Abstract
Purpose:
Alcohol-associated hepatitis (AH) is a unique presentation of cholestatic steatohepatitis with liver dysfunction and malaise preceded by heavy alcohol intake. While it exists on a spectrum, in its most severe form 28-day mortality approaches 50%. Clinical trials of therapeutic interventions over the last 50 years have yielded few durable therapies, none of which convey benefit beyond the short term.
Methods:
A qualitative systematic review was performed via searches of PubMed, International Clinical Trials Registry Platform and ClinicalTrials.gov for therapeutic interventions for AH.
Findings:
Prior to 2005, clinical trial results for AH were identified within PubMed. From 2005-present, trials were well catalogued within online registries and included information regarding trial status (e.g. complete, terminated, actively enrolling). Most clinical trials for AH have utilized existing medications broadly targeting pathogenic themes of AH (inflammation, cell death, etc.) in an off-label manner. The trend of initially promising pilot studies answered by larger trials demonstrating lack of efficacy or safety signals have ended the hopes of many new therapeutics. The emergence of theragnostics to identify patients who may benefit from existing therapies and trials of agents with novel mechanisms of action, including epigenetic modifications and hyaluronic acid signaling, targeted to AH pathogenesis are currently under investigation. Implications: This review of AH treatments details the historical interventions and clinical trials that have led to the current treatment algorithm and active studies shaping the therapeutic pipeline for AH.
Keywords: Clinical trials, corticosteroids, alcoholic hepatitis
Introduction:
While Mallory provided the first histopathology description of AH in 1911 [1], in the 1938 British Medical Journal article “The Physical Basis of Biliousness” Sir Arthur Hurst MD coined the phrase alcoholic hepatosis to describe a man’s symptoms “the morning after the night before” a bout of heavy alcohol drinking [2]. This passage appears to be one of the first to describe the severe, acute presentation of AH however there were no recommendations for treating the patient. Despite its rising prevalence, when compared to other diseases with high mortality such as cancer, little therapeutic progress has been made in the treatment of AH. For perspective, since 1970 over 130 new therapeutics have been Food and Drug Administration (FDA) approved for treating malignancies [3]. Over the same time frame, there have been no FDA approvals for AH, per se, but rather treatment algorithms have been developed using repurposed medications. Since 1979, corticosteroids have remained at the forefront of AH therapies yet show only modest improvement in one month survival [4, 5]. Dozens of historical clinical trials in patients with AH have shown no survival benefit or have proven non-superior to corticosteroids. Many of these trials were performed in an era when clinical trials were less rigorous. For historical vitamin and supplement trials, the quality, purity and sourcing of study drug can often not be verified. Additionally, some historical trials provide scant details of informed consent, blinding or randomization. Few included females, perhaps due to an era in which the FDA restricted females of childbearing age from participating in trials [6]. Patient selection requiring liver biopsy for the diagnosis of AH may have selected for individuals who were less severely ill. Lastly, early studies on individuals with AH must also be viewed from the perspective that this population has historically been marginalized due to stigma associated with alcohol use disorder [7, 8]. While progress has been made in reducing the stigma [9], AH remains a condition of despair and access to trials as well as patient retention are persistent challenges. In the last decade, there has been progress aimed at rationale clinical trial design for AH [10, 11] as well as in the standard of care of patients with AH. This review is not an all-inclusive summary of AH clinical trials but rather aims to provide a historical framework for the current landscape and future trajectory of selected AH therapeutics.
Methods:
A qualitative systematic review was performed via searches of PubMed, International Clinical Trials Registry Platform and ClinicalTrials.gov for therapeutic interventions for alcohol-associated hepatitis.
Results:
The Mainstays: Nutrition and Corticosteroids
Nutrition:
Malnutrition is nearly universal, associates with worse prognosis and plays a role in the pathophysiology of AH [12–14] yet few studies involving AH patients inform nutrition guidelines. The current guideline-based recommendations pertaining to nutrition of patients with AH [15–18] are derived from recommendations for patients with all chronic liver diseases and include a caloric target of 35 to 40 Kcal/kg bw per day and a protein intake of 1.2 to 1.5 g/kg bw per day [19]. The most informative clinical trials to date of AH patients [20, 21] compared enteral nutrition (EN) delivered via nasogastric tube (NGT) to corticosteroids and must be framed by the knowns and unknowns of the respective eras. The Cabre study, performed in 2000 [20], was prior to the knowledge derived from the Lille score described in 2007 [22]. Cabre and colleagues found equivalent 28-day survival in EN and corticosteroid treated patients, with steroid-treated patients more often succumbing to infections in the 6 weeks following their initial 28 day survival [20]. The conclusions of the trial may have been very different had Lille criteria been described and utilized, with some patients stopping steroids at day 7 (or even day 4). The Moreno trial, performed in the post-Lille era [21], was more robust in numbers and multicenter design however it lacked the 2016 National Institute on Alcohol Abuse and Alcoholism (NIAAA) criteria for AH diagnosis [10]. Patient selection utilized biopsy, which tends to select for a population of less sick individuals who can withstand the procedural risk. Patients with severe AH were treated with IV methylprednisolone and randomized to EN for 14 days or conventional nutrition (ad lib by mouth). The study concluded that intensive EN for 14 days was equivalent to conventional nutrition in regards to 6 month survival and also importantly revealed that patients consuming < 21.5 kcal/kg bw per day, regardless of the route, had higher mortality at 6 months (62.9 vs. 34.2%, P<0.001) [21]. Almost 50% of the patients in the EN arm did not complete the 14-day course due to tube intolerance perhaps contributing to the results of the study.
A small trial comparing nil by mouth (N=10) to nasogastric feeding (N=12) for the first 4 days following ligation- or sclerotherapy-stabilized variceal hemorrhage showed no differences in rates of re-bleeding, nutritional status, length of stay [23] though a larger trial may provide reassurance of current practice. Regarding location of enteral feeding, gastric and jejunal tube placement have equally efficacious caloric delivery for this population, demonstrated by a simple but well-designed clinical trial [24]. Finally, in situations where barriers exist to enteral feeding, a small but reassuring study of parenteral nutrition was performed and did not worsen mortality in moderate or severe AH patients but unfortunately also showed no survival benefit [25]. Interestingly, peripheral nutrition appeared to cause less exacerbation of ascites and encephalopathy thus may be favorable in some patients [25]. Moving forward, nutrition trials utilizing NIAAA diagnostic criteria for patient selection [10], refined criteria for steroid-eligible patients [26] and Lille criteria for steroid discontinuance [27] without trepidation of using feeding tubes or parental nutrition will allow for better determination of nutrition goals for AH patients.
Corticosteroids:
In the 1950s, a publication within the Annals of Internal Medicine suggested that hormones from the adrenal cortex may be helpful in the treatment of liver disease [28]. It wasn’t until 1961 that an article titled Acute Alcoholic Hepatitis was published within the British Medical Journal recommending a treatment algorithm including abstinence from alcohol, bedrest, intensive nutrition, and high dose B vitamins [29]. A decade later, the first randomized controlled trial (RCT) evaluating corticosteroids in the treatment of 20 patients with AH was published and failed to show benefit [30]. In the 50+ years following, the efficacy of corticosteroids beyond the short term continues to be controversial, though patient selection and discontinuation criteria have informed their optimal use. A Cochrane review of 16 RCTs showed no difference between corticosteroids and placebo in all-cause mortality, health-related quality of life, and serious adverse events [31] while the STOPAH trial, the largest multicenter RCT of AH patients to date, showed favorable results for corticosteroids. This trial included 1,103 severe AH patients randomized to prednisolone or placebo for 28 days and demonstrated a trend for mortality benefit with corticosteroids (13.8 vs. 18%, P=0.056). The effects of corticosteroids were potentially dampened by overall lower mortality than prior studies, possibly reflecting improved management of patients with decompensated liver disease [4]. Two additional metanalyses have added support for the use of corticosteroids in AH: The first analyzed 3 randomized placebo-controlled clinical trials demonstrating positive results of corticosteroids, with 1-month survival of 84.6% versus 65.1% [32] and the second study, which did include the STOPAH data, concluded that corticosteroids were effective in reducing short-term mortality by 36% [5] thus placing corticosteroids at the cornerstone of modern AH therapeutics.
Given the increased risk of infection with corticosteroid use in this population, studies refining their use including prediction of patients who will benefit from them or who will spontaneously improve without them as well as the optimal duration for use have framed the current treatment guidelines. Maddrey’s discriminant function [33] was historically used to identify the population of patients most likely to respond to corticosteroids but Model of End-Stage Liver Disease (MELD) score is now recommended for determining who should receive steroids. A recent worldwide retrospective multicenter cohort of patients with severe AH showed that a 28-day course of steroids is beneficial in patients with MELD scores ranging between 21–39, with limited benefit in those in the 40–50 range and no benefit above a MELD score of 50 [26]. In predicting patients likely to improve without steroids, the kinetics of a patient’s serum bilirubin may be employed. A recent study stratifying 426 AH patients by change in serum bilirubin as “fast fallers,” “static” and “rapid risers” revealed that fast fallers most improve without steroids and have superior 90-day survival [34], however as the stratification is made 7 days post admission, putting this scoring system into practice may be challenging.
Prediction of steroid responders using advanced molecular techniques has also emerged in the last 5 years. While single markers such as cytokeratin-18 [35] have shown prognostic and theragnostic potential in AH, multimarker transcriptomics related to immunity and the mitochondrial electron transport chain appear to have potential to predict severe AH patients who will be steroid non-responders [36]. Further, the predictive capacities of liver biopsy as well as circulating metabolites and macrovesicles have been explored [37]
In terms of steroid duration, 28-day treatment courses of corticosteroids are standard for responders though the rationale for this duration in the earliest clinical trials was not provided. One could surmise that 28 days was convenient as this duration became the accepted course of care for inpatient stays for alcohol and substance rehabilitation. 30 days also defines a “short” course of steroids for which long term side effects may be avoided [38] though infections from steroids are common and convey risk for poor outcomes extending beyond the treatment course [39]. The Lille model provides guidance for continuing or stopping steroids following 7 days of treatment [40] and more recent studies have shown that utilizing Lille score at day 4 is equally accurate [27, 41].
Finally, the use of prednisolone over prednisone has been favored for AH and early studies showing that prednisolone does not require hepatic conversion are often cited as the rationalization behind this [42, 43]. Most interesting is the better-than-expected outcomes of patients within the AlcHepNet trial, which utilized IV methylprednisolone rather than PO formulations [44]. Head-to-head trials of IV corticosteroid formulations, prednisolone and prednisone have not been performed but may be informative. In summary, corticosteroids have been studied extensively yet have limited utility in patients with alcohol-associated hepatitis, underscoring the need for new therapies.
Foundational work
Metabolic studies on animal models have suggested that modifying the endocrine axis could improve hepatic function [45]. Two initially favorable trials of insulin and glucagon were later answered by additional trials showing lack of survival benefit [46–49] and thyroid blockade with propylthiouracil was likewise found to have no efficacy in AH [50, 51]. Trials of the anabolic steroid oxandrolone showed favorable signals [52, 53] but studies waned in the mid 1990’s and it was not integrated into practice. Small trials of Vitamin E [54] and antioxidant cocktails [55] [56] also proved non-beneficial. A milestone in clinical trials for AH was the first use of a monoclonal antibody (i.e. biologic) targeting the inflammatory cytokine Tumor Necrosis Factor (TNF)-alpha. Given its efficacy in Crohn’s disease and rheumatoid arthritis and the protection that TNF-alpha knockout mice showed against alcohol-induced liver injury [57], studies commenced examining infliximab for AH. While a pilot study showed promise [58], a subsequent RCT [59] was stopped early due to safety signal. An open label trial that followed demonstrated a remarkable 89% 1-month survival with a single dose of infliximab in AH patients [60] however no studies have followed. Etanercept, a soluble TNF receptor targeting TNF-alpha, and beta was the second biologic used in clinical trials for AH and failed to show efficacy [61, 62].
Pentoxifylline (PTX):
PTX is a nonselective phosphodiesterase (PDE) inhibitor and potent inhibitor of TNF-alpha secretion, both of which are implicated in the pathogenesis of AH [63]. The first trial of PTX was presented as an abstract in 1991 [64] describing 22 patients, 12 treated with PTX and 10 controls with 8.3% and 30% 30 day mortality rates respectively. These positive data were followed by a RCT 9 years later: 101 patients with severe AH received 28 days of PTX (400 mg, 3 times daily) or vitamin B 12 tablets (selected as a “placebo”) and demonstrated a remarkable 180-day survival curve showing nearly 80% survival for the PTX arm compared to 50% survival in the placebo arm [65]. While this and other initial studies of PTX showed efficacy, the large STOPAH trial demonstrated no 28-day survival benefit [4] and further studies have shown lack of signal as a salvage therapy for steroid non-responders [22] or as an adjuvant therapy to corticosteroids [66, 67]. The number of trials on PTX have allowed for a metanalysis assuring overall non-benefit of PTX [5] yet many providers continue to use it for AH citing a paucity of available therapies, as well as its availability, safety profile and signal for reducing incidence of hepatorenal syndrome [65, 68, 69]. The conditional recommendation of PTX has since been removed from most treatment algorithms for AH. Given recent studies demonstrating that selective PDE inhibition, particularly that of PDE4, shows efficacy in AH [63, 70], future clinical studies may be considered with specific PDE4 agents.
Era of modern trials
Antibiotics:
Given the incidence of infections in AH patients, particularly following corticosteroid treatment, as well as the common initiation of empiric antibiotics in AH patients given their frequent presentation with SIRS criteria, recent trials studying the utility of antibiotics in AH have been informative. Reduction of gut bacteria using rifaximin [71, 72], amoxicillin-clavulanate [73] and the combination of meropenem, vancomycin, and gentamycin [74] have not shown clinically significant benefit in AH patients. Metadoxine: A complex compound of pyridoxine (a vitamin B 6 precursor) and pyrrolidone carboxylate with multiple proposed mechanisms has shown promise in 2 open label studies published in 2014 and 2015 [75, 76], demonstrating improvements in 90 day and 6-month survival though a RCT has not yet been performed. Anakinra: Anakinra, an IL-1 receptor antagonist which blocks IL-1α and IL-1β signaling showed remarkable preclinical efficacy in a mouse study of alcohol induced liver injury [77] but failed to show survival benefit in the recently published multisite AlcHepNet RCT [44]. No further studies of anakinra are in progress. IL-22: F-652 human interleukin 22 (IL-22) and human Immunoglobulin G2 (IgG2)-Fc was studied in open label format with interesting exploratory endpoints but no survival benefit was demonstrated [78]. There appears to be no further studies in process.
Granulocyte Colony-Stimulating Factor (G-CSF):
G-CSF is a glycoprotein believed to recruit bone-marrow derived stem cells, allowing for hepatocyte cell regeneration by differentiation of bone marrow precursor cells into hepatocytes. The initial study examining G-CSF for the treatment of severe AH in 2008 showed increased CD34+ cells (markers of hematopoietic stem cells) as well as hepatocyte progenitor cells on histologic liver biopsy evaluation. However, there were no significant improvements in liver function [79]. The first clinic trial of G-CSF in AH was reported in 2014 [80] and several subsequent studies have allowed for meta-analyses showing a 90-day survival benefit in studies performed in Asia with a trend for increased mortality found in 2 studies performed in Europe. It has been proposed that study design variability accounted for mixed results [81]. Two subsequent studies since the latest metanalysis have demonstrated the same hemispheric trend. A trial performed in the US revealed that the combination of G-CSF and prednisolone has equivalent 90 day survival compared to prednisolone alone (0.73 vs 0.83 p > 0.05) [82] while another study from Asia comparing G-CSF alone (N=42), prednisolone alone (N=42), and the combination of G-CSF + prednisolone (N=42) showed 64.3%, 78.6% and 88.1% 90 day survival respectively (p =0.03) [83].
Promising Leads
N-Acetylcysteine (NAC):
NAC has been traditionally used for the treatment and prevention of acetaminophen-induced liver injury due to its ability to restore glutathione and enhance hepatic perfusion. The first report of NAC in AH patients was described in a 1991 New England Journal of Medicine article reporting its benefits primarily in acetaminophen overdose however 2 patients with AH were included within the study [84]. NAC has been studied in tandem with and compared against corticosteroids. While initial studies of NAC showed limited efficacy, study designs including NAC within “antioxidant-cocktails” may have obscured benefit. The number of NAC studies accumulating over the years has allowed for a robust network-metanalysis revealing that IV NAC in combination with corticosteroids yielded a remarkable 85% risk reduction of death from AH at 28 days [85] leading to the addition of IV NAC into recent American College of Gastroenterology guidelines for the treatment of AH [86]. Multiple RCTs of NAC are currently in progress.
Fecal microbiota transplant:
In the 1951 journal article titled “Dietetics in alcoholic hepatitis; therapeutic role of cheese” published within Semaine des hôpitaux de Paris may represent the first publication of the positive effects of microbiome modulation for AH [87]. While probiotics have been employed in trials, effects may have been dampened by sample size and patient selection [88, 89]. The first fecal microbiota transplantation (FMT) trial published in 2017 of 8 subjects with AH having contraindications to steroid therapy showed encouraging results [90] and has since been followed by a RCT comparing FMT (N=55) to prednisolone (N=57) with improved 90-day survival in the FMT arm compared to the prednisolone arm (75% vs 57% p=0.044) [91]. As infections from FMT donor stool [92] have led to several FDA alerts, the risks and benefits of FMT must be considered in this relatively immunocompromised patient population. With the recent FDA approval of Rebyota for recurrent C. difficile, the field is theoretically open to its off-label use in AH however drug cost of approximately $9,000 USD per treatment will likely limit the practice.
Unfinished studies:
Several concluded or terminated trials populate clinical trial registries. Among these include emricasan (NCT01912404), DS102 (NCT03452540), vitamin C infusion (NCT03829683), mycophenolate/pilonacept (NCT01903798) and selonsertib (NCT02854631). While data are uploaded demonstrating indications for these unfinished or early terminated studies, peer-reviewed publications with the investigator’s insights have yet to evolve from these. While other endpoints appeared favorable, there was a lack of survival benefit for the IL-1 beta target Canakinumab recently shared in abstract form however the complete trial results have yet to be published [93]
Looking Forward
Sulfated oxysterol (DUR-298):
The first Phase 2a clinical trial of this epigenetic modulator was published in 2023 [94] revealing improvement in MELD score. Results are awaited from a recently completed Phase 2b trial (NCT04563026). Bovine Colostrum: Bovine colostrum, derived from cow milk and rich in immunoglobulins believed to have benefit on gut barrier function and abrogation of endotoxemia, was used “in extremis” and published in a 2015 abstract [95] showing a positive signal. Additional work is in progress (NCT02473341). HA35: Clinical work has demonstrated increased circulating hyaluronic acid (HA) in patients with liver disease [96–98] and preclinical studies of small-specific sized HA (HA35) demonstrate this molecule preserves the intestinal barrier and decreases hepatocyte apoptosis in a mouse model of alcohol-induced liver injury [99]. A study of HA35 is underway in patients with moderate AH (NCT05018481).
Discussion/conclusions:
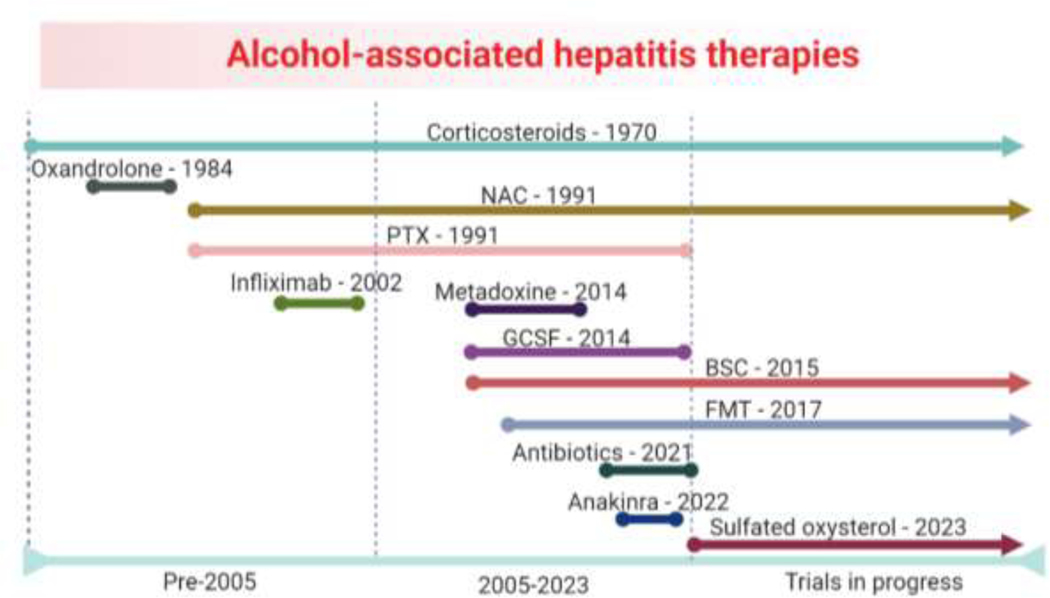
Nearly 90 years ago, Sir Arthur Hurst wrote, “alcohol is the commonest cause of liverishness” [2] and unfortunately his statement remains true to this day. Therapeutic advances for AH have come at a slow pace when compared to other common conditions carrying high mortality and as such severe AH remains a condition that is difficult to treat. While small early trials with design flaws intrinsic to their era may be viewed now as failed studies, their dissemination was critical as they have allowed for larger metanalysis and informed future therapeutic efforts. Cases in point are the dissolution of PTX and adoption of IV NAC in guideline-based care for AH. Corticosteroids remain the pharmacological treatment of choice for severe AH and recent studies have revealed that control arms utilizing steroids have shown better than expected survival when compared to historical cohorts of patients. This may be from improved medical care of AH patients. The demographics of AH have also shifted, with increasing presentations of females and young adults particularly coinciding with the COVID pandemic [100]. These younger patients may present with more physiologic reserves. Nevertheless, AH remains a deadly disease with limited treatment options despite the work that investigators have invested (Figure 1). The commitment of the NIAAA and persistence of investigators in modernizing AH clinical trials cannot be understated and allow for a new era of emerging AH therapies to begin.
Figure 1: Timeline of alcohol-associated hepatitis therapies.
Selected therapies with the year of their first published clinical trial are shown. BSC, bovine serum colostrum; FMT, fecal microbiome transplantation; GCSF, granulocyte colony stimulating factor; NAC, N-acetylcysteine; PTX, pentoxifylline.
Funding:
This work was supported by the National Institutes of Health (NIDDK123381, 2021–2026).
Abbreviations
- AH
Alcohol-Associated Hepatitis
- EN
Enteral Nutrition
- FDA
Food and Drug Administration
- FMT
Fecal Microbiota Transplant
- G-CSF
Granulocyte Colony-Stimulating Factor
- HA
Hyaluronic Acid
- MELD
Model for End-Stage Liver Disease
- NAC
N-Acetylcysteine
- NGT
Nasogastric Tube
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- PDE
Phosphodiesterase
- PTX
Pentoxifylline
- RCT
Randomized Controlled Trial
- TNF
Tumor Necrosis Factor
Footnotes
Declaration of interests: LLJ is a principal investigator for clinical trials sponsored by Abbvie, Galectin, Bausch/Salix and Aldeyra.
Declaration of Competing Interest
LLJ: Principal investigator for clinical trials sponsored by Abbvie, Galectin and Bausch/Salix and is a paid speaker for SC Liver Consortium.
SC: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mallory FB, Cirrhosis of the liver : five different types of lesions from which it may arise. Bull Johns Hopkins Hosp, 1911. 22: p. 69–75. [Google Scholar]
- 2.Hurst A, The Physical Basis of “Biliousness”. Br Med J, 1938. 1(4029): p. 661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, et al. , A systematic analysis of FDA-approved anticancer drugs. BMC Syst Biol, 2017. 11(Suppl 5): p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thursz MR, et al. , Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med, 2015. 372(17): p. 1619–28. [DOI] [PubMed] [Google Scholar]
- 5.Louvet A, et al. , Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology, 2018. 155(2): p. 458–468.e8. [DOI] [PubMed] [Google Scholar]
- 6.Schiebinger L, Women’s health and clinical trials. J Clin Invest, 2003. 112(7): p. 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schomerus G, et al. , The stigma of alcohol-related liver disease and its impact on healthcare. J Hepatol, 2022. 77(2): p. 516–524. [DOI] [PubMed] [Google Scholar]
- 8.Vaughn-Sandler V, et al. , Consequences of perceived stigma among patients with cirrhosis. Dig Dis Sci, 2014. 59(3): p. 681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.; Available from: https://nida.nih.gov/research-topics/addiction-science/words-matter-preferred-language-talking-about-addiction.
- 10.Crabb DW, et al. , Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology, 2016. 150(4): p. 785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comerford M, et al. , Challenges in Patient Enrollment and Retention in Clinical Studies for Alcoholic Hepatitis: Experience of the TREAT Consortium. Alcoholism: Clinical & Experimental Research, 2017. 41(12): p. 2000–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendenhall CL, et al. , Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med, 1984. 76(2): p. 211–22. [DOI] [PubMed] [Google Scholar]
- 13.Mendenhall C, et al. , VA Cooperative Study on Alcoholic Hepatitis. III: Changes in protein-calorie malnutrition associated with 30 days of hospitalization with and without enteral nutritional therapy. JPEN J Parenter Enteral Nutr, 1985. 9(5): p. 590–6. [DOI] [PubMed] [Google Scholar]
- 14.Singal AK, et al. , Nutritional status of patients with alcoholic cirrhosis undergoing liver transplantation: time trends and impact on survival. Transpl Int, 2013. 26(8): p. 788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell MC, Friedman LS, and McClain CJ, Medical Management of Severe Alcoholic Hepatitis: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clinical Gastroenterology & Hepatology, 2017. 15(1): p. 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AK, et al. , ACG Clinical Guideline: Alcoholic Liver Disease. American Journal of Gastroenterology, 2018. 113(2): p. 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. Electronic address, e.e.e. and L. European Association for the Study of the, EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. Journal of Hepatology, 2018. 69(1): p. 154–181. [DOI] [PubMed] [Google Scholar]
- 18.Crabb DW, et al. , Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology, 2020. 71(1): p. 306–333. [DOI] [PubMed] [Google Scholar]
- 19.Plauth M, et al. , ESPEN guideline on clinical nutrition in liver disease. Clinical Nutrition, 2019. 38(2): p. 485–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabre E, et al. , Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology, 2000. 32(1): p. 36–42. [DOI] [PubMed] [Google Scholar]
- 21.Moreno C, et al. , Intensive Enteral Nutrition Is Ineffective for Patients with Severe Alcoholic Hepatitis Treated with Corticosteroids. Gastroenterology, 2016. 150(4): p. 903–910.e8. [DOI] [PubMed] [Google Scholar]
- 22.Louvet A, et al. , Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol, 2008. 48(3): p. 465–70. [DOI] [PubMed] [Google Scholar]
- 23.de Ledinghen V, et al. , Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig Dis Sci, 1997. 42(3): p. 536–41. [DOI] [PubMed] [Google Scholar]
- 24.Bager P., et al., Equal efficacy of gastric and jejunal tube feeding in liver cirrhosis and/or alcoholic hepatitis: a randomised controlled study. British Journal of Nursing, 2020. 29(20): p. 1148–1154. [DOI] [PubMed] [Google Scholar]
- 25.Simon D. and Galambos JT, A randomized controlled study of peripheral parenteral nutrition in moderate and severe alcoholic hepatitis. J Hepatol, 1988. 7(2): p. 200–7. [DOI] [PubMed] [Google Scholar]
- 26.Arab JP, et al. , Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. Journal of Hepatology, 2021. 75(5): p. 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Saenz-de-Sicilia M, et al. , A Day-4 Lille Model Predicts Response to Corticosteroids and Mortality in Severe Alcoholic Hepatitis. American Journal of Gastroenterology, 2017. 112(2): p. 306–315. [DOI] [PubMed] [Google Scholar]
- 28.Webster JJ, Adrenal cortex in liver disease. Ann Intern Med, 1950. 33(4): p. 854–64. [DOI] [PubMed] [Google Scholar]
- 29.Beckett AG, Livingstone AV, and Hill KR, Acute alcoholic hepatitis. Br Med J, 1961. 2(5260): p. 1113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter HP, et al. , Corticosteroid therapy in severe alcoholic hepatitis. A double-blind drug trial. N Engl J Med, 1971. 284(24): p. 1350–5. [DOI] [PubMed] [Google Scholar]
- 31.Pavlov CS, et al. , [Glucocorticosteroids for people with alcoholic hepatitis (Cochrane review)]. Ter Arkh, 2019. 91(8): p. 52–66. [DOI] [PubMed] [Google Scholar]
- 32.Mathurin P, et al. , Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut, 2011. 60(2): p. 255–60. [DOI] [PubMed] [Google Scholar]
- 33.Maddrey WC, et al. , Corticosteroid therapy of alcoholic hepatitis. Gastroenterology, 1978. 75(2): p. 193–9. [PubMed] [Google Scholar]
- 34.Weng F. and Lou L, Trajectory of Serum Bilirubin Predicts Spontaneous Recovery in Patients With Alcoholic Hepatitis. Clinical Gastroenterology and Hepatology, 2022. 20(4): p. e913–e914. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson SR, et al. , In Severe Alcoholic Hepatitis, Serum Keratin-18 Fragments Are Diagnostic, Prognostic, and Theragnostic Biomarkers. American Journal of Gastroenterology, 2020. 115(11): p. 1857–1868. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S, et al. , Differential blood transcriptome modules predict response to corticosteroid therapy in alcoholic hepatitis. JHEP Reports : Innovation in Hepatology / EASL, 2021. 3(3): p. 100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarin SK and Sharma S, Predictors of steroid non-response and new approaches in severe alcoholic hepatitis. Clin Mol Hepatol, 2020. 26(4): p. 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waljee AK, et al. , Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ, 2017. 357: p. j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louvet A, Circulating levels of bacterial DNA and risk of infections in severe alcoholic hepatitis. Clinics and Research in Hepatology and Gastroenterology, 2017. 41(4): p. 354–356. [DOI] [PubMed] [Google Scholar]
- 40.Mathurin P, et al. , Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology, 2003. 38(6): p. 1363–9. [DOI] [PubMed] [Google Scholar]
- 41.Foncea CG, et al. , Day-4 Lille Score Is a Good Prognostic Factor and Early Predictor in Assessing Therapy Response in Patients with Liver Cirrhosis and Severe Alcoholic Hepatitis. Journal of Clinical Medicine, 2021. 10(11): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell LW and Axelsen E, Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut, 1972. 13(9): p. 690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis M, et al. , Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol, 1978. 5(6): p. 501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szabo G, et al. , IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology, 2022. 27: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brockman RP, et al. , Effects of glucagon and insulin on net hepatic metabolism of glucose precursors in sheep. Am J Physiol, 1975. 229(5): p. 1344–9. [DOI] [PubMed] [Google Scholar]
- 46.Baker AL., et al., A randomized clinical trial of insulin and glucagon infusion for treatment of alcoholic hepatitis: progress report in 50 patients. Gastroenterology, 1981. 80(6): p. 1410–4. [PubMed] [Google Scholar]
- 47.Feher J, et al. , A prospective multicenter study of insulin and glucagon infusion therapy in acute alcoholic hepatitis. J Hepatol, 1987. 5(2): p. 224–31. [DOI] [PubMed] [Google Scholar]
- 48.Bird G, et al. , Insulin and glucagon infusion in acute alcoholic hepatitis: a prospective randomized controlled trial. Hepatology, 1991. 14(6): p. 1097–101. [PubMed] [Google Scholar]
- 49.Trinchet JC, et al. , Treatment of severe alcoholic hepatitis by infusion of insulin and glucagon: a multicenter sequential trial. Hepatology, 1992. 15(1): p. 76–81. [DOI] [PubMed] [Google Scholar]
- 50.Orrego H, et al. , Effect of short-term therapy with propylthiouracil in patients with alcoholic liver disease. Gastroenterology, 1979. 76(1): p. 105–15. [PubMed] [Google Scholar]
- 51.Halle P, et al. , Double-blind, controlled trial of propylthiouracil in patients with severe acute alcoholic hepatitis. Gastroenterology, 1982. 82(5 Pt 1): p. 925–31. [PubMed] [Google Scholar]
- 52.Mendenhall CL, et al. , Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. N Engl J Med, 1984. 311(23): p. 1464–70. [DOI] [PubMed] [Google Scholar]
- 53.Mendenhall CL, et al. , A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology, 1993. 17(4): p. 564–76. [DOI] [PubMed] [Google Scholar]
- 54.Mezey E, et al. , A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol, 2004. 40(1): p. 40–6. [DOI] [PubMed] [Google Scholar]
- 55.Phillips M, et al. , Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol, 2006. 44(4): p. 784–90. [DOI] [PubMed] [Google Scholar]
- 56.Stewart S, et al. , A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol, 2007. 47(2): p. 277–83. [DOI] [PubMed] [Google Scholar]
- 57.Yin M, et al. , Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology, 1999. 117(4): p. 942–52. [DOI] [PubMed] [Google Scholar]
- 58.Spahr L, et al. , Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol, 2002. 37(4): p. 448–55. [DOI] [PubMed] [Google Scholar]
- 59.Naveau S, et al. , A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology, 2004. 39(5): p. 1390–7. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P, et al. , Infliximab monotherapy for severe alcoholic hepatitis and predictors of survival: an open label trial. J Hepatol, 2009. 50(3): p. 584–91. [DOI] [PubMed] [Google Scholar]
- 61.Menon KV, et al. , A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol, 2004. 99(2): p. 255–60. [DOI] [PubMed] [Google Scholar]
- 62.Boetticher NC, et al. , A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology, 2008. 135(6): p. 1953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez WE, et al. , Phosphodiesterase 4 Inhibition as a Therapeutic Target for Alcoholic Liver Disease: From Bedside to Bench. Hepatology, 2019. 70(6): p. 1958–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McHutchison J, et al. Pentoxifylline may prevent renal impairment (hepatorenal-syndrome) in severe acute alcoholic hepatitis. in Hepatology. 1991. WB SAUNDERS CO INDEPENDENCE SQUARE WEST CURTIS CENTER, STE 300, PHILADELPHIA: …. [Google Scholar]
- 65.Akriviadis E, et al. , Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology, 2000. 119(6): p. 1637–48. [DOI] [PubMed] [Google Scholar]
- 66.Sidhu SS, et al. , Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial). Dig Dis Sci, 2012. 57(6): p. 1664–71. [DOI] [PubMed] [Google Scholar]
- 67.Mathurin P, et al. , Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA, 2013. 310(10): p. 1033–41. [DOI] [PubMed] [Google Scholar]
- 68.Whitfield K, et al. , Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev, 2009(4): p. CD007339. [DOI] [PMC free article] [PubMed]
- 69.Parker R., et al., Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther, 2013. 37(9): p. 845–54. [DOI] [PubMed] [Google Scholar]
- 70.Ma J, Kumar V, and Mahato RI, Nanoparticle Delivery of Novel PDE4B Inhibitor for the Treatment of Alcoholic Liver Disease. Pharmaceutics, 2022. 14(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimer N, et al. , The impact of rifaximin on inflammation and metabolism in alcoholic hepatitis: A randomized clinical trial. PLoS One, 2022. 17(3): p. e0264278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jimenez C, et al. , Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH). Liver Int, 2022. 42(5): p. 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louvet A, et al. , Effect of Prophylactic Antibiotics on Mortality in Severe Alcohol-Related Hepatitis: A Randomized Clinical Trial. JAMA, 2023. 329(18): p. 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoy S, et al. , No Effect in Alcoholic Hepatitis of Gut-Selective, Broad-Spectrum Antibiotics on Bacterial Translocation or Hepatic and Systemic Inflammation. Clin Transl Gastroenterol, 2021. 12(2): p. e00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Higuera-de la Tijera F, et al. , Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol, 2014. 13(3): p. 343–52. [PubMed] [Google Scholar]
- 76.Higuera-de la Tijera F, et al. , Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol, 2015. 21(16): p. 4975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iracheta-Vellve A, et al. , Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int, 2017. 37(7): p. 968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arab JP, et al. , An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients With Alcohol-associated Hepatitis. Hepatology, 2020. 72(2): p. 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spahr L, et al. , Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology, 2008. 48(1): p. 221–9. [DOI] [PubMed] [Google Scholar]
- 80.Singh V, et al. , Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol, 2014. 109(9): p. 1417–23. [DOI] [PubMed] [Google Scholar]
- 81.Baig M, et al. , Efficacy of Granulocyte Colony Stimulating Factor in Severe Alcoholic Hepatitis: A Systematic Review and Meta-Analysis. Cureus, 2020. 12(9): p. e10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tayek JA, et al. , A phase II, multicenter, open-label, randomized trial of pegfilgrastim for patients with alcohol-associated hepatitis. EClinicalMedicine, 2022. 54: p. 101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mishra AK, et al. , GCSF INCREASES THE STEROID RESPONSIVENESS AND 90 DAY SURVIVAL IN SEVERE ALCOHOLIC HEPATITIS PATIENTS‐ A RANDOMISED CONTROL TRIAL. Hepatology, 2022. 76: p. S150 (Abstract). [Google Scholar]
- 84.Harrison PM, et al. , Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med, 1991. 324(26): p. 1852–7. [DOI] [PubMed] [Google Scholar]
- 85.Singh S, et al. , Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: A systematic review and network meta-analysis. Gastroenterology, 2015. [DOI] [PubMed]
- 86.Jophlin LL, A.K.S., Bataller, Wong RJ, Sauer BG, Terrault NA, and Shah VH, ACG Clinical Guideline: Alcohol-Associated Liver Disease. American Journal of Gastroenterology, 2023. [DOI] [PMC free article] [PubMed]
- 87.Richet C, Andre J, and Bertrand J, [Dietetics in alcoholic hepatitis; therapeutic role of cheese]. Sem Hop, 1951. 27(53–54): p. 2279–86. [PubMed] [Google Scholar]
- 88.Kirpich IA, et al. , Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol, 2008. 42(8): p. 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta H, et al. , Beneficial Shifts in Gut Microbiota by Lacticaseibacillus rhamnosus R0011 and Lactobacillus helveticus R0052 in Alcoholic Hepatitis. Microorganisms, 2022. 10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Philips CA., et al., Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol, 2017. 15(4): p. 600–602. [DOI] [PubMed] [Google Scholar]
- 91.Pande A, et al. , Fecal microbiota transplantation compared with prednisolone in severe alcoholic hepatitis patients: a randomized trial. Hepatol Int, 2023. 17(1): p. 249–261. [DOI] [PubMed] [Google Scholar]
- 92.DeFilipp Z, et al. , Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med, 2019. 381(21): p. 2043–2050. [DOI] [PubMed] [Google Scholar]
- 93.Vergis N, et al. , Il-1beta Signal Inhibition in acute alcoholic hepatitis: a multicentre, randomised, double-blind, placebo-controlled phase 2 trial of canakinumab therapy (ISAIAH). Journal of Hepatology, 2022. 77: p. S34–S35. [Google Scholar]
- 94.Hassanein T, et al. , Safety, Pharmacokinetics, and Efficacy Signals of Larsucosterol (DUR-928) in Alcohol-Associated Hepatitis. Am J Gastroenterol, 2023. [DOI] [PMC free article] [PubMed]
- 95.Sidhu S, et al. , Corticosteroids and Bovine Colostrum in Treatment of Alcoholic Hepatitis ‘In Extremis’: A Pilot Study. Journal of clinical and experimental hepatology, 2015. 5: p. S19–S20. [Google Scholar]
- 96.Hill DB, Deaciuc IV, and McClain CJ, Hyperhyaluronanemia in alcoholic hepatitis is associated with increased levels of circulating soluble intercellular adhesion molecule-1. Alcohol Clin Exp Res, 1998. 22(6): p. 1324–7. [PubMed] [Google Scholar]
- 97.Orasan OH, et al. , Hyaluronic acid as a biomarker of fibrosis in chronic liver diseases of different etiologies. Clujul Medical, 2016. 89(1): p. 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gudowska M, Cylwik B, and Chrostek L, The role of serum hyaluronic acid determination in the diagnosis of liver fibrosis. Acta Biochimica Polonica, 2017. 64(3): p. 451–457. [DOI] [PubMed] [Google Scholar]
- 99.Saikia P, et al. , MicroRNA 181b-3p and its target importin alpha5 regulate toll-like receptor 4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology, 2017. 66(2): p. 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulz P, et al. , Acute Alcohol-Associated Hepatitis in the COVID-19 Pandemic - a Structured Review. Curr Transplant Rep, 2022. 9(4): p. 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]