Abstract
Objective:
Exposure to repetitive head impacts (RHI) is associated with later-life cognitive symptoms and neuropathologies including chronic traumatic encephalopathy (CTE). Cognitive decline in community cohorts is often due to multiple pathologies; however, the frequency and contributions of these pathologies to cognitive impairment in people exposed to RHI is unknown. Here, we examined the relative contributions of 13 neuropathologies to cognitive symptoms and dementia in RHI-exposed brain donors.
Methods:
Neuropathologists examined brain tissue from 571 RHI-exposed donors and assessed for the presence of 13 neuropathologies, including CTE, Alzheimer disease (AD), Lewy body disease (LBD), and TDP-43 inclusions. Cognitive status was assessed by presence of dementia, Functional Activities Questionnaire (FAQ), and Cognitive Difficulties Scale (CDS). Spearman’s rho was calculated to assess intercorrelation of pathologies. Additionally, frequencies of pathological co-occurrence were compared to a simulated distribution assuming no intercorrelation. Logistic and linear regressions tested associations between neuropathologies and dementia status and cognitive scale scores.
Results:
The sample age range was 18–97 (median: 65.0 [IQR 46.0–76.0]). 77.2% of donors had at least one moderate-severe neurodegenerative or cerebrovascular pathology. Stage III-IV CTE was the most common neurodegenerative disease (43.1%), followed by TDP-43 pathology, AD, and hippocampal sclerosis. Neuropathologies were intercorrelated and there were fewer unique combinations than expected if pathologies were independent (p<0.001). The greatest contributors to dementia were AD, neocortical LBD, hippocampal sclerosis, CAA, and CTE.
Interpretation:
In this sample of RHI-exposed brain donors with wide-ranging ages, multiple neuropathologies were common and correlated. Mixed neuropathologies, including CTE, underlie cognitive impairment in contact sport athletes.
Graphical Abstract
Exposure to repetitive head impacts (RHI) is associated with the development of neurodegenerative disease and later-life cognitive symptoms. The most frequent neuropathologies found in a group with a history of RHI included chronic traumatic encephalopathy (CTE), TDP-43 inclusions, cerebral amyloid angiopathy, Alzheimer disease, hippocampal sclerosis, and neocortical Lewy body disease. Neuropathologies were intercorrelated and there were fewer unique combinations than expected if pathologies were independent (p<0.001). Mixed neuropathologies, including CTE, underlie dementia in contact sport athletes exposed to RHI.
INTRODUCTION
Neurodegenerative disease pathologies rarely occur in isolation1 and the importance of mixed neuropathologies underlying cognitive decline in older adults is increasingly recognized.1–6 In previous clinical-pathological studies of older adults from the Religious Orders Study and the Rush Memory and Aging Project (ROSMAP), Boyle et al. found that out of 9 neuropathologies examined, 78% of participants exhibited two or more upon post-mortem examination.5 There were 236 distinct combinations of pathologies, each occurring in <6% of the sample.5,7 Alzheimer Disease (AD) was the most frequent pathology in this sample and also had the strongest effect on cognitive decline of all of the pathologies examined.5 After AD, Lewy Body disease (LBD) and hippocampal sclerosis (HS) were the biggest contributors to cognitive decline, although the contribution of individual pathologies varied greatly at the individual level and depended on the combination present.5 It is in part because of the heterogeneity of underlying neuropathologies that attempts at treating and preventing AD and AD related dementias may have been met with limited success.1,8,9
Chronic traumatic encephalopathy (CTE) is a neurodegenerative tauopathy that is uniquely characterized by the aggregation of hyper-phosphorylated tau (p-tau) in neurons around small blood vessels and at the depths of the sulci. CTE can currently only be diagnosed at autopsy using published neuropathological diagnostic criteria.10,11 CTE is caused by exposure to repetitive head impacts (RHI) such as those from contact sports.12–14 Research diagnostic criteria that describe the clinical syndrome of CTE have been proposed, known as traumatic encephalopathy syndrome (TES). The core clinical features of TES include cognitive impairment (particularly in episodic memory and executive functions) and neurobehavioral dysregulation (e.g., short fuse, impulsivity). There remains uncertainty of the clinical presentation of CTE in part because of the significant heterogeneity in symptom presence, severity, and presentation (e.g., type, age of onset, course). Such heterogeneity may partially be a result of mixed pathologies.
CTE has been associated with multiple other neuropathologies. In those with CTE, common comorbid pathologies include moderate-severe arteriolosclerosis (47%),15 moderate-severe white matter rarefaction (WMR) (46%),15 transactive response DNA-binding protein 43 (TDP-43) inclusions (43%),16 cerebral amyloid angiopathy (CAA) (29%),17 HS (23%),16 AD (13%),18 and neocortical LBD (5%).17,19 Beta-amyloid deposition was found in 52% of brain donors with CTE as predominantly diffuse plaques, while neuritic plaques were less common.20 In addition to CTE, RHI, as measured by years of play, has been associated with multiple neuropathologies, including neocortical LBD,19 frontal CAA,17 hippocampal TDP-43 inclusions,16 and WMR.15 Other studies in small numbers of soccer, rugby, and American football players have also shown frequent comorbid pathologies including AD, CTE, and TDP-43.21,22 All of these pathologies can contribute to dementia. Therefore, the contributions of CTE and other pathologies to cognitive impairment in participants with a history of RHI warrant further investigation.
A systematic examination of co-morbidities and the unique contribution of neuropathologies to cognitive symptoms has yet to be examined in RHI exposed individuals. Here, we investigated the frequency of 13 neuropathological comorbidities in 571 brain donors exposed to RHI. We then examined their unique contributions to informant-reported cognitive and functional symptoms, including antemortem dementia.
METHODS
Brain Donors
The sample included 571 brain donors from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) brain bank who played contact sports during life and had a complete neuropathological examination. Inclusion criteria for brain donors in the UNITE study have been published previously.23 As an overview, inclusion requires a history of RHI (e.g., from contact sports, military service, physical violence, or other sources) and tissue quality sufficient for neuropathological diagnosis using immunohistochemistry. More recently, the criteria were expanded to include a history of moderate to severe traumatic brain injury. For this study, participants were limited to those who played contact sports in order to limit the heterogeneity in brain injury exposure. In most cases, the brain donors’ next-of-kin contacted the CTE Center at the time of the donors’ passing to arrange for brain donation; in other cases, they were referred by medical examiners or recruited by the Concussion Legacy Foundation. Informed consent for brain donation and study enrollment was obtained from the brain donors’ next-of-kin, and approval for all study procedures was obtained from the Boston University Medical Campus Institutional Review Board.
Retrospective Clinical Assessments
Retrospective clinical data were obtained through online surveys and telephone interviews with informants of the brain donor as well as review of medical records. These methods have been described elsewhere.23 Researchers and informants were blind to the neuropathological results. Clinicians with expertise in traumatic brain injury (TBI) and/or neurodegenerative diseases conducted semi-structured interviews and research assistants conducted structured interviews that included administration of standardized clinical scales adapted for informant-based, post-mortem, retrospective administration. The Functional Activities Questionnaire (FAQ) was used to assess instrumental activities of daily living in the year prior to the brain donors’ death.24 It is a 10-item measure and scores range from 0–30, with higher scores representing worse daily function. The Cognitive Difficulties Scale (CDS) is a 39-item instrument that asks about difficulties related to attention, memory, perception, and psychomotor abilities prior to death.25 Responses are made on a 0 (not at all) to 4 (very often) Likert scale and scores are summed to form a global composite (higher scores reflect greater reported cognitive symptoms). Finally, antemortem dementia status was adjudicated through a diagnostic consensus conference of expert clinicians who were blinded to brain donors’ pathological diagnoses. We used adapted DSM-IV-TR criteria for the diagnosis of dementia and the diagnosis was informed by the structured interview conducted by research assistants, the unstructured interview conducted by clinicians, as well as the online surveys. The clinical history (i.e., symptoms, symptom course) and other pertinent history (e.g., medical, family, substance use history) was summarized at the consensus conference.
The UNITE study has evolved over time. Prior to January 2014, only the FAQ was administered for assessing cognitive and functional symptoms, and after 2014, the CDS was added. Demographics, educational attainment, athletic history (type of sports played, level, position, age of first exposure and duration), military history, and traumatic brain injury history were queried during a telephone interview (pre-2014) and/or using an online questionnaire (2014 and on).
Neuropathological Examination
Brain tissue was processed according to previously-published, established procedures.26,27 Neuropathological diagnoses were made blinded to all clinical information about the brain donor. Established criteria were used to diagnose the presence of neurodegenerative pathology including frontotemporal lobar degeneration with tau or TDP-43 inclusions (FTLD-tau; FTLD-TDP), neocortical LBD, CAA, HS, and WMR.28–32 National Institute of Neurological Disorders and Stroke and National Institute of Biomedical Imaging and Bioengineering (NINDS-NIBIB) consensus criteria were used to diagnose CTE,10,11 and stages I-IV were assigned using McKee criteria.33,34 CTE stages III-IV were considered in this study since they have previously been shown to be associated with dementia and to match the disease severity of other diseases such as AD.33,34 Involvement of the hippocampus by CTE pathology in stages III-IV was distinguished from other tauopathies such as primary age-related tauopathy by the preferential involvement of CA2 and CA4.35 CTE in the presence of comorbid AD was determined by the presence of a clear focus of perivascular neurofibrillary tangles with a predilection for greater involvement of the sulcal depths by tau pathology. NIA-Reagan criteria were used to diagnose AD of intermediate or high likelihood.36 TDP-43 inclusions were assessed within the amygdala, entorhinal cortex, hippocampus, and dorsolateral frontal cortex and marked positive if present in any of those regions. Most TDP-43 inclusions were limbic predominant and indistinguishable from limbic-associated TDP-43 encephalopathy-neuropathologic change (LATE-NC); however, TDP-43 deposition has been shown to occur at a younger age and is associated with RHI in CTE.37 Brain tissue was also evaluated for the presence of vascular pathology including gross infarcts, microinfarcts, moderate-severe atherosclerosis, and moderate-severe arteriolosclerosis. Pathologies were dichotomized as present or not (CTE stage III or IV, intermediate or high AD, neocortical LBD, HS, FTLD, TDP-43 inclusions, and gross and micro-infarcts). For pathologies rated on a none-mild-moderate-severe scale (CAA, WMR, atherosclerosis, and arteriolosclerosis), scores were dichotomized as moderate/severe vs none/mild. Primary age-related tauopathy, age-related tau astrogliopathy (ARTAG), argyrophilic grain disease, and LBD other than neocortical are either rare or have not been found to be significant contributors to dementia in previous studies and therefore were not included in our analyses in order to improve power.
Statistical analyses
To assess the intercorrelation of pathologies, the number of unique combinations of pathologies observed in our sample was compared to a distribution of combinations in 10,000 simulated datasets assuming no intercorrelation. The simulated datasets were representative of our sample in terms of sample size and frequencies of each neuropathology. Spearman’s rho was also used to assess correlations between each pathology. We used independent logistic regressions to examine the relationship between each pathology and dementia status. Pathologies were assessed separately because of the high degree of correlation between pathologies and therefore the given β’s do not account for any correlations. Using the regression coefficients, we calculated the percent contribution of each pathology to dementia status to examine the effect of each individual pathology in relation to the other pathologies assessed (β for each pathology / sum of βs for all pathologies). This approach gives the relative contribution of each pathology, but not the fraction of total variance in dementia status. Similarly, we used independent linear regressions to examine the relationships between each pathology and score on the FAQ and the CDS. We used multivariable regression to estimate the total variance in dementia status and FAQ and CDS scale scores explained by all neuropathologies. All regression models were adjusted for age, Black/African American race, and education level. A p-value less than 0.05 was considered statistically significant.
RESULTS
Characteristics of brain donors
Brain donors ranged in age from 18 to 97 (median 65.0 [IQR 46.0–76.0]). Nearly all (565 [98.9%]) of the brain donors were male. Based on informant report, 87 (15.2%) were Black/African American, 466 (81.6%) of the brain donors were White, and 18 (3.2%) were other races. All brain donors previously played contact sports (mean 14.5 years of play [SD 7.5]), and 513 (89.8%) played football. Most of the brain donors had played their primary sport at the professional (n=238 [46.1%]) or college (n=171 [33.1%]) level. 139 (24.3%) of the brain donors had served in the military, and 23 (4.0%) had served in combat. About half of the brain donors (300 [52.5%]) had dementia at the time of their death. The mean FAQ score was 14.2 (SD 12.1) and the mean CDS score was 88.1 (SD 48.6). Additional descriptive data are provided in Table 1.
Table 1.
Descriptive characteristics of brain donors (n=571)
| Variable | Mean (SD) or n (%) |
|---|---|
|
| |
| Age at death, years, median (IQR) (range) | 65.0 (46.0–76.0) (18–97) |
| Education level | |
| Some high school or high school diploma/GED | 38 (6.7) |
| Some college or college degree | 383 (67.1) |
| More than college or graduate degree | 150 (26.3) |
| Male sex | 565 (98.9) |
| Race | |
| White | 466 (81.6) |
| Black/African American | 87 (15.2) |
| Other race | 18 (3.2) |
| Contact sports | |
| Football | 513 (89.8) |
| Soccer | 63 (11.0) |
| Wrestling | 54 (9.5) |
| Hockey | 45 (7.9) |
| Boxing | 36 (6.3) |
| Lacrosse | 22 (3.9) |
| Rugby | 18 (3.2) |
| Mixed martial arts | 5 (0.9) |
| Military | 139 (24.3) |
| Combat | 23 (4.0) |
| Dementia | 300 (52.5) |
| FAQ score | 14.2 (12.1) |
| CDS score | 88.1 (48.6) |
| Neurodegenerative pathology | |
| CTE (stages III-IV) | 246 (43.1) |
| TDP-43 inclusions | 137 (24.0) |
| Alzheimer Disease | 107 (18.7) |
| Hippocampal sclerosis | 96 (16.8) |
| Neocortical Lewy bodies | 34 (6.0) |
| FTLD-TDP | 24 (4.2) |
| FTLD-tau | 24 (4.2) |
| Cerebrovascular pathology | |
| Arteriolosclerosis (mod-sev) | 292 (51.1) |
| CAA (mod-sev) | 123 (21.5) |
| Microinfarcts | 110 (19.3) |
| Atherosclerosis (mod-sev) | 108 (18.9) |
| Gross Infarcts | 77 (13.5) |
| White matter rarefaction (mod-sev) | 251 (44.0) |
FAQ = Functional Activities Questionnaire; CDS = Cognitive Difficulties Scale; CTE = chronic traumatic encephalopathy; FTLD = frontotemporal lobar degeneration; TDP-43 = transactive response DNA-binding protein 43; CAA = cerebral amyloid angiopathy.
Frequency of neuropathologies
The majority of brain donors (441 [77.2%]) met diagnostic criteria for at least one moderate-severe neurodegenerative or cerebrovascular pathology. The most common neurodegenerative disease observed was stage III-IV CTE (246 [43.1%]). Less common, but still frequent, neurodegenerative pathologies included TDP-43 inclusions (137 [24.0%]), AD (107 [18.7%]), and HS (96 [16.8%]). Neocortical LBD (34 [6.0%]), FTLD with TDP-43 inclusions (24 [4.2%]), and FTLD with tau inclusions (24 [4.2%]) were less common. Moderate-severe WMR was observed frequently in our sample (251 [44.0%]), as was moderate-severe cerebrovascular disease including arteriolosclerosis (292 [51.1%]), CAA (123 [21.5%]), and atherosclerosis (108 [18.9%]). Chronic macroscopic infarcts were observed in 77 (13.5%) brain donors and chronic microscopic infarcts were observed in 110 (19.3%) brain donors. Figure S1 displays the frequencies of each unique combination of neuropathologies. The frequency of each unique combination of pathologies ranged from 1–17 people, with the most common combination (arteriolosclerosis + WMR) occurring in only 3% of the sample. The most common combinations of pathologies observed were arteriolosclerosis + WMR and CTE with varying combinations of arteriolosclerosis, WMR, TDP-43 inclusions, and CAA (Table 2).
Table 2:
Most common combinations of pathologies
| Pathology combination | n (%) |
|---|---|
|
| |
| Arteriolosclerosis + WMR | 17 (3.0) |
| CTE + Arteriolosclerosis + WMR | 11 (1.9) |
| CTE + Arteriolosclerosis | 10 (1.8) |
| CTE + WMR | 9 (1.6) |
| CTE + TDP-43 | 6 (1.1) |
| AD + Arteriolosclerosis + WMR | 5 (0.9) |
| Arteriolosclerosis + Microinfarcts + WMR | 5 (0.9) |
| CTE + TDP-43 + Arteriolosclerosis + WMR | 4 (0.7) |
| CTE + Arteriolosclerosis + WMR + CAA | 4 (0.7) |
CTE= chronic traumatic encephalopathy, WMR= white matter rarefaction, AD= Alzheimer disease, CAA= cerebral amyloid angiopathy
Intercorrelation of neuropathologies
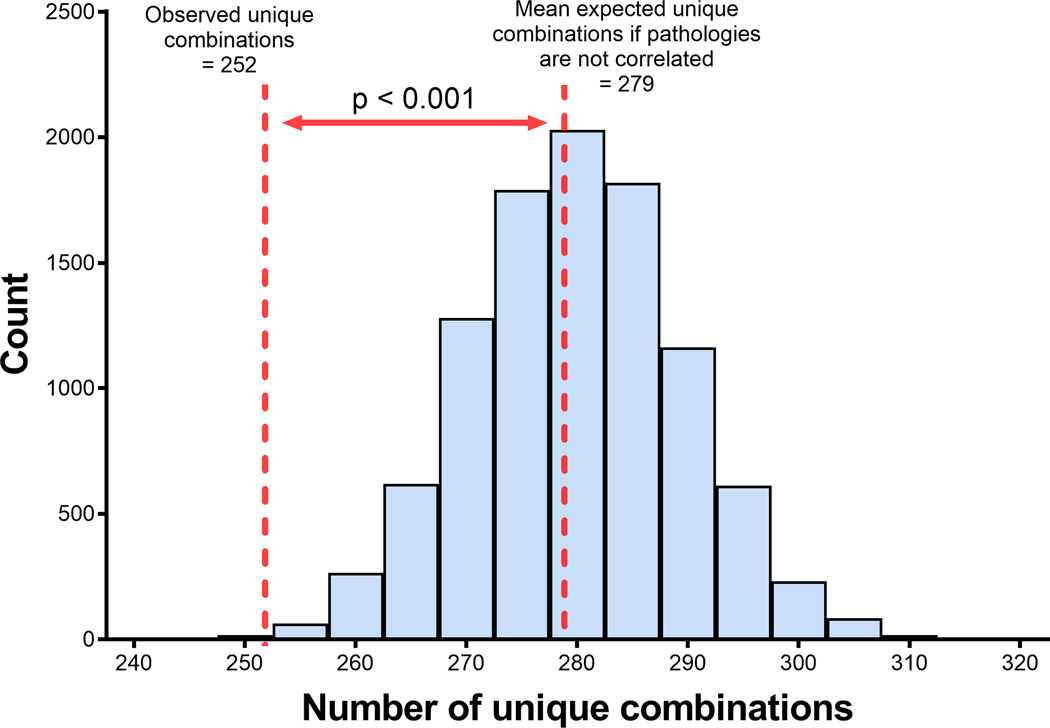
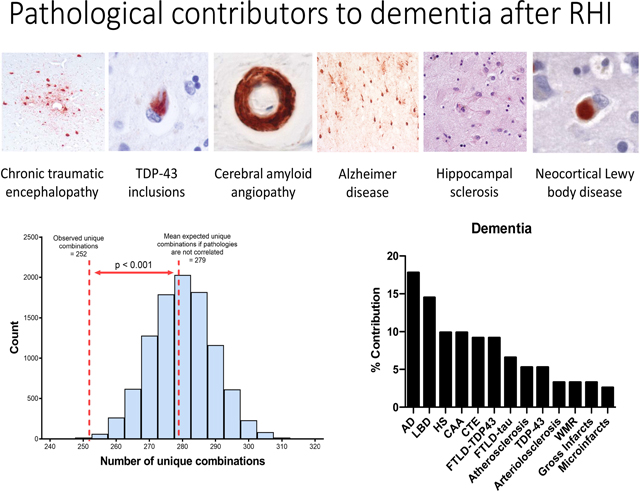
Pathologies frequently occurred in combination in our sample. 357 (62.5%) brain donors had at least two moderate-severe neuropathologies present and 290 (50.8%) had at least three. As shown in Table 3 and Figure 1, the neuropathologies assessed were intercorrelated. Figure 1 shows the expected distribution (mean 278.9 [SD 9.6]) of numbers of unique combinations of independent pathologies, modeled in a simulation of 10,000 datasets representative of our sample in terms of sample size and relative frequencies of each pathology. We observed 252 unique combinations of pathologies in our RHI-exposed sample, which is less than would be expected if the pathologies were completely independent (p<0.001). Table 3 shows the Spearman’s rho correlation between each neuropathology, demonstrating further that most of the pathologies assessed were intercorrelated. When age was accounted for in the analysis, CTE was still significantly correlated with TDP-43 inclusions and HS (Table S3).
Table 3:
Intercorrelations of neuropathologies
| CTE | TDP-43 | AD | HS | LBD | FTLD-TDP | FTLD-tau | Art | CAA | Micro infarcts | AS | Infarcts | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTE | -- | |||||||||||
| TDP-43 | .39 ** | -- | ||||||||||
| AD | .15 ** | .22 ** | -- | |||||||||
| HS | .35 ** | .40 ** | .16 ** | -- | ||||||||
| LBD | .03 * | .10 * | .15 ** | .09 * | -- | |||||||
| FTLD-TDP | .10 * | -.07 | .06 | .33 ** | .06 | -- | ||||||
| FTLD-tau | .05 | .07 | .01 | .05 | .10 * | .04 | -- | |||||
| Art | .31 ** | .21 ** | .23 ** | .23 ** | .06 | .02 | .14 ** | -- | ||||
| CAA | .16 ** | .23 ** | .34 ** | .25 ** | .19 ** | .06 | -.04 | .25 ** | -- | |||
|
Micro
infarcts |
.14 ** | .15 ** | .07 | .12 ** | .09 * | .03 | .08 | .22 ** | .10 * | -- | ||
| AS | .23 ** | .24 ** | .15 ** | .22 ** | .09 * | .06 | .12 ** | .24 ** | .15 ** | .29 ** | -- | |
| Infarcts | .18 ** | .12 ** | .18 ** | .14 ** | .01 | .07 | .02 | .25 ** | .06 | .30 ** | .28 ** | -- |
| WMR | .23 ** | .18 ** | .24 ** | .13 ** | .03 | .13 ** | .03 | .40 ** | .18 ** | .22 ** | .14 ** | .25 ** |
AD = Alzheimer’s disease; Art = Arteriolosclerosis; AS = Atherosclerosis; LBD = neocortical Lewy body disease; HS = hippocampal sclerosis; CAA = cerebral amyloid angiopathy; CTE = chronic traumatic encephalopathy stages III-IV; FTLD = frontotemporal lobar degeneration; TDP-43 = transactive response DNA-binding protein 43; WMR = white matter rarefaction.
Correlation is significant at the 0.05 level (2-tailed), based on Spearman’s rho.
Correlation is significant at the 0.01 level (2-tailed), based on Spearman’s rho.
Figure 1:
Actual and expected unique number of combinations of neuropathologies.
The distribution of numbers of unique combinations of pathologies assuming each is independent is shown from a simulation of 10,000 datasets that are representative of our brain donors in sample size and frequencies of individual pathologies. The dashed lines represent the observed and predicted numbers of unique pathologies observed in our sample.
Association of neuropathologies with dementia and cognitive scales
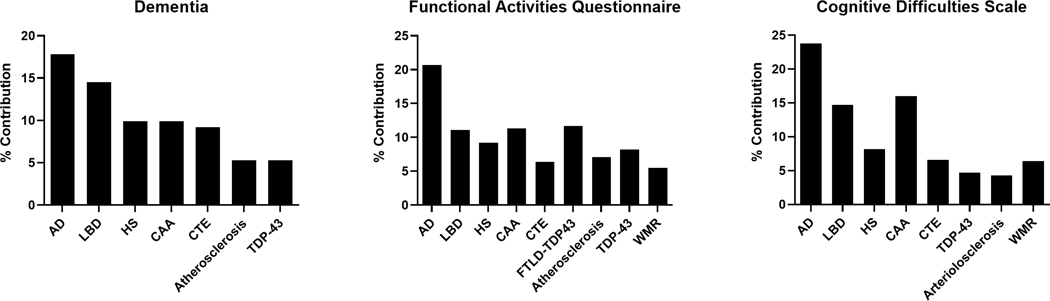
We used independent logistic regression to examine the relationship between each pathology and dementia status. Using the regression coefficients, we calculated the percent contribution of each pathology to dementia status to examine the effect of each individual pathology in relation to the other pathologies assessed. We found that the most influential contributors to dementia status in our sample included the following in rank order: AD (17.8%; β=0.27; p<0.001), neocortical LBD (14.5%; β=0.22; p<0.001), HS (9.9%; β=0.15; p<0.001), CAA (9.9%; β=0.15; p<0.001) and stage III-IV CTE (9.2%; β=0.14; p<0.001).
Similarly, we used independent linear regressions to examine the relationships between each pathology and variation in scores on the FAQ and the CDS. The pathologies contributing most to variance in FAQ scores were AD (20.7%; β=10.50; p<0.001), FTLD-TDP (11.7%; β=5.93; p=0.002), CAA (11.3%; β=5.74; p<0.001), neocortical LBD (11.1%; β=5.64; p<0.001), HS (9.2%; β=4.66; p<0.001), TDP-43 inclusions (8.2%; β=4.16; p<0.001), atherosclerosis (7.1%; β=3.60; p<0.001), and stage III-IV CTE (6.4%; β=3.26; p<0.001); and the pathologies contributing most to variance in CDS scores were AD (23.8%; β=36.63; p<0.001), CAA (16.0%; β=24.62; p<0.001), neocortical LBD (14.7%; β=22.65; p=0.003), FTLD-TDP (9.2%; β=14.21; p=0.13), HS (8.2%; β=12.55; p=0.02), WMR (6.4%; β=9.77; p=0.01), and stage III-IV CTE (6.6%; β=10.1; p=0.02). The regression coefficients and calculated percent contribution to dementia status and cognitive scale scores for each pathology relative to the effect of all pathologies are shown in Table 4. The relative percent contributions to dementia status and cognitive scale scores for each significant pathology are displayed in Figure 2.
Table 4:
The effect and relative contribution of each pathology on dementia and cognitive scale scores
| DEMENTIA | FAQ | CDS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | % cont. | SE | p-value | β | % cont. | SE | p-value | β | % cont. | SE | p-value | |
| AD | .27 | 17.8 | .04 | <.001 | 10.50 | 20.7 | .94 | <.001 | 36.63 | 23.8 | 4.70 | <.001 |
| LBD | .22 | 14.5 | .07 | <.001 | 5.64 | 11.1 | 1.61 | <.001 | 22.65 | 14.7 | 7.51 | .003 |
| HS | .15 | 9.9 | .04 | <.001 | 4.66 | 9.2 | 1.08 | <.001 | 12.55 | 8.2 | 5.14 | .02 |
| CAA | .15 | 9.9 | .04 | <.001 | 5.74 | 11.3 | .98 | <.001 | 24.62 | 16.0 | 4.67 | <.001 |
| CTE | .14 | 9.2 | .04 | <.001 | 3.26 | 6.4 | .89 | <.001 | 10.1 | 6.6 | 4.31 | .02 |
| FTLD-TDP | .14 | 9.2 | .08 | .09 | 5.93 | 11.7 | 1.94 | .002 | 14.21 | 9.2 | 9.32 | .13 |
| FTLD-tau | .10 | 6.6 | .08 | .19 | .94 | 1.9 | 1.89 | .62 | -6.58 | -4.3 | 8.86 | .46 |
| Athero-sclerosis | .08 | 5.3 | .04 | .05 | 3.60 | 7.1 | 1.05 | <.001 | 9.00 | 5.9 | 5.01 | .07 |
| TDP-43 | .08 | 5.3 | .04 | .04 | 4.16 | 8.2 | .96 | <.001 | 7.17 | 4.7 | 4.63 | .12 |
| Arteriolo-sclerosis | .05 | 3.3 | .04 | .14 | 1.31 | 2.6 | .92 | .15 | 6.60 | 4.3 | 4.31 | .13 |
| WMR | .05 | 3.3 | .03 | .15 | 2.77 | 5.5 | .82 | <.001 | 9.77 | 6.4 | 3.92 | .01 |
| Gross Infarcts | .05 | 3.3 | .05 | .31 | 1.59 | 3.1 | 1.16 | .17 | 2.79 | 1.8 | 5.54 | .62 |
| Micro-infarcts | .04 | 2.6 | .04 | .40 | .65 | 1.3 | 1.02 | .52 | 4.31 | 2.8 | 4.84 | .37 |
Multiple linear regressions adjusting for age, Black/AA race, and education level were performed separately for each pathology. The percent contribution (% cont.) shows the contribution of each pathology relative to the total effect of all pathologies. FAQ = functional activities questionnaire; CDS = cognitive difficulties scale; AD = Alzheimer’s disease; LBD = neocortical Lewy body disease; HS = hippocampal sclerosis; CAA = cerebral amyloid angiopathy; CTE = chronic traumatic encephalopathy stages III-IV; FTLD = frontotemporal lobar degeneration; TDP-43 = transactive response DNA-binding protein 43; WMR = white matter rarefaction.
Figure 2:
The relative percent contribution of each neuropathology to variance in dementia status or cognitive scale scores.
The percent contribution of each pathology relative to the total effect of all the pathologies on the variance in dementia status and cognitive scale scores is shown. FAQ = functional activities questionnaire; CDS = cognitive difficulties scale; AD = Alzheimer’s disease; LBD = neocortical Lewy body disease; HS = hippocampal sclerosis; CAA = cerebral amyloid angiopathy; CTE = chronic traumatic encephalopathy stages III-IV; FTLD = frontotemporal lobar degeneration; TDP-43 = transactive response DNA-binding protein 43; WMR = white matter rarefaction.
We used multivariable regression to estimate the total variance in dementia status and cognitive scale scores explained by all neuropathologies. Taken together, all neuropathologies assessed accounted for 46% of variance in dementia status, 57% of variance in FAQ scores, and 39% of variance in CDS scores.
Sensitivity analyses for sex, hypertension, and diabetes were computed because these variables have been previously shown to affect risk for cognitive decline and dementia. The sensitivity analyses showed that inclusion of sex, hypertension, or diabetes diagnosis in these regression models did not impact the results. In addition, we calculated the percent contributions to dementia status and cognitive scale scores for each pathology stratified by age <60 years (Table S1) and ≥60 years (Table S2). The results were similar such that for those age <60 years, AD, neocortical LBD, CAA, CTE, and FTLD-TDP were all significantly associated with dementia while in those age ≥60 years, AD, neocortical LBD, HS, CAA, and CTE were significantly associated with dementia.
DISCUSSION
In this group of participants with contact sports exposure, multiple neuropathologies were common and correlated. High stage CTE was the most frequent neurodegenerative pathology and was commonly present with cerebrovascular pathology, WMR, and TDP-43 inclusions. These findings demonstrate that in patients with a history of RHI, dementia status and cognitive scores were associated with multiple neurodegenerative and cerebrovascular pathologies with the greatest variance explained by AD, neocortical LBD, HS, CAA, TDP-43 inclusions, and CTE, respectively.
Intercorrelation of pathologies secondary to RHI
RHI may be a common cause for multiple neurodegenerations. Clinically, RHI has been linked to cognitive impairment, depression, and behavioral dysregulation18,33,38,39 as well as probable REM sleep behavior disorder.40 Pathologically, years of contact sport play, a proxy for RHI, has been associated with CTE,13 neocortical LBD,19 WMR,15 more severe frontal and leptomeningeal CAA,17 and HS and TDP-43 inclusions.16 Here we found that multiple neuropathologies were intercorrelated and that the number of unique pathology combinations was significantly less than predicted if they were independent in this RHI-exposed group. RHI may be one common cause of multiple neuropathologies. Future studies should examine the effects of RHI, age, and vascular risk factors on the intercorrelation of neuropathologies in different cohorts.
Potency of pathologies
The relative potency of the various neuropathologies on dementia and cognitive scores varied. In other community aging autopsy groups, AD was the most frequent pathology, and AD, neocortical LBD, and HS accounted for the largest percentage of cognitive impairment.5,7 However, unlike in community aging groups, in our RHI-exposed group, CTE was the most frequent neurodegenerative pathology, and AD was less frequent. In addition, neocortical LBD, HS, and CAA were much less frequent than CTE. However, as in community-based groups, when present, AD and neocortical LBD were most strongly associated with dementia. CTE had a similar effect size to HS and CAA, two well-known contributors to dementia and cognitive impairment. Overall, AD, neocortical LBD, HS, CAA, and CTE explained most of the pathology-related variance in dementia and cognitive scores in this RHI-exposed group.
Heterogeneity and frequency of mixed pathologies
Heterogeneity and the presence of multiple neuropathologies is common with advanced age. Here we show that even in a relatively young group of RHI-exposed participants, multiple concurrent neuropathologies are common and varied. This has been previously reported in smaller groups of RHI exposed individuals, including the common comorbidity of HS and TDP-43.16,21,41 In CTE, TDP-43 inclusions can occur in a variety of regions, including at the sulcal depths in the frontal cortex which may represent a CTE-specific form, but more commonly in limbic regions in the pattern described for limbic age-related TDP-43 encephalopathy.16 In addition, we found that CAA showed an association with dementia and cognitive impairment. Our previous study showed that RHI and CTE are associated with more severe frontal leptomeningeal CAA than occurs in aging and AD17 and therefore CAA may partially mediate the association of RHI with dementia. Similarly, neocortical LBD has been linked to a history of traumatic brain injury42 and RHI19 and recent data suggests that high stage CTE is associated with HS.16 The clinical syndrome of CTE, Traumatic encephalopathy syndrome (TES), is evolving43 and the specific causes of the TES phenotypes are uncertain and likely to include tau and non-tau pathologies. Overall, the common presence of mixed pathologies in an RHI exposed group should inform future studies identifying the salient clinical features associated with pathology.
A combination of environmental and genetic factors likely determines the pathologies that develop later in life. Common genetic risk factors such as Apolipoprotein E44 and Transmembrane Protein 106B45 alter the risk for many neurodegenerative pathologies including the severity of CTE. Determining interactions between RHI and genetic risk factors on the development of neurodegenerative disease requires large numbers of individuals and is an ongoing focus in the field.
Importance of factors beyond known pathologies
The average age of the RHI-exposed group was relatively young (median 65 years at death), yet 77.2% had at least one moderate to severe pathology. Nevertheless, this is much less than the reported 94% with at least one neuropathology in an older community group (Religious Orders Study and the Memory and Aging Project (ROSMAP), average age 90 years at death)5 highlighting the importance of age in the development of these pathologies. However, even in the much younger RHI-exposed group the combination of neuropathologies explained 46% of the dementia status, 57% of variance in FAQ scores, and 39% of variance in CDS scores, which is similar to previous reports in ROSMAP.7 Factors that contribute to resilience and resistance to the development of neuropathologies likely at least partially underlie the variance in cognition not explained by the neuropathological combinations. These may include environmental factors previously linked to dementia such as depression, air pollution, physical inactivity, diabetes, hypertension, hearing loss, obesity, excessive alcohol consumption, lack of social contact, and insomnia.46,47 Non-traditional and new measures of pathology such as white matter integrity, synaptic density, and neuroinflammatory measures may mediate some of these effects and require further study.48,49
Limitations
Our sample was primarily comprised of male former American football players who completed at least some college. Although the majority were White, 15% were Black/African American which is high for a brain bank. Nevertheless, these findings may not be generalizable to non-athletes, female athletes, athletes who played other contact sports, athletes with other racial/ethnic backgrounds, or athletes who completed fewer years of education. Brain donors were enrolled in this study at post-mortem, so we did not have access to longitudinal cognitive data, and instead relied on retrospective informant reports of cognitive and functional impairment. The median age of the RHI-exposed group in this study was young compared to other published autopsy groups and the potential modifying effects of age on the association of pathology and cognitive impairment are not well understood. Further, although previously RHI was not found to be associated with ARTAG,50 the role of RHI in the development of primary age-related tauopathy and ARTAG and the association with cognitive impairment requires additional study. Although we were underpowered to examine effects of moderate-severe TBI, comparison of the effects of RHI and TBI is a high priority for future studies. The substantial amount of correlation among pathologies makes statistical modeling difficult. Future studies should examine the roles of age, vascular risk factors, and RHI on the intercorrelations of neuropathologies. In addition, neurobehavioral dysregulation is a common feature among brain donors with CTE, but its specific association with CTE p-tau pathology is uncertain. Future studies that examine associations between neuropathology and mood and behavior symptoms as well as those including longitudinal assessments of cognitive and neurobehavioral functioning are warranted to confirm and extend these findings.
CONCLUSIONS
In an RHI-exposed group, CTE and multiple other pathologies are common and correlated. Cognitive impairment is often associated with multiple neuropathologies, and diagnosis and eventual treatment should target the frequent combinations of pathologies.
Supplementary Material
Figure S1: Frequencies of observed combinations of neuropathologies. The frequency of each of 252 unique combinations of neuropathologies observed in our sample, in order of descending frequency. WMR = white matter rarefaction; CTE = chronic traumatic encephalopathy stages III-IV
Figure S2: Inclusion and exclusion of brain donors.
Figure S3: Brain donor causes of death.
Figure S4: The relative percent contribution of each neuropathology to variance in dementia status or cognitive scale scores stratified by age 60.
Summary for Social Media if Published.
@bu_cte
Exposure to repetitive head impacts (RHI) is associated with later-life cognitive symptoms and neuropathologies including chronic traumatic encephalopathy (CTE). Cognitive decline is often due to multiple pathologies.
The frequency and contributions of these pathologies to cognitive impairment in people exposed to RHI is unknown. Here, we examined the relative contributions of 13 neuropathologies to cognitive symptoms and dementia in RHI-exposed brain donors.
Mixed neuropathologies, including CTE, underlie cognitive impairment in contact sport athletes exposed to repetitive head impacts.
Cognitive impairment is often associated with multiple neuropathologies, and diagnosis and eventual treatment should target the frequent combinations of pathologies.
ACKNOWLEDGMENTS
This work was supported by grant funding from the National Institute on Aging (AG057902, AG06234, RF1AG054156), the National Institute of Neurological Disorders and Stroke (U54NS115266, K23NS102399, RF1NS122854), the National Institute of Aging (P30AG072978); the U.S. Department of Veterans Affairs, Veterans Health Administration (I01BX005161); the Nick and Lynn Buoniconti Foundation, and Boston University Grant Number 1UL1TR001430. The views, opinions and/or findings contained in this article are those of the authors and should not be construed as an official U.S. Department of Veterans Affairs and U.S. Department of Defense position, policy or decision, unless so designated by other official documentation. Funders did not have a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
The authors gratefully acknowledge the use of the resources and facilities at VA Boston Healthcare System (Boston, Massachusetts) and the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, Massachusetts). We also gratefully acknowledge the help of all members of the Boston University CTE Center, the Veterans Affairs-Boston University-Concussion Legacy Foundation Brain Bank, and the Concussion Legacy Foundation (with special thanks to Lisa McHale, EDS, who receives compensation from the Concussion Legacy Foundation to assist with family donor interactions), as well as the individuals and families whose participation and contributions made this work possible.
Footnotes
Data acquisition, analysis, and interpretation: NS, YT, TM, AO, ZB, EY, JP, BM, MU, EN, BA, AS, RN, CN, RC, DD, BD, DK, RS, VA, BH, PB, JS, JM, AM, MA, TS.
Drafting of the manuscript and figures: NS, YT, MA, TS.
POTENTIAL CONFLICTS OF INTEREST
All authors have nothing to report.
DATA AVAILABILITY
Data elements from the Understanding Neurologic Injury and Traumatic Encephalopathy study are available from the publicly available FITBIR data set. Other raw data from this study are available upon reasonable request to the corresponding author.
BIBLIOGRAPHY
- 1.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 2.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol (Berl). 2017;134(2):171–186. doi: 10.1007/s00401-017-1717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86(11):1000–1008. doi: 10.1212/WNL.0000000000002480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol Zurich Switz. 2010;20(1):66–79. doi: 10.1111/j.1750-3639.2008.00244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74–83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84(1):10–22. doi: 10.1002/ana.25246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle PA, Wang T, Yu L, et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain J Neurol. 2021;144(7):2166–2175. doi: 10.1093/brain/awab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The Neuropathology of Probable Alzheimer’s Disease and Mild Cognitive Impairment. Ann Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139(11):2983–2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol (Berl). 2016;131(1):75–86. doi: 10.1007/s00401-015-1515-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieniek KF, Cairns NJ, Crary JF, et al. The Second NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol. 2021;80(3):210–219. doi: 10.1093/jnen/nlab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mez J, Daneshvar DH, Abdolmohammadi B, et al. Duration of American Football Play and Chronic Traumatic Encephalopathy. Ann Neurol. 2020;87(1):116–131. doi: 10.1002/ana.25611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bieniek KF, Blessing MM, Heckman MG, et al. Association between contact sports participation and chronic traumatic encephalopathy: a retrospective cohort study. Brain Pathol Zurich Switz. 2020;30(1):63–74. doi: 10.1111/bpa.12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alosco ML, Stein TD, Tripodis Y, et al. Association of White Matter Rarefaction, Arteriolosclerosis, and Tau with Dementia in Chronic Traumatic Encephalopathy. JAMA Neurol. 2019;76(11):1298–1308. doi: 10.1001/jamaneurol.2019.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicks R, Clement NF, Alvarez VE, et al. Repetitive head impacts and chronic traumatic encephalopathy are associated with TDP-43 inclusions and hippocampal sclerosis. Acta Neuropathol (Berl). Published online January 21, 2023. doi: 10.1007/s00401-023-02539-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Standring OJ, Friedberg J, Tripodis Y, et al. Contact sport participation and chronic traumatic encephalopathy are associated with altered severity and distribution of cerebral amyloid angiopathy. Acta Neuropathol (Berl). 2019;138(3):401–413. doi: 10.1007/s00401-019-02031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA. 2017;318(4):360–370. doi: 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams JW, Alvarez VE, Mez J, et al. Lewy Body Pathology and Chronic Traumatic Encephalopathy Associated With Contact Sports. J Neuropathol Exp Neurol. 2018;77(9):757–768. doi: 10.1093/jnen/nly065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol (Berl). 2015;130(1):21–34. doi: 10.1007/s00401-015-1435-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asken BM, Tanner JA, VandeVrede L, et al. Multi-Modal Biomarkers of Repetitive Head Impacts and Traumatic Encephalopathy Syndrome: A Clinicopathological Case Series. J Neurotrauma. 2022;39(17–18):1195–1213. doi: 10.1089/neu.2022.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol (Berl). 2019;138(3):389–399. doi: 10.1007/s00401-019-02030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mez J, Solomon TM, Daneshvar DH, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimers Res Ther. 2015;7(1):62. doi: 10.1186/s13195-015-0148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- 25.Crook T, Ferris S, Bartus R. Assessment in Geriatric Psychopharmacology. Mark Powley Associates; 1983. [Google Scholar]
- 26.Vonsattel JPG, Amaya M del P, Cortes EP, Mancevska K, Keller CE. 21st Century Brain Banking Practical prerequisites and lessons from the past: The experience of New York Brain Bank – Taub Institute - Columbia University. Cell Tissue Bank. 2008;9(3):247–258. doi: 10.1007/s10561-008-9079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vonsattel JPG, del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol (Berl). 2008;115(5):509–532. doi: 10.1007/s00401-007-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol (Berl). 2010;119(1):1–4. doi: 10.1007/s00401-009-0612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roemer SF, Grinberg LT, Crary JF, et al. Rainwater Charitable Foundation criteria for the neuropathologic diagnosis of progressive supranuclear palsy. Acta Neuropathol (Berl). 2022;144(4):603–614. doi: 10.1007/s00401-022-02479-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain J Neurol. 2019;142(6):1503–1527. doi: 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attems J, Toledo JB, Walker L, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol (Berl). 2021;141(2):159–172. doi: 10.1007/s00401-020-02255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain J Neurol. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alosco ML, Cherry JD, Huber BR, et al. Characterizing tau deposition in chronic traumatic encephalopathy (CTE): utility of the McKee CTE staging scheme. Acta Neuropathol (Berl). 2020;140(4):495–512. doi: 10.1007/s00401-020-02197-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrell K, Iida MA, Cherry JD, et al. Differential Vulnerability of Hippocampal Subfields in Primary Age-Related Tauopathy and Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol. 2022;81(10):781–789. doi: 10.1093/jnen/nlac066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(11):1147–1155. doi: 10.1097/00005072-199911000-00004 [DOI] [PubMed] [Google Scholar]
- 37.Nicks R, Clement NF, et al. Repetitive head impacts and chronic traumatic encephalopathy are associated with TDP-43 inclusions and hippocampal sclerosis. Acta Neuropathol (Berl). 2023;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alosco ML, Tripodis Y, Baucom ZH, et al. Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology. 2020;95(7):e793–e804. doi: 10.1212/WNL.0000000000010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alosco ML, Kasimis AB, Stamm JM, et al. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl Psychiatry. 2017;7(9):e1236. doi: 10.1038/tp.2017.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams JW, Alosco ML, Mez J, et al. Association of probable REM sleep behavior disorder with pathology and years of contact sports play in chronic traumatic encephalopathy. Acta Neuropathol (Berl). 2020;140(6):851–862. doi: 10.1007/s00401-020-02206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol (Berl). 2019;138(3):389–399. doi: 10.1007/s00401-019-02030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane PK, Gibbons LE, Dams-O’Connor K, et al. Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016;73(9):1062–1069. doi: 10.1001/jamaneurol.2016.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz DI, Bernick C, Dodick DW, et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology. 2021;96(18):848–863. doi: 10.1212/WNL.0000000000011850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atherton K, Han X, Chung J, et al. Association of APOE Genotypes and Chronic Traumatic Encephalopathy. JAMA Neurol. 2022;79(8):787–796. doi: 10.1001/jamaneurol.2022.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cherry JD, Mez J, Crary JF, et al. Variation in TMEM106B in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2018;6(1):115. doi: 10.1186/s40478-018-0619-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement J Alzheimers Assoc. 2017;13(4):406–418. doi: 10.1016/j.jalz.2016.07.152 [DOI] [PubMed] [Google Scholar]
- 47.Killin LOJ, Starr JM, Shiue IJ, Russ TC . Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016;16(1):175. doi: 10.1186/s12877-016-0342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alosco ML, Ly M, Mosaheb S, et al. Decreased myelin proteins in brain donors exposed to football-related repetitive head impacts. Brain Commun. 2023;5(2):fcad019. doi: 10.1093/braincomms/fcad019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirsch D, Shah A, Dixon E, et al. Vascular injury is associated with repetitive head impacts and tau pathology in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2023;82(2):127–139. doi: 10.1093/jnen/nlac122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler MLMD, Dixon E, Stein TD, et al. Tau Pathology in Chronic Traumatic Encephalopathy is Primarily Neuronal. J Neuropathol Exp Neurol. 2022;81(10):773–780. doi: 10.1093/jnen/nlac065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Frequencies of observed combinations of neuropathologies. The frequency of each of 252 unique combinations of neuropathologies observed in our sample, in order of descending frequency. WMR = white matter rarefaction; CTE = chronic traumatic encephalopathy stages III-IV
Figure S2: Inclusion and exclusion of brain donors.
Figure S3: Brain donor causes of death.
Figure S4: The relative percent contribution of each neuropathology to variance in dementia status or cognitive scale scores stratified by age 60.
Data Availability Statement
Data elements from the Understanding Neurologic Injury and Traumatic Encephalopathy study are available from the publicly available FITBIR data set. Other raw data from this study are available upon reasonable request to the corresponding author.