Abstract
Tuberculous meningitis (TB meningitis) is the most devastating form of tuberculosis (TB) and there is a critical need to optimize treatment. Linezolid is approved for multidrug resistant TB and has shown encouraging results in retrospective TB meningitis studies, with several clinical trials underway assessing its additive effects on high-dose (35 mg/kg/day) or standard-dose (10 mg/kg/day) rifampin-containing regimens. However, the efficacy of adjunctive linezolid to rifampin-containing first-line TB meningitis regimens and the tissue pharmacokinetics (PK) in the central nervous system (CNS) are not known. We therefore conducted cross-species studies in two mammalian (rabbits and mice) models of TB meningitis to test the efficacy of linezolid when added to the first-line TB regimen and measure detailed tissue PK (multicompartmental positron emission tomography [PET] imaging and mass spectrometry). Addition of linezolid did not improve the bactericidal activity of the high-dose rifampin-containing regimen in either animal model. Moreover, the addition of linezolid to standard-dose rifampin in mice also did not improve its efficacy. Linezolid penetration (tissue/plasma) into the CNS was compartmentalized with lower than previously reported brain and cerebrospinal fluid (CSF) penetration, which decreased further two weeks after initiation of treatment. These results provide important data regarding the addition of linezolid for the treatment of TB meningitis.
Keywords: linezolid, tuberculous meningitis, positron emission tomography, antimicrobial regimens
Graphical Abstract
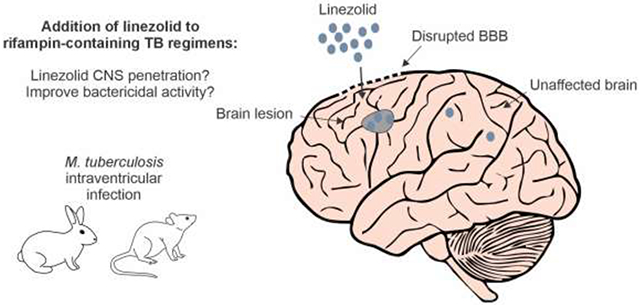
1. Introduction
Tuberculous meningitis (TB meningitis) is the most devastating form of tuberculosis (TB) and is common in young and HIV-infected individuals, especially in TB endemic countries [1-3]. Current treatment regimens have been based on treatments for pulmonary TB and have not been adequately optimized for TB meningitis. Therefore, there is an urgent need to optimize treatment regimens for both drug-susceptible and multidrug-resistant (MDR) TB meningitis.
Many strategies to improve treatment focus on mycobacterial killing and include utilization of repurposed or novel antimicrobials as well as dosage optimization of current TB drugs. Linezolid, an oxazolidinone antimicrobial that inhibits protein synthesis, is a repurposed drug that was approved for MDR-TB by the World Health Organization (WHO) in 2019 [4]. It was initially approved for Gram-positive infections but also has excellent activity against Mycobacterium tuberculosis in culture [5] and animal models of pulmonary TB [6], and demonstrates early bactericidal activity in pulmonary TB patients [7]. Compared to some key TB drugs, such as rifampin, linezolid is thought to have excellent penetration into the central nervous system (CNS) with relatively high tissue/serum ratios reported in neurosurgical patients without infection (brain/serum = 44.66% and cerebrospinal fluid [CSF]/serum = 69.57%) [8] and in patients with staphylococcal ventriculitis (area under the curve [AUC]steady state CSF/plasma = 80%) [9]. However, linezolid causes side effects (i.e., myelosuppression and peripheral neuropathy), especially when administered at 1,200 mg/day doses [10, 11]. Despite these potentially dose-limiting side effects, there is interest in linezolid to treat TB meningitis based on retrospective studies from China, demonstrating improved clinical and neurological parameters in adults [12] and children [13]. Additionally, a recent small retrospective study of rifampin-resistant MDR-TB meningitis showed lower mortality in those treated with linezolid [14]. In fact, there are several clinical trials, NCT03927313 (LASER-TBM, recently completed), NCT04021121 (ALTER), NCT03537495 (SIMPLE), NCT04145258 (INTENSE-TBM), utilizing linezolid with rifampin-containing first-line TB regimens to treat drug-susceptible TB meningitis [15, 16], but only LASER-TBM has reported results which showed no difference in mortality or disability when linezolid was added but a worse cumulative proportion of adverse events and death when linezolid and aspirin were added [17].
Therefore, the efficacy of linezolid when added to the first-line TB regimens for TB meningitis or the tissue pharmacokinetics (PK) in the CNS requires further investigation. Additionally, some data from studies of pulmonary TB suggest that addition of linezolid to the first-line TB regimens may not be beneficial. For instance, the addition of linezolid to the standard-dose rifampin-containing first-line TB regimen was antagonistic, with higher bacterial burden and failure to cure, in a mouse model of pulmonary TB [18]. Similarly, a phase II, multicenter, randomized trial substituting linezolid for ethambutol in the first-line TB regimen for pulmonary TB failed to improve 4-week culture conversion (−1.1%) [19]. Furthermore, current data from clinical studies on antibiotic PK is limited to sampling blood and CSF [20], since access to brain samples is rare due to the inherent risks of brain biopsies [8]. Additionally, sampling is generally limited to a single time-point, even in animal studies. We have therefore utilized noninvasive, molecular imaging technologies to perform cross-species animal and firstin-human studies to measure detailed concentration-time PK of existing and new antibiotics [21], measure changes in tissue penetration during treatment [22-24], and also identify key factors governing tissue penetration of antibiotics [24]. Here, we investigate the contribution of linezolid (at human equipotent dosing of 1,200 mg/day) to the treatment efficacy of high-dose or standard-dose rifampin-containing first-line TB regimens for drug-susceptible TB meningitis in two mammalian (rabbits and mice) models of TB meningitis (Fig. 1). We also measure detailed linezolid tissue PK (multicompartmental positron emission tomography [PET] imaging and mass spectrometry) in the CNS.
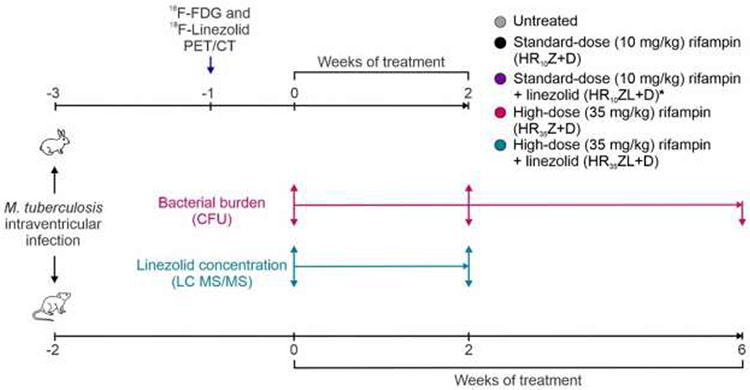
Fig 1. Experimental schematic.
Cross-species studies were performed in rabbits and mice to understand the added effect of linezolid to high-dose or standard-dose rifampin containing first-line TB regimens for TB meningitis. Animals were infected with M. tuberculosis intraventricularly. After a 2-3 weeks incubation, M. tuberculosis-infected animals were randomly allocated into treatment groups. Isoniazid (H), rifampin (R; standard [R10] or high-dose [R35]), pyrazinamide (Z) with or without linezolid (L, at human equipotent dosing of 1,200 mg/day) were administered orally. Dexamethasone (D) was administered for all regimens, as it is the standard of care for the first six weeks of treatment of patients with TB meningitis. Treatment efficacy was assessed by measuring brain bacterial burden (colony forming units, CFU). Dynamic 18F-linezolid PET/CT imaging in live animals as well as postmortem direct measures of linezolid (mass spectrometry, LC MS/MS) were performed to characterize levels in brain lesions, unaffected brain, cerebrospinal fluid and plasma. *Standard-dose rifampin with linezolid regimen was tested in mice only.
2. Material and methods
2.1. Study design
The overall goal of this study was to investigate the contribution of linezolid (at human equipotent dosing of 1,200 mg/day) to the treatment efficacy of high-dose or standard-dose rifampin containing first-line TB regimens for drug-susceptible TB meningitis in two mammalian (rabbits and mice) models of TB meningitis. We also measured detailed linezolid tissue PK using multicompartmental PET imaging, and postmortem direct measures (mass spectrometry) in the CNS. All protocols were approved by the Johns Hopkins University Animal Care and Use (RB19M417, MO19M382), Radiation Safety and Biosafety committees.
2.2. Animal studies
Male and female New Zealand White rabbits (5-7 day old, Robinson Services Inc.; Supplemental Fig. 1) were infected intraventricularly (titrated frozen stock with ~6.5 log10 of M. tuberculosis H37Rv) via the bregma using a 30-gauge insulin syringe as described previously [21,22, 24, 25]. The infection was allowed to incubate for 21 days. Rabbits were weighted at least weekly and death or early sacrifice secondary to symptomatology prior to terminal time-points were noted. Female C3HeB/FeJ mice (7-8 weeks old, Jackson laboratories) were infected intraventricularly (titrated frozen stocks with ~6.5 log10 CFU of M. tuberculosis H37Rv) via a burr hole using a Hamilton syringe (Hamilton, 88000) and stereotaxic instrument (David KOPF instrument, model 900) as described previously [21,24]. The infection was allowed to incubate for 14 days. Bacterial burden (CFUs) in whole brain tissue (rabbits and mice) and spleen/lung (rabbits only) were quantified at two (rabbits and mice) and six weeks (mice only) after treatment initiation using 7H11 plates. Untreated animals were utilized as controls.
2.3. Antimicrobial treatments
All drugs were administered via oral gavage (except dexamethasone in mice), five days a week at human equipotent dosing: rifampin (10 or 35 mg/kg/day), isoniazid (10 mg/kg/day), pyrazinamide (25 mg/kg/day), linezolid (1,200 mg/day) [21, 24] and dexamethasone (0.4 mg/kg) [26-29]. Dexamethasone was administered via the intraperitoneal route in mice [24, 26-29]. Supplemental Table 1 shows human equipotent dosing used in rabbits and mice [13, 19, 24, 30-33]. Experimentally-infected animals were randomly allocated to treatment groups.
2.4. PET imaging
18F-Linezolid was synthesized in-house using methods previously described by us [34]. 18F-FDG was purchased from Sofie Co. Live M. tuberculosis-infected rabbits (n = 4) were imaged in transparent biosafety level 3 (BSL-3)-compliant containers able to sustain animals with an anesthetic-O2 mixture as previously reported [21, 35]. NanoScan PET/CT (Mediso) was used to acquire PET/CT after an ear vein injection as follows: dynamic PET for 60 min after injection of 18F-linezolid (5.5 ± 1.5 MBq) or 15 min static PET, 45 min after injection of 18F-FDG (8 ± 3.4 MBq). Imaging was performed at least two weeks after infection and prior to initiation of treatment with high-dose rifampin plus linezolid (HR35ZL). 18F-Linezolid and 18F-FDG PET were performed on the same rabbits on consecutive days. Given a 109 min physical half-life for F-18, the first tracer would completely decay the day after, and at the time of the second tracer imaging. VivoQuant 2020 (Invicro) was utilized to co-register and analyze PET/CT images. Three-dimensional volumes of interest (VOIs) were drawn to calculate AUC as described previously [21,22, 24]. Plasma was calculated from whole blood VOIs using standard rabbit hematocrit (0.5) [21] but also corrected for red blood cell partition coefficient of linezolid [33].
2.5. Mass spectrometry
For the mouse studies, samples were collected 30 min after oral gavage. For the rabbit studies, linezolid was administered intravenously 30 min prior to sacrifice as described previously [22, 24]. Intravenously administered linezolid (human equipotent dosing 12.5 mg/kg in rabbits) was prepared using Kolliphor HS 15 (Sigma-Aldrich) for solubility. Linezolid levels in plasma, CSF and brain tissues from M. tuberculosis-infected animals were quantified using tandem mass spectrometry (LC-MS/MS) and ultra-high-performance liquid chromatography (UPLC) by the Infectious Diseases Pharmacokinetics Laboratory of the University of Florida (standard curves from 30.00 to 0.03 μg/mL) [21].
2.6. Statistical analysis
Data were analyzed using Prism 9.2 (GraphPad Software Inc.) and presented as median ± IQR except bacterial burden (CFU) that is on a logarithmic scale (base 10) and weight which were presented as mean ± SD. PET-derived AUCs were calculated using the linear trapezoidal rule. Comparisons were made using a t-test (parametric distribution) or Mann-Whitney U test (nonparametric distribution). P values ≤ 0.05 were considered statistically significant.
3. Results
3.1. Bacterial burden
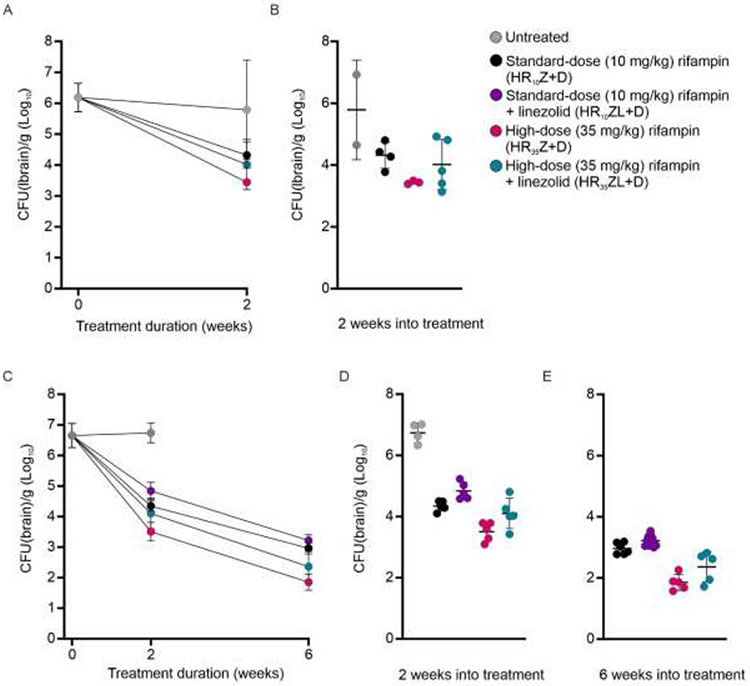
Cross-species studies to evaluate the addition of linezolid (L, at human equipotent dosing of 1,200 mg/day) to the first-line TB regimens were performed in rabbits and mice with experimentally-induced TB meningitis and were conducted as part of our prior studies investigating high-dose rifampin-containing regimens [24]. Dexamethasone was administered for all regimens as it is the standard of care for the first six weeks of treatment of TB meningitis [36, 37]. Since most clinical trials for TB meningitis are evaluating the addition of linezolid to the high-dose rifampin-containing first-line TB regimen, this was evaluated first. Addition of linezolid did not improve the bactericidal activity of the high-dose rifampin-containing first-line TB regimen (HR35Z) in either rabbits or mice. Two weeks after initiation of treatment, the brain bacterial burden was 3.44 ± 0.05 log10 colony forming units (CFU)/g and 4.02 ± 0.81 log10CFU/g in rabbits treated with the HR35Z and HR35ZL regimens, respectively (P = 0.28) and 3.51 ± 0.31 log10CFU/g and 4.10 ± 0.50 log10CFU/g in mice, respectively (P = 0.05) (Fig. 2). This trend was retained at six weeks after treatment initiation in mice, with brain bacterial burdens of 1.85 ± 0.26 log10 CFU/g and 2.36 ± 0.50 log10 CFU/g treated with the HR35Z and HR35ZL regimens, respectively (P = 0.08) (Fig. 2C-E). Similar experiments were performed in mice with standard-dose rifampin-containing first-line TB regimens, where addition of linezolid not only did not improve but potentially worsened bactericidal activity, with brain bacterial burdens of 4.34 ± 0.16 log10 CFU/g and 4.84 ± 0.28 log10 CFU/g in mice treated with the HR10Z and HR10ZL regimens, respectively at two weeks (P < 0.01), and 2.96 ± 0.18 log10 CFU/g and 3.22 ± 0.19 log10 CFU/g respectively at six weeks (P < 0.05) after treatment initiation (Fig. 2C-E).
Fig 2. Addition of linezolid to rifampin containing first-line TB regimens.
(A-B) Three weeks after infection, M. tuberculosis-infected rabbits were randomly allocated to the treatment groups (standard-dose [R10], high-dose rifampin [R35] or high-dose rifampin plus linezolid [R35L] in addition to isoniazid [H], pyrazinamide [Z] and dexamethasone [D]). Two rabbits remained untreated. (A) Bacterial burden overtime and (B) after two weeks of treatment (n = 3-5 rabbits/group). (C-E) Two weeks after infection, M. tuberculosis-infected mice were randomly allocated to the treatment groups, including standard-dose plus linezolid [R10L]. Four mice remained untreated. (C) Bacterial burden overtime and (D) after two and (E) six weeks of treatment (n = 4-10 mice/group). Bacterial burden is shown as colony forming units (CFU) represented as mean ± SD on a logarithmic (base 10) scale. Statistical comparisons were made using two-tailed t-test.
Since linezolid has not been previously evaluated in rabbit models of TB, we also measured lung and spleen bacterial burdens in the same animals to monitor dissemination to the lung and the spleen which has been previously described after brain infection (albeit generating a much lower bacterial burden) [22, 24]. Consistent with previously published data in the mouse model of pulmonary TB [18], the addition of linezolid did not improve the bactericidal activity in lungs or spleens. The lung and spleen bacterial burdens of rabbits treated with the HR35ZL regimen were 0.69 ± 1.55 log10 CFU/g and 1.20 ± 1.86 log10 CFU/g, respectively at two weeks after treatment initiation (Supplemental Fig. 2). In contrast, no bacteria could be cultured from the lungs and spleens of the rabbits treated with the HR35Z regimen, without linezolid. Finally, we monitored total body weight of the rabbits, as a clinical readout, which did not show improvement when linezolid was added to the regimen (Supplemental Fig. 3).
3.2. Linezolid pharmacokinetics
3.2.1. 18F-Linezolid PET
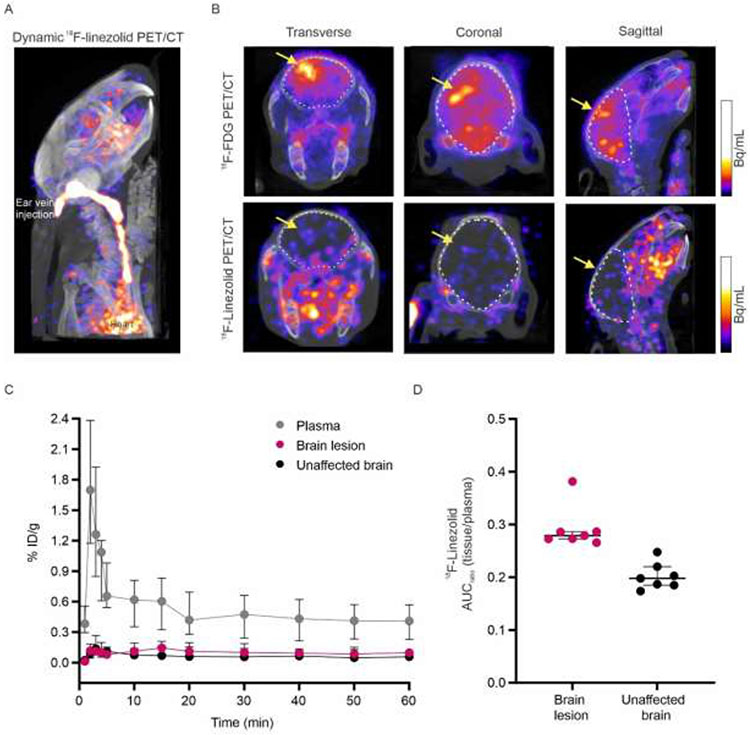
Next, we performed dynamic 18F-linezolid PET imaging to measure multicompartmental time-concentration PK in live rabbits with TB meningitis (Fig. 3). Since linezolid contains a fluorine atom, 18F-linezolid is chemically identical to linezolid, and the radioisotope is retained by the major metabolite [34]. The computed tomography (CT) was used as a guide to draw three dimensional VOIs, which were applied to the dynamic 18F-linezolid PET to measure time activity curves over 60 min and calculate PET-derived area under the curve (AUCtissue/plasma) ratios for brain lesions and unaffected brain tissues (Fig. 3C-D). These data demonstrated a median AUCtissue/plasma ratio for brain lesions and unaffected brain of 0.28 (interquartile range [IQR], 0.27-0.29) and 0.20 (IQR 0.19-0.21; P < 0.01), respectively. 18F-Fluorodeoxyglucose (18F-FDG) PET was also performed in these animals to confirm the presence and location of brain lesions.
Fig 3. Dynamic 18F-linezolid PET/CT in live rabbits.
18F-Fluorodeoxyglucose (18F-FDG) and 18F-linezolid PET/CT imaging was performed in M. tuberculosis-infected rabbits two weeks after infection and before the initiation of treatment. (A) Dynamic 18F-linezolid maximum intensity projection (MIP) PET/CT images at 5-10 min after tracer injection in a representative rabbit showing blood activity in the ear vein and heart. (B) Transverse (left), coronal (middle) and sagittal (right) 18F-FDG PET/CT showing increased signal at the site of the brain lesions (yellow arrow). (C) 18F-linezolid time-activity curve (TAC) for plasma, brain lesion and unaffected brain (n = 4 rabbits). (D) PET-derived area under the curve (AUCtissue/plasma) ratios comparing unaffected brain (n = 7 volumes of interest [VOIs]; n = 4 rabbits) and brain lesions (n = 7 VOIs; n = 4 rabbits; P < 0.01). Each dot represents a VOI. PET data are represented as median ± IQR. Statistical comparisons were made using two-tailed Mann-Whitney U test.
3.2.1. Linezolid mass spectrometry
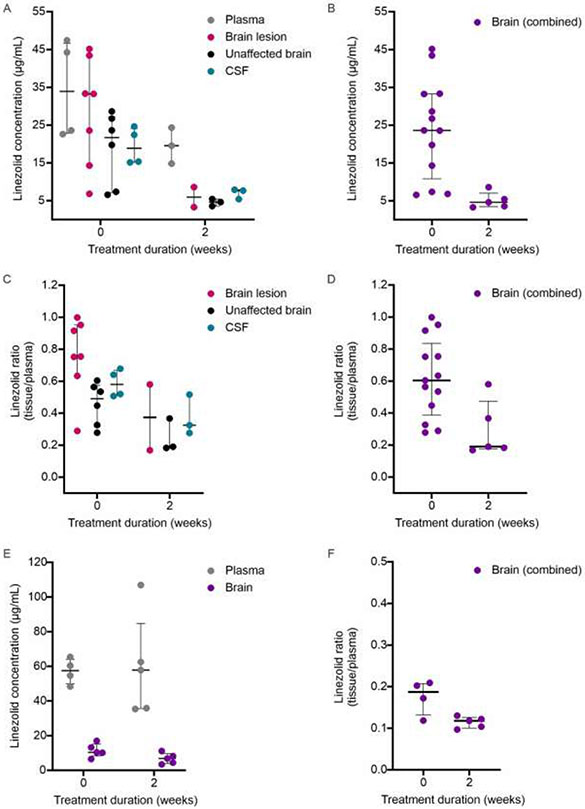
We also performed direct tissue measures of linezolid concentration by mass spectrometry in both the rabbit and the mouse models of TB meningitis (Fig. 4). Key findings in rabbits included that linezolid levels were higher in brain lesion versus the unaffected brain before treatment initiation (P = 0.01) and that linezolid levels in all compartments decreased significantly, two weeks after initiation of treatment (linezolid concentration over time [week 0 to 2]; brain lesion P = 0.06, unaffected brain P = 0.01, combined brain (lesion plus unaffected) P < 0.05, and CSF P < 0.01; Fig. 4A-B). Importantly, the linezolid penetration into all CNS compartments (tissue/plasma) decreased after two weeks of treatment, indicating that decreased plasma levels at two weeks (likely due to drug-drug interations in the rabbits) alone did not account for the decrease in linezolid levels in the CNS (linezolid tissue/plasma over time [week 0 to 2]; brain lesion P = 0.06, unaffected brain P = 0.05, combined brain P = 0.01, and CSF P = 0.06; Fig. 4C-D). Mass spectrometry in the mouse model of TB meningitis also showed a significant decrease in CNS penetration of linezolid, two weeks after initiation of treatment (linezolid brain/plasma ratio over time [week 0 to 2]; P < 0.05; Fig. 4E-F).
Fig 4. Linezolid mass spectrometry.
M. tuberculosis-infected animals were randomly allocated to receive high-dose rifampin [R35] with linezolid [L] in addition to isoniazid [H], pyrazinamide [Z] and dexamethasone [D]) for two weeks. (A-D) Rabbits, n = 7. (A-B) Linezolid tissue concentration (μg/mL) in (A) plasma, brain lesion (P = 0.06), unaffected brain (P = 0.01) and CSF (P < 0.05) and (B) combined brain samples (P < 0.01) at initiation of treatments (week 0) (n = 4 rabbits, 4-7 samples/group) and two weeks after treatment imitation (n = 3 rabbits, 2-3 samples/group) are shown. (C-D) Tissue/plasma ratios in (C) plasma, brain lesion (P = 0.06), unaffected brain (P = 0.05) and CSF (P = 0.06) and (D) combined brain samples (P = 0.01) at initiation of treatments (week 0) (n = 4 rabbits, 4-7 samples/group) and two weeks after treatment imitation (n = 3 rabbits, 2-3 samples/group) are shown. (E-F) Mice, n = 10. (E) Linezolid mass spectrometry concentration (μg/mL) at week 0 (n = 4-5 samples/group) and week 2 (n = 5 samples/group). (F) Mass spectrometry-derived linezolid concentration ratios (tissue/plasma) at week 0 (n = 4 samples/group) and week 2 (n = 5 samples/group; P = 0.03). Mass spectrometry data are represented as median ± IQR. Statistical comparisons were made using one-tailed Mann-Whitney U test.
4. Discussion
Given its excellent activity against M. tuberculosis and good penetration into the CNS, there is substantial interest in using linezolid for treating TB meningitis. In fact, several TB regimens with addition of linezolid to first-line TB treatment are currently being tested in ongoing clinical trials investigating efficacy and PK profiles, the majority of which use high-dose rifampin (35 mg/kg/day) [15, 16]. However, in our studies, linezolid did not improve the bactericidal activity of the high-dose rifampin-containing first-line TB regimen (HR35Z) in either rabbits or mice. In fact, in both animal models, there was a trend toward worse bactericidal activity with the addition of linezolid. These results are consistent with the results of the LASER-TBM clinical trial that showed no mortality benefit with the addition of linezolid to high-dose rifampin, and worse cumulative outcome (death and adverse events) with linezolid and aspirin [17]. Given these paradoxical results, and since several original retrospective studies used standard-dose rifampin [12, 13] in combination with linezolid, we also tested the addition of linezolid to standard-dose rifampin-containing first-line TB regimens in mice and found the same results. That is, addition of linezolid did not improve but potentially worsened bacterial killing of the first-line TB regimen. Importantly, a recent pilot randomized control trial in TB meningitis also showed no mortality benefit when linezolid was added to standard-dose rifampin, although there was a slight improvement in disability [38]. This study and the LASER-TBM study results should be interpreted with caution given their small samples sizes. Although, several factors could be contributing to this paradoxical effect of linezolid, previous studies in the mouse model of pulmonary TB have shown similar antagonism when linezolid was added to the first-line TB regimen, with higher bacterial burden and failure to cure [18]. Importantly, this antagonism was also noted in a phase II clinical trial where linezolid was used instead of ethambutol in the first-line TB regimen for pulmonary TB did not improve (and slightly worsened) the 4-week culture conversion [19].
The reasons for the lack of additive effects of linezolid to high-dose or standard-dose rifampin containing first-line regimens are not clearly understood. Importantly, this finding is not isolated to the CNS, as addition of linezolid did not improve the bactericidal activity in lungs or spleens of rabbits in this study, and as noted above, this antagonism is noted in the treatment of pulmonary TB in both mice and human studies. One explanation is that drug-drug interactions may alter antibiotic activity or PK. Rifampin and linezolid target RNA and protein synthesis, respectively, and thus could in theory interact with each other. However, in vitro [39] and animal studies [39-41] demonstrate that addition of rifampin to linezolid improves the activity of the combination. Nonetheless, co-administration of rifampin can decrease linezolid plasma levels [42, 43], which were also noted in the rabbit studies after two weeks of treatment despite intravenous administration prior to PK sampling. Although it can be hypothesized that myelosuppression secondary to linezolid may affect bacterial burden, linezolid has been effective in neutropenic animals and in pulmonary TB in regimens without rifampin [18, 44, 45].
Additionally, our studies demonstrate that CNS penetration of linezolid is substantially lower (AUCbrain/plasma ratio of 20-29% and CSF/plasma ratio of 52-65% using direct, single time-point measurements) than expected from previously published studies in neurosurgical patients without infection (45-70%) or those with staphylococcal ventriculitis (CSF/plasma 80%) [8, 9], where sampling was also generally limited to a single time-point. The postmortem direct linezolid measurements in both mice and rabbits were supportive of the PET data, although the magnitude varied and which could be explained by sampling differences. For instance, brain/plasma ratios were more variable and generally higher in rabbits than in mice, especially at treatment initiation. While daily antibiotic doses were administered orally in rabbits and mice, for PK studies, linezolid was administrated intravenously in rabbits (but orally in mice) in accordance with our prior studies [22], due to the unique digestive physiology in rabbit leading to variable Tmax (time to reach maximum concentration). Therefore, at the time of sampling (30 min after intravenous dose) plasma values are likely underestimated, overestimating tissue/plasma ratios in the rabbit studies. This is a limitation of single time-point sampling and likely contributed to the higher tissue/plasma ratio in mass spectrometry data compared to AUC ratios from PET data. Unlike mice where whole brain was used for drug quantification, the rabbit brain was dissected to separate brain lesions from the unaffected brain and these sampling differences also likely affected drug levels. Data from our prior 18F-linezolid PET studies [34], as well as direct linezolid measurements from another study assessing the BPaL (bedaquiline, pretomanid, linezolid) regimen for TB meningitis in mice, are consistent with the current studies, and demonstrate low brain tissue and CSF linezolid levels [21]. Importantly, in a recently completed sub-study of a phase II clinical trial (LASER-TBM), adults with HIV associated TB meningitis receiving high-dose rifampin (35 mg/kg) and linezolid (1,200 mg/day for 28 days, then 600 mg/day) with or without aspirin, underwent sampling on day 3 and 28. In this sub-study (currently a preprint), pharmacokinetic modeling showed that linezolid CSF penetration (CSF/plasma) was only 30% [46], correlating with our data. Finally, our studies also demonstrate that absolute linezolid levels as well as CNS penetration (tissue/plasma ratio) substantially decreased by two weeks after treatment initiation in both rabbits and mice. We hypothesize that CNS penetration decreases, at least in part, due to healing of the blood-brain barrier with antibiotic treatments as well as dexamethasone administration, and we have previously described the same phenomenon for rifampin [22, 24]. Importantly, data from the LASER-TBM trial is also supportive of this finding as linezolid levels correlated well with CSF protein levels, which decreased with treatment [46]. This is an important finding, especially given the fact that treatment duration for TB meningitis is 12 months and also because most clinical studies do not perform repeat sampling to measure antibiotic penetration after initiation of treatment.
Our study has some limitations. While following bacterial burden is the standard in determining treatment efficacy in pulmonary TB, TB meningitis is accompanied by neuroinflammation and neurological deficits, thus treatment efficacy needs to account for factors other than bacterial killing. Although we did not measure markers of inflammation with adjunctive linezolid, we previously did not find differences with different rifampin regimens [24]. While rabbit studies utilized both males and females, only female mice were used, and future studies with both males and females would be required to investigate how biological sex may affect linezolid PK. The PET studies administered microdoses (ng-μg) of 18F-linezolid per animal but current evidence suggests that microdosing is a reliable predictor of the drug biodistribution at therapeutic doses [47, 48]. Moreover, mass spectrometry drug levels were measured at Tmax and represent a single time-point, and not time-concentration curves (AUC) as measured by PET. Finally, although a healing blood-brain barrier supports the decreased CNS penetration of linezolid by two weeks of treatment initiation, other mechanisms (e.g., efflux pumps) may be at play, and further research is needed.
5. Conclusion
In conclusion, the results of our cross-species studies in two mammalian (rabbits and mice) models of TB meningitis demonstrate that the addition of linezolid does not improve the bactericidal activity of high-dose (35 mg/kg/day) or standard-dose (10 mg/kg/day) rifampin containing first-line TB regimens. Furthermore, our data demonstrate that CNS penetration of linezolid is more restricted than previously thought and decreases further after two weeks of treatment. Although the reasons for the lack of an additive effect of linezolid are not clearly understood, it is likely that drug-drug interactions specific to rifampin or other drugs in the first-line TB regimen may be at play. Our studies re-emphasize the added value of animal models and advanced imaging technologies for detailed characterization of novel and existing antibiotics and to accelerate the development and prioritization of promising treatments that takes years in clinical trials.
Supplementary Material
Highlights.
Adjunctive linezolid does not improve activity in animal models of TB meningitis
Linezolid penetration into the CNS is compartmentalized and lower than expected
Linezolid penetration decreased within two weeks after initiation of treatments
Funding:
This work was funded by the US National Institutes of Health R01-AI145435-A1 and K08-AI139371.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors have declared that no conflict of interest exists
Ethical Approval: All protocols were approved by the Johns Hopkins University Animal Care and Use (RB19M417, MO19M382), Radiation Safety and Biosafety committees.
Data availability:
Data are available from the corresponding author upon request.
References
- [1].Wilkinson RJ, Rohlwink U, Misra UK, van Crevel R, Mai NTH, Dooley KE, et al. Tuberculous meningitis. Nat Rev Neurol. 2017;13:581–98. [DOI] [PubMed] [Google Scholar]
- [2].Jain SK, Tobin DM, Tucker EW, Venketaraman V, Ordonez AA, Jayashankar L, et al. Tuberculous meningitis: a roadmap for advancing basic and translational research. Nat Immunol. 2018;19:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chiang SS, Khan FA, Milstein MB, Tolman AW, Benedetti A, Starke JR, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:947–57. [DOI] [PubMed] [Google Scholar]
- [4].Organization WH. WHO consolidated guidelines on drug-resistant tuberculosis treatment: World Health Organization; 2019. [PubMed] [Google Scholar]
- [5].Alcala L, Ruiz-Serrano MJ, Perez-Fernandez Turegano C, Garcia De Viedma D, Diaz-Infantes M, Marin-Arriaza M, et al. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother. 2003;47:416–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999;43:1189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dietze R, Hadad DJ, McGee B, Molino LP, Maciel EL, Peloquin CA, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008;178:1180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tsona A, Metallidis S, Foroglou N, Selviaridis P, Chrysanthidis T, Lazaraki G, et al. Linezolid penetration into cerebrospinal fluid and brain tissue. Journal of Chemotherapy. 2010;22:17–9. [DOI] [PubMed] [Google Scholar]
- [9].Beer R, Engelhardt KW, Pfausler B, Broessner G, Helbok R, Lackner P, et al. Pharmacokinetics of intravenous linezolid in cerebrospinal fluid and plasma in neurointensive care patients with staphylococcal ventriculitis associated with external ventricular drains. Antimicrob Agents Chemother. 2007;51:379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang X, Falagas ME, Vardakas KZ, Wang R, Qin R, Wang J, et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis. 2015;7:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun F, Ruan Q, Wang J, Chen S, Jin J, Shao L, et al. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis. Antimicrob Agents Chemother. 2014;58:6297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li H, Lu J, Liu J, Zhao Y, Ni X, Zhao S. Linezolid is associated with improved early outcomes of childhood tuberculous meningitis. Pediatr Infect Dis J. 2016;35:607–10. [DOI] [PubMed] [Google Scholar]
- [14].Fang M-T, Su Y-F, An H-R, Zhang P-Z, Deng G-F, Liu H-M, et al. Decreased mortality seen in rifampicin/multidrug-resistant tuberculous meningitis treated with linezolid in Shenzhen, China. BMC Infect Dis. 2021;21:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maitre T, Bonnet M, Calmy A, Raberahona M, Rakotoarivelo RA, Rakotosamimanana N, et al. Intensified tuberculosis treatment to reduce the mortality of HIV-infected and uninfected patients with tuberculosis meningitis (INTENSE-TBM): study protocol for a phase III randomized controlled trial. Trials. 2022;23:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Davis AG, Wasserman S, Maxebengula M, Stek C, Bremer M, Daroowala R, et al. Study protocol for a phase 2A trial of the safety and tolerability of increased dose rifampicin and adjunctive linezolid, with or without aspirin, for HIV-associated tuberculous meningitis [LASER-TBM]. Wellcome Open Res. 2021;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Davis AG, Wasserman S, Stek C, Maxebengula M, Jason Liang C, Stegmann S, et al. A Phase 2A Trial of the Safety and Tolerability of Increased Dose Rifampicin and Adjunctive Linezolid, With or Without Aspirin, for Human Immunodeficiency Virus–Associated Tuberculous Meningitis: The LASER-TBM Trial. Clinical Infectious Diseases. 2023;76:1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Williams KN, Brickner SJ, Stover CK, Zhu T, Ogden A, Tasneen R, et al. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am J Respir Crit Care Med. 2009;180:371–6. [DOI] [PubMed] [Google Scholar]
- [19].Lee JK, Lee JY, Kim DK, Yoon HI, Jeong I, Heo EY, et al. Substitution of ethambutol with linezolid during the intensive phase of treatment of pulmonary tuberculosis: a prospective, multicentre, randomised, open-label, phase 2 trial. Lancet Infect Dis. 2019;19:46–55. [DOI] [PubMed] [Google Scholar]
- [20].Kempker RR, Smith AGC, Avaliani T, Gujabidze M, Bakuradze T, Sabanadze S, et al. Cycloserine and Linezolid for Tuberculosis Meningitis: Pharmacokinetic Evidence of Potential Usefulness. Clin Infect Dis. 2022;75:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mota F, Ruiz-Bedoya CA, Tucker EW, Holt DP, de Jesus P, Lodge MA, et al. Dynamic 18F-pretomanid PET imaging in animal models of TB meningitis and human studies. Nat Commun. 2022;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tucker EW, Guglieri-Lopez B, Ordonez AA, Ritchie B, Klunk MH, Sharma R, et al. Noninvasive (11)C-rifampin positron emission tomography reveals drug biodistribution in tuberculous meningitis. Sci Transl Med. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ordonez AA, Wang H, Magombedze G, Ruiz-Bedoya CA, Srivastava S, Chen A, et al. Dynamic imaging in patients with tuberculosis reveals heterogeneous drug exposures in pulmonary lesions. Nat Med. 2020;26:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ruiz-Bedoya, Mota F, Tucker EW, Mahmud FJ, Reyes-Mantilla MI, Erice C, et al. High-dose rifampin improves bactericidal activity without increased intracerebral inflammation in animal models of tuberculous meningitis. J Clin Invest. 2022;Mar 15;132(6):e155851. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tucker EW, Pokkali S, Zhang Z, DeMarco VP, Klunk M, Smith ES, et al. Microglia activation in a pediatric rabbit model of tuberculous meningitis. Dis Model Mech. 2016;9:1497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rauchhaus U, Schwaiger FW, Panzner S. Separating therapeutic efficacy from glucocorticoid side-effects in rodent arthritis using novel, liposomal delivery of dexamethasone phosphate: long-term suppression of arthritis facilitates interval treatment. Arthritis Res Ther. 2009;11:R190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Groenink L, Dirks A, Verdouw PM, Schipholt M, Veening JG, van der Gugten J, et al. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry. 2002;51:875–81. [DOI] [PubMed] [Google Scholar]
- [28].Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].FDA. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Food and Drug Administration; 2005. [Google Scholar]
- [30].Pauchard L-A, Blot M, Bruyere R, Barbar S-D, Croisier D, Piroth L, et al. Linezolid and atorvastatin impact on pneumonia caused by Staphyloccocus aureus in rabbits with or without mechanical ventilation. PLoS One. 2017;12:e0187187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Swoboda S, Helbig L, Kommerell M, Simank H, Kees F, Geiss H, et al. Bone tissue and plasma concentrations of linezolid and vancomycin in rabbits with prosthesis-related infection due to MRSA. Pharmazie. 2009;64:407–9. [PubMed] [Google Scholar]
- [32].Srivastava S, Deshpande D, Pasipanodya J, Nuermberger E, Swaminathan S, Gumbo T. Optimal clinical doses of faropenem, linezolid, and moxifloxacin in children with disseminated tuberculosis: Goldilocks. Clin Infect Dis. 2016;63:S102–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hedaya MA, Thomas V, Abdel-Hamid ME, Kehinde EO, Phillips OA. Comparative pharmacokinetic study for linezolid and two novel antibacterial oxazolidinone derivatives in rabbits: Can differences in the pharmacokinetic properties explain the discrepancies between their in vivo and in vitro antibacterial activities? Pharmaceutics. 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mota F, Jadhav R, Ruiz-Bedoya CA, Ordonez AA, Klunk MH, Freundlich JS, et al. Radiosynthesis and biodistribution of 18F-linezolid in Mycobacterium tuberculosis-infected mice using positron emission tomography. ACS Infect Dis. 2020;6:916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Davis SL, Be NA, Lamichhane G, Nimmagadda S, Pomper MG, Bishai WR, et al. Bacterial thymidine kinase as a non-invasive imaging reporter for Mycobacterium tuberculosis in live animals. PLoS One. 2009;4:e6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van Laarhoven A, Dian S, Aguirre-Gamboa R, Avila-Pacheco J, Ricano-Ponce I, Ruesen C, et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: an observational cohort study. Lancet Infect Dis. 2018. [DOI] [PubMed] [Google Scholar]
- [37].Whitworth LJ, Troll R, Pagan AJ, Roca FJ, Edelstein PH, Troll M, et al. Elevated cerebrospinal fluid cytokine levels in tuberculous meningitis predict survival in response to dexamethasone. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sahib A, Bhatia R, Srivastava MP, Singh MB, Komakula S, Vishnu V, et al. Escalate: Linezolid as an add on treatment in the intensive phase of tubercular meningitis. A randomized controlled pilot trial. Tuberculosis. 2023:102351. [DOI] [PubMed] [Google Scholar]
- [39].Baldoni D, Haschke M, Rajacic Z, Zimmerli W, Trampuz A. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrob Agents Chemother. 2009;53:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gordon O, Lee DE, Liu B, Langevin B, Ordonez AA, Dikeman DA, et al. Dynamic PET-facilitated modeling and high-dose rifampin regimens for Staphylococcus aureus orthopedic implant-associated infections. Sci Transl Med. 2021;13:eabl6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thompson JM, Saini V, Ashbaugh AG, Miller RJ, Ordonez AA, Ortines RV, et al. Oral-Only Linezolid-Rifampin Is Highly Effective Compared with Other Antibiotics for Periprosthetic Joint Infection: Study of a Mouse Model. J Bone Joint Surg Am. 2017;99:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hashimoto S, Honda K, Fujita K, Miyachi Y, Isoda K, Misaka K, et al. Effect of coadministration of rifampicin on the pharmacokinetics of linezolid: clinical and animal studies. J Pharm Health Care Sci. 2018;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Okazaki F, Tsuji Y, Seto Y, Ogami C, Yamamoto Y, To H. Effects of a rifampicin pretreatment on linezolid pharmacokinetics. PLoS One. 2019;14:e0214037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xiao J, Gill C, Liang L, Liu J, Wu J, Feng H-P, et al. Use of translational pharmacokinetic/pharmacodynamic infection models to understand the impact of neutropenia on the efficacy of tedizolid phosphate. Antimicrobial Agents and Chemotherapy. 2019;63: 10.1128/aac.00822-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu J, Li S-Y, Almeida DV, Tasneen R, Barnes-Boyle K, Converse PJ, et al. Contribution of pretomanid to novel regimens containing bedaquiline with either linezolid or moxifloxacin and pyrazinamide in murine models of tuberculosis. Antimicrobial agents and chemotherapy. 2019;63: 10.1128/aac.00021-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Abdelgawad N, Wasserman S, Abdelwahab MT, Davis A, Stek C, Wiesner L, et al. Linezolid Population Pharmacokinetic Model in Plasma and Cerebrospinal Fluid Among Patients With Tuberculosis Meningitis. The Journal of Infectious Diseases. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lappin G, Noveck R, Burt T. Microdosing and drug development: past, present and future. Expert Opin Drug Metab Toxicol. 2013;9:817–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Burt T, Young G, Lee W, Kusuhara H, Langer O, Rowland M, et al. Phase 0/microdosing approaches: time for mainstream application in drug development? Nat Rev Drug Discov. 2020;19:801–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon request.