Abstract
Background:
The efficacy of antiplatelet therapy (APT) after aneurysmal subarachnoid hemorrhage (aSAH) remains unclear. We performed a systematic review and meta-analysis to summarize the associations of APT use after aSAH with outcomes.
Methods:
We searched published medical literature to identify cohort studies involving adults with aSAH. The exposure was APT use after aSAH. Outcome measures were good functional outcome (modified Rankin Score 0-2 or Glasgow Outcome Scale 4-5), delayed cerebral ischemia (infarcts on neuroimaging), and intracranial hemorrhage. After assessing study heterogeneity and publication bias, we performed a meta-analysis using random-effects models to assess the strength of association between APT and SAH outcomes.
Results:
A total of 14 studies with 4,228 aSAH patients were included. APT after aSAH was associated with good functional outcome (pooled relative risk, 1.08; 95% confidence interval, [CI], 1.02-1.15; I2 = 45%, p for heterogeneity = 0.04), but there was no relationship with delayed cerebral ischemia (pooled relative risk, 0.80; 95% confidence interval, [CI], 0.63-1.02; I2 = 61%, p for heterogeneity <0.01) or intracranial hemorrhage (pooled relative risk, 1.50; 95% confidence interval, [CI], 0.98-2.31; I2 = 0, p for heterogeneity =0.71). In additional analyses, APT resulted in good functional outcomes in endovascularly-treated patients. When stratified by type of medication, aspirin, clopidogrel, and ticlopidine were associated with good functional outcomes.
Conclusions:
APT after aSAH was associated with a modest improvement in functional outcome, but there was no relationship with delayed cerebral ischemia or intracranial hemorrhage.
Keywords: Subarachnoid hemorrhage, intracranial aneurysm, antiplatelet therapy, disability, delayed cerebral infarction
Introduction
Non-traumatic subarachnoid hemorrhage (SAH) accounts for 5 to 10% of all strokes in the United States, and in 80% of cases occurs from the rupture of an intracranial aneurysm.1 A major cause of death and disability after SAH is delayed cerebral ischemia (DCI), a clinical syndrome of focal neurologic deficits that develops in one third of patients in the first two weeks after aneurysm rupture. 2 Patients with aneurysmal SAH (aSAH) tend to be younger than those affected by other stroke subtypes, which results in a greater loss of productive life.3 Additionally, the course of illness, including treatment of DCI, incurs higher healthcare costs than other forms of stroke.4,5 However, despite technological advances in the surgical treatment of aneurysms, breakthroughs in the prevention of DCI have significantly lagged behind. Current guidelines recommend the use of oral nimodipine, a calcium channel blocker, to improve neurological outcomes should vasospasm occur, and maintenance of euvolemia to prevent vasospasm and subsequent DCI.6,7
Several medical therapies such as statins, magnesium, nicardipine, cilostazol, and clazosentan have been studied as potential interventions to improve SAH outcomes.8-11 With the exception of cilostazol, a medication used primarily for peripheral arterial disease in the U.S., the other therapies have failed to show benefit. DCI and downstream complications of aSAH are purported to result from neuroinflammation and micro-thrombosis, both potential mechanistic targets for antiplatelet therapy (APT).12,13 However, earlier trials and studies of APT found no significant benefit, but these studies were performed at a time when surgical clipping was the mainstay of aneurysm treatment.14,15 In fact, a prior meta-analysis that assessed APT in aSAH patients who mostly underwent surgical clipping, suggested a trend toward better outcome with APT.16 Endovascular therapy is now increasingly preferred for securing ruptured aneurysms with a favorable anatomy17, which sometimes requires APT even in the acute phase of SAH. Emerging single-center observational data suggest that APT may be associated with lower rates of DCI and disability in SAH patients treated with coiling or stenting.18,19 We therefore sought to perform a systematic review and meta-analysis of published literature to first, evaluate the relationship between APT and SAH outcomes, and second, assess if this relationship varies by aneurysm treatment strategy and APT regimen.
Methods
We performed this study in accordance with the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),20 and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE)21 statements. We also prospectively registered our study protocol on the International Prospective Register of Systematic Reviews (PROSPERO registration number CRD42021252189). This study was exempt from approval by the Weill Cornell Medicine Institutional Review Board since the analyses entailed publicly available, de-identified published data.
Data Sources and Searches
We performed comprehensive searches in Ovid MEDLINE, Ovid Embase, and the Cochrane Library from the inception of each database to March 31, 2021. An English language filter was not applied. Following the initial search in Ovid MEDLINE, the search was extended to other databases. Keywords used to query the databases included: ‘subarachnoid hemorrhage’ and a combination of ‘aneurysm’, ‘coiling’, ‘clipping’, ‘antiplatelet’, ‘aspirin’, ‘clopidogrel’, ‘delayed cerebral ischemia’, ‘outcome’, and ‘functional outcome’. Details of the search methodology are listed in the online-only Data Supplement.
Study Selection
We included studies evaluating initiation or resumption of APT during hospitalization for SAH. The inclusion criteria for our study were: (1) studies with aneurysmal SAH as the primary inclusion criteria; (2) studies with documented outcomes of DCI and functional outcomes in the follow-up period; (3) studies with clear documentation of the use of antiplatelet medications; (4) adult patients ≥18 years of age; and (5) sample size ≥10 patients to avoid inclusion of case reports or small case series. In this systematic review, we only included peer-reviewed publications in scientific journals, and not conference proceedings or abstracts since the latter typically do not provide the level of detail needed of rigorous data extraction. In case of multiple publications from a single cohort of patients or institutional database, the study with the largest cohort of patients was selected to avoid duplication of data. The search methodology has been outlined in the online only data supplement.
Data Extraction and Quality Assessment
Two investigators (A.G and K.B) read the title and abstract produced by the initial search, shortlisted articles, reviewed them, and selected articles based on the inclusion criteria and quality of data. Any disagreements were resolved by a third investigator (S.B.M). Data were extracted using a pre-specified collection template. The following study characteristics were extracted: first author, journal of publication, year of publication, and study design. We also collected patient demographics including age, sex, and stroke comorbidities such as hypertension, diabetes mellitus, dyslipidemia, and smoking history. Additionally, we obtained information about the type of antiplatelet medication and type of definitive aneurysm treatment. Outcome data included cases of DCI and functional outcome in the follow-up period.
We adapted risk of bias assessments in previously published meta-analyses on stroke risk, and generated eight specific questions to evaluate for potential selection, detection, reporting, and confounding bias.22,23 Two readers assessed for risk of bias using this questionnaire, with disagreements in assessment resolved by a third tie-breaking evaluator.
Definitions of Outcomes
The main outcomes were (i) good functional outcome, defined as a modified Rankin Score (mRS) 0-2 or a Glasgow Outcome Scale (GOS) 4-5, (ii) DCI, defined as evidence of infarction on neuroimaging (computed tomography of the head or magnetic resonance imaging of the brain), and (iii) any intracranial hemorrhage. DCI was diagnosed during the course of SAH hospitalization, while functional outcomes were ascertained between 2 and 12 months. In the International Subarachnoid Aneurysm Trial (ISAT), rates of good functional outcome in endovascularly treated patients were 74.6% at 2 months and 76.3% at 12 months, suggesting that variation in the timing of disability assessments should have a minimal confounding effect on the relationship between APT and outcomes.24
Data Synthesis and Analysis
We performed a meta-analysis to assess the association between APT and SAH outcomes using the pooled relative risk (RR) as the effect parameter. We used a random-effects (DerSimonian-Laird) model to calculate the pooled RR, and generated forest plots to display the individual study RR and pooled RR.25 The rationale of using the more conservative random-effects model was to account for the variability in effect sizes, design, and follow-up between the individual studies. We assessed heterogeneity using the I2 statistic.26 The presence of publication bias was evaluated using the Begg-Mazumdar rank correlation test. We performed pre-specified subgroup analyses by type of aneurysm treatment, antiplatelet medication, and type of study design, anticipating significant heterogeneity since the studies spanned over 3 decades. Statistical analyses were performed using Stata, version 15 (StataCorp) and R, version 3.6.3 (R Project for Statistical Computing). All tests were two-tailed and p values <0.05 were considered significant.
Results
Study Selection and Characteristics
We screened a total of 752 titles and abstracts, and 14 met criteria for inclusion (Supplemental Figure I). The study from the Magnesium and Acetylsalicylic acid in Subarachnoid Haemorrhage (MASH) trial reported comparisons between APT and no APT groups in the coiling and clipping subgroups.15 We therefore considered the two aneurysm treatment arms as different studies in our analyses. Of the various antiplatelet medications, 8 studies evaluated aspirin monotherapy18,27-32, while 3 studies assessed dual APT.19,32,33 Among aneurysm treatment modalities, predominantly surgical clipping was done in 9 studies, while endovascular treatment was favored in 5 cohorts18,19,27,32,33, and both done equally in 1 study.30 Dual APT was used in 3 studies19,32,33, while the rest preferred antiplatelet monotherapy. Among the included studies, three were from Germany18,27,32, three from the Netherlands15,28,30, two each from the USA31,33 and Japan34,35, and one each from England14, Finland29, and South Korea.19
Association between APT and Good Functional Outcome
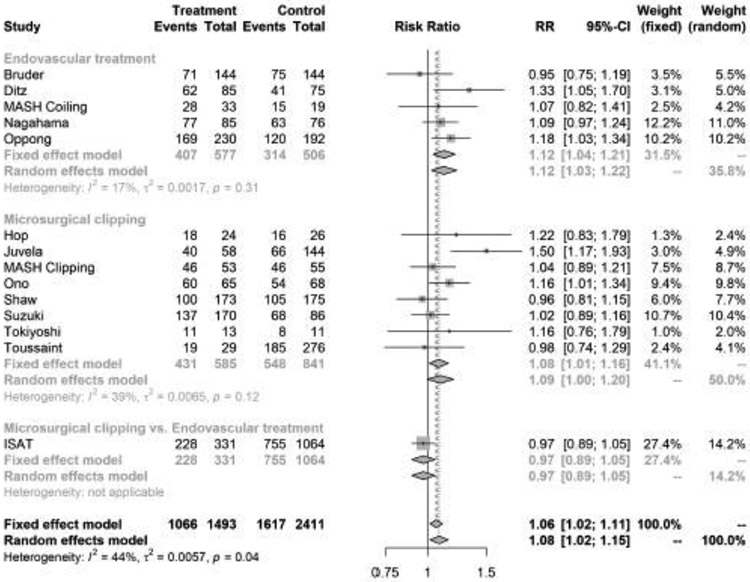
We included 12 studies with 3,904 patients with aSAH. APT was started in 1,493 (38.2%), and not initiated in 2,385 (61.8%) patients. Good functional outcome was observed in 1,067 (71.4%) with APT compared with 1,618 (67.1%) in patients not on APT. In the random effects model, APT was significantly associated with good functional outcome (pooled RR, 1.08; 95% confidence interval, [CI], 1.02-1.15) (Figure 1). There was statistically significant heterogeneity (I2 = 45%; p for heterogeneity = 0.04) but no publication bias (p value for Begg-Mazumdar test = 0.27). The funnel plot for assessment of publication bias is shown in Supplemental Figure II.
Figure 1.
Forest plot of the association between antiplatelet therapy and good functional outcome after aneurysmal subarachnoid hemorrhage, stratified by type of aneurysm treatment. The meta-analysis was calculated using a random-effects model, with the pooled relative risk shown in the forest plot. Each square represents the point estimate of any given study’s effect size. The size of the squares is proportional to the inverse of the variance of the estimate, while the horizontal lines represent each study’s 95% confidence intervals. The diamond represents the pooled estimate with the width of the diamond representing the pooled 95% confidence intervals.
Association between APT and DCI
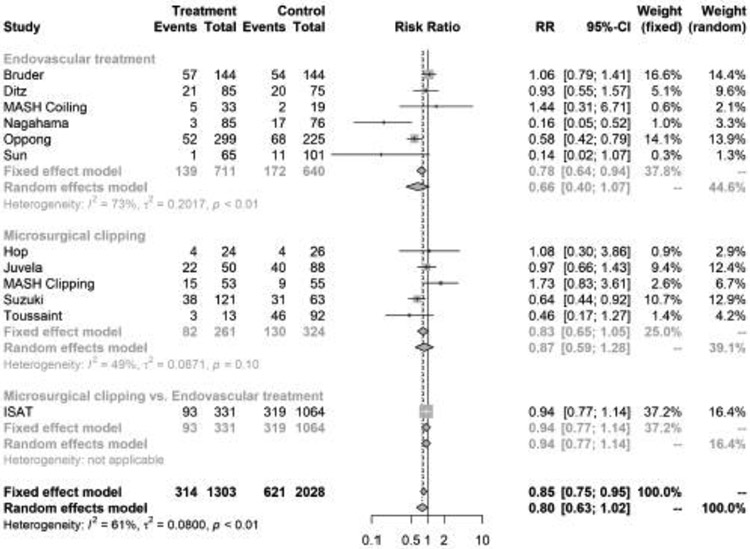
A total of 11 studies were eligible for the meta-analysis on the relationship between APT and DCI. These studies included 3,381 patients with aSAH, of whom 1,303 (38.5%) were started on APT. DCI was observed in 314 (24.1%) patients on APT and 621 (30.6%) who were not started on APT. In the fixed effects model, APT was associated with a lower risk of DCI (pooled RR, 0.85; 95% confidence interval, [CI], 0.75-0.95). However, there was no difference in the risk of DCI in the random effects model (pooled RR, 0.80; 95% CI, 0.63-1.02) (Figure 1). There was significant heterogeneity (I2 = 61%; p for heterogeneity <0.01) but no publication bias was noted (p value for Begg-Mazumdar test = 0.63) (Supplemental Figure III).
Association between APT and intracranial hemorrhage
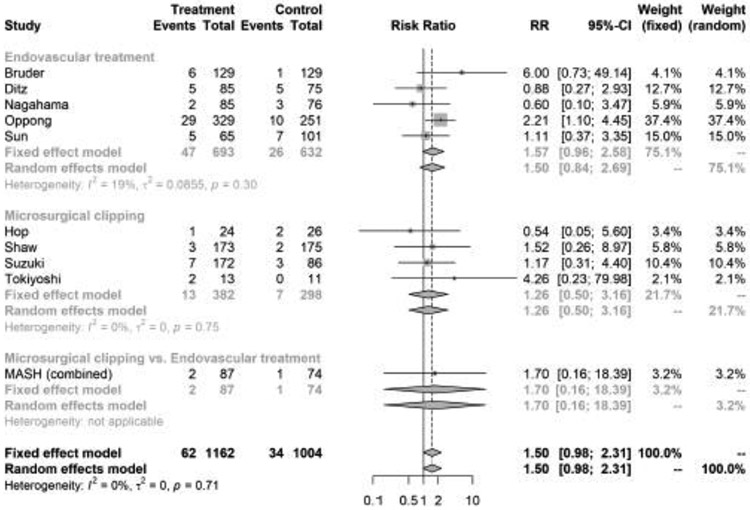
Ten studies reported intracranial hemorrhage, which included a total of 2166 aSAH patients, of whom 1162 (53.6%) were started on APT (Supplemental Table I). In the random effects model, there was no significant relationship between APT and intracranial hemorrhage (pooled RR, 1.50; 95% confidence interval, [CI], 0.98-2.31) (Figure 1). There was statistically significant heterogeneity (I2 = 0; p for heterogeneity = 0.71) but no publication bias (p value for Begg-Mazumdar test = 0.134). The funnel plot for assessment of publication bias is shown in Supplemental Figure IV.
Subgroup Analysis
In pre-specified subgroup analyses by type of aneurysm treatment, APT was associated with favorable functional outcome (RR, 1.12; 95% CI, 1.03-1.22, I2 = 17%, p value for heterogeneity = 0.31), but not with DCI (RR, 0.66; 95% CI, 0.40-1.07, I2 = 73%, p value for heterogeneity <0.01) or intracranial hemorrhage (pooled RR, 1.50; 95% CI, 0.84-2.69, I2 = 19%, p value for heterogeneity 0.30) among patients treated with endovascular interventions (Figures 1-3). However, among SAH patients treated with surgical clipping, APT was not associated with functional outcome, DCI, or intracranial hemorrhage.
Figure 3.
Forest plot of the association between antiplatelet therapy and intracranial hemorrhage after aneurysmal subarachnoid hemorrhage, stratified by type of aneurysm treatment. The meta-analysis was calculated using a random-effects model, with the pooled relative risk shown in the forest plot.
In the subgroup analysis stratified by type of APT, administration of aspirin, clopidogrel, and ticlopidine were associated with favorable functional outcome, while dipyridamole, ozagrel, and OKY-046 were not (Supplemental Figure V). Similarly, ticlopidine was associated with a lower risk of DCI, while aspirin and clopidogrel usage suggested no benefit for DCI (Supplemental Figure VI). There was no relationship between type of APT and intracranial hemorrhage (Supplemental Figure VII).
Finally, in the subgroup analysis stratified by study design, retrospective studies were associated with favorable functional outcomes and lower risk of DCI, but not with intracranial hemorrhage. Prospective studies and randomized clinical trials did not relate to any of the outcomes (Supplemental Figures VIII-X).
Assessment of the Quality of Included Studies
The results from the quality assessment questionnaire are shown in Supplemental Table II. Selection bias was minimized in seven studies through random selection of patients in the setting of a clinical trial. 14,15,28,31,34,36 A majority of the studies adjusted for covariate risk factors in assessing the relationship between APT and aSAH outcomes (Supplemental Table I).
Discussion
In this systematic review and meta-analysis of nearly 4,500 patients with aSAH, initiation of APT was associated with a modestly lower risk of major disability or death, and there was a trend toward a decreased risk of DCI, compared to patients not on APT.
APT has been posited to reduce the risk of aneurysm formation and rupture in healthy subjects, and decrease the risk of embolic events in the periprocedural period in patients undergoing aneurysm treatment, presumably due to anti-inflammatory properties.37 However, data on the efficacy of APT in improving aSAH outcomes is divided. While a few prior studies including randomized trials have shown equivocal benefit of APT for reducing vasospasm and DCI in patients with aSAH undergoing surgical treatment, recent data in primarily endovascularly-treated patients seems to suggest a benefit.8,18 In the context of these conflicting results, our meta-analysis showed a possible benefit for APT after aSAH.
Clinicians may be hesitant to initiate APT after an acute aSAH since majority of these patients require an external ventriculostomy drain (EVD).38 In the secondary analysis of the Clot Lysis: Evaluation of Accelerated Resolution of Intraventricular Hemorrhage Phase III (CLEAR III) trial, prior APT use in the setting of intraventricular alteplase administration only resulted in small, clinically inconsequential EVD tract hemorrhages.39 The APT regimen however appears to influence this bleeding risk. For instance, anecdotal data indicate that flow diversion of ruptured aneurysms in the acute phase of aSAH, an intervention that requires dual APT, is associated with a higher incidence of intracranial hemorrhage, compared to antiplatelet monotherapy.40 The trend toward an increased risk of intracranial hemorrhage in our meta-analysis may have also been driven by dual APT regimen utilized in some of the studies. This is not surprising since several clinical trials in ischemic stroke have demonstrated a higher risk of major bleeding events with dual APT compared to a single agent.41,42 A parallel line of research has suggested that patients with aSAH have a heightened risk of arterial ischemic events such as ischemic stroke, myocardial infarction, and overall cardiovascular mortality, both short- and long-term, further supporting the exploration of APT in these patients.43,44
We observed a trend toward lower DCI risk with APT, particularly in endovascularly treated patients, where this relationship was significant in the fixed-effects model, but not in the random-effects analysis. While symptomatic vasospasm is a more common and reversible complication of aSAH, the definition is subjective and therefore subject to more variation. In the present meta-analysis, some studies used angiographic vasospasm as an outcome, even in the absence of clinical symptoms. To minimize heterogeneity in the definition of vasospasm, we therefore opted to include only DCI, where there was clear evidence of infarction on neuroimaging. From a mechanistic standpoint, APT may help alleviate the severity of vasospasm by targeting neuroinflammation and micro-thrombosis, two pathways believed to result in vasospasm.12,13 Interestingly, ticlopidine appeared to decrease the risk of DCI in our analyses, while the other antiplatelet medications did not. In the Ticlopidine Aspirin Stroke Study, there was a nearly 2-fold reduction in the risk of recurrent stroke in the first year, compared to aspirin among patients with a completed minor stroke.45 Although it is unclear if one type of APT is superior to others in aSAH, further study and careful consideration of the individual antiplatelet medications is warranted. From a mechanistic standpoint, APT may have a role in mitigating DCI. Although the pathophysiology of DCI is not clearly understood, it is purported to involve activation of inflammatory and coagulation cascades, increased platelet aggregation, and endothelial dysfunction, which collectively lead to the formation of microthrombi eventually resulting in infarcts.12,46 APT, particularly aspirin, inhibits platelet aggregation and potentially even decreases vasoconstriction by inhibiting proline-rich tyrosine kinase 2 in vascular smooth muscle cells.47,48 An ongoing clinical trial evaluating the relationship between APT and cognition after SAH may shed more light on the role of APT in future.49
Our meta-analysis has several noteworthy limitations. First, the studies included were published over nearly four decades during which practice changes such as the emergence of endovascular treatment, and improvement in intensive care management changed, and presumably introduced heterogeneity and influenced our results. However, we tried to mitigate this issue by performing subgroup analyses by type of surgical intervention and APT regimen. Second, the follow up period for good functional outcomes varied between studies, ranging from 1-12 months, but rates of good functional outcome in endovascularly treated patients were 74.6% at 2 months and 76.3% at 12 months in the ISAT trial, suggesting that variation in the timing of disability assessments should have had a minimal confounding effect on the relationship between APT and outcomes.24 Third, the findings in this study should be interpreted with caution given the confounding by indication in that SAH severity factors such as presence of a parenchymal hematoma, severe modified Fischer score, and surgical decompression may have influenced clinicians to consider starting or not starting antiplatelet therapy. Fourth, the dose and duration of APT was not available in all studies; therefore, it is unknown whether a specific APT regimen should be targeted in these patients. Additionally, the timing of initiation of APT was also not provided in all studies, which may have influenced outcomes, particularly DCI. Fifth, since some studies evaluated vasospasm regardless of clinical symptoms (i.e. asymptomatic spasm), we chose to use DCI as an outcome, which is objectively easier to diagnose. That said, the diagnosis and treatment of DCI varied across studies and likely introduced bias. Lastly, data on platelet transfusions, a practice shown to potentially worsen outcomes in patients with intracerebral hemorrhage, were not available.50
Conclusion
In this meta-analysis of 14 studies, APT after aSAH was associated with favorable functional outcomes, with a trend toward decreased delayed cerebral ischemia. Increasing use of APT in the context of endovascular therapy for ruptured aneurysms warrants re-exploration of this low-cost intervention to improve outcomes after aSAH.
Supplementary Material
Figure 2.
Forest plot of the association between antiplatelet therapy and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage, stratified by type of aneurysm treatment. The meta-analysis was calculated using a random-effects model, with the pooled relative risk shown in the forest plot.
Table 1:
Overview of the Characteristics of Studies Included in the Meta-Analysis
| Study | Design | Major Inclusion Criteria |
Number of Subjects |
Mean/ Median Follow-up (Mo) |
Type of APT |
Outcomes |
|---|---|---|---|---|---|---|
| Bruder (2018)27 | Prospective cohort | Aneurysmal SAH | 288 | 6 | ASA | Good outcome-mRS 0-2; vasospasm; DCI |
| Ditz (2021)32 | Retrospective cohort | Aneurysmal SAH, endovascular aneurysm treatment only | 160 | 3 | Single and dual APT | Good outcome-mRS 0-2; angiographic vasospasm; DCI |
| Hop (2000)28 | RCT | Aneurysmal SAH; early surgical clipping | 50 | 4 | ASA | Functional outcome; DCI |
| ISAT (2009)30 | Secondary analysis of RCT | Aneurysmal SAH; randomized to surgical clipping vs. endovascular coiling | 1395 | 2 and 12 | Not specified | Good outcome-mRS 0-2; DCI |
| Juvela (1995)29 | Prospective | Aneurysmal SAH | 291 | 12 | NSAIDs, ASA | Functional outcome; cerebral ischemia; DCI |
| MASH Clipping (2009)15 | RCT | Aneurysmal SAH | 108 | N/A | ASA | Good outcome-mRS 0-2; DCI |
| MASH Coiling (2009))15 | RCT | Aneurysmal SAH | 52 | N/A | ASA | Good outcome-mRS 0-2; DCI |
| Nagahama (2018)33 | Retrospective cohort | Aneurysmal SAH | 161 | 6 weeks | DAPT | Good outcome-mRS 0-2; vasospasm; DCI |
| Ono (1984)36 | RCT | Aneurysmal SAH; | 135 | 3 | Ticlopidine | Functional outcome; angiographic vasospasm; mortality |
| Oppong (2019)18 | Retrospective case-control | Aneurysmal SAH; endovascular coiling only | 580 | 6 | ASA | Good outcome-mRS 0-2; DCI |
| Shaw (1985)14 | RCT | Any SAH | 677 | 3 | Dipyridamole | Functional outcome; postoperative complications |
| Sun (2020)19 | Retrospective cohort | Aneurysmal SAH | 166 | None | DAPT | Vasospasm; DCI |
| Suzuki (1989)34 | RCT | Aneurysmal SAH | 258 | 3 | OKY-046 (Ozagrel) | Functional outcome; DCI; intracerebral hemorrhage |
| Tokiyoshi (1991)35 | Prospective cohort | Aneurysmal SAH; Hunt Hess ≤4 | 24 | 1 | Cataclot | Functional outcome; vasospasm |
| Toussaint (2004)31 | Retrospective cohort | Aneurysmal SAH | 305 | 16.4 | ASA | Good outcome-GOS ≤3; vasospasm; permanent deficit; rebleeding |
Abbreviations: ASA, acetyl salicylic acid (aspirin); APT, antiplatelet therapy; DAPT, dual antiplatelet therapy; DCI, delayed cerebral ischemia; GOS, Glasgow Outcome Scale; Mo, months; mRS, modified Rankin Scale; NSAIDs, non-steroidal anti-inflammatory drugs; RCT, randomized clinical trial.
Table 2.
Rates of Good Functional Outcome and Delayed Cerebral Infarction
| Study (Year) | Antiplatelet Therapy | No Antiplatelet Therapy | ||||
|---|---|---|---|---|---|---|
| Good Outcome (%) |
DCI (%) |
Total Population |
Good Outcome (%) |
DCI (%) |
Total Population |
|
| Bruder (2018)27 | 71 (49.3) | 57 (39.5) | 144 | 75 (52.1) | 54 (37.5) | 144 |
| Ditz (2021)32 | 62 (72.9) | 21 (24.7) | 85 | 41 (54.7) | 20 (26.7) | 75 |
| Hop (2000)28 | 18 (75.0) | 4 (16.7) | 24 | 16 (61.5) | 4 (15.4) | 26 |
| ISAT (2009)30 | 228 (68.9) | 93 (28.0) | 331 | 755 (71.0) | 319 (30.0) | 1064 |
| Juvela (1995)29 | 40 (68.9) | 22/50 (44.0) | 58 | 66 (45.8) | 40/88 (45.4) | 144 |
| MASH Clipping (2009)15 | 46 (87.0) | 15 (28.0) | 53 | 46 (84.0) | 9 (16.0) | 55 |
| MASH Coiling (2009))15 | 28 (85) | 5 (15.0) | 33 | 15 (79.0) | 2 (11.0) | 19 |
| Nagahama (2018)33 | 77 (90.6) | 3 (3.5) | 85 | 63 (82.9) | 17 (22.4) | 76 |
| Ono (1984)36 | 60 (92.3) | N/A | 65 | 54 (79.4) | N/A | 68 |
| Oppong (2019)18 | 169/230 (73.5) | 52/299 (17.4) | 329 | 120/192 (62.5) | 68/225 (30.2) | 251 |
| Shaw (1985)14 | 100(58.0) | N/A | 173 | 105 (60.0) | N/A | 175 |
| Sun (2020)19 | N/A | 1 | 65 | N/A | 11 | 101 |
| Suzuki (1989)34 | 138 (81.2) | 38/121 (31.4) | 170 | 69 (80.2) | 31/63 (49.2) | 86 |
| Tokiyoshi (1991)35 | 11 (84.6) | N/A | 13 | 8 (72.7) | N/A | 11 |
| Toussaint (2004)31 | 19 (65.5) | 3/13 (23.0) | 29 | 185 (67.0) | 46/92 (50.0) | 276 |
| Total Events | 1067/1493 | 314/1303 | 1657 | 1618/2411 | 621/2028 | 2571 |
Abbreviations: DCI, delayed cerebral infarction; N/A, not available.
Highlights.
In this meta-analysis of nearly 4,500 aSAH patients, initiation of APT was associated with a modestly lower risk of major disability or death.
There was a trend toward a decreased risk of DCI with the use of APT.
APT was associated with favorable functional outcomes among patients with aneurysms treated with endovascularly compared to those who had surgical clipping.
Funding/Support:
The study was funded by the National Institutes of Health (NIH) to the corresponding author (K23NS105948). The funding entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures:
Dr. Merkler is supported by the American Heart Association (18CDA34110419), the Leon Levy Foundation, and reports personal fees for medicolegal consulting on stroke and neurological disorders. Dr. Kamel reports serving as co-PI for the NIH-funded ARCADIA trial (NINDS U01NS095869) which receives in-kind study drug from the BMS-Pfizer Alliance for Eliquis® and ancillary study support from Roche Diagnostics, serving as Deputy Editor for JAMA Neurology, serving as a steering committee member of Medtronic's Stroke AF trial (uncompensated), serving on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and having served on an advisory board for Roivant Sciences related to Factor XI inhibition. Dr. Knopman serves as a co-PI for the Medtronic-funded Embolization of the Middle Meningeal Artery With ONYX™ Liquid Embolic System for Subacute and Chronic Subdural Hematoma (EMBOLISE) trial. Dr. Murthy reports personal fees for medicolegal consulting on stroke and neurological disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Author Contributions:
Dr. Murthy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Garton, Berger, Murthy.
Acquisition, analysis, or interpretation of data: Garton, Berger, Murthy.
Drafting of the manuscript: Garton, Berger, Murthy.
Critical revision of the manuscript for important intellectual content: Garton, Berger, Merkler, Kamel, Stieg, Knopman, Zhang, Murthy.
Statistical analysis: Zhang, Murthy.
Administrative, technical, or material support: Murthy.
Study supervision: Murthy.
Funding: Murthy.
References
- 1.Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. Aug 2013;73(2):217–22; discussion 212-3. doi: 10.1227/01.neu.0000430290.93304.33 [DOI] [PubMed] [Google Scholar]
- 2.Lawton MT, Vates GE. Subarachnoid Hemorrhage. N Engl J Med. Jul 20 2017;377(3):257–266. doi: 10.1056/NEJMcp1605827 [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. May 1998;50(5):1413–8. doi: 10.1212/wnl.50.5.1413 [DOI] [PubMed] [Google Scholar]
- 4.Yoon S, Yoon JC, Winkler E, Liu C, Lawton MT. Nationwide Analysis of Cost Variation for Treatment of Aneurysmal Subarachnoid Hemorrhage. Stroke. Dec 3 2018:STROKEAHA118023079. doi: 10.1161/STROKEAHA.118.023079 [DOI] [PubMed] [Google Scholar]
- 5.Modi S, Shah K, Schultz L, Tahir R, Affan M, Varelas P. Cost of hospitalization for aneurysmal subarachnoid hemorrhage in the United States. Clin Neurol Neurosurg. Jul 2019;182:167–170. doi: 10.1016/j.clineuro.2019.05.018 [DOI] [PubMed] [Google Scholar]
- 6.Connolly ES Jr., Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. Jun 2012;43(6):1711–37. doi: 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 7.Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care. Sep 2011;15(2):211–40. doi: 10.1007/s12028-011-9605-9 [DOI] [PubMed] [Google Scholar]
- 8.Dorhout Mees SM, Algra A, Vandertop WP, et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet. Jul 7 2012;380(9836):44–9. doi: 10.1016/S0140-6736(12)60724-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD, Collaborators S. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. Jul 2014;13(7):666–75. doi: 10.1016/S1474-4422(14)70084-5 [DOI] [PubMed] [Google Scholar]
- 10.Fujimura M, Joo JY, Kim JS, Hatta M, Yokoyama Y, Tominaga T. Preventive Effect of Clazosentan against Cerebral Vasospasm after Clipping Surgery for Aneurysmal Subarachnoid Hemorrhage in Japanese and Korean Patients. Cerebrovasc Dis. 2017;44(1-2):59–67. doi: 10.1159/000475824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saber H, Desai A, Palla M, Mohamed W, Seraji-Bozorgzad N, Ibrahim M. Efficacy of Cilostazol in Prevention of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage: A Meta-Analysis. J Stroke Cerebrovasc Dis. Nov 2018;27(11):2979–2985. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 12.Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. Nov 2008;28(11):1761–70. doi: 10.1038/jcbfm.2008.74 [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira Manoel AL, Macdonald RL. Neuroinflammation as a Target for Intervention in Subarachnoid Hemorrhage. Front Neurol. 2018;9:292. doi: 10.3389/fneur.2018.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw MD, Foy PM, Conway M, et al. Dipyridamole and postoperative ischemic deficits in aneurysmal subarachnoid hemorrhage. J Neurosurg. Nov 1985;63(5):699–703. doi: 10.3171/jns.1985.63.5.0699 [DOI] [PubMed] [Google Scholar]
- 15.van den Bergh WM, Algra A, Rinkel GJ, Group MS. Magnesium and aspirin treatment in patients with subarachnoid haemorrhage. Comparison of effects after endovascular and neurosurgical aneurysm occlusion. J Neurol. Feb 2009;256(2):213–6. doi: 10.1007/s00415-009-0057-5 [DOI] [PubMed] [Google Scholar]
- 16.Dorhout Mees SM, Rinkel GJ, Hop JW, Algra A, van Gijn J. Antiplatelet therapy in aneurysmal subarachnoid hemorrhage: a systematic review. Stroke. Sep 2003;34(9):2285–9. doi: 10.1161/01.STR.0000083621.44269.3E [DOI] [PubMed] [Google Scholar]
- 17.Maher M, Schweizer TA, Macdonald RL. Treatment of Spontaneous Subarachnoid Hemorrhage: Guidelines and Gaps. Stroke. Apr 2020;51(4):1326–1332. doi: 10.1161/STROKEAHA.119.025997 [DOI] [PubMed] [Google Scholar]
- 18.Darkwah Oppong M, Gembruch O, Pierscianek D, et al. Post-treatment Antiplatelet Therapy Reduces Risk for Delayed Cerebral Ischemia due to Aneurysmal Subarachnoid Hemorrhage. Neurosurgery. Dec 1 2019;85(6):827–833. doi: 10.1093/neuros/nyy550 [DOI] [PubMed] [Google Scholar]
- 19.Sun GU, Park E, Kim DW, Kang SD. Dual antiplatelet treatment associated with reduced risk of symptomatic vasospasm and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Cerebrovasc Endovasc Neurosurg. Sep 2020;22(3):134–140. doi: 10.7461/jcen.2020.22.3.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. Jul 21 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. Apr 19 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A, Giambrone AE, Gialdini G, et al. Silent brain infarction and risk of future stroke: a systematic review and meta-analysis. Stroke. Mar 2016;47(3):719–25. doi: 10.1161/STROKEAHA.115.011889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. Nov 2013;44(11):3071–7. doi: 10.1161/STROKEAHA.113.002551 [DOI] [PubMed] [Google Scholar]
- 24.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. Oct 26 2002;360(9342):1267–74. doi: 10.1016/s0140-6736(02)11314-6 [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. Sep 1986;7(3):177–88. doi:0197-2456(86)90046-2 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. Sep 6 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruder M, Won SY, Wagner M, et al. Continuous Acetylsalicylic Acid Treatment Does Not Influence Bleeding Pattern or Outcome of Aneurysmal Subarachnoid Hemorrhage: A Matched-Pair Analysis. World Neurosurg. May 2018;113:e122–e128. doi: 10.1016/j.wneu.2018.01.188 [DOI] [PubMed] [Google Scholar]
- 28.Hop JW, Rinkel GJ, Algra A, Berkelbach van der Sprenkel JW, van Gijn J. Randomized pilot trial of postoperative aspirin in subarachnoid hemorrhage. Neurology. Feb 22 2000;54(4):872–8. doi: 10.1212/wnl.54.4.872 [DOI] [PubMed] [Google Scholar]
- 29.Juvela S. Aspirin and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Neurosurg. Jun 1995;82(6):945–52. doi: 10.3171/jns.1995.82.6.0945 [DOI] [PubMed] [Google Scholar]
- 30.van den Bergh WM, Kerr RS, Algra A, Rinkel GJ, Molyneux AJ, International Subarachnoid Aneurysm Trial Collaborative G. Effect of antiplatelet therapy for endovascular coiling in aneurysmal subarachnoid hemorrhage. Stroke. Jun 2009;40(6):1969–72. doi: 10.1161/STROKEAHA.108.528802 [DOI] [PubMed] [Google Scholar]
- 31.Toussaint LG 3rd, Friedman JA, Wijdicks EF, et al. Influence of aspirin on outcome following aneurysmal subarachnoid hemorrhage. J Neurosurg. Dec 2004;101(6):921–5. doi: 10.3171/jns.2004.101.6.0921 [DOI] [PubMed] [Google Scholar]
- 32.Ditz C, Machner B, Schacht H, et al. Effects of post-interventional antiplatelet therapy on angiographic vasospasm, delayed cerebral ischemia, and clinical outcome after aneurysmal subarachnoid hemorrhage: a single-center experience. Neurosurg Rev. Jan 25 2021;doi: 10.1007/s10143-021-01477-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagahama Y, Allan L, Nakagawa D, et al. Dual antiplatelet therapy in aneurysmal subarachnoid hemorrhage: association with reduced risk of clinical vasospasm and delayed cerebral ischemia. J Neurosurg. Sep 2018;129(3):702–710. doi: 10.3171/2017.5.JNS17831 [DOI] [PubMed] [Google Scholar]
- 34.Suzuki S, Iwabuchi T, Tanaka T, et al. Prevention of cerebral vasospasm with OKY-046 an imidazole derivative and a thromboxane synthetase inhibitor. A preliminary co-operative clinical study. Acta Neurochir (Wien). 1985;77(3-4):133–41. doi: 10.1007/BF01476216 [DOI] [PubMed] [Google Scholar]
- 35.Tokiyoshi K, Ohnishi T, Nii Y. Efficacy and toxicity of thromboxane synthetase inhibitor for cerebral vasospasm after subarachnoid hemorrhage. Surg Neurol. Aug 1991;36(2):112–8. doi: 10.1016/0090-3019(91)90228-2 [DOI] [PubMed] [Google Scholar]
- 36.Dorhout Mees SM, van den Bergh WM, Algra A, Rinkel GJ. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. Oct 17 2007;(4):CD006184. doi: 10.1002/14651858.CD006184.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Can A, Rudy RF, Castro VM, et al. Association between aspirin dose and subarachnoid hemorrhage from saccular aneurysms: A case-control study. Neurology. Sep 18 2018;91(12):e1175–e1181. doi: 10.1212/WNL.0000000000006200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung DY, Leslie-Mazwi TM, Patel AB, Rordorf GA. Management of External Ventricular Drains After Subarachnoid Hemorrhage: A Multi-Institutional Survey. Neurocrit Care. Jun 2017;26(3):356–361. doi: 10.1007/s12028-016-0352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller A, Mould WA, Freeman WD, et al. The Incidence of Catheter Tract Hemorrhage and Catheter Placement Accuracy in the CLEAR III Trial. Neurocrit Care. Aug 2018;29(1):23–32. doi: 10.1007/s12028-017-0492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natarajan SK, Shallwani H, Fennell VS, et al. Flow Diversion after Aneurysmal Subarachnoid Hemorrhage. Neurosurg Clin N Am. Jul 2017;28(3):375–388. doi: 10.1016/j.nec.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 41.Investigators SPS, Benavente OR, Hart RG, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. Aug 30 2012;367(9):817–25. doi: 10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med. Jul 19 2018;379(3):215–225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parasram M PN, Merkler AE, Ch’ang J, Navi BB, Kamel H, Zhang C, Murthy SB. Long-Term Risk of Ischemic Stroke Among Elderly Survivors of Non-Traumatic Subarachnoid Hemorrhage. Cerebrovascular Diseases. 2021. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieuwkamp DJ, Vaartjes I, Algra A, Rinkel GJ, Bots ML. Risk of cardiovascular events and death in the life after aneurysmal subarachnoid haemorrhage: a nationwide study. Int J Stroke. Dec 2014;9(8):1090–6. doi: 10.1111/j.1747-4949.2012.00875.x [DOI] [PubMed] [Google Scholar]
- 45.Harbison JW. Ticlopidine versus aspirin for the prevention of recurrent stroke. Analysis of patients with minor stroke from the Ticlopidine Aspirin Stroke Study. Stroke. Dec 1992;23(12):1723–7. doi: 10.1161/01.str.23.12.1723 [DOI] [PubMed] [Google Scholar]
- 46.Lauzier DC, Jayaraman K, Yuan JY, et al. Early Brain Injury After Subarachnoid Hemorrhage: Incidence and Mechanisms. Stroke. May 2023;54(5):1426–1440. doi: 10.1161/STROKEAHA.122.040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ying Z, Giachini FR, Tostes RC, Webb RC. Salicylates dilate blood vessels through inhibiting PYK2-mediated RhoA/Rho-kinase activation. Cardiovasc Res. Jul 1 2009;83(1):155–62. doi: 10.1093/cvr/cvp084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, Brecher P. Salicylate Inhibits Phosphorylation of the Nonreceptor Tyrosine Kinases, Proline-Rich Tyrosine Kinase 2 and c-Src. Hypertension. Jan 2001;37(1):148–153. doi: 10.1161/01.hyp.37.1.148 [DOI] [PubMed] [Google Scholar]
- 49.Effect of Antiplatelet Therapy on Cognition After Aneurysmal Subarachnoid Hemorrhage. NCT04548401. https://clinicaltrials.gov/study/NCT04548401?cond=Subarachnoid%20Hemorrhage&intr=antiplatelet&rank=1. Accessed September 1, 2023.
- 50.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. Jun 25 2016;387(10038):2605–2613. doi: 10.1016/S0140-6736(16)30392-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.