalingam V, et al. Age and sex effects on advanced white matter microstructure measures in 15,628 older adults: A UK biobank study. Brain imaging and behavior, 2021. 15(6), 2813–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Coutu JP, Wilkens P, et al. Tract-based analysis of white matter degeneration in Alzheimer’s disease. Neuroscience, 2015; 301: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang Y, Xia Y, et al. Aging of cerebral white matter. Ageing Research Reviews, 2017; 34: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei C, Lee C, Planich M, et al. Using diffusion MRI data acquired with ultra-high gradients to improve tractography in routine-quality data. NeuroImage 2021; 245:118706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo CD, Garcia-Barrera MA, Mazerolle EL, Ritchie LJ, Fisk JD Gawryluk JR for the Alzheimer’s Disease Neuroimaging Initiative. Relationship Between DTI Metrics and Cognitive Function in Alzheimer’s Disease. Frontiers in Aging Neuroscience. 2019; 10:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Hunt S, Jones DK, Leemans A, Aggleton JP, & O’Sullivan MJ Temporal association tracts and the breakdown of episodic memory in mild cognitive impairment. Neurology. 2012; 79(23), 2233–2240. 10.1212/WNL.0b013e31827689e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito R, Raffelt D, Dhollander T, et al. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain. 2018. 141(3), 888–902. 10.1093/brain/awx355 [DOI] [PubMed] [Google Scholar]
- O’Sullivan MRCP, Jones DK, Summers PE, Morris RG, Williams SCR, & Markus HS Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology, 2001; 57(4), 632–638. [DOI] [PubMed] [Google Scholar]
- Shao W, Li X, Zhang J, et al. White matter integrity disruption in the pre‐dementia stages of Alzheimer’s disease: from subjective memory impairment to amnestic mild cognitive impairment. European journal of neurology. 2019; 26(5): 800–807. [DOI] [PubMed] [Google Scholar]
- Sugarman M, Zetterberg H, Blennow K, Tripodis Y, McKee A, Stein T, Martin B, Palmisano J, Steinberg E, Simkin I, Budson A, Killiany R, O’Connor M, Au R, Qiu W, Goldstein L, Kowall N, Mez J, Stern R, Alosco M. A longitudinal examination of plasma neurofilament light and total tau for the clinical detection and monitoring of Alzheimer’s disease. Neurobiol Aging 2020;94:60–70. Epub 2020 May 29. DOI: 10.1016/j.neurobiolaging.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholka A, Grau-Rivera O, Falcon C, et al. Structural connectivity alterations along the Alzheimer’s disease continuum: reproducibility across two independent samples and correlation with cerebrospinal fluid amyloid-β and tau. Journal of Alzheimer’s Disease, 2018; 61(4): 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, & Maguire EA What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009; 10(11), 792–802. 10.1038/nrn2733 [DOI] [PubMed] [Google Scholar]
- Vipin A, Ng KK, Ji F, et al. Amyloid burden accelerates white matter degradation in cognitively normal elderly individuals. Human Brain Mapping. 2019; 40(7), 2065–2075. 10.1002/hbm.24507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Dai Z, Shu H, Liu D, Guo Q, He Y, Zhang Z. Cortical Thickness and Microstructural White Matter Changes Detect Amnestic Mild Cognitive Impairment. J Alzheimers Dis. 2017;56:415–428. [DOI] [PubMed] [Google Scholar]
- West RL An application of prefrontal cortex function theory to cognitiveaging. Psychological bulletin. 1996; 120(2), 272. [DOI] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics. 2011; 5:23. doi: 10.3389/fninf.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the Boston University Alzheimer’s Disease Research Center (https://www.bu.edu/alzresearch/information-for-investigators/)


