Abstract
Background:
Low-titer group O whole blood (LTOWB) or component therapy (CT) may be used to resuscitate hemorrhaging trauma patients. LTOWB has clinical and logistical benefits and may improve survival.
Objectives:
We hypothesized LTOWB would improve 24-hour survival in hemorrhaging patients and would be safe and equally efficacious in non-group O compared to group O patients.
Methods:
Adult trauma patients with massive transfusion protocol activations were enrolled in this observational study. The primary outcome was 24-hour mortality. Secondary outcomes included 72-hour total blood product use. A Cox regression determined the independent associations with 24-hour mortality.
Results:
348 patients were enrolled (CT, n=180; LTOWB, n=168). Demographics were similar between cohorts. Unadjusted 24-hour mortality was reduced in LTOWB vs. CT: 8% vs. 19% (p=0.003), but 6-hour and 28-day mortality were similar. In an adjusted analysis with multivariable Cox regression, LTOWB was independently associated with reduced 24-hour mortality (Hazard Ratio (HR)=0.21; 95% Confidence Interval (CI)=0.07-0.67; p=0.004). LTOWB patients received significantly less 72-hour total blood products (80.9 (41.6-139.3) mL/kg versus 48.9 (25.9-106.9) mL/kg; p<0.001). In stratified 24-hour survival analyses, LTOWB was associated with improved survival for patients in shock or with coagulopathy. LTOWB use in non-group O patients was not associated with increased mortality, organ injury, or adverse events.
Conclusions:
In this hypothesis-generating study, LTOWB use was independently associated with improved 24-hour survival, predominantly in patients with shock or coagulopathy. LTOWB also resulted in a 40% reduction in blood product use which equates to a median 2.4 L reduction in transfused products.
Keywords: Blood Transfusion, Resuscitation, Trauma, Hemorrhage, Mortality
Introduction
Unintentional injury is the fourth leading cause of death in Americans of all ages, and remains the leading cause of death for individuals age 1-44 [1]. Furthermore, death from hemorrhage secondary to trauma persists as the most common cause of preventable mortality [2]. Low-titer group O whole blood (LTOWB) continues to increase in use, both in civilian and military settings, emerging as a safe and efficacious product with logistical benefits [3-6]. There are currently at least 123 verified trauma centers using LTOWB [7] and 10 pediatric hospitals [8] using or planning to use LTOWB in the United States (US).
LTOWB provides a more concentrated product than component therapy (CT) in a 1:1:1 unit ratio due to the reduced volume of anticoagulants and additive solutions in whole blood compared to the amount in each constituent product of an equivalent CT transfusion [9, 10]. LTOWB has increased oxygen carrying capacity (higher hemoglobin concentration), is more hemostatic (higher coagulation factors and platelet concentration) and is less acidotic relative to one unit each of red blood cells (RBCs), plasma, and whole blood derived platelets with CT [11, 12]. In addition, LTOWB contains platelets that have been stored cold (4°C). Cold-stored platelets are more hemostatically active compared to room-temperature stored platelets in vitro, and are currently used in CT [13, 14] for actively bleeding patients in a small number of centers via a variance granted by the US Food and Drug Administration (FDA). Bacterial contamination risk is therefore partially mitigated with the use of LTOWB, and donor exposure is also reduced [15]. In addition, the use of only one bag of LTOWB over the multiple bags and vascular access needed with CT simplifies the logistics of transfusion, potentially resulting in more rapid administration of therapy in the field and at the bedside, therefore LTOWB may ultimately improve outcomes in a situation where every minute counts [15, 16]. This logistic benefit of LTOWB is theoretically amplified in the prehospital phase of resuscitation, where deaths from hemorrhage are most common and providing all blood components is not feasible [2].
We previously published the initial report of this study using data from 86 patients [17]. We now have collected and analyzed data from 384 patients, including the 86 patients previously reported. The primary objective of this hypothesis-generating study was to compare 24-hour mortality between patients transfused with LTOWB or CT, and secondary objectives included comparing organ injury and total amount of all blood products transfused within the first 72 hours from admission. A unique addition in this report is the comparison in differences of measures of safety between group O vs. non-group O recipients of LTOWB. We hypothesized the use of LTOWB would be independently associated with improved 24-hour survival compared to the exclusive use of CT in adult patients with traumatic hemorrhagic shock, and that this benefit would be most apparent in patients with measurable hemostatic dysfunction at time of admission. We also hypothesized that the use of LTOWB in non-group O patients would be safe and not associated with increased adverse outcomes.
Methods
Study Design
This single-center prospective observational study with a historical control was designed in July 2018 with a combination of retrospective and prospective data collection in the CT group. Data were collected from charts of patients with massive transfusion protocol (MTP) activations under a waiver of informed consent (IRB #201909200). Patients were enrolled if they were 18 years of age or older, and their MTP activation was secondary to traumatic injury. On December 12, 2018, the Barnes-Jewish Hospital (BJH) MTP protocol was updated to include LTOWB. Data for the CT group was collected on patients exclusively transfused CT from January 3, 2017 to December 5, 2018. Data collection was retrospective from January 2017 to August 2018 and prospective from August 2018 to December 2018. Patients in the LTOWB group were enrolled from December 12, 2018 to July 15, 2020. Patients were excluded if a blood product was not transfused, which may have been due to early death or if LTOWB was not transfused after December 12, 2018. Patients were also excluded if they expired within 1 hr of arrival due to unsalvageable injury, as has been done in recent similar studies [18]. LTOWB use in the MTP protocol was limited to 8 units, and therefore, patients who were still bleeding and needed additional transfusions in the LTOWB group could have received CT during the MTP activation.
During the study period, LTOWB was supplied to BJH by the American Red Cross; LTOWB was leukoreduced with the TerumoBCT (Lakewood, CO) IMUFLEX ® platelet-sparing filter and stored in CPD. The anti-A and -B titer for all LTOWB units was <200. The shelf life for LTOWB used in this study at the BJH blood bank was 21 days. All LTOWB units were Rh+.
Data Collection
Data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Washington University in St. Louis School of Medicine (St. Louis, MO, US) [19, 20]. Data collected include demographic information including information about the injury sustained, all blood products transfused through 72 hours post-admission, daily laboratory results up through 72 hours post-admission, Multiple Organ Dysfunction Score (MODS), adverse event incidence, and mortality through discharge (time to death defined to the nearest 6 min (0.1 hr)) [21]. Maximum MODS for the 72-hour period were also compared. Admission laboratory values had results generated within 30 minutes of patient arrival; no laboratory values at admission nor during the 72 hours period were collected specifically for the study, but rather only as part of standard care. Blood product data (number of units, product blood type) were extracted from the BJH blood management system database. A small number of patients had labs with values outside of the detectable range, which are summarized in Table S1. Values above or below the detectable limit were taken as the detectable limit to facilitate inclusion in the analysis. ICU-free days were calculated as 28 minus the number of days in the ICU (range, 0 to 28 days), and assigned 0 ICU-free days for death within 28 days [22]. Patients who survived to discharge were assumed alive at 28 days.
Statistical Analysis and Model Selection
Statistical analyses were performed in RStudio (version 2021.09.0; R version 4.1.1; supplemental packages: “survival”, “survminer, “dplyr”, “readxl”, “ggplot2”, “coxphw”, “xlsx”, and “autothresholdr”) [23-29]. Categorical data are displayed as amount over total and percentage of total and compared using Fisher’s exact test. Continuous data are displayed using medians and interquartile ranges and compared using the Wilcoxon Rank Sum test, unless otherwise indicated. Missingness is indicated, if applicable, by the number of patients who had a given test result.
Survival analyses for all mortality outcomes were performed using Kaplan-Meier curves. Survival analyses were performed unadjusted for the entire cohort and also stratified by parameters of interest to examine the impact of LTOWB vs. CT in specific subsets of the patient population. To adjust for survival, a Cox regression model was fit. Univariate Cox regression was performed with 24-hour mortality as outcome. To select the final adjusted model, all parameters from the univariate analysis with p-value ≤0.05 for 24-hour mortality were selected, and Pearson’s R was calculated between all pairs of selected parameters. Multivariable Cox regression was performed with backwards stepwise selection to arrive at a fitted model. Cox models enable investigation of the effects of multiple continuous and categorical covariates on survival, accounting for possible confounders, thereby offering advantages over log-rank tests when comparing survival [30]. Highly co-linear parameters were eliminated from the model selection (R2 > 0.7) prioritizing variables with reduced missingness. Age and injury severity score (ISS) were forced into the initial model given their clinical importance and association with mortality after injury, as well as their consideration as essential for best practice in trauma risk adjustment [31-34]. Patients with any missing covariate(s) were not included in the model. Backwards stepwise selection was then employed to arrive at the final fitted model, by eliminating the covariate with the highest p-value, refitting the new model, and repeating the process until all covariates were significant (p-value cutoff 0.05) aside from forced-in covariates (age and ISS). Model selection is shown in Table S2. The final model included the treatment group, LTOWB use, and covariates ISS, age, admission GCS, admission platelet count, and admission maximum clot firmness (MCF). LTWOB or CT use were then also used as strata with the same covariate selection for the Cox Regression model to generate a model for survival curve visualization out to 28 days.
Results
A total of 384 trauma patients had MTP activations during the study period. 36 patients were excluded due to unsalvageable injuries (expired within 1 hr of hospital arrival). Of the remaining 348 patients included in this study, 180 received hemostatic resuscitation with CT post-MTP activation, and 168 received hemostatic resuscitation with LTOWB post-MTP activation.
Table 1 summarizes the epidemiological information of each cohort. Penetrating injury occurred in 108/180 (60%) and 118/168 (70%) of the CT and LTOWB cohorts, respectively (p=0.089). Most patients were male (CT, 142/180 (79%); LTWOB 129/168 (77%); p=0.699), and the median age was 29 years (interquartile range (IQR) 23-41) in the CT cohort and 32 (24-44; p = 0.089) in the LTOWB cohort. There was also a high frequency (69-74%) of African Americans in both study groups. The ISS was 22 (17-34) and 22 (13-33) in the CT and LTOWB cohorts, respectively (p=0.185). Systolic blood pressure (SBP) and Glasgow Coma Scale (GCS) at admission were not statistically different (p=0.241 and p=0.586, respectively), and the majority of baseline labs were also similar. A small number of baseline parameters were statistically significantly different (abbreviated injury scale (AIS) – lower extremities (p=0.021), partial thromboplastin time (PTT, p=0.023), platelet count (p=0.016), and rotational thromboelastometry (ROTEM) maximum clot formation (MCF; p=0.008). However, we do not anticipate bias in clinical results due to these differences due to the small absolute differences in the medians of these parameters, for example, differences of 26.9 and 25.5 for PTT in the CT and LTOWB groups, respectively.
Table 1. Epidemiology and baseline patient characteristics.
Any p-value that reached significance is shown in bold italic. The reported parameters are split into their respective cohort. Parameters are reported either as n (%) or as median IQR, where indicated. The additional column to the left of parameter values gives the n of patients with a value for a given parameter. Only patients with reported values were included in median (IQR) calculations. CT: component therapy; LTOWB: low-titer group O whole blood; ISS: Injury Severity Score; AIS: Abbreviated Injury Scale; BMI: Body Mass Index; SBP: systolic blood pressure; GCS: Glasgow Coma Score; BUN: blood urea nitrogen; PTT: partial thromboplastin time; PT: prothrombin time; INR: international normalized ratio; Hb: hemoglobin; CT: clotting time; MCF: maximum clot formation; LI30: lysis index at 30 mins.
| Parameter | CT Cohort (N=180) | LTOWB Cohort (N=168) | |||
|---|---|---|---|---|---|
| Patient Descriptors | n | n (%) or median (IQR) | n | n (%) or median (IQR) | p |
| Sex | 0.699 | ||||
| Male | 142/180 (79%) | 129/168 (77%) | |||
| Female | 38/180 (21%) | 39/168 (23%) | |||
| Injury | 0.089 | ||||
| Blunt | 67/180 (37%) | 48/168 (29%) | |||
| Penetrating | 108/180 (60%) | 118/168 (70%) | |||
| Blunt and Penetrating | 3/180 (2%) | 1/168 (1%) | |||
| Blunt and Burn | 2/180 (1%) | 0/168 (0%) | |||
| a | 0/180 (0%) | 0/168 (0%) | |||
| Race | 0.084 | ||||
| Black or African American | 124/180 (69%) | 125/168 (74%) | |||
| White or Caucasian | 54/180 (30%) | 35/168 (21%) | |||
| Asian | 0/180 (0%) | 1/168 (1%) | |||
| Other or Unknown | 2/180 (1%) | 7/168 (4%) | |||
| ISS | 175 | 22 (17-34) | 160 | 22 (14-34) | 0.185 |
| AIS | 175 | 160 | |||
| Head | 0 (0-2) | 0 (0-0) | 0.324 | ||
| Face | 0 (0-1) | 0 (0-0) | 0.529 | ||
| Neck | 0 (0-0) | 0 (0-0) | 0.207 | ||
| Thorax | 2 (0-3) | 3 (0-3) | 0.533 | ||
| Abdomen | 2 (0-4) | 2 (0-3) | 0.368 | ||
| Spine | 0 (0-2) | 0 (0-2) | 0.308 | ||
| Upper Extremities | 0 (0-2) | 0 (0-1) | 0.828 | ||
| Lower Extremities | 1 (0-3) | 0 (0-3) | 0.021 | ||
| External | 0 (0-0) | 0 (0-0) | 0.623 | ||
| Other Trauma | 0 (0-0) | 0 (0-0) | NA | ||
| Admission Variables | n | median (IQR) | n | median (IQR) | p |
| Age, y | 178 | 29 (23-41) | 168 | 32 (24-44) | 0.089 |
| Weight, kg | 172 | 80 (70-95) | 140 | 80 (71-92) | 0.953 |
| BMI | 169 | 26 (23-31) | 134 | 26 (23-30) | 0.647 |
| Baseline Labs | n | median (IQR) | n | median (IQR) | p |
| SBP, mmHg | 152 | 84 (68-102) | 128 | 86 (74-110) | 0.241 |
| GCS | 148 | 13 (3-15) | 157 | 14 (3-15) | 0.586 |
| Creatinine, mg/dL | 134 | 1.21 (1.03-1.41) | 136 | 1.28 (1.11-1.46) | 0.063 |
| BUN, mg/dL | 133 | 12 (9-16) | 135 | 12 (10-15) | 0.991 |
| PTT, sec | 138 | 26.9 (23.3-36.2) | 123 | 25.5 (23.7-29.0) | 0.023 |
| PT, sec | 138 | 13.4 (12.0-14.8) | 122 | 12.7 (11.9-14.0) | 0.051 |
| INR | 138 | 1.21 (1.09-1.35) | 122 | 1.19 (1.10-1.30) | 0.620 |
| Hb, g/dL | 154 | 11.6 (10.1-12.9) | 128 | 11.9 (10.6-12.9) | 0.183 |
| Platelet count, X103 cells/μL | 154 | 202 (160-294) | 128 | 234 (192-285) | 0.016 |
| Base excess, mEq/L | 45 | −7 (−14-−5) | 113 | −6 (−12-−4) | 0.240 |
| Lactate, mmol/L | 120 | 5.9 (3.9-9.1) | 94 | 5.7 (3.6-9.1) | 0.469 |
| ROTEM CT, sec | 136 | 71 (62-86) | 118 | 69 (60-79) | 0.257 |
| ROTEM α, ° | 129 | 69 (63-73) | 116 | 71 (65-74) | 0.109 |
| ROTEM MCF, mm | 135 | 57 (50-63) | 118 | 60 (54-66) | 0.008 |
| ROTEM LI30 | 134 | 100 (99-100) | 118 | 100 (100-100) | 0.281 |
Table 2 summarizes treatments. As expected, the LTOWB group received statistically significantly lower weight-adjusted total volumes of RBCs, platelets, plasma, and cryoprecipitate over 72 hours (units and volumes reported in Table S3). Increased 72-hour saline and albumin volumes were given to patients in the CT cohort. TXA was administered as a 1 gram IV bolus to 56/180 (31%) of CT patients and 41/168 (24%) of LTOWB patients (p=0.189).
Table 2. Treatments.
Any p-value that reached significance is shown in bold italic. Blood products transfused separated by specific component or LTWOB over 72 hours are reported as weight-adjusted totals (median (IQR)). Saline, lactated Ringer’s, total crystalloids, and albumin are shown for each cohort first as the number of patients that received the treatment and then as the median (IQR) total over 72 hours, both outright and weight-adjusted. The number of patients that received TXA and the median infusion is reported, as well as the number of those patients that also received an infusion dose and the median infusion dose. Calcium is reported first as the number of patients that received calcium, and then as both the total over 72-hours and the weight-adjusted 72-hour total. The median (IQR) values include all patients, i.e. patients that did not receive the treatment were reported as 0, with the only exception being TXA doses. CT: component therapy; LTOWB: low-titer group O whole blood; RBCs: red blood cells; TXA: tranexamic acid.
| Parameter | CT Cohort (N=180) | LTOWB Cohort (N=168) | |||
|---|---|---|---|---|---|
|
Blood Product Volumes (72 hours), weight-
adjusted |
n | median (IQR) | n | median (IQR) | p |
| LTOWB, mL/kg | 140 | 31 (13-45) | |||
| RBCs, mL/kg | 172 | 51 (27-83) | 140 | 12 (5-33) | <0.001 |
| Platelets, mL/kg | 172 | 4 (0-11) | 140 | 0 (0-6) | <0.001 |
| Plasma, mL/kg | 172 | 26 (12-45) | 140 | 4 (0-18) | <0.001 |
| Cryoprecipitate, mL/kg | 172 | 0 (0-3) | 140 | 0 (0-0) | 0.040 |
| Other Treatments | n | n (%) or median (IQR) | n | n (%) or median (IQR) | p |
| Normal Saline, n received | 157/180 (87%) | 149/168 (87%) | 0.743 | ||
| Volume, mL | 180 | 2651 (1000-5272) | 168 | 2000 (604-4094) | 0.032 |
| Weight-adjusted volume, mL/kg | 172 | 32 (12-70) | 140 | 23 (8-50) | 0.014 |
| Lactated Ringer's, n received | 147/180 (82%) | 143/168 (85%) | 0.472 | ||
| Volume, mL | 180 | 3113 (1000-6204) | 168 | 3460 (1000-6383) | 0.674 |
| Weight-adjusted volume, mL/kg | 172 | 42 (11-80) | 140 | 37 (12-75) | 0.881 |
| Total Crystalloid Fluids, n received | 164/180 (91%) | 157/168 (93%) | 0.431 | ||
| Volume, mL | 180 | 6964 (3912-11049) | 168 | 6771 (3523-9716) | 0.512 |
| Weight-adjusted volume, mL/kg | 172 | 88 (48-144) | 140 | 79 (42-121) | 0.201 |
| Albumin, n received | 96/180 (53%) | 75/168 (45%) | 0.109 | ||
| Volume, mL | 180 | 250 (0-1000) | 168 | 0 (0-750) | 0.093 |
| Weight-adjusted volume, mL/kg | 172 | 3 (0-11) | 140 | 0 (0-9) | 0.047 |
| TXA, n received | 56/180 (31%) | 41/168 (24%) | 0.189 | ||
| TXA bolus dose, g | 51 | 1 (1-1) | 36 | 1 (1-1) | 0.512 |
| TXA infusion use, n | 13/192 (7%) | 12/192 (6%) | 0.201 | ||
| TXA infusion dose, g | 11 | 1 (1-1) | 8 | 1 (1-1) | 0.416 |
| Calcium, n received | 164/180 (91%) | 148/168 (88%) | 0.383 | ||
| Total, g | 180 | 4 (2-8) | 168 | 4 (2-8) | 0.870 |
| Weight-adjusted 72-hour total dose, mg/kg | 172 | 49 (22-97) | 140 | 44 (17-98) | 0.370 |
Secondary outcomes are listed in Table 3. The LTOWB group received approximately a median of 30mL/kg less total blood products than the CT group (p<0.001) within the first 72 hours after admission (Table 3). 312 (172 CT, 140 LTOWB) patients were included in the transfused weight-normalized volume comparison. ICU-free days and max MODS score were not statistically significantly different between groups (Table 3). The LTOWB cohort had increased survival at 24 hours (13/168 (8%) deaths vs. 34/180 (19%) deaths in the CT group, p=0.003) but not at 6 hours nor at 28 days.
Table 3. Outcomes.
Outcomes are reported as n (%) or median (IQR) where indicated. Significant p-values are shown in bold italic. CT: component therapy; LTOWB: low-titer group O whole blood; ICU: intensive care unit; MODS: Multiple Organ Dysfunction Score.
| CT Cohort (N=180) | LTOWB Cohort (N=168) | ||||
|---|---|---|---|---|---|
| Parameter | n | n (%) or median (IQR) | n | n (%) or median (IQR) | p |
| 6-hour mortality | 20/180 (11%) | 12/168 (7%) | 0.265 | ||
| 24-hour mortality | 34/180 (19%) | 13/168 (8%) | 0.003 | ||
| 28-day mortality | 49/180 (27%) | 33/168 (20%) | 0.102 | ||
| Total blood products, mL/kg | 172 | 82 (44-139) | 140 | 48 (26-108) | <0.001 |
| ICU-free days MODS |
124 | 21 (0-25) | 150 | 19 (2-25) | 0.639 |
| MODS | |||||
| Respiratory | 148 | 1 (0-3) | 151 | 1 (0-3) | 0.953 |
| Renal | 148 | 1 (0-1) | 153 | 1 (0-1) | 0.496 |
| Hepatic | 148 | 0 (0-1) | 151 | 0 (0-1) | 0.905 |
| Cardiologic | 148 | 0 (0-0) | 151 | 0 (0-0) | 0.034 |
| Hematologic | 148 | 1 (1-2) | 151 | 1 (0-2) | 0.036 |
| Neurologic | 148 | 3 (1-4) | 151 | 4 (3-4) | 0.004 |
| Total Score | 148 | 6 (4-8) | 152 | 7 (4-9) | 0.223 |
| Transfusion reaction | 4 (2%) | 3 (2%) | 1.000 | ||
| Stroke within 72 hours | 3 (2%) | 7 (4%) | 0.208 |
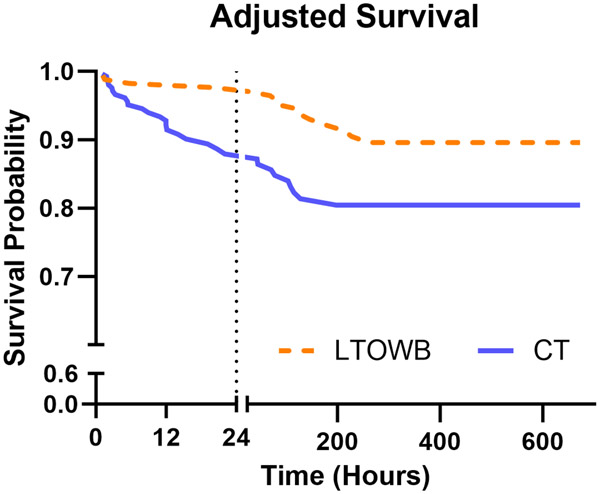
Univariate regression results are shown in Table S4. Univariate regression for the primary outcome of 24-hour mortality was significant for ISS, AIS-head, AIS-face, AIS-abdomen, age, GCS, creatinine, BUN, PTT, PT, INR, platelet count, base deficit, ROTEM CT, ROTEM α, ROTEM MCF, and ROTEM LY30. These parameters were considered for the multivariate Cox regression model. The final selected model (Table 4) found use of LTOWB was associated with decreased hazard of mortality (hazard ratio (HR) 0.21, p=0.004) when adjusted for ISS, age, admission GCS, admission platelet count, and admission MCF. Of the total 348 patients, 193 (97 CT, 96 LTOWB) had a complete set of covariate data, and thus were included in the model. Interaction terms were checked and were not significant and excluded from the final model. The model passes the proportional hazards check (treatment group p=0.20, global p=0.36). The adjusted survival curves for LTOWB vs. CT are shown in Figure 1. The epidemiological data and covariate distribution for the 193 patients included in the model is shown in Table S5.
Table 4. Multivariate Cox Regression results.
The selected model was applied to 24-hour mortality and the resulting HR, 95% CI, and p-values are shown. N=193 (96 component therapy, 97 LTOWB). If p-values achieved significance they are shown in bold italic. GCS: Glasgow Coma Scale; LTOWB: low-titer group O whole blood; Plt: platelet; MCF: maximum clot firmness.
| Cox Regression | 24-hour adjusted mortality | ||
|---|---|---|---|
| Survival Predictor | HR | 95% CI | p |
| LTOWB Use | 0.21 | (0.07, 0.61) | 0.004 |
| ISS | 0.98 | (0.95, 1.01) | 0.124 |
| Age | 1.02 | (0.99, 1.04) | 0.199 |
| GCS | 0.92 | (0.86, 1.00) | 0.043 |
| Plt Count | 0.99 | (0.99, 1.00) | 0.049 |
| MCF | 0.96 | (0.94, 0.99) | 0.004 |
Figure 1. LTOWB use is associated with improved survival in an adjusted model.
Survival adjusted by injury severity score, age, Glasgow Coma Score (GCS), admission platelet count, and admission maximum clot formation (bottom). N=193 (96 CT, 97 LTOWB). LTOWB: low-titer group O whole blood; CT: component therapy.
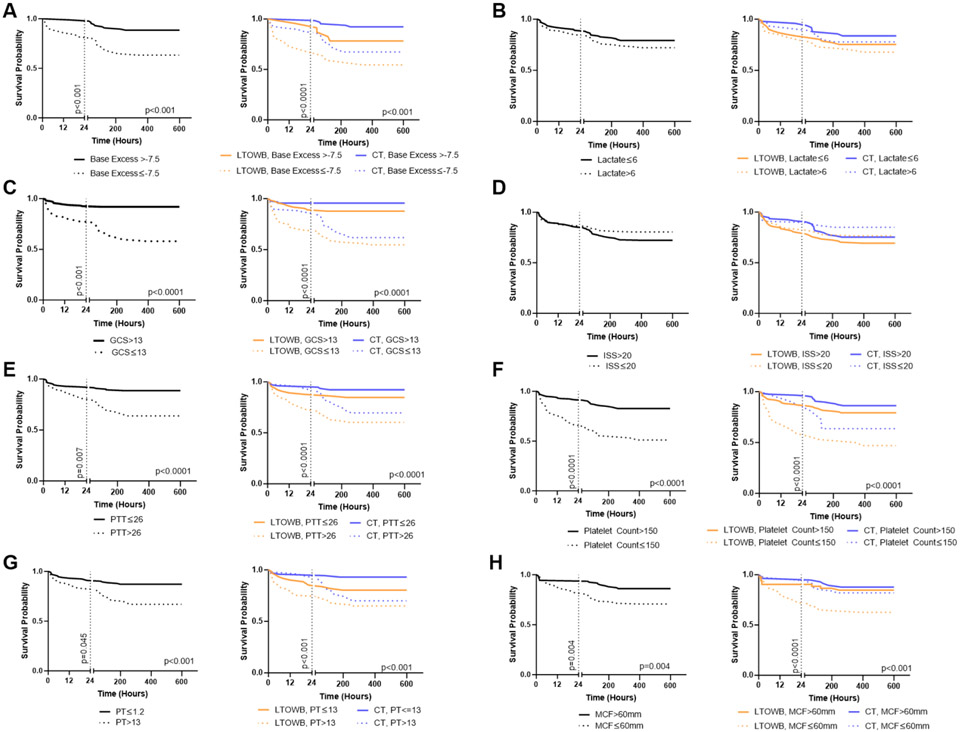
Previously, we identified through stratified unadjusted analysis that MCF served as an indicator of the effectiveness of LTOWB, which had a greater association with survival vs. CT in the coagulopathic population [17]. We therefore wanted to further investigate the effects of important clinical parameters on the effects of LTOWB on survival via stratification to better understand which patient populations might benefit most from this treatment. Patient cohorts were stratified by base excess, lactate, GCS, ISS, PTT, INR, MCF, platelet count, biological sex, and age (Figure 2 and Figure S2). Admission base excess, GCS, PTT, MCF, INR, and platelet count all have distinct significant effects on survival at 24 hours and 28 days in the entire patient population, while lactate, ISS, biological sex, and age did not (Figure 2 and Figure S2). When further stratified, LTOWB vs. CT treatment did not have an association with survival in patients with GCS≤13 vs patients with GCS>13, on patients with ISS≤20 vs. ISS>20, with platelet count>150 vs. platelet count≤150, nor on male vs. female patients. LTWOB vs. CT treatment did have a treatment-specific association, with LTOWB independently associated with improved survival over CT in the patients with shock or coagulopathy; stratified by base excess≤−7.5 vs. base excess>−7.5, patients with PTT>26 vs. PTT≤26 and INR>1.2 vs. INR≤1.2, patients with MCF≤60 vs. MCF>60.
Figure 2. Further stratified survival demonstrates which patient populations may most benefit from use of LTOWB over CT.
Black curves at left stratify survival out to 28 days (672 hours) by A base excess, B lactate, C GCS, D ISS, E PTT, F platelet count, G PT, and H MCF, while the colored curves at right further stratify within those groups by those who received LTOWB vs. CT. The vertical dashed line is at 24 hours. If survival was significantly different at 24 hours, the p-value (log-rank test) is along the dashed line. If survival was significantly different at 28 days, the p-value is shown in the bottom right of the curve. Only significant p-values are shown. LTOWB: low-titer group O whole blood; CT: component therapy; ISS: Injury Severity Score; GCS: Glasgow Coma Scale; PTT: partial thromboplastin time; PT: prothrombin time; MCF: maximum clot firmness; INR: international normalized ratio.
To determine if there was any difference in outcomes between group O and non-group O recipients of LTOWB, we examined differences in outcomes between these two subgroups. Epidemiological information, labs, blood products transfused, and outcomes are summarized in Table 5. There were statistical differences in injury type and age between study groups with increased age and less penetrating injury in the non-group O patients. There were no differences between patient sex, ISS, weight, and BMI between study groups. All baseline laboratory values were not significantly different except for ROTEM α, which was 58 (52-62) in the type O and 61 (58-67) in the non-type O (p=0.021). Maximum creatinine values within 72 hours were not different between subgroups. LTOWB transfused and total blood products transfused over 72 hours were also not different, and no outcomes, including any MODS subscores, were statistically different between type O and non-type O recipients of LTOWB.
Table 5. Type O and non-Type O recipients of LTOWB.
Epidemiology, baseline laboratory values, maximum daily creatinine values and maximum creatinine value over 72 hours, treatments, and outcomes are shown for recipients of LTOWB separated into group O and non-group O sub-cohorts. P-values that reached significance are shown in bold italic. ISS: injury severity score; BMI: body mass index; SBP: systolic blood pressure; GCS: Glasgow Coma Score; BUN: blood urea nitrogen; PTT: partial thromboplastin time; PT: prothrombin time; INR: international normalized ratio; Hb: hemoglobin; CT: clotting time; MCF: maximum clot formation; LI30: lysis index at 30 mins; LTOWB: low-titer group O whole blood; RBC: red blood cells; MODS: Multiple Organ Dysfunction Score.
| parameter | Group O (N=64) | Non-Group O (N=87) | |||
|---|---|---|---|---|---|
| Patient Descriptors | n (%) or median (IQR) | n (%) or median (IQR) | p | ||
| Sex | 0.846 | ||||
| Male | 52/64 (81%) | 68/87 (78%) | |||
| Female | 12/64 (19%) | 19/87 (22%) | |||
| Injury | 0.009 | ||||
| Blunt only | 10/64 (18%) | 33/87 (37%) | |||
| Penetrating only | 52/64 (79%) | 54/87 (63%) | |||
| Blunt and Penetrating | 1/64 (1%) | 0/87 (0%) | |||
| Blunt, Penetrating, and Burn | 0/64 (0%) | 0/87 (0%) | |||
| ISS | 59 | 25 (16-34) | 84 | 22 (13-34) | 0.131 |
| Admission Variables | n | median (IQR) | n | median (IQR) | p |
| Age, y | 64 | 30 (23-41) | 87 | 33 (25-48) | 0.036 |
| Weight, kg | 55 | 80 (73-90) | 76 | 80 (70-94) | 0.465 |
| BMI | 53 | 26 (22-30) | 73 | 25 (23-29) | 0.865 |
| Baseline Labs | n | median (IQR) | n | median (IQR) | p |
| SBP, mmHg | 46 | 84 (69-105) | 72 | 86 (76-108) | 0.373 |
| GCS | 62 | 14 (7-15) | 80 | 14 (3-15) | 0.868 |
| Creatinine, mg/dl | 53 | 1.30 (1.16-1.57) | 76 | 1.28 (1.14-1.41) | 0.215 |
| BUN, mg/dL | 53 | 12 (9-14) | 75 | 12 (10-15) | 0.425 |
| PTT, sec | 47 | 25.7 (24.2-29.3) | 69 | 25.5 (23.0-28.0) | 0.260 |
| PT, sec | 46 | 12.9 (11.9-14.2) | 69 | 12.6 (12.0-13.8) | 0.668 |
| INR | 46 | 1.20 (1.10-1.30) | 69 | 1.18 (1.10-1.30) | 0.621 |
| Hb, g/dL | 50 | 11.8 (10.1-12.8) | 71 | 12.1 (11.3-13.1) | 0.094 |
| Platelet count, X103 cells/μL | 50 | 235 (195-266) | 71 | 233 (185-289) | 0.875 |
| Base excess, mEq/L | 44 | −6 (−14-−4) | 62 | −7 (−12-−4) | 0.890 |
| Lactate, mmol/L | 36 | 6.0 (3.2-9.0) | 50 | 5.2 (3.6-8.9) | 0.937 |
| ROTEM CT, sec | 48 | 72 (59-82) | 64 | 68 (62-78) | 0.467 |
| ROTEM MCF, mm | 47 | 69 (64-74) | 63 | 71 (68-74) | 0.114 |
| ROTEM α, ° | 48 | 58 (52-62) | 64 | 61 (58-67) | 0.021 |
| ROTEM LI30 | 48 | 100 (99-100) | 64 | 100 (100-100) | 0.250 |
| Labs | median (IQR) | median (IQR) | p | ||
| Creatinine Day 1, mg/dL | 58 | 1.26 (0.99-1.99) | 81 | 1.25 (0.97-1.72) | 0.531 |
| Creatinine Day 2, mg/dL | 53 | 1.15 (0.93-2.14) | 73 | 1.07 (0.94-1.52) | 0.242 |
| Creatinine Day 3, mg/dL | 53 | 1.03 (0.86-1.75) | 68 | 0.97 (0.82-1.25) | 0.138 |
| Max Creatinine over 3 Days, mg/dL | 64 | 1.21 (0.98-1.84) | 87 | 1.23 (0.98-1.68) | 0.756 |
| Treatments (Over 3 Days) | median (IQR) | median (IQR) | p | ||
| LTOWB transfused (mL/kg) | 55 | 31 (14-44) | 76 | 27 (13-46) | 0.830 |
| LTOWB+RBC (mL/kg) | 55 | 44 (30-97) | 76 | 39 (23-67) | 0.205 |
| Total blood products transfused (mL/kg) | 55 | 49 (31-139) | 76 | 46 (25-82) | 0.221 |
| Outcomes | n (%) or median (IQR) | n (%) or median (IQR) | p | ||
| 6-hour mortality | 3/64 (5%) | 2/87 (2%) | 0.423 | ||
| 24-hour mortality | 3/64 (5%) | 3/87 (3%) | 0.712 | ||
| 28-day mortality | 9/64 (14%) | 16/87 (18%) | 0.483 | ||
| Max MODS over 3 days | |||||
| Respiratory | 59 | 1 (0-3) | 83 | 1 (0-3) | 0.769 |
| Renal | 61 | 1 (0-1) | 83 | 1 (0-1) | 0.375 |
| Hepatic | 59 | 0 (0-1) | 83 | 0 (0-1) | 0.498 |
| Cardiologic | 59 | 0 (0-0) | 83 | 0 (0-0) | 0.315 |
| Hematologic | 59 | 1 (0-2) | 83 | 1 (0-2) | 0.444 |
| Neurologic | 59 | 4 (3-4) | 83 | 4 (3-4) | 0.754 |
| Total Score | 60 | 6 (4-9) | 83 | 7 (5-9) | 0.982 |
Discussion
We report in an adjusted analysis that use of LTOWB over CT in hemostatic resuscitation of massively bleeding patients resulted in an independent association in reduction of risk of 24-hour mortality (p=0.004) using a Cox regression analysis in our single-center prospective observational study with a historical control. The safety of LTOWB in this population is supported by our results suggesting no differences in transfusion reactions nor stroke, and no difference in organ injury over the same period with the use of LTOWB. This safety signal was also present in non-group O recipients of LTOWB.
Use of LTOWB in hemostatic resuscitation in this patient population also resulted in a 40% reduction in total weight-normalized blood product use over the 72-hour post-admission timeframe. In other terms, the LTOWB group received approximately 30mL/kg less of total blood products. Considering the median weight of patients in this study was 80kg, a 30mL/kg reduction equates to a 2.4-liter reduction in blood products transfused. This is a substantial reduction in blood use and exposure. Using a typical volume of 300mL per unit for RBCs and apheresis unit of platelets, and 250mL for a plasma unit, this equates to about 4 units of RBCs, 4 units of plasma, and 1 apheresis unit of platelets. Additionally, the CT cohort received approximately double the anticoagulant and additive solution volume compared to the LTOWB cohort [10], which could have deleterious consequences for patients in shock experiencing hypocoagulability. The LTOWB cohort also was exposed to on average up to roughly 8 fewer unique donors, potentially reducing infectious and non-infectious risks of transfusion.
Blood management has become a very important principle, due in part to the improved patient outcomes associated with reducing blood product exposure for patients who may not benefit from transfusion. It is now also essential due to the national blood shortage crisis where there is not enough supply of group O RBCs and platelets due to both the ageing donor pool, thereby reducing blood donor availability, and the increased use of blood products for bleeding patients [35, 36]. The risks resulting from a national blood shortage and consequences for patients with traumatic injury have been recently described [35]. Our results demonstrating the use of LTOWB seems to “do more with less” is critical for this patient population with a high rate of preventable death from hemorrhage and increased risk of non-availability of blood products for resuscitation of life-threatening hemorrhage [35, 37].
Our findings of independent association with LTOWB and improved survival while also using fewer total blood products are consistent with other reports of cold-stored LTOWB in adult and pediatric trauma patients [6, 8, 10, 38, 39]. Recently, Brill et al. reported a 4-fold survival benefit and 60% reduction in overall transfusions with use of LTOWB over CT in emergency release blood products in a large (nearly 1380 patients) single-center prospective observational cohort design [6]. There are a few interesting differences between our studies. Of note, the LTOWB used at our institution was leukoreduced (LR) with a platelet-sparing filter, while the LTOWB used in the study by Brill et al. was not. We have previously reported on some minor measurable differences in some in vitro measures of hemostasis in older LR WB (day 14-21) compared to non-LR WB [15]. These differences may or may not be clinically meaningful. As we observed a substantial independent association with improved survival, and two of our final selected covariates (platelet count and MCF) directly implicate the critical role of the platelet in this patient population, this supports the use of a platelet-sparing LR filter. Randomized controlled trials must be done to fully understand the impact of blood product manufacturing methods, to include LR of LTOWB, on in vivo function and clinical outcomes. Use of LTOWB has also been shown to be of benefit in pediatric patients. Use of LTOWB over individual blood components in children with severe traumatic bleeding more rapidly provides RBCs, plasma and platelets, more effectively resolves shock and coagulopathy, is associated with less total amount of blood products administered [39] and mechanical ventilation days, and is independently associated with reduced 72-hour (OR 0.23; [0.08-0.70]), and 28-day mortality (OR 0.41; [ 0.23-0.98]) in a single center retrospective study [8].
The additional stratification in survival analyses indicated there may be patient populations that might benefit most from LTOWB over CT transfusion. We had previously identified MCF as a striking predictor of mortality in preliminary analysis of a small initial subset (approximately 40 patients per group) of these cohorts, and even more interestingly, LTOWB had a distinct substantial benefit specifically in coagulopathic patients (MCF<60) [17]. Interestingly, we see that trend conserved, both with MCF as well as with PTT, where LTOWB use over CT was independently associated with improved survival at 24 hours in patients with low MCF (<60 mm) and high PTT (>26 s). This supports the use of LTOWB for hemostatic resuscitation in this patient population and potentially, especially in the trauma-induced coagulopathy population. This also may indicate a precision medicine application, and that in the case of product shortages, hypocoaguable patients could be prioritized to receive LTOWB. However, it is important to note that access to assays and turnaround time may be a major limiting factor in implementation of such an approach. Pre-hospital labs, if available, would be most informative for hemostatic resuscitation, however, we recognize this is not available at many institutions. Better point-of-care devices that are rapid, easy to use, and could be deployed pre-hospital would help identify patients most in need of transfusion early in their course of care. At minimum, perhaps lab results could help current practitioners tailor an MTP activation, or initiate an MTP if one has not been started already once results are received.
While there is a small association with improved survival at 24 hours due to LTOWB in patients with low platelet counts, it is less in magnitude than seen in the functional hemostatic assays. This is consistent with the hypothesis that one of the mechanisms by which LTWOB increases survival is in rescue of hemostatic mechanism, as a low platelet count alone does not necessarily indicate that platelets are non-functional. Acidotic patients (base excess<−7.5, i.e. those in shock) also had a significant survival benefit in use of LTOWB over CT. The same trend, but to a lesser extent, was seen in patients with high lactate (>6mg/dL). The reduced citrate load with a LTOWB resuscitation that leads to less total blood use is likely contributory to reducing the deleterious effects of acidosis [40]. ISS did not significantly predict survival at any timepoint in our patient population, as seen both in our Cox model and in the unadjusted survival curve, and survival does not appear to be associated with treatment when further stratified by this parameter alone. This may be due to our high penetrating injury case-mix, in which ISS is known to underestimate injury severity [41]. Biological sex was also not impactful on 24-hour and 28-day survival and did not see changes when further stratified by treatment, and age alone was not significant in our analyses; however, again this may be due to case-mix with a relatively young cohort.
Conventional practice historically has dictated that females of childbearing potential (FCP) whom are RhD− should receive RhD− blood products, due to a concern that alloimmunization will result in a risk for the loss of a future RhD+ fetus. Of the 39 women who received CT, five were RhD−. One woman received RhD+ RBC, and that patient was an FCP. At our center, all LTOWB supplied is RhD+. Of the 42 women who received LTOWB, four were RhD−, two of which were FCP. Relative to the use of CT, the implementation of LTOWB only resulted in one additional transfusion of an RhD− incompatible product to a massively bleeding FCP. Notably, recent analysis demonstrates the actual probability of an RhD− FCP experiencing fetal demise or significant neurological morbidity from hemolytic disease of the fetus and newborn (HFDN) due to alloimmunization in this context to be approximately 0.3% [42]. Recent reviews on this topic suggest that the increased use of Rh+ LTOWB which may both reduce the use of total blood products and improve survival justifies exposing FCP to the risk of HDFN [43]. Large surveys of women and parents of female children strongly agree with this concept [44, 45].
There are some limitations to consider in our study. There may have been unmeasured confounders on outcomes that we did not include in our analysis and the lack of randomization increases the risk of bias. The time at which blood products were administered was not able to be collected prospectively, therefore we cannot confirm that all admission labs were collected prior to any transfusion. Of note, admission labs were collected within a half-hour from admission, which minimizes this concern. We did not collect information on pharmacological agents such as antiplatelet medications or anticoagulants. We have presented an adjusted analysis alongside others and direct reporting of unadjusted outcomes to provide a complete review of the data. Due to missingness, the final selected model supporting the benefits of LTOWB contains 193 out of the 348 total patients (55%). While these 97 CT and 96 LTOWB patients included in the model had largely similar epidemiology and had no statistically significant nor clinically significant differences in covariates, the LTOWB did have higher median values for all covariates which may be a source of bias. We also did not fit additional models for secondary outcomes, including other mortality timepoints, and as such, we chose this model as informative with the aim of saving further detailed analysis for a subsequent study or trial. While we did not find any acute safety signal, future studies to further characterize long-term effects of transfusion and different blood types throughout survivor lifetime are needed to fully understand any unknown potential impact. This is a hypothesis-generating study based on our single-center experience with implementation of LTOWB.
Conclusions
LTOWB use in hemostatic resuscitation of trauma patients with life-threatening bleeding was independently associated with reduced 24-hour mortality at our center, while also being transfused with a median of 30mL/kg less of 72-hour total blood products. Use of LTOWB had the greatest association with 24-hour survival in patients with shock or who were in a hypocoagulable state. The use of LTOWB in non-group O patients was not associated with an increased risk of adverse events or organ injury. These results support the use of LTOWB in this patient population. Randomized controlled trials are needed to fully examine the safety and efficacy of this LTOWB for this indication.
Supplementary Material
Essentials.
Low-titer group O whole blood (LTOWB) is increasing in use, yet questions about safety/efficacy remain
Massively bleeding trauma patients received either component therapy (CT) or LTOWB in this observational study
LTOWB was associated with improved survival which was most evident in patients with coagulopathy or shock
Use of LTOWB in non-type O patients was safe as measured by survival, adverse events, and organ dysfunction
Acknowledgements
The authors thank the patients. The authors would also like to acknowledge the bedside teams that treated these patients. The authors thank Stephen R. Wisniewski, PhD, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA for a review of the manuscript and feedback on the statistical approach.
Sources of Support
Department of Pediatrics, Washington University in St. Louis
Department of Surgery, University of Pittsburgh
SMS is supported by K25HL161401.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
P.C. Spinella: Consultant for Hemanext, Cerus, Haima, and Co-Founder and Chief Medical Officer for Kalocyte.
The authors have no other conflicts of interest to disclose.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006; 60: S3–11. 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC. Zero preventable deaths after traumatic injury: An achievable goal. J Trauma Acute Care Surg. 2017; 82: S2–S8. 10.1097/TA.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 3.Yazer MH, Cap AP, Spinella PC. Raising the standards on whole blood. J Trauma Acute Care Surg. 2018; 84: S14–S7. 10.1097/TA.0000000000001778. [DOI] [PubMed] [Google Scholar]
- 4.Spinella PC, Cap AP. Whole blood: back to the future. Curr Opin Hematol. 2016; 23: 536–42. 10.1097/MOH.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 5.Guyette FX, Zenati M, Triulzi DJ, Yazer MH, Skroczky H, Early BJ, Adams PW, Brown JB, Alarcon L, Neal MD, Forsythe RM, Zuckerbraun BS, Peitzman AB, Billiar TR, Sperry JL. Prehospital low titer group O whole blood is feasible and safe: Results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2022; 92: 839–47. 10.1097/TA.0000000000003551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brill JB, Tang B, Hatton G, Mueck KM, McCoy CC, Kao LS, Cotton BA. Impact of Incorporating Whole Blood into Hemorrhagic Shock Resuscitation: Analysis of 1,377 Consecutive Trauma Patients Receiving Emergency-Release Uncrossmatched Blood Products. J Am Coll Surg. 2022; 234: 408–18. 10.1097/XCS.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 7.Hashmi ZG, Chehab M, Nathens AB, Joseph B, Bank EA, Jansen JO, Holcomb JB. Whole truths but half the blood: Addressing the gap between the evidence and practice of pre-hospital and in-hospital blood product use for trauma resuscitation. Transfusion. 2021; 61 Suppl 1: S348–S53. 10.1111/trf.16515. [DOI] [PubMed] [Google Scholar]
- 8.Gaines BA, Yazer MH, Triulzi DJ, Sperry JL, Neal MD, Billiar TR, Leeper CM. Low Titer Group O Whole Blood In Injured Children Requiring Massive Transfusion. Ann Surg. 2021. 10.1097/sla.0000000000005251. [DOI] [PubMed] [Google Scholar]
- 9.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009; 66: S69–76. 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seheult JN, Anto V, Alarcon LH, Sperry JL, Triulzi DJ, Yazer MH. Clinical outcomes among low-titer group O whole blood recipients compared to recipients of conventional components in civilian trauma resuscitation. Transfusion. 2018; 58: 1838–45. 10.1111/trf.14779. [DOI] [PubMed] [Google Scholar]
- 11.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003; 17: 223–31. [DOI] [PubMed] [Google Scholar]
- 12.Zielinski MD, Jenkins DH, Hughes JD, Badjie KS, Stubbs JR. Back to the future: the renaissance of whole-blood transfusions for massively hemorrhaging patients. Surgery. 2014; 155: 883–6. 10.1016/j.surg.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Nair PM, Pandya SG, Dallo SF, Reddoch KM, Montgomery RK, Pidcoke HF, Cap AP, Ramasubramanian AK. Platelets stored at 4 degrees C contribute to superior clot properties compared to current standard-of-care through fibrin-crosslinking. Br J Haematol. 2017; 178: 119–29. 10.1111/bjh.14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, Cap AP. Hemostatic function of apheresis platelets stored at 4 degrees C and 22 degrees C. Shock. 2014; 41 Suppl 1: 54–61. 10.1097/SHK.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas KA, Shea SM, Yazer MH, Spinella PC. Effect of leukoreduction and pathogen reduction on the hemostatic function of whole blood. Transfusion. 2019; 59: 1539–48. 10.1111/trf.15175. [DOI] [PubMed] [Google Scholar]
- 16.Meyer DE, Vincent LE, Fox EE, O'Keeffe T, Inaba K, Bulger E, Holcomb JB, Cotton BA. Every minute counts: Time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg. 2017; 83: 19–24. 10.1097/TA.0000000000001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea SM, Staudt AM, Thomas KA, Schuerer D, Mielke JE, Folkerts D, Lowder E, Martin C, Bochicchio GV, Spinella PC. The use of low-titer group O whole blood is independently associated with improved survival compared to component therapy in adults with severe traumatic hemorrhage. Transfusion. 2020; 60 Suppl 3: S2–S9. 10.1111/trf.15696. [DOI] [PubMed] [Google Scholar]
- 22.Cho H, Wendelberger B, Gausche-Hill M, Wang HE, Hansen M, Bosson N, Lewis RJ. ICU-free days as a more sensitive primary outcome for clinical trials in critically ill pediatric patients. J Am Coll Emerg Physicians Open. 2021; 2: e12479. 10.1002/emp2.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 24.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12: 77. 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005; 21: 3940–1. 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 26.Wickham H, Bryan J. Read Excel Files. 2019. [Google Scholar]
- 27.Heinze G, Ploner M, Dunkler D. coxphw: Weighted Estimation in Cox Regression. R package version 4.0.2 edn, 2020. [Google Scholar]
- 28.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. . New York: Springer, 2000. [Google Scholar]
- 29.Wickham H. Ggplot2: Elegant graphics for data analysis. R package version 3.3.3 edn: Springer International Publishing, 2016. [Google Scholar]
- 30.Deo SV, Deo V, Sundaram V. Survival analysis-part 2: Cox proportional hazards model. Indian J Thorac Cardiovasc Surg. 2021; 37: 229–33. 10.1007/s12055-020-01108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph B, Scalea T. The Consequences of Aging On the Response to Injury and Critical Illness. Shock. 2020; 54: 144–53. 10.1097/SHK.0000000000001491. [DOI] [PubMed] [Google Scholar]
- 32.Hildebrand F, Pape HC, Horst K, Andruszkow H, Kobbe P, Simon TP, Marx G, Schurholz T. Impact of age on the clinical outcomes of major trauma. Eur J Trauma Emerg Surg. 2016; 42: 317–32. 10.1007/s00068-015-0557-1. [DOI] [PubMed] [Google Scholar]
- 33.Haider AH, Hashmi ZG, Zafar SN, Castillo R, Haut ER, Schneider EB, Cornwell EE 3rd, Mackenzie EJ, Efron DT. Developing best practices to study trauma outcomes in large databases: an evidence-based approach to determine the best mortality risk adjustment model. J Trauma Acute Care Surg. 2014; 76: 1061–9. 10.1097/TA.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 34.Haider AH, Saleem T, Leow JJ, Villegas CV, Kisat M, Schneider EB, Haut ER, Stevens KA, Cornwell EE 3rd, MacKenzie EJ, Efron DT. Influence of the National Trauma Data Bank on the study of trauma outcomes: is it time to set research best practices to further enhance its impact? J Am Coll Surg. 2012; 214: 756–68. 10.1016/j.jamcollsurg.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein DM, Upperman JS, Livingston DH, Andrews J, Bulger EM, Cohen MJ, Eastridge BJ, Fontaine MJ, Guillamondegui O, Hess JR, Jenkins DH, Kaups KL, Nance ML, Spinella PC, Zarzaur BL, Zonies D, Coimbra R. National blood shortage: A call to action from the trauma community. J Trauma Acute Care Surg. 2022; 93: e119–e22. 10.1097/TA.0000000000003715. [DOI] [PubMed] [Google Scholar]
- 36.Stubbs JR, Homer MJ, Silverman T, Cap AP. The current state of the platelet supply in the US and proposed options to decrease the risk of critical shortages. Transfusion. 2021; 61: 303–12. 10.1111/trf.16140. [DOI] [PubMed] [Google Scholar]
- 37.Spinella PC, Cap AP. Prehospital hemostatic resuscitation to achieve zero preventable deaths after traumatic injury. Curr Opin Hematol. 2017; 24: 529–35. 10.1097/MOH.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 38.Cotton BA, Podbielski J, Camp E, Welch T, del Junco D, Bai Y, Hobbs R, Scroggins J, Hartwell B, Kozar RA, Wade CE, Holcomb JB, Early Whole Blood I. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013; 258: 527–32; discussion 32-3. 10.1097/SLA.0b013e3182a4ffa0. [DOI] [PubMed] [Google Scholar]
- 39.Anand T, Obaid O, Nelson A, Chehab M, Ditillo M, Hammad A, Douglas M, Bible L, Joseph B. Whole blood hemostatic resuscitation in pediatric trauma: A nationwide propensity-matched analysis. J Trauma Acute Care Surg. 2021; 91: 573–8. 10.1097/ta.0000000000003306. [DOI] [PubMed] [Google Scholar]
- 40.Li K, Xu Y. Citrate metabolism in blood transfusions and its relationship due to metabolic alkalosis and respiratory acidosis. Int J Clin Exp Med. 2015; 8: 6578–84. [PMC free article] [PubMed] [Google Scholar]
- 41.Rowell SE, Barbosa RR, Diggs BS, Schreiber MA, Trauma Outcomes G, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, Dutton RP, Hess JR, Duchesne JC, McSwain NE, Muskat P, Johannigamn J, Cryer HM, Tillou A, Cohen MJ, Pittet JF, Knudson P, De Moya MA, Schreiber MA, Tieu B, Brundage S, Napolitano LM, Brunsvold M, Sihler KC, Beilman G, Peitzman AB, Zenait MS, Sperry J, Alarcon L, Croce MA, Minei JP, Kozar R, Gonzalez EA, Stewart RM, Cohn SM, Mickalek JE, Bulger EM, Cotton BA, Nunez TC, Ivatury R, Meredith JW, Miller P, Pomper J, Marin B. Specific abbreviated injury scale values are responsible for the underestimation of mortality in penetrating trauma patients by the injury severity score. J Trauma. 2011; 71: S384–8. 10.1097/TA.0b013e3182287c8d. [DOI] [PubMed] [Google Scholar]
- 42.Yazer MH, Delaney M, Doughty H, Dunbar NM, Al-Riyami AZ, Triulzi DJ, Watchko JF, Wood EM, Yahalom V, Emery SP. It is time to reconsider the risks of transfusing RhD negative females of childbearing potential with RhD positive red blood cells in bleeding emergencies. Transfusion. 2019; 59: 3794–9. 10.1111/trf.15569. [DOI] [PubMed] [Google Scholar]
- 43.Andrews J, Josephson CD, Young P, Spinella PC, Yazer MH. Weighing the risk of hemolytic disease of the newborn versus the benefits of using of RhD-positive blood products in trauma. Transfusion. 2023. 10.1111/trf.17352. [DOI] [PubMed] [Google Scholar]
- 44.Yu G, Siegler J, Hayes J, Yazer MH, Spinella PC. Attitudes of American adult women toward accepting RhD-mismatched transfusions in bleeding emergencies. Transfusion. 2022; 62 Suppl 1: S211–S7. 10.1111/trf.16981. [DOI] [PubMed] [Google Scholar]
- 45.Morgan KM, Lobo R, Annen K, Villarreal RI, Chou S, Uter S, Leonard JC, Dyer C, Yazer M, Spinella PC, Leeper CM. Parent perceptions of emergent blood transfusion in children. Transfusion. 2023. 10.1111/trf.17334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.