Abstract
dMi-2, the ATPase subunit of the Drosophila nucleosome remodelling and histone deacetylation (dNuRD) complex, was identified in a two-hybrid screen as an interacting partner of the transcriptional repressor, Tramtrack69 (Ttk69). A short region of Ttk69 is sufficient to mediate this interaction. Ttk69, but not the Ttk88 isoform, co-purifies with the dNuRD complex isolated from embryo extracts. dMi-2 and Ttk69 co-immunoprecipitate from embryonic extracts, indicating that they can associate in vivo. Both dMi-2 and Ttk69 co-localize at a number of discrete sites on polytene chromosomes, showing that they bind common target loci. We also demonstrate that dMi-2 and Ttk interact genetically, indicating a functional interaction in vivo. We propose that Ttk69 represses some target genes by remodelling chromatin structure through the recruitment of the dNuRD complex.
INTRODUCTION
The tramtrack locus generates two zinc-finger DNA-binding proteins, Ttk69 and Ttk88, by alternative splicing. Both isoforms share a common N-terminal portion containing a BTB/POZ domain, but have different C-terminal zinc-finger domains and thus DNA-binding specificities (Harrison and Travers, 1990; Brown et al., 1991; Read and Manley, 1992; Xu et al., 2000). BTB/POZ domains are found in a large family of transcription factors, usually in combination with a zinc-finger DNA-binding region, and are thought to mediate protein–protein interactions (Bardwell and Treisman, 1994).
Ttk69 was first characterized as a transcriptional repressor of the pair-rule genes even skipped and fushi tarazu (Harrison and Travers, 1990; Brown et al., 1991; Brown and Wu, 1993). Subsequently, both Ttk69 and Ttk88 were shown to regulate nervous system development. Ttk represses neuronal identity and stabilizes non-neuronal fates in a number of contexts (Xiong and Montell, 1993; Guo et al., 1995; Giesen et al., 1997; Li et al., 1997; Tang et al., 1997; Badenhorst, 2001). However, the pleiotropic nature of ttk mutant phenotypes suggests that Ttk69 and Ttk88 regulate many developmentally important targets. Control of Ttk expression is also complex. The isoforms are subject to transcriptional, translational (Okabe et al., 2001) and post-translational regulation (Li et al., 1997; Tang et al., 1997; Lehembre et al., 2000).
Although the ability of Ttk69 to repress transcription is well established, the mechanisms by which repression is achieved are unclear. Ttk69 interacts genetically with the transcriptional co-repressor dCtBP (Wen et al., 2000), suggesting that recruitment of dCtBP is one way in which Ttk69 represses transcription. However, genetic and biochemical data suggest that there are other mechanisms through which this repression occurs (Wen et al., 2000). To characterize other co-repressors, we performed a yeast two-hybrid screen using Ttk69. Here we show that Ttk69, but not Ttk88, interacts with another co-repressor, dMi-2. We argue that Ttk69 represses transcription by distinct mechanisms at different target promoters.
RESULTS
To identify proteins that mediate transcriptional repression by Ttk69, we performed a two-hybrid screen using full-length Ttk69 as bait to screen a 0–24 h Drosophila cDNA library. We identified 10 clones that interacted strongly and specifically with Ttk69 as judged by both growth and lacZ assays. One positively interacting clone contained sequences derived from the C-terminal region of dMi-2, the Drosophila homologue of the human autoantigen Mi-2 and ATPase subunit of the nucleosome remodelling and histone deacetylation (NuRD) chromatin remodelling complex (Kehle et al., 1998; Brehm et al., 2000). This complex has been shown to establish transcriptional repression of a number of target genes in both vertebrates and invertebrates (Bird and Wolffe, 1999; Solari and Ahringer, 2000). The dMi-2-interacting clones identified in the screen completely overlap with clones isolated in a similar screen using the Drosophila gap gene hunchback (Hb), another transcriptional repressor (Kehle et al., 1998). Interestingly, the C-terminal region of mouse Mi-2 interacts with the Ikaros family of zinc-finger-containing transcription factors (Kim et al., 1999), again suggesting that this region is required for recruitment.
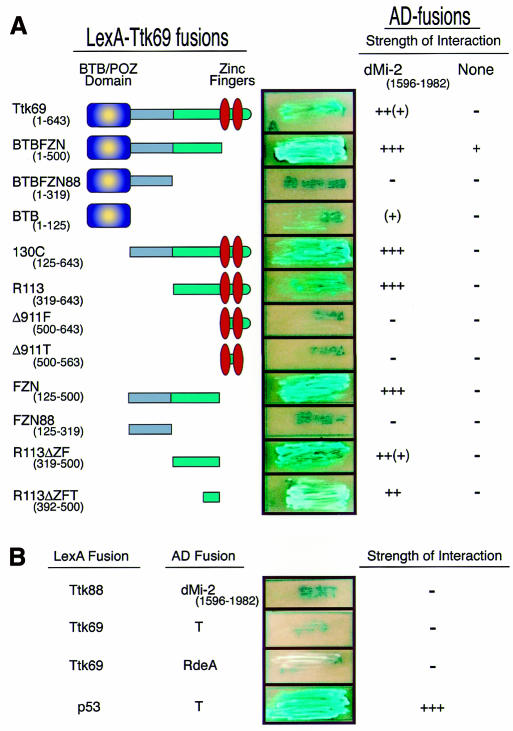
To establish the region of Ttk69 that interacts with dMi-2, we challenged the dMi-2 C-terminal with series of Ttk69 deletions by two-hybrid assay. As shown in Figure 1A, the dMi-2-interacting domain is restricted to an ∼100-amino-acid region immediately upstream of the zinc fingers. This area lies outside the previously characterized BTB/POZ and zinc-finger domains and is found only in Ttk69, but not Ttk88, suggesting that the dMi-2 interaction is isoform specific. We confirmed this by challenging the dMi-2 C-terminal region with full-length Ttk88 in the two-hybrid system (Figure 1B). As expected, Ttk88 did not interact with dMi-2. Moreover, Ttk69 did not interact with the unrelated simian virus 40 (SV40) large T-antigen or the Dictyostelium RdeA proteins, showing that the interaction with dMi-2 is specific.
Fig. 1. Yeast two-hybrid assay maps the Ttk69–dMi-2 interaction domain. (A) A series of Ttk69 truncations fused to the LexA DBD show that Ttk69 interacts with dMi-2 via a 100-amino-acid region (R113ΔZFT). The right-hand column shows the response to a control construct lacking a dMi-2 insert. (B) The complete Ttk88 sequence fused to the LexA DNA binding domain does not interact with dMi-2 in the two-hybrid assay. Negative controls: Ttk69 versus the SV40 large T-antigen or Dictyostelium RdeA proteins. Positive control: p53 versus the SV40 large T-antigen. Representative streaks of strains expressing the two-hybrid fusions (middle) and a summary of the interaction strengths (right) are shown. Interaction strength scale: –, no detectable interaction; +, weak interaction; (+), weak–strong interaction; ++, strong interaction; ++(+), strong–very strong interaction; +++, very strong interaction.
Ttk69 co-purifies with the Drosophila NuRD complex
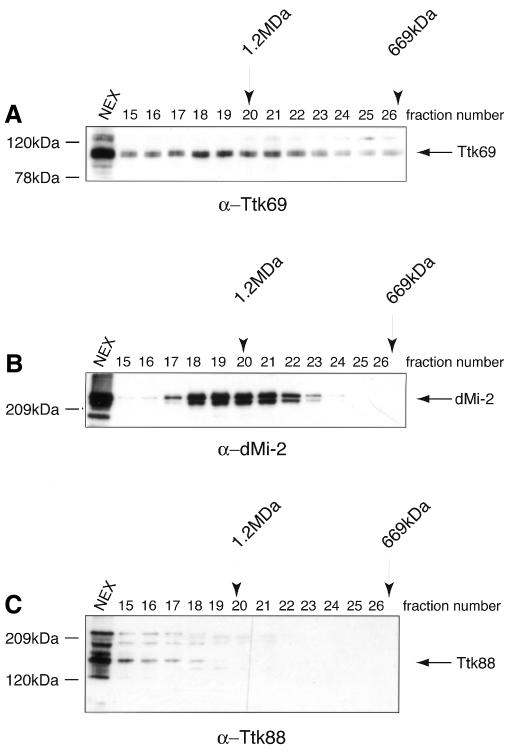
In a complementary experimental approach, we attempted to purify Ttk69-containing complexes from Drosophila embryos. Fractionation of 0–24 h embryonic extracts by gel-filtration chromatography and subsequent western blotting shows that the majority of Ttk69 elutes in a broad peak with a molecular weight of ∼1 MDa, much larger than the predicted monomer size of 69 kDa (Figure 2A). Antibodies directed against the C-terminal region of dMi-2 (Brehm et al., 2000) showed that dMi-2 and Ttk69 are present in an overlapping set of high-molecular-weight fractions (Figure 2B). Ttk88 is not found in the dMi-2 fractions (Figure 2C).
Fig. 2. Ttk69 and dMi-2 co-fractionate in a high-molecular-weight fraction. Western analysis of embryo nuclear extracts fractionated over a Superose-6 gel-filtration column shows that Ttk69 (A) and dMi-2 (B), but not Ttk88 (C), co-elute in overlapping high-molecular-weight fractions. Molecular weights of fractions were determined using standards (Pharmacia) and are displayed above the fractions.
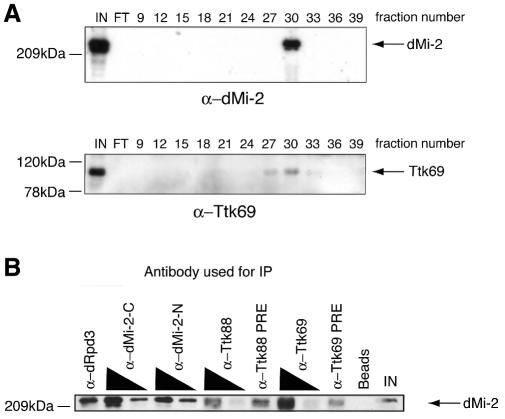
In a separate purification procedure developed to isolate the dMi-2-containing dNuRD complex, Ttk69 consistently co-purified with dMi-2 through multiple fractionation steps. Western analysis confirmed that Ttk69 is present in the purest dMi-2 fractions (Figure 3A). Ttk69 also physically associates with dMi-2 in partially purified nuclear extracts, as shown by co-immunoprecipitation of dMi-2 from these fractions using α-Ttk69 antibodies (Figure 3B). α-Ttk69 antibodies co-precipitated dMi-2 to a significantly greater extent than equivalent amounts of the pre-immune serum. As expected, dMi-2 was also precipitated by α-dMi-2 and α-dRpd3 (HDAC subunit of dNuRD) antibodies. In contrast, no enhanced dMi-2 signal was observed using α-Ttk88 antibodies.
Fig. 3. Ttk69 and dMi-2 associate in vivo. (A) Embryo nuclear extracts were fractionated biochemically to purify the dNuRD complex. Fractions from the final Mini-S column purification step were western blotted and probed using α-dMi-2 and α-Ttk69 antibodies. IN, input from previous column; FT, flow through. (B) dMi-2 and Ttk69 co-immunoprecipitate. Indicated antibodies were added to partially purified fractions and immunoprecipitated material was western blotted and probed with α-dMi-2 antibodies. IN, 15% input material; Beads, protein A beads alone (no antibody); PRE, pre-immune serum.
Ttk69 and dMi-2 co-localize on polytene chromosomes
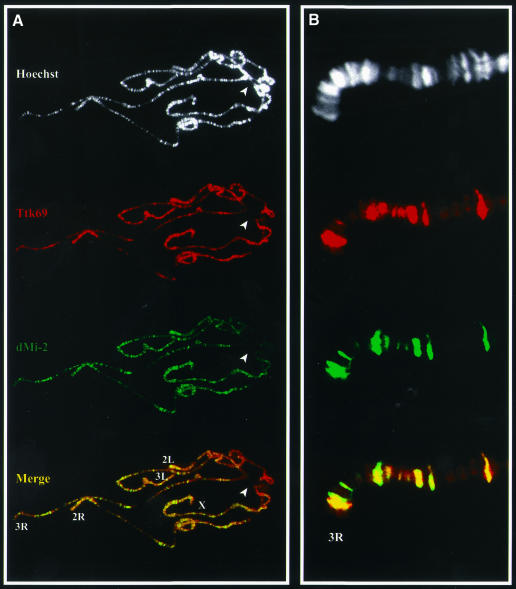
To determine whether dMi-2 and Ttk69 associate on chromatin in vivo, the distribution of both proteins on third instar larval polytene chromosomes was mapped by double-label immunofluorescence (Figure 4). Ttk69 has previously been localized on polytene chromosomes (Lehembre et al., 2000). We confirmed that Ttk69 binds a large number of euchromatic sites (Figure 4A), consistent with the pleiotropic nature of Ttk69 over-expression and loss-of-function phenotypes and developmentally complex Ttk69 expression. A similarly large number of euchromatic sites were stained with antibodies directed against dMi-2. A merge of the Ttk69 and dMi-2 staining patterns revealed a substantial overlap between the Ttk69 and dMi-2 distributions. Importantly, a few Ttk69-reactive sites did not show appreciable accumulation of dMi-2, which suggests that, at some loci, Ttk69 function may not require dMi-2. Reciprocally, dMi-2 was also found to be associated with some sites in the genome that lacked detectable concentrations of Ttk69. dMi-2 was notably absent from the chromocentre (Figure 4A, arrowhead). Common binding sites can clearly be distinguished in a magnified image (Figure 4B). The co-localization of Ttk69 with dMi-2 at many chromosomal sites has a striking parallel in the co-localization of another BTB/POZ zinc-finger protein, GAGA, with the ISWI chromatin remodelling factor (Deuring et al., 2000).
Fig. 4. dMi-2 and Ttk69 bind to a common set of chromosomal locations. (A) Polytene chromosomes were stained using α-dMi-2 (green) and α-Ttk69 (red) antibodies. The two proteins co-localize at a number of sites (yellow bands in Merge). The DNA banding pattern, as revealed by Hoechst staining, is shown in white. The chromocentre is indicated (arrowhead). (B) In a magnified image, common binding sites can clearly be distinguished.
Ttk interacts genetically with dMi-2
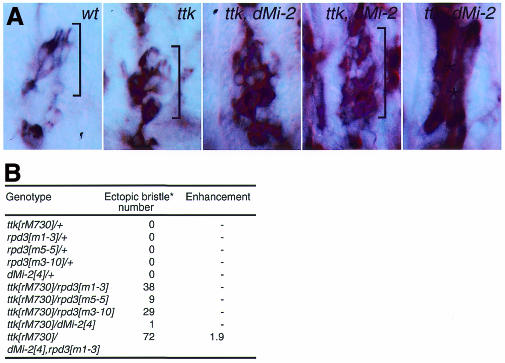
To establish if dMi-2 is required for Ttk69-mediated repression in vivo, we examined whether simultaneous loss of dMi-2 would increase neuron over-production seen in the peripheral nervous system (PNS) of embryos mutant for the hypomorphic ttkrM730 allele. Normally, Ttk is expressed in all non-neuronal cells of PNS sensory organs and prevents these cells from becoming neurons (Guo et al., 1995). In ttk mutants, a variable number of these cells are transformed into neurons, causing excess neurons in the PNS. An antibody against the neuronal antigen 22C10 was used to detect neurons of the lateral pentascolopidial sensory organ of the embryonic PNS. In wild-type embryos, five neurons are present (Figure 5A, bracketed). This number is approximately doubled in ttkrM730 mutants. Although homozygous mutant dMi-24 embryos show no defects in PNS development, loss of dMi-2 in a ttkrM730 mutant background significantly increases neuron number (Figure 5A). Moreover, a number of tissues that are not stained by mAb 22C10 in wild-type and ttkrM730 mutants now express the antigen. Epidermal staining is increased and body-wall muscles stain strongly (asterisks in Figure 5A). mAb 22C10 staining of the somatic musculature has been reported for strong ttk loss-of-function alleles such as ttkD2-50 (Giesen et al., 1997), providing further evidence that loss of dMi-2 increases the strength of ttk mutant phenotypes.
Fig. 5. dMi-2 interacts genetically with tramtrack. (A) mAb 22C10 antibody staining of wild-type, homozygous mutant ttkrM730 and ttkrM730 dMi-24 embryos shows that the number of neurons associated with the lateral pentascolopidial organ (brackets) is increased significantly in ttk dMi-2 double mutants. The extreme-right panel shows mAb 22C10 staining of somatic muscles of ttkrM730 dMi-24 double-mutant embryos. (B) ttkrM730 interacts dominantly with rpd3 and dMi-2 mutant alleles to cause ectopic sensory bristle formation on wing veins of adult flies. Bristle numbers (*) are totals on 20 wings from flies of each genotype. rpd3 alleles correspond to a lethal complementation group defined by Gepner et al. (1996).
ttk also shows dominant interactions with mutations affecting the components of the dNuRD complex. Titration of Ttk function can increase the number of precursor cells that are recruited to initiate sensory-organ development. One manifestation is the production of ectopic bristles along veins of the adult wing (P. Badenhorst and A.A. Travers, unpublished observations). ttkrM730 interacts dominantly with mutants affecting rpd3 to cause bristle de-repression on the adult wing (Figure 5B). Further reduction of dMi-2 levels in the presence of one copy of the null allele dMi-24 increases bristle number synergistically, reflecting functional in vivo interactions between Ttk and both Rpd3 and dMi-2.
DISCUSSION
We have shown that Ttk69, but not Ttk88, interacts and co-purifies with dMi-2, the ATPase subunit of the Drosophila NuRD complex. A yeast two-hybrid assay shows that Ttk69 interacts with the C-terminal region of dMi-2. The dMi-2-interacting site maps immediately N-terminal of the zinc fingers of Ttk69, providing the first described function of this region. These biochemical interactions are confirmed by genetic interaction between ttk and dMi-2. Although the ttk mutant used is a promoter mutant that affects both Ttk69 and Ttk88, the failure of Ttk88 to interact with dMi-2 in either the two-hybrid screen or crude extracts of Drosophila embryos indicates that the most probable explanation for the genetic result is an interaction between Ttk69 and dMi-2 in vivo. Interestingly, the interaction of Ttk69 with the dNuRD complex parallels that of another BTB/POZ-containing protein, GAGA, with the ISWI-containing remodelling complex NURF (Xiao et al., 2001).
A second Drosophila protein, Hb, has also been shown to interact with dMi-2, both genetically and by two-hybrid analysis (Kehle et al., 1998). Hb binds to the same C-terminal region of dMi-2 as does Ttk69, suggesting that this region may be a docking platform for proteins that recruit the dNuRD complex. However, thus far we have been unable to detect Hb in the dNuRD complex. This failure may simply reflect the relative abundance of Ttk69 and Hb in Drosophila embryos. Nevertheless, the association of dMi-2 with more than one Drosophila transcriptional repressor implies that it can be recruited by a number of different proteins. Such recruitment may be a general mechanism by which specific repressors silence their targets. Indeed, MBD2, Ikaros and Aiolos, also known repressors, have been shown to purify with the NuRD complex from mammalian cells (Kim et al., 1999; Ng et al., 1999; Wade et al., 1999).
The association of Ttk69 with the dNuRD complex implies that one mechanism by which Ttk69 may repress its targets is to direct the histone deacetylation and chromatin remodelling activities of the dNuRD complex. Such a function would be consistent with the developmental expression pattern of Ttk69. High levels of Ttk69 expression are detected towards the end of embryogenesis, when most cell types have already been specified and the chosen fate needs to be stabilized (Badenhorst et al., 1996; Badenhorst, 2001). Our results imply in particular that dMi-2 is involved in ttk-mediated neuronal repression. It is noteworthy that all known transcription factors that interact with Mi-2-containing complexes are deployed when particular cell fates or expression patterns need to be maintained. Thus, Hb mediates stable homeotic repression, Ikaros/Aiolos maintains B and T cell fates and MBD2 is required for DNA-methylation-dependent silencing. We speculate that Ttk69 also uses this mechanism stably to repress targets incompatible with determined cell fates.
Our results suggest a model by which Ttk69 interacts with the dMi-2 component of the dNuRD complex and subsequently recruits its repressive activities to target genes. We speculate that in vivo the association of Ttk69 with dMi-2 is probably not the only route by which Ttk69 may repress transcription. It is known that Ttk69 interacts genetically with dCtBP (Wen et al., 2000), another transcriptional co-repressor. Moreover, Ttk69 and dMi-2 do not completely co-localize on polytene chromosomes, suggesting that Ttk69 binds a subset of its targets in the absence of dMi-2. Finally, we note that only a small fraction of the total amount of Ttk69 present in embryonic extracts co-purifies with the dNuRD complex. Taken together, we surmise that Ttk69 is a component of more than one repressive complex and that different target genes may be regulated by different mechanisms.
METHODS
Yeast two-hybrid assays. The yeast two-hybrid screen, domain mapping and specificity tests were performed using the Matchmaker LexA Two-Hybrid System (Clontech) as described by the manufacturer. For the two-hybrid screen, the full-length Ttk69 cDNA was cloned into pLexA202 and used to screen a 0–24 h Drosophila embryonic cDNA library fused to the B42 activation domain of plasmid pJG4-5. For domain-mapping experiments, Ttk69 deletions were cloned into pLexA202 and tested for their interaction with the dMi-2 region fused to the B42 activation domain in plasmid pJG4-5. In order to test whether Ttk88 interacted with dMi-2, the full-length Ttk88 cDNA was cloned into pLexA202 and tested as above. To test the specificity of the Ttk69 interactions, the full-length clone in pLexA202 was tested against the full-length cDNAs of the SV40 large T-antigen and Dictyostelium RdeA genes cloned into pJG4-5.
Preparation of antisera. dMi-2 and dRpd3 antibodies were prepared as previously described (Brehm et al., 2000). Ttk69(aa317–643) and Ttk88(aa289–811) were expressed as His6 fusions in pET15 and pET24, respectively. Both proteins were then purified over Ni–NTA columns and used to raise rabbit or rat polyclonal antisera. The antisera were then affinity purified against the antigens using standard procedures.
Fractionation of Drosophila embryo extracts. To determine whether Ttk69 was present in high-molecular-weight complexes in vivo, crude nuclear extract from Drosophila embryos (Nightingale et al., 1998) was fractionated by fast-performance liquid chromatography (FPLC) using Biorex70 and ResourceQ columns (see below). Ttk69-containing fractions were then applied to a Superose-6 gel-filtration column (HR 10/30, Pharmacia). Proteins were eluted in HEMG100 (25 mM HEPES pH 7.6, 100 mM KCl, 12.5 mM MgCl2, 0.5 mM EDTA, 0.5 mM EGTA, 1 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 10% glycerol). Fractions were subjected to SDS–PAGE and processed for western analysis as described. For purification of dMi-2-containing complexes, crude nuclear extract was separated by FPLC over a Biorex70 (Pharmacia) column using stepwise salt elution. dMi-2-containing fractions were pooled, dialysed against HEMG100 and applied to a ResourceQ (Pharmacia) column. dMi-2 was eluted using a salt gradient. dMi-2-containing fractions were pooled, concentrated with a Centricon concentrator and applied to a Superose-6 sizing column. Fractions containing dMi-2 were pooled and applied to a Mini-S column (Pharmacia). dMi-2 complexes were eluted using a salt gradient. Fractions at each step were analysed by SDS–PAGE and western blotting.
Immunoprecipitations and western analysis. Immunoprecipitations were carried out in HEMG200 (HEMG, 200 mM KCl) supplemented with 0.1% NP-40. Antisera (1 or 10 µl) were added to 75–200 µl of partially purified nuclear extract and incubated for 3 h at 4°C. Protein A beads (Roche) were added and incubation was continued for 1 h. Protein A–antibody complexes were collected by centrifugation and washed four times in 1 ml of HEMG100, 0.1% NP-40. Protein A–antibody complexes were resuspended in loading buffer for SDS–PAGE. For western analysis, immunoprecipitates and column fractions were separated by SDS–PAGE, transferred to Hybond membranes (Amersham) and probed with the relevant antibodies.
Immunocytochemistry. Immunostaining of polytene chromosomes was carried out according to the protocol of Deuring et al. (2000), with the following modifications. Slides were blocked prior to incubation with the primary antibodies by three changes of phosphate-buffered saline (PBS)–10% fetal calf serum. Slides were then incubated overnight with the appropriate primary antibody diluted in blocking solution at 4°C. Rabbit α-dMi-2 was used at 1:1000 dilution and rat α-Ttk69 was used at 1:200 dilution. Slides were washed three times in blocking solution at room temperature prior to incubation with secondary antisera for 30 min at room temperature. Fluorescein isothiocyanate (FITC)-conjugated α-rabbit and Cy3-conjugated α-rat antibodies (Jackson Immunoresearch) were used at 1:200 dilutions in blocking solution. Slides were washed in three changes of PBS-1% Triton X-100 and DNA counterstained by brief incubation in 10 µg/ml Hoechst 33258 (Sigma) prior to mounting in 90% glycerol containing 1 mg/ml phenylenediamine (Sigma). Preparations were viewed using a Zeiss Axiophot microscope and images captured using a cooled CCD camera (Photometrics, Tuscon, AZ). The fluorescent signals were captured separately as grey-scale images and pseudocoloured using the Adobe Photoshop program.
Drosophila embryos were fixed and stained with mAb 22C10 antibodies as described previously (Badenhorst, 2001).
Acknowledgments
ACKNOWLEDGEMENTS
We are most grateful to Dr Jürg Müller for his gift of α-dMi-2 and to Dr James Kennison for advice and fly strains. C.M.M. is supported by a Natural Sciences and Engineering Research Council of Canada studentship, the Cambridge Commonwealth Trust and an Overseas Research Scholarship.
REFERENCES
- Badenhorst P. (2001) Tramtrack controls glial number and identity in the Drosophila embryonic CNS. Development, 128, 4093–4101. [DOI] [PubMed] [Google Scholar]
- Badenhorst P., Harrison, S. and Travers, A. (1996) End of the line? Tramtrack and cell fate determination in Drosophila. Genes Cells, 1, 707–716. [DOI] [PubMed] [Google Scholar]
- Bardwell V.J. and Treisman, R. (1994) The POZ domain: a conserved protein–protein interaction motif. Genes Dev., 8, 1664–1677. [DOI] [PubMed] [Google Scholar]
- Bird A.P. and Wolffe, A.P. (1999) Methylation-induced repression—belts, braces, and chromatin. Cell, 99, 451–454. [DOI] [PubMed] [Google Scholar]
- Brehm A., Längst, G., Kehle, J., Clapier, C.R., Imhof, A., Eberharter, A., Müller, J. and Becker, P.B. (2000) dMi-2 and ISWI chromatin remodelling factors have distinct nucleosome binding and mobilization properties. EMBO J., 19, 4332–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.L. and Wu, C. (1993) Repression of Drosophila pair-rule segmentation genes by ectopic expression of tramtrack. Development, 117, 45–58. [DOI] [PubMed] [Google Scholar]
- Brown J.L., Sonoda, S., Ueda, H., Scott, M.P. and Wu, C. (1991) Repression of the Drosophila fushi tarazu (ftz) segmentation gene. EMBO J., 10, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuring R. et al. (2000) The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol. Cell, 5, 355–365. [DOI] [PubMed] [Google Scholar]
- Gepner J., Li, M., Ludmann, M., Kortas, C., Boylan, K., Iyadurai, S.J., McGrail, M. and Hays, T.S. (1996) Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics, 142, 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen K., Hummel, T., Stollewerk, A., Harrison, S., Travers, A. and Klämbt, C. (1997) Glial development in the Drosophila CNS requires concomitant activation of glial and repression of neuronal differentiation genes. Development, 124, 2307–2316. [DOI] [PubMed] [Google Scholar]
- Guo M., Bier, E., Jan, L.Y. and Jan, Y.N. (1995) tramtrack acts downstream of numb to specify distinct daughter cell fates during asymmetric cell divisions in the Drosophila PNS. Neuron, 14, 913–925. [DOI] [PubMed] [Google Scholar]
- Harrison S.D. and Travers, A.A. (1990) The tramtrack gene encodes a Drosophila finger protein that interacts with the ftz transcriptional regulatory region and shows a novel embryonic expression pattern. EMBO J., 9, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehle J., Beuchle, D., Treuheit, S., Christen, B., Kennison, J.A., Bienz, M. and Müller, J. (1998) dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science, 282, 1897–1900. [DOI] [PubMed] [Google Scholar]
- Kim J. et al. (1999) Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity, 10, 345–355. [DOI] [PubMed] [Google Scholar]
- Lehembre F., Badenhorst, P., Travers, A., Schweisguth, F. and Dejean, A. (2000) Covalent modification of the transcriptional repressor Tramtrack by the ubiquitin-related protein dSmt3 in Drosophila flies. Mol. Cell. Biol., 20, 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Li, Y., Carthew, R.W. and Lai, Z.C. (1997) Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell, 90, 469–478. [DOI] [PubMed] [Google Scholar]
- Ng H.H., Zhang, Y., Hendrich, B., Johnson, C.A., Turner, B.M., Erdjument-Bromage, H., Tempst, P., Reinberg, D. and Bird, A. (1999) MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nature Genet., 23, 58–61. [DOI] [PubMed] [Google Scholar]
- Nightingale K.P., Wellinger, R.E., Sogo, J.M. and Becker, P.B. (1998) Histone acetylation facilitates RNA polymerase II transcription of the Drosophila hsp26 gene in chromatin. EMBO J., 17, 2865–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M., Imai, T., Kurusu, M., Hiromi, Y. and Okano, H. (2001) Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature, 411, 94–98. [DOI] [PubMed] [Google Scholar]
- Read D. and Manley, J.L. (1992) Alternatively spliced transcripts of the Drosophila tramtrack gene encode zinc finger proteins with distinct DNA binding specificities. EMBO J., 11, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari F. and Ahringer, J. (2000) NURD-complex genes antagonise Ras-induced vulval development in Caenorhabditis elegans. Curr. Biol., 10, 223–226. [DOI] [PubMed] [Google Scholar]
- Tang A.H., Neufeld, T.P., Kwan, E. and Rubin, G.M. (1997) PHYL acts to down-regulate Ttk88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell, 90, 459–467. [DOI] [PubMed] [Google Scholar]
- Wade P.A., Gegonne, A., Jones, P.L., Ballestar, E., Aubry, F. and Wolffe, A.P. (1999) Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nature Genet., 23, 62–66. [DOI] [PubMed] [Google Scholar]
- Wen Y., Nguyen, D., Li, Y. and Lai, Z.-C. (2000) The N-terminal BTB/POZ domain and C-terminal sequences are essential for Tramtrack69 to specify cell fate in the developing Drosophila eye. Genetics, 156, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Sandaltzopoulos, R., Wang, H, Hamiche, A., Ranallo, R., Lee, K., Fu, D. and Wu, C. (2001) Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol. Cell, 8, 531–543. [DOI] [PubMed] [Google Scholar]
- Xiong W.C. and Montell, C. (1993) tramtrack is a transcriptional repressor required for cell fate determination in the Drosophila eye. Genes Dev., 7, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Xu C., Kauffmann, R.C., Zhang, J., Kladny, S. and Carthew, R.W. (2000) Overlapping activators and repressors delimit transcriptional response to receptor tyrosine kinase signals in the Drosophila eye. Cell, 103, 87–97. [DOI] [PubMed] [Google Scholar]