Abstract
Sex differences are found across brain regions, behaviors, and brain diseases. Sexual differentiation of the brain is initiated prenatally but it continues throughout life, as a result of the interaction of three major factors: gonadal hormones, sex chromosomes, and the environment. These factors are thought to act, in part, via epigenetic mechanisms, which control chromatin and transcriptional states in brain cells. In this Review, we discuss evidence that epigenetic mechanisms underlie sex-specific neurobehavioral changes during critical organizational periods, across the estrous cycle, and in response to diverse environments throughout life. We further identify future directions for the field that will provide novel mechanistic insights into brain sex differences, inform brain disease treatments and women’s brain health particularly, and apply to people across genders.
Keywords: epigenetics, chromatin, sex difference, gonadal hormones, sex chromosomes, brain structure and function
The role of sex and gender in brain physiology and disease
Few topics in neuroscience are more controversial or less understood than the role of sex and gender in brain physiology and disease. Neuroscience has infamously focused on studying the male brain and behavior [1–3] and this has left current knowledge of the female brain and brain sex differences shallow and in great need for improvement [4–6]. In addition, the definitions of both sex and gender are continuously evolving. Whereas both terms were historically considered binary, it is now widely accepted that gender exists as a spectrum and sex is being redefined including intersex conditions or differences in sex development, beyond males and females [7–9]. For the sake of this article, we will largely consider two main sexes – males and females, as the available knowledge covered in this manuscript is mostly based on binary sex studies in rodents and some clinical information collected from presumably cisgender individuals. However, we will refer to broader categories of sex or gender, whenever possible.
In both rodents and humans, sex differences have been described in gene regulation, neurochemistry, brain structure, behavior, brain disease, and response to psychotropic drugs and other environmental factors [5,10–24]. While effect sizes and the relevance of structural brain differences in humans have been debated [25–28], sex bias in brain disorders has been reported across diseases [5], and sex- and gender-informed treatments could be transformational for neuropsychiatric disorders. For example, depression and anxiety disorders are twice as prevalent in women than in men [16] while autism spectrum disorder is up to four times more common in boys than in girls [29]. Significant sex differences in prevalence and severity of symptoms exist in other conditions such as Alzheimer’s disease, posttraumatic stress disorder (PTSD), anorexia nervosa, attention deficit hyperactivity disorder, multiple sclerosis, Parkinson’s disease, and schizophrenia [5,30–32]. However, in order to develop treatments that account for sex, we need to understand the mechanism(s) underlying sex differences in brain physiology and disease, and those have been largely understudied. Among possible mechanisms, epigenetic mechanisms are a plausible candidate.
In this Review, we first describe the potential sources of brain sex differences, including the effects of gonadal hormones, sex chromosomes, and environmental factors. Next, we review epigenetic regulation in the brain, including chromatin modifications and epigenetic machinery that control chromatin and transcriptional states in cells. We then discuss evidence that epigenetic mechanisms underlie sex-specific neurobehavioral changes during sensitive developmental windows, across the estrous cycle, and in response to diverse environments throughout life. Considering that this is still a fairly nascent area of investigation, we will discuss evidence where available, along with questions that remain to be addressed and suggested directions for future research.
Sex-specific characteristics of the brain: the interaction of gonadal hormones, sex chromosomes, and the environment
Sexual differentiation of the brain (see Glossary) is a dynamic process that is initiated prenatally but it continues throughout life, as a result of the interaction of gonadal hormones, sex chromosomes, and the environment (Figure 1).
Figure 1. Sexual differentiation of the brain.
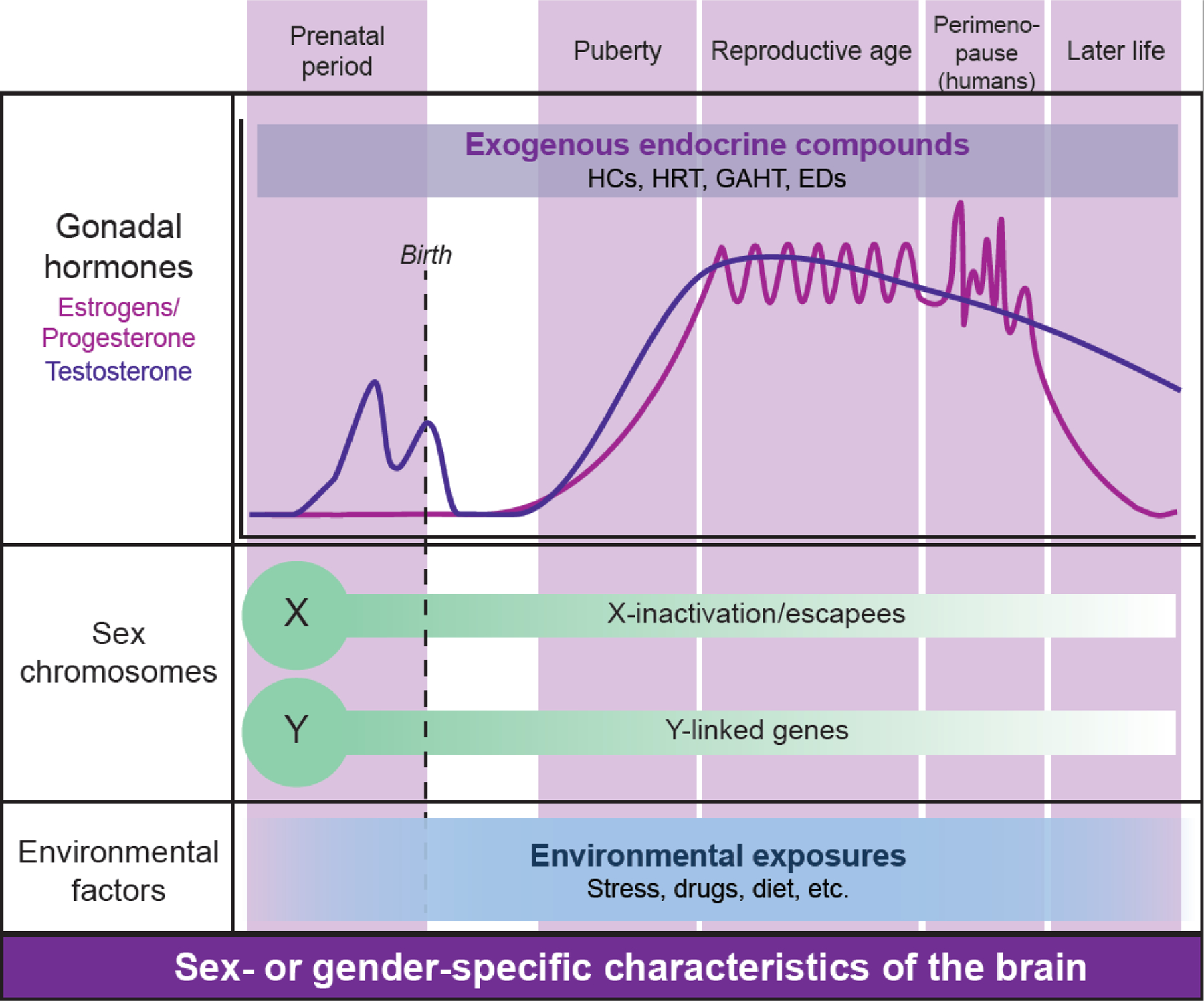
Sex- or gender-specific characteristics of the brain are continously shaped throughout life, by the interactions of three major factors: gonadal hormones, sex chromosomes, and environmental influences. In rodents and humans, hormonal surges occur primarily during two periods in life: during the perinatal period (testosterone in males), and the peripubertal period (testosterone in males; estrogens and progesterone in females). These hormonal surges coincide with two important organizational periods in sexual differentiation of the brain. Later on, during post-puberty, the brain is shaped by hormonal effects that are considered activational in both males and females across species. In humans, cyclical changes in estrogens and progesterone occur until menopause is reached in women and other people with ovaries. Menopause in humans is preceded by menopausal transition or ‘perimenopause’ which is characterized by irregular hormonal changes. Exogenous endocrine compounds, including hormonal contraceptives (HCs), hormonal replacement therapy (HRT), gender-affirming hormone therapy (GAHT), and endocrine disruptors (EDs), will affect hormonal status and sex- and gender-related characteristics of the brain. Sex chromosome complement (most frequently XX or XY, although other combinations such as XXY, XYY, XXX, or XO occur, too) determine gonadal development and, by being present in each brain cell, can affect the cellular environment (e.g. repressor complexes involved in X-chromosome inactivation) or lead to sex-biased gene expression (X-escapees, Y-linked genes). Environmental exposures such as stress, drugs, or diet, can either have sex-independent effects, or increase or decrease brain sex differences. Sex is typically categorized as female, male, or intersex. Gender in humans exists on the spectrum, including (but not limited to) woman, man, non-binary, agender, and gender-fluid individuals. In cisgender individuals, gender corresponds to gender/sex assigned at birth. In transgender individuals, gender differs from gender or sex assigned at birth, and these individuals may or may not choose to go through gender-affirming procedures including gender-affirming hormone therapy.
Gonadal hormones.
Differential secretion of gonadal hormones is thought to represent the major source of sex differences across the lifespan. While gonads are initially undifferentiated, the early prenatal expression of Sry in XY individuals typically initiates the development of testes and secretion of testosterone [33,34]. In rodents, testosterone peaks prenatally, around GD16 in mice and GD18 in rats, and declines shortly after birth (Figure 1). During this critical period, testosterone is converted to estradiol in the brain via the enzyme aromatase; estradiol then “masculinizes” and defeminizes the brain, as reflected in adult behaviors related to reproduction [33,34]. In humans, though, testosterone seems to exert its masculinizing effects on the brain directly, acting through androgen receptors, and the critical period is believed to open and close prenatally [34]. The effect of perinatal surge of testosterone on the male brain is considered the organizational effect that sets up the stage for later (activational) effects of gonadal hormones.
In contrast, female development is considered the default developmental pathway including the development of ovaries and consequent “feminization” of the brain in XX individuals. In females, ovaries do not synthesize steroid hormones during prenatal and early postnatal development (Figure 1), although the default pathway of feminization certainly involves its own drivers and mechanisms, which have been understudied. In fact, recent evidence from rat studies indicates that brain feminization requires active repression of masculinization [35], questioning a long-term belief that feminization is a passive process.
The onset of puberty relies on the activation of the hypothalamic-pituitary-gonadal (HPG) axis [36]. The pulsatile secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus initiates the cells of the pituitary gland to secrete the gonadotropins, luteinizing hormone and follicle-stimulating hormone, which then stimulate the production of estrogens and progesterone from the ovaries in females, and testosterone from the testes in males (Figure 1). The peripubertal period involves significant hormonally-driven shifts in brain organization and is still considered organizational in setting brain sex differences that determine the behavioral potential of the adult organism [37,38].
The high levels of gonadal hormones post-puberty continue to shape the brain and this phenomenon is known as activational effects of hormones, affecting both reproduction-relevant brain areas (e.g. specific hypothalamic nuclei) and areas not directly involved in reproduction (e.g. hippocampal regions). In females, estrogens and progesterone undergo cyclical changes over the ovarian cycle which are associated with dynamic changes in brain structure and function in both mice and humans [6,39–41] until ovarian function reaches senescence in the period known as menopause in humans [42]. Three forms of estrogens are produced in females - estradiol, estrone, and estriol - with estradiol being the major and most potent estrogen during the reproductive period [43]. Reproductive aging in humans is more abrupt in females than in males and this, by itself, creates a sex difference that is reflected in differential brain disease risk following the cessation of reproductive ovarian function [44].
However, the gonadal hormone dynamics described above would be different in intersex individuals and people with gonadal dysfunction. This hormonal dynamic, and consequently the brain, may also be altered throughout life by exogenous compounds that affect gonadal hormone levels and their actions, or have their own endocrine actions, including endocrine disruptors [45], hormonal contraceptives [46], hormone replacement therapy [47], and gender-affirming hormone therapy [48] (Figure 1).
Sex chromosomes.
While sex chromosomes are typically thought to drive sex differences by determining gonadal development and thus hormonal secretions, each cell has XX or XY chromosomal complement that can contribute to sex-specific cellular physiology (see Box 1, Figure 1) [33]. While having a relatively small number of genes, the Y-chromosome contains genes that, beyond sex determination, have other functions [49]. In a female organism, by contrast, cells typically have one active and one inactive X-chromosome, but there are genes that escape X-chromosome inactivation making for the imbalanced expression of X-linked genes in males and females. Studies in mice have contributed to the understanding of sex chromosome-linked genes and their functions. Specifically, the development of the ‘four core genotypes’ mouse model has allowed the distinction between the contribution of sex chromosomes vs. gonadal secretions [50–52], showing the independent contribution of sex chromosomes to multiple neurobehavioral phenotypes including aggression, addiction-related behavior, and nociception (see Box 1) [51]. In addition to two main sex chromosome complements, XX and XY, sex chromosome dosage can also vary among individuals, including combinations such as XXY, XYY, XXX, or XO, with implications for the cellular phenotype and brain structure [53–55].
Box 1. Sex chromosome complement and sex differences in the brain.
Sex chromosome complement typically determines gonadal development (XY - testes; XX - ovaries), so it is challenging to assess the contribution of sex chromosomes to sex differences in the brain independently of gonadal hormones. However, the ‘four core genotypes’ mouse model was developed to allow distinction between the contribution of sex chromosomes and gonadal secretions. In this genetic model, the Sry gene is moved to an autosome, so Y-chromosome (Y-) is no longer testis-determining. This allows the generation of four mouse genotypes with varying sex components, in two of which sex chromosome complement does not correspond to typical gonadal sex: XX – ovaries; XY- Sry – testes; XXSry – testes; and XY- - ovaries (Figure IA) [50–52]. Experiments in these mice, by comparing the phenotypes of the four core genotypes, show gonadal hormone-independent contribution of sex chromosomes to multiple neurobehavioral phenotypes including aggression, addiction-related behavior, and nociception [51].
Generally speaking, the direct contribution of sex chromosome complement to sex differences in the brain comes either from Y-linked genes present in XY individuals (typically males) only, or from double dose of the X-chromosome present in XX individuals (typically females) only (Figure IB). While one X-chromosome in XX individuals is “inactivated”, some genes on the X-chromosome escape inactivation, making for the imbalanced expression of X-linked genes in males and females [100–102]. In addition, the inactive X-chromosome (Xi) has a compact conformation and is associated with repressor complexes in a cell, thus providing a particular cellular environment that exists in XX but not XY cells (Figure IB) [100]. Interestingly, a recent study from our group has shown that Xi in neurons may be more dynamic than anticipated, changing its conformation as a result of ovarian hormone changes [95], with potentially important implications for sex-specific neurobiology and women’s health.
In order to understand X inactivation-related phenomena, there are specific mouse models that allow studying the contribution of Xi and X-escapee genes to brain and behavioral phenotypes. One model includes 39,XO mice which do not have the second (inactive) X-chromosome but are phenotypically females [141,142]. The comparison of these mice with wildtype female (XX) and male (XY) mice of the same genetic background allows for better understanding of the role of Xi in the brain. In addition, the hybrid mouse models that have one mutated copy of Xist allow the distinction between the active and inactive X-chromosomes by allele-specific genomics analyses [143,144].
Figure I (for Box 1). Contribution of sex chromosomes to sex differences in the brain.
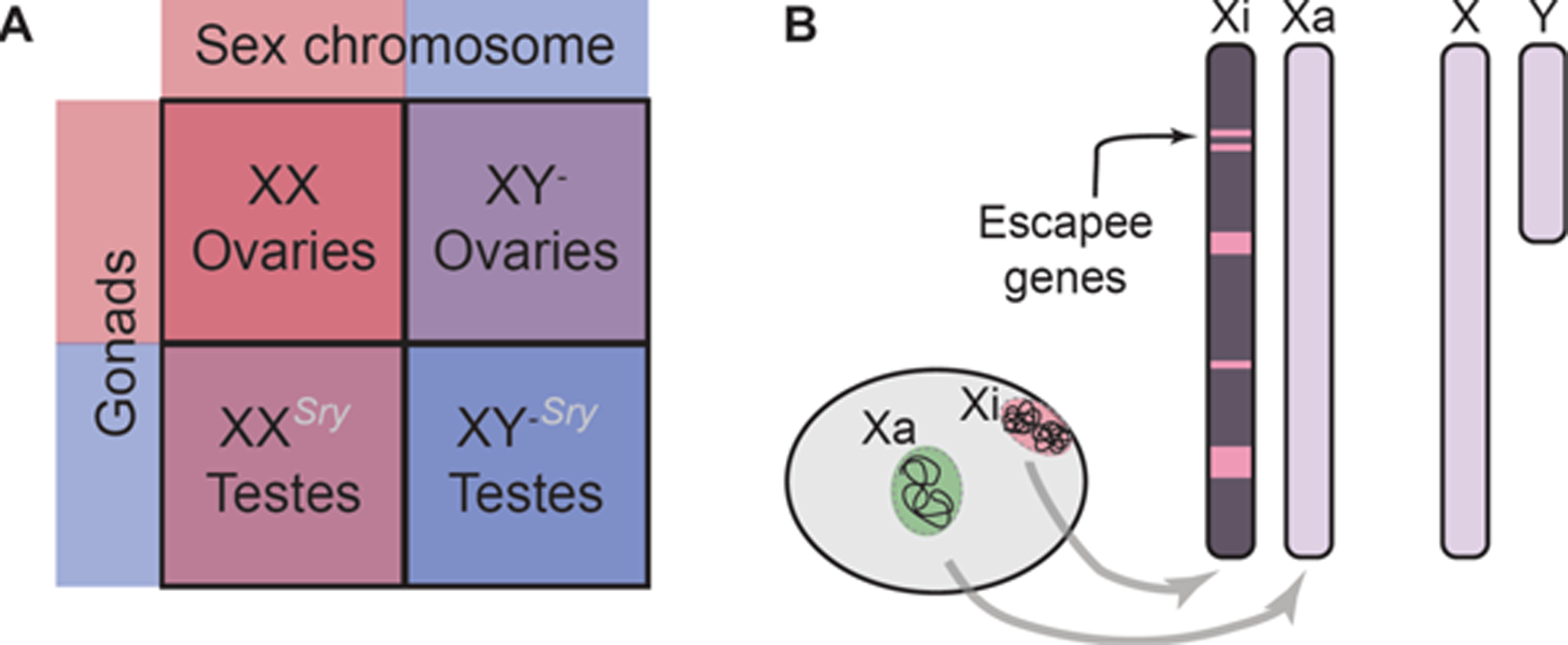
A. The four core genotypes mouse model allows for decoupling of chromosomal and gonadal sex and assessment of the role of sex chromosomes independent of gonadal secretions. B. In females (typically having XX), one of the X-chromosomes is inactivated and has a highly repressive structure but some genes escape inactivation. In males (typically XY), the Y chromosome contains genes that, beyond sex determination, have other functions.
Environmental factors.
Environmental factors can possibly increase or decrease sex differences in the brain. Sex- or gender-specific environments which are typically formed in human society, reflecting different gender stereotypes and expectations (e.g. using gendered toys, gender socialization), are likely to increase sex differences in the brain. However, males and females can also have different responses to the same environmental cue. For instance, in mice, gestational bisphenol A exposure [gestational days (GD) 0–19] affects recognition memory in males but not females [56], while early-life maternal separation [during postnatal days (P) 1–14] more significantly impacts anxiety- and depression-related phenotypes in female than male mice [22]. In contrast, other mouse studies have shown that environmental exposures as different as early-life maternal separation stress (P1-14) [57], adolescent social isolation stress (P22-42) [58] or late-adolescent cocaine exposure (P52-4) [57] can decrease sex differences in nucleus accumbens (NAc) gene expression and addiction-related phenotypes.
In summary, for each individual, the unique interaction of their gonadal hormone status, sex chromosome complement, and environment will determine the state of their sexual differentiation of the brain. It is of interest to understand how epigenetic mechanisms contribute to shaping sex-specific brain physiology and disease.
Epigenetic regulation in the brain: the basics
Many basic principles of epigenetic regulation of gene expression [59–61] apply to neuroscience, although multiple neuron-specific epigenetic phenomena have been described, consistent with highly dynamic gene regulation in neurons [62,63]. Chromatin, a complex structure of DNA and associated proteins, mainly histones, has a central role in epigenetic regulation (Figure 2). At the first level of chromatin organization, one observes nucleosomes or “beads on the string” structure, where ~150 bp of DNA is wrapped around octamers of four canonical histone proteins (H2A, H2B, H3, and H4). Opening or closing of this structure can change the accessibility of regulatory DNA sequences, such as enhancers and promoters, allowing or blocking transcription, respectively. Chromatin accessibility is quite dynamic in neuronal cells, and transcription factors encoded by immediate–early genes, such as AP-1 family members, have been proposed as initiators of chromatin opening [64,65]. In addition to nucleosomes, there are higher levels of chromatin organization including three-dimensional (3D) genome organization that allows interactions of genes with their distant cis-regulatory elements (Figure 2, [60]). This so-called “3D genome” includes chromosome compartments, CTCF loops, and enhancer-promoter interactions and is implicated in neuronal gene regulation [66–69].
Figure 2. Overview of epigenetic regulation.
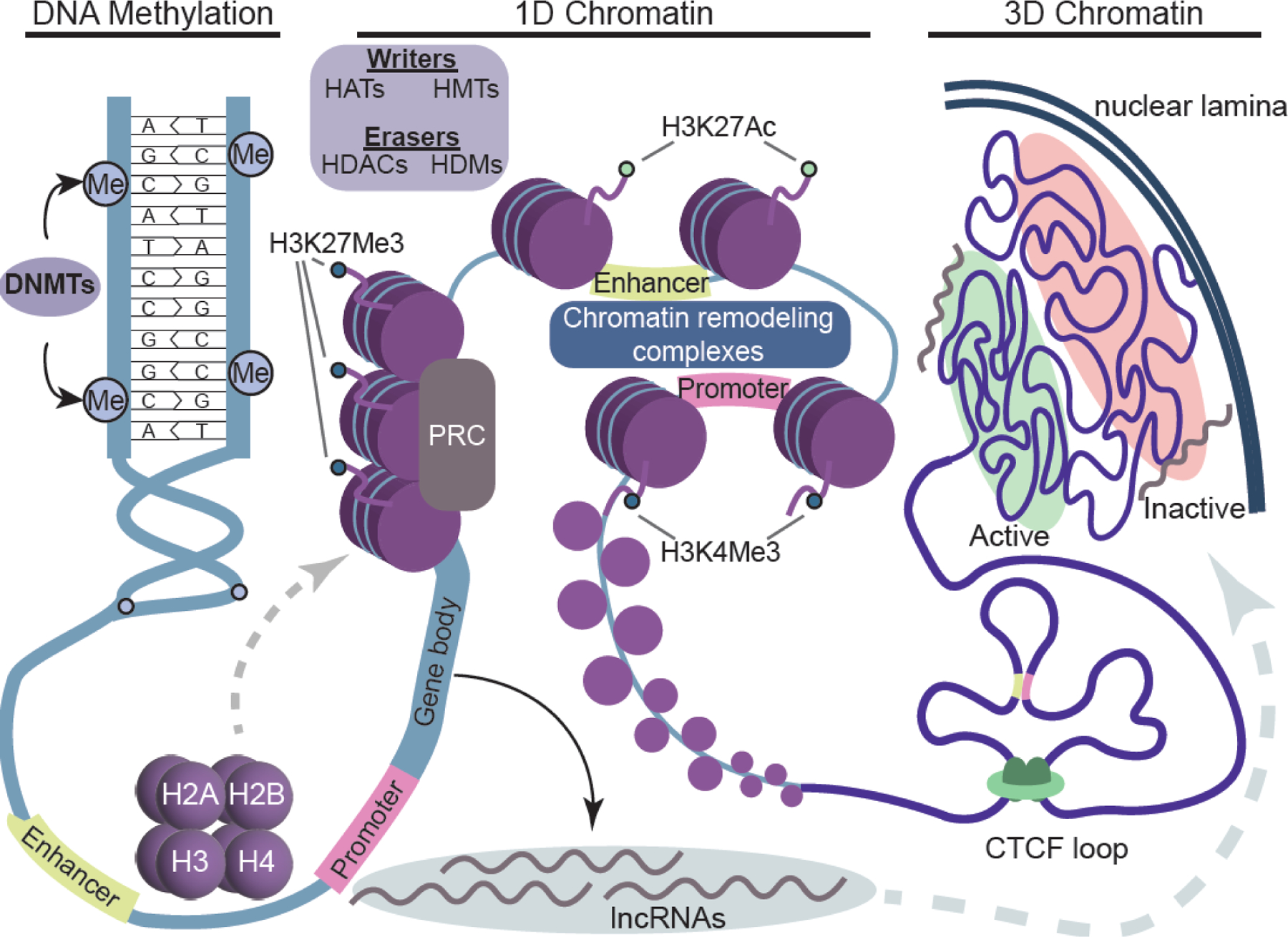
DNA is wrapped around histone proteins forming the highly specialized structure called chromatin. The best established chromatin modifications are DNA methylation and histone acetylation and methylation, all of which are dynamic and could affect chromatin configuration directly or indirectly. Cytosine methylation is catalyzed by DNA methyltransferase (DNMT) and typically inhibits gene transcription. Histone acetylation is “written” by histone acetyltransferase (HAT) and “erased” by histone deacetylase (HDAC). Histone methylation is “written” by residue-specific histone methyltransferase (HMT) and “erased” by histone demethylase (HDM). Active histone modifications mark active enhancers (H3K27ac) and promoters (H3K4me3) and promote chromatin opening and transcription by recruiting ATP-dependent chromatin remodeling complexes. Conversely, Polycomb repressor complexes (PRC) mediate deposition of repressive histone marks (H3K27me3) and induce repressive chromatin state, non-permisive to transcription. Long non-coding RNAs (lncRNA) are another epigenetic mechanism that can recruit chromatin modyfing proteins and change chromatin configuration. At the first level of chromatin organization (1D chromatin), one sees nucleosomes or “beads on the string” type of structure where 147 bp of DNA is wrapped around histone octamers consisting of H2A, H2B, H3, and H4 core histones. Some of these histones can be replaced by histone variants that can affect chromatin properties. The higher level of chromation organization, also known as the “3D genome”, includes a long-range enhancer-promoter interactions, CTCF loops, and chromosome compartments, and is implicated in gene regulation and neuronal function. Typically, activily transcribed genes are located in active compartments (euchromatin) toward inside of the nucleus, while inactive genes are located in inactive compartments (heterochromatin) close to the nuclear lamina. This is examplified by the inactive X-chromosome which is typically located at the nuclear periphery with X escapee genes residing toward the outside of the compacted Xi chromosome (see also Box 1).
Within chromatin, covalent modifications of DNA and histones have been described and implicated in the control of chromatin structure and gene expression. DNA methylation is catalyzed by DNA methyltransferases (DNMTs) and is typically involved in silencing of gene regulatory elements, directly or through recruitment of methyl-CpG-binding proteins and histone modifiers such as histone deacetylases (HDACs) [61]. Unlike in other tissues, DNA methylation in the brain is characterized by unusually high levels of non-CpG methylation and rather frequent occurrence of 5-hydroxy-methylation [70], which is its own epigenetic mark and, via action of Ten eleven translocation (Tet) proteins, is a mediator in DNA demethylation process [71].
Among many histone modifications, histone acetylation and methylation have been mostly studied in the brain, and they can change chromatin in either cis (by changing histone charge) or in trans (by recruiting chromatin modifiers) [72]. Histone acetylation, catalyzed by histone acetyltransferases (HATs), neutralizes positively charged amino groups on histone lysine residues, thus allowing looser binding between histones and negatively charged DNA, and chromatin opening. Regarding the trans mechanism, both histone H3K4 trimethylation (marker of active promoters) and H3K27 acetylation (marker of active enhancers) recruit ATP-dependent chromatin remodeling complexes, opening local chromatin and allowing gene transcription [72]. In contrast, repressive histone marks such as H3K27 trimethylation are mediated by Polycomb Repressor Complexes (PRC1 and 2) that induce repressive chromatin structure, non-permissive for transcription. The PRC2 proteins include the histone methyltransferase enhancer of zeste (EZH2), catalyzing H3K27me3, and its binding partner embryonic ectoderm development (EED), which functions to recruit PRC1 complex to H3K27me3 loci, leading to gene silencing [73].
Additionally, canonical histones can be replaced by histone variants (e.g. H3.3 or macroH2A) that can change chromatin physical properties and affect gene regulation [74]. Finally, long non-coding RNAs (lncRNAs) are another level of epigenetic regulation that has the ability to affect chromatin by recruiting other components of epigenetic machinery, thus affecting transcriptional regulation [75].
In sum, epigenetic mechanisms work in concert to drive chromatin states and gene expression, with critical importance for the regulation of brain structure and function, including in shaping sex differences in the brain.
Sex-specific epigenetic findings in the brain: current knowledge
Here we describe studies that have revealed sex-specific epigenetic findings in the brain, including studies of sex-specific factors that drive brain sexual differentiation, such as gonadal hormones and sex chromosomes, as well as studies of environmental factors that shape the brain epigenome sex-specifically.
Gonadal hormone-mediated epigenetic regulation
Developmental organizational period.
Typical experiments in rodents that examine the organizational role of perinatal testosterone on the brain include injections of testosterone or estradiol in female pups around birth to induce “masculinization” of the female brain, as reflected in more masculine-like sexual behavior and larger, male-sized sexually dimorphic brain regions such as the bed nucleus of the stria terminalis (BNST) in mice and the sexually dimorphic nucleus of the preoptic area (POA) in rats. Females retain sensitivity to the organizational effects of testosterone for the first week of postnatal life, known as the sensitive period [76].
The larger volume of the BNST in male mice seems to be defined by both sex-biased cell number (increased cell survival in males) and gene expression program involving an epigenetic mechanism [77]. A study in mice showed that treatment with an HDAC inhibitor at P1 and P2 counteracted the “masculinizing” effect of testosterone and eliminated sex difference in the mouse BNST, implicating chromatin remodeling in the sexual determination of this brain region [78]. A more recent study indicated that neonatal (P0) estradiol exposure activates estrogen receptor alpha (ERα) and induces male-typical chromatin opening leading to male-biased gene expression program in the BNST of female mouse pups [77]. The details of the machinery involved in ERα-driven chromatin remodeling and link to sex-biased behavior in adulthood remain to be established.
Another study used a similar paradigm in rats and showed that neonatal (P0) estradiol exposure “masculinizes” the POA and sexual behavior via a DNA methylation-dependent mechanism [35]. Estradiol treatment of female rats reduces DNMT activity and decreases DNA methylation in the POA, while neonatal treatment with DNMT inhibitor (P0 and P1) mimics the effect of estradiol on both the POA expression of dendritic spine markers and sexual behavior in female rats, confirming the role of DNA methylation in brain sexual differentiation [35]. Another study using testosterone and DNMT inhibitor in female mice (P0 and P1) also implicated the role of DNA methylation in the increased number of calbindin cells in the POA and reduced number of ERα cells in the ventromedial nucleus of the hypothalamus of males [79]. While “feminization” of the brain is thought as a more passive process, recent findings indicate that rat brain feminization involves active repression of masculinizing genes via DNA-methylation [35], suggesting new opportunities for epigenetic studies.
Puberty.
Puberty is characterized by dramatic changes in brain organization, cognition, risk-taking and social behavior, and represents a period when the emergence of many psychiatric disorders can be triggered [37]. A study in rats showed that the initiation of puberty in females involves epigenetic mechanisms in hypothalamic arcuate nucleus (ARC) neurons [80]. Interestingly, while neonatal (P0-1) DNMT inhibitor treatment masculinizes the brain of female rats [35], the treatment with the same type of compound in the peripubertal period (P22-44) prevents the onset of puberty and estrous cyclicity [80]. In the case of female puberty, DNA methylation is necessary to silence the expression of Polycomb group (PcG) proteins which typically repress the kisspeptin gene (Kiss1), required for the initiation of pulsative secretion of GnrRH. The pubertal increase in Kiss1 expression is accompanied by reduced expression of genes encoding Polycomb proteins (including Eed), EED release from the Kiss1 promoter and enrichment of histone H3K4 methylation [80]. The permissive chromatin configuration at promoter and enhancer regions of puberty-inducing genes is established by the Trithorax group (TrxG) of modifiers, mixed-lineage leukemia (MLL) 1 and 3, serving as H3K4 histone methyltransferases [81]. TrxG dynamically counteracts PcG repression, providing an epigenetic switch from transcriptional repression to activation that seems critical for controlling the timing of female puberty.
While pulsative secretion of GnRH is also important for the initiation of male puberty [36], it is likely that epigenetic mechanisms are different in males and females. A recent study showed extensive changes in DNA methylation from pre- to post-adolescence (10 to 18 years) in humans, with a subset of CpG sites showing sex-specific changes [82]. However, these studies were performed using the DNA isolated from blood, and it will be valuable to perform mechanistic studies of the initiation of male puberty using brain tissue.
Activational period.
After puberty, gonads start secreting high levels of testosterone in males and cyclical levels of estrogens and progesterone in females. While these steroid hormones activate reproductively-relevant neural circuits and behaviors, they continue to shape all the brain regions containing steroid hormone receptors, including the limbic and other cortical regions. Relatedly, the estrous cycle is known to have a significant effect on emotion regulation, metabolic function, reward processing, and memory formation (See Box 2) [13,41,83–93]. Better understanding of the effects of the estrous cycle is critically needed, as many brain disorders such as mood and anxiety disorders can be triggered or exacerbated by ovarian hormones fluctuations [16], especially during the periods of most significant hormonal shifts such as peripubertally, premenstrually, perimenopausally, and postpartum.
Box 2. Ovarian hormone fluctuation as an exemplary sex component.
“Sex” is a complex variable that includes many components (or sex-related variables) [6,137,138]. One such component is ovarian hormone fluctuation occurring across the menstrual cycle (in humans) or the estrous cycle (in rodents) (Figure IA). At puberty, the ovaries start secreting cyclical levels of estrogens and progesterone which are critical for reproduction. Ovarian hormone fluctuation is a context-dependent, female-sex-related variable, present during the reproductive period in individuals with functional ovaries. Ovariectomy or use of hormonal contraceptives abolish hormone fluctuations during the reproductive period. As anyone with functional ovaries can experience ovarian hormone fluctuations, this is a gender-independent variable, relevant to all people who menstruate including cisgender women, non-binary people, and transgender men.
In both rodents and humans, ovarian hormone fluctuations affect numerous neurobehavioral outcomes including gene expression, neurochemistry, neuroplasticity, brain structure, as well as behaviors and brain functions such as feeding, learning, motivation, anxiety and depression-related behaviors [6,13,19,22,39–41,85–97,145–152]. Importantly, in humans, these fluctuations contribute to sex-specific risk for neuropsychiatric conditions, particularly depression and anxiety disorders, which are both about twice as prevalent in women as in men [16].
Studying the ovarian cycle can help identify sex differences in brain and behavior, and gain mechanistic understanding into some of these differences. Since gonadal hormone status is a more precise variable than sex, accounting for the ovarian cycle stage in females can reveal masked sex differences that are missed if only males and females are compared [6]. For instance, in an anxiety-related test in mice, light-dark box, there is lack of sex difference when males and females are compared, with large data overlap between sexes (Figure IB). If the estrous cycle is taken into account, there is a better separation of the data, revealing an increase in anxiety indices following a physiological estrogen drop in females, compared to high-estrogenic females and males ([6,19]; Figure IB). Accordingly, the ability to find sex-specific chromatin features increases when females are segregated based on the estrous cycle phase, allowing improved mechanistic insights into behavioral sex differences [6].
There are different ways to study the effects of ovarian hormone fluctuations on the brain and behavior. In rodents, vaginal cytology allows a non-invasive estrous cycle tracking [153]. Alternatively, serum or brain estradiol and progesterone levels can be measured using ELISA or HPLC-mass spectrometry. The role of specific hormones, e.g. estradiol or progesterone, can be examined by replacing them in ovariectomized animals [154]. Recently, an estrous cycle stage was modelled by overexpressing a transcription factor in the brain [131], expanding the repertoire of tools to study this important variable.
Figure I (for Box 2). Ovarian hormone fluctuation as an exemplary sex component.
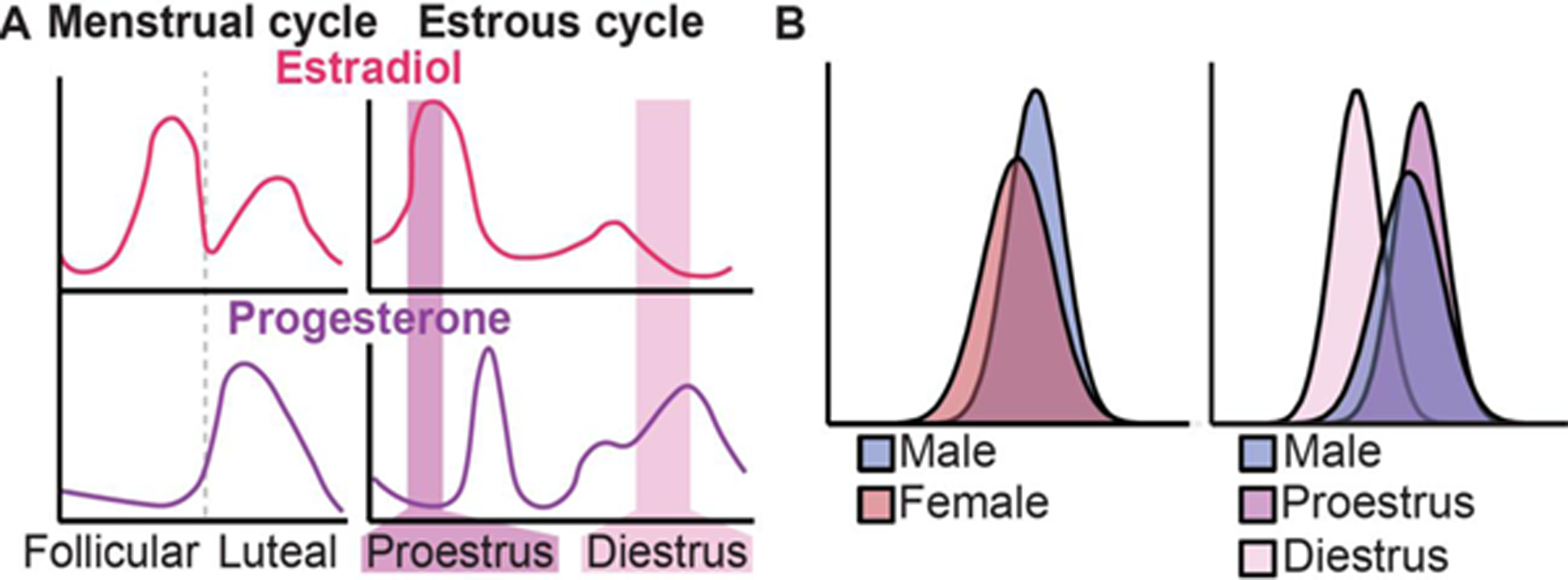
A. Ovarian hormone fluctuation includes cyclical estrogen and progesterone changes across the menstrual cycle (in humans) and the estrous cycle (in rodents); B. In an anxiety-related test in mice, if the estrous cycle is not accounted for, no sex difference is found (left), but when the estrous cycle is included in the analysis, a dynamic sex difference is revealed [6,19]. The sex difference occurs during the low-estrogenic phase (diestrus) but not high-estrogenic phase (proestrus) of the cycle. This dynamic sex difference is clinically-relevant, as hormonal shifts significantly increase depression and anxiety risk in women and other menstruating individuals. Panel B depicts a summary of overlaps in behavioral data between males and females (left), as well as between males, diestrus females, and proestrus females (right) based on [6].
A study from our group aimed to address the increased anxiety and depression risk associated with ovarian hormone fluctuation, by examining the effects of the estrous cycle on anxiety-related behavior and the ventral hippocampus (vHIP), an area that drives these behaviors in adult mice (8–11 weeks old) [16]. Similar to findings in humans [12] and rats [86,89,94], physiological drop in estrogens was associated with increased anxiety indices in mice, and was shown to be linked to a drop in vHIP dendritic spine density [19]. As an underlying mechanism for changes in behavior and synaptic plasticity, extensive changes in chromatin accessibility [19] and 3D genome organization [95] were documented across the mouse estrous cycle, affecting the genes relevant to neuroplasticity, chromatin remodeling, serotonergic transmission, and anxiety. The findings further indicated that the mechanisms involve both nuclear and membrane-bound estrogen receptors. In particular, an estrogen-dependent immediate-early gene product, Egr1, was identified as a candidate regulator of chromatin opening [19] and ERα was implicated in 3D genome organization [95] in vHIP neurons across the cycle.
Another study in mice examined the dorsal hippocampus and learning in ovariectomized females following estradiol treatment [96]. These researchers showed estradiol-induced global changes in histone acetylation in the dorsal hippocampus, implicating epigenetic regulation in sex-specific mechanisms of memory formation. Interestingly, in rats, a study from a different group showed extensive effects of the estrous cycle on gene expression in the prefrontal cortex and implicated the same transcription factor, Egr1, in gene regulation [97], suggesting shared mechanisms of hormone-driven gene regulation across brain regions and behavioral phenotypes.
To our knowledge, neuroepigenetic studies of activational effects of testosterone in males are currently lacking. We hypothesize, however, that testicular testosterone shapes the epigenome of the adult brain with dynamics that is likely to follow diurnal androgen fluctuations [98].
Sex chromosome-related epigenetic effects
The inactivation of X-chromosome is a female-specific process and one of the best studied epigenetic phenomena. The lncRNA Xist orchestrates a multi-step process which results in a random inactivation of one of the two X-chromosomes in each cell with XX complement [99]. Xist is expressed from the X-inactivation center and coats the selected X-chromosome in cis, then recruits several protein complexes, leading to major changes in chromatin organization. Initial loss of histone acetylation, specifically H3K27ac, is mediated by HDAC3, followed by ubiquitination of histone H2AK119 by the PRC1 complex and tri-methylation of H3K27 mediated by the PRC2 complex [100]. Silencing of the inactive X-chromosome (Xi) is locked in by replacement of the canonical histone H2A with macroH2A, and DNA methylation of CpG islands by DNMT3B [100]. Upon X-inactivation, the heterochromatic X-chromosome adopts a compact 3D genome configuration, yielding two chromosomal megadomains, and localizes close to the nuclear periphery or the nucleolus (see Box 1, Figure 2) [101].
However, some gene loci (~3–7% in mice; ~15–25% in humans) are known to escape X-inactivation, although they are located in a constitutively repressed environment. These genes lack epigenetic signatures characteristic of inactivated genes and are located away from repressive genomic elements [100]. The core X-escapee genes escape X-inactivation in most cells in mammals, while other genes can be cell-type-, tissue- or species-dependent escapees [102,103]. Interestingly, some of the core escapees encode for epigenetic regulators such as histone demethylases, providing clear candidates for sex-specific epigenetic regulation in the brain. The escapee Kdm5c encodes lysine H3K4me2/3 demethylase and its mutation in humans results in intellectual disability in males and females, showing dosage sensitivity [104]. By contrast, the X-escapee Kdm6a (or Utx) and its Y-paralog Uty encode lysine H3K27me3 demethylase, and although they are expressed in different parts of the mouse brain, their role in phenotypic sex differences in the brain is unknown. It is known, though, that KDM6A (but not UTY) mutations cause some cases of Kabuki syndrome, a disorder which involves intellectual disability. Since females are less severely affected by Kabuki syndrome than males, this example shows that Uty cannot compensate for the of loss of Kdm6a function in males [105].
While the X-chromosome inactivation process has been mainly studied during development, it is important to note that each cell in the female brain (typically XX) needs to maintain X-chromosome inactivation and has a specific cellular environment containing all Xist-associated repressor complexes not found in cells of the male brain (typically XY). Interestingly, findings from our group have indicated that Xi in neurons is more dynamic than anticipated, with the change in its 3D genome configuration occurring in vHIP neurons across the mouse estrous cycle [95]. The study managed to reproduce X-chromosome-related changes, in part, by estradiol treatment of ovariectomized female mice [95], implying that estradiol interacts with Xi to shape gene regulation and neuronal phenotype. The experiments also revealed accompanied changes in the expression of X-linked genes, indicating the possibility that these may be “the estrous cycle-dependent escapee genes”, representing a hormonally-driven, female-specific mechanism to control gene expression [95]. Moreover, changes in the volume of Xi across the estrous cycle could also induce more global changes in the cellular environment, including changes in the active X-chromosome [106] as well as in autosomal gene expression [95,107].
Interestingly, Xist expression has been shown to be up-regulated by early-life stress [57] and aging [108] in the female mouse brain. It has also been reported that Xist is up-regulated in brains of women with depression [109]. This is an emerging area of sex-specific neurobiology whose future expansion is warranted (see Outstanding questions).
Outstanding questions.
What are the receptors, receptor signaling pathways, and specific epigenetic regulators that drive sex-specific epigenetic programs resulting in sex-specific neurobehavioral phenotypes? Researchers have just begun to dissect these mechanisms, and this understanding will be critical to identify sex-specific targets for treatments.
What are the sex-specific cellular population(s) in the brain that are responsive to hormonal status, sex chromosome complement, and sex-specific environmental effects? With novel cell-specific and single-cell transcriptomics and epigenomics approaches, researchers are better positioned to define cellular populations and, hence, cellular (including epigenetic) mechanisms that drive sex-specific behaviors.
In neurons, what is the role of the inactive X chromosome (Xi) and its possible interaction with the ovarian hormonal cycle? Is increased Xist expression associated with aging and stress a compensatory mechanism or a reflection of a more open Xi chromosome structure, and what are its consequences? Is there another role of Xist beyond X chromosome inactivation?
Menstruating individuals, people who use hormonal contraceptives (HC) or hormonal replacement therapy (HRT), and people who go through gender-affirming hormonal transitions (GAHT), experience important hormonal changes. How do endogenous (puberty, postpartum, (peri)menopausal transition) and exogenous (HRT, HC, GAHT) changes in hormonal status affect the brain epigenome and sex-specific behavioral phenotypes? This information will critically inform women’s health and the health of transgender individuals.
How do environmental factors (such as stress) interact with gonadal hormone- and sex chromosome-mediated epigenetic programs in the brain? Neurons are extremely responsive to both internal and external environments, and it is these interactions that shape the brain and behavior and determine sex-specific phenotypes.
Environmental effects on the brain epigenome
As previously mentioned, environmental factors shape sex-specific characteristics of the brain throughout life, starting from the prenatal period through old age (Figure 1), and increasing evidence shows that epigenetic mechanisms often mediate these processes.
The prenatal period.
The prenatal period is the time of the most dynamic changes in the epigenome [110]. Early candidate gene studies provided a proof of concept that environmentally-induced changes in the embryonic/fetal epigenome can underlie sex- specific effects on the brain and later behavior [111]. One example is maternal stress during pregnancy which, in humans, is associated with increased risk of neuropsychiatric disorders in the offspring, including schizophrenia, depression, and autism. In mice, male (but not female) offspring exposed to chronic stress in early gestation (GD1-7) displayed an increased response of the hypothalamic-pituitary-adrenal (HPA) axis to stress and depressive-like behavior in adulthood [112]. This behavioral phenotype was accompanied with gene expression and epigenetic changes within the two HPA axis-related genes, the glucocorticoid receptor (Nr3c1) and the corticotropin-releasing factor (Crf) gene, in the hippocampus and amygdala, respectively.
In another example, prenatal exposure (during GD0-19) to the endocrine disruptor bisphenol A in mice was shown to induce a male-specific deficit in novel object recognition learning, associated with DNA methylation and gene expression changes in the brain-derived neurotropic factor (Bdnf) gene [56]. None of the effects observed in males were found in female mice. This study and the one discussed earlier provide examples where an environmental factor induces sex-specific phenotypic changes, increasing sex differences compared to baseline conditions. The sources of sex-based variation observed in these studies, however, remain to be clarified: either sex chromosome-linked or gonadal hormone-induced effects may have played a role in the aforementioned sex-specific responses.
Early-life.
Environmental factors can also decrease sex differences in the brain, as exemplified by some early-life environmental exposures. For instance, in mice, early-life maternal separation (P1-14) [113] enhances cocaine-induced conditioned place preference (CPP) in males and females [57]. However, cocaine-induced CPP phenotype is more consistent in males under basal conditions and becomes more similar between the sexes after exposure to maternal separation. Importantly, this stress-induced behavioral effect is accompanied by a sex-biased effect on gene expression in the key reward area, the NAc, with thousands of genes being differentially-expressed in females only, overall reducing the number of genes showing differential expression across sex following early-life stress [57]. Female-specific changes in gene expression include genes important for synaptic function, epigenetic regulation, and X-chromosome inactivation, including up-regulation of Xist and other lncRNAs expressed from the X-inactivation center [57]. Together, these findings imply that epigenetic mechanisms play an important sex-specific role in the effects of early-life stress on gene regulation in the NAc and risk for addiction.
Puberty.
The establishment of puberty is highly sensitive to environmental factors, including stress, nutritional status, exposure to endocrine disruptors, and physical activity, all of which can affect the function of the HPG axis with consequences for reproductive function. While potentially all of these factors can alter the brain epigenome, there is evidence that nutritional status affects epigenetic programming of female puberty [114]. As previously mentioned, the puberty-activating gene, Kiss1, is repressed by Polycomb proteins. In rats, PRCs interact with a special type of NAD+-dependent HDAC, Sirtuin-1 (SIRT1), to restrain female puberty via epigenetic repression of the Kiss1 promoter [81]. As puberty approaches, SIRT1 needs to be released from the Kiss1 promoter to allow for an epigenetic switch from repressive to permissive chromatin state. Importantly, SIRT1 is a fuel-sensing deacetylase [81]. Early-onset overnutrition accelerates SIRT1 release from the Kiss1 promoter and enhances Kiss1 expression, advancing puberty. By contrast, undernutrition delays puberty by raising SIRT1 levels and prolonging Kiss1 repression [81]. These findings exemplify a female-specific epigenetic mechanism by which nutritional cues and obesity influence mammalian puberty. Interestingly, in humans, DNA methylation changes found across puberty were associated with BMI at the age of 10 in females only [82]. Further, in mice, analysis of the hypothalamic ARC during the peri-adolescent period (P12-35) revealed sex-specific DNA methylation changes in genomic regions enriched for BMI heritability in humans [115], implying hormonally-driven, sex-specific epigenetic mechanisms underlying metabolic regulation in rodents and humans.
Similar to early life, puberty is also a vulnerable period when stressful events can initiate psychopathology or prime the brain for psychiatric disorders manifesting later in life [116]. Psychopathology is particularly likely to be triggered during subsequent periods with extreme hormonal shifts, such as pregnancy. As an example, chronic stress around puberty (PD21-35) in female mice was shown to alter HPA axis responsiveness specifically during pregnancy [117]. As an underlying molecular mechanism, pubertal adversity was shown to induce a more dramatic change in chromatin accessibility in the paraventricular nucleus of the hypothalamus in pregnant compared to non-pregnant females, affecting neuronally-relevant genes [117]. This study demonstrates an interaction of pubertal stress and a sex-specific factor (pregnancy) on epigenetic regulation in the brain, providing an example where both the source and mechanism behind sex-specific brain regulation were addressed.
Late adolescence/Adulthood.
Adult stress exposures have a significant effect on the brain and behavior and have been linked to increased psychiatric risk [118]. Early studies strongly implicated epigenetic mechanisms in stress-induced behavioral outcomes, but these studies were done primarily in male mice (reviewed in [119,120]). Other studies in mice of both sexes have shown sex-specific transcriptional effects of both acute [121,122] and chronic [123,124] stress across brain areas of relevance to depression and anxiety disorders, including the hippocampus, the hypothalamus, the nucleus accumbens, and the prefrontal cortex. However, few studies so far explored sex-specific effects of adult stress on the brain epigenome.
In response to acute stress in mice, investigators found sex-specific changes in hippocampal levels of 5-hydroxy-methylcytosine, a mark associated with active transcription [125]. Changes were found in genes linked to stress response and psychiatric disorders and were partially correlated with altered gene expression, providing a new sex-specific epigenetic mechanism for the control of stress response. More recently, a two-hit stress model, combining acute swim stress and acute restraint stress, was used to profile chromatin accessibility in the vHIP of male and female mice in response to adult stress [126]. Interestingly, this paradigm led to decreased anxiety indices in males and no behavioral consequences in females, and this was accompanied with fewer stress-enriched gene pathways identified in males compared to females. Differentially enriched networks after stress in males and females included pathways involved in developmental and immune function, providing a possible mechanism through which stressors can induce sex-specific brain adaptation, which does not necessarily involve visible phenotypic changes. Interestingly, a recent study also implicated histone variant H2A.Z in sex-specific epigenetic regulation of stress-induced fear learning, a PTSD-relevant paradigm [127]. Using conditional-inducible H2A.Z knockout mice, it was shown that deletion of H2A.Z reduced stress-induced sensitization of fear learning preferentially in females, indicating H2A.Z as a sex-specific epigenetic risk factor for PTSD susceptibility.
One of the best described sex-specific effects of environmental factors are the effects of psychostimulants including cocaine. There is evidence that dopamine release and other addiction-related effects of cocaine vary with sex and the estrous cycle [13,93], but the underlying epigenetic mechanisms have been described only recently [57,128]. Cocaine-induced CPP in female mice was shown to vary with the cycle, and preference depended on the mouse estrogen status at the first exposure to cocaine [57]. When females are in the high-susceptibility, low-estrogenic state, they respond to acute cocaine exposure by opening NAc neuronal chromatin enriched for the binding sites of transcription factor ΔFosB [57]. ΔFosB is implicated in chronic cocaine response and addiction [129], and recent evidence of increased ΔFosB expression in the NAc following estrogen withdrawal [130] further supports our hypothesis that a physiological drop in estrogen can “prime” chromatin for cocaine-induced long-term plasticity in the NAc. Conversely, during the high-estrogenic state, females respond to cocaine by preferential closing of neuronal chromatin, providing a mechanism for limiting cocaine-driven chromatin and synaptic plasticity [57]. By using 39,XO female mice that have only one X-chromosome (see Box 1), Xi was shown to be required for this protective effect of estrogens [57]. This estrous cycle’s effect is also erased by early-life stress [57], implying an important interaction of ovarian hormone status, the X-chromosome, and the environment on sex-specific epigenetic regulation and addiction-related phenotype in female mice.
Functional evidence that epigenetic mechanisms drive sex-specific neurobehavioral phenotypes
Epigenetic studies are often correlational, and it can be challenging to provide functional evidence that epigenetic regulation drives sex-specific neurobehavioral phenotypes. Here, we describe three approaches that allow testing a causal link between epigenetic mechanisms and sex-specific behavior in rodents.
Manipulation of candidate epigenetic regulators.
As previously described, estradiol treatment of neonatal female rats masculinizes the brain by reducing DNA methylation in the POA [35] while EED, a PRC2 component, inhibits puberty in mice by repressing the Kiss1 promoter in the ARC. To provide functional evidence for the role of these epigenetic mechanisms in brain sexual differentiation, investigators deleted Dnmt3a in the POA of neonatal female mice [35] and overexpressed EED in the ARC of immature female mice [80], showing that these genetic manipulations were sufficient to masculinize the female brain and delay female puberty, respectively. Similarly, our group has used an approach in which we manipulated Egr1, an immediate-early gene product, which was previously implicated in sex-specific chromatin regulation in the vHIP [19]. Importantly, overexpression of Egr1 in the mouse vHIP partially mimics proestrus, the high-estrogenic phase of the estrous cycle, inducing sex-specific chromatin opening, gene expression, and behavioral changes [131]. These studies provide functional evidence that estrous cycle-driven chromatin changes regulate anxiety-related behavior.
Epigenetic editing of candidate gene loci.
It is sometimes hypothesized that an epigenetic change in a single gene locus is sufficient to drive sex-specific behavior. In these situations, the epigenetic editing approach can be used. For instance, a viral-mediated delivery of engineered zinc-finger proteins was used to target histone H3K9/14 acetylation of the Cdk5 promoter in the hippocampus of male and female mice [132]. This led to attenuated fear memory retrieval in female mice only, revealing a sex-specific epigenetic mechanism that regulates CDK5 expression and influences fear memory formation in mice.
Manipulation of candidate lncRNAs.
Finally, another possible approach is to target a single lncRNA hypothesized to drive sex-specific control of behavior. An example is FEDORA, a lncRNA that was found to be specifically overexpressed in women with depression [133]. An overexpression of FEDORA, either in neurons or oligodendrocytes of the prefrontal cortex, induced depression-like behavioral abnormalities in female mice only, and this was linked to cell type-specific regulation of gene expression, myelin thickness, and synaptic properties [133].
Concluding remarks and future perspectives
As summarized in this Review, there is increasing evidence for the role of epigenetic mechanisms in shaping sex differences in the brain and behavior. Specifically, the major sources of brain sex differences, including gonadal hormones, sex chromosomes, and environmental factors, can all affect epigenetic regulation in the brain with consequences for sex-specific neurobiology and behavior.
Classical studies of sex differences have explored sex-specific factors that drive sexual differentiation of the brain, including perinatal testosterone exposure in males, the initiation of female puberty, post-pubertal ovarian hormone fluctuation, and X-inactivation in females. There is now functional evidence that epigenetic mechanisms underlie sex-specific neurobehavioral changes during critical organizational periods and across the estrous cycle. Still, little is known about the epigenetic regulation of developmental brain “feminization” in females as well as about the initiation of male puberty and post-pubertal activational effects of testosterone on the male brain. In addition, the organizational effects of gonadal hormones have been studied in relation to reproductive-related outcomes, and it is important to understand to what extent other adult behaviors such as anxiety-, learning-, and addiction-related behaviors depend on epigenetic programming effects during perinatal and peripubertal periods (see Outstanding questions).
While the knowledge regarding the epigenetic regulation of the brain by ovarian hormones is advancing rapidly [19,57,95], and there is some progress on pregnancy (e.g. [117]), additional female-specific phenomena need to be explored, including the effects of drastic hormonal changes postpartum and during perimenopause on the brain epigenome, as these studies can provide critical insights into heightened psychiatric risk associated with these periods [134–136]. Also, studies of the estrous cycle’s effect on chromatin regulation in various brain areas including the hypothalamus, the dorsal hippocampus, and the cortex are needed for better understanding of the contribution of epigenetic mechanisms to ovarian hormones’ effects on metabolic function, reward processing, and memory formation. In addition, exposures to exogenous endocrine compounds including hormonal contraceptives, hormone replacement therapy, and gender-affirming hormone therapy, are likely to impact the brain through changes in the epigenome, and this needs to be examined in future work (see Outstanding questions).
Beyond classical studies of sex differences, other studies explored epigenetic mechanisms in males and females and, without a priori sex-based hypotheses, found significant sex differences in the brain epigenome, most frequently in response to different environmental exposures. While the effect of sex provides important information, without knowing the source of sex-based variability, it is difficult to identify the upstream regulators and mechanisms underlying the observed effects. By examining interactions between sex-specific factors (e.g. gonadal hormone status) and environmental factors of interest, we anticipate that future studies will reveal sex-specific receptor mechanisms, epigenetic regulators, and specific cellular populations that drive sex-specific neurobehavioral phenotypes (see Outstanding questions). Considering sex-specific factors or “sex components” rather than “sex” as a single variable improves the ability to identify and understand sex differences, and will generate more inclusive findings applicable across genders in humans (see Box 2) [6,137,138].
The (neuro)epigenetics field has greatly benefitted in recent years from better-targeted and higher-resolution experimental approaches, including epigenetic editing tools [139] and single-cell genomics assays [140]. Coupled with improved study design, these approaches promise to identify critical sex-specific epigenetic mechanisms underlying sex differences in the brain and behavior, and provide novel sex-based targets for treatments that can transform psychiatric practice across genders.
Highlights.
Sex differences in the brain are reflected at the molecular, structural and behavioral levels, and observed across brain disorders.
Brain sexual differentiation is a dynamic process throughout life, resulting from interaction of gonadal hormones, sex chromosomes, and the environment.
Studies of sex-specific factors, such as perinatal testosterone exposure in males and the estrous cycle in females, show clear evidence that epigenetic mechanisms drive sex differences in the brain and behavior.
Studies of environmental impact on the brain epigenome across the lifespan have also revealed important sex differences, but with more limited mechanistic insights.
Future neuroepigenetics studies incorporating sex-specific variables and better targeted, higher resolution approaches are hoped to maximize the benefit of insights gained from basic research for people across genders.
Acknowledgements:
This work was supported by the National Institutes of Health under Award Number R01MH123523 to M.K.
Glossary
- Activational effects
Gonadal hormone or exogenous endocrine compound exposure during adulthood (such as changes over the ovarian hormone cycle) that leads to transient changes in brain structure and function.
- Chromatin
A complex, dynamic structure of DNA and associated proteins, largely histones, with an important role in DNA packaging and gene regulation in the nucleus.
- Chromatin accessibility
Chromatin can exist in either an open (accessible) or closed (repressive) conformation, which determines the availability of a DNA sequence to transcriptional machinery, and allows or inhibits transcription, respectively.
- Chromatin organization
Different levels of chromatin structure impacting gene regulation, from DNA wrapped around histone octamers in a “beads on a string” appearance (1D chromatin) to the location of DNA in physical space in relation to distant sequences and the nuclear lamina (3D chromatin).
- Chromatin remodeling complex
An ATP-dependent protein complex that facilitates an open chromatin configuration and promotes gene transcription.
- DNA methylation
Addition of a methyl group to cytosine in a DNA sequence, within CpG or non-CpG sites, typically resulting in repression of gene expression.
- Enhancer
A region of DNA that can be at any distance from the gene and in any orientation, typically containing transcription factor binding sites, and likely to interact with the gene promoter via looping in order to increase gene transcription.
- Histone modification
Post-translational covalent modifications of histone proteins (e.g. acetylation or methylation) that act to promote or repress gene transcription by changing chromatin structure or providing docking sites for other regulatory proteins.
- Organizational effects
Gonadal hormone or exogenous endocrine compound exposure early in life (during the perinatal or peripubertal period) that leads to permanent changes in brain structure and function.
- Polycomb repressor complex
A multi-protein complex that mediates repressive histone marks and maintains a closed chromatin configuration preventing gene transcription.
- Promoter
A region of DNA upstream of a gene body, on which transcription factors and other proteins can bind in order to initiate gene transcription.
- Sex components
Sex is a multi-layered and context-dependent variable including many components such as gonadal presence, gonadal hormone status, exposure to exogenous endocrine compounds, sex chromosome complement, all (inter)acting within a specific environmental context across the lifespan.
- Sexual differentiation of the brain
A dynamic process through which sex differences in brain structure and function are determined throughout the entire lifespan through interaction between gonadal hormones and exogenous endocrine compounds, sex chromosomes and environmental factors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors declare no competing interests in relation to this work.
References
- 1.Beery AK and Zucker I (2011) Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews 35, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton JA and Collins FS (2014) NIH to balance sex in cell and animal studies. Nature 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Will TR et al. (2017) Problems and Progress regarding Sex Bias and Omission in Neuroscience Research. eNeuro 4, ENEURO. 0278–0217.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lange AG, Jacobs EG and Galea LAM (2021) The scientific body of knowledge: Whose body does it serve? A spotlight on women’s brain health. Frontiers in Neuroendocrinology 60, 100898. [DOI] [PubMed] [Google Scholar]
- 5.Rechlin RK et al. (2022) An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nature Communications 13, 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocks D, Cham H and Kundakovic M (2022) Why the estrous cycle matters for neuroscience. Biology of Sex Differences 13, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ainsworth C (2015) Sex redefined. Nature 518, 288–291. 10.1038/518288a [DOI] [PubMed] [Google Scholar]
- 8.Fausto-Sterling A (2000) Sexing the body: Gender politics and the construction of sexuality. Basic books
- 9.Rosenthal SM (2021) Challenges in the care of transgender and gender-diverse youth: an endocrinologist’s view. Nature Reviews Endocrinology 17, 581–591. [DOI] [PubMed] [Google Scholar]
- 10.Cahill L (2006) Why sex matters for neuroscience. Nature Reviews Neuroscience 7, 477–484. [DOI] [PubMed] [Google Scholar]
- 11.Koss WA and Frick KM (2017) Sex differences in hippocampal function. Journal of Neuroscience Research 95, 539–562. [DOI] [PubMed] [Google Scholar]
- 12.Altemus M, Sarvaiya N and Neill Epperson C (2014) Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology 35, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JB and Chartoff E (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrocco J and McEwen BS (2016) Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci 18, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gogos A et al. (2019) Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: Are gonadal hormones the link? British Journal of Pharmacology 176, 4119–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundakovic M and Rocks D (2022) Sex hormone fluctuation and increased female risk for depression and anxiety disorders: From clinical evidence to molecular mechanisms. Frontiers in Neuroendocrinology 66, 101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galea LAM et al. (2017) Why estrogens matter for behavior and brain health. Neuroscience and Biobehavioral Reviews 76, 363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliva M et al. (2020) The impact of sex on gene expression across human tissues. Science 369, eaba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaric I et al. (2019) Chromatin organization in the female mouse brain fluctuates across the oestrous cycle. Nature Communications 10, 2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeRosa H et al. (2022) Got milk? Maternal immune activation during the mid-lactational period affects nutritional milk quality and adolescent offspring sensory processing in male and female rats. Molecular Psychiatry 27, 4829–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen EY et al. (2015) Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Experimental Neurology 268, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaric I et al. (2019) Sex and Estrous Cycle Effects on Anxiety- and Depression- Related Phenotypes in a Two-Hit Developmental Stress Model. Front Mol Neurosci 12, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savell KE et al. (2020) A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Sci Adv 6, eaba4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parel ST and Peña CJ (2022) Genome-wide Signatures of Early-Life Stress: Influence of Sex. Biol Psychiatry 91, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eliot L et al. (2021) Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neuroscience and Biobehavioral Reviews 125, 667–697. [DOI] [PubMed] [Google Scholar]
- 26.DeCasien AR et al. (2022) Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biology of Sex Differences 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S et al. (2020) Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proceedings of the National Academy of Sciences of the United States of America 117, 18788–18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joel D and McCarthy MM (2017) Incorporating Sex As a Biological Variable in Neuropsychiatric Research: Where Are We Now and Where Should We Be? Neuropsychopharmacology 42, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halladay AK et al. (2015) Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Molecular Autism 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauvais-Jarvis F et al. (2020) Sex and gender: modifiers of health, disease, and medicine. Lancet (London, England) 396, 565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galea LAM et al. (2020) The promises and pitfalls of sex difference research. Frontiers in Neuroendocrinology 56, 100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferretti MT et al. (2018) Sex differences in Alzheimer disease - the gateway to precision medicine. Nature Reviews Neurology 14, 457–469. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy MM and Arnold AP (2011) Reframing sexual differentiation of the brain. Nature Neuroscience 14, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy MM (2023) Sex differences in the brain: Focus on developmental mechanisms. Principles of Gender-Specific Medicine (Fourth Edition), Vol. 11, Elsevier/Academic Press. [Google Scholar]
- 35.Nugent BM et al. (2015) Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience 18, 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manotas MC et al. (2022) Genetic and Epigenetic Control of Puberty. Sexual development 16, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz KM and Sisk CL (2016) The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience and Biobehavioral Reviews 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romeo RD (2003) Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. Journal of Neuroendocrinology 15, 1185–1192. [DOI] [PubMed] [Google Scholar]
- 39.Dubol M et al. (2021) Neuroimaging the menstrual cycle: A multimodal systematic review. Frontiers in Neuroendocrinology 60, 100878. [DOI] [PubMed] [Google Scholar]
- 40.Barth C et al. (2016) In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Scientific Reports 6, 32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocks D and Kundakovic M (2023) Hippocampus-based behavioral, structural, and molecular dynamics across the estrous cycle. J Neuroendocrinol 35, e13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broekmans FJ, Soules MR and Fauser BC (2009) Ovarian aging: mechanisms and clinical consequences. Endocrine Reviews 30, 465–493. [DOI] [PubMed] [Google Scholar]
- 43.Cui J, Shen Y and Li R (2013) Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends in Molecular Medicine 19, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jett S et al. (2022) Endogenous and Exogenous Estrogen Exposures: How Women’s Reproductive Health Can Drive Brain Aging and Inform Alzheimer’s Prevention. Frontiers in Aging Neuroscience 14, 831807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streifer M and Gore AC (2021) Epigenetics, estrogenic endocrine-disrupting chemicals (EDCs), and the brain. Advances in Pharmacology (San Diego, Calif.) 92, 73–99. [DOI] [PubMed] [Google Scholar]
- 46.Tronson NC and Schuh KM (2022) Hormonal contraceptives, stress, and the brain: The critical need for animal models. Frontiers in Neuroendocrinology 67, 101035. [DOI] [PubMed] [Google Scholar]
- 47.Lu DH, Zhou SY and Xu LZ (2023) Association between hormone replacement therapy and sex hormones in postmenopausal women: a systematic review and meta-analysis. European Review for Medical and Pharmacological Sciences 27, 5264–5279. [DOI] [PubMed] [Google Scholar]
- 48.Aghi K et al. (2022) Centering the Needs of Transgender, Nonbinary, and Gender-Diverse Populations in Neuroendocrine Models of Gender-Affirming Hormone Therapy. Biological psychiatry Cognitive Neuroscience and Neuroimaging 7, 1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heydari R et al. (2022) Y chromosome is moving out of sex determination shadow. Cell & Bioscience 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vries GJ et al. (2002) A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. The Journal of Neuroscience 22, 9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold AP and Chen X (2009) What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in Neuroendocrinology 30, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnold AP and Burgoyne PS (2004) Are XX and XY brain cells intrinsically different? Trends in Endocrinology and Metabolism 15, 6–11. [DOI] [PubMed] [Google Scholar]
- 53.Jowhar Z et al. (2018) Effects of human sex chromosome dosage on spatial chromosome organization. Mol Biol Cell 29, 2458–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raznahan A et al. (2018) Sex-chromosome dosage effects on gene expression in humans. Proceedings of the National Academy of Sciences of the United States of America 115, 7398–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guma E et al. (2023) A Cross-Species Neuroimaging Study of Sex Chromosome Dosage Effects on Human and Mouse Brain Anatomy. The Journal of neuroscience 43, 1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kundakovic M et al. (2015) DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences of the United States of America 112, 6807–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rocks D et al. (2023) Early-life stress and ovarian hormones alter transcriptional regulation in the nucleus accumbens resulting in sex-specific responses to cocaine. Cell Rep 42, 113187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker DM et al. (2022) Sex-Specific Transcriptional Changes in Response to Adolescent Social Stress in the Brain’s Reward Circuitry. Biol Psychiatry 91, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klemm SL, Shipony Z and Greenleaf WJ (2019) Chromatin accessibility and the regulatory epigenome. Nature Reviews Genetics 20, 207–220. [DOI] [PubMed] [Google Scholar]
- 60.Rowley MJ and Corces VG (2018) Organizational principles of 3D genome architecture. Nature Reviews Genetics 19, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mattei AL, Bailly N and Meissner A (2022) DNA methylation: a historical perspective. Trends in Genetics 38, 676–707. [DOI] [PubMed] [Google Scholar]
- 62.Gallegos DA et al. (2018) Chromatin Regulation of Neuronal Maturation and Plasticity. Trends in Neurosciences 41, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farrelly LA and Maze I (2019) An emerging perspective on ‘histone code’ mediated regulation of neural plasticity and disease. Current Opinion in Neurobiology 59, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su Y et al. (2017) Neuronal activity modifies the chromatin accessibility landscape in the adult brain. Nature Neuroscience 20, 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vierbuchen T et al. (2017) AP-1 Transcription Factors and the BAF Complex Mediate Signal-Dependent Enhancer Selection. Molecular Cell 68, 1067–1082 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plaza-Jennings A, Valada A and Akbarian S (2022) 3D Genome Plasticity in Normal and Diseased Neurodevelopment. Genes 13, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernandez-Albert J et al. (2019) Immediate and deferred epigenomic signatures of in vivo neuronal activation in mouse hippocampus. Nature Neuroscience 22, 1718–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Girdhar K et al. (2022) Chromatin domain alterations linked to 3D genome organization in a large cohort of schizophrenia and bipolar disorder brains. Nature Neuroscience 25, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu B et al. (2021) Neuronal and glial 3D chromatin architecture informs the cellular etiology of brain disorders. Nature Communications 12, 3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lister R et al. (2013) Global epigenomic reconfiguration during mammalian brain development. Science 341, 1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santiago M et al. (2014) TET enzymes and DNA hydroxymethylation in neural development and function - how critical are they? Genomics 104, 334–340. [DOI] [PubMed] [Google Scholar]
- 72.Bannister AJ and Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Research 21, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blackledge NP and Klose RJ (2021) The molecular principles of gene regulation by Polycomb repressive complexes. Nature Reviews Molecular Cell Biology 22, 815–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martire S and Banaszynski LA (2020) The roles of histone variants in fine-tuning chromatin organization and function. Nature Reviews Molecular Cell Biology 21, 522–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Statello L et al. (2021) Gene regulation by long non-coding RNAs and its biological functions. Nature Reviews Molecular Cell biology 22, 96–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCarthy MM, Herold K and Stockman SL (2018) Fast, furious and enduring: Sensitive versus critical periods in sexual differentiation of the brain. Physiol Behav 187, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gegenhuber B et al. (2022) Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature 606, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murray EK et al. (2009) Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150, 4241–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cisternas CD et al. (2020) Neonatal Inhibition of DNA Methylation Disrupts Testosterone-Dependent Masculinization of Neurochemical Phenotype. Endocrinology 161, bqz022. [DOI] [PubMed] [Google Scholar]
- 80.Lomniczi A et al. (2013) Epigenetic control of female puberty. Nature Neuroscience 16, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toro CA et al. (2018) Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty. Nature Communications 9, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han L et al. (2019) Changes in DNA methylation from pre- to post-adolescence are associated with pubertal exposures. Clinical Epigenetics 11, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taxier LR, Gross KS and Frick KM (2020) Oestradiol as a neuromodulator of learning and memory. Nature Reviews Neuroscience 21, 535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Veen JE et al. (2020) Hypothalamic oestrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nature Metabolism 2, 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berry B, McMahan R and Gallagher M (1997) Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behavioral Neuroscience 111, 267. [DOI] [PubMed] [Google Scholar]
- 86.Gouveia A Jr. et al. (2004) Influence of the estrous cycle on the behavior of rats in the elevated T-maze. Behavioural Processes 67, 167–171. [DOI] [PubMed] [Google Scholar]
- 87.Korol DL et al. (2004) Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior 45, 330–338. [DOI] [PubMed] [Google Scholar]
- 88.Milad MR et al. (2009) Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164, 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mora S, Dussaubat N and Diaz-Veliz G (1996) Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 21, 609–620. [DOI] [PubMed] [Google Scholar]
- 90.Warren SG et al. (1995) LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Research 703, 26–30. [DOI] [PubMed] [Google Scholar]
- 91.Woolley CS and McEwen BS (1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. The Journal of Neuroscience 12, 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Asarian L and Geary N (2002) Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Hormones and Behavior 42, 461–471. [DOI] [PubMed] [Google Scholar]
- 93.Calipari ES et al. (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nature Communications 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marcondes FK et al. (2001) Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 74, 435–440. [DOI] [PubMed] [Google Scholar]
- 95.Rocks D et al. (2022) Sex-specific multi-level 3D genome dynamics in the mouse brain. Nature Communications 13, 3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Z, Fan L and Frick KM (2010) Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proceedings of the National Academy of Sciences of the United States of America 107, 5605–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duclot F and Kabbaj M (2015) The estrous cycle surpasses sex differences in regulating the transcriptome in the rat medial prefrontal cortex and reveals an underlying role of early growth response 1. Genome Biology 16, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harden KP et al. (2016) Diurnal coupling between testosterone and cortisol from adolescence to older adulthood. Psychoneuroendocrinology 73, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu Z, Carter AC and Chang HY (2017) Mechanistic insights in X-chromosome inactivation. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 372, 20160356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang H, Disteche CM and Berletch JB (2019) X Inactivation and Escape: Epigenetic and Structural Features. Frontiers in Cell and Developmental Biology 7, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jégu T, Aeby E and Lee JT (2017) The X chromosome in space. Nature Reviews Genetics 18, 377–389. [DOI] [PubMed] [Google Scholar]
- 102.Peeters SB, Cotton AM and Brown CJ (2014) Variable escape from X-chromosome inactivation: identifying factors that tip the scales towards expression. Bioessays 36, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berletch JB et al. (2015) Escape from X inactivation varies in mouse tissues. PLoS Genetics 11, e1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brookes E et al. (2015) Mutations in the intellectual disability gene KDM5C reduce protein stability and demethylase activity. Human Molecular Genetics 24, 2861–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gažová I, Lengeling A and Summers KM (2019) Lysine demethylases KDM6A and UTY: The X and Y of histone demethylation. Molecular Genetics and Metabolism 127, 31–44. [DOI] [PubMed] [Google Scholar]
- 106.San Roman AK et al. (2023) The human inactive X chromosome modulates expression of the active X chromosome. Cell Genomics 3, 100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wijchers PJ and Festenstein RJ (2011) Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends in Genetics 27, 132–140. [DOI] [PubMed] [Google Scholar]
- 108.Hajdarovic KH et al. (2022) Single-cell analysis of the aging female mouse hypothalamus. Nature Aging 2, 662–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ji B et al. (2015) Over-expression of XIST, the Master Gene for X Chromosome Inactivation, in Females With Major Affective Disorders. EBioMedicine 2, 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reik W (2007) Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447, 425–432. [DOI] [PubMed] [Google Scholar]
- 111.Kundakovic M and Jaric I (2017) The Epigenetic Link between Prenatal Adverse Environments and Neurodevelopmental Disorders. Genes 8, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mueller BR and Bale TL (2008) Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of neuroscience 28, 9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Franklin TB et al. (2010) Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 68, 408–415. [DOI] [PubMed] [Google Scholar]
- 114.Vazquez MJ et al. (2018) SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nature Communications 9, 4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.MacKay H et al. (2022) Sex-specific epigenetic development in the mouse hypothalamic arcuate nucleus pinpoints human genomic regions associated with body mass index. Sci Adv 8, eabo3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrison KE et al. (2014) Epigenetic mechanisms in pubertal brain maturation. Neuroscience 264, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morrison KE et al. (2020) Pubertal adversity alters chromatin dynamics and stress circuitry in the pregnant brain. Neuropsychopharmacology 45, 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McEwen BS and Akil H (2020) Revisiting the Stress Concept: Implications for Affective Disorders. The Journal of neuroscience 40, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nestler EJ et al. (2016) Epigenetic Basis of Mental Illness. The Neuroscientist 22, 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bagot RC et al. (2014) Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci 16, 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marrocco J et al. (2017) A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nature Communications 8, 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brivio E et al. (2023) Sex shapes cell-type-specific transcriptional signatures of stress exposure in the mouse hypothalamus. Cell Reports 42, 112874. [DOI] [PubMed] [Google Scholar]
- 123.Hodes GE et al. (2015) Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. The Journal of Neuroscience 35, 16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Labonte B et al. (2017) Sex-specific transcriptional signatures in human depression. Nat Med 23, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papale LA et al. (2016) Sex-specific hippocampal 5-hydroxymethylcytosine is disrupted in response to acute stress. Neurobiology of Disease 96, 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Caradonna SG, Paul MR and Marrocco J (2022) An allostatic epigenetic memory on chromatin footprints after double-hit acute stress. Neurobiology of Stress 20, 100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramzan F et al. (2020) Sex-specific effects of the histone variant H2A.Z on fear memory, stress-enhanced fear learning and hypersensitivity to pain. Scientific Reports 10, 14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fischer DK et al. (2022) Cocaine regulation of Nr4a1 chromatin bivalency and mRNA in male and female mice. Scientific Reports 12, 15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yeh S-Y et al. (2023) Cell-type-specific whole-genome landscape of ΔFOSB binding in nucleus accumbens after chronic cocaine exposure. Biological Psychiatry 94: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Foster WB et al. (2023) Estradiol withdrawal following a hormone simulated pregnancy induces deficits in affective behaviors and increases ∆FosB in D1 and D2 neurons in the nucleus accumbens core in mice. Hormones and Behavior 149, 105312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rocks D et al. (2023) Egr1 drives estrous cycle-dependent gene regulation and behavioral plasticity. In: Society for Behavioral Neuroendocrinology 27th Annual Meeting Program Book June 26-29, 2023. Tours, France. [Google Scholar]
- 132.Sase AS et al. (2019) Sex-Specific Regulation of Fear Memory by Targeted Epigenetic Editing of Cdk5. Biol Psychiatry 85, 623–634. [DOI] [PubMed] [Google Scholar]
- 133.Issler O et al. (2022) The long noncoding RNA FEDORA is a cell type- and sex-specific regulator of depression. Sci Adv 8, eabn9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bromberger JT and Epperson CN (2018) Depression During and After the Perimenopause: Impact of Hormones, Genetics, and Environmental Determinants of Disease. Obstetrics and Gynecology Clinics of North America 45, 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Culbert KM, Thakkar KN and Klump KL (2022) Risk for midlife psychosis in women: critical gaps and opportunities in exploring perimenopause and ovarian hormones as mechanisms of risk. Psychological Medicine 52, 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hahn-Holbrook J, Cornwell-Hinrichs T and Anaya I (2017) Economic and Health Predictors of National Postpartum Depression Prevalence: A Systematic Review, Meta-analysis, and Meta-Regression of 291 Studies from 56 Countries. Frontiers in Psychiatry 8, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Massa MG and Correa SM (2020) Sexes on the brain: Sex as multiple biological variables in the neuronal control of feeding. Biochimica et Biophysica Acta Molecular Basis of Disease 1866, 165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maney DL (2016) Perils and pitfalls of reporting sex differences. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences 371, 20150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hamilton PJ et al. (2018) Neuroepigenetic Editing. Methods in Molecular Biology 1767, 113–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heumos L et al. (2023) Best practices for single-cell analysis across modalities. Nature Reviews Genetics, 1–23. [DOI] [PMC free article] [PubMed]
- 141.Lynn PM and Davies W (2007) The 39,XO mouse as a model for the neurobiology of Turner syndrome and sex-biased neuropsychiatric disorders. Behavioural Brain Research 179, 173–182. [DOI] [PubMed] [Google Scholar]
- 142.Isles AR et al. (2004) Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner’s syndrome. Human Molecular Genetics 13, 1849–1855. [DOI] [PubMed] [Google Scholar]
- 143.Ma W et al. (2018) X-Chromosome Inactivation and Escape from X Inactivation in Mouse. Methods in Molecular Biology 1861, 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Berletch JB et al. (2015) Identification of genes escaping X inactivation by allelic expression analysis in a novel hybrid mouse model. Data in Brief 5, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iqbal J et al. (2020) Estradiol Alters Hippocampal Gene Expression during the Estrous Cycle. Endocrine Research 45, 84–101. [DOI] [PubMed] [Google Scholar]
- 146.Warren SG and Juraska JM (1997) Spatial and nonspatial learning across the rat estrous cycle. Behavioral Neuroscience 111, 259–266. [DOI] [PubMed] [Google Scholar]
- 147.Young EA and Becker JB (2009) Perspective: sex matters: gonadal steroids and the brain. Neuropsychopharmacology 34, 537–538. [DOI] [PubMed] [Google Scholar]
- 148.Eckel LA (2011) The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 104, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barth C, Villringer A and Sacher J (2015) Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Albert K, Pruessner J and Newhouse P (2015) Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 59, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lisofsky N et al. (2015) Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage 118, 154–162. [DOI] [PubMed] [Google Scholar]
- 152.Sundstrom Poromaa I and Gingnell M (2014) Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Frontiers in Neuroscience 8, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McLean AC et al. (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of Visualized Experiments, e4389. [DOI] [PMC free article] [PubMed]
- 154.Becker JB et al. (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673. [DOI] [PubMed] [Google Scholar]


