Abstract
The p38 mitogen-activated protein kinase (p38MAPK) is activated in response to various stimuli, including cellular stress, inflammatory cytokines and cell surface receptors. The activation of p38MAPK is predominantly mediated by the two upstream MAPK kinases MKK3 and MKK6. To study the role of the p38MAPK pathway in vivo, we generated Mkk6–/– mice. We examined whether T-cell apoptosis is affected in these mice and in our previously reported Mkk3–/– mice. Strikingly, in vivo deletion of double positive thymocytes in Mkk6–/– mice was impaired, whereas Mkk3–/– mice showed no apparent abnormality. Conversely, CD4+T cells from Mkk3–/– but not from Mkk6–/– mice were resistant to activation-induced cell death and cytokine-withdrawal-induced apoptosis. In peripheral CD4+T cells, MKK3 is induced upon stimulation, whereas MKK6 is downregulated. These results suggest a novel mechanism regulating T-cell apoptosis differentially through the p38MAPK pathway by MKK3 and MKK6.
INTRODUCTION
The mitogen-activated protein kinase (MAPK) pathways transduce a variety of extracellular signals through a cascade of protein phosphorylation. In mammals, there are at least three genetically distinct groups of MAPK pathways, including extracellular-signal-regulated kinases (ERKs), the c-Jun N-terminal kinases (JNKs) and the p38MAPKs (Davis, 2000). The JNKs and the p38MAPKs are activated by environmental stress, such as UV irradiation, and by inflammatory cytokines. The p38MAPK pathway has been reported to function in the regulation of cytokine production, T- and B-cell proliferation and differentiation, and apoptosis (Nebreda and Porras, 2000; Rincón et al., 2001).
Molecular cloning studies have identified four mammalian p38MAPK isoforms: p38α, p38β, p38γ and p38δ (Enslen et al., 2000). The specific upstream activator kinases for p38MAPK are MKK3 and MKK6, although MKK4, known to be an activator of JNKs, has been implicated in p38MAPK activation in vitro (Davis, 2000). We reported that Mkk3–/– mice manifested a defect in antigen-presenting cell function (Lu et al., 1999). The existence of multiple upstream kinases for the p38MAPK begs the question whether these activating enzymes have overlapping or distinct functions.
To study the physiological role of p38MAPK and the contribution of MKK3 and MKK6 into T-cell development and cell death, we generated Mkk6–/ – mice. Here, we report that MKK6 is the key in vivo upstream kinase for p38MAPK in the thymus, whereas MKK3 is the predominant activator in activated peripheral CD4+T cells. Thus, these two enzymes function differently, at least in part, in these two compartments of the immune system.
RESULTS
Generation of Mkk6–/– mice
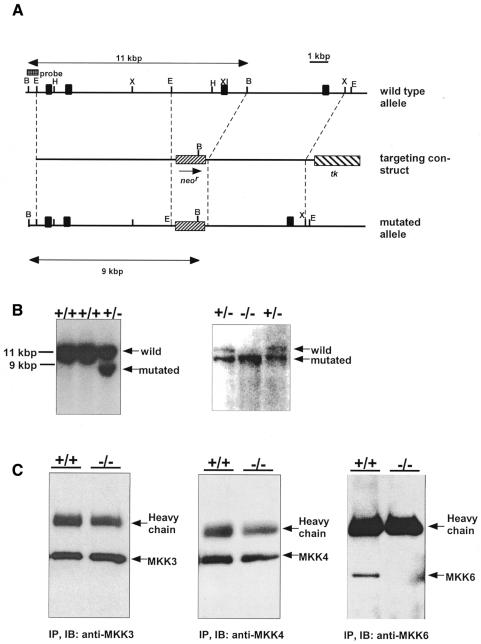
Mice carrying a null mutation in the Mkk6 gene were generated using homologous recombination in embryonic stem (ES) cells (Figure 1A and B, left). Three targeted ES clones were injected into C57BL/6 blastocysts, and all three resulted in chimeras that transmitted the mutated Mkk6 allele through the germline. Crosses of the Mkk6+/– mice resulted in progeny with the expected Mendelian frequencies. Null mutation of MKK6 was confirmed by Southern blot analysis of tail DNA (Figure 1B, right) and western blotting of splenocyte lysate using an anti-MKK6 antibody directed against the N-terminus (Figure 1C, right). Mkk6–/– mice expressed normal levels of MKK3 and MKK4 proteins (Figure 1C) and, therefore, there were no compensatory changes in the expression of these other kinases. The Mkk6–/– mice were viable and fertile, with no detected developmental abnormality. Histological analyses including thymus, lung, spleen, liver and kidney revealed no obvious abnormalities. The knockout mice had a comparable number of thymocytes and splenocytes with similar expression of CD3, CD4, CD8, CD25, CD69 TCRα/β and B220 (data not shown). Therefore, no obvious abnormalities in the lymphoid compartments were observed in Mkk6–/– mice.
Fig. 1. Disruption of the Mkk6 gene by homologous recombination. (A) Structure of the targeted vector and the Mkk6 gene. Restriction enzyme sites (H, HindIII; X, XbaI; B, BamHI; E, EcoRI), exons (closed boxes) and the probe for Southern blots are indicated. Subdomain XI (XI) is a catalytic domain required for MKK6 activation. (B) Southern blots. Genomic DNA from ES cells (left) and tails (right) were digested with BamHI. The wild-type and the mutated allele corresponds to an 11- and a 9-kb fragment, respectively. (C) Western blots. Equal amounts of protein lysates from splenocytes were used.
MKK6 but not MKK3 contributes to the deletion of thymocytes in vivo and in vitro
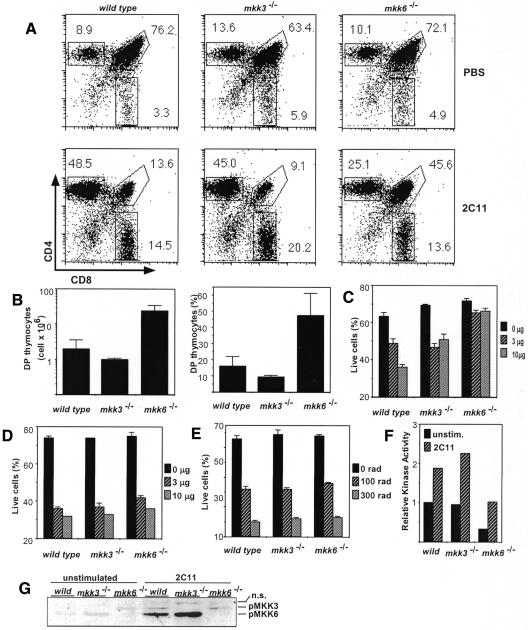
Previous studies indicated a possible association of cell death with the p38MAPK pathway. Since the JNK pathway is known to be involved in thymocyte cell death (Rincón et al., 1998a; Sabapathy et al., 2001), we considered it possible that CD4CD8 double positive (DP) thymocyte apoptosis is also regulated by p38MAPK. To investigate the role of p38MAPK pathways, especially the two MAPK kinases MKK3 and MKK6 in thymocyte apoptosis, we analyzed the deletion of DP thymocytes induced by anti-CD3 mAb treatment in vivo. Forty-eight hours after the administration of antibody, the DP population was extensively eliminated in wild-type mice (Figure 2A, left). In contrast, a higher proportion (∼3- to 4-fold) of DP thymocytes remained viable in Mkk6–/– mice (Figure 2A, right). The fact that the inhibition mediated by MKK6 deficiency was incomplete is interesting, since similar partial inhibition was observed in mice transgenic for a dominant-negative JNK1 (Rincón et al., 1998a) and mice deficient in JNK (Sabapathy et al., 2001). Surprisingly, unlike Mkk6–/– mice, the elimination of DP cells in Mkk3–/– mice was similar to that of wild-type mice (Figure 2A, middle). The inhibition of anti-CD3 mAb-mediated DP cell death in Mkk6–/– mice was ∼3-fold higher, not only in percentage terms but also in the absolute number of surviving DP cells, than that in wild-type and Mkk3–/– mice (Figure 2B). In addition, thymocytes from Mkk6–/– mice were also resistant to in vitro stimulation (Figure 2C). Thymocytes from Mkk6–/– mice showed a severe reduction in p38MAPK activity (Figure 2F). Accordingly, the dominant p38MAPK kinase in wild-type thymocytes was MKK6 (Figure 2G). On the other hand, apoptosis induced by anti-Fas mAb and γ-irradiation was only marginally affected by MKK6 (Figure 2D and E). Thus, thymocytes from Mkk6–/– mice were partially protected from anti-CD3 mAb-mediated deletion in vivo and in vitro.
Fig. 2. (A) Deletion of DP cells by anti-CD3 mAb in vivo administration is impaired in Mkk6–/– but not in Mkk3–/– mice. Mice were intraperitoneally injected with anti-CD3 mAb (2C11, 50 µg). Two days after injection, thymocytes were stained and CD4 and CD8 expression in the live population was analyzed. Numbers represent the percentage of live cells in each gate, CD4+CD8+ (upper right) and single CD8+ (lower) and single CD4+ (upper left). (B) Absolute number (left) and percentage (right) of DP thymocytes 2 days after 2C11 in vivo administration. (C–E) Thymocyte death in vitro by anti-CD3 (C), anti-Fas mAbs (D) and γ-irradiation (E). Thymocytes are stimulated with indicated doses of plate-bound mAb (C and D) or γ-irradiated and analyzed for cell viability 20 h later. (F) Decreased p38MAPK activity in thymocytes from Mkk6–/– mice. Thymocytes were stimulated in vitro by 2C11 for 90 min and subjected to the in vitro kinase assay. (G) MKK3/6 activation by anti-CD3 mAb stimulation in vitro. Activated MKK3/6 was detected by western blotting. pMKK3/6 and n.s. indicate phosphorylated-MKK3/6 and non-specific band signals, respectively.
MKK3 but not MKK6 is involved in activated peripheral CD4+T-cell death
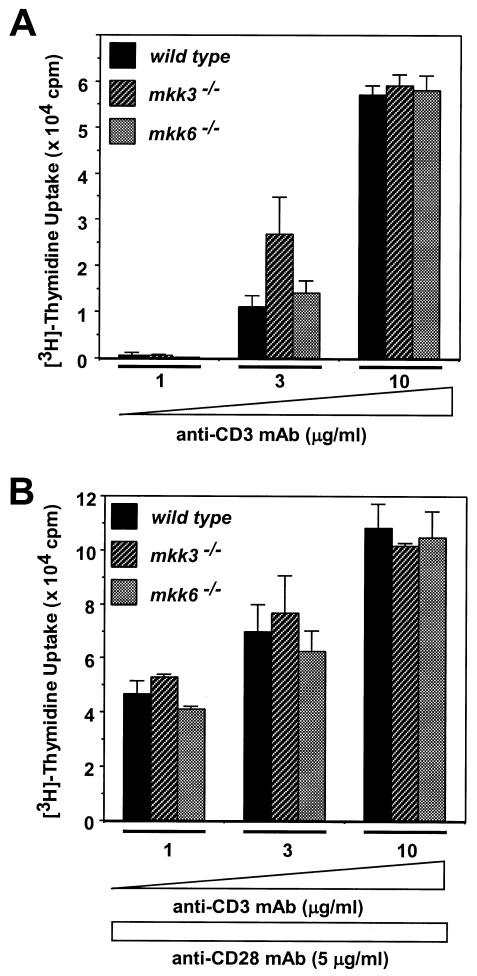
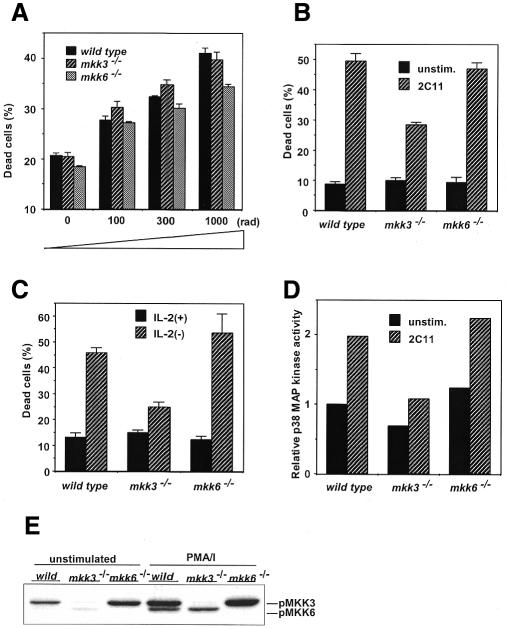
p38MAPK activity has also been implicated in peripheral CD4+T-cell function (Rincón et al., 1998b). To examine the involvement of MKK3 and MKK6 in peripheral T cells, we first analyzed CD4+T-cell proliferation. When optimal amounts of anti-CD3 mAb, or anti-CD3 plus anti-CD28 mAbs, were used, no significant differences in proliferation were observed, suggesting that there was no significant difference in proliferation among Mkk3–/–, Mkk6–/– and wild-type mice (Figure 3). We then examined the involvement of MKK3 and MKK6 in peripheral T-cell death. Freshly prepared peripheral CD4+T cells were γ-irradiated at the indicated doses (Figure 4A). At doses of 300 and 1000 rad, a small but significant difference in the susceptibility to cell death was observed in cells from Mkk6–/– mice. We then analyzed activation-induced cell death (AICD). CD4+T cells were stimulated by plate-bound anti-CD3 and anti-CD28 mAbs. After 3 days, cells were washed extensively and further cultured for one more day in IL-2, since IL-2 is known to induce susceptibility to cell death in AICD. Live cells were stimulated, for a second time, by anti-CD3 mAb. CD4+T cells from wild-type mice showed a cell death frequency of 49% (Figure 4B). However, cells from Mkk3–/– mice were significantly more resistant to cell death (28%). Interestingly, CD4+T cells derived from Mkk6–/– mice showed a similar level of cell death as wild-type mice. These results indicate that AICD of CD4+T cells is regulated by MKK3 but not MKK6. To examine whether MKK3 is involved in CD4+T-cell death mediated by stimuli other than antigen receptor, we analyzed apoptosis induced by cytokine withdrawal. Cells were initially stimulated with plate-bound anti-CD3 and anti-CD28 mAbs. At the end of the culture period these cells are growing in an IL-2-dependent manner and deprivation of IL-2 induces apoptosis. Approximately 47% of the CD4+T cells from wild-type mice underwent apoptosis upon IL-2 withdrawal (Figure 4C). Cells derived from Mkk6–/– mice showed a slightly higher but not statistically significantly different apoptosis rate compared with the wild-type control. However, CD4+T cells derived from the Mkk3–/– mice showed significantly reduced apoptosis (25%) compared with the cells from Mkk6–/– and wild-type control mice. p38MAPK activity in CD4+T cells derived from the Mkk3–/– mice was significantly reduced compared with those from Mkk6–/– and wild-type control mice (Figure 4D), and the dominant p38MAPK kinase in activated CD4+T cells was MKK3 (Figure 4E). These results indicate that, although unstimulated CD4+T-cell death is partially regulated by MKK6, activated CD4+T-cell apoptosis is at least partially regulated by MKK3 but not by MKK6.
Fig. 3. Neither MKK3 nor MKK6 is required for maximal proliferation of CD4+T cells. Cells are stimulated with anti-CD3 mAb alone (A) or anti-CD3 plus anti-CD28 mAbs (B) for 72 h. Proliferation was assayed by determining the incorporation of [3H]thymidine (c.p.m.).
Fig. 4. Differential susceptibility to apoptosis between CD4+T cells from Mkk6–/– and Mkk3–/– mice. (A) Freshly prepared CD4+T cells are γ-irradiated. (B and C) CD4+T cells were stimulated with anti-CD3/CD28 mAbs for 3 days. Cells are restimulated by anti-CD3 mAb (B) or cultured in the absence of IL-2 (C) for 20 h and analyzed for viability. (D) Impaired p38MAPK activity in CD4+T cells from Mkk3–/– mouse. Cells were stimulated by anti-CD3 mAb for 90 min and determined for p38MAPK activity. (E) MKK3 and MKK6 activation by PMA/ionomycin (PMA/I) stimulation. Cells were stimulated in vitro for 15 min and examined for activated MKK3/6.
Differential control mechanism between MKK3 and MKK6 protein in CD4+T cells
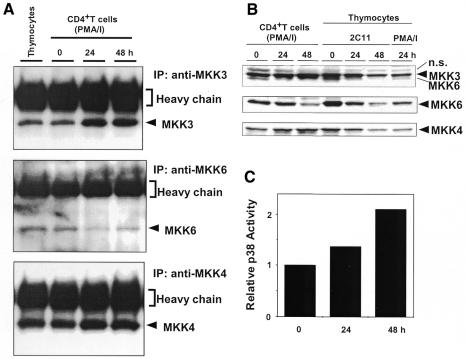
We reported previously that expression of JNK1 and JNK2 was significantly increased when CD4+T cells are stimulated with PMA plus ionomycin, a stimulation that mimics maximal antigen receptor signaling (Weiss et al., 2000). To test whether MKK3 and MKK6 are also regulated, we analyzed the effect of T-cell stimulation on MKK3/6 expression levels using immunoblot analysis. Expression of MKK3 and MKK6 was detected in unstimulated CD4+T cells (Figure 5A and B). However, MKK3 was significantly increased upon stimulation with PMA and ionomycin. Interestingly, in sharp contrast to MKK3, MKK6 was reproducibly decreased. We also examined a JNK kinase, MKK4, which also has some p38MAPK kinase activity. MKK4 was slightly increased upon stimulation. These characteristic changes in the levels of MAPKs result in enhanced p38MAPK activity (Figure 5C). On the other hand, in thymocytes, all three MKK proteins decreased after stimulation (Figure 5B). These results indicate that, in CD4+T cells, there is differential regulation among MAPK kinases: MKK3 and MKK4 are increased upon stimulation, whereas MKK6 is downregulated.
Fig. 5. MKK3 and MKK4 protein are induced, but MKK6 protein is downregulated upon activation in CD4+T cells. Cells from control mice were stimulated with PMA and ionomycin or with anti-CD3 mAb, and the same amounts of lysates were used for immunoprecipitation (A) or total cell extracts (B). (C) Upregulation of p38MAPK activity in CD4+T cells. Cells were stimulated with PMA/I and examined for p38MAPK activity.
DISCUSSION
The present study indicates the involvement of MKK6 in in vivo thymocyte deletion mediated by anti-CD3 mAb. Injection with anti-CD3 mAb has been widely used to study thymocyte apoptosis in non-TCR transgenic mouse models, although other environmental factors including TNF receptors may be involved in cell death (Smith et al., 1989; Rincón et al., 1998a). A previous study has shown that a pharmacological inhibitor for p38MAPK, SB203580, impaired negative selection in fetal thymic organ culture (FTOC) assays and anti-CD3 mAb-mediated DP cell deletion in vitro (Sugawara et al., 1998). Therefore, decreased p38MAPK activity may be critical for DP cell survival. However, the specificity of SB203580 in vivo remains to be established (Whitmarsh et al., 1997). On the other hand, retroviral introduction of wild-type MKK6 into the FTOC mimics negative selection of DP thymocytes (Sugawara et al., 1998). This overexpression study suggests that MKK6 may be one of the molecules that induce a ‘negative selection signal’ for DP cells. Our results clearly demonstrate, for the first time, that in vivo deletion of DP thymocytes is at least partially dependent on signals mediated by MKK6, and the absence of MKK6 results in decreased p38MAPK activity in these cells.
In this study, we further demonstrated that MKK3 is involved in AICD. Previous studies indicated that, in CD4+T cells, p38 activity can be activated by various stimuli including TCR ligation. T-cell apoptosis induced by anti-CD3 mAb is also inhibited by SB203580 (Zhang et al., 2000). MKK3 may therefore exert apoptotic signals through the activation of p38MAPK, which then induces apoptosis. There are two possible mechanisms underlying AICD: Fas- and TNF-α-mediated signaling (Lenardo et al., 1999). Since we were unable to detect significant differences in FasL expression (data not shown), it may be that the absence of MKK3 may partially impair these signaling pathways. Supporting this possibility, primary fibroblast from Mkk3–/– mice showed impaired TNF-α-mediated signaling (Wysk et al., 1999). Our results also showed that activated CD4+T cells from Mkk3–/– mice were more resistant to apoptosis induced by IL-2 withdrawal. Earlier reports suggest an involvement of p38MAPK in NGF withdrawal-induced apoptosis in neuronal cells (Rosini et al., 2000). Since one of the major signaling pathways mediated by IL-2 is the upregulation of anti-apoptotic proteins including Bcl-2 and Bcl-XL, these two molecules may be involved in MKK3-mediated signaling. Indeed, activation of p38MAPK has been shown to downregulate Bcl-2 expression (Merritt et al., 2000) and inactivate Bcl-2 through phosphorylation (Rosini et al., 2000). On the other hand, previous studies demonstrated that p38MAPK activation induces CD8+T-cell apoptosis but not CD4+T-cell death (Merritt et al., 2000). This difference may stem from the different stimulation used to induce apoptosis and the differences between naïve and activated CD4+T cells. Consistent with the latter, transgenic mice expressing dominant-negative p38MAPK show impaired IFNγ secretion from Th1 cells (Rincón et al., 1998b), and the physiological activator for p38MAPK in Th1 cells appears to be MKK3 (Lu et al., 1999).
Activation of MKK3 and MKK6 has been shown previously to be regulated by phosphorylation (Davis, 2000). Here, we demonstrate for the first time that there is strict regulation of MKK3 and MKK6 expression levels during activation of peripheral CD4+T cells. Our results indicate that activated CD4+T cells possess greater amounts of MKK3 and less MKK6; MKK3 may therefore represent most of the p38MAPK kinase activity in these cells. Although both MKK3 and MKK6 seem to be present in the thymus as well as in unstimulated CD4+T cells, only MKK6 is responsible for apoptosis in these cells. Our results clearly demonstrate that thymocyte p38 activity derives primarily from MKK6 rather than MKK3; the converse is true in peripheral CD4+T cells. Furthermore, an MKK6-specific signaling pathway including caspases has been reported (Huang et al., 1997). Collectively, we have shown that MKK3 and MKK6 are differentially involved in apoptosis of activated peripheral CD4+T cells and DP thymocytes, respectively. We propose a novel mechanism of controlling p38MAPK activity through which induction or downregulation of MKK3 and MKK6 defines cell fate. Although further analyses are required to explore the mechanism(s) of T-cell apoptosis, double knockout mice for Mkk3 and Mkk6 should provide important information for understanding the physiological function of p38MAPK pathways.
METHODS
Generation of Mkk6–/– mice. DNA clones corresponding to the Mkk6 locus were cloned from a 129/Sv mouse genomic library (Stratagene). The targeting vector was constructed by inserting a 7.0-kb EcoRI fragment from the 5′ end of the Mkk6 genomic clone, a 1.6-kb PGK-neo cassette, a 4.3-kb BamHI–NotI fragment from the 3′ end and a fragment containing the thymidine kinase gene into pBluescript. The targeting vector was electroporated into W9.5 ES cells. Genomic DNA from transfectants was screened by Southern blot analysis. Heterozygous ES cells were injected into C57BL/6 blastocysts to create chimeric mice, which were crossed to obtain germ-line transmission of the disrupted Mkk6 allele.
Western blot and in vitro kinase assay. Cells were lysed with cell extraction buffer (Cell Signaling). Soluble fractions were immunoprecipitated with the indicated antibodies. The immunoprecipitates were extensively washed, separated by SDS–PAGE and transferred to Immobilon-P membranes (Millipore), and the immunecomplex was detected by chemiluminescence (Pierce). In vitro kinase assays were performed according to the manufacturer’s instructions (Cell Signaling).
Reagents. PMA and ionomycin (Sigma), anti-MKK3/4/6 (Santa Cruz), anti-phospho-MKK3/6 (Cell Signaling), Abs and anti-Fas mAb (Jo-2; PharMingen) were purchased. Anti-CD3 (145-2C11) and anti-CD28 mAbs were described previously (Rincón et al., 1998a). PE-conjugated anti-CD4 and an FITC-conjugated anti-CD8 mAbs (Pharmingen) were used for FACS (Becton Dickinson).
AICD and IL-2 withdrawal experiments. CD4+T cells were prepared by negative selection (Lu et al., 1999). Cells were stimulated with plate-bound anti-CD3 mAb (10 µg/ml), anti-CD28 mAb (5 µg/ml) and IL-2 (20 U/ml) for 3 days and rested for 24 h in the presence of IL-2. Live cells were recovered after Ficoll-gradient centrifugation and then stimulated with anti-CD3 mAb in the presence of IL-2 or cultured in the presence or absence of IL-2 for 20 h. Cells were stained with AnnexinV and 7AAD (Pharmingen) and analyzed by FACS.
Proliferation assay. CD4+T cells were stimulated with indicated mAb(s) for 72 h. Cells were pulsed with [3H]thymidine during the last 6 h of culture.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank L. Evangelisti, C. Hughes and D. Butkus for technical assistance, and F. Manzo and G. Chenell for help with manuscript preparation. R.J.D. and R.A.F. are investigators of the Howard Hughes Medical Institute.
REFERENCES
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Enslen H., Brancho, D.M. and Davis, R.J. (2000) Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J., 19, 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Jiang, Y., Li, Z., Nishida, E., Mathias, P., Lin, S., Ulevitch, R.J., Nemerow, G.R. and Han, J. (1997) Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity, 6, 739–749. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Chan, K.M., Hornung, F., McFarland, H., Siegel, R., Wang, J. and Zheng, L. (1999) Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol., 17, 221–253. [DOI] [PubMed] [Google Scholar]
- Lu H.T., Yang, D.D., Wysk, M., Gatti, E., Mellman, I., Davis, R.J. and Flavell, R.A. (1999) Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J., 18, 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt C., Enslen, H., Diehl, N., Conze, D., Davis, R.J. and Rincon, M. (2000) Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8+ but not CD4+ T cells. Mol. Cell. Biol., 20, 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda A.R. and Porras, A. (2000) p38 MAP kinases: beyond the stress response. Trends Biochem. Sci., 25, 257–260. [DOI] [PubMed] [Google Scholar]
- Rincón M., Whitmarsh, A., Yang, D.D., Weiss, L., Derijard, B., Jayaraj, P., Davis, R.J. and Flavell, R.A. (1998a) The JNK pathway regulates the in vivo deletion of immature CD4+CD8+ thymocytes. J. Exp. Med., 188, 1817–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M., Enslen, H., Raingeaud, J., Recht, M., Zapton, T., Su, M.S., Penix, L.A., Davis, R.J. and Flavell, R.A. (1998b) Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J., 17, 2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M., Flavell, R.A. and Davis, R.J. (2001) Signal transduction by MAP kinases in T lymphocytes. Oncogene, 20, 2490–2497. [DOI] [PubMed] [Google Scholar]
- Rosini P., De Chiara, G., Lucibello, M., Garaci, E., Cozzolino, F. and Torcia, M. (2000) NGF withdrawal induces apoptosis in CESS B cell line through p38 MAPK activation and Bcl-2 phosphorylation. Biochem. Biophys. Res. Comm., 278, 753–759. [DOI] [PubMed] [Google Scholar]
- Sabapathy K., Kallunki, T., David, J.P., Graef, I., Karin, M. and Wagner, E.F. (2001) c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J. Exp. Med., 193, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.A., Williams, G.T., Kingston, R., Jenkinson, E.J. and Owen, J.J. (1989) Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature, 337, 181–184. [DOI] [PubMed] [Google Scholar]
- Sugawara T., Moriguchi, T., Nishida, E. and Takahama, Y. (1998) Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity, 9, 565–574. [DOI] [PubMed] [Google Scholar]
- Weiss L., Whitmarsh, A.J., Yang, D.D., Rincon, M., Davis, R.J. and Flavell, R.A. (2000) Regulation of c-Jun NH2-terminal kinase (Jnk) gene expression during T cell activation. J. Exp. Med., 191, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh A.J., Yang, S.H., Su, M.S., Sharrocks, A.D. and Davis, R.J. (1997) Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell. Biol., 17, 2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysk M., Yang, D.D., Lu, H.T., Flavell, R.A. and Davis, R.J. (1999) Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc. Natl Acad. Sci. USA, 96, 3763–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Gao, J.X., Salojin, K., Shao, Q., Grattan, M., Meagher, C., Laird, D.W. and Delovitch, T.L. (2000) Regulation of fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. J. Exp. Med., 191, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]