Abstract
Heart failure (HF) is a common and serious complication of diabetes mellitus (DM) that remains widely under-recognized. Multidisciplinary management protocols for patients with concurrent DM and HF are not widely utilized in the Middle East/Gulf region, particularly in the United Arab Emirates. Since early identification of patients with DM and HF will likely lead to initiation of therapies known to prevent adverse cardiovascular events and subsequently improve patient prognosis, we aim to highlight the importance of early recognition of HF in diabetic patients. We will also describe existing management challenges in the region, especially the lack of multidisciplinary care and emphasize the role of newer anti-diabetic therapies in preventing and treating HF. Most importantly, this call-to-action proposes a collaborative approach to the care of diabetic patients with HF involving primary care physicians, endocrinologists, and cardiologists.
Keywords: Diabetes mellitus, Heart failure, Middle east
1. Introduction
With the rapidly growing prevalence of obesity, type 2 diabetes mellitus (DM) has drawn specific attention as a major public health problem in the Middle East/Gulf (MEG) region in general and in the United Arab Emirates (UAE) in particular [1,2]. DM is a key risk factor for a host of cardiovascular complications, including development of heart failure (HF) [3]. Whereas at least a third of diabetic patients in western cohorts had a concomitant HF diagnosis [4,5], evidence suggests that HF prevalence among diabetic patients in the MEG region could be even higher [6]. According to the Global Burden of Disease data repository, the prevalence of heart failure in UAE increased significantly from 211 HF cases per 100,000 population (95% UI 164–265) in 1990 to 401 cases per 100,000 population (95% UI 295–527) in 2019, equivalent to a 90% increase in prevalence over time [7]. Similarly, the prevalence of diabetes also increased from 2344 (95% UI 2053–2653) cases per 100,000 population in 1990–7783 (95% UI 2654–6768) cases per 100,000 population in 2019, representing a 232% increase of prevalence over time [7]. The economic burden associated with providing care to more than 1.3 million HF patients across the region highlights the significance of cardiometabolic disorders as major drivers of healthcare expenditure in MEG area [8]. Furthermore, it clearly indicates that curtailing morbidity and mortality associated with these highly prevalent conditions hinges on early and effective treatment of patients with concomitant DM and HF.
Development of HF in patients with DM is multifactorial, and likely involves a complex interplay between myocardial ischemia, abnormal calcium homeostasis, oxidative stress and inflammation [9], ultimately leading to deranged cardiac functions and development of diabetic cardiomyopathy [10]. Additionally, HF may complicate treatment with certain diabetic medications, further compounding the burden of DM in patients with HF [11–14]. Clinically, ventricular systolic or diastolic dysfunction in patients with coexistent DM and HF manifests with a spectrum of clinical symptoms, including shortness of breath, fatigue or diminished physical fitness. Early detection of HF in diabetic patients, however, can be challenging either due to under-recognition of the disease, absence of classical symptoms, or limited utilization of advanced cardiac testing for detection of subclinical stages of cardiac dysfunction. Therefore, there currently exists a need for more standardized and streamlined pathways for evaluation and referral of symptomatic diabetic patients suspected to concurrently have HF for early initiation of effective therapies.
Accordingly, a group of cardiologists and endocrinologists interested in the care of patients across the spectrum of cardiometabolic disorders in the UAE and MEG region collaborated to provide a description of the current status of care for patients with concurrent DM and HF and to propose a framework for initial evaluation and assessment of symptomatic diabetic patients at risk for development of HF.
2. Identification of diabetic patients with concomitant heart failure
Since timely diagnosis of HF in patients with DM may conceivably be associated with improved health status and clinical outcomes, there is ongoing interest in identifying diabetic patients at risk for development of HF. However, it remains unclear as to which patients should be referred for further cardiac testing. The presence of symptoms (dyspnea, orthopnoea, or paroxysmal nocturnal dyspnoea) or physical signs for HF (increased jugular venous pressure, presence of third heart sound, displaced apical impulse, respiratory crackles, or bilateral lower extremity edema) in patients with or without DM should warrant further cardiac evaluation [15]. Furthermore, patients with markers of increased HF risk—including age, hypertension, coronary artery disease, poor glycemic control—may benefit from additional screening for subclinical cardiovascular disease [16].
Relying solely on clinical presentation to identify diabetic patients at risk for HF has some inherent limitations and, therefore, there currently remains a need for additional tools to refine detection of cardiac dysfunction in patients with DM. Electrocardiography (ECG) is a widely available test that provides a simple, non-invasive option to screen for underlying cardiovascular disease, and the presence of ECG abnormalities has relevant prognostic implications in diabetic patients [17]. Biomarkers of cardiac stress and volume overload, including N-terminal pro B-type natriuretic peptide (NT-proBNP) and BNP, have also been suggested to identify diabetic patients at risk for incident HF, especially in outpatient settings [15,18–21]. Albuminuria is another predictor of cardiovascular risk and is marker of diabetic nephropathy and subclinical left ventricular systolic and diastolic dysfunction [22]. The presence of microalbuminuria in asymptomatic diabetic patients is associated with a ~2-fold increase in major adverse cardiovascular events and a ~3-fold increase in risk of HF hospitalization [23]. Reduced glomerular filtration rate (GFR), signifying diabetes-induced target organ damage, is also associated with increased risk of incident HF and impaired GFR was associated with a 52% increase in the risk of HF [24–26].
Echocardiography also plays a pivotal role in noninvasive assessment of cardiac structure and function [27,28]. Left ventricular hypertrophy (LVH) is a common echocardiographic finding in diabetic patients who are at risk for HF [16,29,30]. Left atrial abnormalities, including atrial dilation, and diastolic functional abnormalities are also commonly encountered in diabetic patients. Furthermore, abnormal myocardial deformation, manifesting as reduction in global longitudinal strain (GLS), is another marker of ultrastructural derangements seen in patients with preclinical diabetic cardiomyopathy, and serial changes in GLS may be a marker of response to oral glucose-lowering medications [31].
A summary of recommendations on screening diabetic patients for HF in the UAE is provided in Table 1.
Table 1.
Recommendations for initial screening for heart failure in patients with diabetes in the United Arab Emirates.
| Screening for Heart Failure in Patients with Diabetes Mellitus |
|---|
|
3. Multidisciplinary care for patients with diabetes and heart failure
The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), have identified the main goals of DM treatment to include achievement of glycemic control, prevention of diabetic complications, and maintenance of patients, quality of life [32]. This is achieved through close collaboration between primary care physicians, diabetologists, and cardiologists, who work collaboratively to integrate risk factor control, lifestyle management, and screening for cardiovascular complications [33].
Multidisciplinary management programs have proven successful in overcoming various challenges associated with HF care across various healthcare settings [34,35]. The benefits of multidisciplinary HF management programs include reduction in HF hospitalizations and mortality [36]. As a result, referral of high-risk patients, including those with DM to the multidisciplinary HF clinics has been recommended [35].
Despite clear evidence supporting the role of multidisciplinary HF clinics, adoption of this care model in the Middle East-Gulf region is slow, and there continues to be limited data pertaining to performance of these clinics in the Region. A dedicated HF inpatient service was established at Cleveland Clinic Abu Dhabi on the 1st January 2017, comprising HF cardiologists, clinical pharmacists and HF nurses. The creation of this multidisciplinary program was associated with better adherence to guideline directed medical therapy, reduction in hospital length of stay, lower 30-day readmission, and shorter duration to post-discharge follow up [37].
As such, multidisciplinary care for patients with DM and HF is crucial not only due to the frequent coexistence of both conditions, but also due their complex and systemic nature. Care for diabetic patients with or at-risk for HF has 2 main objectives: (1) identification of symptomatic patients in early stages of diabetic cardiomyopathy, and (2) initiation of effective pharmacotherapy for patients with overt HF in order to improve their health status and long-term survival. Due to the clear benefits of early diagnosis and treatment of HF in diabetic patients, this expert panel has identified several priorities to raise awareness about importance of screening diabetic patients for subclinical diabetic cardiomyopathy (Table 2).
Table 2.
Strategies to improve heart failure management in patients with diabetes in the United Arab Emirates.
| Essential Needs to Improve Care for Diabetic Patients with Heart Failure in the UAE |
|---|
|
4. Diabetes pharmacotherapy from a HF management perspective
Co-existence of DM and HF poses significant challenges with regards to selection of diabetic medications. The use of sulfonylureas or thiazolidinediones should be avoided in patients with symptomatic HF and, similarly, insulins may cause sodium retention in addition to the inherent risk for hypoglycemia. However, new-generation insulins (degludec and glargine) are associated with improved safety profiles. Metiglinide use also has been associated with an increased risk of ischemic complications, especially in patients with known severe coronary artery disease. As such, cardiovascular safety of diabetic medications can have serious implications when selecting diabetic medications.
Earlier studies have shown that more aggressive glycemic control leading to lower HbA1c targets, predominantly with metformin and insulin, was associated with long-term reduction in microvascular diabetic complications, but not in mortality, nonfatal cardiovascular events, or heart failure [38]. As a result, DM pharmacotherapy has moved away from a glucocentric focus to a newer paradigm prioritizing improvement in cardiovascular events and clinical outcomes, facilitated by introduction of novel therapies that made it possible to curtail cardiovascular risk in this population, including glucagon-like peptide 1 (GLP) receptor agonists and sodium glucose co-transporter-2 (SGLT2) inhibitors. Furthermore, the availability of these medications paved the way for the development of cardiometabolic programs, where at-risk diabetic patients are offered lifestyle and pharmacologic interventions with proven benefits in improving long-term prognosis.
GLP-1 receptor agonists, including lixisenatide, liraglutide, semaglutide, exenatide, and albiglutide, represent a class of medications that have transformed treatment for diabetes. Use of these medications is associated with significant metabolic, cardiovascular and renal benefits, including reduction in cardiovascular and all-cause death, fatal and non-fatal stroke and myocardial infarction and HF hospitalizations, in addition other renal outcomes, including blunting of microalbuminuria, slower progression of renal dysfunction, and death due to renal causes [39].
SGLT2 inhibitors are another class of oral medications that exert anti-diabetic effects through blockade of renal tubular glucose reabsorption leading, in return, to glucosuria, natriuresis and osmotic diuresis [40]. Benefits of SGLT2 inhibitors extend beyond improving glycemic control and include modest reduction in body weight and blood pressure, improvement of whole-body sodium balance and volume status, improvement in endothelial function, reduction in vascular stiffness, and reduction in intraglomerular pressure and albuminuria. Several landmark clinical trials have established the role of SGLT2 inhibitors in contemporary management of patients with type 2 diabetes. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) was the first cardiovascular outcomes trial to investigate the effects of SGLT2 inhibition with empagliflozin on cardiovascular outcomes in type 2 DM and established atherosclerotic disease [41]. In this trial, empagliflozin met an exploratory end point of statistically significant reduction in HF hospitalizations versus placebo, with an absolute risk reduction of 1.4% and a relative risk reduction of 35%. The Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial was the first clinical trial powered to examine HF hospitalizations and cardiovascular death as primary end points in 17,160 type 2 diabetic patients with multiple cardiovascular risk factors or established atherosclerotic disease [42]. Dapagliflozin therapy reduced the composite of HF hospitalizations or cardiovascular death compared to placebo, which was primarily driven by a reduction in HF hospitalizations (27% relative risk reduction in compared to placebo). Benefit from dapagliflozin was consistent in patients with and without documented atherosclerotic disease and also in patients with and without prior HF. A meta-analysis of SGLT2 inhibitors landmark clinical trials in patients with diabetes collectively showed reduction in risk of major adverse cardiac events (HR 0.90, 95% [0.85–0.95]), cardiovascular death (HR 0.85, 95% [0.78–0.93]), HF hospitalization (HR 0.68, 95% [0.61–0.76]), and relevant renal endpoints (HR 0.62, 95% [0.56–0.70]) [43].
The consistent signal for improved HF outcomes in patients treated with SGLT2 inhibitors stimulated conduction of additional clinical trial dedicated to examining benefits of this class of medications in patients with HF, with or without presence of DM. The Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF) trial was specifically designed to evaluate the efficacy of SGLT2 inhibitors in patients with HF and reduced ejection fraction and showed 26% reduction in the risk of heart failure or death from cardiovascular causes, regardless of DM status [44]. Following that, results from the Empagliflozin outcome trial in patients with chronic heart failure with reduced ejection fraction, (EMPEROR-Reduced) trial showed that empagliflozin also reduced the composite risk of cardiovascular death and heart failure hospitalisation in patients with known heart failure with reduced ejection fraction, regardless of diabetes status [45].
The SOLOIST-WHF trial was the first to address the utilization of SGLT2 inhibitors in diabetic patients with acute heart failure hospitalization, with an almost equal distribution of sotagliflozin initiation before or after discharge. Once again, the composite end point of cardiovascular death, hospitalization of HF, as well urgent visits for HF was significantly reduced in sotagliflozin-treated patients compared with placebo [46].
Initial success with SGLT2 inhibitors fuelled expansion of their application to patients with HF heart failure and preserved ejection fraction. The EMPEROR-Preserved and the DELIVER trials each included approximately 6000 patients (both diabetic and non-diabetic) with LVEF >40% [47, 48]. Both trials showed significant reductions in end-points combining CV death and worsening HF, with DELIVER including urgent HF visits in the composite end-point and not just hospitalizations. There was a signal towards a decreasing benefit in the EMPEROR-Preserved trial as LVEF approached 60%. However, this was not seen in the DELIVER trial. Importantly, the DELIVER trial allowed the enrollment of hospitalized patients, expanding once again the applicability of this class to the acute setting.
Additionally, SGLT2 inhibitors are associated with improved renal outcomes in patients with DM and existing chronic renal dysfunction. The Dapagliflozin in Patients with Chronic Kidney Disease (DAPACKD) trial also showed a significant reduction in the trial’s primary outcome (a ≥50% decline in GFR, onset of end-stage kidney, or death from renal or cardiovascular years) among patients with chronic kidney disease treated with dapagliflozin, regardless of the presence or absence of diabetes [49].
4.1. Recommendations form major professional societies on treatment of diabetes
Several professional societies, including European Society of Cardiology (ESC), European Association for the Study of Diabetes (EASD), and American Diabetic Association (ADA) have updated their management guidelines for patients with diabetes and cardiovascular disease to include use of novel diabetic medications in diabetic patients and increased cardiovascular risk or those with established cardiovascular diseases [50, 51]. In addition, ADA gave a class A recommendation for use of SGLT2 inhibitors in patients with type 2 diabetes and established atherosclerotic cardiovascular disease, multiple atherosclerotic cardiovascular disease risk factors, or diabetic kidney disease, to reduce the risk of heart failure hospitalizations [50]. The American Association of Clinical Endocrinologists and American College of Endocrinology recommend anti-diabetic therapy based on the patient’s cardiac, cerebrovascular, and renal status, usually with a combination therapy involving agents with complementary mechanisms of action. In this regard, SGLT2i with improved glucosuric effect in terms of decreased A1C, weight, and systolic blood pressure is suggested in the treatment algorithm of diabetic patients with HF regardless of glycemic control.
Moreover, the Heart Failure Association (HFA) of ESC has issued a position paper on the role SGLT2 inhibitors in HF patients. Dapagliflozin or empagliflozin are recommended to reduce the combined risk of HF hospitalization and cardiovascular death in symptomatic patients with HF and reduced ejection fraction, already receiving guideline-directed medical therapy, regardless of the presence of type 2 diabetes mellitus [52].
5. Call to action
The burgeoning number of patients with type 2DM in the Middle East-Gulf region in general, and in the UAE in particular, demands a call to action to improve identification of diabetic patients who are at risk for development of cardiovascular complications, including HF. With recent breakthroughs in the realm of diabetes pharmacotherapies, physicians have a unique opportunity to improve health status and clinical outcomes for at-risk diabetic patients. However, institution of effective therapies in this population is predicated on proper and early identification of at-risk patients, which can only be achieved through collaborative and concerted efforts of various stakeholders involved in the care of diabetic patients.
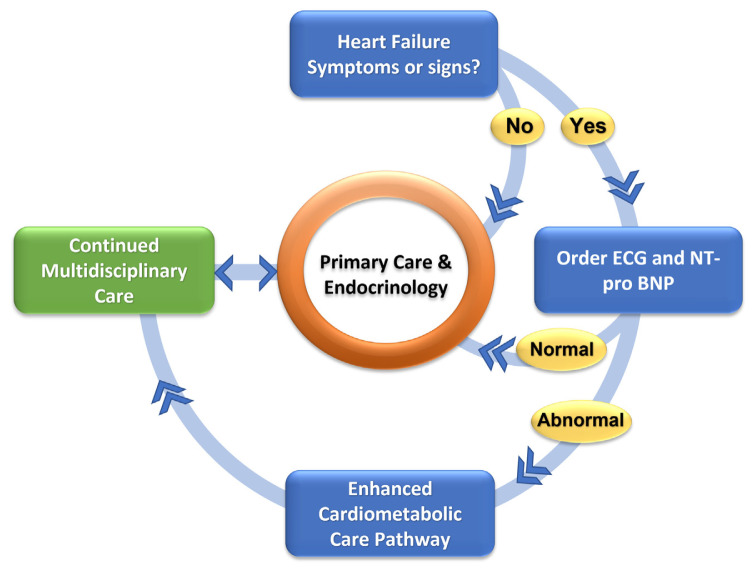
In accordance with these needs, the taskforce on early identification and treatment of HF in diabetic patients in the UAE proposes the framework for a national model of care that can be implemented to encompass most diabetic patients in UAE. This Group also proposes the following care pathway as a general framework for evaluation and management of diabetic patients with a suspected diagnosis of HF (Fig. 1).
Fig. 1.
Proposed framework for a collaborative care pathway for diabetic patients with or at-risk for HF.
Initial treatment for the majority of diabetic patients according to this model will continue to be delivered by primary care physicians and endocrinologists. Symptomatic patients with evidence for increased cardiovascular risk based on initial testing (ECG or cardiac biomarkers) will enter a care pathway that involves prompt evaluation by cardiovascular specialists in addition to intensification of medical therapy according to published practice guidelines and referral for advanced cardiovascular testing, as needed. This pathway will involve routine clinical assessment in addition to laboratory biomarkers of elevated cardiovascular risk (microalbuminuria, BNP and NT pro-BNP) and ECG. Echocardiography will also be utilized once evidence for covert cardiac dysfunction is identified.
Raising awareness about this care model through widespread educational campaigns targeting primary care providers and endocrinologists is of paramount importance. Such activities will highlight recent changes in the treatment paradigm for DM and will emphasize the benefits of early identification of high-risk diabetic patients who may benefit from early institution of effective therapies.
The proposed enhanced cardiometabolic care pathway has several potential implications on diabetic care in the MEG region. Establishing a collaborative relationship between primary care physicians and endocrinologists, on one hand, with cardiologists on the other hand offers an opportunity to optimize outcomes of at-risk diabetic patients through aggressive use of guideline-directed preventative medications, including statins, reninangiotensin-aldosterone system modulators, SGLT2 inhibitors, and anti-platelet medications, when appropriate. Furthermore, this pathway provides diabetic patients access to advanced anatomic and functional cardiac testing, which would facilitate earlier detection of coronary atherosclerosis and sub-clinical stages of cardiac systolic and diastolic dysfunction. Lastly, earlier involvement of various specialties in the care of complex patients, including those with DM and HF, enriches a culture of multidisciplinary care for such patients, placing greater emphasis on patient-centred care and shared decision making.
Naturally, a set of challenges may be encountered with the implementation of the enhanced cardiometabolic pathway. Firstly, adoption of this care pathway will likely be slow and erratic initially. This can be mitigated, however, with intensive educational programs targeting involved primary care physicians and endocrinologists that aim to raise familiarity with this concept, and ultimately broader adoption of the care pathway. Secondly, specialized care might not be readily accessible to all diabetic patients with evidence of early cardiac dysfunction under this care model, due to a variety of financial and geographical obstacles. Strong advocacy for coverage by payors and greater utilization of “virtual visits” can help mitigate these hurdles, respectively.
Whereas this proposed care model is intuitive, its efficacy in correctly identifying patients likely to benefit from further cardiac evaluation, optimizing downstream resource utilization and ultimately improving patients, health status and clinical outcomes needs to be formally tested in dedicated prospective clinical studies. Once this novel pathway is shown to be associated with improved care, it will certainly have a wide-ranging impact on the care of diabetic patients not only in the UAE, but likely in many of the other Gulf countries given similarities in patient demographics and in structure of healthcare systems in the region.
Acknowledgments
The authors acknowledge support from AstraZeneca in providing literature review prior to the writing of this document. The company (AstraZeneca) did not provide any editorial input or influence the recommendations made by the authors. No honoraria were provided for authorship or for contributing to this manuscript.
Abbreviations
- DM
Diabetes mellitus
- HF
Heart failure
- MEG
Middle East Gulf
- SGLT2
Sodium glucose co-transporter-2
Funding Statement
AstraZeneca provided limited support to perform literature review on the topic but did not provide editorial input on the manuscript or influence its content
Footnotes
Disclosures: Dr Wael Al Mahmeed received honoraria from AstraZeneca. The rest of the authors did not have any relevant financial disclosures.
Author contribution: Conception: WAM, FB. Lit review: FAB, LY, SAK, RH, YM. Investigation: not applicable. Resources: FAB, WAM, FB. Data collection: FAB, LY, RH, SAK, WAM, YM, FB. Writing original draft: FAB. Review and editing: LY, RH, SAK, WAM, FB. Visualization: FAB. Supervision: FAB, FB. Project administration: FAB.
Funding: No external funding was provided to support this project and no honoraria were provided for manuscript writing or editorial review. AstraZeneca provided limited support to perform literature review on the topic but did not provide editorial input on the manuscript or influence its content.
References
- 1.Al Busaidi N, Shanmugam P, Manoharan D. Diabetes in the Middle East: government health care policies and strategies that address the growing diabetes prevalence in the Middle East. Curr Diabetes Rep. 2019;19(2):8. doi: 10.1007/s11892-019-1125-6. [published Online First: 2019/02/05] [DOI] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P. Results from the international diabetes federation diabetes atlas. 9th edition. 2021. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045. [DOI] [PubMed] [Google Scholar]
- 3.Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus: impact of glucose-lowering agents, heart failure therapies, and novel therapeutic strategies. Circ Res. 2019;124(1):121–41. doi: 10.1161/CIRCRESAHA.118.311371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American heart association and the heart failure society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7) doi: 10.1161/CIR.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 5.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AlHabeeb W, Akhras K, AlGhalayini K, Al-Mudaiheem H, Ibrahim B, Lawand S, et al. Understanding heart failure burden in Middle East countries: economic impact in Egypt, Saudi Arabia and United Arab Emirates. Value Health. 2018;21:S123. doi: 10.1016/j.jval.2018.04.840. [DOI] [Google Scholar]
- 7. https://vizhub.healthdata.org/gbd-results/
- 8.Hassan M. Gulf CARE: heart failure in the Middle East. Glob Cardiol Sci Pract. 2015;2015(3):34. doi: 10.5339/gcsp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51. doi: 10.1016/j.jacc.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Kasznicki J, Drzewoski J. State of the art paper Heart failure in the diabetic population – pathophysiology, diagnosis and management. AOMS. 2014;3:546–56. doi: 10.5114/aoms.2014.43748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krum H, Skiba M, Wu S, Hopper I. Heart failure and dipeptidyl peptidase-4 inhibitors. Eur J Heart Fail. 2014;16(6):603–7. doi: 10.1002/ejhf.90. [DOI] [PubMed] [Google Scholar]
- 12.Liao HW, Saver JL, Wu YL. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. PubMed. 2021 doi: 10.1136/bmjopen-2016-013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scirica BM, Bhatt DL, Braunwald E. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. PubMed. 2021 [Google Scholar]
- 14.Singh S, Loke YK, Furberg CD. Thiazolidinediones and heart failure: a teleo-analysis. PubMed. 2021 doi: 10.2337/dc07-0141. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27) doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 16.Negishi T, Marwick TH. Prediction of heart failure in patients with type 2 diabetes mellitus-a systematic review and meta-analysis. PubMed. 2021 doi: 10.1016/j.diabres.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 17.de Santiago A, García-Lledó A, Ramos E, Catalina S. Valor pronóstico del electrocardiograma en pacientes con diabetes tipo 2 sin enfermedad cardiovascular conocida. Rev Española Cardiol. 2007;60(10):1035–41. doi: 10.1157/13111235. [DOI] [PubMed] [Google Scholar]
- 18.Huelsmann M, Neuhold S, Resl M. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. PubMed. 2021 doi: 10.1016/j.jacc.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 19.Clodi M, Resl M, Neuhold S. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. PubMed. 2021 doi: 10.1177/1741826711420015. [DOI] [PubMed] [Google Scholar]
- 20.Willeit P, Kaptoge S, Welsh P, Butterworth AS, Chowdhury R, Spackman SA, et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol. 2016;4(10):840–9. doi: 10.1016/S2213-8587(16)30196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarolim P, White WB, Cannon CP. Serial measurement of natriuretic peptides and cardiovascular outcomes in patients with type 2 diabetes in the EXAMINE trial. 2018;41:6. doi: 10.2337/dc18-0109. [DOI] [PubMed] [Google Scholar]
- 22.Liu JE, Robbins DC, Palmieri V, Bella JN, Roman MJ, Fabsitz R, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes. J Am Coll Cardiol. 2003;41(11):2022–8. doi: 10.1016/S0735-1097(03)00403-0. [DOI] [PubMed] [Google Scholar]
- 23.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–6. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 24.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJV, Yusuf S, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113(5):671–8. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 25.Nayor M, Larson MG, Wang N, Santhanakrishnan R, Lee DS, Tsao CW, et al. The association of chronic kidney disease and microalbuminuria with heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2017;19(5) doi: 10.1002/ejhf.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dries DL, Exner DV, Domanski MJ. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. PubMed. 2021 doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 27.Negishi K. Echocardiographic feature of diabetic cardiomyopathy: where are we now? Cardiovasc Diagn Ther. 2018;8(1):47–56. doi: 10.21037/cdt.2018.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jørgensen PG, Jensen MT, Mogelvang R, von Scholten BJ, Bech J, Fritz-Hansen T, et al. Abnormal echocardiography in patients with type 2 diabetes and relation to symptoms and clinical characteristics. Diabetes Vasc Dis Res. 2016;13(5):321–30. doi: 10.1177/1479164116645583. [DOI] [PubMed] [Google Scholar]
- 29.Devereux RB, Roman MJ, Paranicas M, OGrady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the strong heart Study. Circulation. 2000;101(19):2271–6. doi: 10.1161/01.CIR.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 30.Galderisi M, Anderson KM, Wilson PWF, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (The Framingham Heart Study) Am J Cardiol. 1991;68(1):85–9. doi: 10.1016/0002-9149(91)90716-X. [DOI] [PubMed] [Google Scholar]
- 31.Liu JH, Chen Y, Yuen M. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. 2021 doi: 10.1186/s12933-016-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies MJ, DAlessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the Study of diabetes (EASD) Diabetes Care. 2018;41(12):2669–701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bain S, Cummings M, McKay G. Multidisciplinary approach to management and care of patients with type 2 diabetes mellitus. European medical journal. 2021 [Google Scholar]
- 34.Arnold JMO, Howlett JG, Ducharme A, Ezekowitz JA, Gardner MJ, Giannetti N, et al. Canadian Cardiovascular Society Consensus Conference guidelines on heart failure–2008 update: best practices for the transition of care of heart failure patients, and the recognition, investigation and treatment of cardiomyopathies. Can J Cardiol. 2008;24(1):21–40. doi: 10.1016/S0828-282X(08)70545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gravely S, Ginsburg L, Stewart DE, Mak S, Grace SL. Referral and use of heart failure clinics: what factors are related to use? Can J Cardiol. 2012;28(4):483–9. doi: 10.1016/j.cjca.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAlister FA, Stewart S, Ferrua S, McMurray JJJV. Multidisciplinary strategies for the management of heart failure patients at high risk for admission. J Am Coll Cardiol. 2004;44(4):810–9. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 37.Manla Y, Ghalib HH, Badarin FA, Ferrer R, Lee-St John T, Abdalla K, et al. Implementation of a multidisciplinary inpatient heart failure service and its association with hospitalized patient outcomes: first experience from the Middle East and North Africa region. Heart Lung. 2023;61:92–7. doi: 10.1016/j.hrtlng.2023.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [published Online First: 1998/09/22] [PubMed] [Google Scholar]
- 39.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85. doi: 10.1016/s2213-8587(19)30249-9. [published Online First: 2019/08/20] [DOI] [PubMed] [Google Scholar]
- 40.Pradhan A, Vohra S, Vishwakarma P, Sethi R. Review on sodium-glucose cotransporter 2 inhibitor (SGLT2i) in diabetes mellitus and heart failure. J Fam Med Prim Care. 2019;8(6):1855–62. doi: 10.4103/jfmpc.jfmpc_232_19. [published Online First: 2019/07/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 42.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 43.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–58. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [published Online First: 2019/09/20] [DOI] [PubMed] [Google Scholar]
- 45.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomeswith empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 46.Shah SR, Ali A, Ikram S. Sotagliflozin and decompensated heart failure: results of the SOLOIST-WHF trial. Expet Rev Clin Pharmacol. 2021;14(5):523–5. doi: 10.1080/17512433.2021.1908123. [published Online First: 2021/03/26] [DOI] [PubMed] [Google Scholar]
- 47.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61. doi: 10.1056/NEJMoa2107038. [published Online First: 2021/08/28] [DOI] [PubMed] [Google Scholar]
- 48.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98. doi: 10.1056/NEJMoa2206286. [published Online First: 2022/08/27] [DOI] [PubMed] [Google Scholar]
- 49.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 50.9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S98–110. doi: 10.2337/dc20-S009. [published Online First: 2019/12/22] [DOI] [PubMed] [Google Scholar]
- 51.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [published Online First: 2019/09/10] [DOI] [PubMed] [Google Scholar]
- 52.Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(9):1495–503. doi: 10.1002/ejhf.1954. [published Online First: 2020/07/04] [DOI] [PubMed] [Google Scholar]