SUMMARY
Adoptive cellular therapy using chimeric antigen receptor (CAR) T cells has led to a paradigm shift in the treatment of various hematologic malignancies. However, the broad application of this approach for myeloid malignancies and solid cancers has been limited by the paucity and heterogeneity of target antigen expression, and lack of bona-fide tumor-specific antigens that can be targeted without cross-reactivity against normal tissues. This may lead to unwanted on-target off-tumor toxicities that could undermine the desired anti-tumor effect. Recent advances in synthetic biology and genetic engineering have enabled reprogramming of immune effector cells to enhance their selectivity towards tumors, thus mitigating on-target off-tumor adverse effects. In this review, we outline the current strategies being explored to improve CAR selectivity towards tumor cells with a focus on natural killer (NK) cells, and the progress made in translating these strategies to the clinic.
Keywords: Anti-tumor immunotherapy, chimeric antigen receptor (CAR), synthetic immune cell engineering, T cells, natural killer (NK) cells
1. Introduction
In recent decades, the field of adoptive cellular therapy has undergone substantial developments in various clinical settings1. Notably, chimeric antigen receptor (CAR) T cells have emerged as a groundbreaking therapeutic modality for patients with certain types of relapsed and refractory hematologic malignancies2–6. The advent of CAR engineering resulted from extensive research endeavors primarily focused on developing “living drugs” capable of specifically targeting and eliminating tumors. The initial work in the field of adoptive cell therapy consisted of isolating tumor-infiltrating lymphocytes (TILs) with natural specificity against mutated proteins on tumor cells, expanding them ex vivo and re-infusing them to patients. While this strategy showed promising responses in certain types of cancers such as melanoma7, its broad applicability has been limited by the complexity of harvesting TILs and their poor expansion in vitro8. The emergence of sophisticated genetic engineering techniques allowed the modification of T cells to express a T-cell receptor (TCR) against certain tumor antigens. As such, T cells have been engineered to recognize a variety of tumor antigens such as NY-ESO-19,10, PRAME11,12, and selected MAGE-A family members10,13, to treat patients with various cancers such as sarcoma and multiple myeloma9,14,15.
As TCR T cell therapy is restricted by specific human leukocyte antigens (HLA) alleles, and as cancer cells commonly evade TCR recognition by downregulation of their major histocompatibility complex (MHC) proteins, the emergence of CAR-based therapy constituted a major advancement in the field of adoptive cell therapy due to its MHC-independent mechanism of antigen recognition16. In fact, CAR T cell therapy has resulted in remarkable responses in some hematologic cancers and its application has quickly evolved to its current Food and Drug Administration (FDA) approval for B-lymphoid malignancies and multiple myeloma.
However, challenges associated with the autologous nature of these products have limited their widespread implementation. The manufacturing process for CAR T cell therapies is cumbersome and costly, resulting in lengthy collection to administration times, thus posing a challenge for patients who, due to rapidly progressing disease, are in urgent need of treatment17. Moreover, patient-derived T cells may be limited in number or compromised in function, especially in patients who have been heavily treated prior to CAR T administration18,19. These limitations sparked a growing interest in alternative allogeneic cell sources that are off-the-shelf and available for point-of-care use. One approach focuses on developing allogeneic CAR T cells through genome editing to abrogate the endogenous expression of αTCR and/or MHC class I complexes, thus eliminating T cell alloreactivity and reducing the risk of graft-versus-host disease (GvHD)20,21. These ‘universal’ CAR T cells can be manufactured in large scale from healthy donor sources and administered to patients more safely. The first off-the-shelf CAR T cell product to be investigated in clinical trials was UCART19 for the treatment of patients with B-cell acute lymphoblastic leukemia (ALL)22,23. While early results with allogeneic CAR T cells are promising, challenges remain, including allo-rejection of the infused product by the recipient immune system, technical difficulties with achieving 100% editing efficiency and thus the risk of GvHD, and potential risks related to gene editing such as off-target editing, genotoxicity and acquisition of chromosomal abnormalities24. Alternative immune effector cells, such as natural killer (NK) cells25 and invariant NK T (iNKT)/NKT cells26, are actively being explored as vehicles for CAR engineering due to their high cytotoxic potential and low risk of GvHD in the allogeneic setting. Of these, NK cells have been one of the most extensively explored alternative immune cells for adoptive cell therapy.
As with any targeted approach, the broad application of CAR T cell and CAR NK cell therapy has been limited by the paucity of targetable tumor-specific antigens (TSAs). Indeed, many tumor antigens are either inherently expressed at low levels or eventually downregulated as a mechanism of tumor escape from the targeted CAR cell therapy. Moreover, tumor antigens are often also expressed on normal healthy tissues, which might lead to life-threatening on-target off-tumor side-effects of CAR cell therapy19,27–30. To overcome these challenges, extensive translational research has been conducted to design the next generation of CAR immune cells with high potency and tumor selectivity.
In this review, we highlight the current approaches to CAR engineering, with a focus on NK cells, including strategies to enhance their on-target anti-tumor selectivity, and discuss the advances achieved in translating these innovations to the clinic.
2. The modular design of a CAR
Antigen recognition by a CAR is achieved via its extracellular domain, which conventionally employs the binding domain of a single-chain variable fragment (scFv), derived from a monoclonal antibody (mAb), to specifically recognize a tumor antigen. The CAR then transmits an activation signal to the carrier cell, resulting in a potent and targeted cytotoxicity. The CAR molecule consists of three parts: an extracellular domain, a transmembrane domain, and an intracellular domain6. The transmembrane domain is linked by a hinge region to an extracellular domain and is commonly derived from IgG, CD8, or CD28. The transmembrane fraction guides the CAR molecule to the cellular membrane. The intracellular region of the CAR molecule contains activating signaling molecules such as CD28, CD27, 4–1BB, DAP10, or CD3ζ, either individually or in various combinations, and serves to deliver stimulatory signals upon antigen ligation. CAR design has evolved significantly over the past decades to encompass four CAR generations: (i) first generation consisting of a single stimulatory domain (CD3ζ only), (ii) second generation containing a costimulatory domain along with CD3ζ, (iii) third generation containing more than one costimulatory domain in addition to CD3ζ, and (iv) fourth generation incorporating a transgenic protein such as a cytokine with constitutive or inducible expression (referred to as TRUCKs for “T cells redirected for antigen unrestricted cytokine-initiated killing”)31,32. The CAR transgene is introduced into the effector cells through DNA plasmid transfection or viral-based transduction, leading to CAR expression on the plasma membrane.
3. Current limitations of CAR T cell therapies
CAR T cells have led to impressive outcomes in patients with lymphoid malignancies and multiple myeloma6, with six FDA-approved products currently available for eight indications33–43. Despite the remarkable clinical success of CAR T cell therapies in hematologic malignancies, their expanded clinical use has been limited by several factors. In addition to the arduous, time-consuming, and costly manufacturing process of autologous products, CAR T cell therapy has a unique toxicity profile, characterized by cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS)19,44. While more research is needed to determine the risk factors predisposing patients to CAR T cell toxicity, certain associated factors have been established44,45. Other major limitations of CAR T cell therapy pertain to the target antigens themselves, including on-target off-tumor toxicity due to the expression of the target antigens on normal tissues. Hence, identifying an ideal target antigen with the right balance of sensitivity and selectivity for CAR-based cell therapy has been a major challenge for cancers beyond lymphoid malignancies.
CAR NK cells offer several advantages, including a broad range of donor cell source for manufacturing, lower risk of toxicities, and multiple mechanisms of cytotoxicity, making them a promising approach for cell-based immunotherapy. Indeed, CAR NK therapy circumvents the requirement for autologous NK cells due to their reduced risk of alloreactivity and GvHD46. Thus, existing NK cell sources such as NK-92 cell lines, umbilical cord blood (CB), peripheral blood (PB) and induced pluripotent stem cells (iPSCs) can be leveraged for the large-scale production of “off-the-shelf” CAR NK cells. The second major advantage of CAR NK cell therapy is their excellent safety profile with a lower incidence of CRS and neurotoxicity compared to CAR T cell therapy. This difference could be attributed to the distinct profile of cytokines secreted by CAR NK cells, with lower production of cytokines classically associated with CRS such as interleukin (IL)-1β, IL-2 and IL-647. Thirdly, in addition to the targeted cytotoxicity mediated by the CAR, NK cells possess multiple intrinsic mechanisms for tumor recognition as described in the next section. Thus, CAR NK cells could theoretically overcome the challenge of tumor escape through downregulation of the target antigen reported with CAR T cell therapy.
4. NK cell biology
NK cells are part of the innate immune system and unlike T cells, exert their cytotoxicity in an HLA-independent manner and without the need for prior priming48. In fact, multiple preclinical and clinical studies have confirmed the role of NK cells in tumor immunosurveillance and control of metastases49–51. NK cells are characterized by the distinct expression of CD56 and the absence of TCR and CD3 expression52. They are broadly categorized based on the relative expression of the cell surface receptors CD56 and CD16 into two classes: CD56bright CD16low/– NK cells, characterized by immunomodulatory and cytokine-producing properties, and CD56dim CD16+ NK cells that are generally cytotoxic53. Notably, high-parameter cytometry and single-cell proteo-genomics have revealed much greater phenotypic and functional heterogeneity of NK cells than previously appreciated and have enabled the identification of diverse NK cell subpopulations extending far beyond the two known subsets. The CD16 receptor on NK cells binds to the Fc receptor on antibody-coated cells to exert potent antibody-dependent cell cytotoxicity (ADCC)52. Additionally, NK cells can be activated through an intricate interplay between activating and inhibitory germline-encoded receptors present on their cell surface54. These receptors convey signals of either activation (via immunoreceptor tyrosine-based activation motifs or ITAMs) or inhibition (via immunoreceptor tyrosine-based inhibition motifs or ITIMs)55. Notably, cancer cells downregulate MHC class I expression or upregulate stress-induced molecules like MICA/MICB, thereby disengaging inhibitory killer Ig-like receptors (KIRs; the “missing-self” phenomenon) or engaging activating receptors such as NKG2D, respectively. Consequently, NK cells become activated, directing their cytotoxic efforts towards the target cells in a finely orchestrated manner56. Furthermore, NK cells interact with other immune cells through the production of cytokines and chemokines. As such, they have been shown to impact the function of T and B lymphocytes, dendritic cells (DCs), macrophages, and neutrophils57. These diverse roles highlight the complex biological functions of NK cells and underscore their promise for immunotherapy.
While our classical comprehension of NK cells as part of the innate immune system depicts them as fast-acting and short-lived without antigen specificity, exciting discoveries in recent years have defied this convention. In fact, multiple research groups have reported that under specific circumstances, NK cells undergo clonal expansion in response to antigen stimulation to give rise to long-lasting memory cells58. Adaptive NK cells were first studied in murine models of cytomegalovirus (MCMV) infection, revealing that NK cells bearing virus-specific receptors such as Ly49H, Ly49L, and NKR-P1A exhibit rapid proliferation, cytokine production, and clonal expansion upon MCMV reactivation, reminiscent of the memory-like properties typically attributed to adaptive immune cells59. Moreover, Wayne Yokoyama’s group showed that NK cells in mice acquire memory-like features in response to stimulation with inflammatory cytokines60. Similarly, human adaptive NK cells have been identified58 and studied most extensively in the context of human CMV infection61,62. Cytokine-induced memory of human NK cells has also been investigated. In fact, human NK cells that were activated with IL-12, IL-15, and IL-18 and rested for one to three weeks, were shown to exhibit robust anti-tumor response characterized by augmented interferon (IFN)γ production and proliferation in response to cytokines or exposure to K562 leukemia cells63,64. Numerous other research groups have reported analogous memory-like functionality of NK cells in various immunological contexts, challenging the classical delineation of innate and adaptive immunity64,65.
5. Clinical progress of CAR NK cell therapy
CAR NK cell immunotherapy is emerging as an attractive therapeutic option for cancer, with encouraging clinical responses reported in multiple phase I/II trials (Table 1). Given the promising clinical activity and safety of NK cell therapy, it is imperative to define the best NK cell source and CAR design for adoptive cell therapy.
Table 1.
Current clinical trials of CAR NK cell-based anti-tumor therapy.
| CAR strategy | NK cell source | Target antigen | Cancer | Phase | Status | NCT No. |
|---|---|---|---|---|---|---|
| scFv-CAR | Undisclosed | CD33 | AML | I | Recruiting | NCT05008575 |
| NK-92 | CD33 | AML | I/II | Unknown | NCT02944162 | |
| NK-92 | ROBO1 | Pancreatic cancer | I/II | Unknown | NCT03941457 | |
| NK-92& | ROBO1 | Solid tumors | I/II | Unknown | NCT03931720 | |
| NK-92 | ROBO1 | Solid tumors | I/II | Unknown | NCT03940820 | |
| iPSC-NK | PSMA | Prostate cancer | Early I | Recruiting | NCT03692663 | |
| NK-92 | PD-L1 | Gastric cancer/Head and neck cancer | II | Recruiting | NCT04847466 | |
| NK-92 | PD-L1 | Pancreatic cancer | II | Recruiting | NCT04390399 | |
| NK-92 | PD-L1 | Solid tumors | I | Active, not recruiting | NCT04050709 | |
| NK-92 | PD-L1 | Solid tumors | II | Active, not recruiting | NCT03228667 | |
| HSC-NK | MUC1 | Solid tumors | I/II | Unknown | NCT02839954 | |
| iPSC-NK | Mesothelin | Ovarian cancer | Early I | Unknown | NCT03692637 | |
| NK-92 | HER-2 | Glioblastoma | I | Recruiting | NCT03383978 | |
| Primary NK | CLDN6 | Solid tumors | I/II | Recruiting | NCT05410717 | |
| NK-92 | CD7 | Leukemia/Lymphoma | I/II | Unknown | NCT02742727 | |
| CB- NK | CD5 | Hematologic malignancies | I/II | Not yet recruiting | NCT05110742 | |
| iPSC-NK | CD22 | B cell malignancies | Early I | Unknown | NCT03692767 | |
| CB-NK | CD19 | B cell malignancies | I/II | Completed | NCT03056339 | |
| CB-NK | CD19 | B cell malignancies | I | Recruiting | NCT04796675 | |
| CB-NK | CD19 | B cell malignancies | I | Recruiting | NCT04796688 | |
| Primary NK | CD19 | B cell malignancies | I | Completed | NCT00995137 | |
| Primary NK | CD19 | B cell malignancies | I | Recruiting | NCT05410041 | |
| Primary NK | CD19 | B cell malignancies | I | Recruiting | NCT05020678 | |
| Primary NK | CD19 | B cell malignancies | I | Recruiting | NCT05379647 | |
| Primary NK | CD19 | B cell malignancies | I | Suspended | NCT01974479 | |
| NK-92 | CD19 | B cell malignancies | I/II | Unknown | NCT02892695 | |
| iPSC-NK | CD19 | B cell malignancies | I | Recruiting | NCT05336409 | |
| iPSC-NK | CD19 | B cell malignancies | Early I | Unknown | NCT03690310 | |
| iPSC-NK | CD19 | B cell malignancies | Early I | Unknown | NCT03824951 | |
| CB-NK | CD19 | NHL | I | Not yet recruiting | NCT04639739 | |
| CB-NK | CD19 | NHL | II | Recruiting | NCT05020015 | |
| CB-NK | CD19 | NHL | I | Recruiting | NCT05472558 | |
| Primary NK | CD19 | NHL | I | Recruiting | NCT04887012 | |
| NK-92 | BCMA | MM | I/II | Unknown | NCT03940833 | |
| CB-NK | BCMA | MM | I | Recruiting | NCT05008536 | |
| Undisclosed | 5T4 | Solid tumors | I | Recruiting | NCT05194709 | |
| Undisclosed | 5T4 | Solid tumors | I | Recruiting | NCT05137275 | |
| NK-92 | DLL3 | SCLC | I | Recruiting | NCT05507593 | |
| NRB-CAR | CB-NK | CD70 | RCC/Mesothelioma/Osteosarcoma | I/II | Recruiting | NCT05703854 |
| CB-NK | CD70 | Hematologic malignancies | I/II | Recruiting | NCT05092451 | |
| CB-NK | NKG2D ligand | AML | I | Terminated | NCT05247957 | |
| Primary NK | NKG2D ligand | AML | I | Recruiting | NCT04623944 | |
| Undisclosed | NKG2D ligand | Colorectal cancer | I | Recruiting | NCT05213195 | |
| Primary NK | NKG2D ligand | Solid tumors | I | Unknown | NCT03415100 | |
| NK-92 | NKG2D ligand | Solid tumors | I | Recruiting | NCT05528341 | |
| NK-92 | PD-L1 | NSCLC | I | Recruiting | NCT03656705 | |
| Dual-targeting CAR | iPSC-NK | CD19/CD22 | B cell malignancies | Early I | Unknown | NCT03824951 |
| iPSC-NK | CD19/CD22 | B cell malignancies | Early I | Unknown | NCT03824964 | |
| CB-NK | CD33/CLL1 | AML | I | Recruiting | NCT05215015 | |
| Dual-targeting CAR (CAR+hnCD16) | iPSC-NK | CD19/CD20 | B cell malignancies | I | Recruiting | NCT04245722 |
| iPSC-NK | BCMA/CD38 | MM | I | Recruiting | NCT05182073 |
CB: cord blood; scFv: singe-chain variable fragment; NRB: natural receptor-based; hnCD16: high affinity non-cleavable CD16; HSC: hematopoietic stem cell; iPSC: induced pluripotent stem cell; AML: acute myeloid leukemia; MM: multiple myeloma; NSCLC: Non-small cell lung cancer; NHL: non-hodgkin's lymphoma; SCLC: small cell lung cancer; RCC: renal cell carcinoma.
: Combination therapy with anti-ROBO1 CAR T cells
5.1. NK cell sources for clinical use
Current NK cell therapy platforms rely on various sources of cells for therapeutic applications. These include PB, CB, cell lines, hematopoietic stem and progenitor cells (HSPCs), and iPSCs66. Despite their potential for generating scalable and clinically significant NK cell doses for CAR NK cell therapy, each of these sources has distinct characteristics, presenting both unique advantages and challenges.
PB-derived NK cells can be obtained through apheresis from healthy donors and have been extensively studied and widely used in clinical trials of CAR NK cell therapy (e.g., NCT00995137, NCT01974479, NCT05020678, NCT04623944). CB is another valuable source of NK cells for clinical use. The ease of collecting CB units and the ability to cryopreserve them offer unique advantages of this source for NK immunotherapy. Our group has focused on the use of CB-NK cells for CAR engineering, demonstrating the ability to generate over a hundred dose of CAR NK cells from a single CB unit67,68.
NK-92 is an immortalized NK lymphoma cell line that has received Investigational New Drug approval by the FDA for clinical testing. NK-92 cells offer a homogeneous and abundant cell source69 for CAR engineering, however, their cancerous origin necessitates irradiation prior to administration that could limit their in vivo proliferation and persistence. Additionally, without extra engineering steps, cell lines like NK-92 may lack certain functional capabilities, such as the ability to mediate ADCC due to the absence of CD16 expression70.
HSPCs and iPSCs present exciting prospects for NK cell therapy, as they are characterized by clonal growth and high expansion capacity. Differentiation of these stem cells into NK cells allows for the manufacturing of large numbers of homogeneous NK cell products71. However, challenges exist, such as concerns with epigenetic memory of their cellular origin with iPSC-derived NK cells72. Ongoing research is focused on optimizing the use of these cell sources and determining their efficacy and safety in clinical applications.
5.2. Clinical experience with CAR NK cell therapy
In recent years, CAR NK cell therapy has emerged as a promising approach for the immunotherapy of cancer. The clinical safety of administering CAR NK cells was demonstrated in a phase I study in 2018 by a group based in China (NCT02944162)73. The trial used NK-92 cells engineered to express a third-generation CD33-directed CAR construct incorporating CD28 and 4–1BB co-stimulatory domains for the treatment of acute myeloid leukemia (AML)73. While the study reported an excellent safety profile in three patients with relapsed/refractory disease, no durable responses were achieved73. This was mainly attributed to the limited in vivo persistence of the irradiated CAR NK-92 cells73.
Our group investigated the use of cytokine armoring to enhance the in vivo persistence and proliferation of CAR NK cells67. In a first-in-human phase I/II clinical trial, we reported the excellent safety and promising activity of IL-15 armored CB-derived CAR19 NK cells in patients with relapsed/refractory B-lymphoid malignancies (NCT03056339)67. Notably, CAR19 NK cells were detectable up to one year post-infusion, and patients who responded to treatment exhibited substantially higher blood peak copy numbers of CAR19 NK cells67. These results support the incorporation of cytokine armoring to enhance the persistence and clinical activity of NK cells.
iPSC-derived NK cells offer another attractive platform for CAR engineering. FT596 is an engineered iPSC-derived NK cell product that incorporates three genes encoding: (i) a high-affinity non-cleavable Fc receptor (hnCD16) that has been modified to include the 158V variant in combination with an S197P amino acid substitution to prevent cleavage by ADAM1774, (ii) a membrane-bound IL-15/IL-15 receptor (IL-15R) fusion protein, and (iii) an anti-CD19 CAR75. Interim clinical results reported as of June 2021 demonstrated the safety of FT596 with no dose-limiting toxicities76. A total of 20 patients underwent dose escalation treatment, with ten patients receiving FT596 alone (Regimen A) and ten patients receiving FT596 cells combined with rituximab (Regimen B)76. Of the evaluable patients, the overall response rate (ORR) following the first treatment cycle was 5 of 8 patients (62%) in Regimen A and 4 of 9 patients (44%) in Regimen B76. Longer follow-up data will help elucidate the durability of the response and the overall efficacy of this platform.
Similarly, preliminary results from a phase I study (NCT05020678) of off-the-shelf allogeneic CAR19-engineered PB-NK cells expressing a membrane-bound form of IL-15 (NKX019) were recently reported in a press release77. This therapy achieved a complete response rate of 70% (seven of ten patients) in patients with relapsed/refractory non-Hodgkin lymphoma and durable responses of greater than six months in multiple patients77. These encouraging results collectively support the promise of NK cells as alternative immune effectors for CAR cell therapy, with over 45 trials currently registered on clinicatrials.gov (Table 1).
6. The application of CAR engineering for solid tumors
A major challenge in the translation of CAR T cell or CAR NK cell therapies from hematologic malignancies to solid tumors is the identification of target antigens that are widely and homogeneously expressed at high levels on tumor cells while having virtually no expression on normal tissues. TSAs, such as EGFRvIII in glioblastoma78, are considered as ideal targets due to their exclusive expression on tumor cells. However, they also present unique challenges79 primarily due to their heterogeneous levels of expression on tumor cells. Thus, the generation of CARs targeting TSAs would require screening individual patients for target antigen expression and manufacturing a custom product, which is costly and time-consuming79. Due to the paucity of known TSAs, other antigens that have higher expression on tumor cells but are not exclusive to tumor cells (referred to as tumor-associated antigens (TAAs), have been explored. While a myriad of TAAs have been investigated for CAR T cell therapy including HER-280, mesothelin (MSLN)81, CEA82, etc., their clinical success has been limited by concerns related to on-target off-tumor targeting of normal tissues, among others.
The extent and severity of on-target off-tumor toxicity depend on a variety of factors: (i) CAR T or CAR NK cell accessibility to healthy tissues that express the target antigen; (ii) the type of tissue and its physiological activity; (iii) the expression level and cellular localization of the antigen; and (iv) the potency of the CAR-engineered cells. For instance, due to the expression of CD19 on healthy B cells, B cell aplasia has been observed in clinical trials using CAR T cells targeting CD1983–86. Though B cell aplasia is manageable by supplementation with intravenous immunoglobulins (IVIG), long-term B cell depletion and its associated hypogammaglobulinemia increase the risk of severe infections87–89, and are associated with a decreased response to vaccinations (NCT04724642, NCT04410900)90–92. Moreover, ICANS toxicity in CAR19 T cell-treated patients may represent an on-target off-tumor side-effect due to CAR-mediated cytotoxicity targeting low levels of CD19 expressed on brain mural cells93. Similarly, cross-reactivity of CAR-modified immune cells against BCMA-expressing neurons and astrocytes is thought to contribute to the neurocognitive and hypokinetic movement disorders seen after infusion of BCMA CAR T cells94. Treatment of patients with CAR T cells recognizing carbonic anhydrase 9 (CA-IX)95,96, HER-280, ERBB-297, or MSLN81 has been associated with severe side-effects including acute respiratory failure and organ damage, most likely from the expression of these antigens at differing levels on epithelial cells in the lungs and the bile ducts, respectively.
On-target off-tumor toxicity has also been associated with CAR-mediated antigen recognition of a mimotope, which mimics the structure of the targeted epitope but belongs to a distinct antigen expressed on normal cells98. Notably, preclinical murine models may not adequately predict the off-tumor antigen binding and toxicity potential of a given CAR, and more research is needed to develop dedicated in vivo models for the study of on-target off-tumor toxicity99. Some strategies have been devised to curb the deleterious on-target off-tumor toxicities in patients treated with CAR immune therapy, such as the administration of immunosuppressive regimens or activation of safety switches including the inducible Caspase nine suicide (iC9) gene system as used in our iC9/CAR19/IL-15 NK cell clinical trial67. However, these strategies are often merely reactive rather than preventative, and may also compromise the therapeutic benefit of the engineered cells100–103.
Lastly, two important CAR-mediated on-target off-tumor effects are fratricide and trogocytosis. Fratricide occurs when CAR-expressing immune cells kill their sibling cells that also endogenously express the cognate antigen. Examples include CAR T cells targeting CD7104, CD38105,106 or CD70107, antigens that are expressed on normal or activated T cells in addition to cancer cells. This may then result in manufacturing challenges, poor in vivo persistence of the infused product or prolonged immunodeficiency through targeting of normal recipient T cells. Trogocytosis is also an important mediator of fratricide. Trogocytosis corresponds to the receptor-mediated transfer of cognate antigen from tumor cells to the receptor-expressing immune cells108, and contributes to tumor escape and poor responses after CAR T and CAR NK cell therapy by causing antigen loss, NK cell exhaustion and fratricide109–111. Our studies on clinical samples from patients with lymphoid malignancies who received anti-CD19 CAR NK cell treatment confirmed a direct correlation between elevated CD19 levels on CAR NK cells secondary to trogocytosis and reduced CD19 expression on tumor cells, associated with a greater likelihood of relapse109. Taken together, these data indicate the need for innovative and rationally designed CARs to increase tumor specificity while preventing on-target off-tumor toxicities.
6.1. Exploration of novel targeting approaches
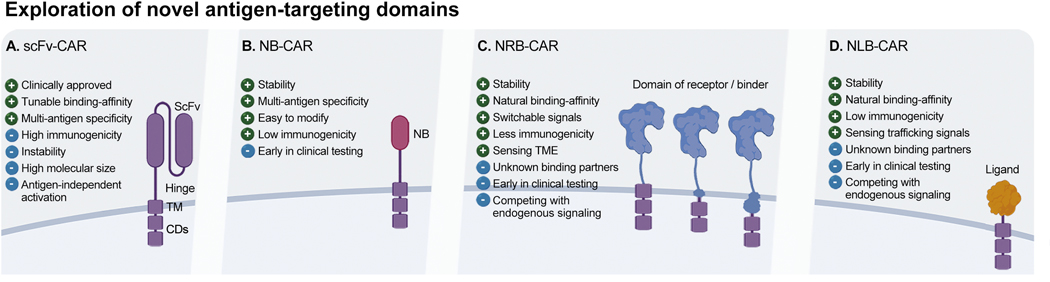
To improve CAR-mediated on-target anti-tumor activity, research and clinical investigations are now focusing on adapting CAR technology to other cancers, while also enhancing potency and/or safety (Table 1 & 2). As scFvs have perceivable limitations such as promoting self-aggregation of the CAR molecule which can in turn lead to premature CAR activation and exhaustion of the transduced immune effector (Fig. 1A)112–114, alternative binding domains have been explored. These include nanobodies (NBs), recombinant antigen-specific scFvs derived from the heavy chain (VHH) of a mAb. An NB has a similar antigen-binding affinity to a conventional or full-length mAb, but due to its smaller size, has greater solubility and more stable physiochemical properties (Fig. 1B)115. Using NBs to generate a CAR could be advantageous due to their enhanced stability, making them more amenable to additional modifications such as multi-targeting116. NB-CAR NK-92 cells targeting CD38 demonstrated targeted cytotoxicity against patient-derived CD38-expressing multiple myeloma cells, but the in vivo efficacy of this approach remains to be validated117. Similarly, CD7-targeted NB-based CAR NK-92 cells mediated strong cytotoxicity in vitro against T-cell leukemia cell lines and primary tumor cells, and in vivo in T-ALL xenograft mouse models118. These and other studies support the further exploration of NBs as the targeting domain of CAR constructs for NK cell immunotherapy.
Table 2.
Current clinical trials of CAR T cell-based anti-tumor therapy using “next-generation” CAR strategies.
| CAR strategy | Target antigen | Cancer type | Phase | Status | NCT No. |
|---|---|---|---|---|---|
| NB-CAR | CD7 | Hematologic malignancies | I | Unknown | NCT04004637 |
| NB-CAR+ tandem CAR | CD19/CD20 | B cell malignancies | I | Unknown | NCT03881761 |
| BCMA | MM | I/II | Unknown | NCT03090659 | |
| NRB-CAR | CD70 | Various*1 | I/II | Suspended | NCT02830724 |
| Fc | Various*2 | NA | Terminated | NCT02840110 | |
| Fc (HER2) | Solid tumors | I | Terminated | NCT03680560 | |
| Fc (CD20) | B cell malignancies | I | Completed | NCT02776813 | |
| Fc (CD20) | B cell malignancies | I | Terminated | NCT03189836 | |
| Fc (BCMA) | MM | I | Terminated | NCT03266692 | |
| NKG2D ligand | Various*3 | I | Not yet recruiting | NCT05302037 | |
| NKG2D ligand | Solid tumors | I | Unknown | NCT04107142 | |
| NKG2D ligand | Solid tumors | I | Recruiting | NCT05131763 | |
| NKG2D ligand | Hematologic malignancies | I | Completed | NCT03612739 | |
| NKG2D ligand | Hematologic malignancies | I | Withdrawn | NCT03018405 | |
| NKG2D ligand | Hematologic malignancies | I/II | Unknown | NCT05248048 | |
| NKG2D ligand | Colorectal cancer | Early I | Recruiting | NCT03310008 | |
| NKG2D ligand | Colorectal cancer | I | Unknown | NCT03692429 | |
| NKG2D ligand | Colorectal cancer | I | Recruiting | NCT04991948 | |
| NKG2D ligand | Colorectal cancer | I | Recruiting | NCT03370198 | |
| NKG2D ligand | Colorectal cancer | I | Unknown | NCT03466320 | |
| NKG2D ligand | AML | I/II | Completed | NCT04167696 | |
| NKG2D ligand | AML | I | Recruiting | NCT05131763 | |
| PSMA | Prostate cancer | I | Recruiting | NCT04768608 | |
| NRB-CAR + OFF-switch | PD-L1 | GBM | I | Unknown | NCT02937844 |
| NLB-CAR | BCMA/TACI | MM | I/II | Terminated | NCT03287804 |
| BCMA/TACI | MM | I | Recruiting | NCT05020444 | |
| BCMA/TACI | MM | Early I | Not yet recruiting | NCT04657861 | |
| ErbB family | HNSCC | I | Recruiting | NCT01818323 | |
| GMR | Hematologic malignancies | I/II | Recruiting | jRCT2033210029 | |
| ICAM-I | Thyroid cancer | I | Recruiting | NCT04420754 | |
| IL-13Rα2 | GBM | I | Active, not recruiting | NCT02208362 | |
| IL-13Rα2 | GBM | I | Recruiting | NCT04003649 | |
| IL-13Rα2 | GBM | I | Recruiting | NCT04510051 | |
| IL-13Rα2 | GBM | I | Recruiting | NCT04661384 | |
| MMP-2 | GBM | I | Recruiting | NCT04214392 | |
| CARVac | CLDN6 | Solid tumors | I/II | Recruiting | NCT04503278 |
| Dual-targeting CAR | BCMA/CS1 | MM | Early I | Unknown | NCT04156269 |
| BCMA/GPRC5D | MM | I | Not yet recruiting | NCT05325801 | |
| CD123/CD33 | Hematologic malignancies | Early I | Unknown | NCT04156256 | |
| CD123/CLL1 | AML | II/III | Unknown | NCT03631576 | |
| CD19/BCMA | MM | I | Recruiting | NCT05412329 | |
| CD19/BCMA | MM | I | Recruiting | NCT04162353 | |
| CD19/BCMA | MM | I/II | Recruiting | NCT03455972 | |
| CD19/BCMA | MM | I | Unknown | NCT03767725 | |
| CD19/BCMA | MM | Early I | Recruiting | NCT04236011 | |
| CD19/BCMA | MM | I/II | Recruiting | NCT04714827 | |
| CD19/BCMA | MM | I | Unknown | NCT04194931 | |
| CD19/BCMA | MM | Early I | Unknown | NCT04182581 | |
| CD19/BCMA | MM | Early I | Unknown | NCT04412889 | |
| CD19/BCMA | MM | I | Unknown | NCT03706547 | |
| Dual-targeting CAR | CD19/BCMA | MM | Early I | Not yet recruiting | NCT04617704 |
| CD19/CD20 | NHL | I | Recruiting | NCT05421663 | |
| CD19/CD20 | NHL | I/II | Unknown | NCT04553393 | |
| CD19/CD20 | NHL | I | Unknown | NCT04693676 | |
| CD19/CD20 | NHL | I | Unknown | NCT04655677 | |
| CD19/CD20 | NHL | I | Unknown | NCT04696432 | |
| CD19/CD20 | NHL | I/II | Recruiting | NCT04697940 | |
| CD19/CD20 | NHL | Early I | Suspended | NCT04697290 | |
| CD19/CD20 | ALL | I | Recruiting | NCT04049383 | |
| CD19/CD20 | B cell malignancies | I | Completed | NCT03019055 | |
| CD19/CD20 | B cell malignancies | I | Completed | NCT04260932 | |
| CD19/CD20 | B cell malignancies | I | Completed | NCT04260945 | |
| CD19/CD20 | B cell malignancies | I/II | Recruiting | NCT04186520 | |
| CD19/CD20 | B cell malignancies | I/II | Completed | NCT03097770 | |
| CD19/CD20 | B cell malignancies | Early I | Unknown | NCT04156178 | |
| CD19/CD20 | B cell malignancies | I | Terminated | NCT04160195 | |
| CD19/CD20 | B cell malignancies | I/II | Unknown | NCT03207178 | |
| CD19/CD20 | DLBCL | I | Recruiting | NCT04215016 | |
| CD19/CD20 | DLBCL | I | Unknown | NCT04486872 | |
| CD19/CD20 | DLBCL | I/II | Unknown | NCT02737085 | |
| CD19/CD20 | Hematologic malignancies | I | Unknown | NCT03271515 | |
| CD19/CD20 | Hematologic malignancies | Early I | Unknown | NCT04700319 | |
| CD19/CD20/CD22 | Hematologic malignancies | I/II | Unknown | NCT03398967 | |
| CD19/CD20/CD22 | Hematologic malignancies | I | Recruiting | NCT05418088 | |
| CD19/CD20/CD22 | B cell malignancies | I | Recruiting | NCT05318963 | |
| CD19/CD20/CD22 | B cell malignancies | I | Recruiting | NCT05094206 | |
| CD19/CD20/CD22 | B cell malignancies | I | Recruiting | NCT05388695 | |
| CD19/CD22 | B cell malignancies | I | Recruiting | NCT04204161 | |
| CD19/CD22 | B cell malignancies | Early I | Unknown | NCT03825731 | |
| CD19/CD22 | B cell malignancies | I | Unknown | NCT03463928 | |
| CD19/CD22 | B cell malignancies | I | Recruiting | NCT04007029 | |
| CD19/CD22 | B cell malignancies | I | Withdrawn | NCT04094766 | |
| CD19/CD22 | B cell malignancies | I | Recruiting | NCT03233854 | |
| CD19/CD22 | B cell malignancies | I/II | Recruiting | NCT04715217 | |
| CD19/CD22 | B cell malignancies | I/II | Recruiting | NCT04782193 | |
| CD19/CD22 | B cell malignancies | I | Terminated | NCT03593109 | |
| CD19/CD22 | B cell malignancies | I/II | Suspended | NCT03098355 | |
| CD19/CD22 | B cell malignancies | I/II | Recruiting | NCT04788472 | |
| CD19/CD22 | B cell malignancies | I | Recruiting | NCT03448393 | |
| CD19/CD22 | DLBCL | I/II | Active, not recruiting | NCT03287817 | |
| CD19/CD22 | HNL | II | Recruiting | NCT04539444 | |
| CD19/CD22 | NHL | Early I | Recruiting | NCT04303247 | |
| CD19/CD22 | NHL | I/II | Not yet recruiting | NCT04626908 | |
| CD19/CD22 | Hematologic malignancies | I | Recruiting | NCT03330691 | |
| CD19/CD22 | Hematologic malignancies | I/II | Not yet recruiting | NCT04029038 | |
| CD19/CD22 | Hematologic malignancies | I | Active, not recruiting | NCT02443831 | |
| CD19/CD22 | Hematologic malignancies | I/II | Unknown | NCT03185494 | |
| CD19/CD22 | ALL | I/II | Recruiting | NCT04714593 | |
| CD19/CD22 | ALL | I/II | Recruiting | NCT04499573 | |
| CD19/CD22 | ALL | I | Recruiting | NCT03919526 | |
| CD19/CD22 | ALL | I/II | Recruiting | NCT04781634 | |
| CD19/CD22 | ALL | I/II | Completed | NCT03289455 | |
| CD19/CD22 | ALL | I | Unknown | NCT04303520 | |
| CD19/CD22 | ALL | I | Not yet recruiting | NCT05223686 | |
| CD19/CD22 | ALL | I | Withdrawn | NCT05168748 | |
| CD19/CD22 | ALL | I/II | Recruiting | NCT04740203 | |
| Dual-targeting CAR | CD19/CD22 | ALL | Early I | Recruiting | NCT04626765 |
| CD19/CD22 | ALL | I | Unknown | ChiCTR-OIB-17013670 | |
| CD19/CD22 | ALL | I | Unknown | ChiCTR1800015575 | |
| CD19/CD22 | ALL | I | Active | EUDRA CT 2016–004680-39 | |
| CD19/CD22 | ALL | Early I | Active, not recruiting | NCT04034446 | |
| CD19/CD22 | ALL | I/II | Recruiting | NCT03614858 | |
| CD19/PD-L1 | Hematologic malignancies | I | Unknown | NCT03932955 | |
| CD19/PD-L1 | B cell malignancies | I | Recruiting | NCT04850560 | |
| CD20/CD22 | Hematologic malignancies | Early I | Recruiting | NCT04283006 | |
| CD33/CLL1 | Hematologic malignancies | Early I | Recruiting | NCT03795779 | |
| CD33/CLL1 | AML | Early I | Not yet recruiting | NCT05016063 | |
| CD33/CLL1 | AML | I | Recruiting | NCT05248685 | |
| CD38/BCMA | MM | I | Recruiting | ChiCTR1800018143 | |
| c-Met/PD-L1 | Hepatocellular Carcinoma | Early I | Unknown | NCT03672305 | |
| EGFR/B7H3 | Lung cancer / TNBC | Early I | Recruiting | NCT05341492 | |
| HER2/PD-L1 | Solid tumors | Early I | Recruiting | NCT04684459 | |
| CD33/CD123/CLL-1 | AML | I/II | Recruiting | NCT04010877 | |
| CD44v6/GD/Her2 | Breast cancer | I/II | Recruiting | NCT04430595 | |
| CD10/CD20/CD22 | ALL | Early I | Recruiting | NCT03407859 | |
| Multiple$1 | MM | I/II | Recruiting | NCT03196414 | |
| Multiple$2 | ALL | I/II | Recruiting | NCT04430530 | |
| Multiple$3 | AML | I/II | Unknown | NCT03222674 | |
| Multiple$4 | Lung cancer | I | Recruiting | NCT03198052 | |
| Multiple$5 | B cell malignancies | I/II | Recruiting | NCT04429438 | |
| Switchable CAR | CD19 | B cell malignancies | I | Recruiting | NCT04450069 |
| CRs-CAR | BCMA | MM | Early I | Not yet recruiting | NCT04727008 |
| CD19 | DLBCL | I | Recruiting | NCT04381741 | |
| CD19 | B cell malignancies | NA | Completed | NCT04833504 | |
| CD19 | B cell malignancies | II | Unknown | NCT03929107 | |
| CD30 | Hematologic malignancies | I | Recruiting | NCT03602157 | |
| EGFR | NSCLC | I | Recruiting | NCT04153799 | |
| EGFR | NSCLC | Early I | Recruiting | NCT05060796 | |
| PSMA# | Prostate cancer | I | Not yet recruiting | NCT04227275 | |
| PSMA# | Prostate cancer | I | Recruiting | NCT03089203 | |
| OFF-Switch CAR | BCMA | MM | I | Active, not recruiting | NCT03070327 |
| CD19 | MCL | II | Recruiting | NCT04484012 | |
| CD19 | Hematologic malignancies | I | Active, not recruiting | NCT03085173 | |
| CD22 | Hematologic malignancies | I | Active, not recruiting | NCT03244306 |
NB: nanobody; NRB: natural receptor-based; NL: natural ligand-based; AML: acute myeloid leukemia; MM: multiple myeloma; HNSCC: Head and neck squamous cell carcinoma; GBM: glioblastoma
: Pancreatic cancer, renal cell carcinoma, breast cancer, melanoma, ovarian cancer
: B cell lymphoma, multiple myeloma, solid tumors
: Relapsed and refractory malignancies
MM: multiple myeloma; NHL: non-hodgkin’s lymphoma; DLBCL: diffuse large B-cell lymphoma; ALL: acute lymphocytic leukemia
CRs: chemokine/cytokine receptor; AML: acute myeloid leukemia; MM: multiple myeloma; DLBCL: diffuse large B-cell lymphoma; ALL: acute lymphocytic leukemia; TNBC: triple negative breast cancer; MCL: mantle cell lymphoma; NSCLC: non-small cell lung cancer
: CD138, BCMA, CD19, and/or other antigens
: CD22, CD123, CD38, CD10, and/or CD20
: CD33, CD38, CD123, CD56, MUC1, and/or CLL1
: GPC3, Mesothelin, Claudin18.2, GUCY2C, B7-H3, PSCA, PSMA, MUC1, TGFβ, HER-2, Lewis-Y, AXL, and/or EGFR
: CD19, CD20, CD22, CD70, CD13, CD79b, GD2 and/or PSMA
: TGFβ-resistant by modifying CAR T cell with a dominant negative TGFβ receptor (TGFβRdn)
Figure 1.
Strategies to explore novel antigen-targeting domains.
An alternative targeting strategy beyond scFvs and NBs is the use of natural receptors and ligands for antigen targeting. To design a natural receptor-based (NRB)-CAR, the ectodomain of the receptor of interest, and often its transmembrane domain and/or signaling endodomain, are incorporated into the CAR construct (Fig. 1C). A similar approach is also applied for natural ligand-based (NLB)-CARs to target the corresponding receptor (Fig. 1D). Natural receptors and ligands used as targeting domains in CAR NK cells include NKG2D (recognizing stress ligands such as MICA, MICB, and ULBP)119, DNAM-1 (targeting PVR/CD155 and Nectin-2/CD112 ligands)120, PD-1 (targeting PD-L1)121, and CD27 (targeting CD70; NCT05092451; NCT05703854), among others (Table 1).
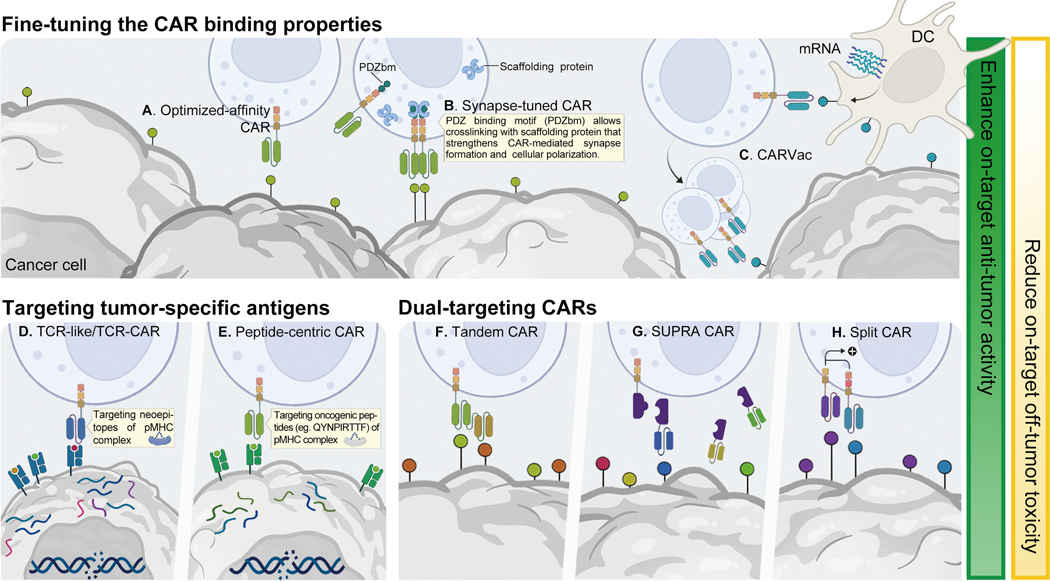
Approaches to fine-tune the binding affinity of the CAR extracellular domain in order to preserve or enhance the recognition of their cognate antigen while minimizing off-tumor recognition have also been explored. An example was the use of an optimized-affinity anti-CD38 CAR capable of targeting cancer cells while sparing CD38-low expressing normal cell populations (Fig. 2A)122. In this study, NK cells transduced with this optimized-affinity anti-CD38 CAR successfully killed primary AML blasts with minimal fratricide against CD38 low-expressing NK cells123. Strategies to optimize the binding of CARs to low antigen expressing targets have also been explored. Recently, innovative synapse-tuned CAR NK cells have been devised by incorporating a PDZ binding motif (PDZbm), important for cell polarization and synapse formation, within the CAR construct (Fig. 2B)124. This modification enhanced the strength of the synapse and the polarization of CAR T and CAR NK cells, resulting in improved effector cell function both in vitro and in vivo124.
Figure 2.
Strategies to advance CAR NK cell mediated on-target anti-tumor activity.
Other emerging approaches to broaden the scope of antigen binding include the use of vaccines to promote antigen display on DCs and thus increase the sensitivity of CAR cells to tumors expressing antigens at low levels (CARVac; Fig. 2C). In one study, an antigen known as claudin 6 (CLDN6), which is not expressed in normal tissues yet is often present at low levels in tumors125, was targeted by a CARVac126. The transient expression of this antigen on DCs was amplified by CLDN6 mRNA vaccine, leading to CAR T cell activation and expansion despite low CLDN6 expression on tumor cells126. This strategy has been investigated in a clinical trial for the treatment of relapsed/refractory testicular, ovarian, and endometrial cancer as well as soft-tissue sarcoma (NCT04503278), with an ORR of 43% and disease control rate of 86% (CT002 - BNT211) (Table 2). It would be very interesting to apply the CARVac strategy with NK cells, given the strong cross-talk between NK cells and DCs127.
Engineering NK cells to express a TCR offers the opportunity to combine the intrinsic, anti-tumor effector functions of NK cells with the TCR’s ability for the recognition of intracellular antigens (Fig. 2D)128–130. In a recent study, NK-92 cells were engineered to express both a TCR specific to the E7 protein of HPV16 and a CAR targeting TROP2130. This combination strategy led to a significant increase in NK cell activation and anti-tumor activity against HPV-driven cancers130. Lastly, in an innovative effort to target oncogenic drivers by CAR therapy, an intracellular unmutated oncogenic driver peptide (QYNPIRTTF) that is commonly expressed in neuroblastoma and presented by HLA was identified and targeted using a peptide-centric CAR that recognizes this TSA (Fig. 2E)131.
6.2. Dual-targeting strategies to improve tumor recognition and reduce toxicity
Dual antigen-targeting CARs have been investigated to overcome antigen escape, and to refocus CAR specificity towards tumor cells and away from normal cells. Targeting two or more antigens with CAR T or CAR NK cells can be achieved in different ways: (i) co-administration of CAR products with different antigen specificities, (ii) engineering a cell product with two or more CAR viral vectors, (iii) introduction of a bicistronic construct encoding for two CARs, or (iv) introduction of a tandem CAR with two different scFvs linked to the same signaling endodomain, whereby antigen recognition by either scFv leads to CAR signal transduction (Fig. 2F)132,133. Dual-targeting CAR T cells have been used in clinical trials for patients with B cell malignancies (targeting CD19/CD20134 and CD19/CD22)135 and in multiple myeloma targeting BCMA and CD38 (ChiCTR1800018143136; Table 1). However, the net clinical benefit of dual-targeting strategies requires further evaluation, with some suggestion that dual-targeting CAR T cells may not sustain their antigen specificity and potency against both antigens135.
Dual-targeting strategies have also been explored with NK cells (Table 2). Notably, in preclinical studies with the previously discussed iPSC NK cell product FT596, dual-targeting against lymphoma was achieved by engineering the cells to express a CAR against CD19 and by combining them with a CD20 mAb that mediated ADCC by binding to hnCD16137. Similarly, FT596 cells engineered to express anti-BCMA CAR and co-administered with anti-CD38 antibodies were shown to mediate strong activity against multiple myeloma in multiple xenogeneic mouse models138. Dual-targeting has also been successfully achieved in preclinical studies with NK-92 cells directed against both CD19 and BCMA139, or CD19 and CD138140.
Dual-targeting strategies may also be an attractive strategy to address the inherent challenge of tumor heterogeneity in solid tumors. As such, dual-targeting CAR NK cells against PD-L1 and ErbB2 were highly effective against solid tumor cell lines expressing both antigens and maintained their cytotoxicity even when one antigen was lost or became inaccessible141. Dual-specificity CAR NK cells that simultaneously recognize two distinct antigens with a shared epitope have also been investigated. For instance, CAR NK cells targeting a shared epitope of EGFR and its mutant form EGFRvIII were shown to be superior to single targeting CAR NK cells in glioblastoma xenografts142. Thus, dual-targeting and dual-specificity CAR NK cells could provide a promising approach to counteract the immune escape mechanisms employed by cancer cells.
Multi-antigen targeting can also be achieved through “target-switchable” CARs, allowing for the recognition of multiple tumor antigens without re-engineering the cell product. One such platform is referred to as split, universal, and programmable (SUPRA) CAR (Fig. 2G), which includes a leucine zipper extracellular domain linked to a conventional CAR signaling endodomain (zipCAR)143. An antigen-recognition module that binds to the leucine zipper can then activate zipCAR expressing T cells upon target engagement143. Other novel receptor design platforms that combine a CAR backbone with a versatile extracellular domain that binds a chemical or a genetic tag linked to a tumor-specific scFv include peptide neoepitope (PNE)-targeting CARs144–146, anti-Tag CARs (e.g., fluorescein isothiocyanate CAR)147, SpyCatcher CAR148, fusion protein CAR149, and Fab-based adaptor CAR (AdCAR)150. Bispecific antibody-binding adaptor CARs151 and “split CARs” that require recognition of both targeted TAAs for full activation (Fig. 2H)152–154 are other dual-targeting approaches shown to increase tumor specificity.
As enhancing on-target activity carries the risk of increasing off-tumor toxicity, extensive research has focused on approaches for CARs to discern tumor vs. healthy tissue targets. These next-generation CARs incorporate novel features such as the capacity to sense signals from the tumor milieu, regulation of CAR expression and activation after drug administration, etc. These designs have been evaluated in mouse models and some are also currently being assessed in clinical trials (Table 1 & 2).
6.3. Inducing CAR activation by cues from the solid tumor microenvironment (TME)
Because the tumor microenvironment (TME) has unique characteristics that are not present on healthy tissues but are essential for tumor growth, targeting its elements [e.g., extracellular matrix (ECM), stroma, vasculature, low pH, immunosuppressive metabolites, etc.] might be an attractive approach to enhance the activity of cell therapies against solid tumors (Fig. 3A)155–157.
Figure 3.
Strategies to overcome CAR NK cell-mediated on-target off-tumor toxicity.
To divert CAR T or CAR NK cell activity away from normal tissues, CARs that rely on sensing certain cues within the TME have been investigated. One such approach is the use of hypoxia-sensing CARs that only activate the CAR response under hypoxic conditions, which is a hallmark of the solid TME158. For example, the promoter of HypoxiCARs contains a hypoxia-response element (HRE), such that the expression of hypoxia-inducible factor 1α (HIF-1-α) that normally increases under low oxygen conditions is mandatory for CAR expression (Fig. 3B)159,160. Another design fused the CAR molecule to the oxygen-dependent degradation domain (ODD) of HIF-1-α, thus, promoting the degradation of CAR molecule under normoxic conditions via ubiquitination160. This CAR T cell system demonstrated high anti-tumor activity in mouse models of solid tumors, with the CAR T cells being exclusively present in the tumor and absent in non-tumor sites160.
Another interesting approach leverages the abundance of proteases in the TME to limit the off-target toxicity of CARs (Fig. 3C). Here, the CAR is modified to express an inhibitory or masking peptide that hinders its ability to bind to its cognate antigen under normal conditions. The inhibitory peptide in a ‘masked CAR’ is susceptible to protease cleavage, such that and in the protease-rich TME, it is cleaved to unmask the CAR antigen binding domain161. The masking approach does not alter the CAR activity, since anti-EGFR masked CAR T cells showed similar in vivo activity to control CAR cells161.
The use of protease-sensitive CARs has also been tested with NK cells. In a preclinical study in glioblastoma, NK cells were engineered to express a dual-targeting GD2-NKG2D CAR that, in response to proteases in the TME, locally released an antibody fragment to block the immunosuppressive purinergic signaling mediated by CD73162. By reducing adenosine levels in the glioblastoma TME, this combinatorial strategy addresses key drivers of glioblastoma resistance to CAR NK cell therapy162. Because normal tissues also express a wide variety of proteases, the safety of this approach requires further evaluation.
Finally, as the TME often contains chemokines and cytokines, modulation of chemokine signaling to improve the trafficking and localization of CAR T and CAR NK cells to tumor sites have also been investigated (Fig. 3D)163. For example, in a preclinical study, EGFRvIII-directed CAR NK cells that also expressed CXCR4 had greater chemotaxis towards glioblastoma cells secreting CXCL12/SDF-1α, resulting in superior tumor control and improved survival in xenograft models164. Similarly, forced expression of the chemokine receptor CXCR1, which is activated by IL-8 (secreted by multiple solid tumors), enhanced the migration and activity of intravenously administered NKG2D CAR NK cells in peritoneal ovarian cancer xenografts165.
6.4. OFF-switch CARs
Alternative strategies to reduce CAR-mediated on-target off-tumor activity consist of regulating the expression and activation of the CAR molecule through drug administration. The more classical models of regulatory CARs employ an inducible caspase “suicide switch” that can be pharmacologically activated leading to the elimination of the CAR T or CAR NK cells100. Our group demonstrated efficient elimination of NK cells expressing a CAR and a suicide switch based on iC9 upon its pharmacologic activation using the small molecule dimerizer AP1903 or Rimiducid both preclinically68 and clinically67. Another suicide system that has been used in CAR NK cells is the herpes simplex virus (HSV) thymidine kinase (HSV TK), which converts ganciclovir into a toxic product166.
Other strategies to control CAR expression incorporate a reversible OFF-switch. For example, CARs engineered with a C2H2 zinc finger degron motif can be induced to interact with an E3 ubiquitin ligase by lenalidomide, leading to CAR proteasomal degradation (Fig. 3E)167. Another drug-controlled system termed signal neutralization by an inhibitable protease (SNIP) incorporates the hepatitis C virus (HCV) NS3 protease (NS3p) together with an NS3p cleavage site at the intracellular end of the transmembrane domain of a CAR to maintain the CAR in an inactive state168. The CAR can in turn be activated upon exposure to an NS3p inhibitor to prevent its proteolytic cleavage (Fig. 3F)168.
6.5. ON-switch CARs
Similar tactics have been used to create ON-switches that control CAR induction and activation by drugs or other chemical or physical factors. For instance, a doxycycline inducible CAR was engineered where the tet response element 3G (TRE3G) was fused to the CAR vector, so that administration of doxycycline induced a conformational change in TRE3G to enable CAR expression169. An inducible system shown to enhance CAR NK cell activation and cytokine production combined the MyD88/CD40 signaling endodomain with anti-CD123 or anti-BCMA-CAR. Mimicking toll-like receptor (TLR) activation in DCs and as a potent costimulatory moiety in T cells, the inducible MyD88/CD40 moiety could be activated with Rimiducid to enhance CAR NK cell function and synergize with IL-15 signaling170. It will be important to validate the benefit of these novel approaches in reducing on-target off-tumor activity without comprising anti-tumor potency in the clinic.
6.6. Synthetic circuits and logic-gating strategies
To further regulate CAR activity, other strategies were developed that required recognition and sequential signaling of multiple antigens for activation. One such strategy that has been widely investigated is the design of synthetic Notch (SynNotch) receptors (Fig. 3G)171. A SynNotch receptor is designed to recognize a tumor antigen of interest, which, upon ligand binding, induces cleavage of an orthogonal transcription factor that in turn induces expression of a second CAR directed towards another tumor antigen172. As such, CAR activity towards a second cognate antigen is only initiated in the presence of the first tumor antigen, thus requiring an ‘AND’ logic of both antigens to be present in order to induce CAR activation173,174. The potential benefits of this strategy were demonstrated in preclinical models of human mesothelioma, ovarian cancer, and glioblastoma through controlling tumor growth, preventing CAR-mediated tonic signaling and maintaining a long-lived memory phenotype173,175. The SynNotch system was also employed to enable an inducible autocrine circuit to drive IL-2 expression in CAR T cells upon engagement with tumor antigens, resulting in more efficient CAR T cell infiltration into solid tumors and enhanced anti-tumor activity176.
The SynNotch system has also been used to increase the secretion of granzyme B (GZMB) and perforin (PRF1) by NK cells and to regulate their intracellular pools by coupling them with pSHP inhibition177. Similarly, NK cells engineered to express a logic-gated GPC3–SynNotch-inducible CD147-CAR were shown to have increased specificity against hepatocellular carcinoma and reduced toxicity in preclinical models, as both antigens were required for the full targeted activity of these CAR NK cells178. However, the immunogenicity potential of the SynNotch receptor, a non-human artificial protein, as well as the high level of background signaling due to ligand-independent receptor activity pose safety concerns171,179. To overcome some of these limitations, a SynNotch system that uses humanized domains with tunable sensing and optimized transcriptional response with the ability to achieve the intended programmed gene regulation (referred to as SNIPR) was recently described179.
An alternative approach to prevent CAR activity against undesirable targets is the use of a “NOT” logic gating strategy. This approach uses an inhibitory CAR (iCAR) directed against a self-antigen expressed on healthy cells linked to the signaling endodomain of a checkpoint molecule (e.g., PD-1 and CTLA-4)180. Recognition of the self-antigen on a heathy cell by the iCAR results in inhibition of CAR T or CAR NK cell activity (Fig. 3H)180. To overcome self-recognition of trogocytic antigen-expressing (TROG+) NK cells by the activating CAR (aCAR), and the resultant fratricide and exhaustion, our research group combined the activity of two CARs — an iCAR directed against a self-antigen expressed on NK cells and an aCAR against a tumor antigen109. This strategy resulted in CAR NK cells receiving a ‘don’t kill me’ signal when interacting with their TROG+ NK siblings, while preserving the function of their aCAR against the tumor target109. By combining the activity of these two CARs, we were able to reduce NK cell exhaustion and fratricide and improve their anti-tumor activity in vivo109.
7. Conclusions and future research
CAR-based cell therapy that combines targeted precision medicine with immunotherapy using living cells has proven therapeutic activity in patients with certain hematologic malignancies. Much progress has been made in designing the next generation of CARs to enhance the effector function, proliferation, persistence, and safety of immune cells. Indeed, we are currently witnessing a burst of innovative cell therapy approaches being explored both preclinically and in the clinic. However, considerable effort is still needed to replicate the success observed with CAR cell therapies in B-lymphoid malignancies in other malignancies. The future will likely focus on developing multi-pronged approaches that leverage our ever-growing scientific and clinical knowledge of tumor immunology and immunotherapy with novel engineering strategies to improve the safety, potency and feasibility of this therapeutic platform.
ACKNOWLEDGEMENTS
Y.L. was supported by the CPRIT Research Training Award RP210028. H.R. was supported by an ASCO Young Investigator Award and the University Cancer Foundation via the Institutional Research Grant program at The University of Texas MD Anderson Cancer Center. This work was supported in part by the generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program, and The Sally Cooper Murray endowment; by Grants (1 R01 CA211044–01, 5 P01CA148600–03) from the National Institutes of Health (NIH), the Cancer Prevention and Research Institute of Texas (CPRIT) grant RP180466, the Leukemia Specialized Program of Research Excellence (SPORE) Grant (P50CA100632), the Specialized Program of Research Excellence (SPORE) in Brain Cancer grant (P50CA127001), the Stand Up To Cancer Dream Team Research Grant (SU2C-AACR-DT-29–19), and the Grant (P30 CA016672) from the NIH to the MD Anderson Cancer Center. Figures were prepared using biorender.com.
Footnotes
COMPETING INTERESTS
Y.L., H.R., K.R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical and Affimed GmbH. K.R. participates on the Scientific Advisory Board for GemoAb, AvengeBio, Virogin Biotech, GSK, Bayer, Navan Technologies, and Caribou Biosciences. K.R. is the scientific founder of Syena. The remaining authors declare that they have no competing interests.
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated
REFERENCES
- 1.Rohaan MW, Wilgenhof S. & Haanen J. Adoptive cellular therapies: the current landscape. Virchows Arch 474, 449–461 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371, 1507–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Stegen SJ, Hamieh M. & Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 14, 499–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guedan S, Ruella M. & June CH Emerging Cellular Therapies for Cancer. Annu Rev Immunol 37, 145–171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June CH & Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med 379, 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betof Warner A, Corrie PG & Hamid O. Tumor-Infiltrating Lymphocyte Therapy in Melanoma: Facts to the Future. Clin Cancer Res 29, 1835–1854 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bethune MT & Joglekar AV Personalized T cell-mediated cancer immunotherapy: progress and challenges. Curr Opin Biotechnol 48, 142–152 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Rapoport AP, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 21, 914–921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groeper C, et al. Cancer/testis antigen expression and specific cytotoxic T lymphocyte responses in non small cell lung cancer. Int J Cancer 120, 337–343 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Orlando D, et al. Adoptive Immunotherapy Using PRAME-Specific T Cells in Medulloblastoma. Cancer Res 78, 3337–3349 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Amir AL, et al. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res 17, 5615–5625 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Morgan RA, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36, 133–151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsimberidou AM, et al. T-cell receptor-based therapy: an innovative therapeutic approach for solid tumors. J Hematol Oncol 14, 102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29, 917–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadelain M, Brentjens R. & Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 3, 388–398 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison RP, Zylberberg E, Ellison S. & Levine BL Chimeric antigen receptor–T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy 21, 224–233 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Young RM, Engel NW, Uslu U, Wellhausen N. & June CH Next-Generation CAR T-cell Therapies. Cancer Discov, OF1–OF14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterner RC & Sterner RM CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11, 69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafiq S, Hackett CS & Brentjens RJ Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nature Reviews Clinical Oncology 17, 147–167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou-el-Enein M, et al. Scalable Manufacturing of CAR T Cells for Cancer Immunotherapy. Blood Cancer Discovery 2, 408–422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qasim W, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Science translational medicine 9, eaaj2013 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Benjamin R, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. The Lancet 396, 1885–1894 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Naeem M, et al. Explorations of CRISPR/Cas9 for improving the long-term efficacy of universal CAR-T cells in tumor immunotherapy. Life Sciences 316, 121409 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Guillerey C, Huntington ND & Smyth MJ Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17, 1025–1036 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Bae EA, Seo H, Kim IK, Jeon I. & Kang CY Roles of NKT cells in cancer immunotherapy. Arch Pharm Res 42, 543–548 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Shah NN & Fry TJ Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol 16, 372–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Donk N, Themeli M. & Usmani SZ Determinants of response and mechanisms of resistance of CAR T-cell therapy in multiple myeloma. Blood Cancer Discov 2, 302–318 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun S, Hao H, Yang G, Zhang Y. & Fu Y. Immunotherapy with CAR-Modified T Cells: Toxicities and Overcoming Strategies. J Immunol Res 2018, 2386187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonifant CL, Jackson HJ, Brentjens RJ & Curran KJ Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 3, 16011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roselli E, Faramand R. & Davila ML Insight into next-generation CAR therapeutics: designing CAR T cells to improve clinical outcomes. J Clin Invest 131(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chmielewski M. & Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther 15, 1145–1154 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Jacobson CA, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol 23, 91–103 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Locke FL, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 20, 31–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neelapu SS, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 377, 2531–2544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abramson JS, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Schuster SJ, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 380, 45–56 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Wang M, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med 382, 1331–1342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maude SL, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 378, 439–448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah BD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398, 491–502 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Munshi NC, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med 384, 705–716 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Berdeja JG, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Donk NWCJv.d., et al. Biological correlative analyses and updated clinical data of ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR-T cell therapy, in patients with multiple myeloma (MM) and early relapse after initial therapy: CARTITUDE-2, cohort B. Journal of Clinical Oncology 40, 8029–8029 (2022). [Google Scholar]
- 44.Brudno JN & Kochenderfer JN Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev 34, 45–55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brudno JN & Kochenderfer JN Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J. & Lanier LL Natural killer cells and cancer. Adv Cancer Res 90, 127–156 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Klingemann H. Are natural killer cells superior CAR drivers? OncoImmunology 3, e28147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myers JA & Miller JS Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol 18, 85–100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imai K, Matsuyama S, Miyake S, Suga K. & Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. The lancet 356, 1795–1799 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Guerra N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28, 571–580 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.López-Soto A, Gonzalez S, Smyth MJ & Galluzzi L. Control of metastasis by NK cells. Cancer cell 32, 135–154 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Liu S, et al. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 14, 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalle J-H, et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatric research 57, 649–655 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK & Kershaw MH Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89, 216–224 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Pende D, et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Frontiers in Immunology 10(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vivier E, Tomasello E, Baratin M, Walzer T. & Ugolini S. Functions of natural killer cells. Nature Immunology 9, 503–510 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Lanier LL Up on the tightrope: natural killer cell activation and inhibition. Nature immunology 9, 495–502 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mujal AM, Delconte RB & Sun JC Natural Killer Cells: From Innate to Adaptive Features. Annual Review of Immunology 39, 417–447 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Sun JC, Beilke JN & Lanier LL Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proceedings of the National Academy of Sciences 106, 1915–1919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rückert T, Lareau CA, Mashreghi M-F, Ludwig LS & Romagnani C. Clonal expansion and epigenetic inheritance of long-lasting NK cell memory. Nature Immunology 23, 1551–1563 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foley B, et al. Human Cytomegalovirus (CMV)-Induced Memory-like NKG2C+ NK Cells Are Transplantable and Expand In Vivo in Response to Recipient CMV Antigen. The Journal of Immunology 189, 5082–5088 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romee R, et al. Cytokine activation induces human memory-like NK cells. Blood 120, 4751–4760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romee R, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Science translational medicine 8, 357ra123–357ra123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shapiro RM, et al. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. The Journal of clinical investigation 132(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laskowski TJ, Biederstädt A. & Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer 22, 557–575 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu E, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. New England Journal of Medicine 382, 545–553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu E, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong J-H, Maki G. & Klingemann HG Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 8, 652–658 (1994). [PubMed] [Google Scholar]
- 70.Kotzur R, et al. NK-92 cells retain vitality and functionality when grown in standard cell culture conditions. PLOS ONE 17, e0264897 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spanholtz J, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PloS one 5, e9221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bar-Nur O, Russ HA, Efrat S. & Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell stem cell 9, 17–23 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Tang X, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. American journal of cancer research 8, 1083 (2018). [PMC free article] [PubMed] [Google Scholar]
- 74.Jing Y, et al. Identification of an ADAM17 cleavage region in human CD16 (FcgammaRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One 10, e0121788 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cichocki F, van der Stegen SJC & Miller JS Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood 141, 846–855 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bachanova V, et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood 138, 823 (2021). [Google Scholar]
- 77.NKARTA ANNOUNCES UPDATED CLINICAL DATA ON ANTI-CD19 ALLOGENEIC CAR-NK CELL THERAPY NKX019 FOR PATIENTS WITH RELAPSED OR REFRACTORY NON-HODGKIN LYMPHOMA. (2022). [Google Scholar]
- 78.O’Rourke DM, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schumacher TN & Schreiber RD Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Hegde M, et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat Commun 11, 3549 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haas AR, et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol Ther 27, 1919–1929 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C, et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers. Mol Ther 25, 1248–1258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3, 95ra73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5, 177ra138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou X, et al. Toxicities of Chimeric Antigen Receptor T Cell Therapy in Multiple Myeloma: An Overview of Experience From Clinical Trials, Pathophysiology, and Management Strategies. Front Immunol 11, 620312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wudhikarn K, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J 10, 79 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wat J. & Barmettler S. Hypogammaglobulinemia After Chimeric Antigen Receptor (CAR) T-Cell Therapy: Characteristics, Management, and Future Directions. J Allergy Clin Immunol Pract 10, 460–466 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tasian SK & Gardner RA CD19-redirected chimeric antigen receptor-modified T cells: a promising immunotherapy for children and adults with B-cell acute lymphoblastic leukemia (ALL). Ther Adv Hematol 6, 228–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walti CS, et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight 6(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu X, Wang L, Shen L, He L. & Tang K. Immune response to vaccination against SARS-CoV-2 in hematopoietic stem cell transplantation and CAR T-cell therapy recipients. J Hematol Oncol 15, 81 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luque Paz D, Sesques P, Wallet F, Bachy E. & Ader F. The burden of SARS-CoV-2 in patients receiving chimeric antigen receptor T cell immunotherapy: everything to lose. Expert Rev Anti Infect Ther (2022). [DOI] [PubMed] [Google Scholar]
- 93.Parker KR, et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell 183, 126–142 e117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Oekelen O, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med 27, 2099–2103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamers CH, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 21, 904–912 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamers CH, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 24, e20–22 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18, 843–851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valton J, et al. A Versatile Safeguard for Chimeric Antigen Receptor T-Cell Immunotherapies. Sci Rep 8, 8972 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donnadieu E, et al. Time to evolve: predicting engineered T cell-associated toxicity with next-generation models. J Immunother Cancer 10(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gargett T. & Brown MP The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol 5, 235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lupo-Stanghellini MT, et al. Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum Gene Ther 21, 241–250 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Vogler I, et al. An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy. Mol Ther 18, 1330–1338 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kieback E, Charo J, Sommermeyer D, Blankenstein T. & Uckert W. A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proc Natl Acad Sci U S A 105, 623–628 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooper ML, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia 32, 1970–1983 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao Z, et al. Blocking CD38-driven fratricide among T cells enables effective antitumor activity by CD38-specific chimeric antigen receptor T cells. J Genet Genomics 46, 367–377 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Naeimi Kararoudi M, et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood 136, 2416–2427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang QJ, et al. Preclinical Evaluation of Chimeric Antigen Receptors Targeting CD70-Expressing Cancers. Clin Cancer Res 23, 2267–2276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joly E. & Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol 4, 815 (2003). [DOI] [PubMed] [Google Scholar]
- 109.Li Y, et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nature Medicine 28, 2133–2144 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamieh M, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olson ML, et al. Low-affinity CAR T cells exhibit reduced trogocytosis, preventing rapid antigen loss, and increasing CAR T cell expansion. Leukemia 36, 1943–1946 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Long AH, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21, 581–590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun W, et al. A combined strategy improves the solubility of aggregation-prone single-chain variable fragment antibodies. Protein Expr Purif 83, 21–29 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Frigault MJ, et al. Identification of Chimeric Antigen Receptors That Mediate Constitutive or Inducible Proliferation of T Cells. Cancer Immunology Research 3, 356–367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem 82, 775–797 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Hegde M, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest 126, 3036–3052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hambach J, et al. Targeting CD38-Expressing Multiple Myeloma and Burkitt Lymphoma Cells In Vitro with Nanobody-Based Chimeric Antigen Receptors (Nb-CARs). Cells 9(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.You F, et al. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am J Cancer Res 9, 64–78 (2019). [PMC free article] [PubMed] [Google Scholar]
- 119.Leivas A, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J 11, 146 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Focaccetti C, et al. DNAM-1-chimeric receptor-engineered NK cells, combined with Nutlin-3a, more effectively fight neuroblastoma cells in vitro: a proof-of-concept study. Frontiers in Immunology 13(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu C, et al. A novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis. Mol Immunol 122, 200–206 (2020). [DOI] [PubMed] [Google Scholar]
- 122.Drent E, et al. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol Ther 25, 1946–1958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gurney M, et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 107, 437–445 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chockley PJ, Ibanez-Vega J, Krenciute G, Talbot LJ & Gottschalk S. Synapse-tuned CARs enhance immune cell anti-tumor activity. Nature Biotechnology (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turksen K. & Troy TC Claudin-6: a novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev Dyn 222, 292–300 (2001). [DOI] [PubMed] [Google Scholar]
- 126.Reinhard K, et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020). [DOI] [PubMed] [Google Scholar]
- 127.Ferlazzo G. & Morandi B. Cross-Talks between Natural Killer Cells and Distinct Subsets of Dendritic Cells. Front Immunol 5, 159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morton LT, et al. T cell receptor engineering of primary NK cells to therapeutically target tumors and tumor immune evasion. Journal for ImmunoTherapy of Cancer 10, e003715 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang G, et al. Retargeting NK-92 for anti-melanoma activity by a TCR-like single-domain antibody. Immunol Cell Biol 91, 615–624 (2013). [DOI] [PubMed] [Google Scholar]
- 130.Quiros-Fernandez I, Poorebrahim M, Marmé F, Burdach SEG & Cid-Arregui A. A costimulatory chimeric antigen receptor targeting TROP2 enhances the cytotoxicity of NK cells expressing a T cell receptor reactive to human papillomavirus type 16 E7. Cancer Letters, 216242 (2023). [DOI] [PubMed] [Google Scholar]
- 131.Yarmarkovich M, et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 599, 477–484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Fernandez de Larrea C, et al. Defining an Optimal Dual-Targeted CAR T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape-Driven Relapse in Multiple Myeloma. Blood Cancer Discov 1, 146–154 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han X, Wang Y, Wei J. & Han W. Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy. J Hematol Oncol 12, 128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shah NN, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med 26, 1569–1575 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Spiegel JY, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nature Medicine 27, 1419–1431 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li C, et al. A Bispecific CAR-T Cell Therapy Targeting Bcma and CD38 for Relapsed/Refractory Multiple Myeloma: Updated Results from a Phase 1 Dose-Climbing Trial. Blood 134, 930–930 (2019). [Google Scholar]
- 137.Cichocki F, et al. Dual antigen–targeted off-the-shelf NK cells show durable response and prevent antigen escape in lymphoma and leukemia. Blood 140, 2451–2462 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cichocki F, et al. Quadruple gene-engineered natural killer cells enable multi-antigen targeting for durable antitumor activity against multiple myeloma. Nat Commun 13, 7341 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roex G, et al. Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells. Journal of Translational Medicine 20, 124 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luanpitpong S, Poohadsuan J, Klaihmon P. & Issaragrisil S. Selective Cytotoxicity of Single and Dual Anti-CD19 and Anti-CD138 Chimeric Antigen Receptor-Natural Killer Cells against Hematologic Malignancies. Journal of Immunology Research 2021, 5562630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eitler J, et al. 216 Dual targeting of CAR-NK cells to PD-L1 and ErbB2 facilitates specific elimination of cancer cells of solid tumor origin and overcomes immune escape by antigen loss. Journal for ImmunoTherapy of Cancer 10, A230-A230 (2022). [Google Scholar]
- 142.Genßler S, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology 5, e1119354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cho JH, Collins JJ & Wong WW Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell 173, 1426–1438 e1411 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rodgers DT, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A 113, E459–468 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raj D, et al. Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut 68, 1052–1064 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peng H, et al. ROR1-targeting switchable CAR-T cells for cancer therapy. Oncogene 41, 4104–4114 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tamada K, et al. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res 18, 6436–6445 (2012). [DOI] [PubMed] [Google Scholar]
- 148.Li L, Fierer JO, Rapoport TA & Howarth M. Structural analysis and optimization of the covalent association between SpyCatcher and a peptide Tag. J Mol Biol 426, 309–317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ambrose C, et al. Anti-CD19 CAR T cells potently redirected to kill solid tumor cells. PLoS One 16, e0247701 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nixdorf D, et al. Adapter CAR T cells to counteract T-cell exhaustion and enable flexible targeting in AML. Leukemia (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Andrea AE, Chiron A, Bessoles S. & Hacein-Bey-Abina S. Engineering Next-Generation CAR-T Cells for Better Toxicity Management. Int J Mol Sci 21(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilkie S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol 32, 1059–1070 (2012). [DOI] [PubMed] [Google Scholar]
- 153.Globerson Levin A, et al. Treatment of Multiple Myeloma Using Chimeric Antigen Receptor T Cells with Dual Specificity. Cancer Immunol Res 8, 1485–1495 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Lanitis E, et al. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res 1, 43–53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rodriguez-Garcia A, Palazon A, Noguera-Ortega E, Powell DJ Jr. & Guedan S. CAR-T Cells Hit the Tumor Microenvironment: Strategies to Overcome Tumor Escape. Front Immunol 11, 1109 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Quail DF & Joyce JA Microenvironmental regulation of tumor progression and metastasis. Nat Med 19, 1423–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neri D. & Bicknell R. Tumour vascular targeting. Nat Rev Cancer 5, 436–446 (2005). [DOI] [PubMed] [Google Scholar]
- 158.Brown JM & Wilson WR Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 4, 437–447 (2004). [DOI] [PubMed] [Google Scholar]
- 159.Juillerat A, et al. An oxygen sensitive self-decision making engineered CAR T-cell. Sci Rep 7, 39833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kosti P, et al. Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumors. Cell Rep Med 2, 100227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han X, et al. Masked Chimeric Antigen Receptor for Tumor-Specific Activation. Mol Ther 25, 274–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang J, et al. Multispecific targeting of glioblastoma with tumor microenvironment-responsive multifunctional engineered NK cells. Proc Natl Acad Sci U S A 118(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Foeng J, Comerford I. & McColl SR Harnessing the chemokine system to home CAR-T cells into solid tumors. Cell Rep Med 3, 100543 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Müller N, et al. Engineering NK Cells Modified With an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1α-secreting Glioblastoma. J Immunother 38, 197–210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ng YY, Tay JCK & Wang S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol Ther Oncolytics 16, 75–85 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raftery MJ, et al. Next Generation CD44v6-Specific CAR-NK Cells Effective against Triple Negative Breast Cancer. International Journal of Molecular Sciences 24, 9038 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jan M, et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci Transl Med 13(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Labanieh L, et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell 185, 1745–1763 e1722 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sakemura R, et al. A Tet-On Inducible System for Controlling CD19-Chimeric Antigen Receptor Expression upon Drug Administration. Cancer Immunol Res 4, 658–668 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Wang X, et al. Inducible MyD88/CD40 synergizes with IL-15 to enhance antitumor efficacy of CAR-NK cells. Blood Advances 4, 1950–1964 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morsut L, et al. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 164, 780–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roybal KT, et al. Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors. Cell 167, 419–432 e416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hyrenius-Wittsten A, et al. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci Transl Med 13(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang ZJ, Yu ZY, Cai YM, Du RR & Cai L. Engineering of an enhanced synthetic Notch receptor by reducing ligand-independent activation. Commun Biol 3, 116 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Choe JH, et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med 13(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Allen GM, et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science 378, eaba1624 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Makaryan SZ & Finley SD An optimal control approach for enhancing natural killer cells’ secretion of cytolytic molecules. APL Bioengineering 4(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tseng HC, et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun 11, 4810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhu I, et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell 185, 1431–1443 e1416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ren J, et al. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin Cancer Res 23, 2255–2266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated