Abstract
We show here that an active Cdk5-p35 kinase is present in Golgi membranes, where it associates with a detergent-insoluble fraction containing actin. In addition, Cdk5-p35-dependent phosphorylation of α-PAK immunoreactive protein species was detected in Golgi membranes, as well as an interaction with the small GTPase, Cdc42. Moreover, antisense oligonucleotide suppression of Cdk5 or p35 in young cultured neurons, as well as inhibition of Cdk5 activity with olomoucine, blocks the formation of membrane vesicles from the Golgi apparatus. Taken together, these results show a novel subcellular localization of this kinase and suggest a role for Cdk5-p35 in membrane traffic during neuronal process outgrowth.
INTRODUCTION
Cyclin-dependent kinase 5 (Cdk5), a small serine/threonine kinase, is required for proper development of the nervous system (Dhavan and Tsai, 2001; Paglini and Cáceres, 2001). To be activated, Cdk5 associates with regulatory subunits such as p35 (Lew et al., 1994; Tsai et al., 1994) or p39 (Tang et al., 1995). The Cdk5-p35 kinase is involved in neuronal morphogenesis, as inactivation of Cdk5 or p35 in cultured neurons inhibits axon elongation (Nikolic et al., 1996; Pigino et al., 1997; Paglini et al., 1998). Moreover, mice lacking Cdk5 or p35 display cortical lamination defects, reduced DiI axonal labeling intensity, a diminished corpus callosum, and aberrant fiber fascicles in the cortex (Ohshima et al., 1996; Chae et al., 1997; Gilmore et al., 1998). In developing neurons, Cdk5 and its activators p35 and p39 are enriched in axonal growth cones, where they associate with the membrane cytoskeleton (Nikolic et al., 1998; Paglini et al., 1998; Humbert et al., 2000). At this location, the kinase phosphorylates proteins, such as MAP1B (Pigino et al., 1997; Paglini et al., 1998) and the p21-activated kinase (PAK1; Nikolic et al., 1998), which have been implicated in the regulation of cytoskeletal dynamics during axon formation (Paglini and Cáceres, 2001). We now report on a novel subcellular localization and function of the Cdk5-p35 kinase in developing neurons.
RESULTS
An active Cdk5-p35 complex is present in Golgi membranes
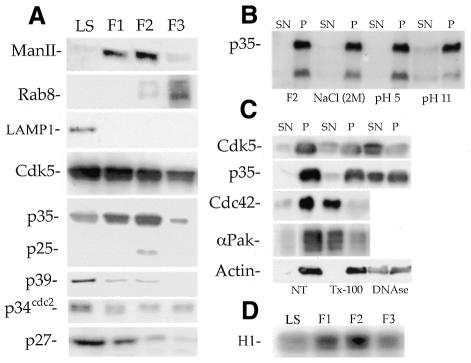
To identify a possible new site of action of the Cdk5-p35 kinase in developing neurons, subcellular fractions enriched in Golgi membranes were obtained from microsomes of postnatal rat cerebral cortex (day 5) by flotation in a discontinuous sucrose density gradient. Figure 1A shows that the F1 and F2 fractions were highly enriched in mannosidase II (Figure 1A), a well-established Golgi marker, while F3 was enriched in rab 8 (Figure 1A), a marker of trans-Golgi transport vesicles (Huber et al., 1995). In contrast, neither F1 nor F2 contain detectable amounts of rab5, a marker of early endosomes, or LAMP1, a marker of lysosomes (Figure 1A). Figure 1A also shows that Cdk5 was present, but not enriched, in all of the Golgi fractions when compared with the levels detected in the total brain homogenate. However, p35 showed a considerable enrichment in F1–F2 and lower levels in F3; interestingly, a 25 kDa immunoreactive protein species was also detected in F2 (Figure 1A). p39, the cdc2p34 kinase and the Cdk inhibitor p27, were almost completely absent from the Golgi fractions (Figure 1A).
Fig. 1. (A) Western blots showing the distribution of several proteins in the low speed supernatant (LS) and Golgi fractions. Note the presence of Cdk5 and p35 in the F1 and F2 fractions. (B) Western blot showing that p35 remains associated with the F2 fraction after treatment with 2 M NaCl or 0.5 M Na2CO3 (pH 5 or 11). (C) Western blots showing that Cdk5 and p35 remain associated with the F2 fraction after treatment with 2% Triton X-100; in contrast, a DNase treatment releases Cdk5-p35 from the pellet. The Cdk5-p35 kinase present in the F1 fraction behaves similarly (not shown). Abbreviations: NT (non-treated), SN (supernatant), P (pellet). (D) Cdk5 histone H1 kinase activity in p35 IPs obtained from the low speed supernatant (LS), and Golgi fractions. For all experiments 30 µg of total protein were loaded in each lane.
Biochemical experiments also showed that binding of Cdk5-p35 to the F1–F2 fractions is resistant to high salt and extreme pH treatment (Figure 1B), suggesting that the kinase may be part of a large complex present in Golgi membranes. Therefore, we examined the ability of detergents to solubilize Cdk5 and p35 from the F2 fraction. As shown in Figure 1C, treatment of the F2 fraction with 2% Triton X-100 does not significantly remove p35 or Cdk5 (not shown) from the pellet fraction; in contrast, this treatment effectively removed Cdc42, a small GTPase which has a Golgi-membrane bound form (McCallum et al., 1998). As detergent insolubility is a characteristic of the actin cytoskeleton, we western blotted for actin and found that the detergent-insoluble pellets also contained actin. In accordance with this, a DNase treatment, which caused depolymerization of actin, removed Cdk5 and p35 from the pellet (Figure 1C).
We also determined whether the Cdk5-p35 complex present in the Golgi fractions had kinase activity. Figure 1D shows the result of a representative experiment that clearly reveals the existence of a high correlation between p35 protein levels and Cdk5 histone H1 activity in Golgi fractions; this activity was inhibited in a dose-dependent fashion by olomoucine or roscovitine (see Supplementary data available at EMBO reports Online).
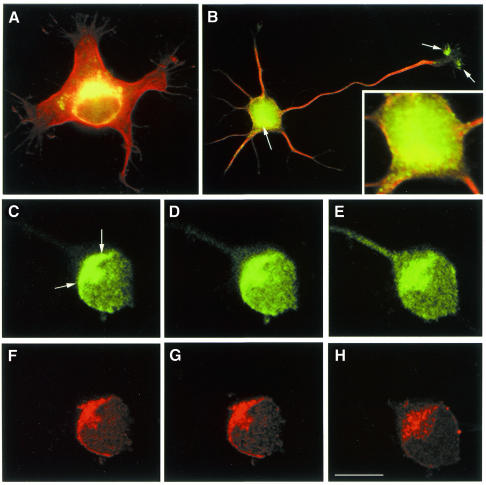
To complement these experiments we analyzed the sub-cellular distribution of p35 in cultured neurons. Staining of hippocampal pyramidal cells with p35 antibodies revealed a strong immunofluorescence signal in a region of the cell body localized in close apposition to the cell nucleus that resembles the Golgi apparatus (Figure 2A and B). In addition, confocal microscopy revealed extensive colocalization of p35 and mannosidase II in the region corresponding to the Golgi apparatus (Figure 2C–H).
Fig. 2. p35 localizes to the Golgi apparatus in developing neurons. (A and B) Red-green overlays of digitized images showing the distribution of tubulin (red) and p35 (green) in cultured hippocampal pyramidal neurons. The cells were fixed prior to detergent extraction with 4% paraformaldehyde–0.12 M sucrose 1 day after plating and processed for immunofluorescence with antibodies against tyrosinated a-tubulin and p35. Note that a high p35 fluorescence signal is detected in a region of the cell body located in close apposition to the nucleus (arrow) and in the axonal growth cone (arrows). The insert in (B) shows a high magnification view of the cell body area. (C–H) A series of confocal images showing the distribution of p35 (C–E) and mannosidase II (F–H) in the cell body of a cultured hippocampal pyramidal neuron. Note the colocalization of both proteins in the region of the Golgi apparatus (arrows). Calibration bar: 10 µm.
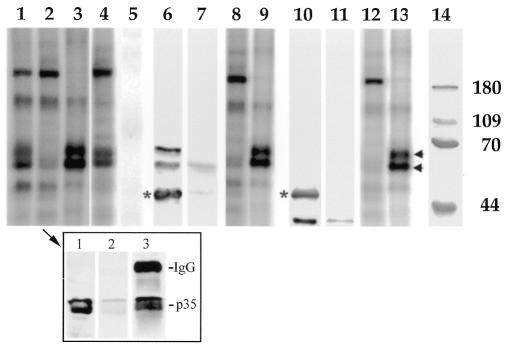
In the next series of experiments we sought to determine possible targets of the kinase in Golgi membranes. When the F2 fraction containing endogenous Cdk5-p35 was incubated with [γ-32P]ATP, several proteins become heavily phosphorylated (Figure 3, lane 1). A similar, but not identical, pattern was detected after phosphorylation of the F2 fraction immunodepleted of either Cdk5 or p35 (Figure 3, lane 2). Depletion of Cdk5 or p35 from the F2 fraction was achieved by two cycles of immunoprecipitation with a rabbit polyclonal antibody against Cdk5 or p35 (Paglini et al., 1998; Figure 3, insert). The major difference between control and immunodepleted F2 fractions was that in the later case we detected a significant decrease in the labeling of two protein bands migrating at ∼65–70 kDa Mr (Figure 3, lane 2). Protein phosphorylation was also analyzed in the Cdk5 or p35 immunoprecipitates (IPs). As expected, we detected the presence of two heavily phosphorylated bands migrating at 65–70 kDa Mr after incubation of the IPs with [γ-32P]ATP (Figure 3, lane 3). Interestingly, phosphorylation of the 65–70 kDa protein bands was significantly inhibited when the IPs were treated with 25 µM olomoucine (not shown). The pattern of protein phosphorylation of the F2 fraction was not altered in control samples previously treated with non-immune serum (not shown) or with an antibody against the mitogen-activated protein kinase, ERK1 (Figure 3, lanes 4 and 5).
Fig. 3. Lanes 1–3: pattern of protein phosphorylation of: (lane 1) control F2 fraction; (lane 2) F2 fraction immunodepleted of Cdk5; (lane 3) Cdk5 immunoprecipitate (IP) obtained from the F2 fraction. Note the absence of two heavily phosphorylated protein bands in the F2 fraction immunodepleted of Cdk5; these two bands are now present in the IP. Lanes 4 and 5: pattern of protein phosphorylation of: (lane 4) F2 fraction immunodepleted of ERK1; (lane 5) ERK1 immunoprecipitate obtained from the F2 fraction. Note that immunodepletion of ERK1 from the F2 fraction does not alter the pattern of protein phosphorylation. Lanes 6 and 7: western blots showing the presence of two α-PAK immunoreactive proteins in a Cdk5 immunoprecipitate obtained from F2 (lane 6); note the absence of α-PAK in the supernatant (lane 7). The asterisk indicates the position of the immunoglobulin heavy chain. Lanes 8 and 9: pattern of protein phosphorylation of: (lane 8) F2 fraction immunodepleted of α-PAK; (lane 9) α-PAK immunoprecipitate obtained from the F2 fraction. Lanes 10 and 11: western blots showing that Cdc42 immunoprecipitates Cdk5 from the F2 fraction. (lane 10) Cdc42 immunoprecipitate; note the absence of Cdk5 in the supernatant (lane 11). The asterisk indicates the position of the immunoglobulin heavy chain. Lanes 12 and 13: pattern of protein phosphorylation of: (lane 12) F2 fraction immunodepleted of Cdc42; (lane 13) Cdc42 immunoprecipitate obtained from the F2 fraction. Note the presence of two heavily phosphorylated protein bands (arrows) in the IPs. Lane 14: molecular weight markers. The insert (arrow in lane 2) shows western blots revealed with a p35 antibody from: (lane 1) control F2 fraction; (lane 2) F2 fraction immunodepleted of p35 after 2 cycles of immunoprecipitation with a Cdk5 antibody; (lane 3) Cdk5 immunoprecipitate from the F2 fraction.
The Cdk5-p35 dependent phosphorylation of the 65–70 kDa proteins prompted us to determine their possible identity. PAK1 has been identified as a major target of the Cdk5/p35 kinase in neuronal growth cones (Nikolic et al., 1998). Interestingly, PAK1 and the related kinases PAK2 and PAK3 have molecular weights similar to those of the phosphorylated bands detected in the Cdk5-p35 IPs. Therefore, the IPs were analyzed by western blotting with a rabbit polyclonal antibody raised against α-PAK, the human homologue of PAK1, which is also crossreactive with β-PAK (PAK2) and γ-PAK (PAK3). The results obtained showed that two α-PAK immunoreactive protein species migrating at 65–70 kDa Mr were present in the IPs (Figure 3, lanes 6 and 7). To complement these experiments, we immunodepleted the F2 fraction with anti α-PAK and the pattern of protein phosphorylation examined in the supernatant and IP; as shown in Figure 3 (lanes 8 and 9), the pattern detected was almost identical to those observed after immunoprecipitation with either Cdk5 or p35 antibodies.
The interaction between α-PAK and Cdk5-p35 at growth cones appears to be mediated by small GTPases (Nikolic et al., 1998). Therefore, since Cdc42 has been localized to the Golgi apparatus (Musch et al., 2001), we sought to determine whether or not such an interaction may occur in Golgi membranes. The results obtained show that an anti-Cdc42 antibody immunoprecipitates Cdk5 (Figure 3, lane 10) and α-PAK (not shown) from F2; in addition, two protein bands migrating at 65–70 kDa Mr become heavily phosphorylated after incubation of the Cdc42 IP with [γ-32P]ATP (Figure 3, lanes 12 and 13).
Inhibition of Cdk5-p35 activity blocks the formation of membrane vesicles from the Golgi apparatus of developing neurons
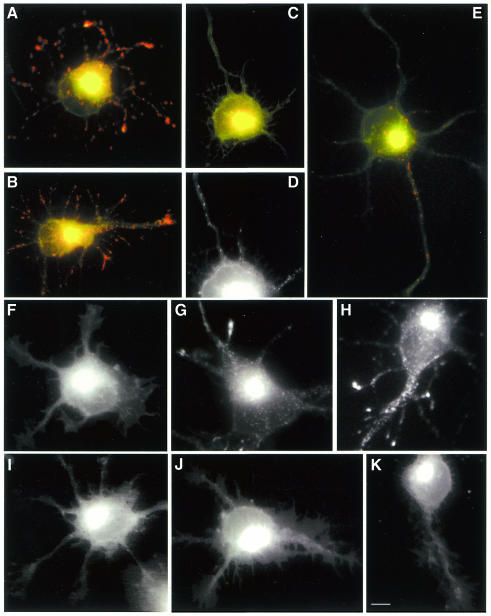
In previous studies we have shown that treatment of cultured cerebellar macroneurons with antisense oligonucleotides against Cdk5 (Pigino et al., 1997) or p35 (Paglini et al., 1998) dramatically and specifically reduces Cdk5 or p35 protein levels and Cdk5 histone H1 activity. This treatment has a similar effect in cultured hippocampal pyramidal neurons (see Supplementary data). Therefore, having shown that an active Cdk5/p35 complex is present in the Golgi apparatus we sought to determine the consequences of Cdk5 or p35 suppression by antisense oligonucleotide treatment on vesicle formation or budding in the Golgi apparatus. Bodipy-ceramide has been used to study vesicle formation in non-neuronal and neuronal cells (Pagano et al., 1991; Huber et al., 1995). With this precursor, the Golgi apparatus, in which ceramide and its metabolites are synthesized, and the post-Golgi transport vesicles show emission at 620 nm (red), whereas structures that accumulate less lipid show emission at 515 nm (green). Addition of Bodipy-ceramide to control or sense-treated young neurons and observation after 60 min at 37°C resulted in the appearance of red labeling in the cell body, with a pattern reminiscent of the Golgi apparatus (Figure 4A and B). The presence of Bodipy-ceramide labeling along neurites was also evident as dot-like structures that accumulate at growth cones. As suggested by Huber et al. (1995), it is likely that the dots represent exocytic vesicles in transit to neuritic tips, where new membrane addition takes place. In contrast, in >90% of the antisense oligonucleotide-treated cells (Figure 4C and D) red fluorescence was found predominantly in the Golgi apparatus. Very few red dots were found dispersed in the cell body, or around the perinuclear Golgi-like structure, or along neurites. A similar effect was observed after treatment with olomoucine (Figure 4E; see also Supplementary data). To complement these experiments control and antisense-treated cells were also labeled with Bodipy-ceramide for 30 min at 4°C and then incubated in dye-free medium at 37°C for different time periods. The results of these pulse–chase experiments show that in control cells, there is an initial accumulation of Bodipy-ceramide labeling (red channel) in the Golgi apparatus followed by the sequential appearance of vesicle-like structures in the cell body and neurites (F–H). In contrast, this phenomenon was blocked in p35 antisense-treated neurons (Figure 4I–K) or when the cells were incubated at 20°C during the chase (not shown); under both conditions the labeling remains confined to the region of the Golgi apparatus.
Fig. 4. Red-green overlays of digitized images of control (A), sense-treated (B) and RP1 antisense-treated (C and D) neurons after labeling with Bodipy-ceramide (60 min at 37°C). (D) A high power view (red channel) of the cell body area of the neuron shown in (C). Note the dramatic decrease in the number of vesicle-like structures (red dots) in the antisense-treated neuron. (E) A similar effect was observed after treatment of control cultures with olomoucine (25 µM) for 5 h. (F–K) A pulse–chase experiment showing the distributions of Bodipy-ceramide (red channel) in control (F–H) and RP1 antisense-treated cells (I–K). For this experiment, cultures were labeled with Bodipy-ceramide for 30 min and then incubated in dye-free medium for 5 (F and I), 30 (G and J) and 60 (H and K) min. Note that in the antisense-treated neurons the labeling remains localized to the region of the Golgi apparatus. Calibration bar: 5 µm.
DISCUSSION
We show here that an active Cdk5-p35 kinase is present in Golgi membranes, where it associates with a detergent insoluble fraction containing actin. This observation is in line with previous studies suggesting that the Cdk5-p35 kinase forms part of a large macromolecular complex (Lee et al., 1996) and that is capable of interacting with elements of the actin subcortical cytoskeleton (Paglini et al., 1998). The possible involvement of an actin matrix in Golgi processes has been the subject of several studies. For example, the identification of Golgi-specific isoforms of spectrin and ankyrin has led to propose a role for actin-binding proteins in sorting events (De Matteis and Morrow, 2000). Moreover, the purification of a detergent-insoluble ‘Golgi matrix’ has yielded the identification of a cytoskeletal-like complex, containing the Golgin proteins (Fritzler et al., 1995; Jung et al., 1996). These proteins have proline-rich regions, which may serve to anchor enzymes or for providing docking sites for vesicles. Golgins may also interact with actin-binding proteins such as IQGAP, which associates with the small GTPases Cdc42 in Golgi membranes (McCallum et al., 1998). Interestingly, recent studies have implicated Cdc42 and PAK4 in the exit of membrane proteins from the Golgi apparatus (Abo et al., 1998; Musch et al., 2001). In this regard, it is interesting to note that our immunoprecipitation experiments suggest an interaction between Cdk5-p35, Cdc42 and α-PAK in Golgi membranes; moreover, a Cdk5-p35 dependent phosphorylation of two α-PAK immunoreactive proteins species was also detected in these membranes. Therefore, it is possible that the association of Cdk5-p35 with a ‘Golgi matrix’ may be important for regulating actin dynamics, and hence Golgi structure and/or membrane traffic.
Thus, our results show that inhibition of Cdk5 or p35 expression with antisense oligonucleotides, or its inactivation with olomoucine, results in the lack of transported exocytic vesicles. The failure to detect Bodipy ceramide-labeled vesicles along neurites in these neurons, plus the subcellular localization data reported here, suggest the involvement of Cdk5-p35 in the early steps of the secretory pathway, regulating either vesicle formation or budding in the Golgi apparatus. Obviously, such a possibility does not exclude its participation in the regulation of carrier movement (see Dhavan and Tsai, 2001). In any case, the severe phenotype of Cdk5-deficient animals is fully consistent with a key role for this kinase in the regulation of membrane traffic.
METHODS
Cell culture and antisense oligonucleotides. Dissociated cultures of hippocampal pyramidal cells from embryonic rat brain tissue were prepared and maintained as described previously (Paglini et al., 1998). The Cdk5 antisense oligonucleotide RK1 (Pigino et al., 1997) and the p35 antisense oligonucleotide RP1 (Paglini et al., 1998) were used in this study (see Supplementary data). For some experiments cultures were treated with the Cdk5 inhibitors olomoucine or roscovitine for 2–5 h.
Subcellular fractionation. Golgi membranes were prepared as described by Jin et al. (1996). Briefly, total brain microsomal membranes were adjusted to 1.24 M sucrose and loaded at the bottom of a 32 ml discontinuous sucrose gradient and centrifuged at 82 000 g for 3 h. Bands at the interface between 0.25M/0.86 M and 0.86 M/1.14 M sucrose, which are enriched in Golgi elements, were collected and designated as Golgi light (F1) and Golgi heavy (F2) fractions. Fraction 1.24 was defined as the residual microsomal fraction (F3). For detergent insolubility experiments the F2 fraction was incubated for 60 min at 4°C with either sucrose–Tris buffer as control or with 2% Triton X-100, 50 mM Tris pH 7.4, 1 mM MgCl2, 1 mM DTT, 250 mM sucrose. In other cases, the F2 fraction was treated with 2 M NaCl or 0.5 M Na2CO3 (pH 5 or 11). For actin depolymerizing experiments, the F2 fraction was treated with either sucrose–Tris buffer or 2% Triton X-100, 1 µM DNase I, 0.2 mM ATP, 250 mM sucrose, 10 mM Tris pH 7.4, 2 mM EDTA for 30 or 60 min (see McCallum et al., 1998). After this, membranes were centrifuged for 30 min at 14 000 r.p.m. in an Eppendorf centrifuge, and supernatants and pellets analyzed by SDS–PAGE followed by western blotting.
Western blotting, immunoprecipitation and assay of kinase activity. The presence of Cdk5 and p35, as well as of several other proteins, in cytosolic and Golgi fractions, were analyzed by western blotting using a chemiluminiscence detection kit as described (Paglini et al., 1998). Immunoprecipitation, phosphorylation of Golgi membranes, and in vitro kinases assays were carried out as described by Tsai et al. (1994) (see also Pigino et al., 1997; Supplementary data).
Primary antibodies. Cdk5, p35 and p39 antibodies were the same ones used in a previous study (Paglini et al., 1998). In addition, we used antibodies (Santa Cruz Biotechnology Inc., CA) against cdc2p34 (C19), Rab 8 (N-20), α-PAK (C-19), Rac1 (C-19), the mitotic inhibitor p27 (clone F-8), actin (l-19), LAMP1 (C20) and cdc42 (clone B-8). We also used a rabbit polyclonal antibody against mannosidase II (Moremen and Touster, 1986), and mAbs against mannosidase II (provided by Dr Carlos Dotti, EMBL, Germany) and Rab 5 (provided by Dr Maria Colombo, University of Mendoza, Argentina).
Vital staining of the Golgi apparatus. For staining the Golgi apparatus in living cells, hippocampal pyramidal cultures were labeled with the sphingolipid Bodipy-ceramide (Pagano et al., 1991) and then examined by fluorescence microscopy as described by Huber et al. (1995). Aliquots of the fluorescent lipid (Molecular Probes, Eugene, OR) were diluted to 40 µM in serum-free medium, and neuronal cultures were labeled with this solution for 60 min. For some experiments, cultures were labeled with Bodipy-ceramide for 30 min at 4°C, and then incubated in dye-free medium at 37°C for periods ranging from 5–60 min. In all cases, cells were observed using a 63× immersion objective. Epifluorescence illumination was attenuated with neutral density filters and images were collected using a CCD camera (Orca 1000, Hamamatsu Corp., Middlesex, NJ) and Metamorph software (Universal Imaging Corporation, West Chester, PA). Photographs were printed using Adobe Photoshop.
Supplementary data. Supplementary data are available at EMBO reports Online.
Acknowledgments
ACKNOWLEDGEMENTS
The authors express their gratitude to Drs Hugo Maccioni, Carlos Dotti and Isabel Colombo for providing some of the antibodies used in this study. This work was supported by grants from CONICET (PICT-PIP 4906), FONCyT (PICT 05-06179), CONICOR, Ministerio Salud de la Nación (Beca ‘Ramón Carrillo-Arturo Oñativia’), Proyecto Cooperacion Iberoamericano and the Howard Hughes Medical Institute.
REFERENCES
- Abo A., Qu, J., Cammarano, M., Dan, C., Fritsch, A., Baud, V., Belisle, B. and Minden, A. (1998) PAK4, a novel effector of Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J., 17, 6527–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae T., Kwon, Y., Bronson, B., Dikkes, P. and Tsai, L. (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron, 18, 29–42. [DOI] [PubMed] [Google Scholar]
- Craig A., Wyborski, R. and Banker, G. (1995) Preferential addition of newly synthesized membrane proteins at axonal growth cones. Nature, 375, 592–594. [DOI] [PubMed] [Google Scholar]
- Dhavan R. and Tsai, L.-H. (2001) A decade of Cdk5. Nature Rev., 2, 749–759. [DOI] [PubMed] [Google Scholar]
- De Matteis M. and Morrow, J. (2000) Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci., 113, 2331–2343. [DOI] [PubMed] [Google Scholar]
- Fritzler M., Lung, C., Hamel, J., Griffith, K. and Chan, E. (1995) Molecular characterization of Golgin-245, a novel Golgi complex protein containing a granin signature. J. Biol. Chem., 29, 31262–31268. [DOI] [PubMed] [Google Scholar]
- Gilmore E., Ohshima, T., Goffinet, A., Kulkarni, A. and Herrup, K. (1998) Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci., 18, 6370–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L., Dupree, P. and Dotti, C. (1995) A deficiency of the small GTPase rab8 inhibits membrane traffic in developing neurons. Mol. Cell Biol., 15, 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert S., Dhaven, R. and Tsai, L. (2000) p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J. Cell Sci., 113, 975–983. [DOI] [PubMed] [Google Scholar]
- Jin M., Saucan, L., Farquhart, M. and Palade, G. (1996) Rab1a and multiple other rab proteins are associated with the transcytotic pathway in rat liver. J. Biol. Chem., 271, 30105–30113. [DOI] [PubMed] [Google Scholar]
- Jung E., Fucini, P., Stewart, M., Noegel, A. and Schleicher, M. (1996) Linking microfilaments to intracellular membranes: the actin-binding and vesicle-associated protein comitin exhibits a mannose-specific lectin activity. EMBO J., 15, 1238–1246. [PMC free article] [PubMed] [Google Scholar]
- Lee K., Rosales, J., Tang, D. and Wang, J. (1996) Interaction of cyclin-dependent kinase 5 (Cdk5) and neuronal Cdk5 activator in bovine brain. J. Biol. Chem., 271, 1538–1542. [DOI] [PubMed] [Google Scholar]
- Lew J., Huang, Q., Zhong, Q., Winkfein, R., Aebersold, R., Hunt, T. and Wang, J. (1994) A brain-specific activator of cyclin-dependent kinase 5. Nature, 271, 423–426. [DOI] [PubMed] [Google Scholar]
- McCallum S., Erikson, J. and Cerione, R. (1998) Characterization of the association of the actin-binding protein, IQGAP, and activated Cdc42 with Golgi membranes. J. Biol. Chem., 273, 22537–22544. [DOI] [PubMed] [Google Scholar]
- Moremen K. and Touster, O. (1986) Topology of mannosidase II in rat liver Golgi membranes and release of the catalytic domain by selective proteolysis. J. Biol. Chem., 261, 10945–10951. [PubMed] [Google Scholar]
- Musch A., Cohen, D., Kreitzer, G. and Rodriguez-Boulan, E. (2001) Cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J., 20, 2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M., Dudek, H., Kwon, Y., Ramos, Y. and Tsai, L. (1996) The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev., 10, 816–825. [DOI] [PubMed] [Google Scholar]
- Nikolic M., Chou, M., Lu, W., Mayer, B. and Tsai, L. (1998) The p35/Cdk5 kinase is a neuron-specific Rac effector and inhibits Pak 1 activity. Nature, 395, 194–198. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Ward, J., Huth, C., Longenecker, G., Veeranna, G., Pant, H., Brady, R., Martin, L. and Kulkarni, A. (1996) Targeted disruption of cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology, and perinatal death. Proc. Natl Acad. Sci. USA, 93, 11173–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R., Martin, O., Kang, H. and Haugland, R. (1991) A novel fluorescent ceramide analog for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J. Cell Biol., 113, 1267–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglini G. and Cáceres, A. (2001) The role of the Cdk5-p35 kinase in neuronal development. Eur. J. Biochem., 268, 1528–1533. [PubMed] [Google Scholar]
- Paglini G., Pigino, G., Kunda, P., Morfini, G., Maccioni, R., Quiroga, S., Ferreira, A. and Cáceres, A. (1998) Evidence for the involvement of the neuron-specific cdk5 activator p35 during laminin-enhanced axonal growth. J. Neurosci., 18, 9858–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G., Paglini, G., Ulloa, L., Avila, J. and Cáceres, A. (1997) The expression and function of cdk5 during process extension in primary cultured neurons. J. Cell Sci., 110, 257–270. [DOI] [PubMed] [Google Scholar]
- Tang D., Young, J., Lee, K.-W., Matsushita, M., Matsui, H., Tomizawa, K., Hatase, O. and Wang, J. (1995) An isoform of the neuronal cyclin-dependent kinase 5 (cdk5) activator. J. Biol. Chem., 270, 26897–26903. [DOI] [PubMed] [Google Scholar]
- Tsai L., Delalle, I., Caviness, V., Chae, T. and Harlow, E. (1994) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature, 371, 419–423. [DOI] [PubMed] [Google Scholar]