Abstract
A bewildering array of proteins containing the caspase recruitment domain (CARD) have now been identified. Previously, CARD–CARD interactions have been shown to be involved in the assembly of protein complexes that promote caspase processing and activation in the context of apoptosis. However, as the family of CARD-containing proteins has grown, it has become apparent that the majority of these proteins do not recruit caspases or promote caspase activation. Instead, many participate in NF-κB signalling pathways associated with innate or adaptive immune responses. Here, we suggest a simplified classification of the CARD proteins based upon their domain structures and discuss the divergent roles of these proteins in the context of host defence.
Introduction
In recent years, a number of related modules have been identified in proteins that participate in signalling pathways leading to apoptosis. These modules include the death domain (DD), death effector domain (DED), caspase recruitment domain (CARD) and the recently described Pyrin domain (PYD) (Hofmann et al., 1997; Aravind et al., 1999; Bertin and DiStefano, 2000; Martinon et al., 2001). All of these modules form a six or seven antiparallel α-helical bundle that has been termed the death domain fold. Homotypic interactions between proteins containing CARDs, DEDs or DDs have been found in divergent signalling pathways that result in caspase (cysteine aspartic acid-specific protease) activation and apoptosis. A classic example is the CD95 (Fas/APO-1) pathway where, upon encounter with its ligand, the CD95 receptor recruits the adaptor Fas-associated protein with death domain (FADD) via DD motifs present in both proteins (Boldin et al., 1996). In turn, FADD recruits caspase-8 to the receptor complex via a single DED motif present in the former, and two tandemly arranged DED motifs present in the latter. This results in caspase-8 activation and propagation of the death signal through a cascade of further proteolytic events.
The CARD module was first identified in a subset of caspases and their adaptor molecules, and notably, CARD–CARD interactions between Apaf-1 and caspase-9 are critical for the assembly of a protein complex (called the apoptosome) that promotes caspase-9 activation during apoptosis (for a recent review, see Adrain and Martin, 2001). Certain CARD-containing caspases have also been implicated in proteolytic processing of cytokines (e.g. caspase-1 is required for maturation of IL-1β and IL-18) that are important in the inflammatory response. However, the family of CARD-containing proteins has expanded dramatically over the past few years and it is now apparent that many of the recently identified CARD-containing proteins do not promote caspase activation. Rather, accumulating evidence suggests that many CARD proteins participate in NF-κB signalling pathways associated with immune responses. Two CARD proteins in particular, Nod1 and Nod2, have been implicated in a pathway that may represent an important pathogen recognition and defence mechanism. These and other recent developments are the subject of this review.
The CARD family: four suits in the deck
For simplicity, we have divided the CARD proteins into four sub-families based on their overall domain structures as follows (Figure 1).
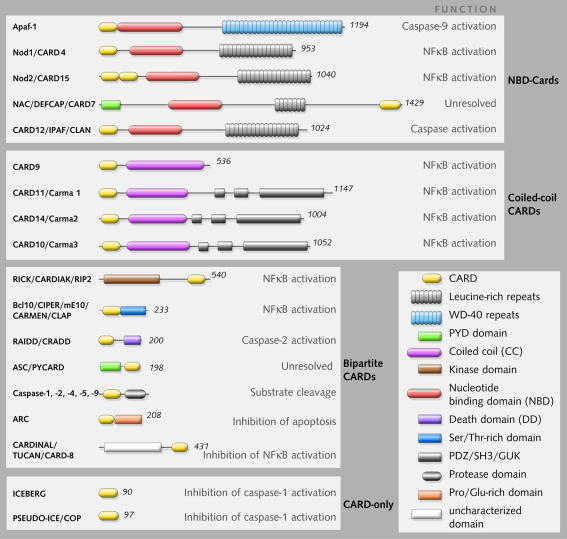
Fig. 1. Schematic representation of the domain structures of human CARD-containing proteins. Proteins have been divided into four sub-groups based upon the domains they share and on their likely function as protein scaffolds, adaptors or decoy molecules (see text).
(i)The NBD-CARDs. These proteins contain a nucleotide-binding domain in addition to a CARD module and most also contain repeats (either leucine-rich or WD-40) within their C-termini. The NBD module most likely acts as an oligomerisation surface (as has been demonstrated for Apaf-1) and the repeat region as a sensory domain that regulates oligomerisation.
(ii)The coiled-coil-CARDs. Proteins within this group are broadly similar in structure to the NBD-CARDs but with the central NBD replaced by a coiled-coil (CC) motif that also most likely acts as an oligomerisation surface. These two subgroups probably act as molecular scaffolds for the assembly of protein activation complexes.
(iii)The bipartite-CARDs. These proteins contain a CARD domain and one other motif such as a kinase domain, a DD motif, a PYD motif or a protease domain. They are frequently recruited by the NBD-CARDs or the CC-CARDs and become activated in the process (e.g. CARD-caspases), or recruit additional effector molecules to the complex, such as the IκB kinase subunit (IKKγ/NEMO).
(iv)The CARD-only proteins. These proteins contain a CARD domain but do not possess any additional recognisable motifs. CARD-only proteins may act as positive or negative regulators of the multi-domain CARDs (NBD-CARDs and CC-CARDs) by regulating recruitment of the bipartite CARDs to the latter molecules. Alternatively, the CARD-only proteins may compete with the bipartite CARDs for binding to their targets.
Although these groupings are somewhat arbitrary, the CARD proteins within each sub-family are likely to act in mechanistically similar ways due to the similarities in their overall domain structures. Furthermore, members of each group tend to act in related signalling pathways.
NBD-CARD proteins
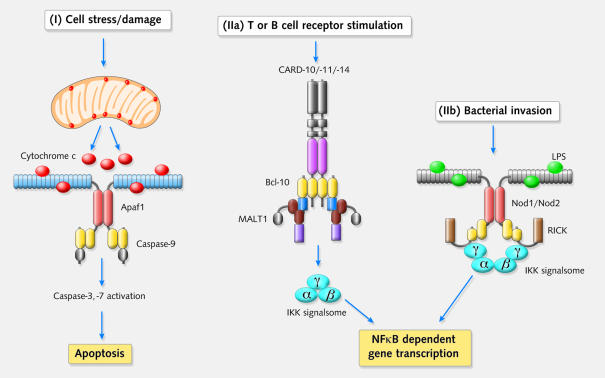
Apaf-1 is a multi-domain protein with an N-terminal CARD, a NBD and multiple WD-40 repeats at its C-terminus (Figure 1). It is well established that Apaf-1 acts as a sensor for cell stress/damage through its activation by cytochrome c released from mitochondria (Adrain and Martin, 2001). Apaf-1 subsequently oligomerises (via its NBD region) and recruits caspase-9 via CARD–CARD interactions between the two proteins (Figure 2). This results in activation of caspase-9 and the propagation of a cascade of further caspase activation events that result in apoptosis.
Fig. 2. Examples of pathways leading to either caspase or NF-κB activation. (I) In response to cell damage or stress, mitochondrial cytochrome c is released into the cytosol to provoke oligomerisation of Apaf-1 and caspase-9 (the apoptosome). Two examples of NF-κB activation pathways involving CARD proteins are shown. (IIa) Upon T- or B-cell receptor stimulation, signalling pathways are initiated that converge upon the bipartite protein Bcl10. Bcl10 promotes NF-κB activation most likely through recruitment of the IKK complex via MALT1. (IIb) A different route to NF-κB activation is initiated by LPS, derived from intracellular bacteria. This may trigger complex formation between Nod1 (or Nod2) and RICK. Recruitment of IKKγ to the complex, via RICK, probably results in assembly and activation of the IKK complex via a mechanism that has not been defined.
Recently, a number of CARD proteins that are structural homologues of Apaf-1 have been identified. Two of these, Nod1 (CARD4) and Nod2 (CARD15), have a similar domain structure to Apaf-1 but contain leucine-rich repeats (LRRs) in place of the C-terminal WD-40 repeats (Figure 1; Bertin et al., 1999; Inohara et al., 1999; Ogura et al., 2001b). Like Apaf-1, the Nod proteins are likely to assemble into large protein complexes upon receipt of an appropriate stimulus, but their role appears to be the activation of NF-κB, rather than caspases.
Both Nod1 and Nod2 appear to drive NF-κB activation through recruitment of the bipartite CARD-kinase protein, RICK (also called CARDIAK/RIP-2; Inohara et al., 1998). RICK can interact with the regulatory subunit of the IκB signalsome, IKKγ/NEMO (Inohara et al., 2000), which probably results in the activation of the IKK complex and the subsequent phosphorylation and proteasome-mediated degradation of IκB (Figure 2). This exposes a nuclear localisation signal in the associated NF-κB, resulting in its translocation to the nucleus and the transcription of a number of genes, many of which are involved in pro-inflammatory processes.
What is the stimulus that drives activation of the Nod proteins? Rather remarkably, studies from Nunez and colleagues indicate that a conserved component of all Gram-negative bacteria, lipopolysaccharide (LPS), may be the culprit (Inohara et al., 2001). LPS is a well-known trigger of the innate immune response through its binding to receptors present on the surfaces of many cell types. Although elusive for many years, the major transmembrane LPS receptor was recently identified as a member of the Toll-like receptor family (TLR4). A wide array of mammalian TLR proteins have now been discovered, all of which appear to be involved in the recognition of different conserved pathogen components by cells of the innate immune system (Aderem and Ulevitch, 2000). However, because intestinal epithelial cells are constantly exposed to large amounts of LPS due to the multitude of gut-resident bacteria, the latter cells neither appear to possess TLR4, nor respond to extracellular LPS by activating NF-κB (Philpott et al., 2000). Presumably, this prevents the chronic inflammation of the gut that would result if colonic epithelial cells were constantly responding to the bacterial flora that surround them. Intriguingly, it has been found that, whereas non-invasive strains of the Gram-negative bacterium Shigella flexneri fail to provoke NF-κB activation in intestinal epithelial cells, invasive strains of the same bacterium do so (Philpott et al., 2000). Moreover, microinjection of LPS into the cytoplasm of epithelial cells (mimicking bacterial invasion) also resulted in NF-κB activation, providing further tantalising evidence that intracellular LPS receptors may exist.
Using the latter observation as a cue, Nunez and colleagues explored whether Nod1 and Nod2 could sensitise otherwise unresponsive cells to bacterial LPS. In a development that parallels the surprising nature of the discovery that cytochrome c can act as a co-factor for Apaf-1 in the caspase-9 activation pathway, LPS was indeed found to drive NF-κB activation in a Nod-dependent manner (Inohara et al., 2001). Furthermore, LPS was found to bind directly to Nod1, most likely through LRRs of the latter (Inohara et al., 2001). Thus, the Nod proteins may function as intracellular pathogen sensors that require a bacterial component (LPS) to trigger their activation (Figure 2). Nod-dependent NF-κB activation may serve to initiate immune responses against intracellular pathogens through transcription of IL-1, IL-8 and other pro-inflammatory cytokines. Interestingly, proteins with similarities to the Nod proteins, although lacking CARD motifs, have been found in plants, where they are associated with resistance to plant pathogens (Dangl and Jones, 2001).
The potential importance of Nod2 (CARD15) as a bacterial pathogen sensor in the gut has been underscored by several recent studies. Inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis, are chronic inflammatory gut disorders of unknown aetiology. However, it has long been suspected that an abnormal immune response to enteric bacteria or food constituents may underlie IBD; genetic linkage studies have previously mapped an IBD susceptibility locus near the centromere on chromosome 16. Thus, when NOD2 was reported to map within this region, at 16q12, several groups explored whether this gene is mutated in individuals with Crohn’s disease or ulcerative colitis (Hampe et al., 2001; Hugot et al., 2001; Ogura et al., 2001a). Remarkably, these studies found frameshift and missense mutations within the LRR region of NOD2, resulting in the expression of a truncated Nod2 protein in a highly significant proportion of Crohn’s individuals. Although the mechanism remains unclear, LRR-truncated Nod2 is likely to be a defective protein that responds to enteric bacteria in an inappropriate way.
Other Apaf-1-like NBD-CARD proteins have also been described recently (Figure 1). These include CARD12 (IPAF/CLAN), which is similar in structure to Nod1 and Nod2 but does not appear to be an NF-κB activating molecule (Figure 1; Geddes et al., 2000). However, initial reports concerning CARD12 function have been contradictory, implicating this molecule in caspase-1 activation and consequent IL-1β processing, as well as in apoptosis.
The NBD and CARD protein, NAC (DEFCAP/CARD7), is similar in structure to the Nod proteins but with some notable differences (Chu et al., 2001; Hlaing et al., 2001). First, unlike all of the other NBD-CARDs, NAC bears the CARD motif at its C-terminus. However, NAC also contains a PYD motif at its N-terminus, which is predicted to have a similar overall tertiary structure to the CARD motif. The PYD motif was initially found in Pyrin, a protein that is mutated in individuals with familial Mediterranean fever. The latter disorder is characterised by frequent occurrences of fever and inflammation, which is consistent with a role for PYD motif-containing proteins in immune response-related signalling pathways. The precise function of NAC remains unclear as it has been implicated in binding directly to caspases (caspase-2 and -9), as well as to Apaf-1, upon simultaneous transient overexpression (Chu et al., 2001; Hlaing et al., 2001). However, the presence of the PYD motif makes it tempting to speculate that NAC functions in an immune response context rather than in apoptosis per se.
The CC-CARDs
The CC-CARD group comprises a number of multi-domain proteins that lack NBDs but possess CC dimerisation motifs in their place (Figure 1). All of the CC-CARD proteins appear to activate NF-κB through recruitment of the bipartite-CARD protein, Bcl10 (Willis et al., 1999).
Is is well established that Bcl10 promotes NF-κB activation, possibly through indirect recruitment of IKKγ via MALT1 (Lucas et al., 2001). Interestingly, MALT1 is a paracapase as it contains a caspase-like domain in addition to a DD. Thus, Bcl10 promotes NF-κB activation by forming a complex with a caspase-like molecule, although how this results in activation of the IKK complex remains to be determined (Lucas et al., 2001). Although initial reports on Bcl10 suggested that this CARD protein may possess pro-apoptotic activity, the phenotype of the BCL10 knockout mouse exhibits few defects that can be attributed to dysregulation of apoptosis (Ruland et al., 2001). A fraction of BCL10 null animals died in utero, apparently due to incomplete neural tube closure, which may reflect some apoptosis-related defect. However, the primary defect in these animals is a profound deficiency in NF-κB activation in the context of T- and B-cell receptor stimulation (Ruland et al., 2001). Surviving BCL10 null animals are highly susceptible to infections due to a failure of lymphocyte populations to undergo clonal expansion in response to antigenic challenge. Thus, Bcl10 appears to act at a convergence point between the T- and B-cell receptor-driven NF-κB signalling pathways. Recent data suggest that, in addition to Bcl10, RICK is also required for appropriate NF-κB signalling downstream of T-cell receptor engagement (Kobayashi et al., 2002).
The intervening steps between the T- and B-cell receptor complexes and Bcl10 may involve additional CC-CARD proteins that have been found to interact with Bcl10. Four CC-CARDs have been reported to date: CARD-9 (Bertin et al., 2000), CARD-10, (Wang et al., 2001), CARD-11 and CARD-14 (Bertin et al., 2001). CARDs -10, -11 and -14 [also called Carma -3, -1, -2, respectively, or Bimp1, -2, -3 in the mouse (Gaide et al., 2001; McAllister-Lucas et al., 2001)] belong to the membrane-associated guanylate kinase (MAGUK) family of proteins, characterised by a tripartite PDZ/SH3/GUK structure that may act as a molecular scaffold to coordinate protein complexes at the cell membrane. CARD-9 is related to the other CC-CARD proteins but lacks the PDZ/SH3/GUK structure. CARD-9 has been shown to oligomerise through its CC domain and it is likely that the other CARDs within this sub-family behave in a similar manner.
Bipartite-CARDs
Most of the CARD proteins mentioned thus far are multi-domain proteins, whereas the bipartite-CARD group of proteins are less complex, comprising a CARD and one additional domain. Proteins within this sub-family appear to function as downstream adaptors for many of the multi-domain CARDs, as discussed above for Bcl10 (a CARD with a Ser/Thr-rich domain) and RICK (a CARD with a kinase domain). Both of these proteins are recruited by several of the multi-domain CARDs to drive NF-κB activation, most likely through their subsequent recruitment of IKKγ into the complex. Interestingly, recent studies using macrophages from RICK null animals have also strongly implicated RICK in NF-κB activation in the context of TLR2, -3 and -4 receptor engagement (Kobayashi et al., 2002). The bipartite-DD protein, RIP, has been shown to function in a similar manner in the TNF receptor pathway (Inohara et al., 2000; Poyet et al., 2000).
Caspases that contain N-terminal CARD (or related DED) motifs also fall into the bipartite-CARD category. CARD-containing caspases are recruited by other CARD (or DED) proteins to initiate caspase signalling cascades. Although the details of this have been worked out for caspase-9/Apaf-1 and caspase-8/FADD, the interaction partners for several of the CARD-containing caspases (caspases -1, -2, -4 and -5) remain unknown.
One bipartite-CARD not discussed thus far is ASC (apoptosis-associated speck-like protein containing a CARD, also called PYCARD; Masumoto et al., 1999). Like NAC, ASC contains a PYD domain along with a CARD motif and so, even though it has been shown to form aggregates in apoptotic cells, it seems more likely to play a role in inflammatory processes. Indeed, ASC has been reported to interact with Pyrin as well as Cryopyrin/Pypaf-1 (Richards et al., 2001; Manji et al., 2002) and to promote caspase-1 activation (Srinivasula et al., 2002). Because the only known multi-domain CARD that contains a PYD motif appears to be NAC, it is tempting to speculate that NAC also interacts with ASC through a PYD–PYD interaction. The CARD motif within ASC would then be free to recruit an additional CARD protein into the complex. Thus, the precise functional role of ASC in apoptosis or the immune response has yet to be resolved.
RAIDD (also called CRADD), one of the first CARDs to be described, also falls into the bipartite-CARD category. RAIDD possesses a DD in addition to a CARD domain and interacts with caspase-2, but the context in which it does so remains obscure. RAIDD is also known to interact with RIP (via DD motifs present in both molecules) and has been proposed to be a component of the TNF receptor signalling complex and therefore to act in an apoptosis pathway (Duan and Dixit, 1997). However, the phenotype of the CASP-2 null mouse strongly suggests that caspase-2 does not play a significant role in TNF receptor signalling (Bergeron et al., 1998). Thus, the function of RAIDD/CRADD remains unresolved.
Apoptosis repressor with CARD (ARC) is another bipartite-CARD and has been implicated as an inhibitor of apoptosis, apparently functioning through direct interaction with caspases-2 and -8 (Koseki et al., 1998). CARDINAL/TUCAN/CARD-8 is also a relatively recently described member of this class which has been implicated as an inhibitor of NF-κB activation and may facilitate apoptosis as a consequence (Bouchier-Hayes et al., 2001; Pathan et al., 2001; Razmara et al., 2002).
CARD-only proteins: the jokers in the pack?
A small number of proteins that contain CARD motifs and little else have been described. This category includes ICEBERG (Humke et al., 2000) and pseudo-ICE/COP (Druilhe et al., 2001; Lee et al., 2001). These molecules are likely to act as decoys that may compete with other CARD proteins for binding to bipartite-CARDs or multi-domain CARDs and in this way may act as inhibitors of either caspase or NF-κB activation pathways. In line with this view, both ICEBERG and pseudo-ICE have been reported to antagonise caspase-1 activation/IL-1β processing (Humke et al., 2000; Druilhe et al., 2001).
Conclusions
From the above discussion of CARD proteins and their functions, it becomes apparent that there are at least three major pathways in which these proteins act: regulation of caspase activation in the context of apoptosis, regulation of caspase activation in the context of inflammation, or regulation of NF-κB activation in the context of innate or adaptive immune responses. Broadly speaking, all of the CARD proteins described thus far have been implicated in one way or another in host defence against infection, environmental stress, or cellular damage. Clearly, there is significant crosstalk between the pathways that result in NF-κB activation and those that lead to caspase-mediated inflammation or apoptosis. Thus, it is not much of a surprise to find that similar protein modules are found repeatedly in proteins from all pathways. Moreover, the CARD motif has also been found within proteins from nematodes, flies and viruses. Clearly, there are a few more hands to be played before we fully understand the complexities of the signalling pathways involving CARD proteins.

Seamus J. Martin & Lisa Bouchier-Hayes
Acknowledgments
Acknowledgements
We thank The Wellcome Trust (047580), The European Union (QLG1-1999-00739) and Science Foundation Ireland (PI1/B038) for their generous support of ongoing work in our laboratory. L.B.-H. was supported by a Wellcome Trust Prize Studentship (055295).
References
- Aderem A. and Ulevitch, R.J. (2000) Toll-like receptors in the induction of the innate immune response. Nature, 406, 782–787. [DOI] [PubMed] [Google Scholar]
- Adrain C. and Martin, S.J. (2001) The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem. Sci., 26, 390–397. [DOI] [PubMed] [Google Scholar]
- Aravind L., Dixit, V.M. and Koonin, E.V. (1999) The domains of death: evolution of the apoptosis machinery. Trends Biochem. Sci., 24, 47–53. [DOI] [PubMed] [Google Scholar]
- Bergeron L. et al. (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev., 12, 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin J. and DiStefano, P.S. (2000) The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ., 7, 1273–1274. [DOI] [PubMed] [Google Scholar]
- Bertin J. et al. (1999) Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J. Biol. Chem., 274, 12955–12958. [DOI] [PubMed] [Google Scholar]
- Bertin J. et al. (2000) CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAPand activates NF-κB. J. Biol. Chem., 275, 41082–41086. [DOI] [PubMed] [Google Scholar]
- Bertin J., Wang, L., Guo, Y., Jacobson, M.D., Poyet, J.L., Srinivasula, S.M., Merriam, S., DiStefano, P.S. and Alnemri, E.S. (2001) CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-κB. J. Biol. Chem., 276, 11877–11882. [DOI] [PubMed] [Google Scholar]
- Boldin M.P., Goncharov, T.M., Goltsev, Y.V. and Wallach, D. (1996) Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell, 85, 803–815. [DOI] [PubMed] [Google Scholar]
- Bouchier-Hayes L., Conroy, H., Egan, H., Adrain, C., Creagh, E.M., MacFarlane, M. and Martin, S.J. (2001) CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF-κB activation pathways. J. Biol. Chem., 276, 44069–44077. [DOI] [PubMed] [Google Scholar]
- Chu Z.L., Pio, F., Xie, Z., Welsh, K., Krajewska, M., Krajewski, S., Godzik, A. and Reed, J.C. (2001) A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. J. Biol. Chem., 276, 9239–9245. [DOI] [PubMed] [Google Scholar]
- Dangl J.L. and Jones, J.D. (2001) Plant pathogens and integrated defence responses to infection. Nature, 411, 826–833. [DOI] [PubMed] [Google Scholar]
- Druilhe A., Srinivasula, S.M., Razmara, M., Ahmad, M. and Alnemri, E.S. (2001) Regulation of IL-1β generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ., 8, 649–657. [DOI] [PubMed] [Google Scholar]
- Duan H. and Dixit, V.M. (1997) RAIDD is a new ‘death’ adaptor molecule. Nature, 385, 86–89. [DOI] [PubMed] [Google Scholar]
- Gaide O., Martinon, F., Micheau, O., Bonnet, D., Thome, M. and Tschopp, J. (2001) Carma1, a CARD-containing binding partner of Bcl10, induces Bcl10 phosphorylation and NF-κB activation. FEBS Lett., 496, 121–127. [DOI] [PubMed] [Google Scholar]
- Geddes B.J. et al. (2000) Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem. Biophys. Res. Commun., 284, 77–82. [DOI] [PubMed] [Google Scholar]
- Hampe J. et al. (2001) Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet, 357, 1925–1928. [DOI] [PubMed] [Google Scholar]
- Hlaing T., Guo, R.F., Dilley, K.A., Loussia, J.M., Morrish, T.A., Shi, M.M., Vincenz, C. and Ward, P.A. (2001) Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins. J. Biol. Chem., 276, 9230–9238. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Bucher, P. and Tschopp, J. (1997) The CARD domain: a new apoptotic signalling motif. Trends Biochem. Sci., 22, 155–156. [DOI] [PubMed] [Google Scholar]
- Hugot J.P. et al. (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature, 411, 599–603. [DOI] [PubMed] [Google Scholar]
- Humke E.W., Shriver, S.K., Starovasnik, M., Fairbrother, W.J. and Dixit, V.M. (2000) ICEBERG: a novel inhibitor of interleukin-1β generation. Cell, 103, 99–111. [DOI] [PubMed] [Google Scholar]
- Inohara N., del Peso, L., Koseki, T., Chen, S. and Nunez, G. (1998) RICK, a novel protein kinase containing a caspase recruitment domain, interacts with CLARP and regulates CD95-mediated apoptosis. J. Biol. Chem., 273, 12296–12300. [DOI] [PubMed] [Google Scholar]
- Inohara N. et al. (1999) Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem., 274, 14560–14567. [DOI] [PubMed] [Google Scholar]
- Inohara N., Koseki, T., Lin, J., del Peso, L., Lucas, P.C., Chen, F.F., Ogura, Y. and Nunez, G. (2000) An induced proximity model for NF-κB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem., 275, 27823–27831. [DOI] [PubMed] [Google Scholar]
- Inohara N., Ogura, Y., Chen, F.F., Muto, A. and Nunez, G. (2001) Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem., 276, 2551–2554. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Inohara, N., Hernandez, L.D., Galan, J.E., Nunez, G., Janeway, C.A., Medzhitov, R. and Flavell, R.A. (2002) RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature, 416, 194–199. [DOI] [PubMed] [Google Scholar]
- Koseki T., Inohara, N., Chen, S. and Nunez, G. (1998) ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc. Natl Acad. Sci. USA, 95, 5156–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Stehlik, C. and Reed, J.C. (2001) Cop, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J. Biol. Chem., 276, 34495–34500. [DOI] [PubMed] [Google Scholar]
- Lucas P.C., Yonezumi, M., Inohara, N., McAllister-Lucas, L.M., Abazeed, M.E., Chen, F.F., Yamaoka, S., Seto, M. and Nunez, G. (2001) Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-κB signaling pathway. J. Biol. Chem., 276, 19012–19019. [DOI] [PubMed] [Google Scholar]
- Manji G.A. et al. (2002) PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-κB. J. Biol. Chem., 277, 11570–11575. [DOI] [PubMed] [Google Scholar]
- Martinon F., Hofmann, K. and Tschopp, J. (2001) The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr. Biol., 11, R118–R120. [DOI] [PubMed] [Google Scholar]
- Masumoto J. et al. (1999) ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem., 274, 33835–33838. [DOI] [PubMed] [Google Scholar]
- McAllister-Lucas L.M. et al. (2001) Bimp1, a MAGUK family member linking protein kinase C activation to Bcl10-mediated NF-κB induction. J. Biol. Chem., 276, 30589–30597. [DOI] [PubMed] [Google Scholar]
- Ogura Y. et al. (2001a) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature, 411, 603–606. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Inohara, N., Benito, A., Chen, F.F., Yamaoka, S. and Nunez, G. (2001b) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem., 276, 4812–4818. [DOI] [PubMed] [Google Scholar]
- Pathan N. et al. (2001) TUCAN, an antiapoptotic caspase-associated recruitment domain family protein overexpressed in cancer. J. Biol. Chem., 276, 32220–32229. [DOI] [PubMed] [Google Scholar]
- Philpott D.J., Yamaoka, S., Israel, A. and Sansonetti, P.J. (2000) Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol., 165, 903–914. [DOI] [PubMed] [Google Scholar]
- Poyet J.L., Srinivasula, S.M., Lin, J.H., Fernandes-Alnemri, T., Yamaoka, S., Tsichlis, P.N. and Alnemri, E.S. (2000) Activation of the IκB kinases by RIP via IKKγ/NEMO-mediated oligomerization. J. Biol. Chem., 275, 37966–37977. [DOI] [PubMed] [Google Scholar]
- Razmara M., Srinivasula, S.M., Wang, L., Poyet, J.L., Geddes, B.J., DiStefano, P.S., Bertin, J. and Alnemri, E.S. (2002) CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J. Biol. Chem., 277, 13952–13958. [DOI] [PubMed] [Google Scholar]
- Richards N., Schaner, P., Diaz, A., Stuckey, J., Shelden, E., Wadhwa, A. and Gumucio, D.L. (2001) Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem., 276, 39320–39329. [DOI] [PubMed] [Google Scholar]
- Ruland J. et al. (2001) Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell, 104, 33–42. [DOI] [PubMed] [Google Scholar]
- Srinivasula S.M., Poyet, J.L., Razmara, M., Datta, P., Zhang, Z. and Alnemri, E.S. (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- Wang L. et al. (2001) CARD10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with bcl10 and activates NF-κB. J. Biol. Chem., 276, 21405–21409. [DOI] [PubMed] [Google Scholar]
- Willis T.G. et al. (1999) Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell, 96, 35–45. [DOI] [PubMed] [Google Scholar]