Abstract
Background:
Components critical to cerebral perfusion have been noted to oscillate over a 24-hour cycle. We previously reported that ischemic core volume has a diurnal relationship with stroke onset time when examined as dichotomized epochs (i.e. Day, Evening, Night) in a cohort of over 1,500 large vessel occlusion (LVO) patients. In this follow-up analysis, our goal was to explore if there is a sinusoidal relationship between ischemic core, collateral status (as measured by HIR), and stroke onset time.
Methods:
We retrospectively examined collection of LVO patients with baseline perfusion imaging performed within 24 hours of stroke onset from four international comprehensive stroke centers. Both ischemic core volume and HIR, were utilized as the primary radiographic parameters. To evaluate for differences in these parameters over a continuous 24-hour cycle, we conducted a sinusoidal regression analysis after linearly regressing out the confounders age and time to imaging.
Results:
A total of 1506 LVO cases were included, with a median ischemic core volume of 13.0 cc (IQR: 0.0–42.0) and median HIR of 0.4 (IQR: 0.2–0.6). Ischemic core volume varied by stroke onset time in the unadjusted (p = 0.001) and adjusted (p = 0.003) sinusoidal regression analysis with a peak in core volume around 7:45PM. HIR similarly varied by stroke onset time in the unadjusted (p = 0.004) and adjusted (p = 0.002) models with a peak in HIR values at around 8:18PM.
Conclusion:
The results suggest that critical factors to the development of the ischemic core vary by stroke onset time and peak around 8PM. When placed in the context of prior studies, strongly suggest a diurnal component to the development of the ischemic core.
Key Terms: Ischemic stroke, Large Vessel Occlusions, Perfusion Imaging, Circadian Rhythm
Introduction
Components critical to cerebral perfusion, including blood pressure1 and cerebral blood flow,2,3 have been noted to oscillate over a 24-hour cycle in both clinical and pre-clinical studies. These discrepancies are thought to be primary drivers of the differences in stroke incidence4,5 and perfusion parameters5,6 over a 24-hour period. We have previously reported5 that ischemic core volume has a diurnal relationship with stroke onset time. In a cohort of over 1,500 large vessel occlusion (LVO) patients, we observed the highest core volumes in the evening. We also found a similar relationship with the Hypoperfusion Intensity Ratio (HIR), a known marker of collateral status.7,8 Our primary conclusions were that factors critical to the establishment to the ischemic core seem to fail most often in the late evening, explaining the large cores observed in this timeframe. However, this analysis evaluated stroke onset time in epochs (i.e. Day, Evening, Night) with an inability to examine the relationship at a more granular level (i.e. over a 24-hour continuous cycle).
A previous study6 has suggested that ischemic core volumes may follow a sinusoidal relationship with stroke onset time, peaking around 11:00PM. The goal of this follow-up analysis was to re-examine the relationship between perfusion parameters critical for the establishment of an ischemic core against a 24-hour continuous cycle. Our primary aim was to evaluate if these radiographic markers follow a sinusoidal curve and secondarily establish a peak in these parameters as a direct comparison to prior work.6 These results would better hone our understanding of when factors critical to perfusion, like collateral status, fail and could guide clinical management, from the development of neuroprotection trials to the patient selection for thrombectomy.
Methods
Full details of the original analysis have been previously reviewed in detail.5 Briefly, this cohort is a retrospective collection of LVO cases (ICA, M1 or M2) with baseline perfusion imaging performed within 24 hours of stroke onset from four comprehensive stroke centers in United States, France, and Switzerland. All sites included both thrombectomy-treated and untreated patients and had approval from local ethics committees in-line with individual guidelines. Stroke onset time was systematically defined5 as the midpoint between last seen well (LSW) and symptom discovery, in line with prior work for unwitnessed strokes.9 Both core (defined as an apparent diffusion coefficient <620 ×10−6 mm2/s on diffusion-weighted imaging or relative cerebral blood flow<30% on CT-perfusion) and HIR (defined as the volume of Tmax>6s divided by Tmax>10s) were utilized as the primary radiographic parameters. To evaluate for differences in these parameters over a continuous 24-hour cycle, we conducted a sinusoidal regression analysis using the ‘R’ package ‘DiscoRhythm’ after linearly regressing out the confounders age and time to imaging similar to previous work.6 All statistics were performed using ‘R’, V.4.2.2.
Results
A total of 1506 LVO cases were included in this subsequent sinusoidal analysis, for which the demographics have been previously described in detail5 and briefly reviewed in Table 1. In our cohort, the median ischemic core volume was 13.0 cc (IQR: 0.0–42.0) and varied by stroke onset time (p = 0.001) with a peak in ischemic core at approximately 8:30PM (Figure 1.A). When adjusted for age and time to image, the relationship remained significant (p = 0.003) with the peak shifted to approximately 7:45PM. Among only the witnessed strokes (n = 1091), a significant sinusoidal relationship (p = 0.002) remained, however the peak was notably later at 10:09PM (Supplementary Figure 1.A). Regarding occlusion site sub-types, there was a significant relationship for the M1 cases (n = 716, p = 0.026) with a peak around 8:45PM (Supplementary Figure 2.C), and a trend for the ICA cases (n = 290, p = 0.061). However, there was no significant relationship when examining exclusively the M2 cases (n = 500, p > 0.05).
Table 1.
Clinical presentation and radiographic features of the total cohort.
| Total Cohort n = 1506 | |
|---|---|
| Age, mean years (IQR) | 74.9 (63.0–84.0) |
| NIHSS, median (IQR) | 14.0 (8.0–20.0) |
| Blood Glucose, median mg/dL (IQR) | 120.6 (104.4–151.0) |
| Occlusion Site, n (%) | |
| ICA | 290 (19.3) |
| M1 | 716 (47.5) |
| M2 | 500 (33.2) |
| Wake-up Stroke, n (%) | 256 (17.0) |
| Medical History*, n (%) | |
| Hypertension | 932 (61.9) |
| Diabetes | 297 (19.7) |
| Dyslipidemia | 700 (46.5) |
| Witnessed Stroke, n (%) | 1091 (72.4) |
| Type of Acquisition, n (%) | |
| MRI Perfusion | 752 (49.9) |
| CT Perfusion | 754 (50.1) |
| Time to Imaging, median (IQR) | 220.0 (104.4–381.3) |
| Core, median (IQR) | 13.0 (0.0–42.0) |
| HIR, median (IQR) | 0.4 (0.2–0.6) |
Data missing for 21 cases.
Figure 1.
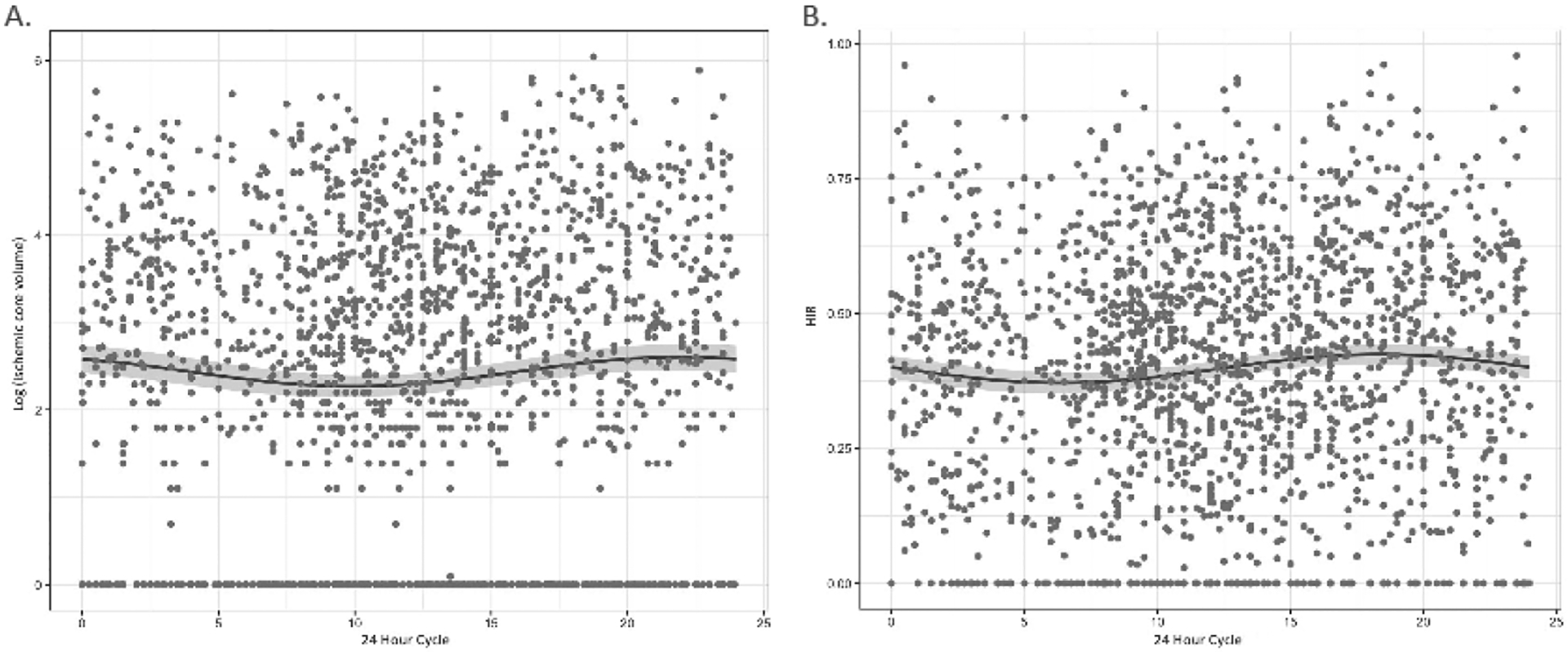
Sinusoidal regression analysis of LVO cases across a 24 hour cycle for both (A) log of ischemic core volume, and (B) HIR.
In regard to collateral status, the median HIR was 0.4 (IQR: 0.2–0.6) in this cohort. The relationship between HIR and stroke onset was also significant in sinusoidal regression analysis (p = 0.004), with a peak in value at approximately 6:22PM (Figure 1.B). Adjusted for age and time to image (p = 0.002), the peak shifted to approximately 8:18PM mirroring the peak for the ischemic core analysis. The peak was similarly around 8:11PM when exclusively examining witnessed strokes (p = 0.034; Supplementary Figure 1.B). Regarding occlusion site sub-types, there was a significant relationship for M1 (p = 0.001) with a peak around 6:04PM (Supplementary Figure 2.D), but not for ICA or M2 cases (both p > 0.05).
Discussion
In this large retrospective analysis, we demonstrate a significant sinusoidal relationship between perfusion imaging parameters and time of stroke onset, with a peak in both ischemic core volume and HIR around 8:00PM. The sinusoidal relationship between ischemic core volume and stroke onset time mirrors a prior study,6 however the noted peak in previous work was approximately 11:00PM. The difference between peaks may be attributable to the differences in patient selection, with previous analyses6 utilizing a cohort with a slightly older population, higher proportion of ICA occlusions, and larger overall core volumes. We also observed within our sub-group analysis of exclusively witnessed strokes a peak closer to this prior work at 10:00PM, which may suggest that the true peak in ischemic core volume exists at some time in the early nighttime period.
This analysis also examined collateral status by examining HIR, where a larger values have been suggested to reflect poor collaterals in prior work.7,8 We observed that HIR values in our cohort peaked in the late evening, mirroring ischemic core volumes. Taken together, results of this cohort suggest that critical factors to the development of the ischemic core vary by stroke onset time and may peak around 8PM. Similar to our prior work,5 these findings support that these radiographic parameters peak in the evening. When placed in the context of prior studies,6 strongly suggest a diurnal component to the development of the ischemic core.
Limitations of these findings include the retrospective study design and assumptions for stroke onset defined in line with prior studies.9 We also chose to combine perfusion imaging techniques, CTP and MR-DWI/PWI, an approach supported by prior work,10 suggesting high correlation between modalities. Reassuringly in our original analysis,5 our core findings were seen in both our witnessed stroke population and among exclusively MRI perfusion scans. Finally, sub-group analyses according to occlusions site were limited by smaller sample size, which may explain the lack of association observed in the ICA and M2 subgroups.
These results continue to expand on our understanding of differences in perfusion imaging of stroke that vary based on onset time. This mirrors work in pre-clinical mouse models suggesting a larger ischemic core during the ‘inactive’ period of mice.2 The underlying physiology has yet to be fully established, but may reflect variations in cerebral blood flow2,3 and/or mitochondrial function3 that also exhibit diurnal variations. A working theory of these findings is that mitochondrial activity is reduced during the ‘inactive’ period (i.e. nighttime for humans), leading to increased susceptibility to neuronal damage which can be exploited during an ischemic stroke further limiting key components of energy generation by the mitochondria. Implications of these findings may have direct results on clinical management of stroke, from understanding the effectiveness of thrombectomy based on time of day,11 to appropriate staffing of stroke units to account for noted diurnal incidence.5
Supplementary Material
Funding/Disclosures
A. Sreekrishnan received salary support from a fellowship grant from StrokeNet (NINDS U24NS107220) and support from the Leducq Trans-Atlantic Network of Excellence On Circadian Effects in Stroke.
JM. Olivot received consulting support for Abbvie and ACticor, and speaker fees from Boehringer Ingelheim and Bristol Myers Squibb.
JJ. Heit is a consultant for Medtronic and MicroVention and a member of the iSchemaView Medical and Scientific Advisory Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Funding
All other authors have nothing to declare.
Ethics Approval and Informed Consent
The individual registry data was approved by the local ethics committee at each center. Data acquired from Switzerland, ethical commission approval and patient consent were not required according to current local and national legislation as all data were anonymized before analysis and as patient’s refusal of scientific use of their data was duly honored. Study design and analysis of this cohort was approved by the Stanford institutional review board (IRB), assurance # FWA00000935 (SU), on 9/15/2022.
References
- 1.Boltze J, Didwischus N, Merrow M, Dallmann R, Plesnila N. Circadian effects on stroke outcome - Did we not wake up in time for neuroprotection? J Cereb Blood Flow Metab. 2021. Mar;41(3):684–686. doi: 10.1177/0271678X20978711. Epub 2020 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo EH, Albers GW, Dichgans M, Donnan G, Esposito E, Foster R, Howells DW, Huang YG, Ji X, Klerman EB, Lee S, Li W, Liebeskind DS, Lizasoain I, Mandeville ET, Moro MA, Ning M, Ray D, Sakadžić S, Saver JL, Scheer FAJL, Selim M, Tiedt S, Zhang F, Buchan AM. Circadian Biology and Stroke. Stroke. 2021. Jun;52(6):2180–2190. doi: 10.1161/STROKEAHA.120.031742. Epub 2021 May 4. [DOI] [PubMed] [Google Scholar]
- 3.Esposito E, Li W, Mandeville ET, Park JH, Şencan I, Guo S, Shi J, Lan J, Lee J, Hayakawa K, Sakadžić S, Ji X, Lo EH. Author Correction: Potential circadian effects on translational failure for neuroprotection. Nature. 2020. Jul;583(7814):E14. doi: 10.1038/s41586-020-2427-1. Erratum for: Nature. 2020 Jun;582(7812):395–398. [DOI] [PubMed] [Google Scholar]
- 4.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998. May;29(5):992–6. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 5.Sreekrishnan A, Seners P, Yuen N, Olivot JM, Mlynash M, Lansberg MG, Heit JJ, Lee S, Michel P, Strambo D, Salerno A, Paredes JBE, Carrera E, Albers GW. Elevated Hypoperfusion Intensity Ratio (HIR) observed in patients with a large vessel occlusion (LVO) presenting in the evening. J Stroke Cerebrovasc Dis. 2023. May 15;32(8):107172. doi: 10.1016/j.jstrokecerebrovasdis.2023.107172. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reidler P, Brehm A, Sporns PB, Burbano VG, Stueckelschweiger L, Broocks G, Liebig T, Psychogios MN, Ricke J, Dimitriadis K, Dichgans M, Kunz WG, Tiedt S. Circadian rhythm of ischaemic core progression in human stroke. J Neurol Neurosurg Psychiatry. 2021. May 26:jnnp-2021–326072. doi: 10.1136/jnnp-2021-326072. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Guenego A, Fahed R, Albers GW, Kuraitis G, Sussman ES, Martin BW, Marcellus DG, Olivot JM, Marks MP, Lansberg MG, Wintermark M, Heit JJ. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol. 2020. May;27(5):864–870. doi: 10.1111/ene.14181. Epub 2020 Mar 13. [DOI] [PubMed] [Google Scholar]
- 8.Wang CM, Chang YM, Sung PS, Chen CH. Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke. J Clin Med. 2021. Mar 21;10(6):1296. doi: 10.3390/jcm10061296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, Kleinig TJ, Wijeratne T, Curtze S, Dewey HM, Miteff F, Tsai CH, Lee JT, Phan TG, Mahant N, Sun MC, Krause M, Sturm J, Grimley R, Chen CH, Hu CJ, Wong AA, Field D, Sun Y, Barber PA, Sabet A, Jannes J, Jeng JS, Clissold B, Markus R, Lin CH, Lien LM, Bladin CF, Christensen S, Yassi N, Sharma G, Bivard A, Desmond PM, Yan B, Mitchell PJ, Thijs V, Carey L, Meretoja A, Davis SM, Donnan GA; EXTEND Investigators. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med. 2019. May 9;380(19):1795–1803. doi: 10.1056/NEJMoa1813046. Erratum in: N Engl J Med. 2021 Apr 1;384(13):1278. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, Parsons MW. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012. Oct;43(10):2648–53. doi: 10.1161/STROKEAHA.112.660548. Epub 2012 Aug 2. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wang X, Ma J, Jia M, Wu L, Li W, Li C, Wu C, Ren C, Chen X, Zhao W, Ji X. Association between the time of day at stroke onset and functional outcome of acute ischemic stroke patients treated with endovascular therapy. J Cereb Blood Flow Metab. 2022. Dec;42(12):2191–2200. doi: 10.1177/0271678X221111852. Epub 2022 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


