Abstract
Introduction:
Right ventricular (RV) dysfunction is associated with increased mortality across a spectrum of cardiovascular diseases. The role of obesity in RV dysfunction and adverse outcomes is unclear.
Methods:
We examined patients undergoing right heart catheterization between 2005–2016 in a hospital-based cohort. Linear regression was used to examine the association of obesity with hemodynamic indices of RV dysfunction [pulmonary artery pulsatility index (PAPi), right atrial pressure: pulmonary capillary wedge pressure ratio (RAP:PCWP), RV stroke work index (RVSWI)]. Cox models were used to examine the association of RV function measures with clinical outcomes.
Results:
Among 8285 patients (mean age 63 years, 40% women), higher BMI was associated with worse indices of RV dysfunction, including lower PAPi (β −0.23, SE 0.01, p <0.001), higher RA:PCWP ratio (β 0.25, SE 0.01, p-value <0.001), and lower RVSWI (β −0.05, SE 0.01, p-value <0.001). Over 7.3 years of follow-up, we observed 3006 mortality and 2004 HF hospitalization events. RV dysfunction was associated with greater risk of mortality (eg PAPi: hazard ratio (HR) 1.11 per 1-standard deviation (SD) increase, 95% CI 1.04–1.18), with similar associations with risk of HF hospitalization. BMI modified the effect of RV dysfunction on mortality (P for interaction <=0.005 for PAPi and RA: PCWP ratio), such that the effect of RV dysfunction was more pronounced at higher BMI.
Conclusions:
Patients with obesity had worse hemodynamic measured indices of RV function across a broad hospital-based sample. While RV dysfunction was associated with worse clinical outcomes including mortality and HF hospitalization, this association was especially pronounced among individuals with higher BMI.
Keywords: obesity, heart failure, pulmonary hypertension
INTRODUCTION
The right ventricle has been historically overshadowed by its counterpart, the left ventricle, in its clinical significance across a broad spectrum of cardiovascular disease and particularly in heart failure (HF). This is in part due the difficulty of understanding the role that right ventricular (RV) dysfunction plays in cardiovascular disease, both anatomically and physiologically [1]. Non-invasive measurements using echocardiography and cardiac magnetic resonance imaging to characterize function are limited by the RV’s unique anatomy, which is highly sensitive to different pressure and volume conditions. From a physiology standpoint, RV dysfunction is intimately related to the pulmonary circulation and is variably affected by a wide range of cardiovascular and pulmonary disease phenotypes. This leads to a heterogenous population with RV dysfunction, which results in challenges in studying the subsequent impact that RV dysfunction may have on adverse outcomes [1,2].
Obesity is a growing problem in the United States with well-recognized contributions to incident cardiovascular disease and heart failure [3, 4]. While obesity has been associated with adverse RV remodeling using echocardiography and cardiac magnetic resonance measurements [5, 6], the association of obesity with invasive hemodynamic indices of RV function that may more accurately reflect hemodynamic consequences of RV function remains unclear. We leveraged a large hospital-based sample of individuals spanning a broad spectrum of cardiopulmonary disease who had undergone clinically indicated right heart catheterization. This provided a unique setting to examine the association of obesity with hemodynamic indices of RV function, and the association of RV function with subsequent clinical outcomes. We hypothesized that obesity is associated with RV dysfunction, and that obesity modifies the association of RV dysfunction with adverse clinical outcomes.
METHODS
Study Sample
Statistical code and analytic methods will be made available upon request. The data are not able to be made available to other researchers for purposes of reproducing the results or replicating the procedure. We examined consecutive ambulatory and hospitalized patients undergoing right heart catheterization (RHC) between 2005 and 2016 at Massachusetts General Hospital. For patients who had multiple RHC procedures during this time period, only the initial RHC was included for analysis, resulting in a total of 10,306 cases. The following clinical exclusion criteria were applied: acute myocardial infarction (MI) occurring on the same day as catheterization, cardiac arrest or shock within 24 hours, presence of mechanical ventilation, presence of intra-aortic balloon pump, history of heart or lung transplant, complicated adult congenital heart disease, history of valvular replacement, or those on dialysis (n=887 excluded). Cases were also excluded if there were missing key clinical covariates (n=484), patient identifier variables (n=398), or hemodynamic parameters (n=252), leading to a final study sample of 8285 for analysis. We defined key clinical covariates as variables included in our multivariable analyses, which included age, sex, hypertension, diabetes mellitus, obstructive sleep apnea (OSA), chronic lung disease, prevalent MI, prevalent HF, and PH, as well as weight and height. This study was approved by the Mass General Brigham Institutional Review Board.
Clinical and RV hemodynamic variables
Clinical characteristics were ascertained from the medical records at the time of RHC, including age, sex, body mass index (BMI), smoking status, and presence of comorbidities (diabetes mellitus, hypertension, history of MI, history of heart failure, prior lung disease, and chronic kidney disease). OSAwas identified by the electronic medical record utilizing appropriate International Classification of Diseases Ninth Revision (ICD-9) or Tenth Revision (ICD-10) codes.
Hemodynamic measures were recorded at the time of RHC, including resting blood pressure, heart rate, mean right atrial (RA) pressure, pulmonary artery (PA) systolic and diastolic pressure, and mean pulmonary capillary wedge pressure (PCWP).
Nonphysiologic parameters were set to missing. The PA pulsatility index (PAPi) was calculated as . In the cases that RA pressure was recorded as zero, the value was set to one for the purposes of PAPi calculation. Otherwise, any negative pressures were deemed non-physiologic and set to missing, which affected <1% of measurements. Cardiac output and index were derived via thermodilution methods utilizing the Mosteller formula for body surface area [7]. RVSWI was derived as 0.0136 x Stroke volume index x (Mean PA pressure -mean RA pressure) utilizing the thermodilution cardiac index measurement.
Clinical Outcomes
All-cause mortality was ascertained using the National Social Security Death Master Index and hospital records, abstracted on 06/10/2020. Due to confidentiality purposes, the precise dates of deaths that occurred between 06/10/2017 and the date of abstraction are protected nationally. To conduct time-to-event analyses, these death dates were imputed at the midpoint of the blanking period (12/10/2018).
Occurrence of a major adverse cardiac event (MACE) was defined as a composite of heart failure (HF) admission, cerebrovascular accident (CVA), transient ischemic attack (TIA) or acute MI with a corresponding ICD-9 or ICD-10 code as the primary discharge diagnosis. A heart failure hospitalization was defined by an ICD-9 or ICD-10 code for heart failure as the primary discharge diagnosis or a current procedural terminology (CPT) code for heart transplantation (OHT) or durable ventricular assist device (VAD). The follow-up period for each participant was defined as time from RHC to death date or date of final encounter in the electronic health record. Patients were censored based on time of last encounter.
Statistical Analysis
Baseline characteristics were summarized across the total sample and according to obesity class (Normal = BMI <25, Overweight = BMI ≥25 and <30, Obesity class 1 = BMI ≥30 and <35, Obesity class 2+3 = BMI ≥35 kg/m2). We examined the cross-sectional association of BMI and obesity class with hemodynamic indices of RV dysfunction, including PAPi, RA:PCWP ratio, and RVSWI using multivariable linear regression. In order to limit leverage of outliers in the analyses, the distribution of PAPi was winsorized by setting the minimum PAPi as 0.3 and the maximum PAPi as 30. PAPi, RA:PCWP ratio, and RVSWI were natural log-transformed as well as scaled to mean 0 and standard deviation 1 due to right-skewed distributions. Models were adjusted for the following clinical covariates: age, sex, hypertension, diabetes mellitus, OSA, chronic lung disease, pulmonary hypertension, prevalent MI, and prevalent HF. Further, we used least squares means to estimate adjusted mean values for untransformed RV function indices across obesity classes after controlling for all covariates.
To investigate the effect of obesity and RV dysfunction and their potential interaction on future clinical outcomes, we first examined the association of RV function measures known to be associated with obesity from primary analyses with clinical outcomes, including all-cause mortality and HF hospitalizations. We used the Kaplan-Meier method to examine the association of RV function measures with outcomes and log rank tests to examine differences between groups. Multivariable Cox models were constructed, adjusting for age, sex, BMI, previous MI, previous HF, the presence of pulmonary hypertension (PH, defined as mean pulmonary arterial pressure ≥20), hypertension, diabetes, and chronic kidney disease. The proportional hazards assumption was tested, and minor violations were found using cumulative Martingale residuals. We therefore included time-varying predictors to account for model fitness, including log-transformed and scaled PAPi, age, BMI, previous HF, and hypertension treatment.
We then examined whether BMI modified the association of RV function with clinical outcomes. We used multiplicative interaction terms (PAPi*BMI, RVSWI*BMI, and RA:PCWP ratio*BMI) entered into multivariable Cox models to examine this. Further, to illustrate effect modification, we plotted estimated adjusted hazard ratio of each RV function measure with clinical outcome (y-axis) by BMI (x-axis). A two-sided p value <0.05 was deemed as statistically significant. Analyses were conducted using SAS software, Version 9.4 (Cary, North Carolina, USA).
RESULTS
We studied 8285 individuals with a mean age of 63 ± 13 years, 39% of whom were women. Comorbid conditions were common including 59% with hypertension, 23% with diabetes mellitus, 16% with chronic lung disease, 14% with obstructive sleep apnea, 19% with previous MI, and 32% with previous HF. Baseline characteristics stratified by obesity class are shown in Table 1. Mean BMI across the sample was 29.3 ± 6.8 kg/m2, and 28% of participants were classified as normal weight, 34% overweight, 21% obesity class 1, and 17% obesity class 2+3. Patients across increasing obesity classes were similar in age, with greater comorbid burden including hypertension, diabetes, prior HF, and obstructive sleep apnea. Of note, natriuretic peptide levels were lower across obesity classes despite higher right- and left-sided filling pressures (Table 1).
Table 1.
Baseline characteristics by obesity class
| Normal (n=2307) | Overweight (n=2793) | Obese Class 1 (n=1732) | Obese Class 2+3 (n=1453) | p-value | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, years | 62 ± 15 | 64 ± 12 | 64 ± 11 | 62 ± 12 | <0.001 |
| Female, n (%) | 1111 (48.2) | 877 (31.4) | 624 (36) | 651 (44.8) | <0.001 |
| Race, n (%) | <0.001 | ||||
| White | 1900 (82.4) | 2377 (85.1) | 1518 (87.6) | 1234 (84.9) | |
| Black | 88 (3.8) | 97 (3.5) | 58 (3.4) | 69 (4.8) | |
| Hispanic | 75 (3.3) | 98 (3.5) | 46 (2.7) | 43 (3.0) | |
| Asian | 86 (3.7) | 54 (1.9) | 18 (1) | 6 (0.4) | |
| Unknown | 158 (6.9) | 167 (6) | 92 (5.3) | 101 (7) | |
| BMI, kg/m2 | 22.2 ± 2.1 | 27.5 ± 1.4 | 32.3 ± 1.4 | 40.5 ± 5.4 | <0.001 |
| Systolic BP, mmHg | 124.5 ± 25.7 | 124.8 ± 23.8 | 128.6 ± 24.3 | 130.2 ± 23.6 | <0.001 |
| Hypertension, n (%) | 1052 (45.6) | 1675 (60) | 1150 (66.4) | 996 (68.6) | <0.001 |
| Diabetes Mellitus, n (%) | 286 (12.4) | 596 (21.3) | 505 (29.2) | 536 (36.9) | <0.001 |
| Previous MI, n (%) | 361 (15.7) | 563 (20.2) | 373 (21.5) | 254 (17.5) | <0.001 |
| Previous HF, n (%) | 706 (30.6) | 860 (30.8) | 587 (33.9) | 503 (34.6) | 0.010 |
| Hypercholesterolemia, n (%) | 1078 (62.9) | 1774 (76.7) | 1151 (80.7) | 974 (84.4) | <0.001 |
| Chronic lung disease, n (%) | 347 (15) | 416 (14.9) | 319 (18.4) | 267 (18.4) | 0.001 |
| OSA, n (%) | 101 (4.4) | 239 (8.6) | 302 (17.4) | 512 (35.2) | <0.001 |
| Chronic kidney disease, n (%) | 54 (2.3) | 85 (3) | 64 (3.7) | 38 (2.6) | 0.070 |
| Current smoker, n (%) | 138 (10.6) | 131 (8.4) | 110 (11.2) | 81 (9.4) | 0.07 |
| NT-proBNP, pg/ml | 2928 (1031, 7346) | 2089 (633, 4997) | 1273 (402, 3177) | 991 (297, 2576) | <0.001 |
| Time to death | 6.8 [3.9, 10] | 7.7 [4.3, 10] | 7.5 [4.3, 10] | 7.3 [4.5, 10] | |
| Time to hospitalization | 3.4 [0.3, 7.1] | 4.3 [0.7, 8.2] | 4.1 [0.9, 8,2] | 4.0 [0.8, 7,4] | |
|
| |||||
| Hemodynamics | |||||
| Heart rate, bpm | 74 ± 16 | 72 ± 15 | 71 ± 15 | 74 ± 16 | <0.001 |
| SVi (mL/m2)* | 37 [29–45] | 37 [30–45] | 37 [30–45] | 36 [29–43] | <0.001 |
| RA mean pressure, mmHg | 5 (3, 8) | 6 (4, 9) | 8 (5, 11) | 10 (7, 14) | <0.001 |
| PA systolic pressure, mmHg | 34 (26, 46) | 35 (27, 47) | 36 (30, 49) | 41 (33, 53) | <0.001 |
| PA diastolic pressure, mmHg | 13 (8, 19) | 13 (9, 20) | 15 (10, 21.5) | 18 (12, 25) | <0.001 |
| PCWP, mmHg | 12 (8, 18) | 13 (9, 19) | 15 (10.5, 20) | 17 (12, 24) | <0.001 |
| Td cardiac output, L/min | 4.5 (3.6, 5.5) | 5 (4.2, 6) | 5.2 (4.4, 6.3) | 5.8 (4.8, 7) | <0.001 |
| PAPi | 4.4 (2.8, 7.8) | 3.6 (2.4, 5.8) | 3 (2, 4.5) | 2.5 (1.7, 3.6) | <0.001 |
| RA:PCWP ratio | 0.2 (0.1, 0.3) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.4 (0.3, 0.4) | <0.001 |
| RVSWI, gm-m/m2/beat | 7.9 (5.8, 11.1) | 8 (5.9, 11) | 8.1 (6.1, 11.3) | 8.4 (6.1, 11.7) | 0.010 |
| Pulmonary hypertension, n(%) | 1261 (54.7) | 1630 (58.4) | 1162 (67.1) | 1155 (79.5) | <0.001 |
Table displays mean ± standard deviation.
denotes variables displayed as median [interquartile range].
Abbreviations: PAPi, pulmonary artery pulsatility index; MI, myocardial infarction; HF, heart failure; OSA, obstructive sleep apnea; BMI, body mass index; NT-proBNP, N-terminal pro-B type natriuretic peptide; PA, pulmonary artery; RVSWI, right ventricular stroke work index (calculated using thermodilution cardiac output), Td, thermodilution method. RVSWI was calculated by thermodilution cardiac output measurement.
BMI and Obesity are Associated with Worse RV function
Higher BMI was associated with worse indices of RV function across all three measures. Specifically, PAPi was lower across obesity classes (4.4 [2.8, 7.8] in normal weight vs 2.5 [1.7, 3.6] in class 2+3 obesity), and RA:PCWP ratio was higher across obesity classes (0.2 [0.1, 0.3] in normal weight vs 0.4 [0.3, 0.4] in class 2+3 obesity). Lastly, RVSWI was lower among those with higher BMI, though there were minimal differences across obesity classes. When applying the clinical cutpoint of PAPi <2 to define RV dysfunction based on previous studies, 12% individuals with normal weight, 16% overweight, 22% class I, and 32% class 2+3 obesity had RV dysfunction [8–10 ] (Table 2).
Table 2.
Associations of obesity classes with indices of RV function
| Predictors | β estimate | Standard error | p value | p-trend |
|---|---|---|---|---|
| PAPi | ||||
| Obesity Class | ||||
| Normal | REF | <0.001 | ||
| Overweight | −0.25 | 0.03 | <0.001 | |
| Class 1 | −0.48 | 0.03 | <0.001 | |
| Class 2+3 | −0.65 | 0.03 | <0.001 | |
|
| ||||
| RA:PCWP ratio | ||||
| Obesity Class | ||||
| Normal | REF | <0.001 | ||
| Overweight | 0.28 | 0.03 | <0.001 | |
| Class 1 | 0.51 | 0.03 | <0.001 | |
| Class 2+3 | 0.69 | 0.03 | <0.001 | |
|
| ||||
| RVSWI | ||||
| Obesity Class | ||||
| Normal | REF | 0.88 | ||
| Overweight | −0.02 | 0.03 | 0.47 | |
| Class 1 | −0.08 | 0.03 | 0.01 | |
| Class 2+3 | −0.15 | 0.03 | <0.001 | |
P-trend was used to examine trend across obesity classes treated as a categorical variable using clinically established cut points and was obtained from the linear regression model, using obesity category as the independent, and right ventricular function measured as the dependent variable. Multivariable models were adjusted for age, sex, hypertension, diabetes mellitus, obstructive sleep apnea, chronic lung disease, prevalent myocardial infarction, prevalent heart failure, and pulmonary hypertension.
Abbreviations: PAPi, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; RA, right atrium/atrial; RV, right ventricle/ventricular; RVSWI, right ventricular stroke work index.
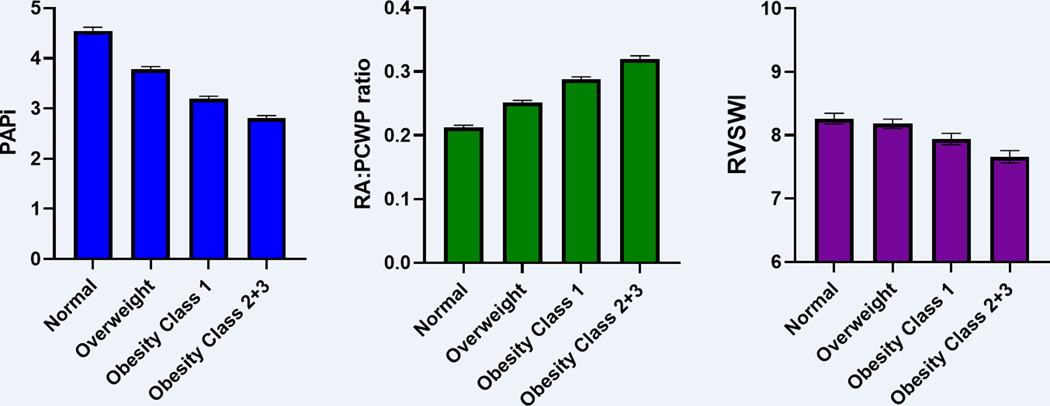
In multivariable-adjusted analyses, we found that higher BMI was associated with worse indices of RV function, including lower PAPi, higher RA:PCWP ratio, and lower RVSWI (Table 3). Specifically, we found that a 1-SD higher BMI was associated with a 0.23 log-unit lower PAPi (β −0.23, SE 0.01, p <0.001). In addition, we examined these effect sizes scaled to per 1-log-unit change which are shown in Supplemental Table 1. In adjusted analyses using least squares means as shown in Figure 1, the mean PAPi among normal weight individuals was 4.5 vs 2.8 among those with class 2+3 obesity. Similarly, a 1-SD higher BMI was associated with a 0.25 log-unit higher RA:PCWP ratio (β 0.25, SE 0.01, p-value <0.001), and mean RA:PCWP ratio among normal weight individuals was 0.21 vs 0.32 among those with class 2+3 obesity (Figure 1). Lastly, a 1-SD higher BMI was associated with a 0.05 log-unit lower RVSWI (β −0.05, SE 0.01, p-value <0.001). Mean RVSWI in normal weight individuals was 8.26 vs 7.66 in those with class 2+3 obesity (Figure 1). In stratified analyses based on urgent vs elective RHC and also based on time era (before vs after 2010), we found that associations of obesity and BMI with measures of RV function were largely consistent. Additionally, in exploratory analyses adjusting for pulmonary effective arterial elastance in place of PH, associations of obesity and BMI with PAPi and RA:PCWP ratio remained largely similar.
Table 3.
Associations of BMI with indices of RV function
| Predictors | β estimate | Standard error | p value |
|---|---|---|---|
| PAPi | |||
| BMI, per 1-SD | −0.23 | 0.01 | <0.001 |
| BMI, per 5 unit change | −0.16 | 0.01 | <0.001 |
|
| |||
| RA:PCWP ratio | |||
| BMI, per 1-SD | 0.25 | 0.01 | <0.001 |
| BMI, per 5 unit change | 0.18 | 0.01 | <0.001 |
|
| |||
| RVSWI | |||
| BMI, per 1-SD | −0.05 | 0.01 | <0.001 |
| BMI, per 5 unit change | −0.03 | 0.01 | <0.001 |
β estimates represent changes in natural log-transformed outcomes per 1-SD increase or 5 unit change in body mass index. Multivariable models were adjusted for age, sex, hypertension, diabetes mellitus, obstructive sleep apnea, chronic lung disease, prevalent myocardial infarction, prevalent heart failure, and pulmonary hypertension. Please see Supplemental Table 7 for effect sizes scaled to per 1-log-unit change.
Abbreviations: BMI, body mass index; PAPi, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; RA, right atrium/atrial; RV, right ventricle/ventricular; RVSWI, right ventricular stroke work index
Figure 1. Hemodynamic indices of RV function across obesity classes.
Least square means represent adjusted mean outcome for each obesity category listed in the table after controlling for all covariates. Multivariable models were adjusted for age, sex, hypertension, diabetes mellitus, OSA, chronic lung disease, prevalent MI, and prevalent HF. BMI categories: Normal = BMI <25, Overweight = BMI >25 and <30, Obesity class 1 = BMI >30 and <35, Obesity class 2+3 = BMI >35.
Abbreviations: PAPi, pulmonary artery pulsatility index; RA:PCWP, – right atrium: pulmonary capillary wedge pressure; RVSWI, right ventricular stroke work index.
Given known associations of BMI and RV function with PH as well as HF, we examined whether PH or HF status may modify the effect of BMI on RV dysfunction in exploratory analyses, shown in Supplemental Tables 2 and 3. Whereas PH did not modify the effect of BMI or obesity on PAPi or RA:PCWP ratio (P for interaction >0.30 for both), we did find that the effect of BMI on RVSWI was more pronounced among those without vs with PH (Pint = 0.02 in multivariable analyses). In stratified analyses, a 1-SD higher BMI was associated with 0.08 lower RVSWI (beta −0.08, SE 0.02, p-value <0.001) among those without PH, compared with 0.04 lower RVSWI among those with PH (β −0.04, SE 0.01, p-value<0.001). We did not find that HF status modified the effect of BMI on RV dysfunction.
In further exploratory analyses, we examined subgroups of individuals including those with HF with reduced ejection fraction (HFrEF) (n=1242) and HF with preserved ejection fraction (HFpEF) (n=820). We found that the association of BMI and obesity class with measures of RV function was similar across these two subgroups (Supplemental Table 4). Interestingly, the association of indices of RV function and outcomes including mortality and HF hospitalization appeared greater among those with HFrEF as compared with HFpEF, acknowledging limited power among the HFpEF subgroup (Supplemental Table5). Furthermore, we also examined PH subgroups including 688 with precapillary, 2488 with postcapillary, and 878 with mixed PH. We found that the association of BMI and obesity class with measures of RV function was similar across PH subgroups (Supplemental Table 6). However, there were some differences in the association of indices of RV function and outcomes including mortality and HF hospitalization among PH subgroups (Supplemental Table 7). Specifically, the association of PAPi and RA:PCWP and mortality was significant in postcapillary and mixed PH, but not in precapillary PH. Conversely, the association between RVSWI and mortality was significant in precapillary PH, but not in postcapillary or mixed PH.
Obesity and RV Function Measures are Associated with Clinical Outcomes
Over 7.3 years of follow-up, we observed 3006 mortality and 2004 heart failure hospitalization events, with Kaplan-Meier curves by obesity class shown in Supplemental Figure 1.
We found that indices of RV function were associated with adverse outcomes in multivariable-adjusted analyses. Specifically, a 1-SD higher PAPi was associated with a 10% lower hazard of all-cause mortality, and a 1-SD higher RA:PCWP ratio was associated with an 8% higher hazard (PAPi: HR 0.90, 95% CI 0.84–0.96; RA:PCWP ratio: HR 1.08 per 1-SD increase, 95% CI 1.02–1.15), with similar associations with HF hospitalization (PAPi: HR 0.79, 95% CI 0.74–0.84; RA:PCWP ratio: HR 1.14 per 1-SD increase, 95% CI 1.07–1.22). Interestingly, while a 1-SD higher RVSWI was associated with a 12% lower hazard of HF hospitalization (HR 0.88, CI 0.83–0.94), it was also associated with a 6% higher hazard of all-cause mortality (HR 1.06, CI 1.02–1.11) (Table 4).
Table 4.
Associations between RV function measures and adverse outcomes
| Predictors | Multivariable Adjusted | |||
|---|---|---|---|---|
| HR | 95% CI | p value | p-intBMI | |
| All-Cause Mortality | ||||
| PAPi | 0.90 | 0.84–0.96 | <0.001 | 0.001 |
| RA:PCWP ratio | 1.08 | 1.02–1.15 | 0. 01 | 0.004 |
| RVSWI | 1.06 | 1.02–1.11 | 0.01 | 0.87 |
|
| ||||
| HF hospitalization | ||||
| PAPi | 0.79 | 0.74–0.84 | <0.001 | 0.24 |
| RA:PCWP ratio | 1.14 | 1.07–1.22 | <0.001 | 0.39 |
| RVSWI | 0.88 | 0.83–0.94 | <0.001 | <0.001 |
Hazard ratios (HR) represent incremental risks per 1-SD increase in the predictors. P-intBMI shows the significance of multiplicative interaction between predictors and body mass index. Multivariable models were adjusted for age, sex, hypertension, diabetes mellitus, obstructive sleep apnea, chronic lung disease, prevalent myocardial infarction, prevalent HF, and pulmonary hypertension.
Abbreviations: HR, hazard ratio; HF, heart failure; PAPi, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; RA, right atrium/atrial; RV, right ventricle/ventricular; RVSWI, right ventricular stroke work index
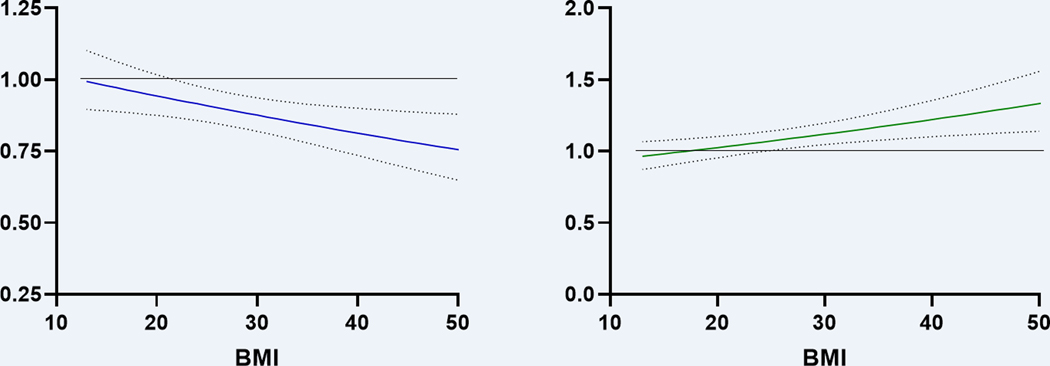
Moreover, we found that BMI modified the effect of RV function on both mortality (P-interaction <=0.005) and heart failure hospitalizations (P-interaction <=0.005) as demonstrated in Figure 2. Specifically, the association of lower PAPi with worse clinical outcomes was more pronounced at higher BMI. For example, at BMI of 20 kg/m2, a 1-SD lower PAPi was associated with a 6% higher hazard of mortality (HR 1.06, 95% CI 0.98–1.1), whereas at a BMI of 40 kg/m2, a 1-SD lower PAPi was associated with a 23% higher hazard of mortality (HR 1.23, 95% CI 1.1–1.4). Similarly, the association of higher RA:PCWP ratio with greater mortality was more pronounced at higher BMI. For example, at BMI of 20 kg/m2, a 1-SD higher RA:PCWP ratio was associated with a 3% higher hazard of mortality (HR 1.03, 95% CI 0.95–1.1), whereas at a BMI of 40 kg/m2, a 1-SD higher RA:PCWP ratio was associated with a 22% higher hazard of mortality (HR 1.22, 95% CI 1.1–1.35).
Figure 2.
BMI modifies the effect of RV function on mortality
HR represents effect on mortality per 1-SD change in log-transformed PAPi or RA:PCWP ratio. Analyses represent PAPi and RA:PCWP as continuous variables with standardized effect sizes expressed to facilitate comparison between different RV function parameters. Multivariable model adjusted for age, sex, hypertension, diabetes, prior myocardial infarction, and prior heart failure. Abbreviations: BMI, body mass index; HR, hazard ratio; PAPi, pulmonary artery pulsatility index; RA:PCWP, right atrium: pulmonary capillary wedge pressure; RV, right ventricle/right ventricular; SD, standard deviation. The horizontal line indicates a hazard ratio of 1 (no effect of RV parameter on outcomes).
DISCUSSION
We investigated the association of obesity and BMI with hemodynamic indices of RV function in a hospital-based sample representing a broad spectrum of cardiopulmonary disease referred for RHC. We found that obesity and higher BMI were consistently associated with hemodynamic measures of RV dysfunction. More importantly, measures of RV dysfunction were associated with an increased risk of mortality and heart failure hospitalization, and obesity significantly heightened the risk RV dysfunction conferred on adverse outcomes. Taken together, these findings underscore the importance of obesity as a risk enhancing factor for RV failure, with important implications on subsequent adverse outcomes. Our findings are of particular interest considering rapidly evolving therapies for obesity, including glucagon-like peptide-1 receptor agonists or dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonists, with potential future implications on the prevention of cardiovascular disease that remain to be elucidated [11, 12].
Prior studies have examined the association of obesity and non-invasive measures of RV structure and function using echocardiography and magnetic resonance imaging (MRI). For example, overweight and obese individuals had lower RV free wall strain and strain rate as well as greater RV free wall thickness and volume as measured by echocardiography compared with individuals with normal BMI in a modest sized case-control study [13]. These findings were corroborated in a larger retrospective study, which found higher BMI was independently associated with abnormal RV free wall longitudinal strain in over one thousand individuals [14]. Similar findings have been observed in the MESA-Right Ventricle study, a cardiac MRI-based study where overweight and higher obesity classes were associated with greater RV mass, larger RV volumes, and slightly lower RV ejection fraction [15]. However, accurate standard echocardiographic assessment is challenged by the complex geometry of the RV, and CMR has practical limitations to widespread use.
Our study adds to previous findings by demonstrating that higher BMI is associated with hemodynamic measures of RV dysfunction including lower PAPi, lower RVSWI, and higher RA:PCWP ratio independent of clinical cardiopulmonary disease. Indeed, when stratified by pre-existing heart failure or PH, we found that higher BMI was still independently associated with lower PAPi and higher RA:PCWP ratio. In this context, utilizing invasive hemodynamic indices of RV function is a strength of our study, allowing for more direct physiologic assessment and leveraging parameters with prognostic implications. Low PAPi has previously been shown to be associated with higher mortality in select samples including post-myocardial infarction, pulmonary arterial hypertension, and advanced heart failure populations [8–10, 16–18]. We recently demonstrated that lower PAPi was associated with increased risk of adverse events including all-cause mortality, MACE, and HF hospitalizations across a much wider spectrum of cardiopulmonary disease [19]. Similarly, RA:PCWP ratio as an index for RV function has also been demonstrated as a marker for in-hospital mortality [20]. Our results of PAPi and RA:PCWP ratio are in keeping with these prior studies but now extend findings across a broader sample of individuals.
Interestingly, our findings related to RVSWI showed a slightly higher hazard of all-cause mortality on one hand, along with a lower hazard of HF hospitalization. Traditionally, RVSWI has been used to assess risk of RV failure in heart transplantation as well as need for mechanical right-sided support following implantation of left ventricular assist devices [21, 22]. However, other populations appear to have more heterogenous clinical outcomes associated with RVSWI. In a retrospective, multi-center registry study examining RV dysfunction in cardiogenic shock from either acute MI or HF, while individuals with greater shock had lower RVSWI consistent with worse RV dysfunction, RVSWI was significantly associated with mortality in the HF cohort but not in the acute MI cohort [23]. Another cross-sectional study found that a higher RVSWI was associated with worse renal function in individuals with HFpEF, which was attributed to the presence of fixed pre-capillary PH in a sub-group leading to increased RV afterload [24]. Given the diverse spectrum of individuals studied in our sample, our seemingly contradictory findings may be due to these same heterogeneous associations of RVSWI with outcomes across different disease states. Further, it is important to recognize that RVSWI is a heterogeneous measure that is highly load dependent, and may be particularly confounded or less useful in obesity, when cardiac output may be high due to other reasons.
Further, we demonstrate that the association of RV dysfunction with adverse outcomes is particularly pronounced at higher BMI. This highlights the importance of obesity as an important effect modifier when considering clinical implications of RV dysfunction. Prior small studies have suggested that adverse RV remodeling may be reversible with weight loss. One study examining echocardiographic parameters of adverse cardiac remodeling in 30 individuals with a starting BMI of >30 suggested that right ventricular dysfunction along with LV diastolic function may be reversible with weight loss over a 3-month period [25]. Another study of 15 females with obesity but no cardiovascular disease found an association with right ventricular dysfunction in obesity and an impaired functional capacity, both which recovered after consistent weight loss [26]. Building on these studies, our results therefore emphasize the potential for greater clinical impact that RV dysfunction has on individuals with higher BMI than previously appreciated.
The mechanisms underlying the relationship between RV dysfunction and obesity remain unclear and are likely multifactorial. This includes a number of possible underlying mechanisms that may lead to “primary” RV dysfunction. For example, excess adipose accumulation has been associated with an expansion in both central and total blood volume, possibly through increased RAAS activation and aldosterone production, thereby also increasing preload which may lead to RV remodeling [27, 28]. In experimental mouse models, excess adiposity has also been shown to increase cardiovascular inflammation and cardiac aging [29, 30]. This effect appears to be modulated through macrophage infiltration, cardiac lipotoxicity from triglyceride accumulation in cardiac myocytes, and increased deposition of extracellular matrix proteins [29–32]. Another possibility is that RV dysfunction may be particularly significant in individuals with obesity as a “secondary” process from left-sided heart failure and pulmonary hypertension. For example, a study by Gorter et al examined 75 individuals with HFpEF and increased epicardial adipose tissue, finding that increased epicardial adipose tissue was associated with a higher BMI as well as higher right-sided filling pressures independent of pulmonary vascular resistance [33]. However, the stratified analyses we performed suggest an association between obesity and RV dysfunction that is present irrespective of pre-existing heart failure or pulmonary hypertension.
Our study has several limitations worth noting. First, this is a hospital-based study of ambulatory and hospitalized patients undergoing right heart catheterization for clinical indications, and both selection bias and confounding by indication may limit generalizability to other samples. Additionally, causal inferences could not be drawn due to the observational nature of the study design and the possibility of residual confounding. Another limitation is the use of code-based diagnoses of HF endpoints which introduce the possibility of misclassification. The potential limitations of right heart catheterization must also be considered as well, particularly in patients with obesity. One study found that obesity confounds estimations of CO and PVR when calculated by the indirect Fick method due to a lower mixed venous oxygen saturation, potentially resulting in inappropriate hemodynamic classification [34]. To avoid this potential confounder, we utilized thermodilution rather than Fick where applicable (eg, with RVSWI). Other limitations related to obesity include intrathoracic pressure fluctuations are exaggerated in individuals with elevated BMI, which may lead to more unreliable estimation of the mean PCWP. Additionally, measurement of requires zeroing a transducer height, which may be more difficult to accurately ascertain in individuals with obesity. Measurement error is also a consideration in the setting of greater respiratory variation, possible effects of arrhythmias, as well as confounded estimations of CO and PVR. Complementary assessment of RV function by echocardiography was not categorically available. Finally, while we accounted for the most common clinical confounders, we acknowledge that residual confounding may remain. For example, pericardial restraint, which can contribute to elevated filling pressures in HFpEF and obesity, may also account for the association of RV dysfunction in obesity found in our study [35].
In sum, individuals with obesity had worse hemodynamic indices of RV function, including lower PAPi and higher RAP:PCWP ratio across a broad hospital-based sample. While RV dysfunction was associated with worse clinical outcomes including mortality and HF hospitalization, this association was especially pronounced among individuals with higher BMI. Our findings highlight the important role of obesity in RV dysfunction and that future studies are needed to further understand the mechanism by which obesity leads to RV dysfunction and adverse outcomes. Further, with rapidly evolving anti-obesity therapies, potential clinical implications with respect to disease prevention are of high interest.
Supplementary Material
Clinical summary.
What is new?
Higher BMI was associated with worse RV function measured using invasive hemodynamic indices
Hemodynamic measures of RV function were associated with an increased risk of mortality and heart failure hospitalization.
Finally, obesity significantly heightened the risk RV dysfunction conferred on adverse outcomes.
What are the clinical implications?
Obesity is associated with worse RV function, and magnifies the effect of RV dysfunction on adverse outcomes including mortality and heart failure hospitalization.
Our findings may carry particular clinical utility in the setting of evolving anti-obesity therapies, with opportunity for disease prevention pertaining to RV dysfunction and heart failure.
Sources of Funding
JEH is supported by NIH grants R01 HL134893, R01 HL140224, R01 HL160003, and K24 HL153669.
Non-standard Abbreviations and Acronyms
- BMI
Body mass index
- CVA
Cerebrovascular accident
- CPT
Current procedural terminology
- HR
Hazard ratio
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- MRI
Magnetic resonance imaging
- MACE
Major adverse cardiac event
- MI
Myocardial infarction
- OSA
Obstructive sleep apnea
- PCWP
Pulmonary capillary wedge pressure
- PA
Pulmonary artery/pulmonary arterial
- PAPi
Pulmonary artery pulsatility index
- PH
Pulmonary hypertension
- SD
Standard deviation
- RA
Right atrium/right atrial
- RHC
Right heart catheterization
- RV
Right ventricle/right ventricular
- RVSWi
Right ventricular stroke work index
- TIA
Transient ischemic attack
- OHT
Orthotopic heart transplantation
- VAD
Ventricular assist device
Footnotes
Disclosures
None
References
- 1.Berglund F, Piña P, Herrera CJ. Right ventricle in heart failure with preserved ejection fraction. Heart 2020;106(23):1798–1804. [DOI] [PubMed] [Google Scholar]
- 2.Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, Crespo-Leiro MG, Guazzi M, Harjola VP, Heymans S, Hill L, Lainscak M, Lam CSP, Lund LH, Lyon AR, Mebazaa A, Mueller C, Paulus WJ, Pieske B, Piepoli MF, Ruschitzka F, Rutten FH, Seferovic PM, Solomon SD, Shah SJ, Triposkiadis F, Wachter R, Tschöpe C, de Boer RA. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20(1):16–37. [DOI] [PubMed] [Google Scholar]
- 3.Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021;143(21):e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–13. [DOI] [PubMed] [Google Scholar]
- 5.Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, Barr RG, Kizer J, Lima JAC, Bluemke DA, Kawut SM. Obesity and right ventricular structure and function: the MESA-Right Ventricle Study. Chest 2012;141(2):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CY, O’Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol 2006;47(3):611–6. [DOI] [PubMed] [Google Scholar]
- 7.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 8.Kochav SM, Flores RJ, Truby LK, Topkara VK. Prognostic Impact of Pulmonary Artery Pulsatility Index (PAPi) in Patients With Advanced Heart Failure: Insights From the ESCAPE Trial. J Card Fail 2018;24(7):453–459. [DOI] [PubMed] [Google Scholar]
- 9.Cesini S, Bhagra S, Pettit SJ. Low Pulmonary Artery Pulsatility Index Is Associated With Adverse Outcomes in Ambulatory Patients With Advanced Heart Failure. J Card Fail 2020;26(4):352–359. [DOI] [PubMed] [Google Scholar]
- 10.Kuwayama T, Morimoto R, Oishi H, Kato H, Kimura Y, Kazama S, Shibata N, Arao Y, Yamaguchi S, Hiraiwa H, Kondo T, Furusawa K, Okumura T, Murohara T. Efficacy of Pulmonary Artery Pulsatility Index as a Measure of Right Ventricular Dysfunction in Stable Phase of Dilated Cardiomyopathy. Circ J 2020;84(9):1536–1543. [DOI] [PubMed] [Google Scholar]
- 11.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021;384(11):989–1002. [DOI] [PubMed] [Google Scholar]
- 12.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A; SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med 2022;387(3):205–216. [DOI] [PubMed] [Google Scholar]
- 13.Wong CY, O’Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol 2006;47(3):611–6. [DOI] [PubMed] [Google Scholar]
- 14.Patel H, Bhutani S, Posimreddy S, Shah P, Rampal U, Gandhi A, Vasudev R, Pullatt R, Virk H, Shamoon F, Bikkina M, Goldfarb I. The obesity paradox: the protective effect of obesity on right ventricular function using echocardiographic strain imaging in patients with pulmonary hypertension. Minerva Cardioangiol 2018;66(5):523–527. [DOI] [PubMed] [Google Scholar]
- 15.Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, Barr RG, Kizer J, Lima JAC, Bluemke DA, Kawut SM. Obesity and right ventricular structure and function: the MESA-Right Ventricle Study. Chest 2012;141(2):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo KB, Mezue K, Ram P, Goyal A, Shah M, Krishnamoorthy P, Gupta S, Pressman GS, Rangaswami J. Echocardiographic and Hemodynamic Parameters Associated with Diminishing Renal Filtration among Patients with Heart Failure with Preserved Ejection Fraction. Cardiorenal Med 2019;9(2):83–91. [DOI] [PubMed] [Google Scholar]
- 17.Rong LQ, Rahouma M, Neuburger PJ, Arguelles G, Emerson J, Mauer E, Tam C, Shore-Lesserson L, Pryor KO, Gaudino M. Use of Pulmonary Artery Pulsatility Index in Cardiac Surgery. J Cardiothorac Vasc Anesth 2020;34(5):1220–1225. [DOI] [PubMed] [Google Scholar]
- 18.Guven G, Brankovic M, Constantinescu AA, Brugts JJ, Hesselink DA, Akin S, Struijs A, Birim O, Ince C, Manintveld OC, Caliskan K. Preoperative right heart hemodynamics predict postoperative acute kidney injury after heart transplantation. Intensive Care Med 2018;44(5):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zern EK, Wang D, Rambarat P, Bernard S, Paniagua SM, Liu EE, McNeill J, Wang JK, Andrews CT, Pomerantsev EV, Picard MH, Ho JE. Association of Pulmonary Artery Pulsatility Index With Adverse Cardiovascular Events Across a Hospital-Based Sample. Circ Heart Fail 2022;15(2):e009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkley DM Jr, Ho KKL, Drazner MH, Kociol RD. The prognostic value of the relationship between right atrial and pulmonary capillary wedge pressure in diverse cardiovascular conditions. Am Heart J 2018;199:31–36. [DOI] [PubMed] [Google Scholar]
- 21.Kato TS, Stevens GR, Jiang J, Schulze PC, Gukasyan N, Lippel M, Levin A, Homma S, Mancini D, Farr M. Risk stratification of ambulatory patients with advanced heart failure undergoing evaluation for heart transplantation. J Heart Lung Transplant 2013;32:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, Hsu AP, Yeager ML, Buda T, Hoercher KJ, Howard MW, Takagaki M, Doi K, Fukamachi K. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation 2002;106(12 Suppl 1):I198–202. [PubMed] [Google Scholar]
- 23.Jain P, Thayer KL, Abraham J, Everett KD, Pahuja M, Whitehead EH, Schwartz BP, Lala A, Sinha SS, Kanwar MK, Garan AR, Hernandez-Monfort JA, Mahr C, Vorovich E, Wencker D, McCabe JM, Jones T, Goud M, Baca P, Harwani N, Burkhoff D, Kapur NK. Right Ventricular Dysfunction Is Common and Identifies Patients at Risk of Dying in Cardiogenic Shock. J Card Fail 2021;27(10):1061–1072. [DOI] [PubMed] [Google Scholar]
- 24.Kanjanahattakij N, Sirinvaravong N, Aguilar F, Agrawal A, Krishnamoorthy P, Gupta S. High Right Ventricular Stroke Work Index Is Associated with Worse Kidney Function in Patients with Heart Failure with Preserved Ejection Fraction. Cardiorenal Med 2018;8(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuksel IO, Akar Bayram N, Koklu E, Ureyen CM, Kucukseymen S, Arslan S, Bozkurt E. Assessment of Impact of Weight Loss on Left and Right Ventricular Functions and Value of Tissue Doppler Echocardiography in Obese Patients. Echocardiography 2016;33(6):854–61. [DOI] [PubMed] [Google Scholar]
- 26.Maniscalco M, Arciello A, Zedda A, Faraone S, Verde R, Giardiello C, Cacciapuoti F, Cacciapuoti F, Sofia M. Right ventricular performance in severe obesity. Effect of weight loss. Eur J Clin Invest 2007. Apr;37(4):270–5. [DOI] [PubMed] [Google Scholar]
- 27.Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev 2012;13(2):136–49. [DOI] [PubMed] [Google Scholar]
- 28.Frigolet ME, Torres N, Tovar AR. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J Nutr Biochem 2013;24(12) [DOI] [PubMed] [Google Scholar]
- 29.Rios FJ, Touyz RM, Montezano AC. Isolation and Differentiation of Murine Macrophages. Methods Mol Biol 2017;1527:297–309. [DOI] [PubMed] [Google Scholar]
- 30.Sawaki D, Czibik G, Pini M, Ternacle J, Suffee N, Mercedes R, Marcelin G, Surenaud M, Marcos E, Gual P, Clément K, Hue S, Adnot S, Hatem SN, Tsuchimochi I, Yoshimitsu T, Hénégar C, Derumeaux G. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 2018;138(8):809–822. [DOI] [PubMed] [Google Scholar]
- 31.Drosatos K, Schulze PC. Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 2013;10(2):109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008;52(22):1793–9. [DOI] [PubMed] [Google Scholar]
- 33.Gorter TM, van Woerden G, Rienstra M, Dickinson MG, Hummel YM, Voors AA, Hoendermis ES, van Veldhuisen DJ. Epicardial Adipose Tissue and Invasive Hemodynamics in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2020;8(8):667–676. [DOI] [PubMed] [Google Scholar]
- 34.Girdharry NR, Bentley RF, Valle FH, Karvasarski E, Osman S, Gurtu V, Kolker S, Mak S. Body Habitus Considerations During Right Heart Catheterization. CJC Open 2021;3(9):1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlaug BA, Reddy YNV. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail. 2019;7(7):574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.