Abstract
In Drosophila, a cascade of maternal, gap, pair-rule and segment polarity genes subdivides the antero/posterior axis of the embryo into repeating segmental stripes. This review summarizes what happens next, i.e. how an intrasegmental pattern is generated and controls the differentiation of specific cell types in the epidermis. Within each segment, cells secreting the signalling molecules Wingless (the homologue of vertebrate Wnt-1) and Hedgehog are found in narrow stripes on both sides of the parasegmental boundary. The Wingless and Hedgehog organizing activities help to establish two more stripes per segment that localize ligands for the Epidermal Growth Factor and the Notch signalling pathways, respectively. These four signals then act at short range and in concert to control epidermal differentiation at the single cell level across the segment. This example from Drosophila provides a paradigm for how organizers generate precise patterns, and ultimately different cell types, in a naïve field of cells.
Introduction
Nüsslein-Volhard and Wieschaus (1980) used the cuticle of the Drosophila embryo in their historic screen to identify patterning mutants because they realized that the exoskeleton pattern of this insect provides a fantastic readout of the underlying genetic program. Twenty years or so later, we are starting to understand how the genes identified in this screen interact to generate this pattern. For example, it is possible to give a sequence of patterning events that take place along the antero/posterior (A/P) axis, starting from egg deposition and continuing down to the specification of epidermal cell types at the end of embryogenesis.
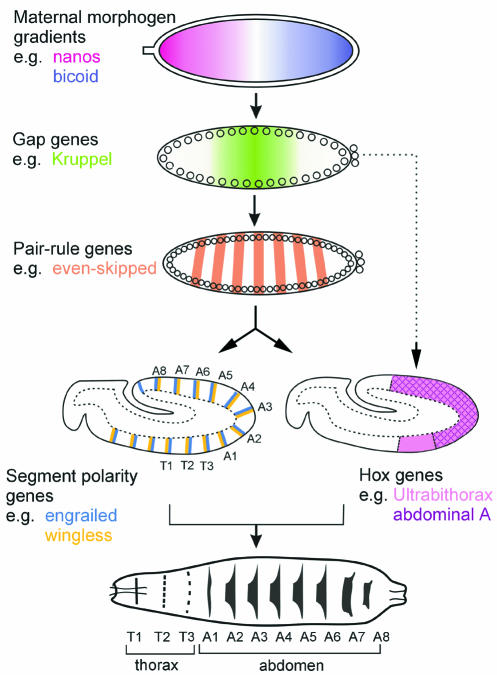
The first steps of embryonic segmentation have been known for some time and involve the progressive subdivision of the A/P axis by maternal, gap, pair-rule and segment polarity genes (reviewed by Ingham and Martinez Arias, 1992; St Johnston and Nüsslein-Volhard, 1992) (Figure 1). Most of these events occur before cellularization of the embryo, in a syncitium where gradients of transcription factors form and regulate downstream genes (reviewed by Pankratz and Jäckle, 1993; Lawrence and Struhl, 1996). Cellularization is completed around the time at which the segment polarity genes divide the A/P axis into 14–15 stripes, prefiguring the future segments of the embryo (Figure 1). The key segment polarity genes are wingless (wg) and hedgehog (hh), the latter being secreted by the cells expressing engrailed (en). It turns out that the other segment polarity genes whose functions have been elucidated are positive or negative regulators of either the Wg or Hh signalling pathways (reviewed by Perrimon, 1994). Pair-rule genes initiate the transcription of wg and en/hh in adjacent domains, and these two domains subsequently regulate each other to stabilize their expression in two adjacent but non-overlapping stripes (reviewed by DiNardo et al., 1994) (Figure 1).
Fig. 1. Patterning along the A/P axis of the Drosophila embryo. A cascade of maternal and zygotic genes is activated in the syncitial embryo to subdivide the ectoderm into smaller domains (see Flybase, http://fly.ebi.ac.uk:7081/, for nomenclature and information about Drosophila genes). The embryo cellularizes and undergoes gastrulation after activation of the pair-rule genes. The segment polarity genes and the Hox genes are activated by the pair-rule genes but a subset of gap genes also influences directly the Hox genes. Both segment polarity and Hox genes are thought to act in concert to control the differentiation of each segment of the future larva.
This review focuses on how Wg and Hh organize the pattern within each segment and how the intrasegmental pattern then allocates distinct identities to single cells. In the ventral ectoderm, after the Wg and En/Hh stripes have stabilized, new stripes form that localize ligands from two other signalling pathways: Serrate (Ser), which activates the Notch receptor, and Spitz (Spi), which activates the Epidermal Growth Factor Receptor (EGFR). The interplay of the four signalling pathways activates downstream genes in stripes as small as single-cell width, and these genes in turn control specific events of epidermal differentiation.
More stripes: generation of an intrasegmental pattern
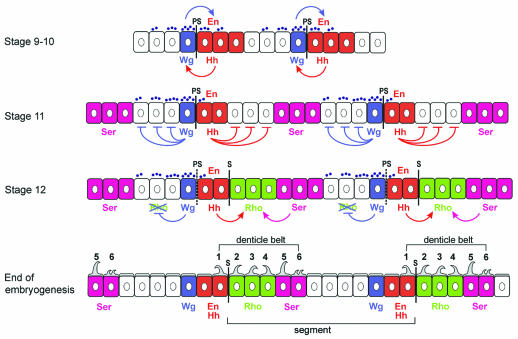
In the ventral ectoderm, the Wg and Hh domains form a bipartite organizer that straddles the parasegment (PS) boundary and patterns cells on both sides (Figure 2). The PS boundary is visible in stage 10–11 embryos as a transient groove at the interface between Wg- and En/Hh-expressing cells. A bit later, at stage 12, a segmental groove (S) forms at the posterior edge of the En/Hh stripe and prefigures the deep segmental grooves evident in the fly larva. The interval between two PS boundaries defines a parasegment, whereas the interval between two S boundaries defines a segment (Figure 2).
Fig. 2. Generation of an intrasegmental pattern in the Drosophila embryo. This sequence is accurate for the ventral side of the abdomen. PS designates the parasegmental boundaries and S the segmental boundaries. The anterior of the embryo is to the left, the posterior to the right. The apical side of the cells is up, the basal side is down. Small violet dots represent the extracellular gradient of Wg protein. At stage 9–10, Wg and En/Hh expression is interdependent, and the Wg gradient symmetrical. At stage 11, Wg and En/Hh expression become independent, and the Wg gradient becomes asymmetric. At the same time, the Ser domain is delimited by the repressive action of both Wg and Hh. This generates one Ser stripe, two to three cells wide, per parasegment. At stage 12, Hh activates Rho expression in two rows of cells posterior to the En/Hh domain, and Ser activates Rho in one row of cells anterior to its domain. This results in a stripe of Rho expression precisely three cells wide. Anterior to the En/Hh domain, Wg signalling represses Rho expression. At the end of stage 12, the PS boundaries are no longer visible, and the segment grooves have formed immediately posterior to the En cells. At the end of embryogenesis, the posterior row of En cells and the Rho and Ser cells secrete denticles which make up the ventral denticle belts of the larval abdomen. Wg signalling specifies smooth cuticle in asymmetric fashion, three to four cell diameters in the anterior direction, but only extending through the first row of En cells to the posterior. The Ser-expressing cells secrete rows 5 and 6 of the denticle belts
Early in embryogenesis, at stage 9–10, the expression of Wg and Hh is interdependent (Figure 2). The Wg protein maintains En expression in adjoining cells, and En-expressing cells secrete Hh, which in turn maintains Wg expression in neighbouring cells (DiNardo et al., 1988; Martinez Arias et al., 1988; Hidalgo and Ingham, 1990). At the end of stage 10, En expression becomes independent of Wg and this is when the roles of Wg and Hh in intrasegmental patterning start. Around stage 11, the segment polarity genes and the Homeobox (Hox) genes (see Figure 1) are responsible for the activation of a single stripe of Ser expression within each abdominal parasegment (Wiellette and McGinnis, 1999) (Figure 2). Overexpression experiments show that Hh and Wg are both repressors of Ser expression (Alexandre et al., 1999). Consistent with this, the Ser stripe is expanded in hh or wg mutants (Alexandre et al., 1999; Gritzan et al., 1999; Wiellette and McGinnis, 1999). Hh, secreted by the En-expressing cells, represses Ser in the posterior direction, two to three cell diameters away, and determines the anterior border of the Ser domain (Figure 2). In the anterior direction, Wg also represses Ser two to three cells away, thus delimiting the posterior border of the Ser stripe. This shows that although the initiation of Ser expression in broad domains is under the control of the Hox genes, the polarity genes wg and hh refine the boundaries of the Ser domain by inactivating its expression in a subset of cells in each parasegment.
At this point, the stage is set for the appearance of another stripe, which expresses the rhomboid gene (rho; also called veinlet). rho codes for a transmembrane protein required for the activation of EGFR ligand Spi (Golembo et al., 1996). As for Ser, the Hox genes are responsible for activating rho in the ventral ectoderm (Szuts et al., 1997). The boundaries of its domain of expression are refined at stage 12 by Ser, Hh and Wg (Alexandre et al., 1999; Gritzan et al., 1999; Sanson et al., 1999) (Figure 2). Ser, through activation of the Notch receptor, stimulates Rho expression in the cells immediately adjacent to its domain. In addition, Hh stimulates Rho expression up to two cell diameters away in both directions. This leads to the activation of a stripe of rho precisely three cells wide posterior to the En/Hh domain (Alexandre et al., 1999). Anterior to the En/Hh domain, rho activation is counteracted by the repressive action of Wg signalling. Together, the activating and repressing activities result in the asymmetric expression of Rho on only one side of the En stripe (OFF anterior and ON posterior), which is important for the polarity of each segment. The key factor in breaking the symmetry is Wg: in wg mutants, Rho stripes are found on both sides of the En domain (Gritzan et al., 1999; Sanson et al., 1999).
Range of each ligand localized by the intrasegmental pattern
At stage 12, the ventral domain of each embryonic segment is thus subdivided into five stripes (Figure 2). Four of the five intrasegmental stripes act as sources of a ligand from a different signalling pathway: Spi (EGFR ligand), Ser (Notch ligand), Wg or Hh. The range of these four ligands is key in positioning the zones of activation of downstream genes. Determining the range of a signalling ligand in a field of cells is a complex issue, in part because of the variety of approaches required for its study. The most direct way of assessing how far a ligand spreads from its source is to examine the distribution of the protein by immunostaining the whole tissue. However, this assay is dependent on the sensitivity of the detection method, and on the accessibility of the epitope, which may vary in different sub-cellular compartments. Another approach is to examine the maximum distance from the source at which a target gene is activated or repressed. One drawback of this is that some target genes may be sensitive to only high doses of ligand, or be unresponsive in some locations due to the input of other signalling pathways.
The range of Wg in the ventral epidermis of the embryo has been studied extensively (reviewed by Howes and Bray, 2000). Strikingly, the shape of the Wg gradient undergoes a transition at mid-embryogenesis (van den Heuvel et al., 1989; Gonzalez et al., 1991; Sanson et al., 1999; Dubois et al., 2001). At stage 9–10, when Wg is required to maintain En expression, the gradient of Wg protein is symmetric, as detected by immunostaining (Figure 2). At stage 11, the Wg protein seems to recede from the En domain (Figure 2). This change of distribution occurs when En expression becomes independent of Wg (Bejsovec and Martinez Arias, 1991; Heemskerk et al., 1991). The asymmetry in Wg distribution is matched by an asymmetry in the activity of Wg signalling; for example, Wg represses rho anterior but not posterior to its source. Unexpectedly, Hh signalling is required for the asymmetric distribution of the Wg ligand (Sanson et al., 1999). In the absence of Hh signalling, and under conditions in which a localized source of Wg is maintained, Wg distribution on both sides of this ectopic source becomes symmetrical (Sanson et al., 1999). Recent studies have shown that the asymmetric distribution of Wg is in part due to the accelerated degradation of the Wg ligand in the En and Rho domains (Dubois et al., 2001). Under normal immunostaining conditions, the Wg protein is mostly undetectable in these domains after stage 11. By fusing the Wg protein to a horseradish peroxidase (HRP) tag, which can be detected intact in the lysosomes, the fate of the Wg-HRP ligand could be traced to the lysosomes in the cells posterior to its source (Dubois et al., 2001). This suggests that some Wg can reach the Rho cells, but is immediately sent to the lysosomes for degradation.
Unlike Wg, Spi and Ser are likely to be distributed symmetrically and they act at a short distance from their sources. The EGFR ligand Spi is expressed uniformly in an inactive form. Rho activates Spi by processing it (Lee et al., 2001). Thus, the Rho stripe is a source of active Spi ligand, and based on its effect on the denticle fate (O’Keefe et al., 1997; Szuts et al., 1997), is likely to have a range of no more than two cell diameters on both sides of the Rho domain. Ser is a transmembrane ligand and is assumed to activate the Notch pathway only in adjacent cells (Fleming et al., 1990).
The situation is more complex with Hh. Judging from its action on its target genes, Hh can signal up to three cell diameters posterior to the En/Hh domain during stage 11. For example, Hh represses Ser expression over three cell diameters in the posterior direction (Alexandre et al., 1999; Gritzan et al., 1999) (Figure 2). Interestingly, Hh signalling is asymmetric in the sense that some of its target genes are expressed posterior, but not anterior, to the En/Hh domain (e.g. rho and stripe, see Gritzan et al., 1999; Hatini and DiNardo, 2001) (Figure 2). This could be explained by a repression of the target genes by Wg signalling anterior to the En/Hh domain, which indeed seems to be the case for rho and stripe (Sanson et al., 1999; Piepenburg et al., 2000; Hatini and DiNardo, 2001). Alternatively, this could result from an asymmetric distribution of the Hh protein after stage 11 but, so far, there is no evidence that this is the case.
From intrasegmental patterning to epidermis differentiation
We now have a relatively clear picture of how an intrasegmental pattern develops and how it establishes a precise distribution of signalling molecules within the ventral ectoderm of the Drosophila embryo. The next problem was to relate this pattern to the differentiation of the different cell types that ultimately make up the epidermis. Recent work has demonstrated how this happens for two events of epidermal differentiation: the specification of the tendon cells and the specification of cells that secrete denticles.
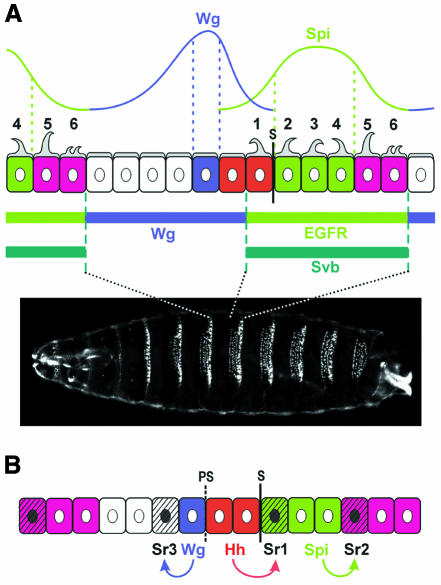
Differentiation of epidermal denticles. At the end of Drosophila embryogenesis, two types of cuticle are made: some cells secrete a smooth cuticle, whereas others secrete hair or denticles of various sizes and shapes (reviewed by Martinez Arias, 1993). On the ventral side of the abdominal epidermis, ‘denticle belts’, which consist of about six rows of denticle-making cells, are formed (Figure 2, end of embryogenesis, and Figure 3A). Each abdominal belt is separated from the next by about six rows of cells secreting a smooth cuticle. The domain of expression of genes in the intrasegmental pattern has been mapped precisely onto the cuticle using β-galactosidase reporter transgenes. Row 1 of each denticle belt is secreted by the second row of En-expressing cells, whereas the anterior En cell secretes a smooth cuticle (Dougan and DiNardo, 1992). Rows 2, 3 and 4 of the denticle belts are secreted by the Rho-expressing cells (Alexandre et al., 1999; Sanson et al., 1999). Wg expression coincides with a single row of smooth cuticle secreting cells, just anterior to the En domain (O’Keefe et al., 1997; Sanson et al., 1999). The Ser-expressing cells secrete rows 5 and 6 of each denticle belt (J.-P. Vincent, personal communication).
Fig. 3. Control of epidermal differentiation by the intrasegmental pattern. (A) Wg and Spi ligands are distributed across two domains of roughly equal size. Their gradients overlap within the En-expressing cells, where a mechanism of competition leads to activation of Wg signalling in the anterior En cell, and to activation of EGFR signalling in the posterior En cell. Svb is expressed in the cells that will secrete denticles on the ventral side of the future larva, and its domain coincides with the domain of EGFR activation. Cells under the influence of Wg signalling repress Svb expression and secrete a smooth cuticle. The photograph shows a larval cuticle visualized by dark field microscopy. (B) The ligands Wg, Hh and Spi act at short-distance and anisotropically to stimulate the expression of the protein Stripe in three rows of cells in the ventral epidermis.
What makes the distinction between cells that secrete denticles and cells that secrete a smooth cuticle? In the ventral epidermis, the cells that later make the denticle belts at the end of embryogenesis express the gene shaven-baby (svb) (Payre et al., 1999; Figure 3A). Payre et al. (1999) showed that svb is necessary and sufficient to direct denticle formation cell-autonomously in the embryo. Consistent with this, orthologues of svb promote hair formation in other species such as mouse (Dai et al., 1998). It has been known for some time that Wg specifies smooth cuticle versus denticle fate, but the details of this induction have remained unclear (Bejsovec and Martinez Arias, 1991; Noordermeer et al., 1992; Lawrence et al., 1996). Payre et al. (1999) demonstrate that Wg specifies smooth cuticle by repressing the expression of Svb. Svb expression becomes ubiquitous in wg mutants, and is repressed when Wg is produced ectopically. Thus, the asymmetric distribution of Wg causes asymmetric repression of Svb on either side of the Wg source, resulting in one row of cells making smooth cuticle posterior to the Wg cells, and three to four cells making smooth cuticle anterior to them (Figure 3A).The expression of Svb seems at the same time to be activated by the EGFR signalling pathway (Payre et al., 1999). The ligands Wg and Spi are distributed in two domains of roughly equivalent size, one in which Wg signalling is activated and a second in which the EGFR pathway is activated (Figure 3A). The zones of influence of the two signalling pathways overlap briefly in the En stripe, where a mechanism of competition resolves them into two non-overlapping domains (Dougan and DiNardo, 1992; O’Keefe et al., 1997; Szuts et al., 1997). The first row of En cells, where the Wg pathway ‘wins’, makes smooth cuticle, whereas the second row, where the EGFR pathway prevails, makes denticles (row 1 of the abdominal denticle belt). As EGFR is active in Rho and Ser cells, the domain of EGFR activation coincides with the domain of Svb expression (Figure 3A). In addition, the EGFR pathway is able to stimulate Svb expression when ubiquitously activated (Payre et al., 1999). There is no available evidence, however, that the EGFR pathway is actually required for Svb expression.
Differentiation of tendon cells. Tendon cells direct the attachment of the muscles to the epidermis. In the ventral part of the ectoderm at mid-embryogenesis, three regularly spaced rows of cells express Stripe (Sr), a determinant of the tendon cell fate (Frommer et al., 1996). The position of these rows is invariable and each one is induced by short-range signalling from one of the signalling pathways active in intrasegmental patterning (Hatini and DiNardo, 2001). This was shown using thermosensitive mutations of the hh, wg and EGFR genes. The first row of Stripe expression (Sr1) is induced by Hh signalling in adjacent posterior cells (Figure 3B), whereas the induction to the anterior is blocked by Wg signalling. Sr2 is induced by EGFR signalling immediately posterior to the Rho domain, presumably through the ligand Spi. Sr3, on the other hand, requires Wg induction and forms adjacent to the Wg source (Figure 3B). It can be one or two (not shown) cells wide. Thus, the induction of each stripe of tendon cells requires a different signalling pathway, each acting at short distance and anisotropically. Moreover, Sr activation seems to occur only at high levels of signalling, explaining why a single row of Sr cells is induced in most cases (Hatini and DiNardo, 2001). However, it remains a mystery why Sr2 and Sr3 induction occurs only anterior to the Wg source, or only posterior to the Spi source, respectively.
Transcriptional control by intrasegmental signalling
Recent work has identified the response elements that trigger the expression of the gene sr in the dorsal and lateral epidermis (Piepenburg et al., 2000). In these regions, only Sr1 is found, Sr2 and Sr3 being restricted to the ventral epidermis (Frommer et al., 1996; see Figure 3B). Piepenburg et al. (2000) found that a small fragment of the sr promoter can drive expression in the Sr1 pattern. Moreover, this small element responds to Hh and Wg signalling in the same way as Sr1: Hh activates its expression posterior to the En domain, whereas Wg represses its expression anterior to the En domain. The reporter element used in these studies contains two binding sites for the downstream effector of Hh signalling, Cubitus interruptus (Ci) (homologue of Gli proteins in vertebrates), and two binding sites for the downstream effector of Wg signalling, Pangolin (Pan) (homologue of TCF factors). Deletion of one Pan binding site is sufficient to trigger ectopic expression of the element anterior to the En domain, indicating that this site is required for Wg repression. Conversely, the deletion of the two Ci sites completely abolishes expression from the element. This work strongly suggests that Hh signalling acts symmetrically and directly to stimulate expression of Sr on both side of the En domain. Wg breaks this symmetry by repressing the expression of Sr anterior to the En cells only, which is consistent with the finding that Wg does not signal far in the posterior direction after stage 11 (Sanson et al., 1999; Dubois et al., 2001).
In light of these results, a direct role of downstream effectors of the Wg pathway in repressing svb transcription is possible. Indeed, Payre et al. (1999) implied that the expression of Svb is controlled directly at the transcriptional level by competition between the Wg and EGFR signalling pathways. Direct evidence for this, however, is still lacking. Moreover, stimulation of Svb expression by EGFR signalling might be indirect. Recent evidence indicates that the fast degradation of the Wg ligand posterior to the En cells is under the control of EGFR signalling (Dubois et al., 2001). Thus, the ectopic activation of Svb observed after ectopic activation of EGFR could be due to an increase in the degradation of the Wg ligand, indirectly blocking Wg-mediated repression. It is conceivable, however, that EGFR signalling acts at several levels, stimulating svb transcription as well as accelerating Wg degradation. Further experiments are needed to distinguish between these possibilities.
Conclusions and perspectives
In the A/P axis of Drosophila, one can now describe a developmental sequence of events from the beginning of axis formation up to the differentiation of single cells at the end of embryogenesis. The sequence is deceptively straightforward: a sheet of cells is subdivided into increasingly smaller stripes in which signals from a handful of pathways are differentially activated. In spite of the simplicity of this mechanism, it has taken years to understand, in part because signals such as Wg perform so many different functions and act at different ranges to regulate their targets. This example provides a paradigm for the study of more complex developing structures such as vertebrate organs or tissues, which generally employ many more signalling pathways.
This example from Drosophila development also points out new fundamental questions that need investigation. For example, there is the problem of ligand trafficking within a developing tissue. How are signalling ligands secreted, processed, transported and recycled? At another level, it will be important to decipher how these events actually modulate patterning. A second problem relates to how the input from various signalling cascades is integrated at the level of transcription within a given cell. The example of Sr1 regulation suggests a combination of modular enhancers working independently (Arnone and Davidson, 1997). A third question is how the activation of genes such as sr and svb leads to the morphogenesis of a tendon or a hair-secreting cell. Both svb and sr code for zinc-finger transcription factors, and thus must regulate a set of target genes able to modify cell shape in a autonomous manner. Finally, each row of denticles in the ventral abdomen has its own polarity and size. It has been hypothesized that the juxtaposition of the different signalling pathways could control the size and polarity of denticles (Alexandre et al., 1999). How the signalling pathways influence these aspects of cell morphogenesis is presently unknown. Most of these questions are quite basic and also valid for other developing structures in other organisms. The small fruitfly provides a wonderful model in which to investigate them.

Bénédicte Sanson
Acknowledgments
Acknowledgements
I would like to thank Daniel St Johnston for contributing to Figure 1 and for readings of the manuscript, Alicia Hidalgo and Alphonso Martinez-Arias for their comments, and the editor and anonymous referees for their input. I also thank Dee Hughes, Ian Bolton and the Anatomy Visual Media Group for help with the artwork. The author is supported by a Career Development Award from the Wellcome Trust (054525/Z/98).
References
- Alexandre C., Lecourtois, M. and Vincent, J. (1999) Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development, 126, 5689–5698. [DOI] [PubMed] [Google Scholar]
- Arnone M.I. and Davidson, E.H. (1997) The hardwiring of development: organization and function of genomic regulatory systems. Development, 124, 1851–1864. [DOI] [PubMed] [Google Scholar]
- Bejsovec A. and Martinez Arias, A. (1991) Roles of wingless in patterning the larval epidermis of Drosophila. Development, 113, 471–485. [DOI] [PubMed] [Google Scholar]
- Dai X., Schonbaum, C., Degenstein, L., Bai, W., Mahowald, A. and Fuchs, E. (1998) The ovo gene required for cuticle formation and oogenesis in flies is involved in hair formation and spermatogenesis in mice. Genes Dev., 12, 3452–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Sher, E., Heemskerk-Jongens, J., Kassis, J.A. and O’Farrell, P.H. (1988) Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature, 332, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Heemskerk, J., Dougan, S. and O’Farrell, P.H. (1994) The making of a maggot: patterning the Drosophila embryonic epidermis. Curr. Opin. Genet. Dev., 4, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan S. and DiNardo, S. (1992) Drosophila wingless generates cell type diversity among engrailed expressing cells. Nature, 360, 347–350. [DOI] [PubMed] [Google Scholar]
- Dubois L., Lecourtois, M., Alexandre, C., Hirst, E. and Vincent, J.P. (2001) Regulated endocytic routing modulates wingless signalling in Drosophila embryos. Cell, 105, 613–624. [DOI] [PubMed] [Google Scholar]
- Fleming R.J., Scottgale, T.N., Diederich, R.J. and Artavanis-Tsakonas, S. (1990) The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev., 4, 2188–2201. [DOI] [PubMed] [Google Scholar]
- Frommer G., Vorbrüggen, G., Pasca, G., Jäckle, H. and Volk, T. (1996) Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J., 15, 1642–1649. [PMC free article] [PubMed] [Google Scholar]
- Golembo M., Raz, E. and Shilo, B.Z. (1996) The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development, 122, 3363–3370. [DOI] [PubMed] [Google Scholar]
- Gonzalez F., Swales, L.S., Bejsovec, A., Skaer, H. and Martinez Arias, A. (1991) Secretion and movement of wingless protein in the epidermis of the Drosophila embryo. Mech. Dev., 35, 43–54. [DOI] [PubMed] [Google Scholar]
- Gritzan U., Hatini, V. and DiNardo, S. (1999) Mutual antagonism between signals secreted by adjacent Wingless and Engrailed cells leads to specification of complementary regions of the Drosophila parasegment. Development, 126, 4107–4115. [DOI] [PubMed] [Google Scholar]
- Hatini V. and DiNardo, S. (2001) Distinct signals generate repeating striped pattern in the embryonic parasegment. Mol. Cell, 7, 151–160. [DOI] [PubMed] [Google Scholar]
- Heemskerk J., DiNardo, S., Kostriken, R. and O’Farrell, P.H. (1991) Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature, 352, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A. and Ingham, P. (1990) Cell patterning in the Drosophila segment: spatial regulation of the segment polarity gene patched. Development, 110, 291–301. [DOI] [PubMed] [Google Scholar]
- Howes R. and Bray, S. (2000) Wingless on the move. Curr. Biol., 10, R222–R226. [DOI] [PubMed] [Google Scholar]
- Ingham P.W. and Martinez Arias, A. (1992) Boundaries and fields in early embryos. Cell, 68, 221–235. [DOI] [PubMed] [Google Scholar]
- Lawrence P.A. and Struhl, G. (1996) Morphogens, compartments, and pattern: lessons from Drosophila? Cell, 85, 951–961. [DOI] [PubMed] [Google Scholar]
- Lawrence P.A., Sanson, B. and Vincent, J.P. (1996) Compartments, wingless and engrailed: patterning the ventral epidermis of Drosophila embryos. Development, 122, 4095–4103. [DOI] [PubMed] [Google Scholar]
- Lee J.R., Urban, S., Garvey, C.F. and Freeman, M. (2001) Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell, 107, 161–171. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A. (1993) Development and patterning of the larval epidermis of Drosophila. In Bate, M. and Martinez Arias, A. (eds), The Development of Drosophila Melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 517–608.
- Martinez Arias A., Baker, N.E. and Ingham, P.W. (1988) Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development, 103, 157–170. [DOI] [PubMed] [Google Scholar]
- Noordermeer J., Johnston, P., Rijsewijk, F., Nusse, R. and Lawrence, P.A. (1992) The consequences of ubiquitous expression of the wingless gene in the Drosophila embryo. Development, 116, 711–719. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. and Wieschaus, E. (1980) Mutations affecting segment number and polarity in Drosophila. Nature, 287, 795–801. [DOI] [PubMed] [Google Scholar]
- O’Keefe L., Dougan, S.T., Gabay, L., Raz, E., Shilo, B.Z. and DiNardo, S. (1997) Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development, 124, 4837–4845. [DOI] [PubMed] [Google Scholar]
- Pankratz M.J. and Jäckle, H. (1993) Blastoderm segmentation.In Bate, M. and Martinez Arias, A. (eds), The Development of Drosophila Melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 467–516.
- Payre F., Vincent, A. and Carreno, S. (1999) ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature, 400, 271–275. [DOI] [PubMed] [Google Scholar]
- Perrimon N. (1994) The genetic basis of patterned baldness in Drosophila. Cell, 76, 781–784. [DOI] [PubMed] [Google Scholar]
- Piepenburg O., Vorbrüggen, G. and Jäckle, H. (2000) Drosophila segment borders result from unilateral repression of Hedgehog activity by wingless signalling. Mol. Cell, 6, 203–209. [PubMed] [Google Scholar]
- Sanson B., Alexandre, C., Fascetti, N. and Vincent, J.P. (1999) Engrailed and hedgehog make the range of Wingless asymmetric in Drosophila embryos. Cell, 98, 207–216. [DOI] [PubMed] [Google Scholar]
- St Johnston D. and Nüsslein-Volhard, C. (1992) The origin of pattern and polarity in the Drosophila embryo. Cell, 68, 201–219. [DOI] [PubMed] [Google Scholar]
- Szuts D., Freeman, M. and Bienz, M. (1997) Antagonism between EGFR and Wingless signalling in the larval cuticle of Drosophila. Development, 124, 3209–3219. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Nusse, R., Johnston, P. and Lawrence, P.A. (1989) Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell–cell communication. Cell, 59, 739–749. [DOI] [PubMed] [Google Scholar]
- Wiellette E.L. and McGinnis, W. (1999) Hox genes differentially regulate Serrate to generate segment-specific structures. Development, 126, 1985–1995. [DOI] [PubMed] [Google Scholar]