SUMMARY
CD4+ T cells are key components of the immune response during lung infections and can mediate protection against tuberculosis (TB) or influenza. However, CD4+ T cells can also promote lung pathology during these infections, making it unclear how these cells control such discrepant effects. Using mouse models of hypervirulent TB and influenza, we observe that exaggerated accumulation of parenchymal CD4+ T cells promotes lung damage. Low numbers of lung CD4+ T cells, in contrast, are sufficient to protect against hypervirulent TB. In both situations, lung CD4+ T cell accumulation is mediated by CD4+ T cell-specific expression of the extracellular ATP (eATP) receptor P2RX7. P2RX7 upregulation in lung CD4+ T cells promotes expression of the chemokine receptor CXCR3, favoring parenchymal CD4+ T cell accumulation. Our findings suggest that direct sensing of lung eATP by CD4+ T cells is critical to induce tissue CD4+ T cell accumulation and pathology during lung infections.
Graphical Abstract
In brief
Santiago-Carvalho et al. demonstrate that, in response to severe lung infections, sensing of extracellular ATP through P2RX7 leads to exacerbated numbers of lung parenchymal CD4+ T cells that promote pathology and impair host survival. Their results suggest a possible explanation for how tissue danger signals can inadvertently support pathogenic lung immune responses.
INTRODUCTION
The magnitude of immunopathology caused by infectious diseases is linked to the intensity and duration of the immune response.1 The strength of the immune response is also determined by tissue sensitivity to damage and its regenerative capacity. In the lung there is a fine line between effective or harmful immune responses.1,2 A dysregulated lung inflammatory response can cause severe tissue damage and compromise respiratory physiology.2,3 CD4+ T cells are key players in the immune response of lung diseases,4-7 which include infections such as tuberculosis (TB), one of the world’s deadliest bacterial diseases,8 and influenza virus, a major inducer of patient hospitalizations and deaths worldwide.9 During these infections, accumulation of distinct CD4+ T cell subsets mediates protection or worsening of the disease.10,11 Effector CD4+ T cells can either remain in the circulation and populate the lung intravascular regions or infiltrate the lung parenchyma.7,12 The T cell-intrinsic mechanism underlying these homing choices is based on differential chemokine receptor expression. In response to virulent H37Rv Mycobacterium tuberculosis (Mtb), CD4+ T cells expressing the fractalkine receptor CX3CR1 stay within the vasculature and produce high levels of interferon-γ (IFN-γ), while cells expressing the CXC motif chemokine receptor 3 (CXCR3) establish parenchymal residency and are more effective in containing infection, despite lower IFN-γ production on a per-cell basis.7,13 CXCR3 also promotes lung CD4+ T cell residency in response to influenza.14 The balance between protective immune responses promoted by CD4+ T cells, which are by definition pleiotropic, and their unintended effects in the lung parenchyma defines whether parenchymal CD4+ T cells are protective or harmful.15
The influence of lung microenvironmental factors in the establishment of parenchymal CD4+ T cells is less understood. Influenza and Mtb infect distinct parenchymal cells (epithelial cells and macrophages, respectively) and consequently induce different local immune responses and changes in the lung microenvironment.16,17 Mtb often induces the generation of complex immune/parenchymal cell structures called granulomas,18,19 while flu leads to diffuse accumulation of immune cells around infected areas.20,21 In both infections, however, inflammatory responses are triggered by pathogen load, local cell death, and immune cell infiltration. An important inflammation primer is the release of damage-associated molecular patterns (DAMPs), which contribute to the dysregulation of lung microenvironment and immune responses.22,23 Extracellular ATP (eATP) is one of the most abundant DAMPs released in these processes.24,25 High eATP levels are recognized by the low-affinity purinergic receptor P2RX7, which is mostly expressed on immune cells, including CD4+ T cells.26-28 We have previously demonstrated that P2RX7 expression in hematopoietic cells contributes to severe pulmonary TB disease.29,30 Moreover, pharmacological blockade of P2RX7 prevents the onset of severe TB.31 The biomedical importance of P2RX7 as a promoter of lung disease is further suggested by a previous report in influenza-infected global P2RX7-knockout (KO) mice.32 Whether P2RX7 serves as a microenvironmental sensor for lung CD4+ T cell accumulation, and whether P2RX7-expressing CD4+ T cells are the main promoters of lung damage rather than other cell types, are not understood. We have found that transient P2RX7 blockade led to reduced numbers of CD69+ lung parenchymal CD4+ T cells and concomitant protection from disease.31 P2RX7 is known to play an important role in the establishment of tissue-resident memory (TRM) CD8+ T cells in response to systemic viruses.33,34
Here, we report that cell-intrinsic P2RX7 promotes the lung parenchymal accumulation of effector CD4+ T cells in response to TB and influenza. Exaggerated (but not low) numbers of P2RX7+ parenchymal CD4+ T cells lead to lung inflammation and severe disease. Our data indicate that P2RX7-expressing CD4+ T cells display increased ability to infiltrate lung parenchyma in a CXCR3-dependent way, contributing to their increased tissue accumulation. Overall, our results suggest that sensing of lung-derived eATP is crucial to inducement of parenchymal CD4+ T cell establishment and exemplifies how the lung microenvironment shapes tissue-localized CD4+ T cell responses.
RESULTS
Parenchymal CD4+ T cells aggravate pulmonary TB caused by hypervirulent mycobacteria
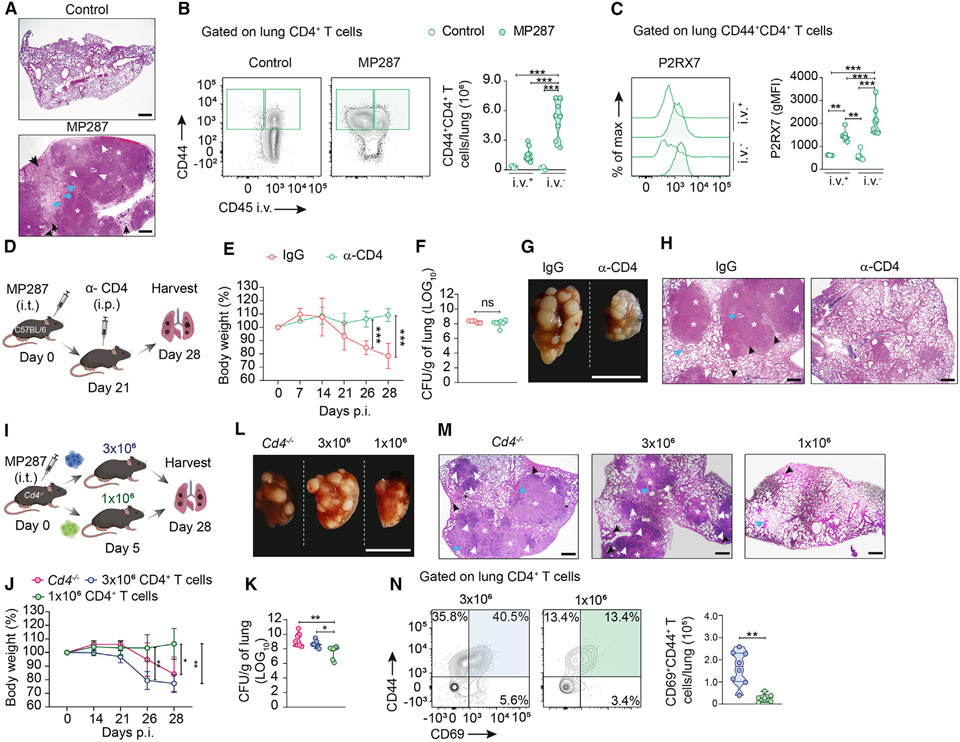
We first infected C57BL/6 (wild-type [WT]) mice with the hypervirulent mycobacterial Mycobacterium bovis (Mbv) MP287 strain.30,31 At day 28 post infection (p.i.), MP287 infection induced severe lung damage associated with extensive inflammatory infiltrates, granulomatous necrotic lesions, and areas of alveolitis with bronchial obstruction (Figure 1A). Alveoli were filled with inflammatory components, including innate immune cells, lymphocytes, fibrin, and perifocal exudative reaction areas surrounding TB foci. We then assessed intravascular and parenchymal CD44+CD4+ T cells in the lungs using intravenous anti-CD45 labeling for their distinction12 (gate strategy in Figure S1A). At 28 days p.i., we observed increased numbers of parenchymal (CD45i.v.−) compared to intravascular (CD45i.v.+) lung CD4+ T cells (Figure 1B). Phenotypic analysis of antigen-experienced (CD44+) CD4+ T cells revealed that KLRG1 and CX3CR1 were mostly expressed on intravascular cells, while CD69 and CXCR3 were primarily expressed on parenchymal cells (Figures S2A-S2C), similar to what was previously described during Mtb H37Rv infection.7 Lung parenchymal CD4+ T cells expressed higher levels of P2RX7 than intravascular cells in MP287-infected mice (Figure 1C).
Figure 1. Excessive accumulation of parenchymal CD4+ T cells induces lung severe disease in response to hypervirulent mycobacteria.
C57BL/6 (WT) or CD4-KO mice (Cd4−/−) were infected with ~100 CFU of Mbv MP287 strain. Non-infected mice were used as controls.
(A) Representative lung sections of WT mice stained with hematoxylin and eosin (H&E). White asterisks indicate necrotic areas, white arrows indicate alveolitis, blue arrows indicate bronchial obstruction, and black arrows indicate perifocal exudative reaction. Scale bars, 500 μm.
(B) Left: flow-cytometry plots of CD44 and CD45i.v. in WT CD4+ T cells. Right: average numbers of CD45i.v.+ and CD45i.v.− CD44+CD4+ T cells/lung.
(C) Left: histograms of P2RX7 expression in CD45i.v.+ and CD45i.v.− WT CD44+CD4+ T cells. Right: average geometric mean fluorescence intensity (gMFI) of P2RX7.
(D) CD4+ T cell depletion protocol (BioRender.com). Infected WT mice were treated with control immunoglobulin G or α-CD4 antibody.
(E) Average body weights (percentages related to day 0) of mice from (D).
(F) Lung CFU numbers of mice from (D).
(G) Macroscopic images of right upper lung lobes of mice from (D). Scale bar, 1 cm.
(H) Representative H&E lung sections of mice from (D). Scale bars, 500 μm.
(I) Adoptive transfer protocol (BioRender.com). Infected CD4-KO mice were transferred with 1 × 106 or 3 × 106 WT CD4+ T cells.
(J) Average body weights (percentages related to day 0) of mice from (I).
(K) Lung CFU numbers of mice from (I).
(L) Macroscopic images of right upper lung lobes of mice from (I). Scale bar, 1 cm.
(M) Representative lung sections stained with H&E of mice from (I). Scale bars, 500 μm.
(N) Left: flow-cytometry plots of CD44 and CD69 in CD4+ T cells of mice from (I). Right: average numbers of CD45i.v.+ and CD45i.v.− CD44+CD4+ T cells/lung.
Data from three independent experiments, n = 3–5/experimental group per experiment. Data shown as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA (Tukey post tests) (B, C, J, and K) or unpaired t tests (E, F, and N).
The contribution of CD44+CD4+ T cells to the worsening of TB was assessed by depleting CD4+ T cells with a high dose of α-CD4 antibody on day 21 p.i. (Figures 1D and S2D), when disease onset occurs in MP287-infected mice.29 CD4+ T cell depletion prevented weight loss induced by MP287 infection (Figure 1E). Despite this, CD4+ T cell depletion did not change pulmonary colony-forming units (CFU) (Figure 1F). Lung weights and total cell numbers were reduced in CD4+ T cell-depleted mice (Figures S2E and S2F). The size of the upper right lobe of the lung and the presence of granuloma nodules were also decreased by CD4+ T cell depletion (Figure 1G) as well as areas of pneumonia, alveolitis, bronchial obstruction, and necrosis (Figure 1H).
To investigate whether lung parenchymal CD4+ T cells quantitatively regulate the onset of severe TB in mice, MP287-infected CD4-KO (Cd4−/−) were adoptively transferred with intermediate (3 × 106) or low (1 × 106) numbers of CD4+ T cells (Figures 1I and S2G). The CD4+ T cell numbers were considered intermediate or low in comparison to those of infected WT mice. Both non-transferred and 3 × 106 CD4+ T cell-transferred CD4-KO mice lost significant body weight and developed severe disease compared to mice that received lower cell numbers, which were more efficient in controlling lung damage and bacterial load (Figures 1J-1M, S2H, and S2I). As expected, the lung CD69+CD44+CD4+ T cell population was larger in infected mice that received 3 × 106 CD4+ T cells (Figure 1N). Of note, TB in CD4-KO mice transferred with 1 × 106 CD4+ T cells was much less severe than in WT mice (Figure 1A). These results suggest that excessive accumulation of lung parenchymal CD4+ T cells contributes to severe TB disease caused by hypervirulent mycobacteria.
T cell-specific P2RX7 promotes lung parenchymal CD4+ T cell accumulation in response to hypervirulent mycobacteria
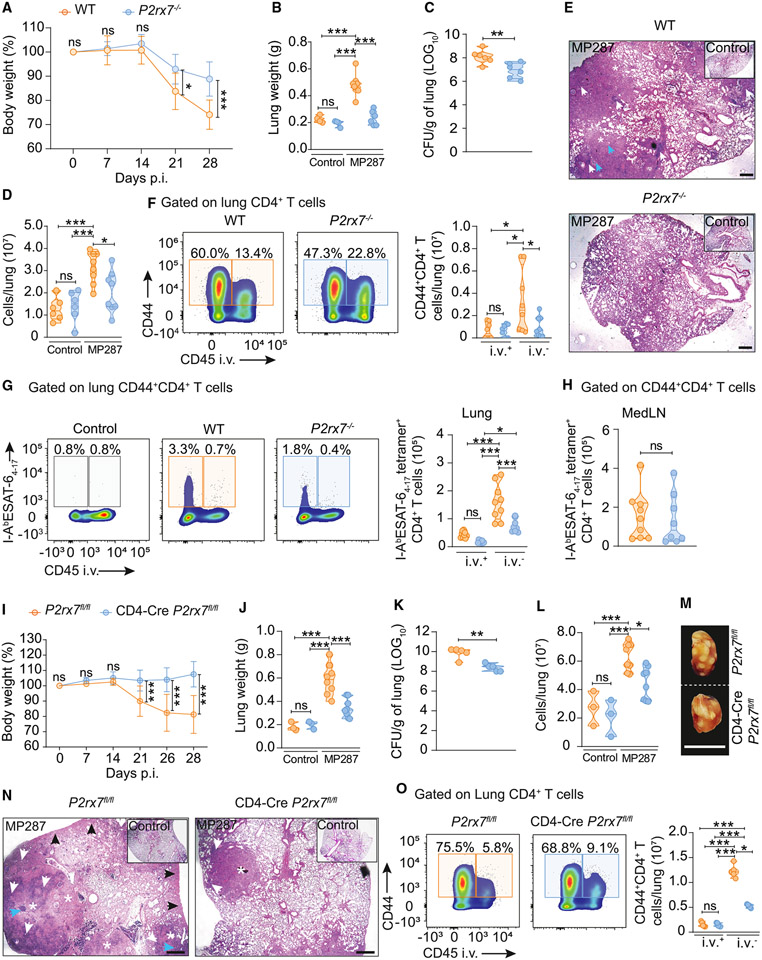
We have previously defined that P2RX7 promotes CD8+ T cell residency in non-lymphoid tissue,33,34 but whether the same is true for CD4+ T cells is less clear. Therefore, the lung parenchymal and intravascular CD4+ T cell responses to severe TB were investigated in WT and P2RX7-KO (P2rx7−/−) mice. P2RX7-KO mice displayed decreased body weight loss, lighter lungs, less lung cellularity, and milder lung pathology compared to WT mice (Figures 2A-2E). The numbers of lung parenchymal bulk CD4+ T cells were reduced in infected P2RX7-KO mice compared to infected WT mice, while comparable numbers were observed in the lung vasculature (Figure 2F). Infected P2RX7-KO mice also had reduced numbers of I-AbESAT-64-17 TB tetramer-specific CD4+ T cells in the lung parenchyma (Figure 2G), while no difference was observed in mediastinal lymph nodes (medLNs) (Figures 2H and S3A). P2RX7 promotes the survival of regulatory T cells (Tregs),35 but Foxp3+CD4+ T cell numbers in the lung parenchyma of infected WT and P2RX7-KO mice were equal (Figures S1 and S2B).
Figure 2. T cell-specific P2RX7 promotes lung parenchymal CD4+ T cell accumulation and severe TB caused by hypervirulent mycobacteria.
WT (C57BL/6 or P2rx7fl/fl), P2RX7-KO (P2rx7−/−), or T cell-P2RX7-KO (CD4-Cre P2rx7fl/fl) mice were infected with ~100 CFU of Mbv MP287. Non-infected mice were used as controls.
(A and I) Percentages of body weights in relation to day 0 of WT, P2RX7-KO, or T cell-P2RX7-KO mice.
(B and J) Lung weight values of WT, P2RX7-KO, or T cell-P2RX7-KO mice.
(C and K) CFU numbers per lung of WT, P2RX7-KO, or T cell-P2RX7-KO mice.
(D and L) Total lung cell numbers of WT, P2RX7-KO, or T cell-P2RX7-KO mice.
(E) Representative lung sections stained with H&E of WT or P2RX7-KO mice. Scale bars, 500 μm.
(F) Left: flow-cytometry plots of CD44 and CD45i.v. in lung CD4+ T cells. Right: average numbers of CD45i.v.+ and CD45i.v.− CD44+CD4+ T cells/lung.
(G) Left: flow-cytometry plots of I-AbESAT-64-17 and CD45i.v. expression in lung CD4+ T cells. Right: average numbers of CD45i.v.+ and CD45i.v.− I-AbESAT-64-17+CD44+CD4+ T cells/lung.
(H) Average numbers of medLN I-AbESAT-64-17+CXCR3+CD4+ T cells.
(M) Macroscopic images of right upper lung lobes of WT and T cell-P2RX7-KO mice. Scale bars, 1 cm.
(N) Representative lung sections stained with H&E of WT and T cell-P2RX7-KO mice. Scale bars, 500 μm.
(O) Left: flow-cytometry plots of CD44 and CD45i.v. in lung CD4+ T cells of WT and T cell-P2RX7-KO mice. Right: average numbers of CD45i.v.+ and CD45i.v.− CD44+CD4+T cells/lung.
Data from three independent experiments: n = 3–5/experimental group per experiment. Data shown as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA (Tukey post tests) (B, D, F, G, J, L, and O) or unpaired t tests (A, C, H, I, and K).
In addition to CD4+ T cells (Figure 1C), innate immune cells also express P2RX7 in the lungs of MP287-infected WT mice29,30 and can mediate lung CD4+ T cell accumulation in severe TB. To evaluate whether P2RX7-mediated lung parenchymal CD4+ T cell accumulation is T cell intrinsic or not, we used a T cell-specific P2RX7-KO (T cell-P2RX7-KO; CD4-CreP2rx7fl/fl) model. After MP287 infection, T cell-P2RX7-KO mice were resistant to severe TB disease if compared to WT mice (P2rx7fl/fl), evidenced by decreased values of body weight loss (Figure 2I), lung weight (Figure 2J), CFU (Figure 2K), lung cellularity (Figure 2L), and lung inflammation (Figures 2M and 2N). Finally, T cell-P2RX7-KO mice had significantly lower numbers of lung parenchymal CD4+ T cells in comparison to WT mice (Figure 2O). Together, these data indicate that T cell-intrinsic P2RX7 promotes the accumulation of lung parenchymal CD4+ T cells and subsequent development of severe TB in response to MP287 infection.
P2RX7 is required for restricted numbers of lung parenchymal CD4+ T cells to protect against hypervirulent mycobacteria
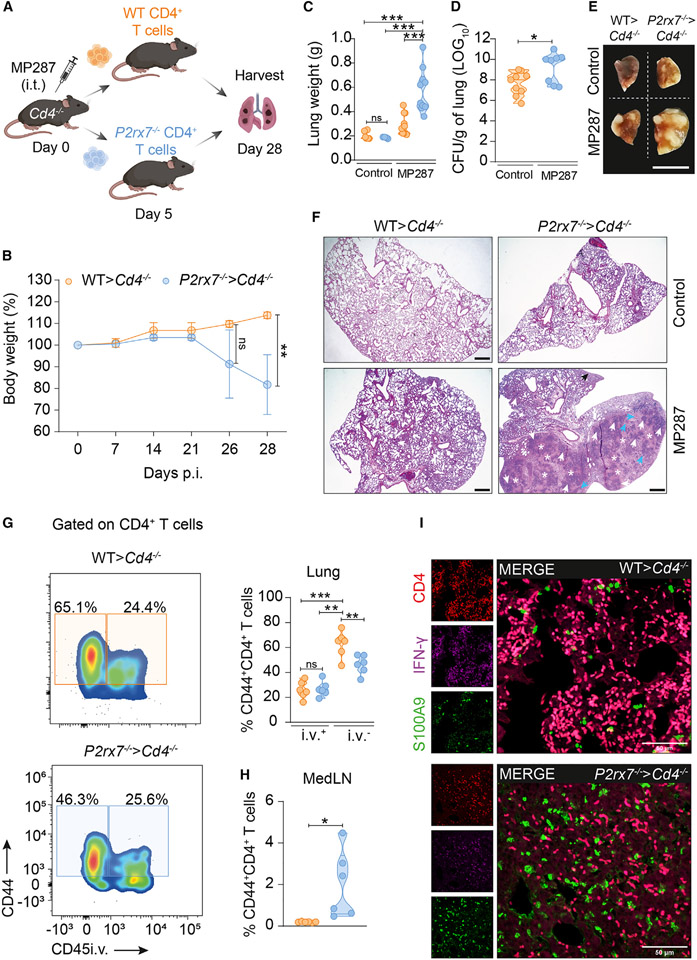
We then investigated whether the previously observed protective role of restricted accumulation of lung parenchymal CD4+ T cells (Figures 1I-1N) is controlled by P2RX7. MP287-infected CD4-KO hosts were adoptively transferred with 1 × 106 WT CD4+ T cells (WT>Cd4−/−) or P2RX7-KO CD4+ T cells (P2rx7−/−>Cd4−/−) (Figure 3A). P2rx7−/− > Cd4−/− progressively lost body weight from day 21 p.i., which was not observed for WT>Cd4−/− mice (Figure 3B). Likewise, we found increased lung weights in P2rx7−/−>Cd4−/− mice compared to WT>Cd4−/− mice (Figure 3C). Supporting a crucial role for P2RX7 in CD4+ T cell effector function, higher lung bacterial loads were observed in P2rx7−/−>Cd4−/− mice (Figure 3D) as well as increased numbers of granulomatous lesions and necrotic areas (Figures 3E and 3F).
Figure 3. Restricted numbers of P2RX7-KO CD4+ T cells fail to transfer protection against severe TB.
CD4-KO (Cd4−/−) mice were infected with ~100 CFU of Mbv MP287. Non-infected and infected CD4-KO mice were used as controls. At day 5 p.i., mice received splenic CD4+ T cells (1 × 106) from WT and P2RX7-KO (P2rx7−/−) mice.
(A) Experimental protocol (BioRender.com).
(B) Average body weights (percentages related to day 0).
(C) Lung weights/mice.
(D) Lung CFU numbers.
(E) Macroscopic images of right upper lung lobes. Scale bar, 1 cm.
(F) Representative lung sections stained with H&E. Scale bars, 500 μm.
(G) Left: flow-cytometry plots of CD44 and CD45i.v. on CD4+ T cells. Right: average frequencies of CD45i.v.+ and CD45i.v.− cells in CD44+CD4+ T cells.
(H) Average numbers of CD44+CD4+ T cells in the medLNs.
(I) Representative confocal images of CD4, IFN-γ, and S100A9 expression in infected lung tissues. Scale bars, 50 μm.
Data from three independent experiments, n = 3–5/experimental group per experiment. Data shown as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA (Tukey post tests) (C, D, and G) or unpaired t tests (B and H).
We next characterized CD44+CD4+ T cells from the lung parenchyma, lung vasculature, and medLN of WT>Cd4−/− and P2rx7−/−>Cd4−/− mice. WT cells were preferentially found in lung parenchyma compared to vasculature, while lower frequencies of lung parenchymal P2RX7-KO cells were present (Figure 3G). In contrast, P2RX7-KO cells were significantly increased in medLNs compared to WT cells (Figure 3H). Moreover, fewer P2RX7-KO IFN-γ-producing CD4+ T cells infiltrated the lungs of infected mice compared to WT cells (Figure 3I). In addition, S100A9 alarmin signal was more abundant in infected P2rx7−/−>Cd4−/− mice (Figure 3I), suggesting increased lung tissue damage. These results indicate that CD4+ T cell-intrinsic P2RX7, despite promoting lung damage when exaggeratedly present in the lung parenchyma, is paradoxically required for restricted numbers of CD4+ T cells to protect against MP287 infection.
Cell-intrinsic P2RX7 expression leads to increased numbers of proliferating CXCR3+CD4+ T cells in the MP287-infected lung parenchyma
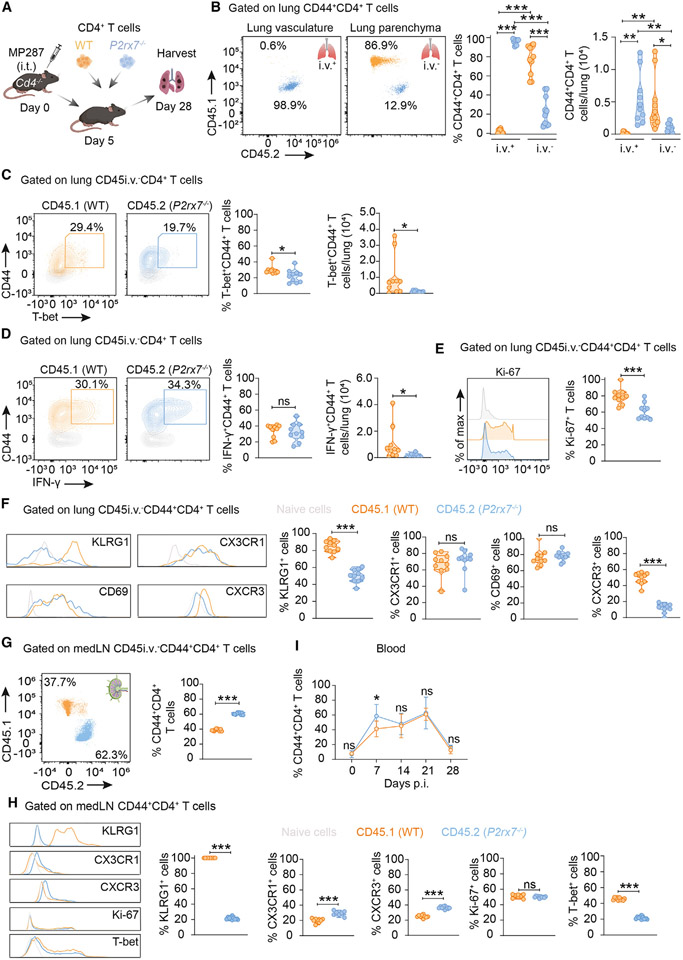
To evaluate how WT and P2RX7-KO CD4+ T cells respond to MP287 infection in a competitive environment, WT (CD45.1+) and P2RX7-KO (CD45.2+) CD4+ T cells were co-transferred to CD4-KO mice at 5 days p.i. (Figure 4A). On day 28 p.i., WT CD4+ T cells accumulated mainly in the lung parenchyma, whereas P2RX7-KO counterparts predominated in the lung vasculature (Figure 4B). Additionally, lung parenchymal WT CD4+ T cells had higher T-bet expression frequency and total numbers compared to P2RX7-KO counterparts (Figures 4C and S1B). No difference was observed in the frequencies of WT and P2RX7-KO lung IFN-γ+ CD4+ T cells, but lung WT cells had higher numbers of this population (Figures 4D and S1B).
Figure 4. Cell-intrinsic P2RX7 is required for CD4+ T cell lung parenchymal establishment in response to severe TB.
CD4-KO (Cd4−/−) mice were infected with ~100 CFU of the Mbv MP287 strain. On day 5 p.i., splenic CD4+ T cells (1 × 106) from WT (CD45.1+) and P2RX7-KO (CD45.2+; P2rx7−/−) mice were co-transferred (1:1) to infected CD4-KO mice.
(A) Experimental protocol (BioRender.com).
(B) Left: flow-cytometry plots of CD45.1 and CD45.2 on CD45i.v.+ and CD45i.v.− CD44+CD4+ T cells. Right: average frequencies and numbers of CD45i.v.+ and CD45i.v.− CD44+CD4+ T cells.
(C) Left: flow-cytometry plots of CD44 and T-bet on CD45i.v.−CD4+ T cells. Right: average frequencies and numbers of lung intravascular and parenchymal T-bet+CD45i.v.−CD44+CD4+ T cells.
(D) Left: flow-cytometry plots of CD44 and IFN-γ on CD45i.v.−CD4+ T cells. Right: average frequencies and numbers of lung intravascular and parenchymal IFN-γ+CD45i.v.−CD44+CD4+ T cells.
(E) Left: histograms showing Ki-67 on CD45i.v.−CD44+CD4+ T cells. Right: average frequencies of CD45i.v.−Ki-67+CD4+ T cells.
(F) Left: histograms of KLRG1, CX3CR1, CD69 and CXCR3 in CD45i.v.−CD44+CD4+ T cells. Right: average frequencies of KLRG1+, CX3CR1+, CD69+ and CXCR3+ CD45i.v.−CD44+CD4+ T cells.
(G) Left: flow cytometry plots of CD45.1 and CD45.2 on CD45i.v.−CD44+CD4+T cells in the medLNs. Right: average frequencies of CD45i.v.−CD44+CD4+T cells in medLNs.
(H) Left: histograms of KLRG1, CX3CR1, CD69 and CXCR3 in CD45i.v.−CD44+CD4+ T cells in medLNs. Right: average frequencies of KLRG1+, CX3CR1+, CD69+ and CXCR3+ CD45i.v.−CD44+CD4+ T cells in medLNs.
(I) Average frequency of CD44+CD4+ T cells in the blood over the weeks after infection.
Data from two independent experiments, n = 5/experimental group per experiment. Data shown as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA (Tukey post tests) (B) or unpaired t tests (C–I).
We also analyzed cell-proliferation rates of parenchymal CD44+CD4+ T cells in response to MP287 infection. P2RX7-KO cells showed lower positivity for the proliferation marker Ki-67 (Figure 4E). We then evaluated the expression of molecules associated with tissue homing. In the lung parenchyma, WT cells expressed higher levels of CXCR3 and KLRG1 compared to P2RX7-KO cells, whereas CD69 and CX3CR1 were at similar levels (Figure 4F). Considering the low frequency (0.09%) of WT CD44+CD4+ T cells in the lung vasculature, it was not feasible to compare the expression of surface molecules in the intravascular compartment.
To investigate whether the effects of P2RX7 on CD4+ T cells were induced prior to lung infiltration, WT and P2RX7-KO CD4+ T cells were analyzed in the medLNs and blood of co-transferred, MP287-infected CD4-KO mice. P2RX7-KO cells were over-represented in the medLNs compared to WT cells (Figure 4G), but the frequencies of T-bet+ and KLRG1+ cells were higher among the WT cell population (Figure 4H). In contrast to the lung, medLN P2RX7-KO cells expressed higher levels of CXCR3 and CX3CR1 than WT cells, making it unlikely that their lung phenotypes were acquired during priming. No difference was observed in WT and P2RX7-KO cell proliferation in the medLNs. In the blood, the frequency of P2RX7-KO cells is higher on day 7 p.i., but no difference was observed over subsequent weeks (Figure 4I). Together, these results demonstrate that both WT and P2RX7-KO CD4+ T cells leave medLNs with the ability to localize either in the vasculature or in the lung parenchyma during MP287 infection, but cell-intrinsic P2RX7 expression leads to accumulation of proliferating CXCR3+CD4+ T cells in the lung parenchyma.
P2RX7 signaling promotes the establishment of lung parenchymal CD4+ T cells in response to H37Rv virulent mycobacteria
Previous studies in P2RX7-KO mice reported a protective or no role for P2RX7 during infection with the virulent Mtb H37Rv strain,30,36,37 but no analysis has been conducted in WT and P2RX7-KO CD4+ T cells using this strain, specifically in a competitive setup. Using co-transfer of WT and P2RX7-KO CD4+ T cells to H37Rv-infected CD4-KO mice (Figure S4A), we found that lung parenchymal CXCR3+ WT CD4+ T cells were more abundant than P2RX7-KO counterparts (Figures S4B and S4C). In contrast, P2RX7-KO CD4+ T cells had increased accumulation in the lung vasculature (Figure S4B) and medLNs (Figure S4D). These data show that even in response to non-hypervirulent mycobacteria, P2RX7 favors CD4+ T cell establishment in the lung parenchyma. Thus, the contribution of P2RX7 in mild TB may be less evident than in severe disease, ranging from a protective role to no role, possibly because a small- to intermediate-sized population of lung parenchymal CD4+ T cells is equally efficient in controlling mycobacteria.
T cell-intrinsic P2RX7 promotes the accumulation of lung parenchymal CD4+ T cells during severe influenza infection
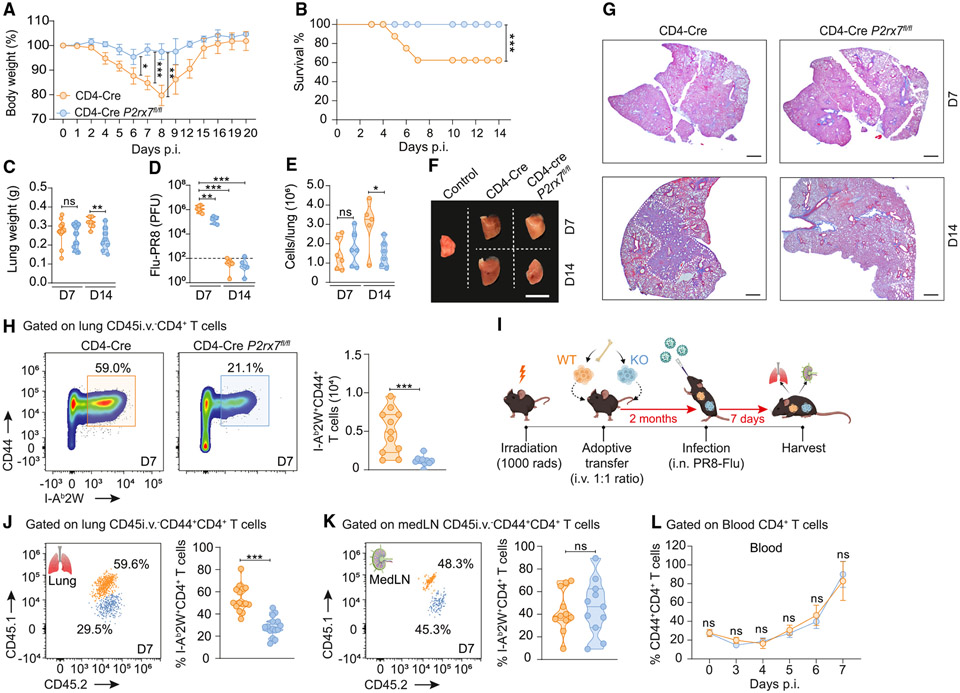
We then tested whether the role of CD4-specific P2RX7 in response to TB is infection specific or is generalizable to other lung infections, using influenza virus (PR8 strain) expressing the model antigen I-Ab-2W.38 T cell-P2RX7-KO mice infected with influenza lost less body weight (Figure 5A), had 100% survival in comparison to 50% in WT mice (Figure 5B), and had decreased lung weight compared to WT mice (Figure 5C). On day 7 p.i., lung viral loads were higher in WT mice than in T cell-P2RX7-KO mice, with no differences at day 14 p.i. (Figure 5D). Lower cell infiltration (Figure 5E) and smaller lung sizes (Figure 5F) were also observed in T cell-P2RX7-KO mice. This indicates faster recovery of lung tissue, which is further evidenced by reduced lung inflammation and fibrosis in T cell-P2RX7-KO mice (Figure 5G). The location of PR8-specific CD4+ T cells in infected lungs was determined during acute disease (day 7 p.i.) rather than after viral control, when memory immune responses are already initiated and could be a confounding factor. WT mice had increased numbers of lung parenchymal flu-specific CD4+ T cells compared to T cell-P2RX7-KO mice (Figure 5H). The decreased lung pathology observed in T cell-P2RX7-KO mice was not due to increased accumulation of lung Tregs, since this population was larger at day 7 p.i. in WT mice (Figure S5A).
Figure 5. T cell-intrinsic P2RX7 promotes the accumulation of lung parenchymal CD4+ T cells and lung inflammation in response to influenza virus.
WT (CD4-Cre) and T cell-P2RX7-KO (CD4-Cre P2rx7fl/fl) mice were infected with influenza virus (PR8-2W, LD50 ~ 1,400 plaque-forming units).
(A) Average body weights (related to day 0).
(B) Survival curves over time.
(C) Average lung weights.
(D) Average viral loads.
(E) Average lung cell numbers.
(F) Macroscopic images of right upper lung lobes. Scale bar, 1 cm.
(G) Representative lung sections stained with Masson’s trichrome. Scale bars, 20 μm.
(H) Left: flow-cytometry plots of CD44 and CD45i.v. on I-Ab2W+CD4+ T cells. Right: average numbers of CD45i.v.+ and CD45i.v.− I-Ab2W+CD44+CD4+ T cells.
(I–L) In some experiments, WT (CD45.1+) mice were lethally irradiated and reconstituted with a 1:1 mix of WT (CD45.1/2+) and T cell-P2RX7-KO (CD45.2+) bone marrow cells. After 2 months, mice were infected with PR8-2W.
(I) Experimental protocol (BioRender.com).
(J) Left: flow-cytometry plots of CD45.1 and CD45.2 on lung CD45i.v.− I-Ab2W+CD4+CD44+ T cells. Right: average frequencies of CD45.1+ and lung CD45.2+ I-Ab2W+CD4+CD44+ T cells. (K) Left: flow-cytometry plots of CD45.1 and CD45.2 on medLN I-Ab2W+CD4+CD44+ T cells. Right: average frequencies of medLN CD45.1+ and CD45.2+ I-Ab2W+CD4+CD44+ T cells. (L) Average frequency of CD44+CD4+ T cells in the blood over acute infection.
Data from three independent experiments, n = 3–5/experimental group per experiment. Data shown as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA (Tukey post tests) (C–E), log-rank survival analysis (B), or unpaired t tests (A, H, and J–L).
To test the role of P2RX7 for lung CD4+ T cell accumulation in response to influenza in a competitive setup, mixed bone marrow chimeras33 were generated and infected with PR8-2W (Figures 5I and S5B). At day 7 p.i., flu-specific WT CD4+ T cells were enriched in the lung parenchyma compared to P2RX7-KO counterparts (Figure 5J). No significant difference between WT and P2RX7-KO cells in the medLNs and blood was found (Figures 5K and 5L). Together, these data demonstrate that T cell-intrinsic P2RX7 leads to lung parenchymal CD4+ T cell establishment and exacerbation of lung inflammation in response to influenza.
P2RX7 promotes the transcription of genes related to tissue-tropic chemotaxis in response to influenza
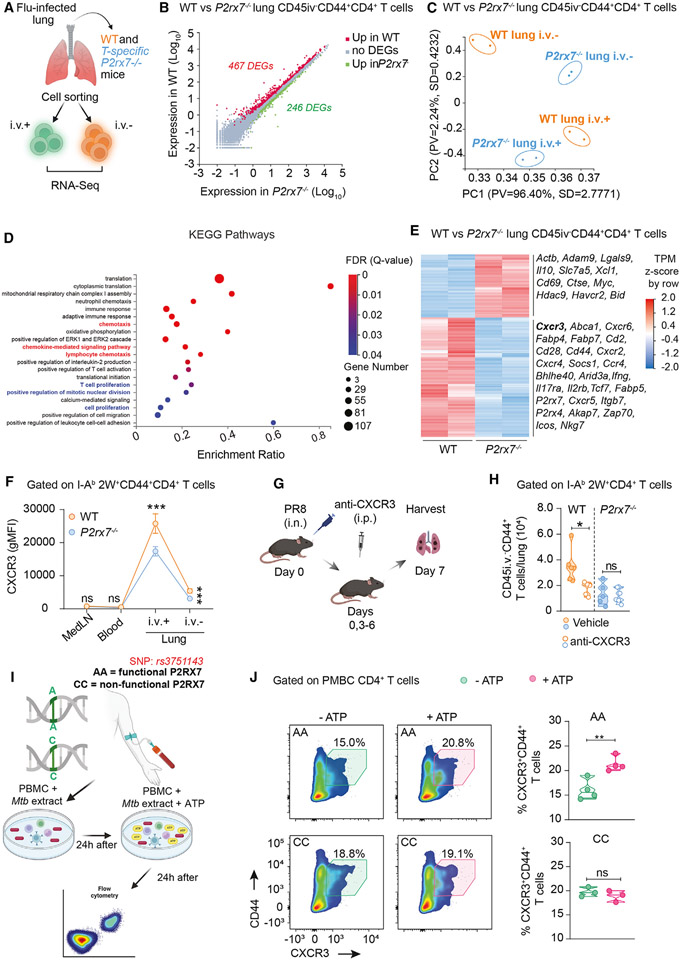
Next, we assessed the molecular pathways controlled by P2RX7 in lung CD4+ T cells. We sorted lung CD45i.v.−, lung CD45i.v.+, and spleen CD4+ T cells from PR8-infected mice for RNA-sequencing (RNA-seq) analysis (Figure 6A). We detected >700 differentially expressed genes between WT and T cell-P2RX7-KO cells in i.v.+ and i.v.− lung compartments, and <100 genes between spleen cell populations (Figures 6B, S5C, and S5D). The few forming lung parenchymal P2RX7-KO cells clustered apart from lung parenchymal WT cells in principle component analysis (Figure 6C). We then performed pathway enrichment analyses of these experimental groups. In comparison to lung parenchymal P2RX7-KO cells, WT cells had increased expression of genes related with migration and cell infiltration such as chemokine-mediated signaling and lymphocyte chemotaxis pathways (Figure 6D). Indeed, several chemokine receptor genes were upregulated in parenchymal WT CD4+ T cells, such as Cxcr3, Cxcr2, Cxcr4, Cxcr5, and Cxcr6 (Figure 6E). All these chemokine receptors are associated with the establishment of CD4+ T cells in the lung parenchyma, but CXCR3 is considered crucial for the entry and residence of these cells in the tissue.7,14 We also observed that cell proliferation and cell survival (metabolism, cell activation, and adaptive response) pathways were increased in WT CD4+ T cells, and this may also contribute to accumulation in the lung parenchyma.
Figure 6. CXCR3 mediates the P2RX7-induced lung CD4+T cell accumulation in influenza and is upregulated by P2RX7/eATP in human CD4+ T cells.
WT (CD4-Cre) and T cell-P2RX7-KO (CD4-Cre P2rx7fl/fl) mice were infected with PR8-2W. At day 7 p.i., lung CD45i.v.− and CD45i.v.+ cells and spleen CD44+CD4+ T cells were sorted and RNA-seq analysis performed.
(A) Experimental protocol (BioRender.com).
(B) Scatterplot with differentially expressed genes (DEGs) highlighted between WT and P2RX7-KO lung CD45i.v.−CD4+ T cells.
(C) Principal component (PC) analysis plots representing the relative transcriptional signatures of WT and P2RX7-KO lung CD45i.v.− and CD45i.v.+ CD4+ T cells.
(D) Transcriptional pathways enriched in lung WT CD45i.v.−CD4+ T cells in comparison to P2RX7-KO counterparts.
(E) Heatmap of DEGs between WT and P2RX7-KO lung CD45i.v.−CD4+ T cells; representative DEGs higher on WT or P2RX7-KO CD4+ T cells are shown.
(F) Average gMFI values of CXCR3 on I-Ab2W+CD45i.v.−CD44+CD4+ T cells in the indicated organs at day 7 p.i.
(G) Experimental protocol (BioRender.com). WT and P2RX7-KO mice were infected with influenza and treated or not intraperitoneally with anti-CXCR3 antibody on days 0, 3, 4, and 6 of infection.
(H) Average numbers of CD45i.v.−CD44+CD4+ T cells per lung.
(I) Experimental protocol (BioRender.com). PBMCs (1 × 106) from seven patients with leprosy, three of them carrying a loss-of-function P2RX7 SNP (rs3751143 A>C, “CC”; controls are “AA”) were stimulated with Mtb extract for 24 h. Cells were then cultured with ATP (50 μM) for 24 h and analyzed by flow cytometry.
(J) Left: flow-cytometry plots of CD44 and CXCR3 in Mtb extract-stimulated CD4+ T cells cultured with or without ATP. Right: average frequencies of CXCR3+CD44+CD4+ T cells.
Each replicate is a pool of sorted CD4+ T cells from five mice: n = 2 replicates per experimental group. Data shown as means ± SD. *p < 0.05, unpaired t tests (F–J).
Therefore, in response to influenza, P2RX7 leads to transcriptional modifications in lung parenchymal CD4+ T cells. Among these changes, increase in expression of the lung-homing chemokine receptor CXCR3 (also observed in response to MP287 infection) suggests that some of these P2RX7-induced CD4+ T cell adaptations in the lung tissue are common to multiple lung infections.
P2RX7/eATP induces the accumulation of lung parenchymal CD4+ T cells through induction of CXCR3 expression
We next measured CXCR3 protein expression in influenza-specific WT and P2RX7-KO CD4+ T cells from multiple organs. CXCR3 expression in the medLNs and blood was low, with no differences between WT and P2RX7-KO cells (Figure 6F). CXCR3 expression is maximal in the lung vasculature, where WT cells showed considerably higher levels of this chemokine receptor than P2RX7-KO cells. In the lung parenchyma, CXCR3 decreased in relation to the vasculature but remained at higher levels in WT cells. To investigate whether P2RX7 promotes lung CD4+ T cell accumulation through CXCR3, PR8-infected WT and P2RX7-KO mice were treated with anti-CXCR3 blocking antibodies during acute infection (Figure 6G). CXCR3 blockade reduced the PR8-specific CD4+ T cell population in the lung parenchyma, leading the WT mice to display numbers equal to treated or untreated P2RX7-KO mice (Figure 6H). Therefore, in response to acute influenza infection, PR8-specific CD4+ T cells depend on P2RX7 activation to upregulate CXCR3 and accumulate in high numbers in the lung parenchyma.
We then investigated whether P2RX7 activation regulates CXCR3 expression and proliferation of CD4+ T cells from human subjects with past exposure to leprosy, an infection caused by Mycobacterium leprae. This was performed with rare samples from three donors with a loss-of-function P2RX7 single-nucleotide polymorphism (SNP rs3751143 CC, non-functional P2RX7) and four non-polymorphic donors (AA, functional P2RX7)39 (Figure 6I). Peripheral blood mononuclear cells (PBMCs) were pre-stimulated with an Mtb extract and then cultured with eATP to mimic the eATP-rich environment that cells find in the lung parenchyma. Adding eATP to cell cultures increased the CXCR3+CD44+CD4+ T cell population for the AA donors but not for the CC donors (Figure 6J).
Considering the differences in cell-cycle pathways observed in the RNA-seq analyses, we also evaluated CD4+ T cell proliferation in distinct organs. Ex vivo assays of medLN PR8-specific CD4+ T cells showed increased proliferation of P2RX7-KO CD4+ T cells (Figures S5F and S5G). We then used intranasal carboxyfluorescein succinimidyl ester (CFSE) to track the proliferation of lung WT and P2RX7-KO CD4+ T cells, using FTY720 to stop leukocyte trafficking and prevent contamination with cells from the vasculature40 (Figure S5H). No differences were observed in the proliferation of lung WT and P2RX7-KO CD4+ T cells (Figure S5I). We also evaluated the proliferation of mycobacterial-stimulated human CD4+ T cells. Addition of eATP to human PBMC cultures equally induced proliferation of CD4+ T cells from P2RX7-AA and P2RX7-CC donors (Figure S5J). These results indicate that, in contrast to CXCR3 induction, P2RX7 does not induce proliferation of lung flu-specific CD4+ T cells or human mycobacterial-stimulated CD4+ T cells.
T cell-intrinsic P2RX7 contributes to the generation of flu-specific Tfh-like resident memory CD4+ T cells
Our RNA-seq data showed differences in some follicular T cell (Tfh)-related genes, such as Cxcr5 and Icos. This could be reminiscent of the recently described Tfh-like tissue-resident CD4+ T helper (TRH) population.40,41 Two different subpopulations of parenchymal CD4+ T cells (PSGL-1+FR4− and PSGL-1+FR4+ cells) were observed in the lung at day 14 p.i. (Figure S6A). Based on past studies,40,41 these populations were identified as T helper 1 (Th1)-like cells and Tfh-like/TRH cells, respectively. Tfh-like/TRH cells expressed higher levels of P2RX7 compared to Th1-like cells. Comparing WT and P2RX7-KO CD4+ T cells in a competitive setup (WT:P2RX7-KO mixed bone marrow chimeras), no differences were observed in the frequency of Tfh-like cells 7 days p.i., but there was a progressive reduction of Tfh-like cells among P2RX7-KO CD4+ T cells in the early and late memory phases (day 14 and day 42, respectively) (Figures S6B and S6C). Therefore, T cell-intrinsic P2RX7 expression is important for the generation of lung Tfh-like CD4+ T cells after control of influenza virus. Future mechanistic studies are needed to understand the effects of signaling via P2RX7 on flu-specific memory CD4+ T cells.
DISCUSSION
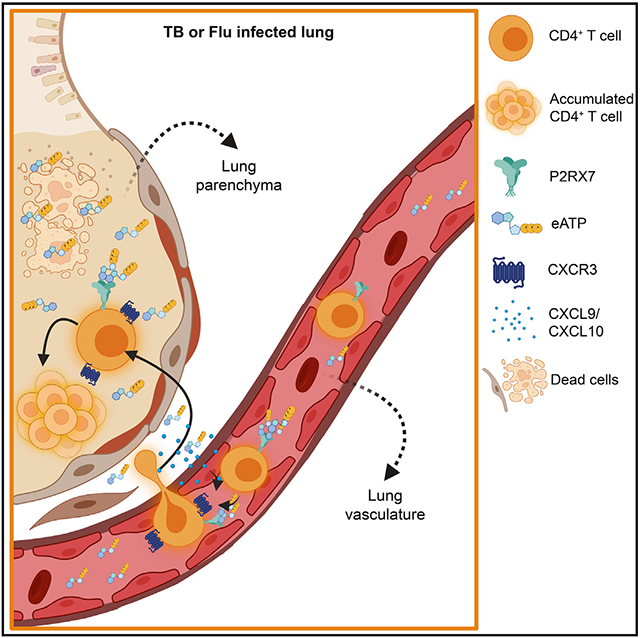
CD4+ T cells promote host defense against pulmonary infections, being a featured vaccine target.42,43 However, there is a great dilemma involving the CD4+ T cell response during pulmonary infections44: despite providing protection, excessive effector CD4+ T cell responses can induce irreversible damage in tissues with low regenerative capacity.10 Our work suggests that the magnitude of lung parenchymal CD4+ T cell accumulation, controlled by P2RX7 expression, determines the positive versus negative outcome of their effect in response to lung severe infections such as TB caused by hypervirulent strains and severe influenza.
Infection with the hypervirulent MP287 mycobacteria is an efficient system for the development of severe lung damage.29-31 Differences between Mbv MP287 and standard Mtb H37Rv are not limited to pathology induction: lung CD4+ T cells infiltrate and localize in different lung tissue compartments when comparing these two infections. In H37Rv-infected mice, TB-specific IFN-γ-producing CD4+ T cells are located mostly in the pulmonary vasculature,7 with low-to-intermediate numbers accumulating in the parenchyma. MP287 infection, in contrast, induces high numbers of lung parenchymal CD4+ T cells. These comparisons raised the question that excessive lung parenchyma CD4+ T cell accumulation could be the cause and not the consequence of the worsening of MP287 disease. Depletion and adoptive transfer experiments indicated this is indeed the case. Moreover, these results evidenced that, even in the context of MP287 infection, low-to-intermediate accumulation of lung parenchymal CD4+ T cells is sufficient to promote protection. The fact that CD4+ T depletion was incomplete, allowing the persistence of low numbers of parenchymal CD4+ T cells, may explain why bacterial loads did not increase after anti-CD4 treatment. When the treatment effect wanes, the CD4+ T cell population could expand again and worsen lung pathology. It is also important to note that, even in intact MP287-infected WT mice, there was extensive lung accumulation of non-ESAT6-specific CD4+ T cells. It is possible that the immune responses promoted by these cells contribute to exacerbated lung pathology. Our data support a working model in which a goldilocks intensity of lung CD4+ T cell infiltration is necessary to protect against lung-tropic infections while avoiding excessive tissue damage. Mechanistically, this may be explained by differential production of CD4+ T cell-derived pro-inflammatory cytokines, such as IFN-γ or tumor necrosis factor α (TNF-α). High levels of these cytokines promote exacerbated recruitment and in situ activation of innate immune cells, exaggerated host cell death, or increased pulmonary fibrosis.30,45,46 In contrast, low-to-intermediate numbers of CD4+ T cells would lead to optimal levels of IFN-γ and TNF-α for the elimination of viruses or bacteria, with off-target effects in the lung tissue being limited.
We found a crucial role for P2RX7 in the generation of CD8+ TRM cells,33,34 and CD4+ TRM cells in several non-lymphoid tissues are P2RX7,47 including a subset of lung CD4+ TRM cells observed long after influenza clearance.40,41 Our current work demonstrates that P2RX7 is required for the establishment of infection-induced CD4+ T cells in the lung parenchyma. Multiple immune cells and lung epithelial cells express P2RX7, and our group has previously shown that pharmacological P2RX7 inhibition led to decreased CD4+ T cell numbers in the lung parenchyma in response to MP287 infection.31 Our work expands these findings by identifying that infection-induced accumulation of bulk and antigen-specific lung parenchymal CD4+ T cells depends on P2RX7 specifically on T cells. The P2RX7-mediated increase in lung parenchymal CD4+ T cells is also a causal factor of lung damage and development of severe TB or influenza infections. On the flip side, transfer of limited numbers of P2RX7-KO CD4+ T cells was insufficient to transfer protection against MP287 infection. This further suggests the diminished ability of P2RX7-KO CD4+ T cells to enter the lung parenchyma. Moreover, it indicates that P2RX7, by decreasing the CD4+ T cell-intrinsic threshold for lung infiltration, may help define the beneficial versus deleterious nature of lung CD4+ T cell responses in severe lung infections while not affecting the severity of milder infections such as H37Rv.
Not only P2RX7 but its ligand eATP may be a controlling factor for that definition. MP287 infection induces more extensive macrophage death than H37Rv infection,48 and this is linked to increased lung eATP accumulation. It is possible that deleterious effects of P2RX7 on lung pathology may only occur in severe infections that cause exacerbated tissue damage and eATP release, such as TB-MP287 and influenza-PR8, but not TB-H37Rv.30,36,37 Our data support the notion that, regardless of lung damage, P2RX7/eATP controls the lung accumulation of CD4+ T cells. In response to severe infections, intense activation of P2RX7 promotes massive accumulation of parenchymal CD4+ T cells and worsens pulmonary pathology. In milder infections, P2RX7 still promotes CD4+ T cell accumulation in the lung tissue, however at lower numbers, consequently not affecting lung tissue health. In future studies, it will be important to evaluate the respective roles of overall lung pathogen load versus infection of specific lung cell types. It is important to remember that influenza and mycobacteria infect different lung cell types16,17 and induce distinct inflammation modalities,18,20 yet the role of CD4+ T cell-specific P2RX7 is similar. Therefore, lung pathogen load may be the decisive factor for eATP release in the lung.
P2RX7 promotes CD8+ T cell tissue residency through transforming growth factor β (TGF-β) signaling.34 However, tissue-resident CD4+ T cells do not express TGF-β signaling or induced molecules and form independently of TGF-βRII.47,49 In contrast to TGF-βRII and CD103, tissue-resident CD4+ T cells express the chemokine receptor CXCR3.47 This is also true in response to mild Mtb infection, where CXCR3 helps determine the lung parenchymal homing of CD4+ T cells.50 In PR8-flu infection, CXCR3 deficiency prevents the infiltration of immune cells into the lung, increasing the survival of PR8-infected mice.51 We found that lung parenchymal P2RX7-KO CD4+ T cells expressed lower levels of CXCR3 than WT counterparts and that CXCR3 blockade reduced WT CD4+ T cells in lung parenchyma, with no effects on P2RX7-KO CD4+ T cells. Activation of this pathway may occur upon entry into the lung vasculature, where CXCR3 upregulation is maximal, similar to recent findings for skin-infiltrating CD4+ T cells.52 In contrast, P2RX7-KO CD4+ T cells from blood and draining lymph nodes express normal levels of CXCR3 and CX3CR1, which induce, respectively, parenchymal and intravascular T cell positioning.7,53 Instead of a pre-determination of tissue homing during priming, our results suggest that sensing of lung eATP determines this fate by upregulation of CXCR3. This was also observed in Mtb-responding human CD4+ T cells. It is still to be determined whether eATP/P2RX7 directly alters the transcriptional and protein landscape of effector CD4+ T cells or if it merely serves as a de facto chemotactic signal to attract CD4+ T cells into the parenchyma. In contrast, lung P2RX7-KO CD4+ T cells (and eATP-responding human CD4+ T cells) did not have major issues in proliferation, reinforcing the idea that P2RX7 mostly contributes to lung parenchymal accumulation through chemokine signaling. Overall expansion of antigen-specific CD4+ T cells is also not affected by P2RX7-KO, although more studies will be important to confirm the lack of this effect at an intracellular signaling level. Previous studies, however, have demonstrated that eATP can induce the proliferation of human CD4+ T cells.26 It is possible, in this scenario, that other eATP receptors specifically induce lung CD4+ T cell proliferation, for example P2RX4.54 Future studies are still needed to pinpoint the role of eATP sensing for in situ CD4+ T cell proliferation.
Overall, our work suggests that, in response to lung infections, P2RX7 favors lung CD4+ T cell parenchymal establishment via induction of CXCR3. The magnitude of this effect may depend on the P2RX7 expression level by CD4+ T cells and on the amount of lung eATP. Future research will be needed to test whether this is generalizable for tissue-resident CD4+ T cells in other non-lymphoid tissues. Our study opens the perspective for therapeutic interventions aimed at reducing the worsening of infectious diseases that attack the lung without affecting the ability of the adaptive immune system to fight off these infections.
Limitations of the study
Infections caused by Mbv MP287, Mtb H37Rv, and influenza-PR8 are not fully comparable. Besides their distinct etiology, at the doses used here H37Rv and MP287 infections generate 0% versus 100% mortality within 30 days p.i.,29,30 whereas PR8 virus induces recovery of approximately 50% of mice after acute infection (Figure 5B). Nevertheless, in both scenarios P2RX7 played a similar role in lowering the threshold for CD4+ T cell lung parenchymal accumulation. Previous reports had suggested a positive role for P2RX7 in the protection against milder forms of TB,37,55 which may help solidify the notion that, more than species-specific nuances, it is the overall lung damage induced by pathogen load that dictates the role of P2RX7 in CD4+ T cells. Additional research to test these nuances will be the subject of future research by us.
Another limitation is related to experiments with patient samples. The samples used in this study are rare, which explains why we did not obtain a high number of donors. These samples come from patients who had leprosy, and as this condition is associated with poverty, many of the genotyped patients could not be contacted. Additionally, our results in murine models would have been best compared to lung tissue biopsies or bronchoalveolar lavage, but these methods are very intrusive and not easy to obtain. In the future, we will establish collaborations with clinical researchers and explore this particular avenue.
Our T cell-P2RX7-KO mouse model is also P2RX7 deficient on CD8+ T cells. We have reported that P2RX7 promotes CD8+ TRM cells in response to systemic viruses.33,34 However, the role of P2RX7 in the initial CD8+ T cell seeding of the lung parenchyma in response to influenza, where these cells play a central role,56 has not been defined (and is being currently studied by our group). To study the effects of P2RX7 deficiency only on CD4+ T cells, we used either mixed bone marrow chimeras33 or, in other experiments, adoptive transfer of CD4+ T cells into CD4-KO infected mice, as previously done by us.57
Finally, our intranasal CFSE method, although capturing a window of proliferation at a population level, does not lead to uniform CFSE staining between lung CD4+ T cells, which limits our data interpretation. Future experiments with alternative strategies will be necessary to further test the in situ proliferation of lung-resident CD4+ T cells.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Henrique Borges da Silva (borgesdasilva.henrique@mayo.edu).
Materials availability
No new unique reagents were generated in this study.
Data and code availability
The data that support the findings of this study are available from the corresponding authors on request.
All sequencing reads generated in this study and processed RNA expression matrices are deposited in NCBI’s GEO and are publicly available as of the date of publication. The accession number GSE244194 is listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE I-Ab Mycobacterium tuberculosis ESAT-64-1 tetramer | NIH Tetramer Core Facility | https://tetramer.yerkes.emory.edu/ |
| APC I-Ab Influenza A 2W1S tetramer | NIH Tetramer Core Facility | https://tetramer.yerkes.emory.edu/ |
| FITC anti-mouse CD45 | Biolegend | Cat# 147709; RRID:AB_2563542 |
| BUV395 anti-mouse CD4 | BD Biosciences | Cat# 563790; RRID:AB_2738426 |
| BV510 anti-mouse/human CD44 | Biolegend | Cat# 103043; RRID:AB_2561391 |
| PE anti-mouse P2X7 | Biolegend | Cat# 148703; RRID:AB_2650951 |
| BV786 anti-mouse P2X7 | BD Biosciences | Cat# 744724; RRID:AB_2742436 |
| BV711 anti-mouse/human KLRG1 | Biolegend | Cat# 138427; RRID:AB_2629721 |
| BV786 anti-mouse CX3CR1 | Biolegend | Cat# 149029; RRID:AB_2565938 |
| PE/Cyanine7 anti-mouse CD69 | Biolegend | Cat# 104511; RRID:AB_493565 |
| BV650 anti-mouse CXCR3 | Biolegend | Cat# 126531; RRID:AB_2563160 |
| PE anti-mouse/human Ki-67 | Biolegend | Cat# 151209; RRID:AB_2716014 |
| BV650 anti-mouse IFN-y | Biolegend | Cat# 505831; RRID:AB_11142685 |
| PE-CF594 anti-mouse FOXP3 | BD Biosciences | Cat# 562466; RRID:AB_11151905 |
| PerCP-Cyanine5.5 anti-mouse T-bet | Biolegend | Cat# 644805; RRID:AB_1595593 |
| BV421 anti-mouse CD45.1 | Tongo Biosciences | Cat# 75-0453-U025; RRID:AB_2621949 |
| APC anti-mouse CD45.2 | Biolegend | Cat# 109813; RRID:AB_389210 |
| Percp anti-mouse CD45.2 | Biolegend | Cat# 109825; RRID:AB_893351 |
| BV421 anti-mouse/human CD4 | BD Biosciences | Cat# 562425; RRID:AB_11154221 |
| APC-Cyanine7 anti-mouse/human CD44 | Biolegend | Cat# 103027; RRID:AB_830784 |
| PE anti-mouse-human CXCR3 | BioLegend | Cat# 353705; RRID:AB_10959652 |
| PE-Cyanine7 anti-human Ki-67 | BioLegend | Cat# 350525; RRID:AB_2562871 |
| Anti-mouse S100A9 | Bio-Techne | Cat# AF2065; RRID:AB_2184263 |
| PE anti-mouse CD4 | Biolegend | Cat# 100407; RRID:AB_312693 |
| FITC anti-rabbit antibody | BD Biosciences | Cat# 554020; RRID:AB_395212 |
| APC anti-mouse IFN-y | Biolegend | Cat# 505809; RRID:AB_315403 |
| InVivoMAb anti-mouse CD4 | BioXCell | Cat# BE0003-1; RRID:AB_1107636 |
| InVivoMAb anti-mouse CXCR3 (CD183) | BioXCell | Cat# BE0249; RRID:AB_2687730 |
| Anti-ARTC2.2 Treg-protector nanobodie | Biolegend | Cat# 149802; RRID:AB_2565485 |
| Pe/Cyanine5 anti-mouse CD69 | Biolegend | Cat# 104510; RRID:AB_313113 |
| BV785 anti-mouse/human CD44 | Biolegend | Cat# 103041; RRID:AB_11218802 |
| Bacterial and virus strains | ||
| Mycobacterium bovis MP287/03 | José Soares Ferreira Neto, PhD | Grown in house |
| Mycobacterium tuberculosis H37Rv | American Type Culture Collection | Grown in house |
| Influenza A PR8/34 | Jie Sun, PhD | Grown in house |
| Influenza A PR8/2W | Ryan Langlois, PhD | Grown in house |
| Chemicals, peptides, and recombinant proteins | ||
| Middlebrook 7H9 medium | Becton Dickinson | Cat# 271310 |
| Middlebrook 7H10 Agar | Becton Dickinson | Cat# 262710 |
| Middlebrook ADC Growth Supplement | Sigma-Aldrich | Cat# M0553-1VL |
| Middlebrook OADC Growth Supplement | Sigma-Aldrich | Cat# M0678-1VL |
| RPMI 1640 Medium | Gibico | Cat# 11875093 |
| Ketamine | Syntec | N/A |
| Xylazine | Syntec | N/A |
| CFSE | Abcam | Cat# ab113853 |
| CellTrace Violet | Thermo Fisher Scientific | Cat# C34557 |
| Fingolimod (FTY720) | Selleck Chemicals | Cat# S5002 |
| Collagenase type IV | Gibico | Cat# 17104019 |
| ACK Lysing Buffer | This paper | Made in house |
| PBS (Phosphate Buffered Saline) 1x | Corning | Cat# 21-040-CV |
| ProLong Gold antifade | Invitrogen | Cat# P36934 |
| Bovine serum albumin (BSA) | Sigma-Aldrich | Cat# A9418 |
| Fetal Bovine Serum | Gibico | Cat# A52567-01 |
| Critical commercial assays | ||
| EasySep Mouse CD4+ T cell isolation Kit | Stem Cell | Cat# 19765 |
| True Nuclear Transcription Factor Buffer Set | Biolegend | Cat# 424401 |
| BD Cytofix/Cytoperm Plus | BD Biosciences | Cat# 554715 |
| RNA isolation RNeasy Kits | QIAGEN | Cat# 74004 |
| Deposited data | ||
| Raw and analyzed data | This paper | GEO: GSE244194 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Jackson Laboratory | Cat# 000664; RRID:IMSR_JAX:000664 |
| CD45.1 congenic mice | Jackson Laboratory | Cat# 002014; RRID:IMSR_JAX:002014 |
| Cd4−/− mice | Jackson Laboratory | Cat# 002663; RRID:IMSR_JAX:002663 |
| P2rx7−/− mice | Jackson Laboratory | Cat# 005576; RRID:IMSR_JAX:005576 |
| CD4-Cre | Jackson Laboratory | Cat# 022071; RRID:IMSR_JAX:022071 |
| P2rx7 flox/flox | György Haskó - Columbia University | N/A |
| Oligonucleotides | ||
| PR8-NP fwd: 5′-GATTGGTGGAATTGGACGAT-3′ | Thermo Fisher Scientific | N/A |
| PR8-NP rev: 5′-AGAGCACCATTCTCTCTATT-3′ | Thermo Fisher Scientific | N/A |
| Software and algorithms | ||
| FlowJo 10.7.1 | BD Biosciences | https://www.flowjo.com/; RRID:SCR_008520 |
| Prism 9 | Graphpad Software | https://www.graphpad.com/; RRID:SCR_002798 |
| Fiji Java 8 | ImageJ | https://github.com/imagej/ImageJ.git; RRID:SCR_002285 |
| Biorender | Biorender | https://Biorender.com; RRID:SCR_018361 |
| BGI | N/A | https://www.bgi.com/global/dr-tom/; RRID:SCR_011114 |
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Mice
Specific-pathogen-free (SPF) mice of 6-8-week-old were used. Male mice were used for experiments of severe TB and female mice for experiments with Influenza virus. Mice were kept in the mouse facilities of the Institute of Biomedical Sciences of the University of São Paulo (USP) (São Paulo, Brazil), the Center for Biosciences and Biotechnology of the State University of Norte Fluminense (UENF) (Rio de Janeiro, Brazil) or at the Department of Immunology, Mayo Clinic Arizona. All TB experiments were done at the Biosafety Level 3 (BSL-3) laboratories of the Institute of Pharmaceutical Sciences at USP or the Laboratory of Biology of Recognition at UENF. Influenza experiments were done in the Biosafety Level 2 (BSL-2) laboratory of the Mayo Clinic Arizona. In all experiments, mice were randomly assigned to experimental groups. All experimental procedures were approved by the institutional animal care and use committee of the institutions involved in the work (CEUA 5611150818, CEUA 402/2021 and IACUC A00005542-20).
Mycobacterial preparations
Mycobacterial strains were kept and frozen at −80°C in Middlebrook 7H9 medium (Becton Dickinson, US) supplemented with ADC (albumin, dextrose and catalase) (Difco, US). Monitoring of mycobacterial growth was carried out by measuring Optical Density (OD 600nm) by spectrophotometry (Biochrom, model Libra S6). To carry out the experiments, the mycobacterial strain was removed from −80°C, thawed and added to Middlebrook 7H9 medium plus 10% of ADC enrichment medium. The suspension culture was vortexed (Biomatic, Brazil) and sonicated in an ultrasound bath (Ultrasonic Maxi cleaner 800 – Unique, Brazil) for 1 min to disperse the lumps. The culture was incubated at 37°C and 5% of CO2 for 5–7 days.
METHOD DETAILS
Infections
Infections with Mbv MP287 and Mtb H37Rv were performed intratracheally (i.t.), by surgical incision, with ~100 CFU in 90 μL of final volume. To be infected, the mice were anesthetized with a solution of ketamine (110 mg/kg) and xylazine (15 mg/kg), being inoculated 100 μL of anesthetic intraperitoneally. For Influenza virus infections the mice were anesthetized with isoflurane and 20–30 μL of viral inoculum (~1400 PFU) was injected intranasally (i.n.).
CD4+ T cells purification and adoptive transfer
Splenic CD4+ T cells were isolated from C57BL/6 and P2rx7−/− mice by negative selection using the EasySep Mouse CD4+ T cell Isolation Kit (STEMCELL), following the manufacturer’s protocol. Next, CD4+ T cells (1 × 106 or 3 × 106) were transferred (1 × 106 or 3 × 106 per mouse) by i.v. to Cd4−/− mice 5 days after infection with the MP287 Mbv strain.
Intravascular labeling
Mice were anesthetized and given intravenous (i.v.) injections of 2.5 μg of a fluorophore-labeled monoclonal antibody (mAb) anti-CD45. The lungs were harvested after 3 min, as described previously.12
CD4+ T cell depletion
C57BL/6 mice were infected with the Mbv MP287 strain and on day 21 p.i. received a single i.p. inoculation of purified anti-α-CD4 antibody GK1.5 (250 μg) (Bio X Cell). This day was chosen for the treatment, because it is when the aggravation of the disease in the mice begins. On day 28 p.i., mice were euthanized, and depletion was verified in the lung and blood by flow cytometry (Figure S2D).
Tissue processing for flow cytometry
In all experiments, 50 μg of Treg-protector (anti-ARTC2.2) nanobodies (BioLegend) were injected intravenously 30 min before tissue harvesting to prevent cell death as previously described.58 The lungs were harvested, and the lobes were processed and digested with collagenase type IV (0.5 mg/mL) (Sigma-Aldrich) at 37°C for 40 min under agitation (200 rpm).59 Lymph nodes were mechanically processed in a cell strainer. Blood was collected and kept in heparin until the time of lysing the red blood cells. The cell suspension obtained from the tissues was homogenized and filtered through cell filters (Corning) and incubated with ACK Lysing Buffer (Thermo Fisher Scientific) at room temperature for 2 min to deplete the erythrocytes. The cell suspensions were washed with PBS 10% fetal calf serum (Gibco, US). The cell suspension was centrifuged at 1,200 rpm for 5 min and resuspended in FACS buffer until the time of staining.
Flow cytometry analysis
Bulk and antigen-specific CD4+ T cells from lung, lymph nodes and blood cells were stained with I-Ab M. tuberculosis ESAT-64-17 (QQWNFAGIEAAASA) and I-Ab Influenza A 2W1S (EAWGALANWAVDSA) tetramers (NIH – Tetramer Core Facility) following previously published protocols.38,59 Next, cell suspension was stained with fluorochrome-labeled monoclonal antibodies (see key resources table) diluted in FACS buffer and incubated at room temperature for 40 min. For ex vivo intracellular IFN-γ staining, lung cells were incubated with monensin (2 μM) for 5 h at 37°C in 5% CO2, fixed, and permeabilized with BD Cytofix/Cytoperm kit (BD Biosciences). Live/dead dye was used to stain dead cells. Cells were analyzed using FACS Symphony A5 flow cytometer (BD Biosciences) and Cytek Aurora (Cytek Biosciences). Conventional and spatial distribution by t-distributed stochastic neighbor embedding analysis (tSNE) was performed using FlowJo software (BD Biosciences).60
Histological analysis
The right upper lung lobe was collected, washed in PBS and maintained in 10% paraformaldehyde for 24 h. Subsequently, histological paraffin sections of approximately 4–5 μm were stained with Hematoxylin-eosin (H&E) or Masson’s trichrome (MT) for microscopic visualization and then photographed.
CFU determination
The lung suspension was undergoing serial dilution in PBS and soon after it was seeded in petri dishes with Middlebrook 7H10 Agar (Becton Dickinson, US) supplemented with OADC enrichment medium (oleate, albumin, dextrose and catalase) (Difco, US). The plates were sealed and kept in an oven at 37°C and 5% CO2 for 21 days. After this period, colonies were counted to determine the CFU.
Viral load determination
Viral loads in the experiments with the PR8 strain were defined by quantitative PCR (qPCR), comparing the amplification of lung supernatant samples with the amplification of viral cDNA from a sample of known concentration of PFU (standard curve method). The primers used for virus cDNA amplification were: PR8-NP, 5′-GATTGGTGGAATTGGACGAT-3′ and 5′-AGAGCACCATTCTCTCTATT-3′.
Confocal microscopy
Paraffinized sections of approximately 10 μm were made with the right upper lobe of the lung. After dewaxing and antigen retrieval, the sections were submitted to antigenic recovery. Subsequently, the sections were blocked with 2% BSA in PBS for 30 min, and then incubated overnight at 4°C with primary anti-S100A9 antibody (Bio-Techne). After 24 h, the sections were stained with fluorochrome-labeled anti-rabbit antibody and anti-CD4 and anti-IFN-γ conjugated antibodies (BD Biosciences).61 The slides were treated with the Sudan Black B (Sigma-Aldrich) reagent to remove the natural autofluorescence of the tissue. Next, the slides were rinsed in PBS and coverslipped with ProLong Gold antifade reagent (Invitrogen). After being stained and treated, slides were washed, and coverslips were inserted. The high resolution SP5 confocal microscope (Leica) was used to view slides and image analysis performed in Fiji software.
Mixed bone marrow chimeras
C57BL/6 (CD45.2) mice were irradiated with 1000 rads and reconstituted with the 1:1 mixture of CD4-Cre (CD45.1/2) and CD4-Cre P2rx7fl/fl (CD45.2) mouse bone marrow. Reconstitution was followed weekly by flow cytometry and approximately 2 months later the chimeras were ready for experimentation.
RNA-seq analysis
WT or CD4-Cre P2rx7fl/fl mice were infected with PR8/2W. At day 7 post-infection, CD44+ CD4+ T cells from lungs (i.v.+ and i.v.− compartments) and spleen were isolated by cell sorting. RNA was extracted using the RNeasy Plus Mini Kit (QIAgen). Library preparation and RNA-seq (DNBseq platform, PE 100bp pair-end read length) was done by BGI Americas. RNA-seq reads were mapped and raw count matrix was generated. DEG analysis was done using DESeq2, and genes with >2-fold changes and FDR <0.05 were considered for gene cluster analysis. Heatmaps, PCA plots and Pathway Enrichment Analyses were generated using the R-based BGI Dr.Tom online analysis toolkit (https://www.bgi.com/global/dr-tom/).
In vivo CXCR3 inhibition
For anti-CXCR3 treatment, WT and P2rx7−/− mice were infected with Influenza virus PR8/34 strain (~1400 PFU) and inoculated intraperitoneally with 200 μg of anti-mouse CXCR3 antibody (BioXCell, Lebanon/NH, clone CXCR3-173) on days 0, 3, 4 and 6 p.i. On day 7 p.i. the lung was harvested for analysis by flow cytometry.
In vivo and ex vivo proliferation assays
For proliferation assays, WT and CD4-Cre P2rx7fl/fl mice were infected with Influenza virus PR8/34 strain (~1400 PFU) and treated intraperitoneally with 20 μg of Fingolimod (FTY720) (Selleck Chemicals) on days 5, 6 and 7 p.i. On day 6 p.i. infected mice also received CFSE intranasally to stain infiltrated lung parenchyma cells. Lung and medLN harvest were done at 24 h and 48 h after CFSE staining. MedLNs were processed and cells stained (per manufacturer’s protocol) with CellTrace Violet (Thermo Fisher Scientific). After staining, cells were plated and incubated at 37°C and 5% CO2 for 24 h and 48 h. After this period the cells were stained and analyzed by flow cytometry.
In vitro experiments with human PBMC
For in vitro experiments using human samples the blood was collected by vacuum venipuncture in a tube containing anticoagulant heparin. PBMCs were obtained using density gradient centrifugation with Ficoll and plated. The isolated PBMCs from 6 individuals (4 AA and 3 CC), previously characterized by having or not P2RX7 single-nucleotide polymorphisms (rs3751143 A>C),39 were isolated and plated (1 × 105). Both donor groups already had the active form of leprosy, caused by Mycobacterium leprae. They were healthy at the time of blood withdrawal, after drug treatment. To generate a specific response against mycobacteria, we activated the PBMCs with mycobacterial extract (1 μg/mL) at the time of plating. Non-activated cells were used as a control. The cells were kept at 37°C and 5% CO2 and 24 h later part of the cells received supplementation (50 μM) of Adenosine 5-triphosphate (ATP - Sigma-Aldrich). After 24 h the cells were stained and subjected to flow cytometry analysis to assess the expression of Live/dead markers CD4, CD44, CXCR3 and Ki-67 (BioLegend). Project submitted and approved to the Ethics and Research Committee of the Faculty of Medicine of Campos under CAAE number: 19679119.8.0000.5244.
QUANTIFICATION AND STATISTICAL ANALYSIS
Details of the statistical analyses used for each experiment can be found in the figure legends. Statistical analyses were performed using the GraphPad Prism 9 software. Data were described as mean with error bars indicating the SD t-tests were used to assess differences between only two groups. One-way ANOVA tests and Tukey’s post hoc tests were used to assess the effects of only 1 parameter among more than 2 groups. Differences between groups were considered significant when p < 0.05 (*), p < 0.01 (**) or p < 0.001 (***).
Supplementary Material
Highlights.
eATP sensing via P2RX7 promotes lung CD4+ T cell residency in response to infection
P2RX7-induced lung CD4+ T cells lead to tissue pathology and poor host survival
P2RX7 induces lung CD4+ T cell establishment through CXCR3 expression
ACKNOWLEDGMENTS
We thank José Israel Lima, Silvana Silva, Maria Áurea, Daniel Bihnam, Juliana Azevedo, and Verônica Lanes for technical assistance. We thank the support from the Flow Cytometry Cores at the Institute of Biomedical Sciences, University of São Paulo and Mayo Clinic Arizona. This work was funded by Research Support of São Paulo (FAPESP) (M.R.D.-L.: 2019/24700-8 and 2015/20432-8), National Council for Scientific and Technological Development (CNPq) (M.R.D.-L.: 408909/2018-8 and 303810/2018-1), and the National Institute of Allergy and Infectious Diseases (NIAID) (H.B.d.S.: R00 AI139381, R01 AI170649).
INCLUSION AND DIVERSITY
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community.
Footnotes
DECLARATION OF INTERESTS
H.B.d.S. is an advisor for the International Genomics Consortium.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.113448.
REFERENCES
- 1.Medzhitov R, Schneider DS, and Soares MP (2012). Disease tolerance as a defense strategy. Science 335, 936–941. 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravimohan S, Kornfeld H, Weissman D, and Bisson GP (2018). Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur. Respir. Rev 27, 170077. 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klomp M, Ghosh S, Mohammed S, and Nadeem Khan M (2021). From virus to inflammation, how influenza promotes lung damage. J. Leukoc. Biol 110, 115–122. 10.1002/JLB.4RU0820-232R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DM, Román E, and Swain SL (2004). CD4 T cell responses to influenza infection. Semin. Immunol 16, 171–177. 10.1016/j.smim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.McDermott AJ, and Klein BS (2018). Helper T-cell responses and pulmonary fungal infections. Immunology 155, 155–163. 10.1111/imm.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, Dejnirattisai W, Rostron T, Supasa P, Liu C, et al. (2020). Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol 21, 1336–1345. 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, and Barber DL (2014). Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol 192, 2965–2969. 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO (2021). Global tuberculosis report 2021, 1, pp. 1–25. [Google Scholar]
- 9.Paget J, Spreeuwenberg P, Charu V, Taylor RJ, Iuliano AD, Bresee J, Simonsen L, and Viboud C; Global Seasonal Influenza-associated Mortality Collaborator Network and GLaMOR Collaborating Teams* (2019). Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 9, 020421. 10.7189/jogh.09.020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moguche AO, Musvosvi M, Penn-Nicholson A, Plumlee CR, Mearns H, Geldenhuys H, Smit E, Abrahams D, Rozot V, Dintwe O, et al. (2017). Antigen Availability Shapes T Cell Differentiation and Function during Tuberculosis. Cell Host Microbe 21, 695–706.e5. 10.1016/j.chom.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riberdy JM, Christensen JP, Branum K, and Doherty PC (2000). Diminished primary and secondary influenza virus-specific CD8(+) T-cell responses in CD4-depleted Ig(−/−) mice. J. Virol 74, 9762–9765. 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, and Masopust D (2014). Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc 9, 209–222. 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallin MA, Sakai S, Kauffman KD, Young HA, Zhu J, and Barber DL (2017). Th1 Differentiation Drives the Accumulation of Intravascular, Non-protective CD4 T Cells during Tuberculosis. Cell Rep. 18, 3091–3104. 10.1016/j.celrep.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhume K, Finn CM, Strutt TM, Sell S, and McKinstry KK (2019). T-bet optimizes CD4 T-cell responses against influenza through CXCR3-dependent lung trafficking but not functional programming. Mucosal Immunol. 12, 1220–1230. 10.1038/s41385-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers S. (1999). Immunity to tuberculosis: a delicate balance between protection and pathology. FEMS Immunol. Med. Microbiol 23, 149–158. 10.1111/j.1574-695X.1999.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, and Berry MPR (2013). The immune response in tuberculosis. Annu. Rev. Immunol 31, 475–527. 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 17.Thomas PG, Keating R, Hulse-Post DJ, and Doherty PC (2006). Cell-mediated protection in influenza infection. Emerg. Infect. Dis 12, 48–54. 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, and Behar SM (2014). In search of a new paradigm for protective immunity to TB. Nat. Rev. Microbiol 12, 289–299. 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders BM, Frank AA, and Orme IM (1999). Granuloma formation is required to contain bacillus growth and delay mortality in mice chronically infected with Mycobacterium tuberculosis. Immunology 98, 324–328. 10.1046/j.1365-2567.1999.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damjanovic D, Divangahi M, Kugathasan K, Small CL, Zganiacz A, Brown EG, Hogaboam CM, Gauldie J, and Xing Z (2011). Negative regulation of lung inflammation and immunopathology by TNF-alpha during acute influenza infection. Am. J. Pathol 179, 2963–2976. 10.1016/j.ajpath.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiris JSM, Hui KPY, and Yen HL (2010). Host response to influenza virus: protection versus immunopathology. Curr. Opin. Immunol 22, 475–481. 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong T, Liu L, Jiang W, and Zhou R (2020). DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol 20, 95–112. 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 23.Patel S. (2018). Danger-Associated Molecular Patterns (DAMPs): the Derivatives and Triggers of Inflammation. Curr. Allergy Asthma Rep 18, 63. 10.1007/s11882-018-0817-3. [DOI] [PubMed] [Google Scholar]
- 24.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MAM, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. (2007). Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med 13, 913–919. 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 25.Kono H, and Rock KL (2008). How dying cells alert the immune system to danger. Nat. Rev. Immunol 8, 279–289. 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, and Grassi F (2008). Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal 1, ra6. 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 27.Surprenant A, Rassendren F, Kawashima E, North RA, and Buell G (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738. 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 28.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, and Falzoni S (2017). The P2X7 Receptor in Infection and Inflammation. Immunity 47, 15–31. 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Bomfim CCB, Amaral EP, Cassado ADA, Salles ÉM, do Nascimento RS, Lasunskaia E, Hirata MH, Álvarez JM, and D’Império-Lima MR (2017). P2X7 Receptor in Bone Marrow-Derived Cells Aggravates Tuberculosis Caused by Hypervirulent Mycobacterium bovis. Front. Immunol 8, 435. 10.3389/fimmu.2017.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaral EP, Ribeiro SCM, Lanes VR, Almeida FM, de Andrade MRM, Bomfim CCB, Salles EM, Bortoluci KR, Coutinho-Silva R, Hirata MH, et al. (2014). Pulmonary infection with hypervirulent Mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog. 10, e1004188. 10.1371/journal.ppat.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santiago-Carvalho I, de Almeida-Santos G, Bomfim CCB, de Souza PC, Silva JCSE, de Melo BMS, Amaral EP, Cione MVP, Lasunskaia E, Hirata MH, et al. (2021). P2x7 Receptor Signaling Blockade Reduces Lung Inflammation and Necrosis During Severe Experimental Tuberculosis. Front. Cell. Infect. Microbiol 11, 672472. 10.3389/fcimb.2021.672472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyva-Grado VH, Ermler ME, Schotsaert M, Gonzalez MG, Gillespie V, Lim JK, and García-Sastre A (2017). Contribution of the Purinergic Receptor P2X7 to Development of Lung Immunopathology during Influenza Virus Infection. mBio 8, e00229–17. 10.1128/mBio.00229-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, et al. (2018). The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559, 264–268. 10.1038/s41586-018-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borges da Silva H, Peng C, Wang H, Wanhainen KM, Ma C, Lopez S, Khoruts A, Zhang N, and Jameson SC (2020). Sensing of ATP via the Purinergic Receptor P2RX7 Promotes CD8(+) Trm Cell Generation by Enhancing Their Sensitivity to the Cytokine TGF-beta. Immunity 53, 158–171.e156. 10.1016/j.immuni.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, and Grassi F (2011). ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal 4, ra12. 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 36.Myers AJ, Eilertson B, Fulton SA, Flynn JL, and Canaday DH (2005). The purinergic P2X7 receptor is not required for control of pulmonary Mycobacterium tuberculosis infection. Infect. Immun 73, 3192–3195. 10.1128/IAI.73.5.3192-3195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos AA Jr., Rodrigues-Junior V, Zanin RF, Borges TJ, Bonorino C, Coutinho-Silva R, Takyia CM, Santos DS, Campos MM, and Morrone FB (2013). Implication of purinergic P2X7 receptor in M. tuberculosis infection and host interaction mechanisms: a mouse model study. Immunobiology 218, 1104–1112. 10.1016/j.imbio.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, and Jenkins MK (2007). Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213. 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souza RDC, Louvain de Souza T, Ferreira CDS, Nascimento LS, Nahn EP Jr., and Peixoto-Rangel AL (2021). Associations Between the Purinergic Receptor P2X7 and Leprosy Disease. Front. Genet 12, 730991. 10.3389/fgene.2021.730991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Son YM, Cheon IS, Wu Y, Li C, Wang Z, Gao X, Chen Y, Takahashi Y, Fu YX, Dent AL, et al. (2021). Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci. Immunol 6, eabb6852. 10.1126/sciimmunol.abb6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swarnalekha N, Schreiner D, Litzler LC, Iftikhar S, Kirchmeier D, Künzli M, Son YM, Sun J, Moreira EA, and King CG (2021). T resident helper cells promote humoral responses in the lung. Sci. Immunol 6, eabb6808. 10.1126/sciimmunol.abb6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sant AJ, DiPiazza AT, Nayak JL, Rattan A, and Richards KA (2018). CD4 T cells in protection from influenza virus: Viral antigen specificity and functional potential. Immunol. Rev 284, 91–105. 10.1111/imr.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner DL, and Farber DL (2014). Mucosal resident memory CD4 T cells in protection and immunopathology. Front. Immunol 5, 331. 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barber DL (2017). The Helper T Cell’s Dilemma in Tuberculosis. Cell Host Microbe 21, 655–656. 10.1016/j.chom.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Hillaire MLB, Rimmelzwaan GF, and Kreijtz JHCM (2013). Clearance of influenza virus infections by T cells: risk of collateral damage? Curr. Opin. Virol 3, 430–437. 10.1016/j.coviro.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Hirahara K, Kokubo K, Aoki A, Kiuchi M, and Nakayama T (2021). The Role of CD4(+) Resident Memory T Cells in Local Immunity in the Mucosal Tissue - Protection Versus Pathology. Front. Immunol 12, 616309. 10.3389/fimmu.2021.616309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beura LK, Fares-Frederickson NJ, Steinert EM, Scott MC, Thompson EA, Fraser KA, Schenkel JM, Vezys V, and Masopust D (2019). CD4(+) resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med 216, 1214–1229. 10.1084/jem.20181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrade MR, Amaral EP, Ribeiro SC, Almeida FM, Peres TV, Lanes V, D’Império-Lima MR, and Lasunskaia EB (2012). Pathogenic Mycobacterium bovis strains differ in their ability to modulate the proinflammatory activation phenotype of macrophages. BMC Microbiol. 12, 166. 10.1186/1471-2180-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonseca R, Burn TN, Gandolfo LC, Devi S, Park SL, Obers A, Evrard M, Christo SN, Buquicchio FA, Lareau CA, et al. (2022). Runx3 drives a CD8(+) T cell tissue residency program that is absent in CD4(+) T cells. Nat. Immunol 23, 1236–1245. 10.1038/s41590-022-01273-4. [DOI] [PubMed] [Google Scholar]
- 50.Hoft SG, Sallin MA, Kauffman KD, Sakai S, Ganusov VV, and Barber DL (2019). The Rate of CD4 T Cell Entry into the Lungs during Mycobacterium tuberculosis Infection Is Determined by Partial and Opposing Effects of Multiple Chemokine Receptors. Infect. Immun 87, e00841–18. 10.1128/IAI.00841-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fadel SA, Bromley SK, Medoff BD, and Luster AD (2008). CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur. J. Immunol 38, 3376–3387. 10.1002/eji.200838628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prizant H, Patil N, Negatu S, Bala N, McGurk A, Leddon SA, Hughson A, McRae TD, Gao YR, Livingstone AM, et al. (2021). CXCL10(+) peripheral activation niches couple preferred sites of Th1 entry with optimal APC encounter. Cell Rep. 36, 109523. 10.1016/j.celrep.2021.109523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, and von Andrian UH (2016). The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity 45, 1270–1284. 10.1016/j.immuni.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, and Junger WG (2010). Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116, 3475–3484. 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng X, Li T, Chen Y, Pan H, Zhang Z, Dai Y, and Wang J (2017). Genetic polymorphisms of the P2X7 gene associated with susceptibility to and prognosis of pulmonary tuberculosis. Infect. Genet. Evol 53, 24–29. 10.1016/j.meegid.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, and Kaech SM (2014). CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41, 633–645. 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Salles ÉM, Raeder PL, Angeli CB, Santiago VF, de Souza CN, Ramalho T, Câmara NOS, Palmisano G, Álvarez JM, and D’Império Lima MR (2023). P2RX7 signaling drives the differentiation of Th1 cells through metabolic reprogramming for aerobic glycolysis. Front. Immunol 14, 1140426. 10.3389/fimmu.2023.1140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borges da Silva H, Wang H, Qian LJ, Hogquist KA, and Jameson SC (2019). ARTC2.2/P2RX7 Signaling during Cell Isolation Distorts Function and Quantification of Tissue-Resident CD8(+)T Cell and Invariant NKT Subsets. J. Immunol 202, 2153–2163. 10.4049/jimmunol.1801613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amaral EP, Machado de Salles É, Barbosa Bomfim CC, Salgado RM, Almeida FM, de Souza PC, Alvarez JM, Hirata MH, Lasunskaia EB, and D’Império-Lima MR (2019). Inhibiting Adenosine Receptor Signaling Promotes Accumulation of Effector CD4+ T Cells in the Lung Parenchyma During Severe Tuberculosis. J. Infect. Dis 219, 964–974. 10.1093/infdis/jiy586. [DOI] [PubMed] [Google Scholar]
- 60.Barbosa Bomfim CC, Pinheiro Amaral E, Santiago-Carvalho I, Almeida Santos G, Machado Salles É, Hastreiter AA, Silva do Nascimento R, Almeida FM, Lopes Biá Ventura Simão T, Linhares Rezende A, et al. (2021). Harmful Effects of Granulocytic Myeloid-Derived Suppressor Cells on Tuberculosis Caused by Hypervirulent Mycobacteria. J. Infect. Dis 223, 494–507. 10.1093/infdis/jiaa708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva de Melo BM, Veras FP, Zwicky P, Lima D, Ingelfinger F, Martins TV, da Silva Prado D, Schärli S, Publio G, Hiroki CH, et al. (2023). S100A9 Drives the Chronification of Psoriasiform Inflammation by Inducing IL-23/Type 3 Immunity. J. Invest. Dermatol 143, 1678–1688.e8. 10.1016/j.jid.2023.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on request.
All sequencing reads generated in this study and processed RNA expression matrices are deposited in NCBI’s GEO and are publicly available as of the date of publication. The accession number GSE244194 is listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE I-Ab Mycobacterium tuberculosis ESAT-64-1 tetramer | NIH Tetramer Core Facility | https://tetramer.yerkes.emory.edu/ |
| APC I-Ab Influenza A 2W1S tetramer | NIH Tetramer Core Facility | https://tetramer.yerkes.emory.edu/ |
| FITC anti-mouse CD45 | Biolegend | Cat# 147709; RRID:AB_2563542 |
| BUV395 anti-mouse CD4 | BD Biosciences | Cat# 563790; RRID:AB_2738426 |
| BV510 anti-mouse/human CD44 | Biolegend | Cat# 103043; RRID:AB_2561391 |
| PE anti-mouse P2X7 | Biolegend | Cat# 148703; RRID:AB_2650951 |
| BV786 anti-mouse P2X7 | BD Biosciences | Cat# 744724; RRID:AB_2742436 |
| BV711 anti-mouse/human KLRG1 | Biolegend | Cat# 138427; RRID:AB_2629721 |
| BV786 anti-mouse CX3CR1 | Biolegend | Cat# 149029; RRID:AB_2565938 |
| PE/Cyanine7 anti-mouse CD69 | Biolegend | Cat# 104511; RRID:AB_493565 |
| BV650 anti-mouse CXCR3 | Biolegend | Cat# 126531; RRID:AB_2563160 |
| PE anti-mouse/human Ki-67 | Biolegend | Cat# 151209; RRID:AB_2716014 |
| BV650 anti-mouse IFN-y | Biolegend | Cat# 505831; RRID:AB_11142685 |
| PE-CF594 anti-mouse FOXP3 | BD Biosciences | Cat# 562466; RRID:AB_11151905 |
| PerCP-Cyanine5.5 anti-mouse T-bet | Biolegend | Cat# 644805; RRID:AB_1595593 |
| BV421 anti-mouse CD45.1 | Tongo Biosciences | Cat# 75-0453-U025; RRID:AB_2621949 |
| APC anti-mouse CD45.2 | Biolegend | Cat# 109813; RRID:AB_389210 |
| Percp anti-mouse CD45.2 | Biolegend | Cat# 109825; RRID:AB_893351 |
| BV421 anti-mouse/human CD4 | BD Biosciences | Cat# 562425; RRID:AB_11154221 |
| APC-Cyanine7 anti-mouse/human CD44 | Biolegend | Cat# 103027; RRID:AB_830784 |
| PE anti-mouse-human CXCR3 | BioLegend | Cat# 353705; RRID:AB_10959652 |
| PE-Cyanine7 anti-human Ki-67 | BioLegend | Cat# 350525; RRID:AB_2562871 |
| Anti-mouse S100A9 | Bio-Techne | Cat# AF2065; RRID:AB_2184263 |
| PE anti-mouse CD4 | Biolegend | Cat# 100407; RRID:AB_312693 |
| FITC anti-rabbit antibody | BD Biosciences | Cat# 554020; RRID:AB_395212 |
| APC anti-mouse IFN-y | Biolegend | Cat# 505809; RRID:AB_315403 |
| InVivoMAb anti-mouse CD4 | BioXCell | Cat# BE0003-1; RRID:AB_1107636 |
| InVivoMAb anti-mouse CXCR3 (CD183) | BioXCell | Cat# BE0249; RRID:AB_2687730 |
| Anti-ARTC2.2 Treg-protector nanobodie | Biolegend | Cat# 149802; RRID:AB_2565485 |
| Pe/Cyanine5 anti-mouse CD69 | Biolegend | Cat# 104510; RRID:AB_313113 |
| BV785 anti-mouse/human CD44 | Biolegend | Cat# 103041; RRID:AB_11218802 |
| Bacterial and virus strains | ||
| Mycobacterium bovis MP287/03 | José Soares Ferreira Neto, PhD | Grown in house |
| Mycobacterium tuberculosis H37Rv | American Type Culture Collection | Grown in house |
| Influenza A PR8/34 | Jie Sun, PhD | Grown in house |
| Influenza A PR8/2W | Ryan Langlois, PhD | Grown in house |
| Chemicals, peptides, and recombinant proteins | ||
| Middlebrook 7H9 medium | Becton Dickinson | Cat# 271310 |
| Middlebrook 7H10 Agar | Becton Dickinson | Cat# 262710 |
| Middlebrook ADC Growth Supplement | Sigma-Aldrich | Cat# M0553-1VL |
| Middlebrook OADC Growth Supplement | Sigma-Aldrich | Cat# M0678-1VL |
| RPMI 1640 Medium | Gibico | Cat# 11875093 |
| Ketamine | Syntec | N/A |
| Xylazine | Syntec | N/A |
| CFSE | Abcam | Cat# ab113853 |
| CellTrace Violet | Thermo Fisher Scientific | Cat# C34557 |
| Fingolimod (FTY720) | Selleck Chemicals | Cat# S5002 |
| Collagenase type IV | Gibico | Cat# 17104019 |
| ACK Lysing Buffer | This paper | Made in house |
| PBS (Phosphate Buffered Saline) 1x | Corning | Cat# 21-040-CV |
| ProLong Gold antifade | Invitrogen | Cat# P36934 |
| Bovine serum albumin (BSA) | Sigma-Aldrich | Cat# A9418 |
| Fetal Bovine Serum | Gibico | Cat# A52567-01 |
| Critical commercial assays | ||
| EasySep Mouse CD4+ T cell isolation Kit | Stem Cell | Cat# 19765 |
| True Nuclear Transcription Factor Buffer Set | Biolegend | Cat# 424401 |
| BD Cytofix/Cytoperm Plus | BD Biosciences | Cat# 554715 |
| RNA isolation RNeasy Kits | QIAGEN | Cat# 74004 |
| Deposited data | ||
| Raw and analyzed data | This paper | GEO: GSE244194 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Jackson Laboratory | Cat# 000664; RRID:IMSR_JAX:000664 |
| CD45.1 congenic mice | Jackson Laboratory | Cat# 002014; RRID:IMSR_JAX:002014 |
| Cd4−/− mice | Jackson Laboratory | Cat# 002663; RRID:IMSR_JAX:002663 |
| P2rx7−/− mice | Jackson Laboratory | Cat# 005576; RRID:IMSR_JAX:005576 |
| CD4-Cre | Jackson Laboratory | Cat# 022071; RRID:IMSR_JAX:022071 |
| P2rx7 flox/flox | György Haskó - Columbia University | N/A |
| Oligonucleotides | ||
| PR8-NP fwd: 5′-GATTGGTGGAATTGGACGAT-3′ | Thermo Fisher Scientific | N/A |
| PR8-NP rev: 5′-AGAGCACCATTCTCTCTATT-3′ | Thermo Fisher Scientific | N/A |
| Software and algorithms | ||
| FlowJo 10.7.1 | BD Biosciences | https://www.flowjo.com/; RRID:SCR_008520 |
| Prism 9 | Graphpad Software | https://www.graphpad.com/; RRID:SCR_002798 |
| Fiji Java 8 | ImageJ | https://github.com/imagej/ImageJ.git; RRID:SCR_002285 |
| Biorender | Biorender | https://Biorender.com; RRID:SCR_018361 |
| BGI | N/A | https://www.bgi.com/global/dr-tom/; RRID:SCR_011114 |