Abstract
Targeted therapies have revolutionized treatment for metastatic non-small cell lung cancer (NSCLC) with oncogenic driver mutations. However, challenges arise in managing concurrent mutations and overcoming resistance. We present the case of a patient with epidermal growth factor receptor (EGFR) (L747_A750delinsP exon19 deletion) and mesenchymal-epithelial transition factor (MET) mutations (D1228H, D1228N, D1228Y, Y1230H, MET amplification) who achieved a durable response to amivantamab (14 months ongoing) after progression on multiple lines of therapy including platinum-based chemotherapy, EGFR tyrosine kinase inhibitors (TKI) and combination TKI and MET inhibitors. This case highlights the utility of longitudinal next-generation sequencing (NGS) testing to identify acquired resistance and the need for continued research into understanding mechanisms of resistance to help develop future treatment strategies.
Keywords: Non-small cell lung cancer, amivantamab, EGFR mutation, MET mutation
Introduction:
Targeted therapies are integral in managing metastatic non-small cell lung cancer (NSCLC) with oncogenic driver mutations. Roughly one-third of patients with advanced NSCLC harbor epidermal growth factor receptor (EGFR) mutations.1 Mutations in EGFR lead to constitutive activation of tyrosine kinase (TK) signaling pathways causing unregulated cell growth. Targeting mutated EGFR with tyrosine kinase inhibitors (TKI) is the preferred first-line treatment for advanced NSCLC. However, challenges arise over the eventual resistance of targeted therapies.
Various mechanisms of acquired resistance to classes of EGFR TKI have been established. Roughly 5–22% of patients develop resistance through MET amplification, bypassing TK through activation of the mesenchymal-epithelial transition (MET) kinases pathway.2 Strategies for overcoming this resistance include EGFR and MET concomitant inhibition. However, resistance often develops even with dual inhibition. Acquired MET kinase domain mutations, as seen in the MET D1228N mutation, lead to resistance by affecting the binding and stabilization of MET kinase inhibitors.3 In a case report, MET D1228N was described as a mechanism for acquired resistance to dual TKI and MET inhibition.4
While there is no standard treatment after progression on dual EGFR and MET inhibition, amivantamab may be able to fill that role in the future. Amivantamab is a fully humanized bispecific monoclonal antibody that concurrently binds to EGFR and MET factor leading to receptor inactivation and inhibition of cell signaling pathways.5 In May 2021, it received FDA approval for EGFR exon 20 insertional for patients who progressed after treatment with platinum-based chemotherapy based on results of the CHRYSALIS study, which showed improvement in overall response rate and duration of response.5
Here, we present the case of a patient with EGFR and MET mutations who achieved a durable response to amivantamab after progression on multiple lines of therapy, including platinum-based chemotherapy, TKIs, and combination TKI and MET inhibitors.
Case presentation
A 47-year-old woman with no smoking history but a family history of lung cancer was diagnosed with lung adenocarcinoma after several months of dyspnea, dry cough, and failed treatment with antibiotics for presumed pneumonia. A computed tomography (CT) scan showed a 2.5 cm left lower lobe nodule, and a biopsy was performed in late 2015, as shown in Figure 1. Pathology showed the nodule was adenocarcinoma that was +TTF1, +NapsinA, - p63, and had an EGFR exon 19 deletion detected by polymerase chain reaction (PCR) in 80% of the tumor. She completed staging with a positron emission tomography (PET) scan, endobronchial ultrasonography, and fine need aspirate that showed disease in one 4R lymph node, giving her an initial stage of IIIB (T4N3M0).
Figure 1. Patient Timeline.
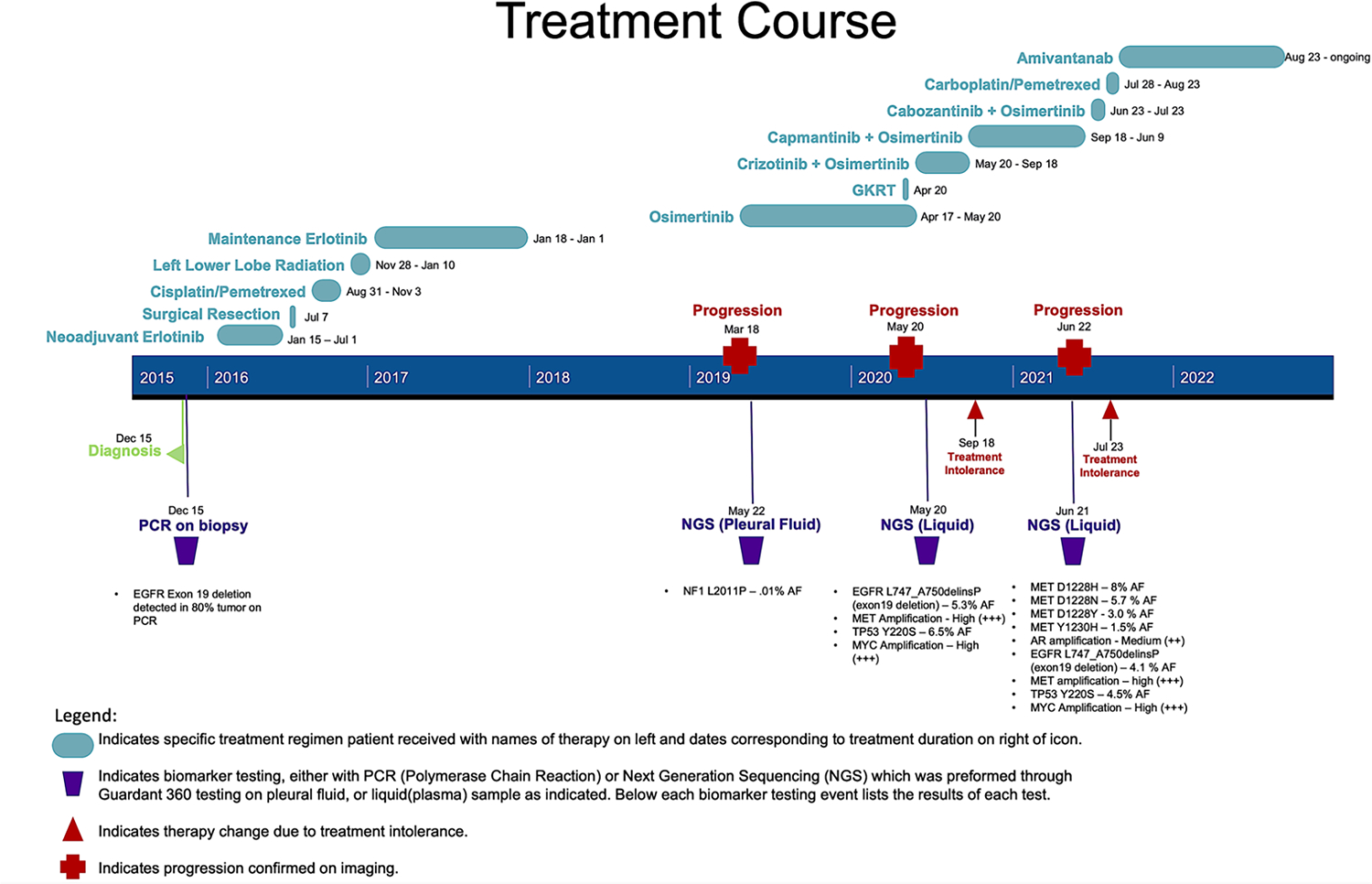
This timeline displays the pertinent diagnoses, DNA sequencing, treatments, procedures, and progressions of this patient’s course.
Her case was presented at a multi-disciplinary thoracic tumor board, and she was started on neoadjuvant erlotinib. Her 3-month PET showed excellent treatment response with no uptake in the previous hypermetabolic nodes. Because of her response, she underwent a left lower lobectomy and mediastinal lymph node dissection to attempt to remove any remaining tumor. Unfortunately, pathology from her lymph nodes showed residual ypT1aN1 disease in 3/4 nodes. After surgery, she underwent four cycles of consolidation chemotherapy with cisplatin/pemetrexed followed by radiation to the left lung and mediastinum. She then completed one year of maintenance erlotinib.
After completing this year of erlotinib, she had 13 months without any signs of recurrence. Unfortunately, she developed a persistent cough that prompted a CT chest and PET scan, which confirmed the presence of recurrent disease with mediastinal adenopathy and a new pleural effusion. She underwent thoracentesis, and cytology of the pleural fluid was positive for adenocarcinoma. Guardant was sent on pleural fluid, which did not reveal any mutations. One month later, she developed headaches, and an MRI brain showed cerebellar lesions consistent with metastatic disease. Given her presumed acquired resistance to first-generation EGFR-TKIs, she was started on Osimertinib and underwent gamma knife radiation therapy for brain lesions.
After initiating osimertinib, she had a partial response and 13 months of progression-free disease. However, she then developed back pain, and an MRI spine revealed suspicious new soft tissue lesions near her thoracic spine. A PET scan confirmed extensive metastatic disease with new hypermetabolic activity in the left lung pleural along with left axillary, left supraclavicular, para-aortic, right internal mammary, and juxtadiaphragmatic lymph nodes. There were also nodules with increased activity in the superficial soft tissues of the left flank and posterior abdominal wall musculature.
Due to concern that the patient had developed another resistance mechanism, next-generation sequencing (NGS) was sent with a guardant 360 liquid biopsy. Results of NGS identified a new mutation with a MET amplification, so crizotinib was added to her current regimen of osimertinib. She had significant diarrhea and fatigue with this combination, and after four months, she transitioned from crizotinib to capmantinib to try to alleviate her symptoms.
On the combination of osimertinib and capmantinib, she had stable disease for nine months until she developed new dyspnea. PET scan done at this time showed mixed response to her treatment, but overall disease progression given new hypermetabolic lesions in the neck, thorax, abdomen, pelvis, and peritoneum. Given her progression, repeat NGS with guardant 360 liquid was sent, which identified a MET amplification and new MET mutations in D1228 and Y1230. Based on this, capmantinib was replaced with cabozantinib, and she continued osimertinib. However, she experienced severe diarrhea, and treatment was stopped after one month.
With limited remaining options to target her EGFR mutation and multiple MET mutations, she started amivantamab. Due to delays in insurance approval of this drug, she received one cycle of carboplatin/pemetrexed before initiating amivantamab. Since starting amivantamaba, she has had a partial response based on surveillance imaging and continues to do well with no evidence of recurrence. At the time of this article, she has been on amivantamab for 14 months.
Discussion:
In this case report, we describe a patient with an EGFR mutation who developed multiple mechanisms of acquired resistance to targeted treatment with MET amplification followed by MET mutations. Dynamic NGS testing allowed these changes to be monitored and her treatment regimen to be tailored based on her resistance.
While patients with EGFR mutations have improved survival when treated with TKI, they inevitably develop resistance. Repeating NGS after progression or resistance is identified can help identify resistance mechanisms and make appropriate therapy changes to overcome them.
MET amplification has been identified as a common resistance mechanism to EGFR TKI by activating downstream RAS/ERK/MAPK and PI3K-AKT signaling pathways allowing for cell proliferation and continued survival.6 There are several strategies for overcoming resistance to MET amplification and mutations described in the literature. The TATTON trial showed that combining EGFR TKI with MET inhibition, for example, with osimertinib and savolitinib, improves patient outcomes.6 A case report by Wang et al. provides another potential way to overcome MET mutations as they described a short-term clinical benefit in a patient initially treated with cabozantinib after they developed resistance to crizotinib through a MET D1228N mutation.7
Amivantamab is another promising way to overcome MET mutations. It is a bispecific antibody to EGFR and MET that inhibits ligand-induced activation by blocking both EGFR and cMET and causing receptor degradation. While it is FDA approved for NSCLC with an EGFR exon 20 insertion, data from the Phase I CHRYLASIS trial also showed a benefit in patients with a MET exon 14 skip mutation, showing that it has a MET specific mechanism as well, which may expand its clinical utility. 8–9 The treatment responses seen in the trial are consistent with the benefit presented in our case report.
Novel drugs are currently being designed to overcome MET resistance which develops in EGFR mutated NSCLC. Telisotuzumab vedotin, is an antibody-drug conjugate (ADC), targeting cMET conjugated to a microtubule inhibitor.10 Phase I studies showed a response rate of 18% in patients with c-MET positive squamous cell NSCLC.11 Recent results from the phase II SWOG S1400K trial with Telisotuzumab vedotin failed to meet appropriate response rates for continuing.11 Other ADCs designed to overcome MET resistance in EGFR mutated patients are currently being tested in preclinical studies, including REGN5093-M114, a MET×MET biparatopic antibody.12 In preclinical studies, patient derived xenografts (PDX) were developed from samples with acquired MET amplifications in EGFR mutated NSCLC. Treatment with REGN5093-M114 in those PDX led to tumor regression.12 More data needs to be obtained to determine if patients with similar resistance mechanism will derive the same benefit.
In this case report, we demonstrated a patient with EGFR mutated NSCLC who developed complex MET and obtained a durable response after initiating treatment with amivantamab. More research is needed to develop novel treatments for patients with these resistance mechanisms, however, there is hope other patients will experience similar clinical benefit to the currently available targeted treatments.
Figure 2:
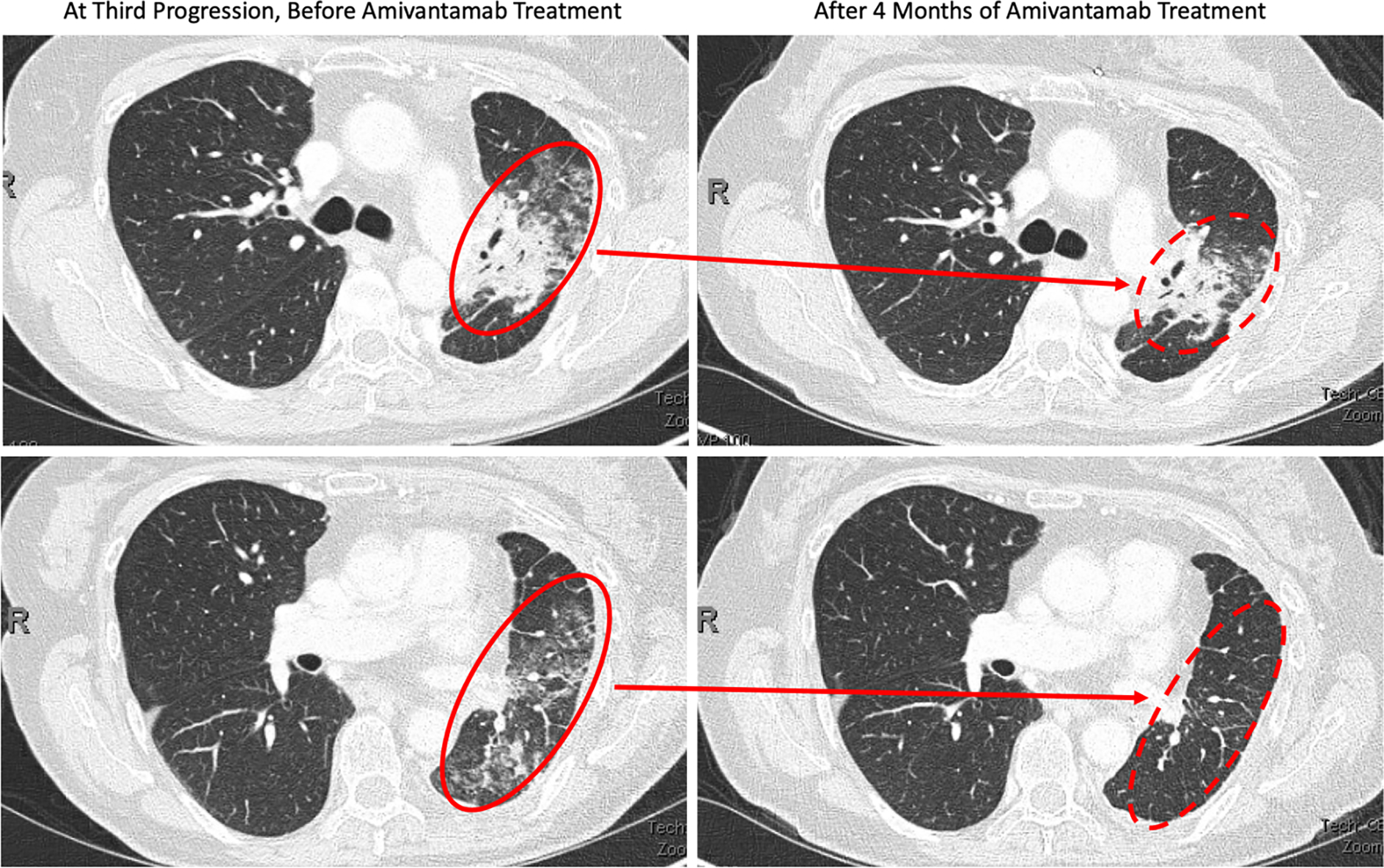
The above CT Chest images were obtained during the patient’s treatment course. The two images on the left show significant groundglass opacities in the left lung (solid red circle) corresponding with progression of disease in June 2021. The two images on the right were obtained in Dec 2021 after 4 months of amivantamab treatment. The top right image shows decrease size of disease related opacities (dashed red circle) and the bottom right image shows near resolution of previous disease findings (dashed red circle).
Highlights from our case report include:
The use of dynamic next-generation sequencing from various sources over the course of the disease to guide treatment against new mutations.
The patient’s response to amivantamab suggest it does have activity against MET mutations as well.
This patient’s exceptional response to amivantamab after failing many prior treatments may help guide other clinicians who face similar challenges.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. Nov 29 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Yang S, Wang K, Sun SY. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. Jun 21 2019;12(1):63. doi: 10.1186/s13045-019-0759-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recondo G, Bahcall M, Spurr LF, et al. Molecular Mechanisms of Acquired Resistance to MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14-Mutant NSCLC. Clin Cancer Res. Jun 1 2020;26(11):2615–2625. doi: 10.1158/1078-0432.CCR-19-3608 [DOI] [PubMed] [Google Scholar]
- 4.Piper-Vallillo AJ, Halbert BT, Rangachari D, Kobayashi SS, Costa DB. Acquired Resistance to Osimertinib Plus Savolitinib Is Mediated by MET-D1228 and MET-Y1230 Mutations in EGFR-Mutated MET-Amplified Lung Cancer. JTO Clin Res Rep. Nov 1 2020;1(4):100071. doi: 10.1016/j.jtocrr.2020.100071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syed YY. Amivantamab: First Approval. Drugs. Jul 2021;81(11):1349–1353. doi: 10.1007/s40265-021-01561-7 [DOI] [PubMed] [Google Scholar]
- 6.He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rdgeneration EGFRTKI resistance in advanced non-small cell lung cancer (Review). Int J Oncol. Nov 2021;59(5)doi: 10.3892/ijo.2021.5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Chen Z, Han X, Li J, Guo H, Shi J. Acquired MET D1228N Mutations Mediate Crizotinib Resistance in Lung Adenocarcinoma with ROS1 Fusion: A Case Report. Oncologist. Mar 2021;26(3):178–181. doi: 10.1002/onco.13545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazel D, Nagasaka M. Spotlight on Amivantamab (JNJ-61186372) for EGFR Exon 20 Insertions Positive Non-Small Cell Lung Cancer. Lung Cancer (Auckl). 2021;12:133–138. doi: 10.2147/LCTT.S337861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol. 2021;39(30):3391–3402. doi: 10.1200/JCO.21.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard N. New Strategies and Novel Combinations in EGFR TKI-Resistant Non-small Cell Lung Cancer. Curr Treat Options Oncol. 2022;23(11):1626–1644. doi: 10.1007/s11864-02201022-7 [DOI] [PubMed] [Google Scholar]
- 11.Waqar SN, Redman MW, Arnold SM, et al. A Phase II Study of Telisotuzumab Vedotin in Patients With c-MET-positive Stage IV or Recurrent Squamous Cell Lung Cancer (LUNG-MAP Sub-study S1400K, NCT03574753). Clin Lung Cancer. 2021;22(3):170–177. doi: 10.1016/j.cllc.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh SY, Lee YW, Lee EJ, et al. Preclinical Study of a Biparatopic METxMET Antibody-Drug Conjugate, REGN5093-M114, Overcomes MET-driven Acquired Resistance to EGFR TKIs in EGFR-mutant NSCLC. Clin Cancer Res. 2023;29(1):221–232. doi: 10.1158/1078-0432.CCR-22-2180 [DOI] [PubMed] [Google Scholar]


