Abstract
Antiretroviral therapy controls immunodeficiency in people with HIV but many develop mild neurocognitive disorder. Here we investigated HIV brain disease by infecting mice with the chimeric HIV, EcoHIV, and probing changes in brain gene expression during infection and reversal with polyinosinic-polycytidylic acid (poly I:C). EcoHIV-infected C57BL/6 mice were treated with poly I:C and monitored by assay of learning in radial arm water maze, RNAseq of striatum, and QPCR of virus burden and brain transcripts. Poly I:C reversed EcoHIV-associated cognitive impairment and reduced virus burden. Major pathways downregulated by infection involved neuronal function, these transcriptional changes were normalized by poly I:C treatment. Innate immune responses were the major pathways induced in EcoHIV-infected, poly I:C treated mice. Our findings provide a framework to identify brain cell genes dysregulated by HIV infection and identify a set of innate immune response genes that can block systemic infection and its associated dysfunction in the brain.
Keywords: HIV, Innate immunity, RNAseq, Neuropathogenesis
1. Introduction
Innate immunity is an ancient system of self/non-self-discrimination and defense against pathogens present in eukaryotes ranging through plants, invertebrates, and vertebrates (Ausubel, 2005). Remarkably, some elements are broadly shared among diverse organisms including protein motifs, intracellular signaling, and transcriptional regulation (Bryant and Monie, 2012; Dzik, 2010). The Toll and Toll-like receptor (TLR) pair present in Drosophila and mammals, respectively, is among the best studied (Kimbrell and Beutler, 2001). In the fly, Toll serves as a receptor that upon ligation to fungal or bacterial components activates a transcriptional pathway remarkably similar to NFκB-IκB in mammals (Valanne et al., 2011). In mammals, there are multiple TLR, both surface membrane bound and intracellular, that recognize ligands derived from bacteria, fungi, and viruses and signal for changes in cellular gene expression. By administering ligands to single TLR, we have investigated TLR responses that control HIV infection in vitro (Wang et al., 2011) and in vivo (Dong et al., 2020). The present study focuses upon transcriptional changes in the brain during HIV-associated neurocognitive impairment (NCI) and its reversal by responses to the TLR3 ligand, poly inosinic-polycytidylic acid (poly I:C), in mice.
To facilitate experimental studies of the HIV life cycle and its control, we constructed a chimeric HIV that infects rodents and not humans, EcoHIV. In EcoHIV the gp120 coding region of HIV was replaced with the ecotropic murine leukemia virus envelope gene that converts EcoHIV tropism, providing both means to model infection in a widely available small animal and safety for research personnel and the public (Potash et al., 2005). EcoHIV can infect immunocompetent mice and multiple mutant mouse strains (Dong et al., 2020; Gu et al., 2018; He et al., 2014) and replicates only in CD4+ T cells, macrophages, and microglia in mice; it induces both adaptive and innate antiviral responses that control infection (Gu et al., 2018; He et al., 2014; Potash et al., 2005). Multiple independent investigators and the present authors have demonstrated that EcoHIV-infected mice and rats suffer NCI resembling HIV NCI seen in people with HIV (PWH) on suppressive ART, including maintenance of virus in the brain and behavioral defects (Bertrand et al., 2019; He et al., 2014; Jones et al., 2016; Kelschenbach et al., 2019; Kim et al., 2019; Li et al., 2021; Nedelcovych et al., 2019; Nedelcovych et al., 2017; Olson et al., 2018). EcoHIV-infected mice fail to perform in two functionally independent tests of cognitive ability (Gu et al., 2018; Kelschenbach et al., 2019; Kim et al., 2019), fear conditioning test of associative memory (Johansen et al., 2011; LeDoux, 2003) and the radial arm water maze (RAWM) task that requires functional working and long-term spatial memory (Diamond et al., 1999; Puzzo et al., 2014), that frequently underlie HIV–NCI in PWH on ART (reviewed in (Carroll and Brew, 2017; Saylor et al., 2016)). Investigators including ourselves have identified compounds with different mechanisms of action that prevent and/or reverse EcoHIV NCI in mice (Jaureguiberry-Bravo et al., 2021; Kim et al., 2019; Murphy et al., 2022; Nedelcovych et al., 2017, 2019; Olson et al., 2018); including amplifying an innate immune response by poly I:C treatment (Dong et al., 2020).
Innate immunity poses an active barrier to HIV replication in vivo, the virus has evolved to carry three genes, constituting roughly 14% of its genome, that function to evade innate immune responses: vif to antagonize APOBEC3G, vpu to control tetherin, and nef to inhibit SerinC3/5 (Dubé et al., 2010; Malim, 2009; Usami et al., 2015). Moreover, during HIV sexual transmission itself, viral species that establish infection, transmitted-founder clones, exhibit increased type I interferon (IFN–I)-resistance relative to viruses found in the infected sexual partner (Fenton-May et al., 2013; Parrish et al., 2013). We found a similar phenomenon in mice: knockout of the IFN-I receptor, a form of IFN-I resistance, increases EcoHIV infection (Dong et al., 2020; He et al., 2014). Treatment of mice by poly I:C either by intraperitoneal (IP) injection, intranasal application or topical administration to the vaginal surface prior to EcoHIV infection induces near sterilizing immunity, reverse transcription is blocked so the infection is never established (Dong et al., 2020). As shown recently, innate immune responses can control established SHIV infection. Neutralizing anti-envelope antibodies and a TLR7 ligand reduced virus burden in macaques; the virus was cleared in some animals that received both treatments (Borducchi et al., 2018). The protective role of the TLR7 ligand is unlikely to be adjuvant since no adaptive response was induced. Rather the TLR7 ligand likely activated cells, the authors speculate that NK cells were involved (Borducchi et al., 2018). We found that poly I:C as a single agent reduces chronic EcoHIV burden in multiple peripheral tissues and reverses virus-associated cognitive disease (Dong et al., 2020). Here we extend this work to the molecular level by conducting RNAseq to identify transcriptional changes in the brain caused by infection and those that accompany the therapeutic activity of poly I:C in treated, EcoHIV-infected mice.
2. Materials and methods
Mouse infection, RAWM, and measurement of virus burden
All animal studies were conducted under protocol IACUC-2014-0124 with the approval of the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee in full compliance with the U.S. Animal Welfare Act and Public Health Service (PHS) policies. Four-week-old male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were maintained under standard mouse husbandry conditions. Discomfort, distress, and injury to the animals were minimized. We followed our previous protocols: EcoHIV/NDK was constructed, prepared, and administered by IP injection (Potash et al., 2005), poly I:C was administered (Dong et al., 2020), RAWM was conducted (Kelschenbach et al., 2019), and virus burden measured in spleen and macrophage by QPCR (Hadas et al., 2007). In brief, five-week-old mice were infected by EcoHIV/NDK at 106 pg p24, a dose yielding roughly103 copies of HIV gag DNA per 106 spleen cells during chronic infection (Gu et al., 2018). Four weeks after infection 50 μg poly I:C was administered by IP injection to mice thrice weekly for two weeks, RAWM assay of learning was conducted during the last week of poly I:C treatment, at time when EcoHIV NCI was established, (Kim et al., 2023). The assay was conducted until uninfected, PBS-treated mice achieve 0 or 1 error to find the hidden platform. Mice were then euthanized to collect tissues for measurement of virus burden, RNAseq, and select host transcripts.
RNA-sequencing and analysis.
Upon euthanasia, mouse brain was dissected from three mice per group and striatum obtained and frozen immediately in liquid nitrogen. Total RNA from fresh frozen brain samples were sent to Novogene Co. (Durham, NC) for next-generation sequencing (mRNA sequencing). All detailed information is described in their website: https://www.novogene.com. Briefly, the workflow included initial PolyA selection-based mRNA enrichment, mRNA fragmentation followed by random priming with subsequent first- and second-strand complementary DNA (cDNA) synthesis, library preparation and sequencing using an Illumina NovaSeq 6000 sequencing system and 150 bp paired-end reads. The resulting data was checked for its quality, aligned to the mouse reference genome GRCm39 and differentially expressed genes (DEGs) were obtained from Novogene Co. Hierarchical Clustering analysis (Average Linkage Clustering) and heatmap visualization were performed using normalized read counts generated by DESeq2 and the online tool Heatmapper (Babicki et al., 2016). Gene ontology analysis were performed using Enrichr analysis tool (Kuleshov et al., 2016) and the Reactome database (Fabregat et al., 2017). Volcano plots representing significant DEGs were generated using DESeq2.
QPCR of select cellular transcripts.
Cellular transcripts from the brain were measured by QPCR using TaqMan transcript specific kits obtained from Thermo Fisher Scientific. Assays were conducted according to the manufacturer’s instructions.
Statistics.
Findings from QPCR of viral DNA and RNA are presented as mean and standard error of the mean, the differences between systems was evaluated by Student’s t-test. Findings from QPCR of cellular transcripts are presented as mean and standard error of the mean, the differences between system was evaluated by Student’s t-test. Asterisks over a bar indicate differences between uninfected, PBS-treated mice and the indicated system, gallows indicate differences between the two groups indicated.
Significantly differentially expressed genes in RNA sequencing analysis were identified using DESeq2 (Love et al., 2014) and selected as significant when the p value calculated by the Wald test was ≤0.05. DEG was considered upregulated when the log FC was >0 and downregulated with less FC < 0. Average linkage analysis was used for Hierarchical Clustering. Gene ontology enrichment tool uses Fisher’s exact test to assess significance (Chen et al., 2013).
3. Results
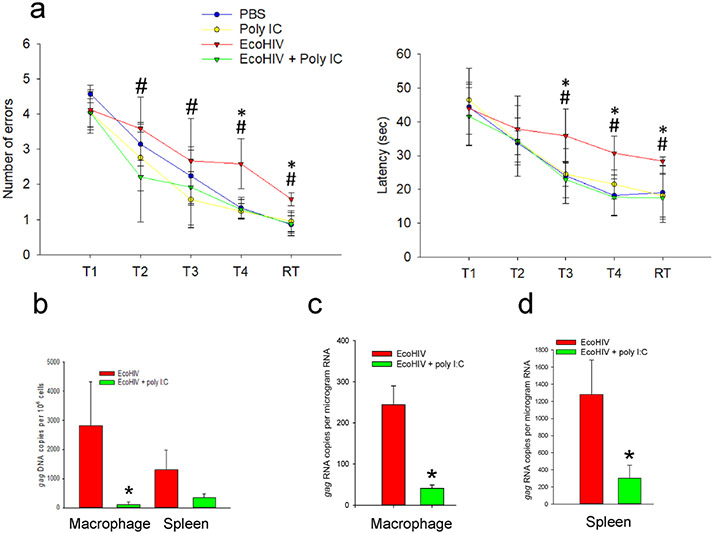
To evaluate the changes in brain RNA expression during poly I:C treatment of EcoHIV-infected mice, we first confirmed the cognitive dysfunction of infected mice and its normalization with poly I:C treatment. The experimental format followed that in our previous study (Dong et al., 2020) and included four experimental groups 1) uninfected + PBS, 2) uninfected + poly I:C, 3) infected + PBS, and 4) infected + poly I:C. C57BL/6 mice were infected by EcoHIV by IP injection, rested for three weeks, and then treated thrice weekly for two weeks with poly I:C. Mice were tested in RAWM during poly I:C administration four weeks after infection (Fig. 1A), then were euthanized and tissue harvested. Viral DNA and RNA were measured in spleen and macrophages by QPCR (Fig. 1B-D) and striatum was collected and subjected to RNAseq (Fig. 2). EcoHIV infection impaired learning in the RAWM at the level of errors in finding a hidden platform or time to do so as we previously reported (Gu et al., 2018; Jaureguiberry-Bravo et al., 2021; Kelschenbach et al., 2012, 2019; Kim et al., 2019, 2023; Murphy et al., 2022) but limited poly I:C administration restored cognitive function in infected mice (Dong et al., 2020) (Fig. 1A). Viral RNA burden in macrophages and spleen cells was significantly reduced by poly I:C treatment of infected mice, viral DNA while significantly reduced in macrophages, did not reach significance in spleen (Fig. 1B-D). This suggests that the response to poly I:C blocked virus transcription by both cell types efficiently but blocked virus spread among macrophages and new viral DNA synthesis more efficiently than among spleen cells. With the confirmation of EcoHIV NCI and its reversal by poly I:C in this cohort we conducted RNAseq in striatum from the four mouse groups to link brain cell gene expression to cognitive function or antiviral immunity.
Fig. 1. Virus burden and behavioral competence in uninfected and EcoHIV-infected mice with and without poly I:C treatment.
a. Results of RAWM showing errors (left panel) and time to find (right panel) a submerged platform. Significance was assessed using two ways ANOVA (Errors: F = 15.726 p < 0.001; Time: F = 20.078 p < 0.001). Pairwise comparisons were performed using Student’s t-test p < 0.05; *EcoHIV vs. PBS, #EcoHIV + poly I:C vs. EcoHIV. b. QPCR of HIV gag DNA burden in peritoneal macrophages or spleen from infected mice. c. QPCR of HIV gag RNA burden in peritoneal macrophages or d. spleen from infected mice. Pairwise comparisons were performed using Student’s t-test *p < 0.05, **p < 0.01.
Fig. 2. Transcriptional characterization using RNA-sequencing of brain tissue of EcoHIV infected and uninfected mice treated with poly I:C and controls.
a. Clustering analysis was performed using the normalized read counts of significantly up and downregulated transcripts in the striatum of EcoHIV-infected mice vs. PBS controls but not in EcoHIV + poly I:C vs. PBS. Significant up and downregulated genes in EcoHIV vs. PBS. b. and EcoHIV + poly I:C vs. PBS. c. were subjected to gene ontology analysis to identify the most dysregulated pathways in each group. d. Heatmap visualization of a selection of neuronal-related genes downregulated in EcoHIV vs. PBS but not in EcoHIV + Poly I:C vs. PBS. e. Heatmap selection of immune-related genes that are upregulated in EcoHIV + poly I:C vs. PBS but not in untreated EcoHIV.
Bulk RNA sequencing was performed in striatum from three mice of each experimental group. After sequencing quality control was assessed (Supplementary Table 1), significant DEGs were calculated and selected by using a cutoff of ∣log Fold Change∣ > 0 and p-value <0.05. The total numbers of up and downregulated DEGs are represented in Volcano Plots in Supplementary Fig. 1. The heatmap was constructed to illustrate transcripts from striatum significantly different between EcoHIV infected versus PBS (control) and EcoHIV infected versus EcoHIV infected, poly I:C treated with clustering by average linkage and Pearson distance measurement. With these parameters, roughly 1500 transcripts from EcoHIV-infected mouse brains show up and downregulation in opposite directions from that of both control and poly I:C treated mice (Fig. 2A). Infected mice downregulated expression of roughly 800 transcripts and poly I:C treatment reduced this to roughly 300 transcripts (not shown). Using the Enrichr pathway analysis tool and the Reactome database, we found that the ten pathways in striatum most significantly downregulated by EcoHIV infection involve synaptic function and associated neurotransmitters consistent with our observations of NCI in EcoHIV-infected mice (Fig. 2B). The antiviral action of poly I:C treatment in infected mice is indicated in that the ten most significantly upregulated pathways involving IFN-I signaling and other aspects of innate immunity (Fig. 2C). Specific transcripts within these pathways are shown in Fig. 2D-E. The dysregulation in neurotransmitter release, glutamatergic responses, and axonal guidance among others associated with EcoHIV infection are largely reversed by poly I:C treatment (Fig. 2D). In complementary fashion, consistent with our therapeutic intervention, innate immune related genes are upregulated by poly I:C, independently of infection (Fig. 2E). Systemic EcoHIV infection under the conditions reported here does not induce innate immune responses in the striatum, possibly because the virus burden in the brain at this time after IP injection is at the limit of detection (Kim et al., 2023).
To confirm and quantify the extent of changes in gene expression in the brain induced by poly I:C treatment of infected mice, we performed QPCR of four transcripts associated with NCI in both SIV-infected macaques and HIV-infected humans (Winkler et al., 2012) as well as other immune-related transcripts and two neuronal genes downregulated in EcoHIV-infected mouse brains (Kim et al., 2019) (Fig. 3). A comprehensive report of brain gene modulation by EcoHIV infection is being prepared (A. Borjabad, personal communication). With the exception of IRF7, Kalirin, and CAMK2a, the expression of all transcripts tested was increased in EcoHIV-infected mice treated with poly I:C compared to control mice. All four transcripts found in common in humans and macaques with HIV/SIV NCI, β2-microglobulin, IFI44, IFIT3, and Mx1, were also significantly increased with poly I:C treatment of infected mice compared to infected, untreated mice. Both Kalirin and CAMK2a were significantly reduced in infected mice compared to controls, as we previously observed (Kim et al., 2019) but were not reversed by poly I:C. Adaptive antiviral immune responses may also be activated in the infected brain by poly I:C as suggested in the induction of CD8, granzyme B, and CCL5. The observed increase in CD8 expression in infected, poly I:C treated mice indicates an increase in the number of cytotoxic T cells in the brain. Granzyme B is essential for cytotoxicity by both CD8+ T cells and NK cells (Lord et al., 2003), CCL5 is a major chemokine activating brain entry for T cells as well as other cell types (Winkler et al., 2012). Overall, poly I:C is a powerful activator of immune responses in EcoHIV-infected mice for treatment of infection and correction of transcriptional dysfunction and disease in the brain.
Fig. 3.
QPCR of select transcripts from striatum of uninfected and EcoHIV-infected mice with and without poly I:C treatment. Pairwise comparisons were performed using Student’s t-test *p < 0.05, **p < 0.01 indicated group versus uninfected, PBS-treated mice. Gallows indicate comparisons between other groups: *p < 0.05, **p < 0.01.
4. Discussion
Results reported here demonstrate that during systemic EcoHIV infection of wildtype mice, animals exhibit defects in learning (Dong et al., 2020; Gu et al., 2018; Kim et al., 2019, 2023) and significantly downregulate roughly 800 genes in striatum. All of the most significantly downregulated pathways involve neuronal gene expression indicating that the major effect of HIV in this analysis is loss of function as observed in previous studies of the brain transcriptome from PWH by others and ourselves (Borjabad et al., 2011; Gelman et al., 2004). As previously reported and reproduced here, systemic infection induces behavioral defects (Dong et al., 2020; Gu et al., 2018; Kim et al., 2019, 2023), likewise it induces subtle changes in selected brain transcripts (Kim et al., 2019) and as shown here, widespread alteration in gene expression in the striatum despite the very low virus burden present in the brain (Kim et al., 2023; Murphy et al., 2022). The primary effect of poly I:C is to reduce HIV expression and spread of infection in macrophages and lymphocytes, presumably by its induction of IFN-I pathways (Dong et al., 2020; Wang et al., 2011) as shown here, reducing the extent of HIV-associated pathogenic effects. Limited systemic poly I:C effectively treats brain disease at the behavioral level (Dong et al., 2020) and as shown here significantly reduces transcriptional downregulation in striatum to roughly 300 genes. What is apparent at the gene expression level is that EcoHIV infection and poly I:C treatment each initiates a broad program of gene modulation that can serve as an overall map to virally driven dysfunction and its reversal with innate immune stimulation. At the level of individual, downregulated transcripts infected mice show reduced expression of neurotransmitter release, potassium channels, glutamatergic and GABAergic response genes among other genes related to neuronal function, consistent with their impaired spatial memory. PBS-treated mice, uninfected mice and infected, poly I: C treated mice that all maintain learning and memory competence, show little down regulation of the transcripts tested. In complementary fashion, infected mice treated with poly I:C show intense innate immune responses exceeding that seen in uninfected, treated mice while infected, untreated mice resemble control mice in the limited expression of transcripts assayed.
Although the present study shows little innate immune response in the brain by infected mice, our previous studies using IC inoculation of virus found EcoHIV-mediated activation of innate immunity (He et al., 2014; Kelschenbach et al., 2019). This prominent response likely reflects both the large brain virus burden following IC inoculation (He et al., 2014; Kelschenbach et al., 2019) and the capacity of all resident brain cell types to mount TLR3-driven innate immune responses (Hanke and Kielian, 2011). An analogous phenomenon observed here is the increase in innate immune-related transcripts in infected, poly I:C-treated mice compared to uninfected, poly I:C-treated mice.
In chronically-infected mice, poly I:C reduces the levels of both viral DNA and RNA suggesting antiviral activity upon productively infected rather than latently infected spleen cells or macrophages (Dong et al., 2020) findings reproduced here (Fig. 1B-C). Blocks at different stages of the HIV life cycle imposed by select gene products were significantly induced in brains of infected, treated mice, these include IFIT3, IFI44, granzyme B, and Mx1. IFIT3 blocks HIV entry and therefore initial DNA synthesis (Chemudupati et al., 2019), IFI44 reduces LTR activity and therefore RNA transcription (Power et al., 2015), granzyme B promotes apoptosis in productively infected cells (Afonina et al., 2010). Although Mx1 is induced in both uninfected and infected cells by poly I: C, the gene has an extensive deletion in C57BL/6 mice that renders it dysfunctional (Staeheli et al., 1988) and the homolog in human beings, MxA, does not inhibit HIV infection (Goujon et al., 2013). Sialoadhesin, Siglec 1, although induced by poly I:C does not antagonize virus replication, rather it can promote HIV infection (Akiyama et al., 2017). These findings indicate that attributing antiviral function to specific gene products in mice requires mutation, knockout, or specific intervention and an associated loss of function to affect EcoHIV infection. We suggest that changes in gene expression, like those observed here, can serve as markers for pathogenic processes with common drivers, including virus, IFN-I, and antiviral therapy in different settings as described (Borjabad et al., 2011; Winkler et al., 2012).
During chronic infection by IP EcoHIV injection, peritoneal macrophages from mice show increases in innate responses including CCL5, CXCL10, and IFN-I β1 that were not affected by poly I:C (Dong et al., 2020). Using IC EcoHIV inoculation of mice, some transcripts measured here were highly induced in the brain including CD8, CXCL10 (IP10), and IRF7 (He et al., 2014; Kelschenbach et al., 2019). In contrast in the present study, infection alone was insufficient to induce these transcripts in the brain. The simplest explanation for this discrepancy is a vast difference in virus burden in the brain. IC infection with a dose of 1 μg viral p24 EcoHIV, as used here, results in roughly 80,000 copies of viral DNA per million brain cells (Kelschenbach et al., 2019) while IP infection at a dose of 2 μg viral p24 yields only 80 copies of viral DNA per million brain cells (Kim et al., 2023). However, the present study reveals apparent synergy between EcoHIV and poly I:C in induction of specific genes: CD8, CXCL10, and IFIT3 each trended to higher expression in infected, poly I:C-treated cells than in uninfected, treated cells.
The observed increase in CD8 transcripts may have additional, adaptive immune antiviral effects in the brain. We have previously shown that during EcoHIV infection loss of T cells, as observed in athymic mice, actually increases virus burden in macrophages presumably due to a loss of antiviral, CD8+ T cells (Gu et al., 2018). Transfer of CD8+ cells from infected to uninfected mice prevents their subsequent EcoHIV infection (Kelschenbach et al., 2012). In macaques chronically infected by SIV, depletion of CD8+ cells from the central nervous system increases brain virus burden and microglial activation (Marcondes et al., 2015). The observation here of increased CD8 expression in the brains of infected, poly I:C-treated mice leads to the possibility that poly I:C acts as an adjuvant to ongoing adaptive responses to promote antiviral CD8+ cell activity in the brain. Consistent with this interpretation, a recent report demonstrates that innate immune responses enhance the function of anti-HIV CD8+ cells (Cabral-Piccin et al., 2023). Such a dual immune system activation during pathogen infection by a single agent, here poly I: C, can have significant impact on host protection and requires further investigation.
Although poly I: C treatment has some beneficial effects during HIV infection of mice, due to its instability in primate serum compared to mouse serum (Nordlund et al., 1970) poly I:C is not suitable for use in human beings. However, a modified poly I:C, poly ICLC, is stable in humans (Stahl-Hennig et al., 2009) and has been shown in clinical trial to induce transient expression of CXCL10, IFI44, IFIT3, and Mx1 in peripheral blood cells with no adverse reactions in PWH (Saxena et al., 2019) consistent with its possible future application.
5. Conclusions
The findings reported here demonstrate that systemic EcoHIV infection of mice dysregulates the brain transcriptome, primarily at the level of neuronal function. However, poly I:C induces a robust innate antiviral immune response in the brain of chronically-infected mice that accompanies a reduction in systemic virus burden and a reversal of cognitive disease. Further studies are required to identify the specific gene products responsible for the therapeutic effects observed.
Supplementary Material
Acknowledgements
The authors thank Ilene Totillo for help in manuscript preparation.
Funding
Funding for the research and publication costs was supplied by PHS, United States grants: R21 NS129460 (mjp), R01 NS094146 (mjp), U01 DA053629 (djv) U01 DA056003 (djv).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virol.2023.109917.
Data availability
The complete RNAseq database will be deposited at the Gene Expression Omnibus at ncbi.nih.gov accession number GSE245947 upon publication.
References
- Afonina IS, Cullen SP, Martin SJ, 2010. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol. Rev 235, 105–116. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Ramirez NP, Gibson G, Kline C, Watkins S, Ambrose Z, Gummuluru S, 2017. Interferon-inducible CD169/siglec1 attenuates anti-HIV-1 effects of alpha interferon. J. Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, 2005. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol 6, 973–979. [DOI] [PubMed] [Google Scholar]
- Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS, 2016. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 44, W147–W153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L, Méroth F, Tournebize M, Leda AR, Sun E, Toborek M, 2019. Targeting the HIV-infected brain to improve ischemic stroke outcome. Nat. Commun 10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu W-H, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, Jetton D, Peter L, McMahan K, Moseley ET, Bekerman E, Hesselgesser J, Li W, Lewis MG, Alter G, Geleziunas R, Barouch DH, 2018. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjabad A, Morgello S, Chao W, Kim S-Y, Brooks AI, Murray J, Potash MJ, Volsky DJ, 2011. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 7, e1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CE, Monie TP, 2012. Mice, men and the relatives: cross-species studies underpin innate immunity. Open Biol 2, 120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral-Piccin MP, Papagno L, Lahaye X, Perdomo-Celis F, Volant S, White E, Monceaux V, Llewellyn-Lacey S, Fromentin R, Price DA, Chomont N, Manel N, Saez-Cirion A, Appay V, 2023. Primary role of type I interferons for the induction of functionally optimal antigen-specific CD8+ T cells in HIV infection. EBioMedicine 91, 104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A, Brew B, 2017. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Res 6, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemudupati M, Kenney AD, Bonifati S, Zani A, McMichael TM, Wu L, Yount JS, 2019. From APOBEC to ZAP: diverse mechanisms used by cellular restriction factors to inhibit virus infections. Biochim. Biophys. Acta Mol. Cell Res 1866, 382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A, 2013. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 14, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM, 1999. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 9, 542–552. [DOI] [PubMed] [Google Scholar]
- Dong B, Borjabad A, Kelschenbach J, Chao W, Volsky DJ, Potash MJ, 2020. Prevention and treatment of HIV infection and cognitive disease in mice by innate immune responses. Brain Behav. Immun. Health 3, 100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M, Bego MG, Paquay C, Cohen ÉA, 2010. Modulation of HIV-1-host interaction: role of the Vpu accessory protein. Retrovirology 7, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzik JM, 2010. The ancestry and cumulative evolution of immune reactions. Acta Biochim. Pol 57, 443–466. [PubMed] [Google Scholar]
- Fabregat A, Sidiropoulos K, Viteri G, Forner O, Marin-Garcia P, Arnau V, D’Eustachio P, Stein L, Hermjakob H, 2017. Reactome pathway analysis: a high-performance in-memory approach. BMC Bioinf. 18, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton-May AE, Dibben O, Emmerich T, Ding H, Pfafferott K, Aasa-Chapman MM, Pellegrino P, Williams I, Cohen MS, Gao F, Shaw GM, Hahn BH, Ochsenbauer C, Kappes JC, Borrow P, 2013. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 10, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Schuenke KW, Keherly MJ, Holzer C 3rd, Richey FJ, Lahart CJ, 2004. Acquired neuronal channelopathies in HIV-associated dementia. J. Neuroimmunol 157, 111–119. [DOI] [PubMed] [Google Scholar]
- Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, Hue S, Barclay WS, Schulz R, Malim MH, 2013. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502, 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Kim B-H, Chao W, Arancio O, Suh J, Polsky B, McMillan J, Edagwa B, Gendelman HE, Potash MJ, Volsky DJ, 2018. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 14, e1007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadas E, Borjabad A, Chao W, Saini M, Ichiyama K, Potash MJ, Volsky DJ, 2007. Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. AIDS 21, 905–909. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Kielian T, 2011. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci. (Lond.) 121, 367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Sharer LR, Chao W, Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Ichiyama K, Do M, Potash MJ, Volsky DJ, 2014. Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses. J. Neuropathol. Exp. Neurol 73, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaureguiberry-Bravo M, Kelschenbach J, Murphy A, Carvallo L, Hadas E, Tesfa L, Scott TM, Rivera-Mindt M, Cunningham CO, Arnsten JH, Volsky DJ, Berman JW, 2021. Treatment with buprenorphine prior to EcoHIV infection of mice prevents the development of neurocognitive impairment. J. Leukoc. Biol 109, 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE, 2011. Molecular mechanisms of fear learning and memory. Cell 147, 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LD, Jackson JW, Maggirwar SB, 2016. Modeling HIV-1 induced neuroinflammation in mice: role of platelets in mediating blood-brain barrier dysfunction. PLoS One 11, e0151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelschenbach J, He H, Kim BH, Borjabad A, Gu CJ, Chao W, Do M, Sharer LR, Zhang H, Arancio O, Potash MJ, Volsky DJ, 2019. Efficient expression of HIV in immunocompetent mouse brain reveals a novel nonneurotoxic viral function in hippocampal synaptodendritic injury and memory impairment. mBio 10, e00591–00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelschenbach JL, Saini M, Hadas E, Gu CJ, Chao W, Bentsman G, Hong JP, Hanke T, Sharer LR, Potash MJ, Volsky DJ, 2012. Mice chronically infected with chimeric HIV resist peripheral and brain superinfection: a model of protective immunity to HIV. J. Neuroimmune Pharmacol 7, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B-H, Hadas E, Kelschenbach J, Chao W, Gu C-J, Potash MJ, Volsky DJ, 2023. CCL2 is required for initiation but not persistence of HIV infection mediated neurocognitive disease in mice. Sci. Rep 13, 6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B-H, Kelschenbach J, Borjabad A, Hadas E, He H, Potash MJ, Nedelcovych MT, Rais R, Haughey NJ, McArthur JC, Slusher BS, Volsky DJ, 2019. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS 33, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell DA, Beutler B, 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet 2, 256–267. [DOI] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A, 2016. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 2003. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol 23, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, McLaurin KA, Illenberger JM, Mactutus CF, Booze RM, 2021. Microglial HIV-1 Expression: Role in HIV-1 Associated Neurocognitive Disorders. Viruses 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord S, Rajotte R, Korbutt G, Bleackley RC, 2003. Granzyme B: a natural born killer. Immunol. Rev 193, 31–38. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, 2009. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos. Trans. R. Soc. Lond. B Biol. Sci 364, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Morsey B, Emanuel K, Lamberty BG, Flynn CT, Fox HS, 2015. CD8+ T cells maintain suppression of simian immunodeficiency virus in the central nervous system. J. Infect. Dis 211, 40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Kelschenbach J, He H, Chao W, Kim B-H, Volsky DJ, Berman JW, 2022. Buprenorphine reverses neurocognitive impairment in EcoHIV infected mice: a potential therapy for HIV-NCI. Front. Immunol 13, 1004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcovych MT, Kim B-H, Zhu X, Lovell LE, Manning AA, Kelschenbach J, Hadas E, Chao W, Prchalová E, Dash RP, Wu Y, Alt J, Thomas AG, Rais R, Kamiya A, Volsky DJ, Slusher BS, 2019. Glutamine antagonist JHU083 normalizes aberrant glutamate production and cognitive deficits in the ecoHIV murine model of HIV-associated neurocognitive disorders. J. Neuroimmune Pharmacol 14, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcovych MT, Tenora L, Kim B-H, Kelschenbach J, Chao W, Hadas E, Jančařík A, Prchalová E, Zimmermann SC, Dash RP, Gadiano AJ, Garrett C, Furtmüller G, Oh B, Brandacher G, Alt J, Majer P, Volsky DJ, Rais R, Slusher BS, 2017. N-(Pivaloyloxy)alkoxy-carbonyl prodrugs of the glutamine antagonist 6-Diazo-5-oxo-l-norleucine (DON) as a potential treatment for HIV associated neurocognitive disorders. J. Med. Chem 60, 7186–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund JJ, Wolff SM, Levy HB, 1970. Inhibition of biologic activity of poly I: poly C by human plasma. Proc Soc Exp Biol Med 133, 439–444. [DOI] [PubMed] [Google Scholar]
- Olson KE, Bade AN, Namminga KL, Potash MJ, Mosley RL, Poluektova LY, Volsky DJ, Gendelman HE, 2018. Persistent EcoHIV infection induces nigral degeneration in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-intoxicated mice. J. Neurovirol 24, 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP Jr., Bjorkman PJ, Wilen CB, Doms RW, O’Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH, 2013. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. U. S. A 110, 6626–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ, 2005. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. U.S.A 102, 3760–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power D, Santoso N, Dieringer M, Yu J, Huang H, Simpson S, Seth I, Miao H, Zhu J, 2015. IFI44 suppresses HIV-1 LTR promoter activity and facilitates its latency. Virology 481, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Lee L, Palmeri A, Calabrese G, Arancio O, 2014. Behavioral assays with mouse models of Alzheimer’s disease: practical considerations and guidelines. Biochem. Pharmacol 88, 450–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena M, Sabado RL, La Mar M, Mohri H, Salazar AM, Dong H, Correa Da Rosa J, Markowitz M, Bhardwaj N, Miller E, 2019. Poly-ICLC, a TLR3 agonist, induces transient innate immune responses in patients with treated HIV-infection: a randomized double blinded placebo controlled trial. Front. Immunol 10, 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC, 2016. HIV-associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat. Rev. Neurol 12, 234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P, Grob R, Meier E, Sutcliffe JG, Haller O, 1988. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell Biol 8, 4518–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl-Hennig C, Eisenblatter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, Salazar AM, Uberla K, Nieto K, Kleinschmidt J, Schulte R, Gissmann L, Muller M, Sacher A, Racz P, Steinman RM, Uguccioni M, Ignatius R, 2009. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 5, e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami Y, Wu Y, Göttlinger HG, 2015. SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature 526, 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valanne S, Wang J-H, Ramet M, 2011. The Drosophila Toll signaling pathway. J. Immunol 186, 649–656. [DOI] [PubMed] [Google Scholar]
- Wang X, Chao W, Saini M, Potash MJ, 2011. A common path to innate immunity to HIV-1 induced by Toll-like receptor ligands in primary human macrophages. PLoS One 6, e24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler JM, Chaudhuri AD, Fox HS, 2012. Translating the brain transcriptome in neuroAIDS: from non-human primates to humans. J. Neuroimmune Pharmacol 7, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete RNAseq database will be deposited at the Gene Expression Omnibus at ncbi.nih.gov accession number GSE245947 upon publication.