Abstract
Background:
The adverse impact of Zika (ZIKV), dengue (DENV), and chikungunya (CHIKV) virus infection in pregnancy has been recognized in Latin America and Asia but is not well studied in Africa. Although originally discovered in sub-Saharan Africa the non-specific clinical presentation of arbovirus infection may have hampered our detection of adverse clinical outcomes and outbreak.
Objective:
This prospective study of arbovirus infection in pregnant women in north-central Nigeria sought to characterize the prevalence of acute arbovirus infection and determine the impact on pregnancy and infant outcomes.
Methods:
In Nigeria, we screened 1006 pregnant women for ZIKV, DENV and CHIKV IgM/IgG by rapid test (2019–2022). Women with acute infection were recruited for prospective study and infants were examined for any abnormalities from delivery through six months. A subset of rapid test-reactive samples were confirmed using virus-specific ELISAs and neutralization assays.
Results:
The prevalence of acute infection (IgM+) was 3.8%, 9.9% and 11.8% for ZIKV, DENV and CHIKV, respectively; co-infections represented 24.5% of all infections. The prevalence in asymptomatic women was twice the level of symptomatic infection. We found a significant association between acute maternal ZIKV/DENV/CHIKV infection and any gross abnormal birth outcome (p=0.014).
Conclusions:
Over three rainy seasons, regular acute infection with ZIKV, DENV, and CHIKV was observed with significantly higher rates in pregnant women without symptoms. The potential association arbovirus infection with abnormal birth outcome warrants further prospective study to ascertain the clinical significance of these endemic arboviruses in Africa.
Keywords: Zika, dengue, chikungunya virus, arbovirus, pregnancy, microcephaly, West Africa, Nigeria
Introduction
The emergence and re-emergence of Zika (ZIKV), dengue (DENV), and chikungunya (CHIKV) viruses have caused significant human disease outbreaks globally. Phylogenetic studies have delineated the evolution of multiple Asian or American arbovirus lineages from ancestral African strains [1]. Across the globe, 90% of ~200 million annual pregnancies occur in regions with endemic arboviruses [2]. The 2015–16 ZIKV outbreak in the Americas involving the Asian strain identified the teratogenicity of ZIKV and renewed interest in the impact of other arboviruses on pregnancy and infant outcomes. Arbovirus studies have shown an increased risk of severe disease in pregnant women as well as fetal loss, prematurity, microcephaly and other birth defects or severe neonatal infection [2]. While arbovirus infections may have similar clinical presentations, they exert temporally distinct impacts over the course of pregnancy. Antenatal ZIKV has been associated with microcephaly, antenatal DENV with preterm delivery and stillbirth, and intrapartum CHIKV with neonatal disease and neurodevelopmental delays.
With the largest population in Africa at over 200 million and over 70% inhabiting rural areas, Nigeria has been identified as the African nation at highest risk for ZIKV transmission [3]. Previously, we reported the circulation of ZIKV in West Africa over two decades [4]. The seroprevalence of ZIKV IgM was 6.4% in Nigerian patients and phylogenetic analysis identified ZIKV strains from the African lineage. The WHO considers a linkage between a ZIKV strain circulating in Africa and microcephaly would significantly impact global risk assessments [5].
In recent decades, DENV has emerged as a public health threat in Asia and Latin America. DENV has been described in Africa since the late 19th century where the Aedes mosquito vector is ubiquitous. Halstead and others have considered genetic factors may be protective from disease based on differential disease manifestations by race, as seen in outbreaks in Cuba [6]. Others have suggested that circulating endemic flaviviruses in Africa may provide cross-protection from severe dengue disease [7]. In a meta-analysis, symptomatic dengue disease in pregnant women was associated with increased risks of miscarriage, stillbirth, preterm birth, and low birthweight for gestational age [8].
Chikungunya is an alphavirus transmitted by Aedes mosquitoes, like ZIKV and DENV. Aedes have been shown to be infected with and transmit combinations of CHIKV, DENV-2 and ZIKV [9,10], yet the different clinical consequences of multiple co-infections are unknown [11]. The 2005–6 CHIKV outbreak on Reunion Island was estimated to have infected one-third of the population (>250,000). The risk of CHIKV vertical transmission was highest in the intrapartum period at ~50% transmission [12]. Severe neonatal disease was also noted with sepsis-like illness, encephalitis, convulsions, and death; cases of fetal demise were attributed to in utero CHIKV [13]. In follow-up, 51% of CHIKV-exposed children had neurodevelopmental delays compared to 15% of controls [14].
Given the high risk for arbovirus infection in Africa, our prospective study sought to better characterize the epidemiology of arbovirus infection in West Africa and its potential impact on pregnancy and infant outcomes.
Methods
Study design and participants
Pregnant women ≥18 years of age attending the antenatal clinics at Jos University Teaching Hospital and Our Lady of Apostles Hospital in Jos, north-central Nigeria were recruited from April 1, 2019 to January 31, 2022. Women presenting with any of six common Zika symptoms (fever ≥37.5°C, rash, headache, arthralgia, conjunctivitis, or myalgia) were recruited for a screening questionnaire and rapid test. One asymptomatic woman was recruited for every four symptomatic women. All women provided written informed consent for the screening, and separate informed consent if participating in the prospective follow-up study. The study was approved by the institutional review boards of the Jos University Teaching Hospital and the Harvard T.H. Chan School of Public Health.
Participating women provided a finger prick sample for the CE-marked DPP® ZCD IgM/IgG rapid test (ChemBio, Medford, NY). They were administered a questionnaire that collected information on participant demographics, clinical history, exposure, and symptoms and had a brief clinical examination. The rapid test result was provided to the women within 30 minutes with appropriate counseling. The DPP® ZCD IgM/IgG rapid test is CE-marked with 99–100% sensitivity and specificity for each virus and antibody isotype. All rapid test IgM and IgM/IgG positive women were recruited for the observational prospective study. A subset of enrolled women was tested by ELISA, neutralization tests and for nucleic acid detection.
At delivery, infants were examined for abnormal clinical outcomes, including microcephaly and congenital Zika syndrome. Any adverse pregnancy outcomes, including miscarriage, stillbirth, and premature delivery were documented. Infants were followed at their routine follow-up visits: 6, 10, 14 weeks, and 6 months. Microcephaly and low birth weight were defined as head circumference or weight, respectively, with Z-score less than or equal to −2 standard deviations (SD). Z-score was calculated as the difference between the observed value and the median reference value divided by the SD, derived from sex- and age-specific reference populations used in the WHO Multicentre Growth Reference Study [15]. Infants were preterm if delivered earlier than 37 weeks gestational age; Fenton growth charts were used to calculate Z-scores for preterm infants [16].
Confirmation of arbovirus serology
Anti-ZIKV and -DENV IgM and IgG were determined by previously described ELISAs using DENV and ZIKV envelope protein fusion-loop (FL) mutated virus-like particles (VLP) as antigens, which greatly reduced flavivirus cross-reactivities [17]. ELISA sensitivities/specificities were 75.0%/95.8% and 100.0%/93.0% for DENV FL-VLP-based IgM and IgG, respectively, and 90.0%/99.3% and 100.0%/83.3% for ZIKV FL-VLP-based IgM and IgG, respectively. ZIKV and DENV NS1 IgG ELISAs were also used to distinguish previous DENV and ZIKV infections [18].
Microneutralization tests using Vero cells and DENV (DENV1-Hawaii, DENV2-NGC, DENV3-H87, DENV4-H241), or ZIKV (PRVABC59 strain) were performed in 96-well plates as previously described, and 90% neutralization (NT90) titers to DENV1–4 and ZIKV were determined [19]. NT90 titers <10 to all 5 flaviviruses tested were designated as DENV- and ZIKV-naïve, ≥10 to only one virus as primary infection, ≥10 to two or more viruses as multiple flavivirus infections. The CHIKV neutralization test used a CHIKV pseudovirus assay [20]. The % neutralization was determined and NT50 titer was the serum dilution that reached 50% neutralization using 4-parameter nonlinear regression analysis.
We analyzed the sensitivity and specificity of the DPP® ZCD IgM/IgG rapid test using 146 convalescent-phase samples from RT-PCR-confirmed DENV, ZIKV and CHIKV cases, and naïve samples [20, 21]. The sensitivity/specificity of CHIKV-, ZIKV- and DENV-IgM was 90.9%/95.1%, 81.0%/94.2% and 80.0%/82.0%, respectively (Supplementary Table 1).
Statistical analysis
Descriptive statistics characterized the study population, and associations with arbovirus infection detected by Χ2 test. Variables significant at p≤0.20 in bivariate analyses were included in the multiple logistic regression model, and backwards elimination used to build the final model predicting acute arbovirus infection, which retained variables significant at p≤0.05.
Outcomes of infants of arbovirus IgM-positive mothers were compared with outcomes recorded in the JUTH and OLA delivery registers using the two-sided Fisher’s exact test; this analysis was repeated with just the infants born to ELISA- and NT-confirmed arbovirus-positive mothers. Stata v.15.1 (StataCorp, College Station, TX, USA) software was used for analyses.
Results
From April 2019 to January 2022, we recruited 1006 pregnant women, 478 at JUTH and 528 at OLA. One-third of women (312/1006, 31.0%) showed antibody responses to one or more arboviruses, with 19.9% IgM+ or IgM+/IgG+ and 11.1% IgG+ only (Figure 1). Of 1006 women, acute/recent arbovirus prevalence (IgM+ or IgM+/IgG+) was: ZIKV 1.4%, DENV 6.0%, CHIKV 7.7%, ZIKV+DENV 0.7%, ZIKV+CHIKV 0.9%, DENV+CHIKV 2.5% and ZIKV+CHIKV+DENV 0.8% (Table 1).
Figure 1:
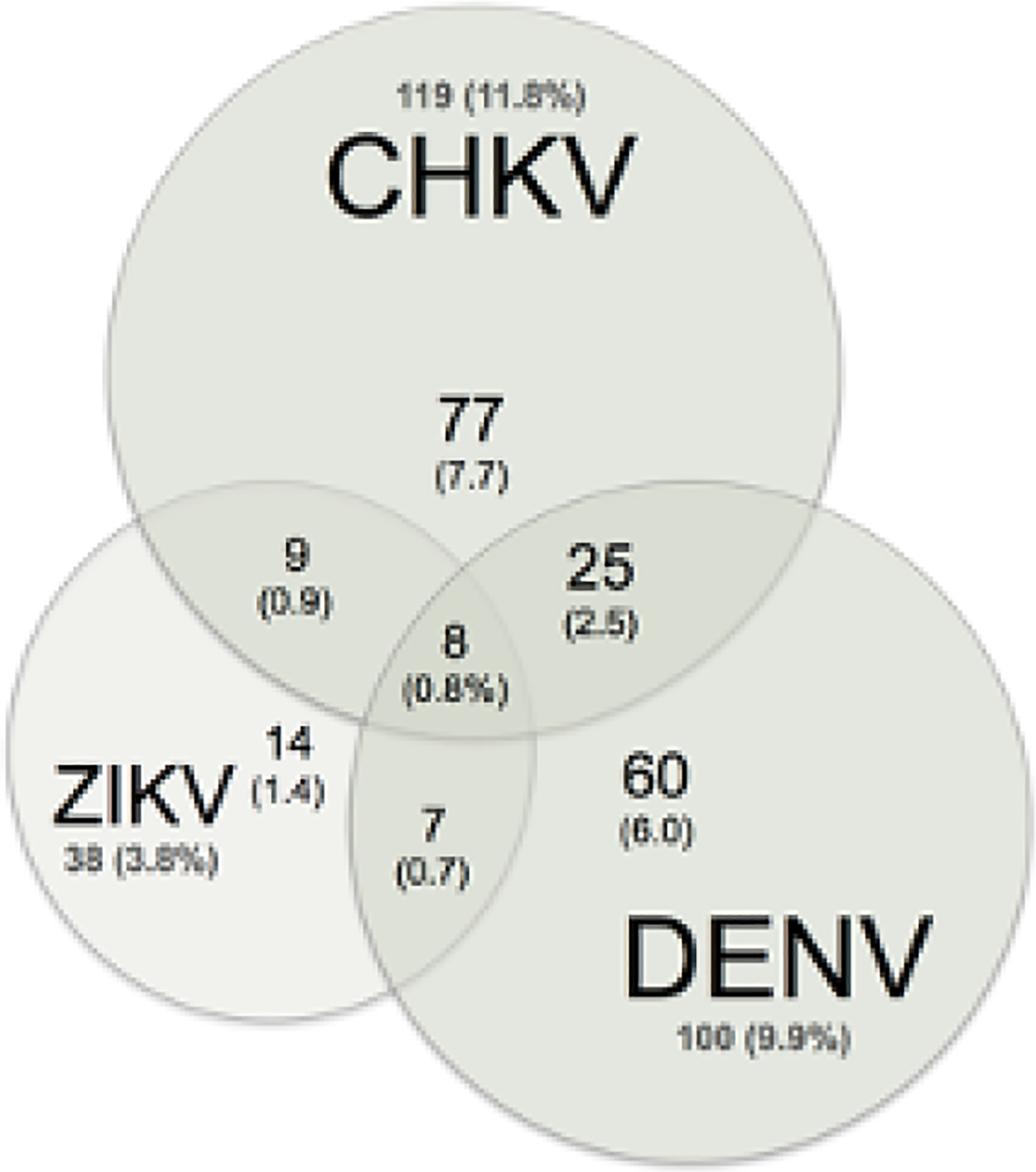
ZIKV, DENV and CHIKV Seroprevalence
Venn diagram of acute (IgM) arbovirus status. Women with any IgM reactivity (200, 19.9%) by arbovirus.
Table 1.
Prevalence of Zika, chikungunya, and dengue infection (Chembio IgM+) among pregnant women at Jos University Teaching Hospital and Our Lady of Apostles Hospital, Jos, Nigeria, April 2019–January 2022*
| Any IgM+ (with or without IgG+) | IgG+-only | |||||
|---|---|---|---|---|---|---|
| Symptomatic, no. (%) | Asymptomatic, no. (%) | Total, no. (%) | Symptomatic, no. (%) | Asymptomatic, no. (%) | Total, no. (%) | |
| Total | 787 | 219 | 1006 | 787 | 219 | 1006 |
| ZIKV | 10 (1.3%) | 4 (1.8%) | 14 (1.4%) | 4 (0.5%) | 0 (0.0%) | 4 (0.4%) |
| CHIKV | 53 (6.7%) | 24 (11.0%) | 77 (7.7%) | 38 (4.8%) | 12 (5.5%) | 50 (5.0%) |
| DENV | 39 (5.0%) | 21 (9.6%) | 60 (6.0%) | 26 (3.3%) | 5 (2.3%) | 31 (3.1%) |
| ZIKV & CHIKV | 6 (0.8%) | 3 (1.4%) | 9 (0.9%) | 3 (0.4%) | 0 (0.0%) | 3 (0.3%) |
| ZIKV & DENV | 5 (0.6%) | 2 (0.9%) | 7 (0.7%) | 5 (0.6%) | 3 (1.4%) | 8 (0.8%) |
| CHIKV & DENV | 12 (1.5%) | 13 (5.9%) | 25 (2.5%) | 10 (1.3%) | 4 (1.8%) | 14 (1.4%) |
| ZIKV, CHIKV, & DENV | 7 (0.9%) | 1 (0.5%) | 8 (0.8%) | 2 (0.3%) | 0 (0.0%) | 2 (0.2%) |
| Arbovirus reactive | 132 (16.8%) | 68 (31.1%) | 200 (19.9%) | 88 (11.2%) | 24 (11.0%) | 112 (11.1%) |
IgM, immunoglobulin M; IgG, immunoglobulin G; ZIKV, Zika virus; CHIKV, chikungunya virus; DENV, dengue virus.
Acute infected women (IgM+) showed significant variation by arbovirus and time. Higher recruitment of symptomatic women corresponded to the three rainy seasons over the study period (Figure 2). To evaluate temporal trends, the study was divided into three time periods: arbovirus IgM+ prevalence was 26.7% (88/330) from April 2019-March 2020, and reduced to 16.0% (59/368) from April 2020-March 2021 and 17.2% (53/308) from April 2021-January 2022. Age-specific arbovirus prevalence demonstrated a characteristic pattern of endemic infection (Supplementary Figure S1).
Figure 2:
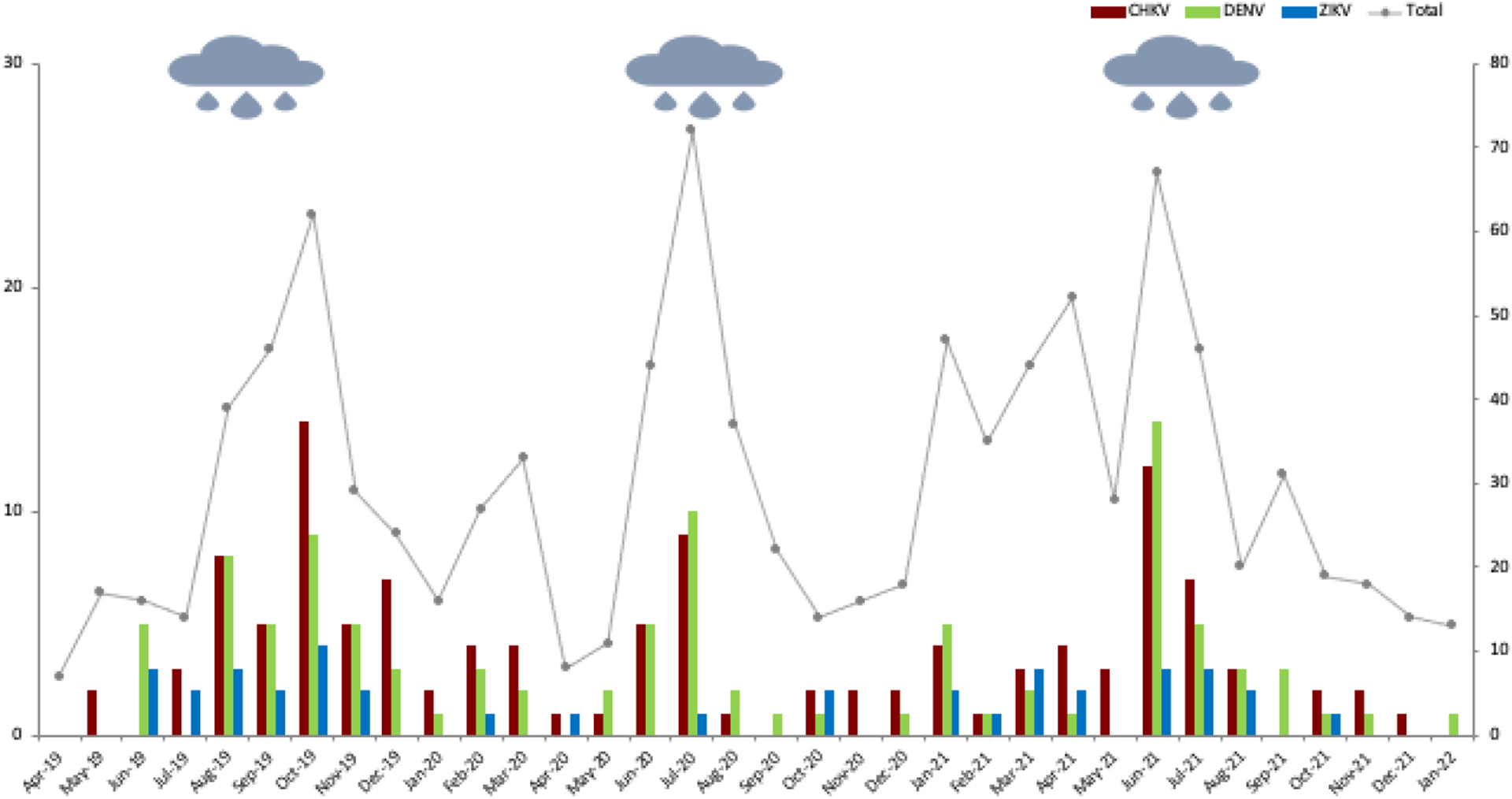
Acute arbovirus infection (2019–22)
Seroprevalence (left axis) and number of women tested (right axis); rainy season indicated by rain cloud. Acute CHKV (red), DENV (green), ZIKV (blue) and Total (grey) infections
We analyzed baseline characteristics and exposure of pregnant women to identify risks for acute (IgM+) arbovirus infection (Table 2). In bivariate analysis, OLA clinic, enrollment 2019–2020, age 25–34 years, Igbo/other ethnicity, and unemployment status were associated with acute infection. Questionnaire data also found that majority of daytime outdoors, travel to known mosquito locales, and mosquito bites were associated with acute infection. In multiple logistic regression, age 25–34 years, enrollment 2019–2020, unemployment status, majority outdoor day environment, travel to known mosquito locales, and prior mosquito bites (>2 weeks) remained statistically significant.
Table 2.
Baseline characteristics and risk factors for Zika, dengue, and chikungunya infection (Chembio IgM+) among pregnant women in Jos, Nigeria, April 2019-January 2022*
| Bivariate analysis | Logistic regression analysis | ||||||
|---|---|---|---|---|---|---|---|
| ZIKV/DENV/CH IKV negative or IgG+ only, no. (%)† |
ZIKV/DENV/CHI KV IgM+, no. (%) |
Chi-square p value |
Unadjusted OR (95% CI)‡ |
Chi-square p value |
Adjusted OR (95% CI) |
Chi-square p value |
|
| Demographic characteristics | |||||||
| Clinic | |||||||
| JUTH | 420 (87.9) | 58 (12.1) | <.001 | Ref | |||
| OLA | 385 (72.9) | 143 (27.1) | 2.69 (1.92–3.76) | <.001 | |||
| Year | |||||||
| Apr 2019–Mar 2020 | 241 (73.0) | 89 (27.0) | .001 | Ref | Ref | ||
| Apr 2020–Mar 2021 | 309 (84.0) | 59 (16.0) | .52 (.32–.83) | .007 | .49 (.31–.78) | .002 | |
| Apr 2021–Jan 2022 | 255 (82.8) | 53 (17.2) | .56 (.15–2.13) | .398 | .50 (.16–1.62) | .249 | |
| Age, years | |||||||
| 18–24 | 243 (85.3) | 42 (14.7) | .039 | Ref | Ref | ||
| 25–34 | 415 (77.9) | 118 (22.1) | 1.65 (1.25–2.17) | <.001 | 1.29 (1.12–1.49) | <.001 | |
| 35–45 | 138 (80.7) | 33 (19.3) | 1.38 (.86–2.22) | .178 | 1.16 (.86–1.55) | .329 | |
| Trimester (weeks) | |||||||
| 1 (≤12) | 87 (79.8) | 22 (20.2) | .520 | Ref | |||
| 2 (13–27) | 425 (81.3) | 98 (18.7) | .91 (.64–1.30) | .608 | |||
| 3 (≥28) | 286 (78.1) | 80 (21.9) | 1.11 (.71–1.74) | .660 | |||
| Ethnic group | |||||||
| Hausa/Fulani | 299 (85.7) | 50 (14.3) | .018 | Ref | |||
| Yoruba | 36 (81.8) | 8 (18.2) | 1.33 (.49–3.57) | .573 | |||
| Igbo | 102 (75.6) | 33 (24.4) | 1.93 (1.14–3.27) | .014 | |||
| Other | 356 (77.9) | 101 (22.1) | 1.70 (1.57–1.84) | <.001 | |||
| Occupation | |||||||
| Student | 121 (80.1) | 30 (19.9) | .144 | Ref | Ref | ||
| Housewife | 268 (84.0) | 51 (16.0) | .77 (.45–1.31) | .332 | .85 (.69–1.05) | .133 | |
| Employed | 387 (78.0) | 109 (22.0) | 1.14 (.84–1.54) | .408 | .94 (.80–1.11) | .461 | |
| Unemployed | 13 (72.2) | 5 (27.8) | 1.55 (.28–8.65) | .616 | 2.21 (1.05–4.67) | .038 | |
| Marital status | |||||||
| Single | 8 (88.9) | 1 (11.1) | .525 | Ref | .528 | ||
| Married | 770 (80.5) | 187 (19.5) | 1.94 (.25–15.30) | ||||
| Education | |||||||
| None/primary | 32 (78.1) | 9 (22.0) | .916 | Ref | |||
| Secondary | 334 (80.5) | 81 (19.5) | .86 (.59–1.26) | .445 | |||
| Tertiary | 410 (79.8) | 104 (20.2) | .90 (.41–1.97) | .795 | |||
| Mosquito exposure | |||||||
| Season | |||||||
| Dry (Nov 1–Mar 31) | 274 (82.3) | 59 (17.7) | .207 | Ref | |||
| Rainy (Apr 1–Oct 31) | 531 (78.9) | 142 (21.1) | 1.24 (1.24–1.25) | <.001 | |||
| Neighborhood | |||||||
| City | 352 (80.7) | 84 (19.3) | .557 | Ref | |||
| Suburb | 414 (80.4) | 101 (19.6) | 1.02 (.62–1.68) | .931 | |||
| Rural | 31 (73.8) | 11 (26.2) | 1.49 (.71–3.12) | .293 | |||
| Work setting | |||||||
| City | 352 (80.2) | 87 (19.8) | .321 | Ref | |||
| Suburb | 415 (81.1) | 97 (19.0) | .95 (.60–1.49) | .81 | |||
| Rural | 30 (71.4) | 12 (28.6) | 1.62 (.80–3.27) | .18 | |||
| Majority day environment | |||||||
| Indoor | 466 (85.4) | 80 (14.7) | <.001 | Ref | Ref | ||
| Outdoor | 326 (74.1) | 114 (25.9) | 2.04 (1.65–2.51) | <.001 | 1.78 (1.61–1.97) | <.001 | |
| Travel to area with mosquitos | |||||||
| No | 646 (81.5) | 147 (18.5) | .053 | Ref | Ref | ||
| Yes | 122 (74.9) | 41 (25.2) | 1.48 (.66–3.33) | .347 | 1.43 (1.01–2.03) | .043 | |
| Recent mosquito bite(s) (<2 weeks) | |||||||
| No | 316 (85.4) | 54 (14.6) | .001 | Ref | |||
| Yes | 471 (76.2) | 147 (23.8) | 1.83 (.99–3.38) | .055 | |||
| Prior mosquito bite(s) (>2 weeks) | |||||||
| No | 227 (85.3) | 39 (14.7) | .007 | Ref | Ref | ||
| Yes | 557 (77.6) | 161 (22.4) | 1.68 (1.02–2.78) | .042 | 2.16 (2.15–2.17) | <.001 | |
IgM, immunoglobulin M; ZIKV, Zika virus; DENV, dengue virus; CHIKV, chikungunya virus; IgG, immunoglobulin G; OR, odds ratio; CI, confidence interval; JUTH, Jos University Teaching Hospital; Ref, reference category; OLA, Our Lady of Apostles Hospital.
Row percentages shown.
Site used as cluster variable in logistic regression analyses.
Unexpectedly, lower rates of acute arbovirus infection were observed in symptomatic women at screening at 132/787 (16.8%) compared to 68/219 (31.1%) in asymptomatic women (Fishers exact p<0.001), ratio of symptomatic to asymptomatic of 1:2. We failed to detect hyper-endemic arbovirus infection, with relatively low levels of IgG reactivity without IgM to all three arboviruses (1.7%−6.9%).
We compared birth outcomes for 101 ZIKV/DENV/CHIKV rapid test IgM-positive women to 5930 JUTH and OLA delivery records from May 2019 to May 2021(Table 3). We found potential associations between maternal ZIKV/DENV/CHIKV infection and macerated stillbirth (p=0.017), asymmetric intrauterine growth retardation (p=0.017), microcephaly (p<0.001), cleft lip/palate (p=0.017), polydactyly (p=0.017), and multiple congenital anomalies (p<0.001). We found a significant association between maternal ZIKV/DENV/CHIKV infection and any abnormal gross birth outcome (p=0.014).
Table 3.
Abnormal delivery outcomes among study infants born to mothers with any acute Zika, dengue, or chikungunya infection versus infants in birth registers at Jos University Teaching Hospital and Our Lady of Apostles Hospital, Jos, Nigeria, May 2019–May 2021*†
| Birth registers, no.(%)‡ | Study infants born to any Chembio ZIKV/DENV/CHIKV IgM+ mother, no.(%) | Fisher’s exact p value |
Study infants born to any ZIKV/DENV/CHIKV IgM-confirmed mother, no. (%) | Fisher’s exact p value |
|
|---|---|---|---|---|---|
| Total | 5930 | 101 | 37 | ||
| Macerated stillbirth | 117 (2.0%) | 6 (5.9%) | .017 | 3 (8.1%) | .038 |
| Fresh stillbirth | 81 (1.4%) | 0 (0.0%) | .647 | 0 (0.0%) | 1 |
| Preterm | 294 (5.0%) | 6 (5.9%) | .640 | 4 (10.8%) | .111 |
| Asymmetric intrauterine growth retardation | 0 (0.0%) | 1 (1.0%) | .017 | 0 (0.0%) | … |
| Neural tube defects | 7 (0.1%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Anencephalocel§ | 3 (0.1%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Spina bifida | 2 (0.0%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Encephalocele | 1 (0.0%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Holoprosencephaly | 1 (0.0%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Other CNS abnormalities | 34 (0.6%) | 6 (5.9%) | <.001 | 2 (5.4%) | .021 |
| Microcephaly | 32 (0.5%) | 6 (5.9%) | <.001 | 2 (5.4%) | .019 |
| Hydrocephalus | 2 (0.0%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Cleft lip/palate | 0 (0.0%) | 1 (1.0%) | .017 | 1 (2.7%) | .006 |
| Gastrointestinal defects | 4 (0.1%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Omphalocele | 2 (0.0%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Duodenal atresia | 2 (0.0%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Limb defects | 1 (0.0%) | 1 (1.0%) | .033 | 1 (2.7%) | .012 |
| Clubbed foot | 1 (0.0%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Polydactyly | 0 (0.0%) | 1 (1.0%) | .017 | 1 (2.7%) | .006 |
| Multiple gross congenital anomalies¶ | 3 (0.1%) | 3 (3.0%) | <.001 | 2 (5.4%) | <.001 |
| Any abnormality# | 541 (9.1%) | 17 (16.8%) | .014 | 9 (24.3%) | .005 |
ZIKV, Zika virus; DENV, dengue virus; CHIKV, chikungunya virus; IgM, immunoglobulin M.
Data shown includes adverse and gross abnormal outcomes recorded in hospital birth registers; other outcomes, such as neonatal sepsis and jaundice, are not shown. 101 study infants born to mothers with a Chembio IgM+ rapid test result during the time period were observed at birth. Among them, 37 of the mothers had their IgM+ status confirmed by ELISA or neutralization assay.
Column percentages shown.
Italicized outcomes are subsets of their larger category. Numbers, percentages, and p values are shown for each outcome and category, if applicable.
Among the three study infants with multiple congenital anomalies, one also had cleft lip/palate, asymmetric microcephaly, craniocynostosis, hypotonia, and died on day 2; another also had loss of consciousness, seizures, hypotonia, and sustained microcephaly through 6 months; another was a stillbirth with facial dysmorphia.
Study infants that fell under multiple abnormal categories were only counted once under “Any abnormality.”
On a subset of samples from arbovirus IgM-positive women, we conducted additional serologic tests using ELISAs or neutralization assays and confirmed 37 samples. We re-compared the delivery outcomes of these confirmed arbovirus-infected women to the hospital control deliveries and found confirmed maternal IgM-positive status remained associated with microcephaly (p=0.019) and with any gross abnormal birth outcome (p=.005) (Table 3). We described 33 infants from May 2019-May 2021 with any abnormal observations recorded from birth through six months, including some abnormalities not recorded in the birth registers, with the mother’s rapid test results (Supplementary Table S2).
Finally, we explored maternal characteristics associated with risk of abnormal delivery outcomes (n=17) among the 101 ZIKV/DENV/CHIKV IgM+ pregnant women whose infants were examined at delivery (Supplementary Table S4). We found no associations with statistical significance between abnormal delivery outcomes and demographic characteristics or arbovirus responsible for infection but found potential associations with spending majority of day indoors (p=.087), anemia (p=.091), hypertension (p=.063), and vaginal infection (.044) during pregnancy.
Discussion
Our study of pregnant women provides new insights into the epidemiology of arbovirus infection in a West African setting. Spanning three rainy seasons, we detected regular peaks in symptomatic pregnant women enrollment coincident with rainy seasons and heightened risk for mosquito transmission. Acute ZIKV, DENV and CHIKV infection occurred continuously throughout the 34-month study, with an overall IgM prevalence of 3.8% ZIKV, 9.9% DENV, and 11.8% CHIKV.
Geographic regions with endemic Aedes sp. may be at risk for co-circulation of ZIKV, DENV and CHIKV, and related viruses such as Yellow Fever virus (YFV), West Nile virus (WNV), Usutu virus and the alphavirus O’nyong-nyong. The non-distinguishing clinical presentations and cross-reactive diagnostic assays hinder surveillance efforts. Comprehensive arbovirus surveillance is rarely performed in most African settings where inadequate infrastructure and/or the cost of testing are prohibitive. As a result, most studies from Asia and Latin America are outbreak focused on symptomatic patients that focus on a single viral pathogen, limiting our understanding of arbovirus co-infection. We found significant ZIKV, DENV, and CHIKV co-infections comprising 24.5% of all IgM-positive infections. Among co-infections, 67.3% included CHIKV and DENV (+/− ZIKV) and 18.4% ZIKV and CHIKV. In Brazil and Colombia, multiple arbovirus infections among pregnant women have been described [22, 23]. Case reports of congenital CHIKV and ZIKV co-infection described increased disease and miscarriage [24].
Although DENV, ZIKV and CHIKV have origins in Africa, severe arbovirus disease outbreaks have been only rarely described. For ZIKV, the rapid geographic dispersal in the 2015–16 epidemic in the Americas and the 2015 Cape Verde and 2016 Guinea Bissau outbreaks indicate its re-emergence potential [5]. We reported a 6% seroprevalence in febrile West Africans (2004–2016) with African lineage ZIKV [4], similar to the reported 6.2% IgM seroprevalence by ZIKV NS1-based ELISA in north-central Nigeria in 2018 [25]. This low prevalence may be explained by the mosquito vector competence: lower ZIKV transmission potential has been described for native A. aegpyti species compared to sub-species from Asia or the Americas, also seen with YFV and DENV [26].
Worldwide, DENV is considered one of the most widely distributed arboviruses with over 100 million annual infections. In 2020, data from 23 countries in southeast Asia and Latin America described reduced dengue transmission associated with COVID-19 restrictions accounting for seasonal dengue cycles and underreporting [27]. We similarly saw a reduction in acute IgM-positive arbovirus infections in pregnant women from 26.7% to 16.0% and 17.2% in 2020–2022, coinciding with the COVID-19 pandemic. The Nigerian governmental pandemic response began in March 2020, with closed national and state borders and week-long stay-at-home orders, followed by curfews and public health restrictions limiting population movement and gathering. As a result, antenatal attendance dropped by 50%.
Most studies of ZIKV and DENV are based on hospital-based disease surveillance whereby patients with fever or other significant flavivirus symptoms present to healthcare settings. By contrast, our study population consisted of healthy and mildly symptomatic pregnant women attending antenatal clinics (Supplementary Table S3). We found a two-fold higher rate of acute arbovirus infection in asymptomatic women (31.1%) compared with mildly symptomatic women (16.8%). Other arbovirus studies have reported that most infections are asymptomatic, with the ratio of symptomatic to inapparent infection ranging from 1:1 to 1:19 with DENV and 1:0.13 to 1:1.2 with CHIKV [28–30]. In the 2007 ZIKV outbreaks in Yap Island, household surveillance estimated a ratio of 1:4.4 symptomatic to asymptomatic infected individuals [31]. Endemic exposure in Africa and Asia may limit recognized outbreaks via herd immunity, which could be imparted by multiple cross-reacting flaviviruses.
Microcephaly in ZIKV-infected infants in the 2015–16 outbreak of the Americas increased interest in the pathologic effects of these previously poorly studied flaviviruses. Our study identified microcephaly in 6/101 (5.9%) acute arbovirus-infected women compared to 32/5930 (0.5%) control hospital deliveries, (p<0.001). We were unable to confirm arbovirus transmission in all infants born to IgM+ mothers, so are cautious in interpreting these results. Nonetheless, the significant differences in infants born to arbovirus-infected women compared to the control population warrant future study. Acute arbovirus infection was significantly associated with gross abnormal birth outcomes in both rapid test and confirmed subset analysis (p=0.014 and 0.005, respectively). The significant associations with multiple congenital anomalies (p<0.001) should be studied further.
Our study has several limitations. The low rates of arbovirus mono-infections and high rates of co-infections made direct associations with maternal and infant outcomes difficult. We were unable to confirm all rapid test IgM/IgG samples with other confirmatory assays. Study strengths included the prospective study design and evaluation of multiple arboviruses. We were able to describe acute and convalescent-phase arbovirus infection, frequent co-infection, and significant acute asymptomatic infection. Most importantly, we describe a significant association of birth abnormalities in women with acute arbovirus infection. Larger studies are needed to verify these associations and identify risk factors for infant abnormalities given arboviral infection/coinfection during pregnancy.
ZIKV, DENV and CHIKV are arboviruses of global public health importance. While their epidemiology and association with clinical disease are actively studied in the Americas and Asia, the consequences of these endemic viruses in Africa remain poorly understood. Our study demonstrated low-level but persistent infection of all three arboviruses in pregnant women in Nigeria. The significant asymptomatic infection rates and potential association with abnormal birth outcomes warrant further epidemiologic research to reveal the disease burden, transmission risks, and immunopathogenesis linked to disease outcomes of these viruses on the continent.
Supplementary Material
Figure S1: Age-specific arbovirus seroprevalence: DENV, ZIKV and CHIKV
Highlights.
ZIKV, DENV and CHIKV infection in pregnancy in Africa is poorly understood.
Low level of acute ZIKV, DENV and CHIKV infection over three rainy seasons.
Asymptomatic arbovirus infection was twice the level of symptomatic infection.
Acute maternal arbovirus infection associated with gross abnormal birth outcomes.
Acknowledgments
We acknowledge the invaluable support of our clinic and laboratory staff at JUTH and OLA that enabled this study.
Role of the funding source
Research reported in this publication was supported by the National Institutes of Health under award number R21AI137840 (Kanki) and R01AI149502 (Wang). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval
All women provided written informed consent for the screening, and separate informed consent for participation with their infants in the prospective follow-up study. The study was approved by the institutional review boards of the Jos University Teaching Hospital and the Harvard T.H. Chan School of Public Health.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Lanciotti RS, Lambert AJ. Phylogenetic Analysis of Chikungunya Virus Strains Circulating in the Western Hemisphere. Am J Trop Med Hyg. 2016;94(4):800–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Charlier C, Beaudoin MC, Couderc T, Lortholary O, Lecuit M. Arboviruses and pregnancy: maternal, fetal, and neonatal effects. Lancet Child Adolesc Health. 2017;1(2):134–46. [DOI] [PubMed] [Google Scholar]
- [3].Messina JP, Kraemer MU, Brady OJ, et al. Mapping global environmental suitability for Zika virus. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Herrera BB, Chang CA, Hamel DJ, et al. Continued Transmission of Zika Virus in Humans in West Africa, 1992–2016. J Infect Dis. 2017;215(10):1546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].WHO. Zika situation report. Zika virus microcephaly Guillain-Barré syndrome [Available from: http://www.who.int/emergencies/zika-virus/situation-report/10-march-/en/2017.
- [6].Bravo JR, Guzman MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81(5):816–20. [DOI] [PubMed] [Google Scholar]
- [7].Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comparative immunology, microbiology and infectious diseases. 2004;27(5):319–30. [DOI] [PubMed] [Google Scholar]
- [8].Paixao ES, Teixeira MG, Costa M, Rodrigues LC. Dengue during pregnancy and adverse fetal outcomes: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):857–65. [DOI] [PubMed] [Google Scholar]
- [9].Vazeille M, Mousson L, Martin E, Failloux AB. Orally co-Infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl Trop Dis. 2010;4(6):e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ruckert C, Weger-Lucarelli J, Garcia-Luna SM, et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun. 2017;8:15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Waggoner JJ, Gresh L, Vargas MJ, et al. Viremia and Clinical Presentation in Nicaraguan Patients Infected With Zika Virus, Chikungunya Virus, and Dengue Virus. Clin Infect Dis. 2016;63(12):1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramful D, Carbonnier M, Pasquet M, et al. Mother-to-child transmission of Chikungunya virus infection. Pediatr Infect Dis J. 2007;26(9):811–5. [DOI] [PubMed] [Google Scholar]
- [13].Touret Y, Randrianaivo H, Michault A, et al. [Early maternal-fetal transmission of the Chikungunya virus]. Presse Med. 2006;35(11 Pt 1):1656–8. [DOI] [PubMed] [Google Scholar]
- [14].Gerardin P, Barau G, Michault A, et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Reunion. PLoS Med. 2008;5(3):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].WHO. WHO Child Growth Standards: World Health Organization; 2007. [Available from: http://www.who.int/childgrowth/standards/second_set/technical_report_2.pdf.
- [16].Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tsai WY, Youn HH, Brites C, et al. Distinguishing Secondary Dengue Virus Infection From Zika Virus Infection With Previous Dengue by a Combination of 3 Simple Serological Tests. Clin Infect Dis. 2017;65(11):1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsai WY, Driesse K, Tsai JJ, et al. Enzyme-linked immunosorbent assays using virus-like particles containing mutations of conserved residues on envelope protein can distinguish three flavivirus infections. Emerg Microbes Infect. 2020;9(1):1722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsai WY, Chen HL, Tsai JJ, et al. Potent Neutralizing Human Monoclonal Antibodies Preferentially Target Mature Dengue Virus Particles: Implication for Novel Strategy for Dengue Vaccine. J Virol. 2018;92(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sagay SA, Hsieh S-C, Dai Y-C, et al. Association of recent Chikungunya virus infection of pregnant women with intrauterine transmission and abnormal infant outcomes in Nigeria. medRxiv. 2023. [Google Scholar]
- [21].Tyson J, Tsai WY, Tsai JJ, et al. Combination of Nonstructural Protein 1-Based Enzyme-Linked Immunosorbent Assays Can Detect and Distinguish Various Dengue Virus and Zika Virus Infections. J Clin Microbiol. 2019;57(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brasil P, Pereira JP Jr., Moreira ME, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Villamil-Gomez W, Alba-Silvera L, Menco-Ramos A, et al. Congenital Chikungunya Virus Infection in Sincelejo, Colombia: A Case Series. J Trop Pediatr. 2015;61(5):386–92. [DOI] [PubMed] [Google Scholar]
- [24].Barr KL, Vaidhyanathan V. Chikungunya in Infants and Children: Is Pathogenesis Increasing? Viruses. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mathe P, Egah DZ, Muller JA, et al. Low Zika virus seroprevalence among pregnant women in North Central Nigeria, 2016. J Clin Virol. 2018;105:35–40. [DOI] [PubMed] [Google Scholar]
- [26].Aubry F, Dabo S, Manet C, et al. Enhanced Zika virus susceptibility of globally invasive Aedes aegypti populations. Science. 2020;370(6519):991–6. [DOI] [PubMed] [Google Scholar]
- [27].Sasmono RT, Santoso MS. Movement dynamics: reduced dengue cases during the COVID-19 pandemic. Lancet Infect Dis. 2022;22(5):570–1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Montoya M, Gresh L, Mercado JC, et al. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis. 2013;7(8):e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Endy TP, Anderson KB, Nisalak A, et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2011;5(3):e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bettis AA, L’Azou Jackson M, Yoon IK, et al. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Negl Trop Dis. 2022;16(1):e0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Age-specific arbovirus seroprevalence: DENV, ZIKV and CHIKV


