Abstract
Most vertebrate mRNAs with premature termination codons (PTCs) are specifically recognized and degraded by a process referred to as nonsense-mediated mRNA decay (NMD) while still associated with the nucleus. However, it is still a matter of debate whether PTCs can be identified by intranuclear scanning or only by ribosomes on the cytoplasmic side of the nuclear envelope. Here we show that inhibition of mRNA export by two independent approaches does not affect the downregulation of PTC-containing T-cell receptor β transcripts in the nuclear fraction of mammalian cells, providing strong evidence for intranuclear NMD. Our results are fully consistent with recently reported evidence for nuclear translation and suggest that an important biological role for nuclear ribosomes is the early elimination of nonsense mRNA during a pioneer round of translation.
INTRODUCTION
Nonsense-mediated mRNA decay (NMD) serves as a post-transcriptional quality control system that rids eukaryotic cells of premature termination codon-containing mRNAs (PTC+ mRNAs), thereby preventing the production of truncated, potentially deleterious proteins (Li and Wilkinson, 1998; Frischmeyer and Dietz, 1999; Hentze and Kulozik, 1999; Hilleren and Parker, 1999; Maquat, 2000; Wilusz et al., 2001). In accordance with the necessity to read the genetic code to identify PTCs, translation or a translation-like process is a prerequisite for NMD. For example, inhibiting translation by ribosome-binding drugs such as cycloheximide, emetine or puromycin (Carter et al., 1995; Noensie and Dietz, 2001), or translation inhibition by a secondary structure (Belgrader et al., 1993) or an iron-responsive element (Thermann et al., 1998) in the 5′-untranslated region of mRNA, abolishes NMD. Furthermore, expression of suppressor tRNAs partially reverse NMD (Belgrader et al., 1993; Li et al., 1997). Given the translational dependence of NMD, it is surprising that most mammalian PTC+ mRNAs are apparently degraded prior to their release from the nucleus, based on the reduced steady-state PTC+ mRNA levels detected in the nuclear fraction. Examples for nucleus-associated NMD are mRNAs encoding immunoglobulin κ light chain (Lozano et al., 1994; Aoufouchi et al., 1996), T-cell receptor β (TCR-β) (Carter et al., 1995, 1996; Li et al., 1997), dihydrofolate reductase (Urlaub et al., 1989), adenine phosphoribosyl-transferase (Kessler and Chasin, 1996), triose phosphate isomerase (Cheng and Maquat, 1993; Belgrader et al., 1994), mouse major urinary protein (Belgrader and Maquat, 1994) and β-globin (Baserga and Benz, 1992).
Two fundamentally different models have been proposed to solve the apparent paradox of a translation-dependent process (NMD) in the nuclear fraction of cells (Maquat, 1995; Li and Wilkinson, 1998; Frischmeyer and Dietz, 1999; Brogna, 2001; Wilkinson and Shyu, 2002). The co-translational export model posits that ribosomes on the cytoplasmic side of the nuclear membrane translate mRNAs that are in transit through the nuclear pore. Thus, PTCs would be recognized and NMD elicited while the mRNA is still physically attached to the nucleus. According to this model, reduced PTC+ mRNA levels are detected in the nuclear fraction even though NMD actually occurs in the cytoplasm. In contrast, the nuclear scanning model posits that PTC recognition occurs inside the nucleus, prior to mRNA export, by a nuclear frame reader, most likely a nuclear ribosome. An experimental approach to distinguish between these two models is to interfere with mRNA export and analyze the effect on NMD. According to the nuclear scanning model, NMD should remain unaffected upon inhibition of mRNA export and, hence, PTC+ mRNA levels in the nuclear fraction are predicted to stay low. In contrast, if the co-translational export model is correct, the PTC+ and PTC– versions of a given mRNA species should be at the same level inside the nucleus, as this model posits that NMD occurs in the cytoplasm. The co-translational export model therefore predicts a substantial increase in the steady-state level of PTC+ mRNA in the nuclear fraction upon mRNA export inhibition. According to this model, if mRNA export could be blocked completely, nuclear PTC+ mRNA would rise to the same level as the corresponding PTC– mRNA.
In this study, we inhibited mRNA export by the vesicular stomatitis virus matrix protein (VSV M) (von Kobbe et al., 2000) or by overexpression of UAP56 (Luo et al., 2001) and show that, in both cases, PTC+ TCR-β mRNA levels in the nuclear fraction were not affected. These results are incompatible with the co-translational export model, but demonstrate instead that NMD can occur within the nucleus proper, which is consistent with the recently reported evidence for nuclear translation in mammalian cells (Iborra et al., 2001). Moreover, our results suggest that an important biological role for nuclear ribosomes is the early elimination of nonsense mRNA during a pioneer round of translation (Ishigaki et al., 2001).
RESULTS AND DISCUSSION
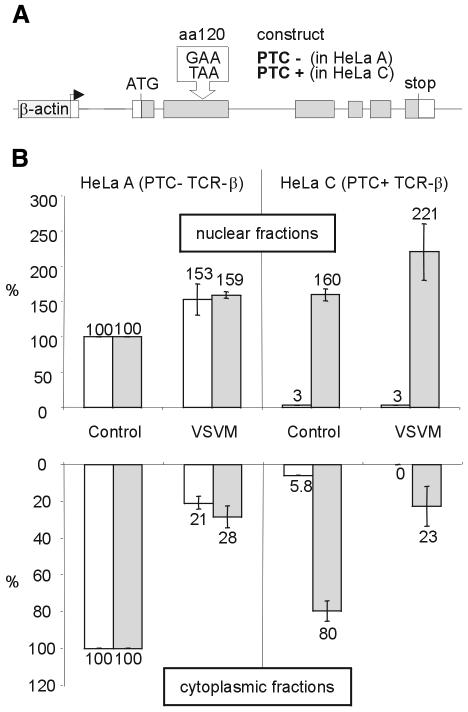
To address the question of the subcellular localization of NMD, and to specifically distinguish between the nuclear scanning and the co-translational export models, we sought ways to inhibit mRNA export to the cytoplasm. The first approach that we used to accomplish this was to express VSV M, a known inhibitor of mRNA export in vertebrate cells (Her et al., 1997; Petersen et al., 2000; von Kobbe et al., 2000). When injected into Xenopus laevis oocytes, VSV M inhibits nuclear export of U snRNA and mRNA by binding to Nup98 (von Kobbe et al., 2000), a component of the nuclear pore complex basket (Radu et al., 1995; Fontoura et al., 1999). As a reporter system for efficient nucleus-associated NMD, we used HeLa cells stably transfected with either a TCR-β minigene without a PTC (PTC–, in HeLa A) or an identical construct harboring a PTC at amino acid position 120 (PTC+, in HeLa C; Figure 1A) (Muhlemann et al., 2001). These cells were transfected with a plasmid encoding VSV M N-terminally fused to GFP (von Kobbe et al., 2000) or GFP only as control (Figure 1B). Thirty hours after transfection, nuclear and cytoplasmic RNA were isolated, reverse transcribed and analyzed by real-time PCR using specific TaqMan probes and primers to quantify TCR-β and GAPDH mRNA, as well as 18S rRNA as an internal control. Relative to control transfections, expression of VSV M decreased the levels of endogenous GAPDH and PTC– TCR-β mRNA in the cytoplasm by 3- to 5-fold, indicating that VSV M inhibited mRNA export in our assay. This extent of cytoplasmic mRNA decrease is similar to the previously reported reduction in β-galactosidase and CAT activities upon VSV M expression in human 293 cells (von Kobbe et al., 2000). Strikingly, the nuclear retention caused by VSV M did not reduce the degree of downregulation of PTC+ TCR-β mRNA in the nuclear fraction (Figure 1B). This result strongly suggests that the PTC+ transcripts are degraded inside the nucleus. If, instead, NMD occurred in the cytoplasm, the 3- to 5-fold inhibition of nuclear mRNA export imposed by VSV M should have increased the nuclear levels of PTC+ TCR-β mRNA to 60–80% of the PTC– TCR-β mRNA levels.
Fig. 1. Expression of VSV M inhibits nuclear export of PTC– and PTC+ TCR-β mRNA, but does not affect the extent of NMD in the nuclear fraction. (A) Schematic representation of the PTC– and PTC+ TCR-β constructs stably expressed in HeLa A and C, respectively, under the control of the human β-actin promotor (Muhlemann et al., 2001). (B) Relative amounts of TCR-β (white bars) and endogenous GAPDH mRNA (gray bars) in the nuclear (upper histogram) and in the cytoplasmic fractions (lower histogram) of cells expressing a VSV M–GFP fusion protein (VSVM) or GFP (Control) were determined by real-time RT–PCR. The indicated values are normalized to 18S rRNA levels and corrected for background from untransfected cells (see Methods). Average values of three real-time PCR runs are shown.
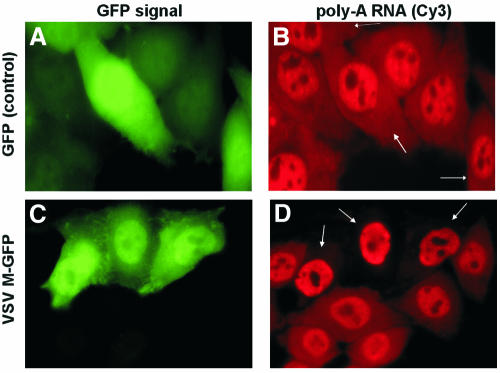
In order to confirm the VSV M-mediated mRNA export inhibition by an independent method, HeLa C cells transfected with a plasmid encoding the VSV M–GFP fusion protein were analyzed by fluorescent in situ hybridization (FISH) using a Cy3-labeled oligo (dT)70 (Figure 2). In cells expressing the VSV M–GFP fusion protein, the Cy3 signal in the cytoplasm was greatly reduced when compared with neighboring cells that did not express VSV M–GFP (Figure 2C and D), confirming that the VSV M–GFP protein inhibits general export of polyadenylated [poly(A)] RNA to the cytoplasm. However, a correspondingly large accumulation of poly(A) RNA in the nucleus was not detected. This is consistent with the moderate increase in nuclear GAPDH and PTC– TCR-β mRNA measured by real-time PCR (Figure 1B), and might be due to elevated general nuclear mRNA turnover under conditions of nuclear mRNA accumulation (Moore, 2002).
Fig. 2. Reduction of poly(A) RNA in the cytoplasm of VSV M–GFP-expressing HeLa C cells detected by FISH. Cells were transfected with a plasmid encoding GFP (A and B) or VSV M–GFP (C and D), fixed after 24 h and hybridized with a Cy3-labeled oligo (dT)70 probe to analyze the subcellular distribution of poly(A) RNA (B and D). To easily distinguish them from untransfected cells, the cells expressing VSV M–GFP fusion protein (C) or GFP (A) are marked by arrows in (D) and (B), respectively.
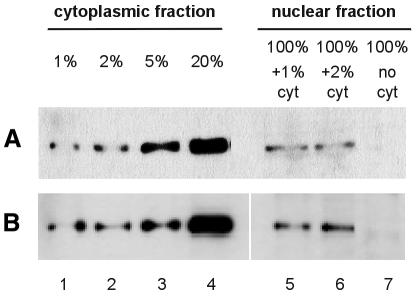
Because our experimental approach involves biochemical separation of nuclear and cytoplasmic components, potential contamination of the nuclear fractions with cytoplasmic RNA was a concern. Therefore, we used western blot analysis to examine the nuclear and cytoplasmic fractions of untransfected and VSV M-expressing HeLa A cells for the presence of tyrosine-tubulin, an exclusively cytoplasmic protein. While tyrosine-tubulin was readily detected when we loaded only 1% cell equivalents of cytoplasmic fraction, even in the presence of 100% cell equivalents of nuclear fraction, this cytoplasmic marker could not be detected when we loaded 100% of nuclear fraction alone (Figure 3). We conclude that the nuclear fractions used in this study are virtually free (<1%) of cytoplasmic contamination.
Fig. 3. Nuclear fractions contain <1% contamination with cytoplasmic tyrosine-tubulin. Nuclear and cytoplasmic fractions of untransfected (A) and VSV M–GFP-expressing HeLa cells (B) were separated by SDS–PAGE, transferred onto nylon membrane and probed with a monoclonal antibody against tyrosine-tubulin. For comparison with the nuclear fractions, cytoplasmic fractions corresponding to 1, 2, 5 and 20% (lanes 1–4) of the number of cells represented in the nuclear fractions (lanes 5–7) were loaded, and nuclear fractions were spiked with 1 or 2% cytoplasm (lanes 5 and 6).
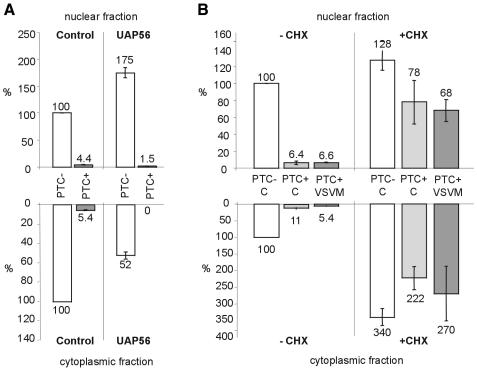
Since pleiotropic effects have been reported in connection with VSV M expression (Ahmed and Lyles, 1998; Kopecky et al., 2001), we wanted to inhibit mRNA export by a different method in order to validate the results obtained by VSV M expression. Recently, a role in mRNA export has been reported for the human splicing factor UAP56 and its homologs in yeast (Sub2) and Drosophila melanogaster (HEL) (Gatfield et al., 2001; Jensen et al., 2001; Luo et al., 2001; Strasser and Hurt, 2001). UAP56 directly interacts with Aly, an export-promoting mRNP component, and injection of excess UAP56 into X. laevis oocytes efficiently inhibits mRNA export in a dose-dependent manner (Luo et al., 2001). To test whether UAP56 overexpression also leads to inhibition of mRNA export in mammalian cells, we transfected a plasmid encoding a GFP–UAP56 fusion protein under the control of the CMV promotor (Gatfield et al., 2001). Upon UAP56 overexpression, PTC– TCR-β mRNA was reduced 2-fold in the cytoplasmic fraction and increased 1.75-fold in the nuclear fraction relative to the control, while PTC+ TCR-β mRNA decreased ∼3-fold in the nuclear fraction and could not be detected in the cytoplasm (Figure 4A). We conclude that under our experimental conditions, UAP56 overexpression in HeLa cells also reduced export of TCR-β mRNA, although to a lesser extent than VSV M. Most importantly, the UAP56-induced reduction of TCR-β mRNA export did not inhibit the extent of NMD in the nuclear fraction. This confirms the results obtained with VSV M that nucleus-associated NMD is not dependent on export of the PTC+ mRNA. If NMD was restricted to the cytoplasmic side of the nuclear membrane, as proposed by the co-translational export model, an increase in nuclear PTC+ TCR-β mRNA to ∼50% of the nuclear PTC– mRNA level would be expected when mRNA export is reduced 2-fold. In contrast to the effect of VSV M, UAP56 overexpression did not cause a general mRNA export inhibition. No significant change in the subcellular distribution of poly(A) RNA was detected by FISH (data not shown), and also GAPDH mRNA levels remained unaffected by UAP56 overexpression (data not shown). This interference with export of only a subset of mRNAs greatly reduces the likelihood of indirect effects influencing the result.
Fig. 4. Nucleus-associated NMD of TCR-β mRNA is not disturbed by UAP56-mediated reduction of export, but is sensitive to translation inhibition. (A) Relative amounts of PTC– (white bars) and PTC+ (gray bars) TCR-β mRNA in the nuclear (upper histogram) and cytoplasmic fractions (lower histogram) of cells expressing UAP56–GFP fusion protein or GFP (Control) were determined by real-time RT–PCR. (B) Twenty-eight hours after transfection with GFP (C) or VSV M–GFP (VSV M), cells were exposed to 100 µg/ml cycloheximide for 2 h (+CHX) or left in normal medium (–CHX). Relative amounts of PTC– (white bars) and PTC+ (light and dark gray bars) TCR-β mRNA in the nuclear (upper histogram) and cytoplasmic fractions (lower histogram) were measured by real-time RT–PCR. Values in (A) and (B) are normalized to 18S rRNA levels and were corrected for background from untransfected cells in (A). Average values of three real-time PCR runs are shown.
To determine whether the PTC+ TCR-β mRNA in the nuclear fraction is at low levels because of NMD, we used the protein synthesis inhibitor cycloheximide, a potent inhibitor of NMD (Carter et al., 1995; Noensie and Dietz, 2001). We found that cycloheximide dramatically increased the level of PTC+ TCR-β mRNA in the nuclear fraction to similar levels as measured for PTC– mRNA, both in cells transfected with VSV M–GFP and with GFP (Figure 4B). Thus, cycloheximide efficiently reduced the extent of intranuclear NMD of TCR-β mRNA from 15-fold to <2-fold. It is noteworthy that PTC+ TCR-β mRNA increased equally dramatically in the cytoplasmic fraction. One possible explanation is that PTC+ TCR-β mRNA molecules escaping intranuclear NMD are subject to NMD in the cytoplasm.
Although indirect effects of the ribosome-binding drug cycloheximide cannot be ruled out, the simplest interpretation of the dramatic increase in nuclear PTC+ TCR-β mRNA in the presence of cycloheximide is that translation mediated by nuclear ribosomes is required for NMD of TCR-β mRNA. Recently, direct evidence for coupled transcription and translation within nuclei of mammalian cells has been demonstrated (Iborra et al., 2001), and PTC+ mRNA bound by the predominantly nuclear cap binding complex CBP80/CBP20 has been shown to be the substrate for NMD (Ishigaki et al., 2001). Notably, a significant fraction of the translation initiation factor eIF4G is also present in the nucleus and co-immunoprecipitates with splicing complexes (McKendrick et al., 2001). Our conclusion that PTC+ mRNA can be degraded inside the nucleus is fully consistent with these data and suggests a role for nuclear translation in proofreading newly synthesized mRNA and eliminating aberrant transcripts before they exit the nucleus.
Our results are also consistent with a cyto-nuclear feedback model, in which PTC recognition during translation in the cytoplasm transmits a signal to the nucleus for degradation of nascent nuclear transcripts derived from the same gene allele (Frischmeyer and Dietz, 1999). However, there is currently no evidence for such a cyto-nuclear signal transduction mechanism, and it is conceptually difficult to envisage how such a feedback signal could function in an allele-specific manner. Therefore, we believe that the simplest interpretation of our results is that a scanning mechanism operating in the nucleus proper is responsible for nonsense codon recognition and subsequent mRNA decay.
METHODS
Transfections, cell fractionation and RNA isolation. HeLa A and HeLa C cells (Muhlemann et al., 2001) were transfected with pEGFPN3-VSVM (von Kobbe et al., 2000) or pEGFPC1-UAP56 (Gatfield et al., 2001) using LipofectAmine (Invitrogen). Thirty hours post-transfection, nuclear–cytoplasmic fractionation was performed as described elsewhere (Carneiro and Schibler, 1984), except that RNase A treatment and the sucrose cushion were omitted. Instead, the nuclei were washed twice in lysis buffer. Cytoplasmic RNA was isolated by standard phenol/chloroform extraction and ethanol precipitation; nuclear RNA was isolated using Tri-Reagent (Molecular Research Center).
Reverse transcription and real-time PCR. RNA (1 µg) was reverse transcribed with 50 ng of random hexamers and 100 U of Superscript II (Invitrogen). For real-time PCR, Platinum Quantitative PCR SuperMix-UDG (Invitrogen), TaqMan PDARs (Applied Biosystems) and cDNA corresponding to 40 ng or 400 pg of RNA were used to measure GAPDH mRNA or 18S rRNA, respectively. TCR-β mRNA was measured similarly, using 40 ng of cDNA, 200 nM TaqMan probe (5′-FAM-CTCGAGGATCTGAGAAATGTGACTCCACC-TAMRA-3′) and 200 nM of each primer (5′-GCGGTGCAGAAACGCTGTA-3′; 5′-TGGCTCAAACAAGGAGACCTT-3′). The GeneAmp 5700 Sequence Detection System (Applied Biosystems) was used for PCR and fluorescence read. Relative RNA levels were calculated from CT values according to the ΔCT method (Applied Biosystems), and relative TCR-β and GAPDH mRNA levels were normalized to 18S rRNA levels. Background signal from untransfected cells was subtracted using the equation: xcorr = {100 × x – (100 – tx) × c}/tx, where x and c are the normalized relative mRNA levels (in percent) from cells transfected with the VSV M–GFP and the GFP control plasmid, respectively. The transfection efficiency of VSV M–GFP-transfected cells (tx) was determined by measuring GFP in cells from each transfection by flow cytometry, and ranged between 60 and 80%.
FISH. Twenty-four hours after transfection, HeLa cells grown on coverslips were fixed in 4% paraformaldehyde for 30 min and permeabilized with 70% ethanol at 4°C overnight. After rehydration in 2× SSC/50% formamide, in situ hybridization was performed with 100 ng of Cy3-labeled oligo (dT)70 in 15% formamide at 37°C for 2–3 h.
Western blotting. Nuclear and cytoplasmic fractions were electrophoresed by 12% SDS–PAGE, transferred to reinforced nitrocellulose (Schleicher & Schuell) and probed with monoclonal anti-tyrosine-tubulin antibody (Sigma). Tyrosine-tubulin was then detected using horseradish peroxidase-conjugated anti-mouse IgG antibody (Promega) followed by the ECL+ Plus western blotting detection system (Amersham).
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Elisa Izaurralde and Torben Heick Jensen for VSV M and UAP56 plasmids and for the Cy3-labeled oligo (dT), respectively. We also thank Daniel Schümperli and Elisa Izaurralde for discussions and comments on the manuscript. This work was supported by a grant from the Swiss National Science Foundation to O.M. and by the Kanton Bern.
REFERENCES
- Ahmed M. and Lyles, D.S. (1998) Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III. J. Virol., 72, 8413–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoufouchi S., Yelamos, J. and Milstein, C. (1996) Nonsense mutations inhibit RNA splicing in a cell-free system: recognition of mutant codon is independent of protein synthesis. Cell, 85, 415–422. [DOI] [PubMed] [Google Scholar]
- Baserga S.J. and Benz, E.J.,Jr (1992) β-globin nonsense mutation: deficient accumulation of mRNA occurs despite normal cytoplasmic stability. Proc. Natl Acad. Sci. USA, 89, 2935–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrader P. and Maquat, L.E. (1994) Nonsense but not missense mutations can decrease the abundance of nuclear mRNA for the mouse major urinary protein, while both types of mutations can facilitate exon skipping. Mol. Cell. Biol., 14, 6326–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrader P., Cheng, J. and Maquat, L.E. (1993) Evidence to implicate translation by ribosomes in the mechanism by which nonsense codons reduce the nuclear level of human triosephosphate isomerase mRNA. Proc. Natl Acad. Sci. USA, 90, 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrader P., Cheng, J., Zhou, X., Stephenson, L.S. and Maquat, L.E. (1994) Mammalian nonsense codons can be cis effectors of nuclear mRNA half-life. Mol. Cell. Biol., 14, 8219–8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogna S. (2001) Pre-mRNA processing: insights from nonsense. Curr. Biol., 11, R838–R841. [DOI] [PubMed] [Google Scholar]
- Carneiro M. and Schibler, U. (1984) Accumulation of rare and moderately abundant mRNAs in mouse L-cells is mainly post-transcriptionally regulated. J. Mol. Biol., 178, 869–880. [DOI] [PubMed] [Google Scholar]
- Carter M.S., Doskow, J., Morris, P., Li, S., Nhim, R.P., Sandstedt, S. and Wilkinson, M.F. (1995) A regulatory mechanism that detects premature nonsense codons in T-cell receptor transcripts in vivo is reversed by protein synthesis inhibitors in vitro. J. Biol. Chem., 270, 28995–29003. [DOI] [PubMed] [Google Scholar]
- Carter M.S., Li, S. and Wilkinson, M.F. (1996) A splicing-dependent regulatory mechanism that detects translation signals. EMBO J., 15, 5965–5975. [PMC free article] [PubMed] [Google Scholar]
- Cheng J. and Maquat, L.E. (1993) Nonsense codons can reduce the abundance of nuclear mRNA without affecting the abundance of pre-mRNA or the half-life of cytoplasmic mRNA. Mol. Cell. Biol., 13, 1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura B.M., Blobel, G. and Matunis, M.J. (1999) A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J. Cell Biol., 144, 1097–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer P.A. and Dietz, H.C. (1999) Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet., 8, 1893–1900. [DOI] [PubMed] [Google Scholar]
- Gatfield D., Le Hir, H., Schmitt, C., Braun, I.C., Kocher, T., Wilm, M. and Izaurralde, E. (2001) The DExH/D box protein HEL/UAP56 is essential for mRNA nuclear export in Drosophila. Curr. Biol., 11, 1716–1721. [DOI] [PubMed] [Google Scholar]
- Hentze M.W. and Kulozik, A.E. (1999) A perfect message: RNA surveillance and nonsense-mediated decay. Cell, 96, 307–310. [DOI] [PubMed] [Google Scholar]
- Her L.S., Lund, E. and Dahlberg, J.E. (1997) Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science, 276, 1845–1848. [DOI] [PubMed] [Google Scholar]
- Hilleren P. and Parker, R. (1999) Mechanisms of mRNA surveillance in eukaryotes. Annu. Rev. Genet., 33, 229–260. [DOI] [PubMed] [Google Scholar]
- Iborra F.J., Jackson, D.A. and Cook, P.R. (2001) Coupled transcription and translation within nuclei of mammalian cells. Science, 293, 1139–1142. [DOI] [PubMed] [Google Scholar]
- Ishigaki Y., Li, X., Serin, G. and Maquat, L.E. (2001) Evidence for a pioneer round of mRNA translation. mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell, 106, 607–617. [DOI] [PubMed] [Google Scholar]
- Jensen T.H., Boulay, J., Rosbash, M. and Libri, D. (2001) The DECD box putative ATPase Sub2p is an early mRNA export factor. Curr. Biol., 11, 1711–1715. [DOI] [PubMed] [Google Scholar]
- Kessler O. and Chasin, L.A. (1996) Effects of nonsense mutations on nuclear and cytoplasmic adenine phosphoribosyltransferase RNA. Mol. Cell. Biol., 16, 4426–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky S.A., Willingham, M.C. and Lyles, D.S. (2001) Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol., 75, 12169–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. and Wilkinson, M.F. (1998) Nonsense surveillance in lymphocytes? Immunity, 8, 135–141. [DOI] [PubMed] [Google Scholar]
- Li S., Leonard, D. and Wilkinson, M.F. (1997) T cell receptor (TCR) mini-gene mRNA expression regulated by nonsense codons: a nuclear-associated translation-like mechanism [published erratum appears in J. Exp. Med. (1997) 185, 1883]. J. Exp. Med., 185, 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano F., Maertzdorf, B., Pannell, R. and Milstein, C. (1994) Low cytoplasmic mRNA levels of immunoglobulin κ light chain genes containing nonsense codons correlate with inefficient splicing. EMBO J., 13, 4617–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M.L., Zhou, Z., Magni, K., Christoforides, C., Rappsilber, J., Mann, M. and Reed, R. (2001) Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature, 413, 644–647. [DOI] [PubMed] [Google Scholar]
- Maquat L.E. (1995) When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA, 1, 453–465. [PMC free article] [PubMed] [Google Scholar]
- Maquat L.E. (2000) Nonsense-mediated RNA decay in mammalian cells: a splicing-dependent means to down-regulate the levels of mRNAs that prematurely terminate translation. In Sonenberg, N., Hershey, J.W.B. and Mathews, M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 849–868.
- McKendrick L., Thompson, E., Ferreira, J., Morley, S.J. and Lewis, J.D. (2001) Interaction of eukaryotic translation initiation factor 4G with the nuclear cap-binding complex provides a link between nuclear and cytoplasmic functions of the m7 guanosine cap. Mol. Cell. Biol., 21, 3632–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.J. (2002) Nuclear RNA turnover. Cell, 108, 431–434. [DOI] [PubMed] [Google Scholar]
- Muhlemann O., Mock-Casagrande, C.S., Wang, J., Li, S., Custodio, N., Carmo-Fonseca, M., Wilkinson, M.F. and Moore, M.J. (2001) Precursor RNAs harboring nonsense codons accumulate near the site of transcription. Mol. Cell, 8, 33–43. [DOI] [PubMed] [Google Scholar]
- Noensie E.N. and Dietz, H.C. (2001) A strategy for disease gene identification through nonsense-mediated mRNA decay inhibition. Nat. Biotechnol., 19, 434–439. [DOI] [PubMed] [Google Scholar]
- Petersen J.M., Her, L.S., Varvel, V., Lund, E. and Dahlberg, J.E. (2000) The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol., 20, 8590–8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A., Moore, M.S. and Blobel, G. (1995) The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell, 81, 215–222. [DOI] [PubMed] [Google Scholar]
- Strasser K. and Hurt, E. (2001) Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature, 413, 648–652. [DOI] [PubMed] [Google Scholar]
- Thermann R., Neu-Yilik, G., Deters, A., Frede, U., Wehr, K., Hagemeier, C., Hentze, M.W. and Kulozik, A.E. (1998) Binary specification of nonsense codons by splicing and cytoplasmic translation. EMBO J., 17, 3484–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Mitchell, P.J., Ciudad, C.J. and Chasin, L.A. (1989) Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol. Cell. Biol., 9, 2868–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kobbe C., van Deursen, J.M., Rodrigues, J.P., Sitterlin, D., Bachi, A., Wu, X., Wilm, M., Carmo-Fonseca, M. and Izaurralde, E. (2000) Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin nup98. Mol. Cell, 6, 1243–1252. [DOI] [PubMed] [Google Scholar]
- Wilkinson M.F. and Shyu, A-B. (2002) RNA surveillance by nuclear scanning? Nat. Cell Biol., 4, E144–E147. [DOI] [PubMed] [Google Scholar]
- Wilusz C.J., Wang, W. and Peltz, S.W. (2001) Curbing the nonsense: the activation and regulation of mRNA surveillance. Genes Dev., 15, 2781–2785. [DOI] [PubMed] [Google Scholar]