Introduction
STAT3DN disease causes an autosomal dominant Hyper IgE syndrome (AD-HIES; Job’s syndrome) characterized by elevated immunoglobin E (IgE), recurrent lung and skin infections, eczematous dermatitis, mucocutaneous candidiasis, and skeletal, connective tissue, and vascular abnormalities. Since genetic etiology identification in 20071,2, diagnosis of infants with a family history of STAT3DN can be confirmed in newborns or prenatally. Although antibiotic prophylaxis targeting Staphylococcus aureus is known to decrease infectious complications in STAT3DN3,4, the impact of early diagnosis and treatment specifically in infants with antimicrobials and antiseptics is unexamined. We retrospectively reviewed medical histories of 18 STAT3DN pediatric patients to compare the clinical course for those with and without a family history of STAT3DN.
Methods
Eighteen STAT3DN pediatric patients born in 2008 or later were identified in our National Institute of Allergy and Infectious Diseases IRB-approved natural history study; guardian consent, with assent when appropriate, was obtained for all patients. Demographics, clinical features, laboratory results, and treatments were compared between those with and without (probands) a family history of STAT3DN. Patient data were analyzed and reported for descriptive statistics. For continuous variables, means and/or medians were determined and t-tests were performed. For categorical data, chi-squared tests were performed. P-values < 0.05 denoted significance.
Results (Table 1)
Table 1:
Clinical characteristics of STAT3DN pediatric patients
| Total n = 18 | Family History (n= 8, 44%) | Proband (n= 10, 56%) | P-value |
|---|---|---|---|
| Female | 6 (75%) | 5 (50%) | 0.28 |
| Current age (years, median) | 9 | 13 | 0.32 |
| Age at diagnosis (years, median) | 0.2 | 2 | 0.001* |
| STAT3 mutation domain | |||
| DNA | 5 (63%) | 5 (50%) | 0.60 |
| SH2 | 3 (38%) | 4 (40%) | 0.91 |
| TA | 0 (0%) | 1 (10%) | 0.36 |
| CLINICAL FEATURES | Family History | Proband | P-value |
| 6,865 | 13,317 | 0.31 | |
| Abs eosinophil count (cells/μL, mean) | 1,023 | 1,182 | 0.73 |
| HIES score (median) | 32 | 60.5 | 0.001* |
| Skin abscesses (>4) | 1 (13%) | 9 (90%) | 0.001* |
| Parenchymal lung abnormalities | 1 (13%) | 5 (50%) | 0.09 |
| Bronchiectasis | 1 (13%) | 5 (50%) | 0.09 |
| Pneumatoceles | 1 (13%) | 3 (30%) | 0.37 |
| Eczema | |||
| None | 1 (13%) | 0 (0%) | 0.25 |
| Mild | 3 (38%) | 0 (0%) | 0.03* |
| Moderate | 3 (38%) | 4 (40%) | 0.91 |
| Severe | 1 (13%) | 6 (60%) | 0.04* |
| Recurrent pneumonia | 1 (13%) | 9 (90%) | 0.001* |
| Chronic lung infection | 0 (0%) | 3 (30%) | 0.04* |
| Pulmonary Aspergillus | 0 (0%) | 2 (20%) | 0.07 |
| Lifetime Hospitalizations (mean) | 0.875 | 6.3 | 0.02* |
| Severe Infections ** | 0 (0%) | 4 (40%) | 0.04* |
| Mucocutaneous candidiasis | 6 (75%) | 9 (90%) | 0.40 |
| Oral | 4 (50%) | 8 (80%) | 0.18 |
| Nail | 0 (0%) | 3 (30%) | 0.09 |
| Groin/vaginal | 4 (50%) | 4 (40%) | 0.67 |
| Sinus infections | 3 (38%) | 7 (70%) | 0.17 |
| Fractures | 2 (25%) | 6 (60%) | 0.14 |
| Ear infections/tympanostomy tubes | 0 (0%) | 6 (60%) | 0.01* |
| Newborn rash | 6 (75%) | 10 (100%) | 0.09 |
| Allergies | |||
| Drug allergy | 1 (13%) | 2 (20%) | 0.67 |
| Food allergy | 2 (25%) | 4 (40%) | 0.50 |
| TREATMENTS | |||
| Antiseptic washes | |||
| Chlorhexidine usage | 6 (75%) | 3 (30%) | 0.06 |
| Bleach bath usage | 4 (50%) | 10 (100%) | 0.01* |
| Age initiated chlorhexidine washes (years, mean) | 0.2 | 4.7 | 0.001* |
| Age initiated bleach baths (years, mean) | 2 | 4.4 | 0.28 |
| Immunoglobin Replacement Therapy (igRT) | 1 (13%) | 6 (60%) | 0.04* |
| Lung surgery (VATS) | 0 (0%) | 4 (40%) | 0.04* |
| Hematopoietic stem cell transplant (HSCT) | 0 (0%) | 1 (10%) | 0.36 |
| Prophylactic antifungal medications | 2 (25%) | 6 (60%) | 0.14 |
| Prophylactic antibiotics | 8 (100%) | 10 (100%) | |
| Dupilumab | 2 (25%) | 3 (30%) | 0.81 |
denotes statistically significant features
excludes severe bacterial pneumonias
Eight patients with STAT3DN family history (median age 9 years; range 4-14) and 10 probands (median age 13 years; range 5-14) were identified (Table 1). The time of genetic diagnosis in those with family history were in utero (n = 1), within the first 2 months (n=6), and at 2 years (n=1). Probands were diagnosed at a median age of 2 years (range 1-6 years). Patients with a family history had lower median HIES scores as compared to probands (32 vs. 61 points respectively; p = 0.001)5. Mean peak serum IgE and peripheral eosinophilia were comparable in patients with or without a family history. Extended lymphocyte phenotyping at comparable ages were not available.
Patients with family history started antiseptic washes (commonly chlorhexidine) at a younger age than probands (mean 0.2 vs. 4.7 years, p = 0.001). Dilute bleach baths were initiated at a mean of 2 years for 4 patients with family history, while 100% of probands started this therapy at a mean age of 4.4 years (p = 0.01). All initiated prophylactic antibiotics, commonly trimethoprim/sulfamethoxazole (TMP/SMX); those with family history started earlier than probands (mean 0.4 vs. 3.0 years respectively, p = 0.00005). Four probands received chronic antifungals for mold infections; mold infections were not observed in those with family history, but one initiated itraconazole prophylaxis. Immunoglobulin replacement was prescribed less frequently in patients with family history (13% vs. 60%, p = 0.04). Dupilumab was prescribed for eczema at comparable rates between those with and those without a family history (25% vs 30%).
Patients with family history had significantly fewer hospitalizations (mean 0.9 vs 6.3 times for probands, p = 0.02) and fewer bacterial pneumonias (13% vs 90%, p = 0.001). The majority of infection-related hospitalizations for both groups were at ages 6 years or younger. Three of eight patients with family history had hospitalizations outside the newborn period for viral bronchiolitis (2), cellulitis (1), and bacterial pneumonia (1). Nine of ten probands had a total of 16 hospitalizations for infection, including sepsis (1), hemoptysis (1), pneumonia (bacterial and Aspergillus, 5), prolonged bronchopleural fistula after lung resection (2) and pneumatocele-related pneumothorax (1), Fusarium skin infection (2), severe thrush (1), skin abscess (2), and mastoiditis with epidural abscess (1). Mechanical ventilation for pneumonia was required for two probands (newborn and at 4 years) versus one newborn with family history. One proband underwent hematopoietic stem cell transplantion at 7 years after recurrent severe infections and pneumatocele development despite optimal prophylaxis.
Patients with family history had reduced rates of recurrent skin abscesses (13% vs 90%, p = 0.001), recurrent ear infections and/or tympanostomy tube placement (0% vs 60%, p = 0.01), and severe eczema (13% vs 60%, p = 0.04) (Supplemental Figure 1). The only patient with family history with recurrent abscesses and severe eczema did not start therapy (bleach baths and TMP/SMX) until 2 years of age. Chronic mucocutaneous candidiasis (CMC) was frequent in both groups. While parenchymal lung abnormalities (e.g. bronchiectasis and pneumatoceles) did not reach clinical significance (13% in patients with family history vs. 50% in probands, p = 0.09), more significant lung disease and lung surgery (40%) was seen in probands versus none in patients with family history (Figure 1). Fractures and joint hyperextensibility were comparable in those with and without family histories. Young age prohibited analysis of retained primary teeth and scoliosis.
Figure 1.
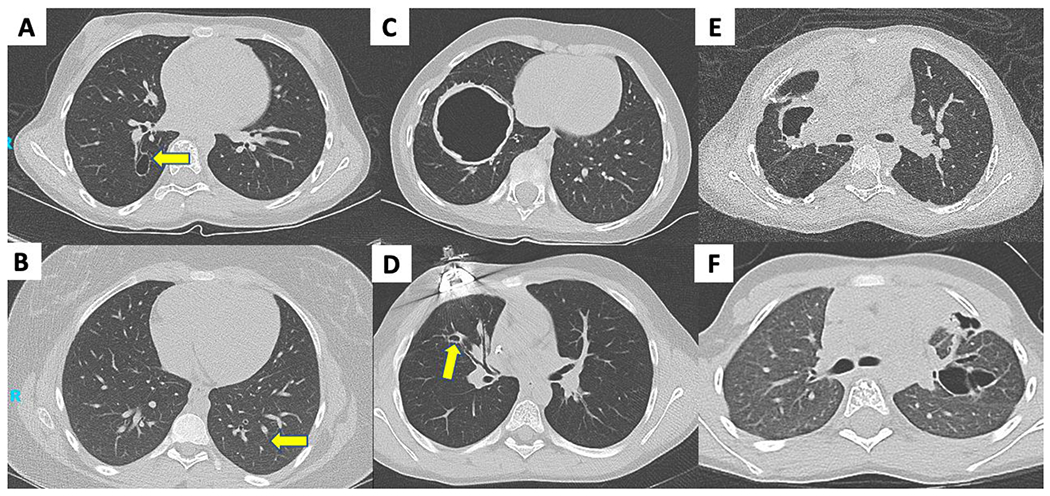
Parenchymal lung findings on CT. (A) small pneumatocele in 12-year-old with family history; (B) subtle bronchiectasis in 13-year-old with family history (C) large pneumatocele in 7-year-old proband; (D) bronchiectasis and small pneumatocele in 10-year-old proband with prior lobectomy; (E) pneumatocele in 9-year-old with prior lobectomy; (F) cystic bronchiectasis in 6-year-old with prior pneumothorax and bronchopleural fistula.
Discussion
With earlier diagnosis and treatment, patients with STAT3DN are living longer with increased potential for childbearing, and as an autosomal dominant disease, there is 50% risk of passing the mutant allele to offspring. Genetic testing has allowed prenatal/newborn diagnosis, facilitating interventions with antiseptics and prophylactic antibiotics. Sixteen years after the genetic identification of STAT3DN, we believe these early interventions significantly reduce infection, hospitalization and surgery rates, positively impacting disease natural history.
Pulmonary complications contribute greatly to STAT3DN morbidity and mortality3,4,6,7. Our study found lung surgery performed only in probands and hospitalization outside of the newborn period was predominantly seen in probands, suggesting early interventions has a positive impact on clinical outcomes. Long-term follow-up should determine whether pulmonary morbidity reduction is sustained as patients age, since our overall cohort has a 70% prevalance of parachymmal lung abnormalities. We believe these differences in outcomes are from medical interventions (i.e. antiseptics and prophylactic antimicrobials) at younger ages, although family experience managing HIES may play a role.
We recommend chlorhexidine wash initiation during the newborn period, transitioning to dilute bleach baths in early childhood8. We also initiate TMP/SMX around 1-2 months old, with attention to hyperbilirubinemia risk. These measures in newborn and prenatally-diagnosed infants were associated with significantly fewer infections than probands, whose disease was later identified from such infections. Although no patietnts developed Pneumocystis jirovecii pneumonia, this risk in STAT3DN infants provides additional rationale for TMP/SMX prophylaxis9. More probands received immunoglobin replacement therapy, likely reflecting their more severe course. Affected children with refractory eczema can consider dupilumab10.
Early diagnosis and intervention were associated with reduced STAT3DN disease severity and improved clinical outcomes. Despite a similar age at comparison, infectious complications were markedly reduced in those with family history receiving early interventions.
We believe these clinical outcome improvements enhance quality of life, lead to less work and school missed, decrease healthcare costs, and may alter the natural history of STAT3DN.
Supplementary Material
Clinical Implications.
Early genetic diagnosis of signal transduction and activation of transcription 3 dominant negative (STAT3DN) disease allows for early intervention, including initiation of antiseptic washes and antimicrobial prophylaxis. These early interventions may reduce long-term infectious, pulmonary, and dermatologic complications.
Acknowledgements
We thank the referring physicians for allowing us to participate in the study and care of the STAT3DN patients, as well as the patients and their families for their participation. This project has been funded, in part, by the NIH Intramural Research Program of NIAID and NIAMS. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
K. Williams has research support from GlaxoSmithKline, ADMA Biologics, and Cogent Bio; and has served on the medical advisory board with Horizon Therapeutics, Pharming, Kenota Health, Pfizer and Enzyvant. All other authors and reviewers reported no relevant financial relationships.
Abbreviations
- STAT3DN
Signal transducer and activator of transcription 3 dominant-negative
- AD-HIES
Autosomal dominant hyper IgE syndrome
- IgE
Immunoglobulin E
- DNA
Deoxyribonucleic acid binding domain
- SH2
Src-homology 2 domain
- TA
Transactivation domain
- TMP/SMX
Trimethoprim/sulfamethoxazole
- RSV
Respiratory syncytial virus
- CMC
Chronic mucocutaneous candidiasis
- IgRT
Immunoglobin replacement therapy
- VATS
Video-assisted thoracoscopic surgery
- HSCT
Hematopoietic stem cell transplantation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 Mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–19. [DOI] [PubMed] [Google Scholar]
- 2.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–1062. [DOI] [PubMed] [Google Scholar]
- 3.Carrabba M, Dellepiane RM, Cortesi M, Baselli LA, Soresina A, Cirillo E, et al. Long term longitudinal follow-up of an AD-HIES cohort: The impact of early diagnosis and enrollmenet to IPINet centers on the natural history of Job’s syndrome. Allergy Ashtma Clin Immunol 2023; 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine. 2012;91(4):e1–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimbacher B, Schaffer AA, Holland SM, Davis J, Gallin JI, Malech HL, et al. Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genetics 1999; 65: 735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman AF, Kleiner DE, Nadiminti H, Davis J, Quezado M, Anderson VL, et al. Causes of death in hyper IgE syndrome. J Allergy Clin Immunol 2007; 119: 1234–40. [DOI] [PubMed] [Google Scholar]
- 7.Freeman AF, Renner ED, Henderson C, Langenbeck A, Olivier KN, Hsu AP, et al. Lung parenchyma surgery in autosomal dominant hyper-IgE syndrome. J Clin Immunol 2013; 33: 896–902. [DOI] [PubMed] [Google Scholar]
- 8.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009; 123: e808–14. [DOI] [PubMed] [Google Scholar]
- 9.Freeman AF, Davis J, Anderson VL, Barson W, Darnell DN, Puck JM, et al. Pneumocystis jiroveci infection in patients with hyper-immunoglobulin E syndrome. Pediatrics 2006; 118: e127105. [DOI] [PubMed] [Google Scholar]
- 10.Staudacher O, Kruger R, Kolsch U, Thee S, Gratopp A, Wahn V, et al. Relieving Job: dupilumab in autosomal dominant STAT3 hyper-IgE syndrome. J Allergy Clin Immunol Pract 2022; 10: 349–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


