Abstract
5-Aminolevulinic acid (ALA) has been approved by the U. S. FDA for fluorescence-guided resection of high-grade glioma and photodynamic therapy (PDT) of superficial skin precancerous and cancerous lesions. As a prodrug, ALA administered orally or topically is metabolized in the heme biosynthesis pathway to produce protoporphyrin IX (PpIX), the active drug with red fluorescence and photosensitizing property. Preferential accumulation of PpIX in tumors after ALA administration enables the use of ALA for PpIX-mediated tumor fluorescence diagnosis and PDT, functioning as a photo-theranostic agent. Extensive research is currently underway to further enhance ALA-mediated PpIX tumor disposition for better tumor visualization and treatment. Particularly, the discovery of PpIX as a specific substrate of ATP binding cassette subfamily G member 2 (ABCG2) opens the door to therapeutic enhancement with ABCG2 inhibitors. Studies with human tumor cell lines and human tumor samples have demonstrated ABCG2 as an important biological determinant of reduced ALA-PpIX tumor accumulation, inhibition of which greatly enhances ALA-PpIX fluorescence and PDT response. These studies strongly support targeting ABCG2 as an effective therapeutic enhancement approach. In this review, we would like to summarize current research of ABCG2 as a drug efflux transporter in multidrug resistance, highlight previous works on targeting ABCG2 for therapeutic enhancement of ALA, and provide future perspectives on how to translate this ABCG2-targeted therapeutic enhancement strategy from bench to bedside.
Keywords: 5-Aminolevulinic acid (ALA), Protoporphyrin IX, ATP binding cassette subfamily G member 2 (ABCG2), Photodynamic therapy (PDT), Lapatinib
Graphical Abstract

1. Introduction
5-aminolevulinic acid (ALA) and its derivatives have received worldwide approval for photodynamic therapy (PDT) and fluorescence-guided tumor resection (1). As a natural metabolite and prodrug, ALA has neither fluorescence property nor photosensitizing activity on its own. It needs to be metabolized in the heme biosynthesis pathway to produce protoporphyrin IX (PpIX), the active drug with strong photosensitizing activity and red fluorescence upon light activation (Fig. 1). The heme biosynthesis pathway begins with the synthesis of ALA from succinyl-CoA and glycine by ALA synthase (ALAS) in mitochondria, continues with four consecutive enzymatic steps in the cytosol, and ends with the chelation of PpIX and ferrous iron to produce heme by ferrochelatase (FECH) back in the mitochondria (2). The pathway is tightly regulated by the end product heme, which imposes a negative feedback inhibition on ALAS, the rate-limiting enzyme of the pathway. Because of this tight regulation, intracellular PpIX is normally maintained at a very low level (3). Exogenously administered ALA bypasses the rate-limiting step and causes increased biosynthesis of PpIX that cannot be converted to heme in a timely manner, resulting in PpIX intracellular accumulation. This ALA-stimulated PpIX accumulation is particularly pronounced in some tumor tissues due to tumor-associated metabolic and micro-environmental changes (4). As PpIX is the only metabolite in the heme biosynthesis pathway with fluorescence and photosensitizing property, ALA-induced PpIX tumor accumulation enables the use of ALA as a prodrug for PpIX fluorescence-guided tumor resection and PDT for tumor destruction.
Figure 1.
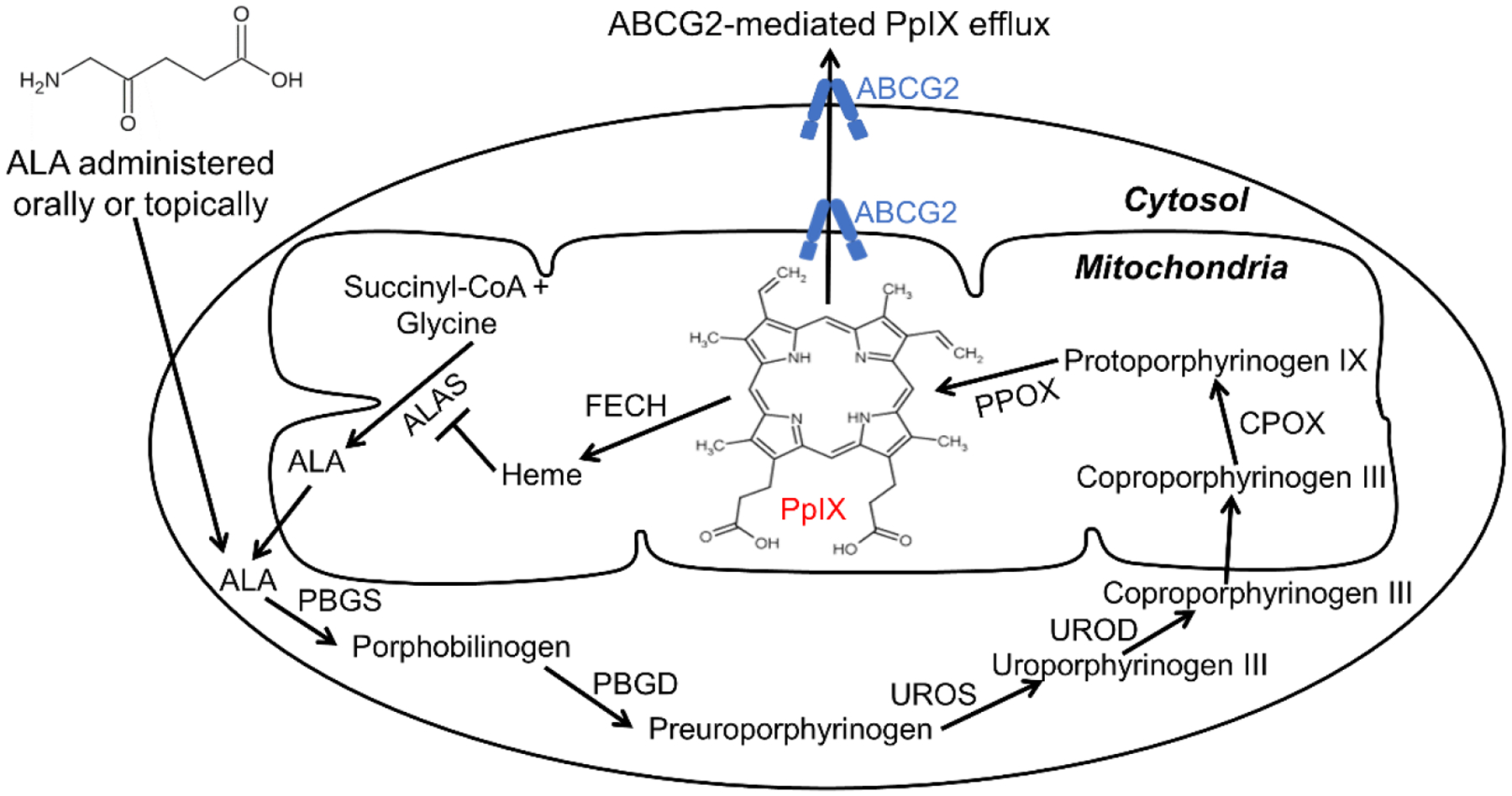
ALA administered orally or topically as a prodrug is metabolized in the heme biosynthesis pathway to produce the active drug PpIX with strong photosensitizing activity and red fluorescence upon light activation. The heme biosynthesis pathway is composed of eight enzymes including ALA synthase (ALAS), porphobilinogen synthase (PBGS), porphobilinogen deaminase (PBGD), uroporphyrinogen III synthase (UROS), uroporphyrinogen III decarboxylase (UROD), coproporphyrinogen III oxidase (CPOX), protoporphyrinogen IX oxidase (PPOX) and ferrochelatase (FECH). PpIX produced in the heme biosynthesis pathway is pumped out of the cell by the ABCG2 transporter.
A successful use of ALA for tumor visualization and treatment relies on sufficient and preferential PpIX drug disposition in the tumor tissue compared to the surrounding normal tissue. However, low and heterogeneous PpIX tumor disposition has been shown in skin and brain cancer patient samples (5–7), which may limit the use of ALA for tumor fluorescence diagnosis and PDT and calls for therapeutic enhancement strategies. Fig. 1 indicates that the level of PpIX in tumor cells following ALA administration depends on three dynamic biological processes, 1) ALA uptake and subsequent PpIX biosynthesis catalyzed by six heme biosynthetic enzymes, 2) PpIX to heme (with no fluorescent and photosensitizing property) bioconversion catalyzed by the FECH, and 3) PpIX outward transport. The first process results in enhanced PpIX biosynthesis after ALA, while the latter two reduce PpIX level intracellularly. Intracellular PpIX can be enhanced by increasing PpIX biosynthesis and/or suppressing PpIX bioconversion and outward transport.
Although activating ALA-PpIX biosynthetic enzymes may increase intracellular PpIX, there is evidence indicating that overexpression of a single enzyme involved in PpIX biosynthesis does not significantly increase intracellular PpIX (8, 9), suggesting the necessity for simultaneous activation of multiple enzymes in order to enhance PpIX biosynthesis. However, selective and simultaneous activation of multiple enzymes for PpIX biosynthesis in tumor cells remains to be a significant technological difficulty. In contrast, targeting two PpIX-reducing processes has turned out to be both pharmacologically feasible and therapeutically effective (1). Particularly, the identification of PpIX as a specific substrate of ATP-binding cassette (ABC) subfamily G member 2 (ABCG2) about twenty years ago stimulated the exploration of targeting ABCG2 to enhance ALA-PpIX tumor fluorescence and PDT. In this review, we describe the discovery of ABCG2 transporter and its substrate PpIX, summarize current understanding of ABCG2 structure and function, and highlight research progresses on targeting ABCG2 for therapeutic enhancement of ALA. Research over the past twenty years has laid a solid foundation for the clinical translation of this ABCG2-targeted therapeutic enhancement strategy.
2. Discovery of ABCG2
In search for multidrug resistance (MDR) mechanisms to anticancer drug doxorubicin (adriamycin) other than the involvement of permeability glycoprotein (P-gp) encoded by ABCB1 or MDR1, Chen and colleagues selected doxorubicin-resistant MCF-7 breast cancer cells in the presence of P-gp inhibitor verapamil (10). The resultant MCF-7/AdrVp subline exhibited drug resistance to doxorubicin and other chemotherapeutic drugs including daunorubicin and melphalan without the overexpression of P-gp. Western blot analysis revealed an unknown cell membrane protein overexpressed in this MDR subline, the level of which was not only correlated with drug resistance but also found high in tumor samples from patients refractory to doxorubicin treatment. These results suggested a novel cell membrane protein involved in the MDR, although its identity was still a mystery. The newly established MCF-7/AdrVp cells did not overexpress multidrug resistance-associated protein 1 (MRP1, ABCC1) either, another ABC transporter involved in the MDR (11). However, the obervation that intracellular accumulation of daunorubicin and rhodamine in MCF-7/AdrVp cells was reduced in an ATP-dependent manner suggested that this mysterious protein was a new ABC transporter.
Using RNA fingerprinting method, Doyle et al. identified a 2.4-kb mRNA overexpressed in MCF-7/AdrVp cells, which indeed encoded for a new member of the ABC superfamily (12). Overexpression of this 663-amino acid protein in the wild type MCF-7 cells rendered multidrug resistance to doxorubicin, daunorubicin and mitoxantrone by reducing drug accumulation in an ATP-dependent manner. This novel ABC transporter responsible for the multidrug resistance phenotype was named breast cancer resistance protein (BCRP) due to its involvement in mediating drug resistance in MCF-7 breast cancer cells. Around the same time from 1998 to 1999, two other groups also independently reported the discovery of the same gene. One group screened through the human genome and found a new ABC transporter gene named as ABCP (P for placenta) because it was highly expressed in the placenta (13). The other group isolated and cloned a mitoxantrone-resistant gene MXR from S1-M1-80 human colon carcinoma cells resistant to mitoxantrone, which turned out to be the same ABC gene overexpressed in the MCF-7/AdrVp cells (14). BCRP, ABCP and MXR all share the same gene sequence and are now named as ABC subfamily G member 2 (ABCG2).
3. Genetics, structure & functions of ABCG2
Human ABCG2 gene is located on chromosome 4q22 and expands over 66 kilo bases including 16 exons and 15 introns (15). Its promoter region, spanning over a few hundred base pairs upstream the transcriptional start site, contains the hormone response elements (16, 17), hypoxia response element (18), and antioxidant response element (19), resulting in transcriptional activation of ABCG2 by estrogen/progesterone, hypoxia and oxidative stress, respectively. It also has the binding sites of transcriptional factors including specificity protein (SP)-1, −3, activator protein (AP)-1 and −2 (15, 20). Several kilo base pairs upstream the promoter region, there are xenobiotic and peroxisome proliferator-activated receptor gamma (PPARγ) response elements, which lead to ABCG2 transcriptional activation when aryl hydrocarbon receptor (21) and PPARγ (22) are activated by xenobiotics such as drugs and environmental toxins. Furthermore, ABCG2 gene amplification (23, 24), promoter demethylation (25) and histone modifications (26) have all been shown to activate ABCG2 transcription. Mutation and single nucleotide polymorphism (SNP) of ABCG2 are other genetic alternations that can have significant effects on the gene expression, protein level and function of ABCG2 (27). For instance, mutation of arginine at amino acid number 482 to threonine (R482T) or glycine (R482G) changes substrate specificity, whereas SNP 421C>A (causing Q141K change in amino acid) is a common variant of ABCG2 in Asian population and known for reducing ABCG2 protein level and function (28).
ABCG2 gene encodes for an ABC transporter protein with N-terminal nucleotide binding domain (NBD) for ATP binding and hydrolysis, and C-terminal transmembrane domain (TMD) for substrate binding and transport in the order of N-NBD-TMD-C (12). This protein sequence is different from most plasma membrane-associated ABC transporters such as ABCB1 and ABCC1 where the sequence is N-TMD-NBD-TMD-NBD-C (29). Not only the protein sequence is reversed in ABCG2, it has only one TMD and one NBD instead of two of each in a full ABC transporter. Therefore, ABCG2 is considered a half transporter and requires dimerization or oligomerization for its function. Although the structure of ABCG2 by X-ray crystallography is not yet available, studies of ABCG2 structure by high-resolution cryo-electron microscopy indicate the homodimerization of two ABCG2 molecules to form a channel-like structure across the membrane (30–33). In the presence of substrate molecules, ABCG2 dimers have been captured in two conformations, the inward-facing and outward-facing states as illustrated in Fig. 2 (31). In the inward-facing state, two intracellular NBDs face away from each other, which creates a substrate-binding cavity within the TMDs for substrate binding. The binding of two ATP molecules to the NBDs causes two NBDs to dimerize, resulting in the outward-facing conformation in which the substrate-binding cavity is collapsed and an opening at the extracellular site is generated for substrate release. ATP hydrolysis causes this outward-facing conformation switched back to the inward-facing conformation. It is hypothesized that rapid cycling from the inward- to the outward-facing conformation and back to the inward-facing conformation propels the peristatic movement of substrate molecules across the membrane. This substance transport model is further substantiated by a recent revelation of ABCG2 structure in a transient turnover conformation where substrate and ATP molecules were all bound to ABCG2 (33). Without the presence of substrate molecules, ABCG2 has been shown to adopt an apo-closed conformation where the substrate-binding cavity is blocked by the TMDs (Fig. 2), which suggests the induction of ABCG2 from the apo state to the substrate-binding conformation by substrate molecules (32).
Figure 2.
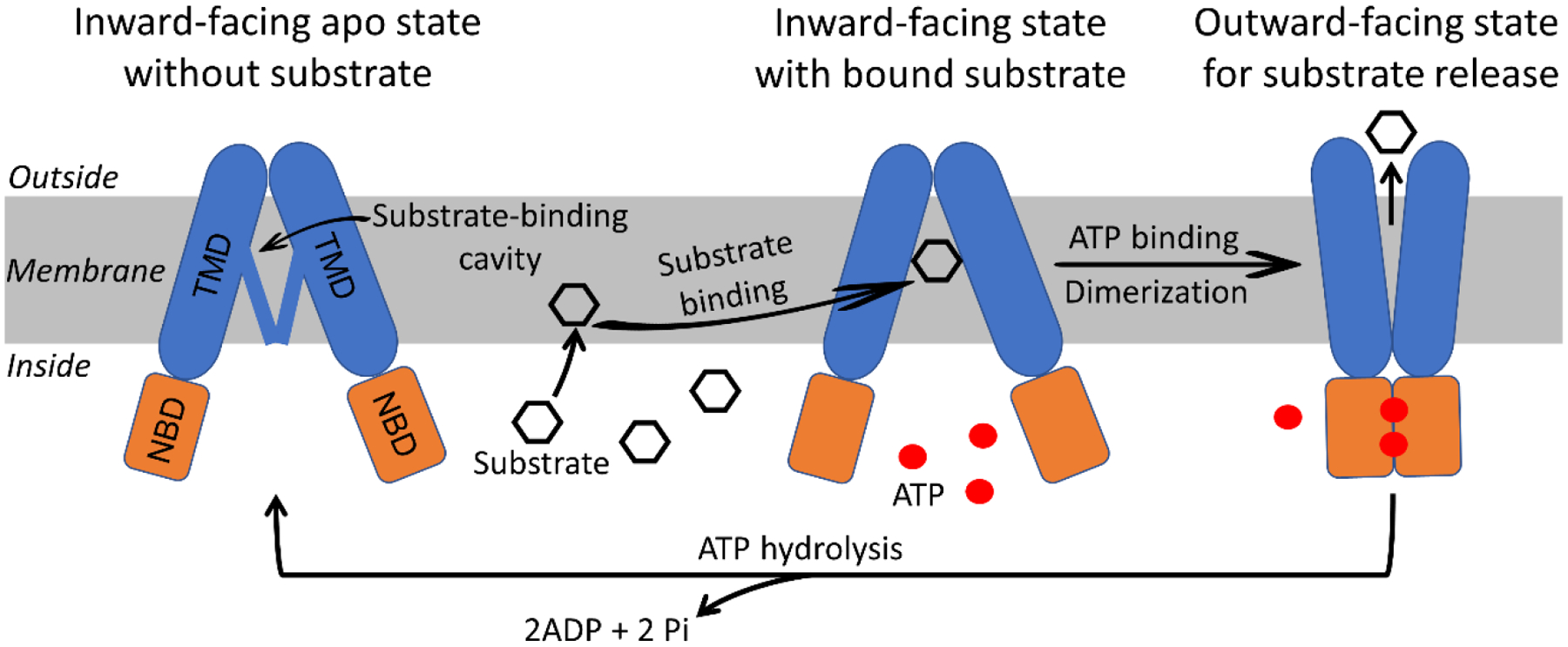
Schematic illustration of ABCG2 structure and transport cycle. ABCG2 is shown as the homodimer of two subunits that is composed of a transmembrane domain (TMD) for substrate binding and transport and a nucleotide binding domain (NBD) for ATP binding and hydrolysis. Without the presence of substrate, it occurs in the inward-facing apo conformation where two NBDs are away from each other and the substrate-binding cavity is closed by the TMDs. In the presence of substrate molecules, the substrate-binding cavity is accessible for substrate binding due to substrate-induced conformational change of the TMD, although ABCG2 still exists in the inward-facing state. The binding of two ATP molecules to the NBD triggers the dimerization and induces the outward-facing conformation in which the substrate-binding cavity is collapsed and an extracellular cavity is created for substrate release. The cycle ends with the hydrolysis of ATP that resumes ABCG2 molecules to the inward-facing state.
As an ATP-dependent efflux transporter, ABCG2 has a variety of structurally unrelated substrates including physiological substances (e.g., estrone-3-sulfate, uric acid), environmental carcinogens (e.g., benzo[a]pyrene conjugates, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), and hundreds of drugs such as chemotherapeutic drugs and kinase inhibitors (34). ABCG2 transporter is widely distributed in the human body with high expression in placental syncytiotrophoblasts (forming blood placental barrier), cerebral endothelial cells (forming blood brain barrier), small intestine and colon epithelium (apical membranes), hepatocytes (canalicular membranes), kidney proximal tubule epithelial cells (apical membranes) and stem cells (27, 35). The ability of ABCG2 to transport both endogenous and exogenous substances with diverse chemical structures together with its localization at the entry and exit sites of the body and the barrier structure of tissue compartments suggest its primary physiological function, i.e., protecting tissues/bodies from being exposed to potentially harmful substances. This is well illustrated by the finding that the loss of function of ABCG2 in intestinal epithelium and kidney proximal tubule cells due to SNP 421C>A results in the accumulation of urate in the blood, causing hyperuricemia and gout (36, 37). Because ABCG2 has hundreds of drugs as its substrates, it therefore affects the pharmacokinetics and intracellular levels of these substrate drugs, notably anticancer drugs (38). Its important role in mediating drug resistance by reducing anticancer drug cellular uptake as well as its high expression in cancer stem cells makes it an appealing therapeutic target for cancer treatment, which has stimulated the discovery of ABCG2 inhibitors and the exploration of using these inhibitors to overcome cancer drug resistance.
4. ABCG2 inhibitors
Fumitremorgin C (FTC) is the first potent ABCG2 inhibitor discovered with the goal of overcoming multidrug resistance. It was identified during the screening of a library of microorganism extracts for restoring the drug sensitivity in mitoxantrone-resistant colon cancer cells, even before the identity of ABCG2 was uncovered (39). FTC suppresses ABCG2 activity by inhibiting its ATPase activity (40). However, because FTC causes severe neurotoxic symptoms in animals, its analogues such as Ko143 with better toxicity profile were developed (41). Ko143 is able to reverse drug resistance in ABCG2-mediated resistant cell lines at nanomolar concentrations and increase the oral availability of topotecan in mice with no apparent toxicity (41). Although Ko143 is a potent ABCG2 inhibitor, it has been shown to inhibit P-gp and MRP1 at micromolar concentrations (41, 42). In addition, its instability in the plasma and rapid elimination by the liver prevent its application in vivo (42, 43). Nevertheless, due to its high potency, Ko143 is commonly used as a pharmacological tool compound for ABCG2 inhibition.
To develop clinically useful ABCG2 inhibitors, extensive screening of existing chemicals and synthesis of new chemical entities have been undertaken, which results in the identification of many chemicals with ABCG2 inhibitory activity (44). For instance, high-throughput screening of 89,229 natural product extracts has led to the identification of botryllamides that increase drug penetration into the brain by inhibiting ABCG2 (45, 46). New chemical entities such as flavonoids (47), pyrimidopyrimidines (48), quinazoline derivatives (49) and heteroarylphenyl derivatives (50) have been synthesized and evaluated as potential ABCG2 inhibitors. Despite the discovery of many chemicals with ABCG2 inhibitory activity, clinical applications of these inhibitors are limited largely due to issues such as normal tissue toxicity and poor pharmacokinetic property. There is currently no ABCG2 inhibitors available for the clinical application (51)
As some approved drugs are both ABCG2 substrates and inhibitors (34), repurposing these existing drugs as possible ABCG2 inhibitors is becoming an efficient and promising strategy for developing clinical ABCG2 inhibitors because the safety and pharmacokinetic profile of these drugs have been well established. Applying a ligand-based in silico classification model to screen the DrugBank database with 1780 compounds has generated 129 hits as possible ABCG2 inhibitors including drugs such as cisapride and roflumilast with confirmed ABCG2 inhibitory activity (52). Febuxostat, a drug used for hyperuricemia, has been shown to be a potent ABCG2 inhibitor (53). Inhibitors developed for ABCB1 such as elacridar and tariquidar are ABCG2 inhibitors as well (54, 55). Some tyrosine kinase inhibitors including imatinib and gefitinib have been reported to inhibit ABCG2 (56). Mechanistically, ABCG2 inhibitors including Ko143, tariquidar and kinase inhibitor imatinib have all been shown to bind to the ABCG2 substrate-binding sites and stabilize the inward-facing conformation, which blocks the access and transport of substrate molecules (57, 32).
5. Discovery of PpIX as a specific ABCG2 substrate
The finding that exogenously applied ALA stimulates the biosynthesis and accumulation of endogenous PpIX with potent phototoxicity (58) led to the development of ALA as a prodrug for PpIX-mediated PDT in cancer treatment (59). It was noted that PpIX produced in tumor cells after ALA could rapidly leak out into the cell culture medium (60, 61). Although the passive diffusion was expected due to the lipophilicity of PpIX, more rapid leakage seen in some tumor cell lines over the others suggested the existence of an active outward transport mechanism. As ABC transporters were already known to cause tumor cell MDR to chemotherapy, studies were initiated to determine whether these transporters were involved in transporting PpIX as well, which may cause tumor cross-resistance to ALA-PDT. The findings that PpIX outward transport was not blocked by ABCB1 inhibitor verapamil in bladder cancer cells (61) and there was no difference in ALA-PpIX fluorescence and PDT response between ABCB1-mediated drug resistant ovarian tumor cell line and its parental line (62) suggest that ABCB1 is not involved in transporting PpIX. Comparing the level of intracellular and extracellular PpIX and PDT response in ABCB1-overexpressing leukemia cell lines versus the parental lines, Li et al. also concluded that ABCB1 had no effect on PpIX transport and PDT effect (63). Robey et al. not only confirmed this finding in ABCB1 drug-resistant breast cancer cell lines, but further showed that ABCC1 was not involved in transporting PpIX either (64).
The first evidence suggesting PpIX as an ABCG2 substrate came up unexpectedly from the Abcg2 knockout study in 2002 (65). Mice without Abcg2 function were found to develop protoporphyria with increased PpIX in erythrocytes and plasma, which could be cured by bone marrow transplantation from the wild type mice. Abcg2-knockout mice also exhibited a severe skin photosensitivity to pheophorbide a (Pha), a chlorophyll-derived dietary phototoxin. This study demonstrated that Pha was transported by Abcg2/ABCG2 in mouse and human cell lines, and suggested the involvement of ABCG2 in transporting PpIX based on the similarity in structure between the two, one (Pha) from the green pigment of life chlorophyll and the other (PpIX) from the red pigment of life heme. A subsequent study confirmed that bone marrow cells from Abcg2-knockout mice had higher PpIX fluorescence than those from the wide type mice (18). Moreover, overexpression of ABCG2 in acute myeloid leukemia cells reduced PpIX fluorescence, further supporting the notion that ABCG2 is involved in PpIX efflux. By measuring the intracellular and extracellular PpIX in both parental and ABCG2-overexpressing cell lines with or without ABCG2 inhibitor Ko143, Zhou et al. provided unequivocal evidence that PpIX is indeed an ABCG2 substrate (66).
6. Inhibition of ABCG2 to enhance ALA-PpIX fluorescence and PDT
Identification of PpIX as a specific substrate of ABCG2 leads to the use of ABCG2 inhibitors to enhance ALA-PpIX fluorescence and PDT response. Robey et al. first showed that ABCG2 inhibitor fumitremorgin C significantly increased ALA-PpIX fluorescence and PDT-induced cytotoxicity in ABCG2-transfected cells (64). Furthermore, cells transfected with mutant ABCG2 (R482T, R482G) exhibited similar fluorescence as cells transfected with the wild type ABCG2 (R482), suggesting that mutation of arginine at amino acid number 482 to threonine (R482T) or glycine (R482G) has no effect on PpIX transport. Fumitremorgin C was also shown to increase intracellular PpIX and reduce extracellular PpIX levels after ALA treatment in T24 human urothelial cancer cell line, but not in its ABCG2-knockdown counterpart, highlighting the importance of ABCG2 expression in PpIX efflux (67). The study further showed that PpIX efflux in T24 cells was dependent on the presence of fetal bovine serum, bovine serum albumin in particular. This result, which was reported previously (60), is in agreement with the finding that ABCG2 transports its porphyrin substrates including heme and PpIX to the albumin molecules with specific heme-binding sites through its large extracellular loop (68). Without the presence of albumin in the medium to receive PpIX, PpIX molecules remain to be associated with the cell membrane. In addition to the T24 urothelial cancer cell line, the effectiveness of fumitremorgin C in enhancing ALA-PpIX/PDT has also been demonstrated in gastric cancer (69), prostate cancer (70), ovarian cancer (71) and other cancer cell lines (72).
Due to the neurotoxicity of fumitremorgin C, its analogues including Ko143 without this adverse effect were synthesized (41). Ko143 has been extensively studied as an ABCG2 inhibitor for enhancing ALA-PpIX fluorescence and PDT in a variety of tumor cell lines. HaCaT human keratinocytes show strong ABCG2 expression when cells are in the proliferative state and little ABCG2 expression when in the differentiated condition (73). Therefore, proliferative HaCaT cells display lower ALA-PpIX fluorescence, which can be significantly enhanced by Ko134 (an analogue of Ko143 with lower activity of ABCG2 inhibition (41), whereas differentiated HaCaT cells exhibit higher PpIX fluorescence and little response to Ko134. These results suggest that ALA-PpIX fluorescence and the effect of Ko134 is related to the ABCG2 expression. Similar results have also been reported in the U-251 glioblastoma cell line (74). In a panel of five human cancer cell lines from five different histological origins, Kobuchi et al. found that ALA-PpIX fluorescence was inversely correlated with the ABCG2 protein level and had no correlation with peptide transporter 1 & 2 (PEPT1 & 2) that are involved in ALA uptake or the FECH that converts PpIX to heme with no fluorescence (75). Additionally, the study showed that ABCG2 was primarily localized in the mitochondria rather than on the cell membrane, and treatment with Ko143 caused PpIX accumulation in mitochondria.
Multiple studies with different tumor cell line panels have led to the conclusion that ABCG2 expression causes intrinsic tumor resistance to ALA-PpIX/PDT, which can be reversed by ABCG2 inhibitors such as Ko143 (70, 71, 76). Importantly, tumor cell response to Ko143 for the enhancement of ALA-PpIX/PDT is cell line dependent (76, 3, 77, 78). Cell lines with high ABCG2 activity or expression are more likely to show a good response. For instance, Ko143 increases intracellular PpIX fluorescence and reduces PpIX efflux into the medium (extracellular) in Caki-2 kidney tumor cells with robust ABCG2 activity (77). In contrast, it has no effect on PpIX fluorescence in A704 kidney tumor cells with no ABCG2 activity.
Given that some clinically used drugs such as tyrosine kinase inhibitors are both ABCG2 substrates and inhibitors (34), combining these existing cancer drugs with ALA have been explored to enhance PpIX accumulation in tumor cells through the inhibition of ABCG2. Imatinib, an ABCG2 substrate and inhibitor (79, 80), is the first kinase inhibitor shown to significantly increase intracellular PpIX and sensitize tumor cells to ALA-PDT in Colo 26 murine colon cancer and BCC-1 human basal cell carcinoma cells (81). Gefitinib is another approved kinase inhibitor with activity of enhancing ALA-PpIX fluorescence and PDT response in four human glioma cell lines (82). We have identified six FDA-approved small molecule inhibitors including lapatinib, gefitinib, sunitinib, vismodegib, vemurafenib, and sorafenib that significantly increase ALA-PpIX fluorescence in tumor cells via suppressing ABCG2-mediated PpIX efflux (77). Particularly, lapatinib, a dual kinase inhibitor of human epidermal growth factor receptor 1 (EGFR) & 2 (HER2), exhibits the highest efficacy. ALA in combination with lapatinib not only enhance ALA-PpIX fluorescence in human breast cancer (76, 83), kidney cancer (77), and glioblastoma (78) cell lines, but also sensitize tumor cells to apoptotic cell death induced by ALA-PDT. Competitive inhibition study suggests that lapatinib and Ko143 share similar binding sites in ABCG2, although it is less efficacious than Ko143 (78). Research on ABCG2 and its inhibitors has culminated in the identification of these FDA-approved drugs with strong activity for enhancing ALA-PpIX/PDT (Fig. 3), which paves the way of using these clinical agents for therapeutic enhancement of ALA.
Figure 3.

Timeline depicting important studies leading to therapeutic enhancement of ALA with clinically relevant ABCG2 inhibitors for better tumor visualization and PDT.
7. Clinical evidence that ABCG2 is involved in transporting PpIX
Clinical evidence suggesting the involvement of ABCG2 in PpIX efflux after ALA is also emerging. Human bladder tumor samples with strong ALA-induced PpIX fluorescence exhibited lower ABCG2 expression than the carcinoma-in-situ tissue with weak PpIX fluorescence, whereas normal bladder tissues from the same patient with no visible PpIX fluorescence had the highest ABCG2 expression (84). This trend of negative correlation between ABCG2 expression and ALA-PpIX fluorescence, which has been shown in the in vitro studies described above, was also observed in non-small cell lung cancer patient tumor samples (85). Comparing the gene expression and protein level of heme synthesis enzymes and ABCG2 in 19 human glioma tumor samples with strong ALA-induced PpIX fluorescence versus 21 glioma samples without ALA-induced fluorescence, Mischkulnig et al. recently found that both ABCG2 gene expression and protein level were significantly reduced in glioma samples with strong PpIX fluorescence, highlighting the importance of ABCG2-mediated PpIX efflux in reducing tumor PpIX fluorescence (86).
8. Conclusions & future perspectives
Since the discovery of PpIX as an ABCG2 substrate in 2002 (65) and the first report demonstrating the enhancement of ALA-PpIX/PDT by an ABCG2 inhibitor in 2005 (64), a plethora of in vitro studies with different tumor cell lines and emerging clinical studies have established ABCG2 as the most important biological determinant of reduced ALA-PpIX tumor disposition. Targeting ABCG2-mediated PpIX efflux with ABCG2 inhibitors has consistently yielded good therapeutic response in tumor cell lines with ABCG2 activity or expression. All these studies have unequivocally pointed to the conclusion that targeting ABCG2 is an effective and promising therapeutic enhancement strategy for ALA. With the identification of FDA-approved small molecule drugs that greatly enhance ALA-PpIX fluorescence and PDT by suppressing ABCG2 activity, the field is now supplied with clinically relevant ABCG2 inhibitors such as lapatinib and imatinib for further evaluation and possible clinical translation. Future research should focus on studying the mechanism, pharmacological response, and therapeutic indications of this ABCG2-targeted therapeutic enhancement strategy at molecular, cellular, tissue, and whole-body levels.
At the molecular level, it is necessary to determine the molecular mechanism of ABCG2 inhibition exerted by these ABCG2-interacting drugs. Although competing with PpIX for the same transporter is certainly a contributing factor as they are all ABCG2 substrates, not all ABCG2 substrates are effective transporter inhibitors, and the structural study has revealed that imatinib is particularly effective in stabilizing ABCG2 in the inward-facing conformation (32). Such specific molecular interactions slow down the dynamic movement of substrates through the transporter, rendering imatinib an effective ABCG2 inhibitor. Better understanding the interaction between ABCG2 and clinical ABCG2 inhibitors at the molecular level is not only necessary for determining the mechanism of ABCG2 inhibition, but also important for developing future ABCG2 inhibitors with higher selectivity and potency.
At the cellular level, the pharmacological effects of clinical ABCG2 inhibitors on ALA-PpIX and PDT need to be characterized in tumor cells lines with varied ABCG2 activity. It is clear that this ABCG2-targeted therapeutic enhancement strategy will not likely bring in much benefit in tumors with no or little ABCG2 activity. But how different tumor cell lines with detectable and varied ABCG2 expression react to ALA in combination with clinical ABCG2 inhibitors remains to be determined. Are tumor cells with higher ABCG2 expression more sensitive to this combination treatment than the ones with lower ABCG2 expression? Given the fact that human cancers exhibit highly varied ABCG2 expression (Fig. 4), answers to this question will have important implications in choosing the optimal dose of clinical ABCG2 inhibitors and the appropriate types of cancer for the application of this enhancement approach.
Figure 4.

The Cancer Genome Atlas (TCGA) study showing the expression of ABCG2 in human cancers. Data are obtained from the cBioPortal website (www.cbioportal.org). The type of cancer is sorted based on the median expression level and shown from the lowest on the left (leukemia) to the highest on the right (glioma).
It is imperative to determine whether this ABCG2-targeted combination treatment results in PpIX fluorescence enhancement and better tumor response in vivo as there is currently no published study that evaluates this strategy in an animal tumor model. Studies in different tumor models with varied ABCG2 expression should not only address whether ABCG2 inhibitors actually block PpIX efflux and enhance PDT tumor response in vivo, but also identify possible biomarkers that may predict tumor response to this combination regimen. In addition to assessing how clinical ABCG2 inhibitors affect PpIX tumor disposition and PDT response at tumor tissue level, effects of inhibitors on ALA absorption, PpIX tissue distribution in the whole body, and skin photosensitivity also need to be investigated.
Lastly, an important lesson learned from several decades’ research on overcoming cancer drug resistance with ABC transporter inhibitors is that patient stratification based on molecular characterization of tumor ABC transporter expression is key to obtain therapeutic benefits of these transporter inhibitors (51). The advent of genomic analysis has made it possible to measure the expression of ABC transporters in human tumor samples. As shown in Fig. 4, a wide range of ABCG2 expression in human tumors revealed by the RNA-Seq analysis from the Cancer Genome Atlas (TCGA) database (87) suggests that future clinical use of ABCG2 inhibitors for therapeutic enhancement of ALA needs to be tailored to the ABCG2 expression in patients’ tumors. The multi-level studies outlined above are expected to address whether ABCG2 expression is a predictive biomarker for guiding the personalized use of this ABCG2-targeted therapeutic enhancement strategy for ALA.
Acknowledgments
We would like to appreciate previous lab members Xue Yang, Pratheeba Palasuberniam, Matthew Mansi, Lolwah Alsalamah for their valuable contributions to this work. This study is supported in part by the NIH grant R15CA268200.
List of the abbreviations
- ABC
ATP binding cassette
- ABCB1
ATP binding cassette subfamily B member 1
- ABCC1
ATP binding cassette subfamily C member 1
- ABCG2
ATP binding cassette subfamily G member 2
- ALA
5-aminolevulinic acid
- ALAS
ALA synthase
- BCRP
breast cancer resistance protein
- CPOX
coproporphyrinogen III oxidase
- FECH
ferrochelatase
- FTC
fumitremorgin C
- MDR
multidrug resistance
- MRP1
multidrug resistance-associated protein 1
- NBD
nucleotide binding domain
- PBGD
porphobilinogen deaminase
- PBGS
porphobilinogen synthase
- PDT
photodynamic therapy
- PpIX
protoporphyrin IX
- P-gp
permeability glycoprotein
- Pha
pheophorbide a
- PPOX
protoporphyrinogen IX oxidase
- SNP
single nucleotide polymorphism
- TMD
transmembrane domain
- UROD
uroporphyrinogen III decarboxylase
- UROS
uroporphyrinogen III synthase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
None
References
- 1.Howley R, Chandratre S and Chen B 5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy. Bioengineering (Basel) 10 (4) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponka P Cell biology of heme. Am J Med Sci 318 (4) (1999), 241–256. [DOI] [PubMed] [Google Scholar]
- 3.Barron GA, Moseley H and Woods JA Differential sensitivity in cell lines to photodynamic therapy in combination with ABCG2 inhibition. J Photochem Photobiol B 126 (2013), 87–96. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Palasuberniam P, Kraus D and Chen B Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement. Int J Mol Sci 16 (10) (2015), 25865–25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suero Molina E, Kaneko S, Black D and Stummer W 5-Aminolevulinic Acid-Induced Porphyrin Contents in Various Brain Tumors: Implications Regarding Imaging Device Design and Their Validation. Neurosurgery 89 (6) (2021), 1132–1140. [DOI] [PubMed] [Google Scholar]
- 6.Kamp MA, Fischer I, Buhner J, Turowski B, Cornelius JF, Steiger HJ, Rapp M, Slotty PJ and Sabel M 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget 7 (41) (2016), 66776–66789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanick SC, Davis SC, Zhao Y, Hasan T, Maytin EV, Pogue BW and Chapman MS Dual-channel red/blue fluorescence dosimetry with broadband reflectance spectroscopic correction measures protoporphyrin IX production during photodynamic therapy of actinic keratosis. J Biomed Opt 19 (7) (2014), 75002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauder A, Feuerstein T and Malik Z The centrality of PBGD expression levels on ALA-PDT efficacy. Photochem Photobiol Sci 10 (8) (2011), 1310–1317. [DOI] [PubMed] [Google Scholar]
- 9.Hilf R, Havens JJ and Gibson SL Effect of delta-aminolevulinic acid on protoporphyrin IX accumulation in tumor cells transfected with plasmids containing porphobilinogen deaminase DNA. Photochem Photobiol 70 (3) (1999), 334–340. [PubMed] [Google Scholar]
- 10.Chen YN, Mickley LA, Schwartz AM, Acton EM, Hwang JL and Fojo AT Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J Biol Chem 265 (17) (1990), 10073–10080. [PubMed] [Google Scholar]
- 11.Lee JS, Scala S, Matsumoto Y, Dickstein B, Robey R, Zhan Z, Altenberg G and Bates SE Reduced drug accumulation and multidrug resistance in human breast cancer cells without associated P-glycoprotein or MRP overexpression. J Cell Biochem 65 (4) (1997), 513–526. [PubMed] [Google Scholar]
- 12.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK and Ross DD A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 95 (26) (1998), 15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V and Dean M A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58 (23) (1998), 5337–5339. [PubMed] [Google Scholar]
- 14.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T and Bates SE Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 59 (1) (1999), 8–13. [PubMed] [Google Scholar]
- 15.Bailey-Dell KJ, Hassel B, Doyle LA and Ross DD Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim Biophys Acta 1520 (3) (2001), 234–241. [DOI] [PubMed] [Google Scholar]
- 16.Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD and Beck WT Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res 64 (4) (2004), 1247–1251. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Lee EW, Zhou L, Leung PC, Ross DD, Unadkat JD and Mao Q Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol Pharmacol 73 (3) (2008), 845–854. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurthy P, R. D, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 279 (23) (2004), 24218–24225. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Wu H, Zhang P, Happel C, Ma J and Biswal S Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Mol Cancer Ther 9 (8) (2010), 2365–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang WJ, Song MJ, Park EY, Lee JJ, Park JH, Park K, Park JH and Kim HP Transcription factors Sp1 and Sp3 regulate expression of human ABCG2 gene and chemoresistance phenotype. Mol Cells 36 (4) (2013), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tompkins LM, Li H, Li L, Lynch C, Xie Y, Nakanishi T, Ross DD and Wang H A novel xenobiotic responsive element regulated by aryl hydrocarbon receptor is involved in the induction of BCRP/ABCG2 in LS174T cells. Biochem Pharmacol 80 (11) (2010), 1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, Jeney V, Ozvegy-Laczka C, Szanto A, Barta E, Balla J, Sarkadi B and Nagy L Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem 281 (33) (2006), 23812–23823. [DOI] [PubMed] [Google Scholar]
- 23.Rao VK, Wangsa D, Robey RW, Huff L, Honjo Y, Hung J, Knutsen T, Ried T and Bates SE Characterization of ABCG2 gene amplification manifesting as extrachromosomal DNA in mitoxantrone-selected SF295 human glioblastoma cells. Cancer Genet Cytogenet 160 (2) (2005), 126–133. [DOI] [PubMed] [Google Scholar]
- 24.Bram EE, Ifergan I, Grimberg M, Lemke K, Skladanowski A and Assaraf YG C421 allele-specific ABCG2 gene amplification confers resistance to the antitumor triazoloacridone C-1305 in human lung cancer cells. Biochem Pharmacol 74 (1) (2007), 41–53. [DOI] [PubMed] [Google Scholar]
- 25.Bram EE, Stark M, Raz S and Assaraf YG Chemotherapeutic drug-induced ABCG2 promoter demethylation as a novel mechanism of acquired multidrug resistance. Neoplasia 11 (12) (2009), 1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.To KK, Polgar O, Huff LM, Morisaki K and Bates SE Histone modifications at the ABCG2 promoter following treatment with histone deacetylase inhibitor mirror those in multidrug-resistant cells. Mol Cancer Res 6 (1) (2008), 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyes N, Kapoor P and Kerr ID Polymorphisms of the Multidrug Pump ABCG2: A Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics. Drug Metab Dispos 46 (12) (2018), 1886–1899. [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, Miki Y and Sugimoto Y C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther 1 (8) (2002), 611–616. [PubMed] [Google Scholar]
- 29.Eckenstaler R and Benndorf RA 3D structure of the transporter ABCG2-What’s new? Br J Pharmacol 177 (7) (2020), 1485–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H and Locher KP Structure of the human multidrug transporter ABCG2. Nature 546 (7659) (2017), 504–509. [DOI] [PubMed] [Google Scholar]
- 31.Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H and Locher KP Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 563 (7731) (2018), 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlando BJ and Liao M ABCG2 transports anticancer drugs via a closed-to-open switch. Nat Commun 11 (1) (2020), 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Q, Ni D, Kowal J, Manolaridis I, Jackson SM, Stahlberg H and Locher KP Structures of ABCG2 under turnover conditions reveal a key step in the drug transport mechanism. Nat Commun 12 (1) (2021), 4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao Q and Unadkat JD Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. AAPS J 17 (1) (2015), 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H and Sorrentino BP The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7 (9) (2001), 1028–1034. [DOI] [PubMed] [Google Scholar]
- 36.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB and Kottgen M Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A 106 (25) (2009), 10338–10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, Yamanashi Y, Kasuga H, Nakashima H, Nakamura T, Takada Y, Kawamura Y, Inoue H, Okada C, Utsumi Y, Ikebuchi Y, Ito K, Nakamura M, Shinohara Y, Hosoyamada M, Sakurai Y, Shinomiya N, Hosoya T and Suzuki H Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun 3 (2012), 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CA, O’Connor MA, Ritchie TK, Galetin A, Cook JA, Ragueneau-Majlessi I, Ellens H, Feng B, Taub ME, Paine MF, Polli JW, Ware JA and Zamek-Gliszczynski MJ Breast cancer resistance protein (ABCG2) in clinical pharmacokinetics and drug interactions: practical recommendations for clinical victim and perpetrator drug-drug interaction study design. Drug Metab Dispos 43 (4) (2015), 490–509. [DOI] [PubMed] [Google Scholar]
- 39.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T and Greenberger LM Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res 58 (24) (1998), 5850–5858. [PubMed] [Google Scholar]
- 40.Robey RW, Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T and Bates SE A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim Biophys Acta 1512 (2) (2001), 171–182. [DOI] [PubMed] [Google Scholar]
- 41.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ and Schinkel AH Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1 (6) (2002), 417–425. [PubMed] [Google Scholar]
- 42.Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, Mulder J, Gottesman MM, Innis RB and Hall MD The Inhibitor Ko143 Is Not Specific for ABCG2. J Pharmacol Exp Ther 354 (3) (2015), 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu K, Zhu J, Huang Y, Li C, Lu J, Sachar M, Li S and Ma X Metabolism of KO143, an ABCG2 inhibitor. Drug Metab Pharmacokinet 32 (4) (2017), 193–200. [DOI] [PubMed] [Google Scholar]
- 44.Pena-Solorzano D, Stark SA, Konig B, Sierra CA and Ochoa-Puentes C ABCG2/BCRP: Specific and Nonspecific Modulators. Med Res Rev 37 (5) (2017), 987–1050. [DOI] [PubMed] [Google Scholar]
- 45.Henrich CJ, Robey RW, Takada K, Bokesch HR, Bates SE, Shukla S, Ambudkar SV, McMahon JB and Gustafson KR Botryllamides: natural product inhibitors of ABCG2. ACS Chem Biol 4 (8) (2009), 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strope JD, Peer CJ, Sissung TM, Hall OM, Huang PA, Harris EM, Gustafson KR, Henrich CJ, Sigano DM, Pauly GT, Schneider JP, Bates SE and Figg WD Botryllamide G is an ABCG2 inhibitor that improves lapatinib delivery in mouse brain. Cancer Biol Ther 21 (3) (2020), 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang S, Yang X, Coburn RA and Morris ME Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem Pharmacol 70 (4) (2005), 627–639. [DOI] [PubMed] [Google Scholar]
- 48.Dakhlaoui I, Vahdati S, Maalej E, Chabchoub F, Wiese M, Marco-Contelles J and Ismaili L Synthesis and biological assessment of new pyrimidopyrimidines as inhibitors of breast cancer resistance protein (ABCG2). Bioorg Chem 116 (2021), 105326. [DOI] [PubMed] [Google Scholar]
- 49.Krapf MK, Gallus J, Spindler A and Wiese M Synthesis and biological evaluation of quinazoline derivatives - A SAR study of novel inhibitors of ABCG2. Eur J Med Chem 161 (2019), 506–525. [DOI] [PubMed] [Google Scholar]
- 50.Kohler SC, Vahdati S, Scholz MS and Wiese M Structure activity relationships, multidrug resistance reversal and selectivity of heteroarylphenyl ABCG2 inhibitors. Eur J Med Chem 146 (2018), 483–500. [DOI] [PubMed] [Google Scholar]
- 51.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE and Gottesman MM Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18 (7) (2018), 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montanari F, Cseke A, Wlcek K and Ecker GF Virtual Screening of DrugBank Reveals Two Drugs as New BCRP Inhibitors. SLAS Discov 22 (1) (2017), 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toyoda Y, Takada T and Suzuki H Inhibitors of Human ABCG2: From Technical Background to Recent Updates With Clinical Implications. Front Pharmacol 10 (2019), 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P and Bates SE Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res 64 (4) (2004), 1242–1246. [DOI] [PubMed] [Google Scholar]
- 55.Allen JD, Brinkhuis RF, Wijnholds J and Schinkel AH The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 59 (17) (1999), 4237–4241. [PubMed] [Google Scholar]
- 56.Beretta GL, Cassinelli G, Pennati M, Zuco V and Gatti L Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur J Med Chem 142 (2017), 271–289. [DOI] [PubMed] [Google Scholar]
- 57.Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH and Locher KP Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol 25 (4) (2018), 333–340. [DOI] [PubMed] [Google Scholar]
- 58.Malik Z and Lugaci H Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer 56 (5) (1987), 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy JC and Pottier RH Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B 14 (4) (1992), 275–292. [DOI] [PubMed] [Google Scholar]
- 60.Fukuda H, Batlle AM and Riley PA Kinetics of porphyrin accumulation in cultured epithelial cells exposed to ALA. Int J Biochem 25 (10) (1993), 1407–1410. [DOI] [PubMed] [Google Scholar]
- 61.Iinuma S, Farshi SS, Ortel B and Hasan T A mechanistic study of cellular photodestruction with 5-aminolaevulinic acid-induced porphyrin. Br J Cancer 70 (1) (1994), 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossi FM, Campbell DL, Pottier RH, Kennedy JC and Dickson EF In vitro studies on the potential use of 5-aminolaevulinic acid-mediated photodynamic therapy for gynaecological tumours. Br J Cancer 74 (6) (1996), 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W, Zhang WJ, Ohnishi K, Yamada I, Ohno R and Hashimoto K 5-Aminolaevulinic acid-mediated photodynamic therapy in multidrug resistant leukemia cells. J Photochem Photobiol B 60 (2–3) (2001), 79–86. [DOI] [PubMed] [Google Scholar]
- 64.Robey RW, Steadman K, Polgar O and Bates SE ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol Ther 4 (2) (2005), 187–194. [PubMed] [Google Scholar]
- 65.Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH and Schinkel AH The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A 99 (24) (2002), 15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou S, Zong Y, Ney PA, Nair G, Stewart CF and Sorrentino BP Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels. Blood 105 (6) (2005), 2571–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogino T, Kobuchi H, Munetomo K, Fujita H, Yamamoto M, Utsumi T, Inoue K, Shuin T, Sasaki J, Inoue M and Utsumi K Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells. Mol Cell Biochem 358 (1–2) (2011), 297–307. [DOI] [PubMed] [Google Scholar]
- 68.Desuzinges-Mandon E, Arnaud O, Martinez L, Huche F, Di Pietro A and Falson P ABCG2 transports and transfers heme to albumin through its large extracellular loop. J Biol Chem 285 (43) (2010), 33123–33133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagiya Y, Endo Y, Yonemura Y, Takahashi K, Ishizuka M, Abe F, Tanaka T, Okura I, Nakajima M, Ishikawa T and Ogura S Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn Ther 9 (3) (2012), 204–214. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto S, Fukuhara H, Seki H, Kawada C, Nakayama T, Karashima T, Ogura SI and Inoue K Predictors of therapeutic efficacy of 5-aminolevulinic acid-based photodynamic therapy in human prostate cancer. Photodiagnosis Photodyn Ther 35 (2021), 102452. [DOI] [PubMed] [Google Scholar]
- 71.Teshigawara T, Mizuno M, Ishii T, Kitajima Y, Utsumi F, Sakata J, Kajiyama H, Shibata K, Ishizuka M and Kikkawa F Novel potential photodynamic therapy strategy using 5-Aminolevulinic acid for ovarian clear-cell carcinoma. Photodiagnosis Photodyn Ther 21 (2018), 121–127. [DOI] [PubMed] [Google Scholar]
- 72.Kitajima Y, Ishii T, Kohda T, Ishizuka M, Yamazaki K, Nishimura Y, Tanaka T, Dan S and Nakajima M Mechanistic study of PpIX accumulation using the JFCR39 cell panel revealed a role for dynamin 2-mediated exocytosis. Sci Rep 9 (1) (2019), 8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bebes A, Nagy T, Bata-Csorgo Z, Kemeny L, Dobozy A and Szell M Specific inhibition of the ABCG2 transporter could improve the efficacy of photodynamic therapy. J Photochem Photobiol B 105 (2) (2011), 162–166. [DOI] [PubMed] [Google Scholar]
- 74.Muller P, Abdel Gaber SA, Zimmermann W, Wittig R and Stepp H ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J Photochem Photobiol B 210 (2020), 111963. [DOI] [PubMed] [Google Scholar]
- 75.Kobuchi H, Moriya K, Ogino T, Fujita H, Inoue K, Shuin T, Yasuda T, Utsumi K and Utsumi T Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS One 7 (11) (2012), e50082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palasuberniam P, Yang X, Kraus D, Jones P, Myers KA and Chen B ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy. Sci Rep 5 (2015), 13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howley R, Mansi M, Shinde J, Restrepo J and Chen B Evaluation of aminolevulinic acid-mediated protoporphyrin IX fluorescence and enhancement by ABCG2 inhibitors in renal cell carcinoma cells. J Photochem Photobiol B 211 (2020), 112017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mansi M, Howley R, Chandratre S and Chen B Inhibition of ABCG2 transporter by lapatinib enhances 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence and photodynamic therapy response in human glioma cell lines. Biochem Pharmacol 200 (2022), 115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E and Traxler P Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res 64 (7) (2004), 2333–2337. [DOI] [PubMed] [Google Scholar]
- 80.Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G and Nooter K Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 104 (9) (2004), 2940–2942. [DOI] [PubMed] [Google Scholar]
- 81.Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, Pandey RA and Oseroff AR The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res 13 (8) (2007), 2463–2470. [DOI] [PubMed] [Google Scholar]
- 82.Sun W, Kajimoto Y, Inoue H, Miyatake S, Ishikawa T and Kuroiwa T Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells. Photodiagnosis Photodyn Ther 10 (1) (2013), 42–50. [DOI] [PubMed] [Google Scholar]
- 83.Palasuberniam P, Kraus D, Mansi M, Howley R, Braun A, Myers K and Chen B Small molecule kinase inhibitors enhance aminolevulinic acid-mediated protoporphyrin IX fluorescence and PDT response in triple negative breast cancer cell lines. J Biomed Opt 26 (9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hagiya Y, Fukuhara H, Matsumoto K, Endo Y, Nakajima M, Tanaka T, Okura I, Kurabayashi A, Furihata M, Inoue K, Shuin T and Ogura S Expression levels of PEPT1 and ABCG2 play key roles in 5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin IX (PpIX) accumulation in bladder cancer. Photodiagnosis Photodyn Ther 10 (3) (2013), 288–295. [DOI] [PubMed] [Google Scholar]
- 85.Omoto K, Matsuda R, Nakai Y, Tatsumi Y, Nakazawa T, Tanaka Y, Shida Y, Murakami T, Nishimura F, Nakagawa I, Motoyama Y, Nakamura M, Fujimoto K and Hiroyuki N Expression of peptide transporter 1 has a positive correlation in protoporphyrin IX accumulation induced by 5-aminolevulinic acid with photodynamic detection of non-small cell lung cancer and metastatic brain tumor specimens originating from non-small cell lung cancer. Photodiagnosis Photodyn Ther 25 (2019), 309–316. [DOI] [PubMed] [Google Scholar]
- 86.Mischkulnig M, Roetzer-Pejrimovsky T, Lotsch-Gojo D, Kastner N, Bruckner K, Prihoda R, Lang A, Martinez-Moreno M, Furtner J, Berghoff A, Woehrer A, Berger W, Widhalm G and Kiesel B Heme Biosynthesis Factors and 5-ALA Induced Fluorescence: Analysis of mRNA and Protein Expression in Fluorescing and Non-fluorescing Gliomas. Front Med (Lausanne) 9 (2022), 907442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C and Schultz N Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6 (269) (2013), pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]


