Abstract
Preclinical data strongly suggest that myocardial steatosis leads to adverse cardiac remodelling and left ventricular dysfunction. Using 1H cardiac magnetic resonance spectroscopy, similar observations have been made across the spectrum of health and disease. The purpose of this brief review is to summarize these recent observations. We provide a brief overview of the determinants of myocardial triglyceride accumulation, summarize the current evidence that myocardial steatosis contributes to cardiac dysfunction, and identify opportunities for further research.
Keywords: magnetic resonance, myocardial triglyceride content, steatosis
-
What is the topic of this review?
The role of myocardial steatosis on cardiac morphology and function.
-
What advances does it highlight?
Myocardial steatosis contributes to adverse cardiac remodelling and left ventricular dysfunction.
Preclinical data strongly suggest that myocardial steatosis leads to adverse cardiac remodelling and ventricular dysfunction. Evidence is reviewed of clinical translation using 1H magnetic resonance spectroscopy. Figure created using BioRender.com.
1. INTRODUCTION
More than two decades have passed since the seminal discovery that intracellular triglyceride accumulation within the hearts (myocardial steatosis) of obese Zucker Diabetic Fatty rats leads to adverse cardiac remodelling and left ventricular dysfunction (Zhou et al., 2000), via lipotoxic pathways (Goldberg et al., 2012; Schulze et al., 2016; Wende & Abel, 2010). Clinical translation of this important work was largely limited to clinical biopsies and post‐mortem analyses (Anderson et al., 2009; Björnson et al., 2020; Gizurarson et al., 2015; Knapp et al., 2020; Liu et al., 2012; Mazzali et al., 2015), but greatly expanded following the advent of 1H cardiac magnetic resonance spectroscopy (den Bakermans et al., 2021; Hollander et al., 1994; Reingold et al., 2005; Szczepaniak et al., 2003), as previously reviewed (J. M. McGavock et al., 2006; Szczepaniak et al., 2007). Since these early clinical observations, the field has continued to evolve, with myocardial steatosis now described across the spectrum of health and disease. This brief review summarizes these later observations while identifying remaining knowledge gaps and opportunities for future research.
2. MYOCARDIAL TRIGLYCERIDE CONTENT AS A MASS (IM)BALANCE
Myocardial triglyceride content is ultimately determined by the balance between substrate availability and substrate utilization (Figure 1). Under fasting conditions (Figure 1a), the majority of energy is derived from the oxidation of free fatty acids, with a limited pool of stored triglyceride. Indeed, data from 10 separate studies in young, normal weight, healthy adults suggest myocardial triglyceride content is approximately 0.5% fat/water (Gaborit, Kober, et al., 2012; Hammer, van der Meer, Lamb, Schar et al., 2008; Oneglia et al., 2023; Petritsch et al., 2016; Sai et al., 2013, 2015; Smajis et al., 2020; van der Meer et al., 2007, van der Meer, Hammer et al., 2008; Wolf et al., 2021). Moreover, myocardial triglyceride content is relatively stable within the small variations in substrate availability/utilization that may occur with day‐to‐day changes in dietary or exercise habits (Aengevaeren et al., 2020; Bucher et al., 2014; Ith et al., 2014; Smajis et al., 2020; van der Meer, Hammer et al., 2008). However, it is possible to shift the balance between lipid oxidation and storage, such that myocardial triglyceride content can change, even in healthy adults. For example, reducing circulating free fatty acids with acipimox (an anti‐lipolytic agent) reduces myocardial triglyceride content (Figure 1b) (Lehto et al., 2012; Winhofer et al., 2015). In contrast, multiple lines of evidence highlight acute increases in myocardial triglyceride content in the healthy myocardium when substrate availability exceeds utilization (Figure 1c,d), such as during periods of severe caloric restriction and fasting (Hammer, van der Meer, Lamb, Schar et al., 2008; Oneglia et al., 2023; van der Meer et al., 2007), following aerobic exercise in a fasted state (Bilet et al., 2011), and during hyperinsulinaemia–hyperglycaemia (Winhofer et al., 2012). Among these latter examples, substrate availability exceeds utilization in clear and close relation to nutritional state. Indeed, low nutrient intake depletes glycogen stores so that the body must instead rely on free fatty acids and ketones for energy, both of which increase their circulating concentrations via lipolysis of adipose tissue. The effect on myocardial triglyceride content can be seen in as little as 4 h post‐exercise and 48‐h of fasting (Bilet et al., 2011; Oneglia et al., 2023).
FIGURE 1.
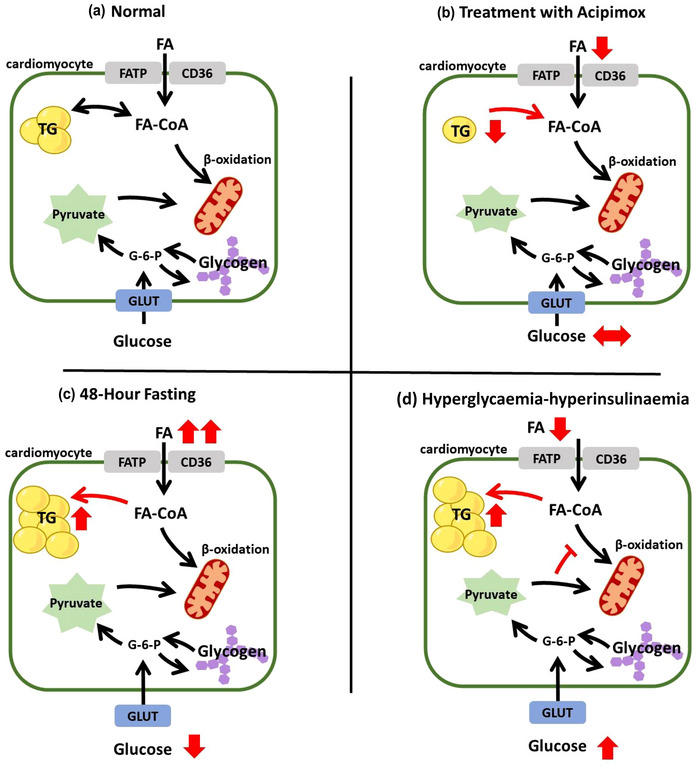
Schematic representation of a cardiomyocyte under normal conditions (a), during systemic treatment with acipimox (b, a lipid lowering agent), during 48‐h fasting (c), and in response to a whole‐body hyperglycaemic–hyperinsulinaemic clamp (d). FA, free fatty acids; G‐6‐P, glucose‐6‐phosphate; GLUT, glucose transporter; TG, triglyceride.
3. AGE AND SEX
Several studies suggest that myocardial triglyceride content increases with age (Petritsch et al., 2015; Sarma et al., 2013; van der Meer, Rijzewijk et al., 2008); however, the total sample size available to support this conclusion is relatively small, with very few reports measuring myocardial triglyceride content in individuals >65 years of age. Sex does not appear to influence myocardial triglyceride content in young, normal weight, healthy adults (Oneglia et al., 2023; Petritsch et al., 2016; Winhofer et al., 2012); however, the interaction between sex and metabolic dysfunction/disease has not been thoroughly investigated. Where available, sex as a biological variable is described in each of the following sections.
4. OBESITY AND DIABETES
Mounting evidence support a stepwise increase in myocardial triglyceride content with obesity, impaired glucose tolerance, and type 2 diabetes (T2D) (Dong et al., 2023; Gaborit, Kober, et al., 2012; Iozzo et al., 2009; J. M. McGavock et al., 2007). Our interpretation of this stepwise increase is illustrated in Figure 2, where obesity‐mediated increases in myocardial triglyceride content are largely explained by an associated increase in substrate availability (Figure 2a) (Banerjee et al., 2015; Gaborit, Kober et al., 2012; Hannukainen et al., 2018; Lai et al., 2015; Liu et al., 2014; Rayner et al., 2018, 2019). This effect appears to occur in both adults and adolescent boys (Banerjee et al., 2015). However, obesity is rarely defined by a simple increase in circulating free fatty acids, but rather also involves varying degrees of insulin resistance. Therefore, abnormal substrate utilization, in addition to an obesity‐related increase in substrate availability, augments myocardial triglyceride content among those at risk of developing T2D and those with diagnosed T2D (Figure 2b) (Gao et al., 2020; Graner et al., 2013; Levelt, Mahmod et al., 2016, Levelt, Pavlides et al., 2016; Muniyappa et al., 2015; Nyman et al., 2013; Rijzewijk et al., 2008).
FIGURE 2.
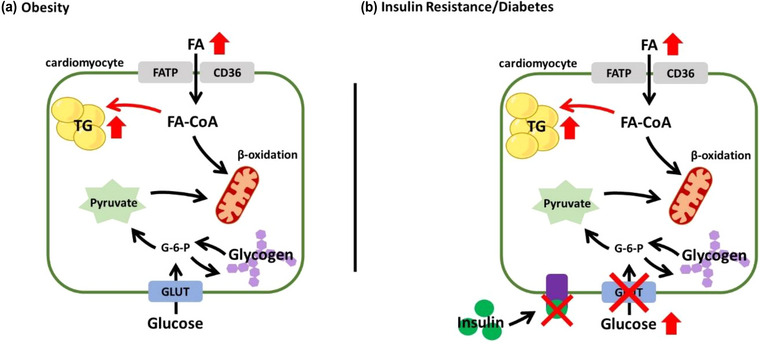
Schematic representation of a cardiomyocyte during obesity (a) and insulin resistance/diabetes (b). CD36, fatty acid translocase; FA, free fatty acids; FATP, fatty acid transport protein; G‐6‐P, glucose‐6‐phosphate; GLUT, glucose transporter; TG, triglyceride.
While myocardial triglyceride content may be associated with ectopic fat deposition elsewhere in the body, including visceral adiposity (Gaborit, Kober et al., 2012; Graner et al., 2013; Iozzo et al., 2009; Liu et al., 2014; Rayner et al., 2018), pericardial fat content (Gaborit, Kober et al., 2012; Graner et al., 2013; Iozzo et al., 2009) and hepatic steatosis (Graner et al., 2013; Liu et al., 2014), these associations are not perfect, emphasizing the importance of measuring myocardial triglyceride content directly. Results from interventional trials further demonstrate this point, as summarized in Table 1. This is perhaps best exemplified following bariatric surgery, where myocardial triglyceride content remains elevated 6 months after surgery, despite a ∼20% reduction in body mass, and marked reductions in pericardial, visceral and subcutaneous fat (Gaborit, Jacquier et al., 2012; Hannukainen et al., 2018; van Schinkel et al., 2014). We interpret this dissociative pattern to reflect a shift in myocardial substrate utilization, whereby improvements in myocardial glucose utilization, without changes in myocardial fatty acid utilization, result in a preservation of myocardial triglyceride content. Myocardial steatosis decreases only when fatty acid utilization exceeds fatty acid supply, so that the heart may instead rely on stored triglycerides. This could also explain why the majority of the T2D literature finds no direct relationship between myocardial steatosis and whole body insulin sensitivity/resistance (Graner et al., 2013; Iozzo et al., 2009; Kosi‐Trebotic et al., 2017; Krssak et al., 2011; Muniyappa et al., 2015; Winhofer, Krssak et al., 2014), and a number of glucose‐lowering drug trials have improved glycaemic control without altering myocardial triglyceride content (Bizino et al., 2020; Dutour et al., 2016; Gaborit et al., 2021; Hiruma et al., 2021; Hsu et al., 2019; J. McGavock et al., 2012; Paiman et al., 2020; van der Meer et al., 2009).
TABLE 1.
Myocardial triglyceride content response to interventional trials in obesity and diabetes mellitus.
| Reference | Subjects (m/f) | Age (years) | Study design | Medications | Results |
|---|---|---|---|---|---|
| Exercise intervention | |||||
| Schrauwen‐Hinderling et al. (2010) | 14 overweight to obese subjects (14/—) | Mean: 58 (SEM: 1) | 12‐week, combination aerobic and resistance, training programme | N/A | • mTG ↓ mean 45%, but weight and body fat % ↔, after exercise training |
| Schrauwen‐Hinderling et al. (2011) | 11 T2D patients (11/—) | Mean: 60 (SEM: 1) | 12‐week, combination aerobic and resistance, training programme | Oral glucose‐lowering, lipid‐lowering, anti‐hypertensive and/or blood diluent agents were continued |
• mTG, weight, body fat %, and total body fat all ↔ after exercise training |
| Honkala et al. (2017) | 28 healthy vs. 16 IGT subjects, 13 of which were T2D patients (44/—) |
Healthy range: 40–55 IGT range: 43–53 |
Randomized, 2‐week HIIT or MICT | Oral glucose‐lowering agents were continued |
• mTG tends to ↓ with HIIT, but not MICT, irrespective of glucose tolerance • Epi‐ and pericardial fat ↓ in both exercise conditions, irrespective of glucose tolerance |
| Dietary Intervention | |||||
| Utz et al. (2013) | 38 overweight and obese subjects (—/38) | Mean: 43 (SD: 9) | Randomized, 6‐month reduced‐CHO or reduced‐fat hypocaloric dietary interventions | N/A |
• mTG ↓ mean 25%, along with a ↓ in weight and total fat mass, irrespective of diet type |
| Andersson et al. (2016) | 68 overweight to obese subjects (—/68) |
Palaeolithic diet Mean: 60 (SD: 6) Nordic diet Mean: 60 (SD: 6) |
Randomized, 24‐month ad libitum Palaeolithic or Nordic Nutrition Recommendation dietary interventions | N/A |
• mTG ↔, but weight ↓, 6 and 24 months after diet initiation, irrespective of diet type |
| Hammer, van der Meer, Lamb, de Boer et al. (2008) | 11 T2D patients (11/—) | Mean: 58 (SD: 5) | 3‐day regular diet vs. randomized VLCD and VLCD + acipimox treatment | Metformin was continued |
• mTG ↑ mean 48% after VLCD, but ↔ after VLCD + acipimox • weight ↓, but HTG ↔, in both VLCD conditions |
| Hammer, Snel et al. (2008) | 12 T2D patients (7/5) | Mean: 48 (SEM: 3) | 16‐week VLCD | Glucose lowering therapy was discontinued | • mTG ↓ mean 27%, along with a ↓ in weight and HTG |
| Jonker et al. (2014) | 14 T2D patients with obstructive CAD and/or myocardial perfusion defects (7/7) | Mean: 57 (SEM: 3) | regular diet vs. 3‐day VLCD | Glucose‐lowering therapy was adjusted to maintain comparable glucose levels between study days | • mTG ↑ mean 33% after VLCD, but weight ↓ while VAT, SAT, HTG and pericardial fat ↔ |
| Airhart et al. (2016) | 16 T2D patients (4/12) |
MCFA diet Mean: 48 (SEM: 3) LCFA diet Mean: 52 (SEM: 3) |
Double‐blind, randomized 2‐week MCFA or LCFA rich eucaloric dietary intervention | Medication use was continued, but no subject was taking insulin |
• mTG, waist/hip ratio, and HTG ↔, but weight ↓, irrespective of diet type |
| Other | |||||
| Jankovic et al. (2012) | 10 T2D patients (6/4) | Mean: 58 (SEM: 3) | 10 days’ standardized insulin therapy | Metformin, lipid‐lowering and/or anti‐hypertensive agents were continued | • mTG ↑ mean 80% |
| Hammer, Jonker et al. (2008) | 10 T1D patients (5/5) | Mean: 41 (SEM: 3) | Randomized, euglycaemic and 24‐h hyperglycaemic conditions | Hyperglycaemic condition was achieved by reducing basal and bolus insulin infusions ∼50% | • mTG ↔ after hyperglycaemia compared to euglycaemia |
| Abdesselam et al. (2015) | 21 morbidly obese subjects (4/17) | Mean: 42 (SD: 2) | 32‐month follow‐up visit after bariatric surgery | N/A |
• mTG ↓ 32 months post‐surgery, whereas VAT, SAT, EAT, HTG, PTG all ↓ 6 months post‐surgery |
| Wolf et al. (2016) | 8 T2D patients (6/2) | Mean: 56 (SD: 11) | 6‐h randomized, placebo‐controlled acipimox treatment | Glucose‐lowering therapy and statin therapy were omitted |
• mTG ↓ mean 41% after acipimox treatment • mTG ↔ after placebo treatment |
Abbreviations: CAD, coronary artery disease; CVD, cardiovascular disease; EAT, epicardial adipose tissue; HIIT, high‐intensity interval training; HTG, hepatic triglyceride content; IGT, impaired glucose tolerance; LCFA, long‐chain fatty acid; MCFA, medium‐chain fatty acid; MICT, moderate‐intensity continuous training; mTG, myocardial triglyceride content; N/A, not available; PTG, pancreatic triglyceride content; SAT, subcutaneous adipose tissue; T1D, type‐1 diabetes mellitus; T2D, type‐2 diabetes mellitus; VAT, visceral adipose tissue; VLCD, very‐low calorie diet.
In a relatively small sample, Iozzo et al. (2009) first reported that myocardial triglyceride content was lower in obese women than men but was comparable between sexes with impaired glucose tolerance or T2D, suggesting that women may be protected from obesity‐related increases in myocardial triglyceride content. Studies conducted since, however, suggest otherwise (Dong et al., 2023; Gaborit, Kober et al., 2012). Indeed, myocardial triglyceride content is still elevated in overweight and obese women compared to normal weight and overweight men (Liu et al., 2014). Furthermore, sex does not influence the independent relationship between obesity and myocardial steatosis among adults across a range of obesity (Banerjee et al., 2015). As such, sex does not appear to influence myocardial triglyceride content in obesity.
The functional consequence of myocardial triglyceride accumulation in obesity and T2D remains incompletely understood. Although multiple cross‐sectional studies have identified an independent association between myocardial steatosis and cardiac function (Banerjee et al., 2015; Dong et al., 2023; Gao et al., 2020; Korosoglou et al., 2012; Levelt, Mahmod et al., 2016; Ng et al., 2010; Rijzewijk et al., 2008) and/or left ventricular (LV) concentric remodelling (Banerjee et al., 2015; Jankovic et al., 2012; Jonker et al., 2014; Levelt, Mahmod et al., 2016), improvements in cardiac function do not always follow reductions in myocardial triglyceride content and vice versa (van der Meer et al., 2009; Zib et al., 2007). These latter observations have contributed to the understanding that cardiac function is not related to myocardial steatosis per se, but rather the accumulation of toxic intermediates from lipid metabolism (Schulze et al., 2016; Wende & Abel, 2010; Goldberg et al., 2012).
5. HEART DISEASE
Myocardial triglyceride content varies across different forms of heart disease (Graner et al., 2014; Nakae et al., 2010; Sai et al., 2017). Myocardial ischaemia appears to promote the accumulation of triglycerides, likely related to a metabolic switch away from free fatty acid oxidation. Indeed, myocardial triglyceride content is elevated in patients with ischaemic coronary artery disease (CAD), but not patients with non‐ischaemic CAD or non‐CAD controls (Hannukainen et al., 2016). Moreover, myocardial steatosis was elevated in women with ischaemia but non‐obstructed coronary arteries, and inversely related to LV diastolic function (Wei et al., 2016). Notably, in this latter cohort, triglyceride content was not elevated elsewhere in the body (Hannukainen et al., 2016; Wei et al., 2016), arguing against systemic metabolic disease and in support of the ischaemia hypothesis. Myocardial triglyceride content was also elevated in heart failure with preserved ejection fraction (Mahmod et al., 2018; Wu et al., 2020), but not heart failure with reduced ejection fraction (Wu et al., 2020) or end‐stage heart failure (Chokshi et al., 2012). However, these latter two cohorts were primarily non‐obese and non‐diabetic, which may have influenced the results (Sharma et al., 2004).
Interventional studies aimed at regressing myocardial steatosis in heart disease are limited. In one investigation, 1 year of high‐intensity exercise training, without dietary intervention, promoted positive cardiac and vascular remodelling in adults at risk for heart failure, but did not affect myocardial steatosis (Hearon et al., 2022). Other investigations targeted myocardial lipid composition in heart failure, rather than total content per se, but research in this area is extremely limited (Chang et al., 2020; Liao et al., 2016). Hata et al. 2022 are the only group to investigate the longitudinal effects of myocardial steatosis on LV diastolic function. Using computed tomography, patients with no or mild coronary artery stenosis were grouped by the presence or absence of myocardial fat deposition at baseline. Echocardiography was then performed at baseline, 1–2 years and 2–3 years after the baseline assessment. When compared to patients without excess myocardial fat deposition, those with excess myocardial fat deposition had worse LV diastolic function at baseline and follow‐up. Moreover, the decline in LV diastolic function with ageing appeared accelerated in patients with excess myocardial fat deposition. While these data support the negative effect of myocardial steatosis on LV function, a direct cause‐and‐effect relationship between the two is limited by the study's retrospective design. Therefore, future prospective, longitudinal studies are needed to confirm these results.
6. HUMAN IMMUNODEFICIENCY VIRUS
The influence of human immunodeficiency virus (HIV) and associated highly active anti‐retroviral therapy has received considerable attention with respect to myocardial steatosis and its influence of future cardiovascular disease risk. Indeed, myocardial triglyceride content is often elevated among individuals with HIV, and associated with worsening LV systolic and diastolic function (Holloway et al., 2013; Nelson et al., 2014; Shitole et al., 2023; Thiara et al., 2015; Toribio et al., 2019). Several mechanisms may contribute to an increase in myocardial triglyceride content with HIV, including age (Chew et al., 2017; Neilan et al., 2020; Toribio et al., 2019), body mass index (Lai et al., 2017; Neilan et al., 2020), ectopic body fat deposition (Chew et al., 2017; Diaz‐Zamudio et al., 2015; Howard et al., 2016; Thiara et al., 2015) and even duration of anti‐retroviral therapy (Lai et al., 2017; Nelson et al., 2014). Sex does not appear to influence this association, with myocardial triglyceride content elevated in both men and women with HIV. Studies aimed at regressing myocardial triglyceride content in HIV are actively underway (NCT02344290) (Grinspoon et al., 2019).
7. SPECIAL POPULATIONS
Investigations into other clinical populations not yet described are summarized in Table 2, ranging from valvular heart disease to metabolic disease (e.g., non‐alcoholic fatty liver disease, generalized lipodystrophy, Cushing's syndrome). Where correlation analyses were performed, the accumulation of myocardial steatosis is often associated with adverse ventricular remodelling and/or cardiac dysfunction, except in non‐alcoholic fatty liver disease.
TABLE 2.
Myocardial triglyceride content in special populations.
| References | Clinical population | Results |
|---|---|---|
| Mahmod et al. (2013) | 39 aortic stenosis patients vs. 20 matched controls |
• mTG ↑ in severe aortic stenosis • mTG ↓ in patients after aortic valve replacement to concentrations similar to controls |
| Graner et al. (2015) | 75 non‐alcoholic fatty liver disease patients divided into tertiles according to hepatic TG |
• mTG ↑ with ↑ adiposity (BMI, hepatic TG, VAT, SAT, epi‐ and pericardial fat) |
| Brittain et al. (2016) | 6 pulmonary arterial hypertension vs. 8 controls |
• mTG ↑ in PAH • Despite higher FFA availability, PAH experience impaired FA utilization so that lipotoxicity ensued |
| Gizurarson et al. (2015) | 34 patients with atrial fibrillation vs. 17 controls | • mTG ↓ in atrial fibrillation when assessed directly from atrial biopsies |
| Nelson et al. (2013) | 6 generalized lipodystrophy patients vs. 6 matched controls | • mTG ↑ in generalized lipodystrophy with concurrent concentric LV hypertrophy |
| Wolf et al. (2021) | 23 Cushing's syndrome patients vs. 27 matched controls |
• mTG ↔ despite greater epi‐ and pericardial fat mass in patients • mTG ↔ after treatment and remission |
| Scherer et al. (2014) | 10 hypothyroidism patients vs. 10 matched controls |
• mTG ↔ • After levothyroxine treatment, mTG ↓ with concurrent improvements in LV morphology and filling dynamics |
| Winhofer, Wolf et al. (2014) | 10 acromegaly patients vs. 10 matched controls |
• mTG and hepatic TG ↔ but pericardial fat ↓ in acromegaly • After treatment, mTG and hepatic TG ↔ but pericardial fat ↑ |
| Wolf et al. (2014) | 8 familial hypocalcuric hypercalcaemia patients vs. 9 matched controls |
• mTG, hepatic TG, VAT, and SAT ↔ |
| Knottnerus et al. (2020) | 14 patients with long‐chain fatty acid β oxidation disorders vs. 14 matched controls |
• mTG ↔ |
| Gastl et al. (2019) | 11 cardiac amyloidosis patients vs. 11 matched controls | • mTG ↓ in cardiac amyloidosis |
BMI, body mass index; FA, fatty acid; FFA, free fatty acid; mTG, myocardial triglyceride content; PAH, pulmonary arterial hypertension; SAT, subcutaneous adipose tissue; TG, triglyceride; VAT, visceral adipose tissue.
8. CONCLUSION
Myocardial triglyceride content is determined by the (im)balance between free fatty acid substrate availability and utilization. Myocardial steatosis increases with obesity and insulin‐resistance, is elevated in multiple disease states, and may be an important source of cardiac dysfunction and/or adverse remodelling. More work is still needed, however, to address remaining gaps in the literature, including the influence of age and sex, and the independent role myocardial steatosis plays in the development and progression of heart disease.
AUTHOR CONTRIBUTIONS
Conception or design of the work: Andrew P. Oneglia, Lidia S. Szczepaniak, Vlad G. Zaha, Michael D. Nelson . Drafting the work or revising it critically for important intellectual content: Andrew P. Oneglia, Lidia S. Szczepaniak, Vlad G. Zaha, Michael D. Nelson. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Oneglia, A. P. , Szczepaniak, L. S. , Zaha, V. G. , & Nelson, M. D. (2024). Myocardial steatosis across the spectrum of human health and disease. Experimental Physiology, 109, 202–213. 10.1113/EP091566
Handling Editor: Toby Mundel
REFERENCES
- Abdesselam, I. , Pepino, P. , Troalen, T. , Macia, M. , Ancel, P. , Masi, B. , Fourny, N. , Gaborit, B. , Giannesini, B. , Kober, F. , Dutour, A. & Bernard, M. (2015). Time course of cardiometabolic alterations in a high fat high sucrose diet mice model and improvement after GLP‐1 analog treatment using multimodal cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 17(1), 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aengevaeren, V. L. , Froeling, M. , van den Berg‐Faay, S. , Hooijmans, M. T. , Monte, J. R. , Strijkers, G. J. , Nederveen, A. J. , Eijsvogels, T. M. H. , & Bakermans, A. J. (2020). Marathon running transiently depletes the myocardial lipid pool. Physiological Reports, 8(17), e14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airhart, S. , Cade, W. T. , Jiang, H. , Coggan, A. R. , Racette, S. B. , Korenblat, K. , Spearie, C. A. , Waller, S. , O'Connor, R. , Bashir, A. , Ory, D. S. , Schaffer, J. E. , Novak, E. , Farmer, M. , Waggoner, A. D. , Davila‐Roman, V. G. , Javidan‐Nejad, C. , & Peterson, L. R. (2016). A diet rich in medium‐chain fatty acids improves systolic function and alters the lipidomic profile in patients with type 2 diabetes: A pilot study. Journal of Clinical Endocrinology and Metabolism, 101(2), 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, E. J. , Kypson, A. P. , Rodriguez, E. , Anderson, C. A. , Lehr, E. J. , & Neufer, P. D. (2009). Substrate‐specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. Journal of the American College of Cardiology, 54(20), 1891–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. , Mellberg, C. , Otten, J. , Ryberg, M. , Rinnstrom, D. , Larsson, C. , Lindahl, B. , Hauksson, J. , Johansson, B. , & Olsson, T. (2016). Left ventricular remodelling changes without concomitant loss of myocardial fat after long‐term dietary intervention. International Journal of Cardiology, 216, 92–96. [DOI] [PubMed] [Google Scholar]
- Bakermans, A. J. , Boekholdt, S. M. , de Vries, D. K. , Reckman, Y. J. , Farag, E. S. , de Heer, P. , Uthman, L. , Denis, S. W. , Zuurbier, C. J. , Houtkooper, R. H. , Koolbergen, D. R. , Kluin, J. , Planken, R. N. , Lamb, H. J. , Webb, A. G. , Strijkers, G. J. , Beard, D. A. , Jeneson, J. A. L. & Nederveen, A. J. (2021). Quantification of myocardial creatine and triglyceride content in the human heart: Precision and accuracy of in vivo proton magnetic resonance spectroscopy. Journal of Magnetic Resonance Imaging, 54(2), 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, R. , Rial, B. , Holloway, C. J. , Lewandowski, A. J. , Robson, M. D. , Osuchukwu, C. , Schneider, J. E. , Leeson, P. , Rider, O. J. & Neubauer, S. (2015). Evidence of a direct effect of myocardial steatosis on LV hypertrophy and diastolic dysfunction in adult and adolescent obesity. JACC Cardiovasc Imaging, 8(12), 1468–1470. [DOI] [PubMed] [Google Scholar]
- Bilet, L. , van de Weijer, T. , Hesselink, M. K. , Glatz, J. F. , Lamb, H. J. , Wildberger, J. , Kooi, M. E. , Schrauwen, P. & Schrauwen‐Hinderling, V. B. (2011). Exercise‐induced modulation of cardiac lipid content in healthy lean young men. Basic research in cardiology, 106(2), 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizino, M. B. , Jazet, I. M. , de Heer, P. , van Eyk, H. J. , Dekkers, I. A. , Rensen, P. C. N. , Paiman, E. H. M. , Lamb, H. J. & Smit, J. W. (2020). Placebo‐controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: A pre‐specified secondary study on ectopic fat accumulation. Diabetologia, 63(1), 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnson, E. , Östlund, Y. , Ståhlman, M. , Adiels, M. , Omerovic, E. , Jeppsson, A. , Borén, J. , & Levin, M. C. (2020). Lipid profiling of human diabetic myocardium reveals differences in triglyceride fatty acyl chain length and degree of saturation. International Journal of Cardiology, 320, 106–111. [DOI] [PubMed] [Google Scholar]
- Brittain, E. L. , Talati, M. , Fessel, J. P. , Zhu, H. , Penner, N. , Calcutt, M. W. , West, J. D. , Funke, M. , Lewis, G. D. , Gerszten, R. E. , Hamid, R. , Pugh, M. E. , Austin, E. D. , Newman, J. H. & Hemnes, A. R. (2016). Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation, 133(20), 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, J. , Krusi, M. , Zueger, T. , Ith, M. , Stettler, C. , Diem, P. , Boesch, C. , Kreis, R. & Christ, E. (2014). The effect of a single 2 h bout of aerobic exercise on ectopic lipids in skeletal muscle, liver and the myocardium. Diabetologia, 57(5), 1001–1005. [DOI] [PubMed] [Google Scholar]
- Chang, K. F. , Lin, G. , Huang, P. C. , Juan, Y. H. , Wang, C. H. , Tsai, S. Y. , Lin, Y. C. , Wu, M. T. , Liao, P. A. , Yang, L. Y. , Liu, M. H. , Lin, Y. C. , Wang, J. J. , Ng, K. K. & Ng, S. H. (2020). Left ventricular function and myocardial triglyceride content on 3T cardiac MR predict major cardiovascular adverse events and readmission in patients hospitalized with acute heart failure. Journal of Clinical Medicine, 9(1), 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, K. W. , Liu, C. Y. , Ambale‐Venkatesh, B. , Liao, D. , Horwich, T. B. , Lima, J. A. C. , Bluemke, D. A. , Paul Finn J., Butt, A. A. & Currier, J. S. (2017). Subclinical myocardial disease by cardiac magnetic resonance imaging and spectroscopy in healthy HIV/Hepatitis C virus‐coinfected persons. Journal of International Medical Research, 45(6), 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokshi, A. , Drosatos, K. , Cheema, F. H. , Ji, R. , Khawaja, T. , Yu, S. , Kato, T. , Khan, R. , Takayama, H. , Knoll, R. , Milting, H. , Chung, C. S. , Jorde, U. , Naka, Y. , Mancini, D. M. , Goldberg, I. J. & Schulze, P. C. (2012). Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation, 125(23), 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz‐Zamudio, M. , Dey, D. , LaBounty, T. , Nelson, M. , Fan, Z. , Szczepaniak, L. S. , Hsieh, B. P. , Rajani, R. , Berman, D. , Li, D. , Dharmakumar, R. , Hardy, W. D. & Conte, A. H. (2015). Increased pericardial fat accumulation is associated with increased intramyocardial lipid content and duration of highly active antiretroviral therapy exposure in patients infected with human immunodeficiency virus: A 3T cardiovascular magnetic resonance feasibility study. Journal of Cardiovascular Magnetic Resonance, 17, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, X. , Strudwick, M. , Wang, W. Y. , Borlaug, B. A. , van der Geest, R. J. , Ng, A. C. , Delgado, V. , Bax, J. J. & Ng, A. C. (2023). Impact of body mass index and diabetes on myocardial fat content, interstitial fibrosis and function. The International Journal of Cardiovascular Imaging, 39(2), 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutour, A. , Abdesselam, I. , Ancel, P. , Kober, F. , Mrad, G. , Darmon, P. , Ronsin, O. , Pradel, V. , Lesavre, N. , Martin, J. C. , Jacquier, A. , Lefur, Y. , Bernard, M. & Gaborit, B. (2016). Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes, Obesity & Metabolism, 18(9), 882–891. [DOI] [PubMed] [Google Scholar]
- Gaborit, B. , Ancel, P. , Abdullah, A. E. , Maurice, F. , Abdesselam, I. , Calen, A. , Soghomonian, A. , Houssays, M. , Varlet, I. , Eisinger, M. , Lasbleiz, A. , Peiretti, F. , Bornet, C. E. , Lefur, Y. , Pini, L. , Rapacchi, S. , Bernard, M. , Resseguier, N. , Darmon, P. , …, Dutour, A. (2021). Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: The EMPACEF study. Cardiovasc Diabetol, 20(1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaborit, B. , Jacquier, A. , Kober, F. , Abdesselam, I. , Cuisset, T. , Boullu‐Ciocca, S. , Emungania, O. , Alessi, M. C. , Clement, K. , Bernard, M. & Dutour, A. (2012). Effects of bariatric surgery on cardiac ectopic fat: Lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. Journal of the American College of Cardiology, 60(15), 1381–1389. [DOI] [PubMed] [Google Scholar]
- Gaborit, B. , Kober, F. , Jacquier, A. , Moro, P. J. , Cuisset, T. , Boullu, S. , Dadoun, F. , Alessi, M. C. , Morange, P. , Clement, K. , Bernard, M. & Dutour, A. (2012). Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: Relationship to metabolic profile, cardiac function and visceral fat. International Journal of Obesity, 36(3), 422–430. [DOI] [PubMed] [Google Scholar]
- Gao, Y. , Ren, Y. , Guo, Y. K. , Liu, X. , Xie, L. J. , Jiang, L. , Shen, M. T. , Deng, M. Y. & Yang, Z. G. (2020). Metabolic syndrome and myocardium steatosis in subclinical type 2 diabetes mellitus: A (1)H‐magnetic resonance spectroscopy study. Cardiovasc Diabetol, 19(1), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastl, M. , Peereboom, S. M. , Gotschy, A. , Fuetterer, M. , von Deuster, C. , Boenner, F. , Kelm, M. , Schwotzer, R. , Flammer, A. J. , Manka, R. & Kozerke, S. (2019). Myocardial triglycerides in cardiac amyloidosis assessed by proton cardiovascular magnetic resonance spectroscopy. Journal of Cardiovascular Magnetic Resonance, 21(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizurarson, S. , Ståhlman, M. , Jeppsson, A. , Shao, Y. , Redfors, B. , Bergfeldt, L. , Borén, J. & Omerovic, E. (2015). Atrial fibrillation in patients admitted to coronary care units in western Sweden—focus on obesity and lipotoxicity. Journal of Electrocardiology, 48(5), 853–860. [DOI] [PubMed] [Google Scholar]
- Goldberg, I. J. , Trent, C. M. & Schulze, P. C. (2012). Lipid metabolism and toxicity in the heart. Cell metabolism, 15(6), 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graner, M. , Nyman, K. , Siren, R. , Pentikainen, M. O. , Lundbom, J. , Hakkarainen, A. , Lauerma, K. , Lundbom, N. , Nieminen, M. S. & Taskinen, M. R. (2015). Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circulation: Cardiovascular Imaging, 8(1), e001979. [DOI] [PubMed] [Google Scholar]
- Graner, M. , Pentikainen, M. O. , Nyman, K. , Siren, R. , Lundbom, J. , Hakkarainen, A. , Lauerma, K. , Lundbom, N. , Nieminen, M. S. , Petzold, M. & Taskinen, M. R. (2014). Cardiac steatosis in patients with dilated cardiomyopathy. Heart, 100(14), 1107–1112. [DOI] [PubMed] [Google Scholar]
- Graner, M. , Siren, R. , Nyman, K. , Lundbom, J. , Hakkarainen, A. , Pentikainen, M. O. , Lauerma, K. , Lundbom, N. , Adiels, M. , Nieminen, M. S. & Taskinen, M. R. (2013). Cardiac steatosis associates with visceral obesity in nondiabetic obese men. Journal of Clinical Endocrinology and Metabolism, 98(3), 1189–1197. [DOI] [PubMed] [Google Scholar]
- Grinspoon, S. K. , Fitch, K. V. , Overton, E. T. , Fichtenbaum, C. J. , Zanni, M. V. , Aberg, J. A. , Malvestutto, C. , Lu, M. T. , Currier, J. S. , Sponseller, C. A. , Waclawiw, M. , Alston‐Smith, B. , Cooper‐Arnold, K. , Klingman, K. L. , Desvigne‐Nickens, P. , Hoffmann, U. & Ribaudo, H. J ,, Douglas PS & Investigators R . (2019). Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). American Heart Journal, 212, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, S. , Jonker, J. T. , Lamb, H. J. , van der Meer, R. W. , Zondag, W. , Sepers, J. M. , de Roos, A. , Smit, J. W. & Romijn, J. A. (2008). Short‐term hyperglycemic dysregulation in patients with type 1 diabetes does not change myocardial triglyceride content or myocardial function. Diabetes Care, 31(8), 1613–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, S. , Snel, M. , Lamb, H. J. , Jazet, I. M. , van der Meer, R. W. , Pijl, H. , Meinders, E. A. , Romijn, J. A. , de Roos, A. & Smit, J. W. (2008). Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. Journal of the American College of Cardiology, 52(12), 1006–1012. [DOI] [PubMed] [Google Scholar]
- Hammer, S. , van der Meer, R. W. , Lamb, H. J. , de Boer, H. H. , Bax, J. J. , de Roos, A. , Romijn, J. A. & Smit, J. W. (2008). Short‐term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. American Journal of Physiology. Endocrinology and Metabolism, 295(3), E714‐E718. [DOI] [PubMed] [Google Scholar]
- Hammer, S. , van der Meer, R. W. , Lamb, H. J. , Schar, M. , de Roos, A. , Smit, J. W. & Romijn, J. A. (2008). Progressive caloric restriction induces dose‐dependent changes in myocardial triglyceride content and diastolic function in healthy men. Journal of Clinical Endocrinology and Metabolism, 93(2), 497–503. [DOI] [PubMed] [Google Scholar]
- Hannukainen, J. C. , Lautamaki, R. , Mari, A. , Parkka, J. P. , Bucci, M. , Guzzardi, M. A. , Kajander, S. , Tuokkola, T. , Knuuti, J. & Iozzo, P. (2016). Elevated glucose oxidation, reduced insulin secretion, and a fatty heart may be protective adaptions in ischemic CAD. Journal of Clinical Endocrinology and Metabolism, 101(7), 2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannukainen, J. C. , Lautamaki, R. , Parkka, J. , Strandberg, M. , Saunavaara, V. , Hurme, S. , Soinio, M. , Dadson, P. , Virtanen, K. A. , Gronroos, T. , Forsback, S. , Salminen, P. , Iozzo, P. & Nuutila, P. (2018). Reversibility of myocardial metabolism and remodelling in morbidly obese patients 6 months after bariatric surgery. Diabetes, Obesity & Metabolism, 20(4), 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata, Y. , Koike, Y. , Kimura, N. , Mochizuki, J. , Okamoto, S. , Matsumi, H. & & Hashimoto, K. (2022). Longitudinal effect of myocardial fat deposition on left ventricular diastolic function: A retrospective cohort study. The International Journal of Cardiovascular Imaging, 38, 955–961. [DOI] [PubMed] [Google Scholar]
- Hearon, C. M., Jr. , Dias, K. A. , MacNamara, J. P. , Hieda, M. , Mantha, Y. , Harada, R. , Samels, M. , Morris, M. , Szczepaniak, L. S. , Levine, B. D. & Sarma, S. (2022). 1 Year HIIT and Omega‐3 fatty acids to improve cardiometabolic risk in stage‐a heart failure. JACC Heart Fail, 10(4), 238–249. [DOI] [PubMed] [Google Scholar]
- Hiruma, S. , Shigiyama, F. , Hisatake, S. , Mizumura, S. , Shiraga, N. , Hori, M. , Ikeda, T. , Hirose, T. & Kumashiro, N. (2021). A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early‐stage type 2 diabetes: The ASSET study. Cardiovasc Diabetol, 20(1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander, J. A. den , Evanochko, W. T. & Pohost, G. M. (1994). Observation of cardiac lipids in humans by localized 1H magnetic resonance spectroscopic imaging. Magnetic Resonance in Medicine, 32(2), 175–180. [DOI] [PubMed] [Google Scholar]
- Holloway, C. J. , Ntusi, N. , Suttie, J. , Mahmod, M. , Wainwright, E. , Clutton, G. , Hancock, G. , Beak, P. , Tajar, A. , Piechnik, S. K. , Schneider, J. E. , Angus, B. , Clarke, K. , Dorrell, L. & Neubauer, S. (2013). Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation, 128(8), 814–822. [DOI] [PubMed] [Google Scholar]
- Honkala, S. M. , Motiani, K. K. , Eskelinen, J. J. , Savolainen, A. , Saunavaara, V. , Virtanen, K. A. , Loyttyniemi, E. , Kapanen, J. , Knuuti, J. , Kalliokoski, K. K. & Hannukainen, J. C. (2017). Exercise training reduces intrathoracic fat regardless of defective glucose tolerance. Medicine and Science in Sports and Exercise, 49(7), 1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, L. C. , Liu, C. Y. , Purdy, J. B. , Walter, P. , Bluemke, D. A. & Hadigan, C. (2016). Lipolytic rate associated with intramyocardial lipid in an HIV cohort without increased lipolysis. Journal of Clinical Endocrinology and Metabolism, 101(1), 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, J. C. , Wang, C. Y. , Su, M. M. , Lin, L. Y. , & Yang, W. S. (2019). Effect of empagliflozin on cardiac function, adiposity, and diffuse fibrosis in patients with type 2 diabetes mellitus. Scientific Reports, 9(1), 15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo, P. , Lautamaki, R. , Borra, R. , Lehto, H. R. , Bucci, M. , Viljanen, A. , Parkka, J. , Lepomaki, V. , Maggio, R. , Parkkola, R. , Knuuti, J. & Nuutila, P. (2009). Contribution of glucose tolerance and gender to cardiac adiposity. Journal of Clinical Endocrinology and Metabolism, 94,11, 4472–4482. [DOI] [PubMed] [Google Scholar]
- Ith, M. , Stettler, C. , Xu, J. , Boesch, C. & Kreis, R. (2014). Cardiac lipid levels show diurnal changes and long‐term variations in healthy human subjects. Nmr in Biomedicine, 27(11), 1285–1292. [DOI] [PubMed] [Google Scholar]
- Jankovic, D. , Winhofer, Y. , Promintzer‐Schifferl, M. , Wohlschlager‐Krenn, E. , Anderwald, C. H. , Wolf, P. , Scherer, T. , Reiter, G. , Trattnig, S. , Luger, A. , Krebs, M. & Krssak, M. (2012). Effects of insulin therapy on myocardial lipid content and cardiac geometry in patients with type‐2 diabetes mellitus. PLoS ONE, 7(12), e50077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker, J. T. , Djaberi, R. , van Schinkel, L. D. , Hammer, S. , Bus, M. T. , Kerpershoek, G. , Kharagjitsingh, A. V. , Romijn, J. A. , Bax, J. J. , Jukema, J. W. , de Roos, A. , Smit, J. W. & Lamb, H. J. (2014). Very‐low‐calorie diet increases myocardial triglyceride content and decreases diastolic left ventricular function in type 2 diabetes with cardiac complications. Diabetes Care, 37(1), e1‐e2. [DOI] [PubMed] [Google Scholar]
- Knapp, M. , Górski, J. , Lewkowicz, J. , Lisowska, A. , Gil, M. , Wójcik, B. , Hirnle, T. , Chabowski, A. & Mikłosz, A. (2020). The gene and protein expression of the main components of the lipolytic system in human myocardium and heart perivascular adipose tissue. Effect of coronary atherosclerosis. International Journal of Molecular Sciences, 21(3), 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knottnerus, S. J. G. , Bleeker, J. C. , Ferdinandusse, S. , Houtkooper, R. H. , Langeveld, M. , Nederveen, A. J. , Strijkers, G. J. , Visser, G. , Wanders, R. J. A. , Wijburg, F. A. , Boekholdt, S. M. & Bakermans, A. J. (2020). Subclinical effects of long‐chain fatty acid beta‐oxidation deficiency on the adult heart: A case‐control magnetic resonance study. Journal of Inherited Metabolic Disease, 43(5), 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosoglou, G. , Humpert, P. M. , Ahrens, J. , Oikonomou, D. , Osman, N. F. , Gitsioudis, G. , Buss, S. J. , Steen, H. , Schnackenburg, B. , Bierhaus, A. , Nawroth, P. P. & Katus, H. A. (2012). Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. Journal of Magnetic Resonance Imaging, 35(4), 804–811. [DOI] [PubMed] [Google Scholar]
- Kosi‐Trebotic, L. , Thomas, A. , Harreiter, J. , Chmelik, M. , Trattnig, S. , & Kautzky‐Willer, A. (2017). Gliptin therapy reduces hepatic and myocardial fat in type 2 diabetic patients. European Journal of Clinical Investigation, 47(11), 829–838. [DOI] [PubMed] [Google Scholar]
- Krssak, M. , Winhofer, Y. , Gobl, C. , Bischof, M. , Reiter, G. , Kautzky‐Willer, A. , Luger, A. , Krebs, M. , & Anderwald, C. (2011). Insulin resistance is not associated with myocardial steatosis in women. Diabetologia, 54(7), 1871–1878. [DOI] [PubMed] [Google Scholar]
- Lai, S. , Gerstenblith, G. , Li, J. , Zhu, H. , Bluemke, D. A. , Liu, C. Y. , Zimmerman, S. L. , Chen, S. , Lai, H. , & Treisman, G. (2015). Chronic cocaine use and its association with myocardial steatosis evaluated by 1H magnetic resonance spectroscopy in African Americans. Journal of Addiction Medicine, 9(1), 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, S. , Gerstenblith, G. , Moore, R. D. , Celentano, D. D. , Bluemke, D. A. , Treisman, G. , Liu, C. Y. , Li, J. , Chen, S. , Kickler, T. & , & Lai, H. (2017). Cocaine use may modify HIV/ART‐associated myocardial steatosis and hepatic steatosis. Drug and Alcohol Dependence, 177, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto, H. R. , Parkka, J. , Borra, R. , Tuunanen, H. , Lepomaki, V. , Parkkola, R. , Knuuti, J. , Nuutila, P. , & Iozzo, P. (2012). Effects of acute and one‐week fatty acid lowering on cardiac function and insulin sensitivity in relation with myocardial and muscle fat and adiponectin levels. Journal of Clinical Endocrinology and Metabolism, 97(9), 3277–3284. [DOI] [PubMed] [Google Scholar]
- Levelt, E. , Mahmod, M. , Piechnik, S. K. , Ariga, R. , Francis, J. M. , Rodgers, C. T. , Clarke, W. T. , Sabharwal, N. , Schneider, J. E. , Karamitsos, T. D. , Clarke, K. , Rider, O. J. , & Neubauer, S. (2016). Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes, 65(1), 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt, E. , Pavlides, M. , Banerjee, R. , Mahmod, M. , Kelly, C. , Sellwood, J. , Ariga, R. , Thomas, S. , Francis, J. , Rodgers, C. , Clarke, W. , Sabharwal, N. , Antoniades, C. , Schneider, J. , Robson, M. , Clarke, K. , Karamitsos, T. , Rider, O. , & Neubauer, S. (2016). Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. Journal of the American College of Cardiology, 68(1), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, P. A. , Lin, G. , Tsai, S. Y. , Wang, C. H. , Juan, Y. H. , Lin, Y. C. , Wu, M. T. , Yang, L. Y. , Liu, M. H. , Chang, T. C. , Lin, Y. C. , Huang, Y. C. , Huang, P. C. , Wang, J. J. , Ng, S. H. , & Ng, K. K. (2016). Myocardial triglyceride content at 3 T cardiovascular magnetic resonance and left ventricular systolic function: A cross‐sectional study in patients hospitalized with acute heart failure. Journal of Cardiovascular Magnetic Resonance, 18(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. Y. , Bluemke, D. A. , Gerstenblith, G. , Zimmerman, S. L. , Li, J. , Zhu, H. , Lai, S. , & Lai, H. (2014). Myocardial steatosis and its association with obesity and regional ventricular dysfunction: Evaluated by magnetic resonance tagging and 1H spectroscopy in healthy African Americans. International Journal of Cardiology, 172(2), 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. Y. , Liu, Y. C. , Venkatesh, B. A. , Lima, J. A. , Bluemke, D. A. , & Steenbergen, C. (2012). Heterogeneous distribution of myocardial steatosis–An ex vivo evaluation. Magnetic Resonance in Medicine, 68(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmod, M. , Bull, S. , Suttie, J. J. , Pal, N. , Holloway, C. , Dass, S. , Myerson, S. G. , Schneider, J. E. , De Silva, R. , Petrou, M. , Sayeed, R. , Westaby, S. , Clelland, C. , Francis, J. M. , Ashrafian, H. , Karamitsos, T. D. , & Neubauer, S. (2013). Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circulation: Cardiovascular Imaging, 6(5), 808–816. [DOI] [PubMed] [Google Scholar]
- Mahmod, M. , Pal, N. , Rayner, J. , Holloway, C. , Raman, B. , Dass, S. , Levelt, E. , Ariga, R. , Ferreira, V. , Banerjee, R. , Schneider, J. E. , Rodgers, C. , Francis, J. M. , Karamitsos, T. D. , Frenneaux, M. , Ashrafian, H. , Neubauer, S. , & Rider, O. (2018). The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: A cardiovascular magnetic resonance study. Journal of Cardiovascular Magnetic Resonance, 20(1), 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzali, G. , Fantin, F. , Zoico, E. , Sepe, A. , Bambace, C. , Faccioli, S. , Pedrotti, M. , Corzato, F. , Rizzatti, V. , Faggian, G. , Micciolo, R. , Cinti, S. , Santini, F. , & Zamboni, M. (2015). Heart fat infiltration in subjects with and without coronary artery disease. Journal of Clinical Endocrinology and Metabolism, 100(9), 3364–3371. [DOI] [PubMed] [Google Scholar]
- McGavock, J. , Szczepaniak, L. S. , Ayers, C. R. , Abdullah, S. M. , See, R. , Gore, M. O. , Drazner, M. H. , de Lemos, J. A. , & McGuire, D. K. (2012). The effects of rosiglitazone on myocardial triglyceride content in patients with type 2 diabetes: A randomised placebo‐controlled trial. Diabetes and Vascular Disease Research, 9(2), 131–137. [DOI] [PubMed] [Google Scholar]
- McGavock, J. M. , Lingvay, I. , Zib, I. , Tillery, T. , Salas, N. , Unger, R. , Levine, B. D. , Raskin, P. , Victor, R. G. , & Szczepaniak, L. S. (2007). Cardiac steatosis in diabetes mellitus: A 1H‐magnetic resonance spectroscopy study. Circulation, 116(10), 1170–1175. [DOI] [PubMed] [Google Scholar]
- McGavock, J. M. , Victor, R. G. , Unger, R. H. , & Szczepaniak, L. S , (2006). American college of P & the American Physiological S. Adiposity of the heart, revisited. Annals of Internal Medicine, 144(7), 517–524. [DOI] [PubMed] [Google Scholar]
- Muniyappa, R. , Noureldin, R. , Ouwerkerk, R. , Liu, E. Y. , Madan, R. , Abel, B. S. , Mullins, K. , Walter, M. F. , Skarulis, M. C. , & Gharib, A. M. (2015). Myocardial fat accumulation is independent of measures of insulin sensitivity. Journal of Clinical Endocrinology and Metabolism, 100(8), 3060–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae, I. , Mitsunami, K. , Yoshino, T. , Omura, T. , Tsutamoto, T. , Matsumoto, T. , Morikawa, S. , Inubushi, T. , & Horie, M. (2010). Clinical features of myocardial triglyceride in different types of cardiomyopathy assessed by proton magnetic resonance spectroscopy: Comparison with myocardial creatine. Journal of Cardiac Failure, 16(10), 812–822. [DOI] [PubMed] [Google Scholar]
- Neilan, T. G. , Nguyen, K. L. , Zaha, V. G. , Chew, K. W. , Morrison, L. , Ntusi, N. A. B. , Toribio, M. , Awadalla, M. , Drobni, Z. D. , Nelson, M. D. , Burdo, T. H. , Van Schalkwyk, M. , Sax, P. E. , Skiest, D. J. , Tashima, K. , Landovitz, R. J. , Daar, E. , Wurcel, A. G. , Robbins, G. K. , … Zanni, M. V. (2020). Myocardial steatosis among antiretroviral therapy‐treated people with human immunodeficiency virus participating in the REPRIEVE trial. Journal of Infectious Diseases, 222(Supplement_1), S63–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, M. D. , Szczepaniak, L. S. , LaBounty, T. M. , Szczepaniak, E. , Li, D. , Tighiouart, M. , Li, Q. , Dharmakumar, R. , Sannes, G. , Fan, Z. , Yumul, R. , Hardy, W. D. , Conte, A. H. (2014). Cardiac steatosis and left ventricular dysfunction in HIV‐infected patients treated with highly active antiretroviral therapy. JACC Cardiovasc Imaging, 7(11), 1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, M. D. , Victor, R. G. , Szczepaniak, E. W. , Simha, V. , Garg, A. , & Szczepaniak, L. S. (2013). Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging. American Journal of Cardiology, 112(7), 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, A. C. , Delgado, V. , Bertini, M. , van der Meer, R. W. , Rijzewijk, L. J. , Hooi Ewe, S. , Siebelink, H. M. , Smit, J. W. , Diamant, M. , Romijn, J. A. , de Roos, A. , Leung, D. Y. , Lamb, H. J. , & Bax, J. J. (2010). Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation, 122(24), 2538–2544. [DOI] [PubMed] [Google Scholar]
- Nyman, K. , Graner, M. , Pentikainen, M. O. , Lundbom, J. , Hakkarainen, A. , Siren, R. , Nieminen, M. S. , Taskinen, M. R. , Lundbom, N. , & Lauerma, K. (2013). Cardiac steatosis and left ventricular function in men with metabolic syndrome. Journal of Cardiovascular Magnetic Resonance, 15(1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oneglia, A. P. , Szczepaniak, L. S. , Jaffery, M. F. , Cipher, D. J. , McDonald, J. G. , Haykowsky, M. J. , Moreau, K. L. , Clegg, D. J. , Zaha, V. , & Nelson, M. D. (2023). Myocardial steatosis impairs left ventricular diastolic‐systolic coupling in healthy humans. The Journal of Physiology, 601(8), 1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiman, E. H. M. , van Eyk, H. J. , van Aalst, M. M. A. , Bizino, M. B. , van der Geest, R. J. , Westenberg, J. J. M. , Geelhoed‐Duijvestijn, P. H. , Kharagjitsingh, A. V. , Rensen, P. C. N. , Smit, J. W. A. , Jazet, I. M. , & Lamb, H. J. (2020). Effect of liraglutide on cardiovascular function and myocardial tissue characteristics in type 2 diabetes patients of south Asian descent living in the Netherlands: A double‐blind, randomized, placebo‐controlled trial. Journal of Magnetic Resonance Imaging, 51(6), 1679–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petritsch, B. , Gassenmaier, T. , Kunz, A. S. , Donhauser, J. , Goltz, J. P. , Bley, T. A. , & Horn, M. (2015). Age dependency of myocardial triglyceride content: A 3T high‐field 1H‐MR spectroscopy study. Rofo, 187(11), 1016–1021. [DOI] [PubMed] [Google Scholar]
- Petritsch, B. , Kostler, H. , Gassenmaier, T. , Kunz, A. S. , Bley, T. A. , & Horn, M. (2016). An investigation into potential gender‐specific differences in myocardial triglyceride content assessed by 1H‐Magnetic Resonance Spectroscopy at 3Tesla. Journal of International Medical Research, 44(3), 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner, J. J. , Abdesselam, I. , Peterzan, M. A. , Akoumianakis, I. , Akawi, N. , Antoniades, C. , Tomlinson, J. W. , Neubauer, S. , & Rider, O. J. (2019). Very low calorie diets are associated with transient ventricular impairment before reversal of diastolic dysfunction in obesity. International Journal of Obesity (Lond), 43(12), 2536–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner, J. J. , Banerjee, R. , Holloway, C. J. , Lewis, A. J. M. , Peterzan, M. A. , Francis, J. M. , Neubauer, S. , & Rider, O. J. (2018). The relative contribution of metabolic and structural abnormalities to diastolic dysfunction in obesity. International Journal of Obesity (Lond), 42(3), 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold, J. S. , McGavock, J. M. , Kaka, S. , Tillery, T. , Victor, R. G. , & Szczepaniak, L. S. (2005). Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: Reproducibility and sensitivity of the method. American Journal of Physiology. Endocrinology and Metabolism, 289(5), E935‐E939. [DOI] [PubMed] [Google Scholar]
- Rijzewijk, L. J. , van der Meer, R. W. , Smit, J. W. , Diamant, M. , Bax, J. J. , Hammer, S. , Romijn, J. A. , de Roos, A. , & Lamb, H. J. (2008). Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. Journal of the American College of Cardiology, 52(22), 1793–1799. [DOI] [PubMed] [Google Scholar]
- Sai, E. , Shimada, K. , Yokoyama, T. , Hiki, M. , Sato, S. , Hamasaki, N. , Maruyama, M. , Morimoto, R. , Miyazaki, T. , Fujimoto, S. , Tamura, Y. , Aoki, S. , Watada, H. , Kawamori, R. , & Daida, H. (2017). Myocardial triglyceride content in patients with left ventricular hypertrophy: Comparison between hypertensive heart disease and hypertrophic cardiomyopathy. Heart and Vessels, 32(2), 166–174. [DOI] [PubMed] [Google Scholar]
- Sai, E. , Shimada, K. , Yokoyama, T. , Sato, S. , Miyazaki, T. , Hiki, M. , Tamura, Y. , Aoki, S. , Watada, H. , Kawamori, R. , & Daida, H. (2013). Association between myocardial triglyceride content and cardiac function in healthy subjects and endurance athletes. PLoS ONE, 8(4), e61604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai, E. , Shimada, K. , Yokoyama, T. , Sato, S. , Nishizaki, Y. , Miyazaki, T. , Hiki, M. , Tamura, Y. , Aoki, S. , Watada, H. , Kawamori, R. , & Daida, H. (2015). Evaluation of myocardial triglyceride accumulation assessed on 1H‐magnetic resonance spectroscopy in apparently healthy Japanese subjects. Internal Medicine, 54(4), 367–373. [DOI] [PubMed] [Google Scholar]
- Sarma, S. , Carrick‐Ranson, G. , Fujimoto, N. , Adams‐Huet, B. , Bhella, P. S. , Hastings, J. L. , Shafer, K. M. , Shibata, S. , Boyd, K. , Palmer, D. , Szczepaniak, E. W. , Szczepaniak, L. S. , & Levine, B. D. (2013). Effects of age and aerobic fitness on myocardial lipid content. Circulation: Cardiovascular Imaging, 6(6), 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, T. , Wolf, P. , Winhofer, Y. , Duan, H. , Einwallner, E. , Gessl, A. , Luger, A. , Trattnig, S. , Hoffmann, M. , Niessner, A. , Baumgartner‐Parzer, S. , Krssak, M. , & Krebs, M. (2014). Levothyroxine replacement in hypothyroid humans reduces myocardial lipid load and improves cardiac function. Journal of Clinical Endocrinology and Metabolism, 99(11), E2341‐E2346. [DOI] [PubMed] [Google Scholar]
- Schrauwen‐Hinderling, V. B. , Hesselink, M. K. , Meex, R. , van der Made, S. , Schar, M. , Lamb, H. , Wildberger, J. E. , Glatz, J. , Snoep, G. , Kooi, M. E. , & Schrauwen, P. (2010). Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. Journal of Clinical Endocrinology and Metabolism, 95(4), 1932–1938. [DOI] [PubMed] [Google Scholar]
- Schrauwen‐Hinderling, V. B. , Meex, R. C. , Hesselink, M. K. , van de Weijer, T. , Leiner, T. , Schar, M. , Lamb, H. J. , Wildberger, J. E. , Glatz, J. F. , Schrauwen, P. , & Kooi, M. E. (2011). Cardiac lipid content is unresponsive to a physical activity training intervention in type 2 diabetic patients, despite improved ejection fraction. Cardiovasc Diabetol, 10(1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze, P. C. , Drosatos, K. , & Goldberg, I. J. (2016). Lipid use and misuse by the heart. Circulation Research, 118(11), 1736–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Adrogue, J. V. , Golfman, L. , Uray, I. , Lemm, J. , Youker, K. , Noon, G. P. , Frazier, O. H. , & Taegtmeyer, H. (2004). Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb Journal, 18(14), 1692–1700. [DOI] [PubMed] [Google Scholar]
- Shitole, S. G. , Naveed, M. , Wang, Z. , Wang, T. , Kato, Y. , Ambale‐Venkatesh, B. , Kaplan, R. C. , Tien, P. C. , Anastos, K. , Lazar, J. M. , Lima, J. A. C. , Qi, Q. , & Kizer, J. R. (2023). Metabolomic profiling of cardiac fibrosis and steatosis in women with or at risk for HIV. Journal of Acquired Immune Deficiency Syndromes, 92,(2), 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smajis, S. , Gajdosik, M. , Pfleger, L. , Traussnigg, S. , Kienbacher, C. , Halilbasic, E. , Ranzenberger‐Haider, T. , Stangl, A. , Beiglbock, H. , Wolf, P. , Lamp, T. , Hofer, A. , Gastaldelli, A. , Barbieri, C. , Luger, A. , Trattnig, S. , Kautzky‐Willer, A. , Krssak, M. , Trauner, M. , & Krebs, M. (2020). Metabolic effects of a prolonged, very‐high‐dose dietary fructose challenge in healthy subjects. American Journal of Clinical Nutrition, 111(2), 369–377. [DOI] [PubMed] [Google Scholar]
- Szczepaniak, L. S. , Dobbins, R. L. , Metzger, G. J. , Sartoni‐D'Ambrosia, G. , Arbique, D. , Vongpatanasin, W. , Unger, R. , & Victor, R. G. (2003). Myocardial triglycerides and systolic function in humans: In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magnetic Resonance in Medicine, 49(3), 417–423. [DOI] [PubMed] [Google Scholar]
- Szczepaniak, L. S. , Victor, R. G. , Orci, L. , & Unger, R. H. (2007). Forgotten but not gone: The rediscovery of fatty heart, the most common unrecognized disease in America. Circulation Research, 101(8), 759–767. [DOI] [PubMed] [Google Scholar]
- Thiara, D. K. , Liu, C. Y. , Raman, F. , Mangat, S. , Purdy, J. B. , Duarte, H. A. , Schmidt, N. , Hur, J. , Sibley, C. T. , Bluemke, D. A. , & Hadigan, C. (2015). Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV‐infected adults. Journal of Infectious Diseases, 212(10), 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribio, M. , Neilan, T. G. , Awadalla, M. , Stone, L. A. , Rokicki, A. , Rivard, C. , Mulligan, C. P. , Cagliero, D. , Fourman, L. T. , Stanley, T. L. , Ho, J. E. , Triant, V. A. , Burdo, T. H. , Nelson, M. D. , Szczepaniak, L. S. , & Zanni, M. V. (2019). Intramyocardial triglycerides among women with vs without HIV: Hormonal correlates and functional consequences. Journal of Clinical Endocrinology and Metabolism, 104(12), 6090–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz, W. , Engeli, S. , Haufe, S. , Kast, P. , Bohnke, J. , Haas, V. , Hermsdorf, M. , Wiesner, S. , Pofahl, M. , Traber, J. , Luft, F. C. , Boschmann, M. , Jordan, J. , & Schulz‐Menger, J. (2013). Moderate dietary weight loss reduces myocardial steatosis in obese and overweight women. International Journal of Cardiology, 167(3), 905–909. [DOI] [PubMed] [Google Scholar]
- van der Meer, R. W. , Hammer, S. , Lamb, H. J. , Frolich, M. , Diamant, M. , Rijzewijk, L. J. , de Roos, A. , Romijn, J. A. , & Smit, J. W. (2008). Effects of short‐term high‐fat, high‐energy diet on hepatic and myocardial triglyceride content in healthy men. Journal of Clinical Endocrinology and Metabolism, 93(7), 2702–2708. [DOI] [PubMed] [Google Scholar]
- van der Meer, R. W. , Hammer, S. , Smit, J. W. , Frolich, M. , Bax, J. J. , Diamant, M. , Rijzewijk, L. J. , de Roos, A. , Romijn, J. A. , & Lamb, H. J. (2007). Short‐term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes, 56(12), 2849–2853. [DOI] [PubMed] [Google Scholar]
- van der Meer, R. W. , Rijzewijk, L. J. , de Jong, H. W. , Lamb, H. J. , Lubberink, M. , Romijn, J. A. , Bax, J. J. , de Roos, A. , Kamp, O. , Paulus, W. J. , Heine, R. J. , Lammertsma, A. A. , Smit, J. W. , & Diamant, M. (2009). Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high‐energy phosphate metabolism in patients with well‐controlled type 2 diabetes mellitus. Circulation, 119(15), 2069–2077. [DOI] [PubMed] [Google Scholar]
- van der Meer, R. W. , Rijzewijk, L. J. , Diamant, M. , Hammer, S. , Schar, M. , Bax, J. J. , Smit, J. W. , Romijn, J. A. , de Roos, A. , & Lamb, H. J. (2008). The ageing male heart: Myocardial triglyceride content as independent predictor of diastolic function. European Heart Journal, 29(12), 1516–1522. [DOI] [PubMed] [Google Scholar]
- van Schinkel, L. D. , Sleddering, M. A. , Lips, M. A. , Jonker, J. T. , de Roos, A. , Lamb, H. J. , Jazet, I. M. , Pijl, H. , & Smit, J. W. (2014). Effects of bariatric surgery on pericardial ectopic fat depositions and cardiovascular function. Clinical Endocrinology, 81(5), 689–695. [DOI] [PubMed] [Google Scholar]
- Wei, J. , Nelson, M. D. , Szczepaniak, E. W. , Smith, L. , Mehta, P. K. , Thomson, L. E. , Berman, D. S. , Li, D. , Bairey Merz, C. N. , & Szczepaniak, L. S. (2016). Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. American Journal of Physiology. Heart and Circulatory Physiology, 310(1), H14‐H19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende, A. R. , & Abel, E. D. (2010). Lipotoxicity in the heart. Biochimica Et Biophysica Acta, 1801(3), 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhofer, Y. , Krssak, M. , Jankovic, D. , Anderwald, C. H. , Reiter, G. , Hofer, A. , Trattnig, S. , Luger, A. , & Krebs, M. (2012). Short‐term hyperinsulinemia and hyperglycemia increase myocardial lipid content in normal subjects. Diabetes, 61(5), 1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhofer, Y. , Krssak, M. , Wolf, P. , Anderwald, C. H. , Geroldinger, A. , Heinze, G. , Baumgartner‐Parzer, S. , Marculescu, R. , Stulnig, T. , Wolzt, M. , Trattnig, S. , Luger, A. , & Krebs, M. (2015). Free fatty acid availability is closely related to myocardial lipid storage and cardiac function in hypoglycemia counterregulation. American Journal of Physiology. Endocrinology and Metabolism, 308(8), E631‐E640. [DOI] [PubMed] [Google Scholar]
- Winhofer, Y. , Krssak, M. , Wolf, P. , Tura, A. , Anderwald, C. H. , Kosi, L. , Reiter, G. , Pacini, G. , Trattnig, S. , Luger, A. , Krebs, M. , & Kautzky‐Willer, A. (2014). Hepatic rather than cardiac steatosis relates to glucose intolerance in women with prior gestational diabetes. PLoS ONE, 9(3), e91607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhofer, Y. , Wolf, P. , Krssak, M. , Wolfsberger, S. , Tura, A. , Pacini, G. , Gessl, A. , Raber, W. , Kukurova, I. J. , Kautzky‐Willer, A. , Knosp, E. , Trattnig, S. , Krebs, M. , & Luger, A. (2014). No evidence of ectopic lipid accumulation in the pathophysiology of the acromegalic cardiomyopathy. Journal of Clinical Endocrinology and Metabolism, 99(11), 4299–4306. [DOI] [PubMed] [Google Scholar]
- Wolf, P. , Krssak, M. , Winhofer, Y. , Anderwald, C. H. , Zwettler, E. , Just Kukurova, I. , Gessl, A. , Trattnig, S. , Luger, A. , Baumgartner‐Parzer, S. , & Krebs, M. (2014). Cardiometabolic phenotyping of patients with familial hypocalcuric hypercalcemia. Journal of Clinical Endocrinology and Metabolism, 99(9), E1721‐E1726. [DOI] [PubMed] [Google Scholar]
- Wolf, P. , Marty, B. , Bouazizi, K. , Kachenoura, N. , Piedvache, C. , Blanchard, A. , Salenave, S. , Prigent, M. , Jublanc, C. , Ajzenberg, C. , Droumaguet, C. , Young, J. , Lecoq, A. L. , Kuhn, E. , Agostini, H. , Trabado, S. , Carlier, P. G. , Feve, B. , Redheuil, A. , … Kamenicky, P. (2021). Epicardial and pericardial adiposity without myocardial steatosis in cushing syndrome. Journal of Clinical Endocrinology and Metabolism, 106(12), 3505–3514. [DOI] [PubMed] [Google Scholar]
- Wolf, P. , Winhofer, Y. , Krssak, M. , Smajis, S. , Harreiter, J. , Kosi‐Trebotic, L. , Furnsinn, C. , Anderwald, C. H. , Baumgartner‐Parzer, S. , Trattnig, S. , Luger, A. , & Krebs, M. (2016). Suppression of plasma free fatty acids reduces myocardial lipid content and systolic function in type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases, 26(5), 387–392. [DOI] [PubMed] [Google Scholar]
- Wu, C. K. , Lee, J. K. , Hsu, J. C. , Su, M. M. , Wu, Y. F. , Lin, T. T. , Lan, C. W. , Hwang, J. J. , & Lin, L. Y. (2020). Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. European Journal of Heart Failure, 22(3), 445–454. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. T. , Grayburn, P. , Karim, A. , Shimabukuro, M. , Higa, M. , Baetens, D. , Orci, L. , & Unger, R. H. (2000). Lipotoxic heart disease in obese rats: Implications for human obesity. PNAS, 97(4), 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zib, I. , Jacob, A. N. , Lingvay, I. , Salinas, K. , McGavock, J. M. , Raskin, P. , & Szczepaniak, L. S. (2007). Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin‐treated patients with type 2 diabetes. Journal of Investigative Medicine, 55(5), 230‐236. [DOI] [PubMed] [Google Scholar]


