Abstract
Background:
Depression has frequently been associated with smaller hippocampal volume. The hippocampus varies in function along its anterior-posterior axis, with the anterior hippocampus more strongly associated with stress and emotion processing. The goals of this study were to examine the associations among parental history of anxiety/depression, polygenic risk scores for depression (PGS-DEP), and anterior and posterior hippocampal volumes in children and adolescents. To examine specificity to PGS-DEP, we examined associations of educational attainment polygenic scores (PGS-EA) with anterior and posterior hippocampal volume.
Methods:
Participants were 350 3- to 21-year-olds (46% female). PGS-DEP and PGS-EA were computed based on recent, large-scale genome-wide association studies. High-resolution, T1-weighted magnetic resonance imaging (MRI) data were acquired, and a semi-automated approach was used to segment the hippocampus into anterior and posterior subregions.
Results:
Children and adolescents with higher polygenic risk for depression were more likely to have a parent with a history of anxiety/depression. Higher polygenic risk for depression was significantly associated with smaller anterior but not posterior hippocampal volume. PGS-EA was not associated with anterior or posterior hippocampal volumes.
Limitations:
Participants in these analyses were all of European ancestry.
Conclusions:
Polygenic risk for depression may lead to smaller anterior but not posterior hippocampal volume in children and adolescents, and there may be specificity of these effects to PGS-DEP rather than PGS-EA. These findings may inform the earlier identification of those in need of support and the design of more effective, personalized treatment strategies.
Keywords: genetics, depression, hippocampus, brain structure
Introduction
Depression is prevalent and a leading cause of disability worldwide, making it a major public health issue (Herrman et al., 2022). The core symptoms of depressive disorders are depressed mood and anhedonia. Although depression is relatively rare in childhood, it increases in prevalence starting in adolescence (Avenevoli et al., 2015; Kessler et al., 2005). The etiology of depression is multifactorial and includes a robust genetic component (Flint & Kendler, 2014). At the neural level, depression has been repeatedly associated with smaller hippocampal volume (Belleau et al., 2019; Schmaal et al., 2020), although null findings have been reported (Ge et al., 2019; Shengli et al., 2022; Vakili et al., 2000). The hippocampus is crucial to learning, memory, and emotion processes and highly susceptible to chronic stress (Kaul et al., 2021; McEwen et al., 2016). Polygenic scores derived from genome-wide association studies (GWAS) have been used to elucidate genetic influences on a range of phenotypes (Armstrong-Carter et al., 2021). Polygenic risk scores for depression (PGS-DEP), computed via weighted sums of the single nucleotide polymorphisms (SNPs) associated with depression, have been found to predict depressive symptoms and risk for depression in multiple studies (Howard et al., 2019; Wray et al., 2018). Yet, the associations between PGS-DEP and hippocampal structure are not well understood. As such, one main goal of this study was to examine the associations between PGS-DEP and hippocampal structure in children and adolescents.
PGS-DEP and Depression
Several large-scale GWAS of depression have been conducted (Howard et al., 2019; Wray et al., 2018). One of the largest and most recent included meta-analyzed data on 807,553 individuals and identified 102 independent genetic variants associated with depression (Howard et al., 2019). PGS-DEP derived from these GWAS significantly predict depressive symptoms and risk for depression in independent samples of adults (Mitchell et al., 2021) and children and adolescents (Halldorsdottir et al., 2019; Kwong et al., 2021; Rice et al., 2019). An important next step is to build an understanding of how PGS-DEP influences brain structure and function, leading to variability in mental health outcomes.
Anterior and Posterior Hippocampal Function
The hippocampus varies in function along its anterior-posterior axis (Grady, 2020; Poppenk et al., 2013; Strange et al., 2014). In rodent studies, the dorsal subregion (posterior in humans) shows stronger associations with spatial learning and memory, while the ventral subregion (anterior in humans) shows stronger associations with emotion processing (Bannerman et al., 2003, 2004; Fanselow & Dong, 2010; Kjelstrup et al., 2002; Levone et al., 2021; Maren & Holt, 2004; Trivedi & Coover, 2004). Human functional neuroimaging studies have yielded findings consistent with these results (Kumaran et al., 2009; Nadel et al., 2013; Poppenk & Moscovitch, 2011). These distinct roles are thought to be due to differential connectivity patterns, with the posterior hippocampus strongly connected to regions such as the retrosplenial cortex, cuneus, precuneus, cingulate cortex, and inferior parietal cortex (Dalton et al., 2019; Poppenk & Moscovitch, 2011; Tang et al., 2020) and the anterior hippocampus strongly connected to emotion processing regions (e.g., amygdala, medial prefrontal cortex) (Adnan et al., 2016; Blessing et al., 2016; Satpute et al., 2012).
Depression and Anterior and Posterior Hippocampal Structure
While major depressive disorder (MDD) has been associated with volumetric reductions in both anterior and posterior hippocampal subregions (Belleau et al., 2019; Liu et al., 2021; Malykhin et al., 2010), there is evidence that the anterior hippocampus might be particularly vulnerable. In animal studies, chronic stress, which often precedes depression, has stronger effects on the ventral compared to dorsal hippocampus (Hawley et al., 2012; Hawley & Leasure, 2012). Moreover, both depression and antidepressants have stronger effects on ventral compared to dorsal hippocampal morphology (O’Leary & Cryan, 2014; Tanti & Belzung, 2013; Willard et al., 2009). Specifically, chronic stress reduces neurogenesis in the ventral hippocampus and antidepressants reverse these effects (Levone et al., 2015). In humans, treatment for depression (electroconvulsive therapy) has been associated with increases in anterior hippocampal volume (Gyger et al., 2021; Joshi et al., 2016). Although we focus on hippocampal subregional structure, depression has also been associated with differences in hippocampal subregional functional connectivity, often with different patterns found for the anterior vs. posterior hippocampus (Fateh et al., 2019; Ge et al., 2019; Hu et al., 2021; Shengli et al., 2022; Zhou et al., 2022). Taken together, this work in both humans and nonhuman animals raises the possibility that higher PGS-DEP may lead to volumetric reductions that are stronger in the anterior compared to the posterior hippocampus.
PGS-DEP and Hippocampal Structure
Despite the link between depression and smaller hippocampal volumes, large-scale studies have not found that PGS-DEP are associated with total hippocampal volumes in adults (Reus et al., 2017) or children (Alemany et al., 2019). These non-significant findings may reflect variability in the strength of associations by hippocampal subregion. To our knowledge, only one study has examined the associations between PGS-DEP and hippocampal subregional volumes. In this large-scale study, PGS-DEP were unrelated to hippocampal head, body, or tail volumes in children (Pine et al., 2023). The hippocampal head corresponds to the anterior hippocampus, while hippocampal body and tail are considered parts of the posterior hippocampus (Botdorf et al., 2022). However, Pine et al. (2023) was restricted to 9- to 11-year-olds and fully automatically segmented the hippocampus. Importantly, in contrast to Pine et al. (2023), we used a manual segmentation approach to subdivide the automatically segmented hippocampal labels into anterior and posterior portions (Decker et al., 2020). In addition, our sample included adolescents, an age range in which the effects of PGS-DEP might be more likely to manifest compared to childhood (Avenevoli et al., 2015).
Parental History of Depression and PGS-DEP in Children
Parental history of depression heightens risk for depression in children and adolescents (Pagliaccio et al., 2020; Weissman et al., 2016), likely because of a combination of genetic and environmental mechanisms. Parental history of depression has also been associated with smaller hippocampal volume in children and adolescents, although findings have been inconsistent (Kemp et al., 2022; Nazarova et al., 2022), with some studies showing significant associations (Chen et al., 2010; Rao et al., 2010) and others showing no associations (Lupien et al., 2011; Mannie et al., 2014; Pagliaccio et al., 2020). In one study, maternal history of depression was associated with reduced bilateral hippocampal head volume and increased left hippocampal body volume in children (Hubachek et al., 2021). Parents contribute genetics to their children while also shaping the rearing environments their children experience. Parents with depression may pass down a genetic predisposition for depression to their children and provide more stressful rearing environments to their children compared to parents without depression (Goodman & Gotlib, 1999). Yet, less is understood about whether polygenic risk for depression represents a pathway through which parental history of depression may lead to reduced hippocampal volume in children.
In sum, the current study addresses novel questions about associations of PGS-DEP with anterior and posterior hippocampal volume in a non-clinical sample of children and adolescents. It is critical to understand these associations in young individuals to shed light on how these effects unfold prior to the onset or worsening of depression. Understanding the early emergence of these associations could support the earlier identification of those at risk for depression and guide decision-making that leads to more effective prevention and intervention strategies.
Current Study
The main goals of this study were to investigate the associations between PGS-DEP and anterior and posterior hippocampal volume and whether PGS-DEP mediated the association of parental history of anxiety/depression with anterior or posterior hippocampal volume in children and adolescents. We also examined the associations of educational attainment polygenic scores (PGS-EA) with anterior and posterior hippocampal volume to discern whether patterns of results found for PGS-DEP were specific to PGS-DEP rather than PGS-EA. Participants were 3- to 21-year-olds (M = 12.07 years; N = 350; 46% female). High-resolution, T1-weighted MRI data were acquired, and anterior and posterior hippocampal subregions were segmented using a semi-automated approach (Decker et al., 2020). PGS-DEP and PGS-EA were computed using summary statistics from recent, well-powered GWAS of depression (Howard et al., 2019) and educational attainment (Lee et al., 2018), respectively. We hypothesized that PGS-DEP would be more strongly associated with anterior compared to posterior hippocampal volume. We also expected that anterior hippocampal volume would be more strongly associated with PGS-DEP compared to PGS-EA. PGS-DEP-by-age interactions were examined for hippocampal subregional volumes because the effects of PGS-DEP on hippocampal structure may be stronger in older adolescents who have already gone through puberty (Avenevoli et al., 2015).
Methods
Participants
This study utilized publicly-available data from the Pediatric Imaging, Neurocognition, and Genetics (PING) study, which enrolled typically-developing children and adolescents (Jernigan et al., 2016). For this study, participants were recruited across nine sites in the United States. Individuals with neurological disorders; history of head trauma; preterm birth; diagnosis of autism spectrum disorder, bipolar disorder, schizophrenia, or intellectual disability; pregnancy; daily illicit drug use by the mother for more than one trimester; or contraindications for MRI were excluded from the study (Jernigan et al., 2016). More common forms of psychopathology such as anxiety, depression, and attention-deficit/hyperactivity disorder were not excluded because the recruitment strategy was designed to be representative of the general population (Newman et al., 2015). Participants in this investigation ranged from 3 to 21 years of age, and 46% were female (see Table 1 for sample characteristics and Figure S1 for a histogram showing the age distribution).
Table 1.
Descriptive statistics for sample characteristics and anterior and posterior hippocampal volume (N = 350)
| M | SD | Range | |
|---|---|---|---|
|
| |||
| Age (years) | 12.07 | 4.71 | 3.58–21.00 |
| Family income (U.S. dollars) | 117,980.00 | 74,515.54 | 4,500.00-325,000.00 |
| Parental education (years) | 15.62 | 1.90 | 8.00–18.00 |
| Anterior hippocampal volume (mm3) | 2425.84 | 323.59 | 1645.52–3337.18 |
| Posterior hippocampal volume (mm3) | 2322.83 | 276.18 | 1504.27–3175.46 |
| % | n | Range | |
| Sex (female) | 46.29 | 162 | -- |
| Parental history of anxiety/depression | 29.14 | 102 | -- |
| Acquisition site | |||
| University of California, Davis | 17.43 | 61 | -- |
| Kennedy Krieger Institute/Johns Hopkins | 15.43 | 54 | -- |
| Massachusetts General Hospital/Harvard University | 16.86 | 59 | -- |
| University of California, San Diego | 28.57 | 100 | -- |
| University of Massachusetts | 11.71 | 41 | -- |
| Yale University | 10.00 | 35 | -- |
Note. The data for anterior and posterior hippocampal volume are adjusted for intracranial volume (ICV). U.S., United States;
, not applicable
Written informed consent was provided by parents for all participants younger than 18 years of age and by the participants themselves if they were 18 years or older. Child assent was obtained for 7- to 17-year-old participants. Each site’s Institutional Review Board approved the study.
Genomic Data
The PING dataset includes 550,000 SNPs genotyped from saliva samples using Illumina Human660W-Quad BeadChip (Jernigan et al., 2016). Computation of polygenic scores followed procedures similar to those of our previous studies (Khundrakpam et al., 2020; Merz et al., 2022). Steps included preparation of the data for imputation using the “imputePrepSanger” pipeline (https://hub.docker.com/r/eauforest/imputeprepsanger/) and implemented on CBRAIN (Sherif et al., 2014) using Human660W-Quad_v1_A-b37-strand chip as reference. The next step involved data imputation with Sanger Imputation Service (McCarthy et al., 2016) using default settings and the Haplotype Reference Consortium (http://www.haplotype-reference-consortium.org/) as the reference panel. Using PLINK 1.9 (Chang et al., 2015), the imputed SNPs were then filtered with the inclusion criteria: SNPs with unique names, only ACTG, and minor allele frequencies > .05. All SNPs that were included had INFO scores R2 > .9 with PLINK 2.0. Next, using PRSice 2.1.2 (Euesden et al., 2015) additional ambiguous variants were excluded, resulting in 4,696,385 variants being available for polygenic scoring. We filtered individuals with .95 loadings to the European principal component (GAF_Europe variable provided with the PING data), resulting in 526 participants. These participants were then used to compute 10 principal components with PLINK 1.9. PGS-DEP computed based on Howard et al (2019), and PGS-EA based on the EA3 GWAS (Lee et al., 2018) were used in analyses. We clumped the data as per PRSice default settings (clumping distance = 250 kb, threshold r2 = 0.1). PGS-DEP calculated at nine p-value thresholds (1 × 10−6, 1 × 10−5, .0001, .001, .01, .05, .1, .5, and 1), which correspond to the level of significance needed for SNPs to be included in the polygenic score, were used in this study. The number of SNPs included at each of these p-value thresholds is provided in Table S1.
Image Acquisition and Processing
Each PING study site used a standardized structural MRI protocol, with data collected using 3-Tesla scanners manufactured by General Electric, Siemens, and Philips (Jernigan et al., 2016). The scanning session included a high-resolution 3D T1-weighted RF-spoiled gradient echo sequence. Details of the image acquisition can be found in Jernigan et al. (2016). The raw T1-weighted imaging data for the PING study are publicly available for a subset of the sample (https://nda.nih.gov). These data were used in this study.
Hippocampal volumetric segmentation was conducted for a previous study (Decker et al., (2020), which provides a full description of these methods. Prior to segmentation, the images were visually inspected to check for indicators of motion using Display (version 2.0), an MRI image viewing software (http://www.bic.mni.mcgill.ca/software/Display/Display.html). Images were rated based on the degree to which signs of motion were detectable on a scale ranging from 1 (no signs of motion) to 4 (excessive motion). Images with either clear or excessive signs of motion were excluded from analyses (Decker et al., 2020).
Hippocampal Segmentations
We used a combination of automatic and manual methods to define the anterior and posterior hippocampus. First, we segmented the whole hippocampus automatically using the Multiple Automatically Generated Templates for different brains algorithm (MAGeT Brain) (Pipitone et al., 2014), which has been validated in clinical and healthy samples and generates labels for the whole hippocampus that are comparable to existing automated methods (Herten et al., 2019; Pipitone et al., 2014). The MAGeT Brain algorithm uses a set of manually labeled hippocampal atlases as inputs to segment unlabeled T1 images in a dataset. We used five pre-existing, manually segmented hippocampal atlases that included definitions of hippocampal subfields as inputs (Winterburn et al., 2013), which have previously been used in analyses validating MAGeT Brain (Pipitone et al., 2014) and span the length of the anterior-posterior hippocampal axis. The MAGeT Brain algorithm registers these manually labeled atlas labels via nonlinear image registration to a subset of MR images in the sample specified as template images. Each of the newly generated labels on each template image is then registered to the entire dataset of MR images. The labels on each MR image are then fused using a voxel voting procedure, in which the most commonly occurring label at each voxel is retained as part of the final label. By registering the atlases to a subset of the sample, the templates, and then using the template labels to segment the entire dataset, labeling errors that might arise due to anatomical differences between the atlases and subject images are minimized (Pipitone et al., 2014). Hippocampal atlases developed by the same group that are based on adult anatomy and used in conjunction with MAGeT (Pipitone et al., 2014) have been validated in developmental samples (Guo et al., 2015; Herten et al., 2019). These results suggest that using adult atlases to segment the developing hippocampus with the MAGeT algorithm results in labels that have acceptable accuracy relative to manually derived labels.
After automated segmentation of the whole hippocampus, we combined the subfield labels (CA1, CA2–3, CA4-DG, subiculum, SRSLSM) into a single label for the left and right hippocampus. We then visually inspected each label to ensure that the label covered the hippocampus. Data were included if the segmentations covered the entire hippocampus on each slice that the hippocampus was visible or most of the hippocampus on each slice that the hippocampus was visible. Otherwise, data were excluded. Two labels for which the segmentations only covered a few slices of hippocampus were excluded.
A trained rater (A.L.D.) then identified the slice that subdivided the anterior and posterior segments by identifying the slice corresponding to the uncal apex, which is a commonly used landmark for the anterior-posterior hippocampus boundary (Weiss et al., 2005). Importantly, this additional step has advantages over fully automated approaches, such as more precise and reliable delineation of the subregions (Daugherty et al., 2015; Herten et al., 2019; Schoemaker et al., 2016). The caudal-most slice of the anterior hippocampus corresponded to the last slice at which the uncal apex was visible, and the rostral most slice of the posterior hippocampus corresponded to the first slice at which the uncal apex was no longer visible. To ensure the accuracy of this boundary, a second trained rater re-identified the anterior-posterior boundary in an overlapping 10% of the labels. For the anterior-posterior boundary, the researchers identified the same slice in 94% of cases, and the same or a slice that differed by one slice in 98% of cases. Both raters were blind to demographic information relevant to this study. After identifying the boundary slice, any part of a subfield label (CA1, CA2/3, CA4/DG, subiculum, SRLM) rostral to the uncus was counted towards the volume of the anterior hippocampus, whereas any part of a label that was caudal to the uncus was counted towards the volume of the posterior hippocampus. Thus, the volume of the anterior and posterior segments, respectively, reflected the total voxels covered by any subfield label that was rostral or caudal to the boundary slice. Thus, the subfield labels were largely treated as though they were a single label: we ignored the divisions between them, and they were not used to inform the boundary between anterior and posterior segments. Data for anterior and posterior hippocampal volume were then adjusted for intracranial volume (ICV) using a regression-based approach (Jack et al., 1989) described by Decker et al. (2020) (see also Supplemental Materials). These adjusted volumetric data were used in analyses.
Of the 526 participants with polygenic score data, 350 had anterior and posterior hippocampal volume data, and 522 had parental history of anxiety/depression data. Thus, 350 participants were included in analyses of associations between the polygenic scores and anterior and posterior hippocampal volume, and 522 were included in analyses of associations between parental history of anxiety/depression and PGS-DEP.
Parental History of Anxiety/Depression
Respondents indicated whether the biological mother and/or father of the participant had a history of anxiety or depression. These responses were summed to create a measure of the number of parents with a known history of anxiety or depression (0, 1, or 2). A dichotomous variable was then created in which 0 = no parental history of anxiety/depression and 1 = one or two parents with a history of anxiety/depression.
Statistical Analyses
Multiple linear regression analyses in SAS (version 9.4) were conducted using the general linear model procedure to examine associations of PGS-DEP and PGS-EA (at nine different p-value thresholds) with anterior and posterior hippocampal volume. False discovery rate (FDR) corrections were employed to control for multiple comparisons (Benjamini & Hochberg, 1995). Covariates included age, sex, family income, and scanner/site. In the PING subsample that was the focus of this study (n = 350), six sites were represented (see Table 1), with one scanner per site. Age-squared was initially included as a covariate in analyses of hippocampal volume but was not included in the final models because it was not significant. The first 10 principal components (PC1–10) were also included as covariates in all analyses involving PGS-DEP or PGS-EA (Price et al., 2006). Including PC1–10 in analyses controls for population stratification or random differences in population genomic signatures that may explain outcomes (Price et al., 2006). Effect sizes (ηp2) are presented, with values of .01, .06, and .14 indicating small, medium, and large effects, respectively (Cohen, 1988). We also tested whether the correlation between PGS-DEP and anterior hippocampal volume was statistically different than the correlation between PGS-DEP and posterior hippocampal volume using Meng, Rosenthal, and Rubin’s z test (Meng et al., 1992) via the cocor package in R (Diedenhofen & Musch, 2015).
We then examined whether PGS-DEP mediated the association between parental history of anxiety/depression and anterior and/or posterior hippocampal volume in children and adolescents. First, we examined associations between parental history of anxiety/depression and PGS-DEP while controlling for age, sex, PC1–10, and scanner/site. If these associations were significant and significant associations were found between PGS-DEP and anterior or posterior hippocampal volume, we then tested the significance of the indirect effect (ab path) using bias-corrected bootstrapping via the PROCESS macro in SAS (Hayes, 2013; MacKinnon et al., 2002). Indirect effects were significant if the 95% confidence intervals (CIs) did not include zero (Preacher & Hayes, 2008).
Results
Descriptive statistics for anterior and posterior hippocampal volume are provided in Table 1, and zero-order correlations between PGS-DEP, parental history of anxiety/depression, and anterior and posterior hippocampal volume are provided in Table S2. As shown in Table S2, higher PGS-DEP was significantly correlated with a parental history of anxiety/depression and smaller anterior hippocampal volume.
PGS-DEP and Anterior and Posterior Hippocampal Volume
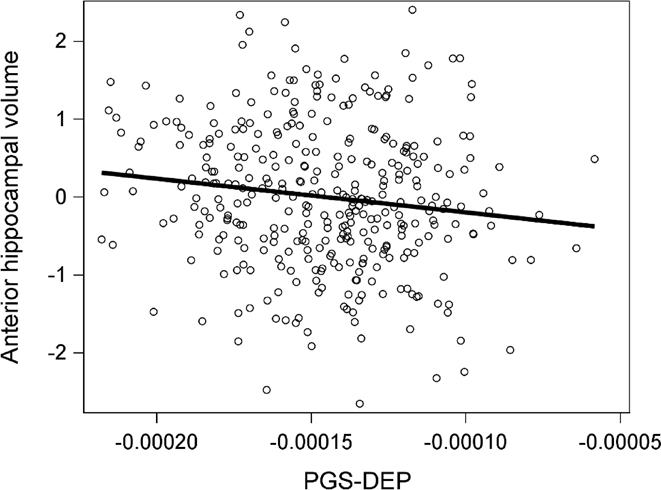
Higher PGS-DEP at p-value thresholds of .10, .05, and .001 were significantly associated with reduced anterior hippocampal volume (see Table 2 and Figure 1). The effect size (ηp2) was .02 for PGS-DEP computed at all three p-value thresholds. PGS-DEP was not significantly associated with posterior hippocampal volume. The correlation between PGS-DEP and anterior hippocampal volume was significantly different than that between PGS-DEP and posterior hippocampal volume for PGS-DEP at p-value thresholds of .10 (z = −2.13, p = .03) and .05 (z = −2.02, p = .04) but not for PGS-DEP at a p-value threshold of .001 (z = −1.49, p = .14). There were no significant PGS-DEP-by-age interactions for anterior or posterior hippocampal volume.
Table 2.
Multiple linear regression results showing associations between PGS-DEP and anterior and posterior hippocampal volume
| PGS-DEP p-value threshold | Anterior hippocampal volume |
Posterior hippocampal volume |
||||
|---|---|---|---|---|---|---|
| β | p | FDR-corrected p | β | p | FDR-corrected p | |
|
| ||||||
| p < 1 | −.10 | .0608 | .0914 | .05 | .3917 | .7088 |
| p < .5 | −.10 | .0618 | .0914 | .04 | .4401 | .7088 |
| p < .1 | −.14 | .0085 | .0306 | .02 | .7672 | .8631 |
| p < .05 | −.15 | .0054 | .0306 | −.001 | .9845 | .9845 |
| p < .01 | −.10 | .0711 | .0914 | −.03 | .5513 | .7088 |
| p < .001 | −.14 | .0102 | .0306 | −.03 | .5511 | .7088 |
| p < .0001 | −.07 | .1946 | .1946 | −.09 | .0900 | .4266 |
| p < 1 x 10−5 | −.08 | .1501 | .1689 | −.08 | .1404 | .4266 |
| p < 1 x 10−6 | −.10 | .0688 | .0914 | −.08 | .1422 | .4266 |
Note. PGS-DEP, polygenic risk score for depression; FDR, false discovery rate
Figure 1.
Higher polygenic risk for depression (PGS-DEP) was significantly associated with smaller anterior hippocampal volume. Data for PGS-DEP computed at a p-value threshold of .10 are displayed. Anterior hippocampal volume data were adjusted for covariates.
PGS-EA and Anterior and Posterior Hippocampal Volume
There were no significant associations between PGS-EA and anterior or posterior hippocampal volume (see Table S3).
Parental History of Anxiety/Depression, PGS-DEP, and Anterior Hippocampal Volume
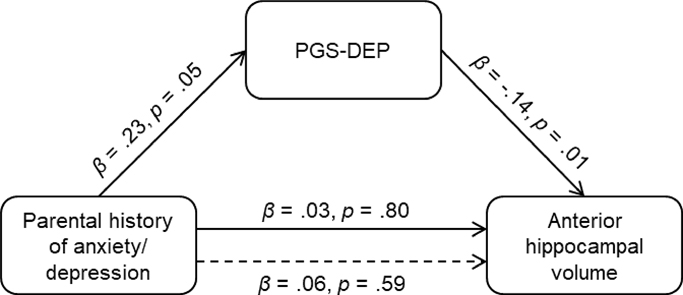
Having a parent with a history of anxiety/depression was associated with higher PGS-DEP at p-value thresholds of .05 (β = .22, p = .03, ηp2 = .01) and .10 (β = .24, p = .02, ηp2 = .01) but not at a p-value threshold of .001 (β = .15, p = .14). In addition, there was a significant indirect effect of parental history of anxiety/depression on anterior hippocampal volume via PGS-DEP, ab = −.03, 95% CI: −.0949, −.0004. Having a parent with a history of anxiety/depression was associated with higher PGS-DEP which was in turn associated with smaller anterior hippocampal volume (see Figure 2). Analyses were re-run using ComBat-GAM (Pomponio et al., 2020) to control for scanner effects, and the pattern of results was the same.
Figure 2.
Significant indirect effect of parental history of anxiety/depression on anterior hippocampal volume via polygenic risk for depression (PGS-DEP; p-value threshold: .10), ab = −.03, 95% CI: −.0949, −.0004. Having a parent with a history of anxiety/depression was associated with higher polygenic risk for depression, which was associated with smaller anterior hippocampal volume in children and adolescents.
Discussion
Here, we investigated the associations among parental history of anxiety/depression, PGS-DEP, and anterior and posterior hippocampal volume in a large sample of children and adolescents. Higher PGS-DEP was significantly associated with reduced anterior but not posterior hippocampal volume, consistent with the idea that anterior hippocampal volumes are particularly vulnerable in individuals who are at higher genetic risk for depression. There was also a significant indirect association between parental history of anxiety/depression and smaller anterior hippocampal volume via higher PGS-DEP. Having a parent with a history of anxiety/depression was associated with higher PGS-DEP, which was in turn associated with smaller anterior hippocampal volume.
Higher PGS-DEP is Associated with Smaller Anterior Hippocampal Volume
The few previous studies that have investigated associations between PGS-DEP and total hippocampal volume have yielded non-significant findings (Alemany et al., 2019; Reus et al., 2017). And, one previous study did not find significant associations between PGS-DEP and hippocampal head, body, or tail volumes in children (Pine et al., 2023). The difference between our results and those of Pine et al. (2023) could be partially due to differences in sample characteristics. More specifically, Pine et al. (2023) focused on 9- to 11-year-olds, whereas our sample included adolescents. In addition, we manually subdivided automatically generated hippocampal labels into anterior and posterior portions (Decker et al., 2020).
Higher genetic risk for depression may lead to reductions in anterior hippocampal volume that then contribute to increased risk for depression. Both animal models and human studies have indicated that the anterior hippocampus is heavily involved in emotion processing (Adnan et al., 2016; Blessing et al., 2016; Fanselow & Dong, 2010) likely due in part to functional connections with regions such as the amygdala and ventromedial prefrontal cortex (Adnan et al., 2016; Blankenship et al., 2017; Poppenk & Moscovitch, 2011). Genetic effects on anterior hippocampal morphology may lead to altered emotional memory and regulation in ways that increase risk for depression. In addition, altered anterior hippocampal morphology may lead to reduced negative feedback control over the hypothalamic-pituitary-adrenal (HPA) axis, leading to a disinhibited stress response which in turn increases risk for depression (Blessing et al., 2016; Frodl & O’Keane, 2013). In this way, genetic effects leading to reduced anterior hippocampal morphology may contribute to increases in vulnerability to chronic stress (Anacker et al., 2018; Jones et al., 2022; Levone et al., 2015). Given that the anterior hippocampus likely plays many roles, there may be additional implications of volumetric changes in this structure for emotion processing and aspects of memory functioning (Strange et al., 2014).
While findings from previous studies are consistent with the notion that smaller hippocampal volume may precede the onset of depression (Chen et al., 2010; Rao et al., 2010), in studies of MDD, recurrent major depressive episodes and prolonged duration of the disorder have also been associated with reductions in hippocampal volume (Belleau et al., 2019; Schmaal et al., 2020). The PING sample is a non-clinical sample of children and adolescents. However, participants with depression were not excluded, and depressive symptoms were not measured for the full sample. Thus, although many participants likely did not have a clinically significant depressive disorder, some probably did (Avenevoli et al., 2015; Kessler et al., 2005). Smaller hippocampal volume may be both a cause and consequence of depression. Patterns of altered hippocampal subregional volumes may be different in high-risk individuals prior to the onset of depression compared to after recurrent depressive episodes. Reductions in anterior hippocampal volume may emerge prior to more widespread reductions across the longitudinal axis of the hippocampus (Hubachek et al., 2021).
Effect sizes were small; after taking the covariates into account, PGS-DEP uniquely explained 2% of the variability in anterior hippocampal volume. Although genetic risk, chronic stress, and possibly reduced anterior hippocampal volume are risk factors for depression, it is how these risk factors relate to one another (e.g., diathesis-stress model) and accumulate that explains the most variability in mental health outcomes (Belleau et al., 2019; McEwen et al., 2016). Future longitudinal MRI studies of PGS-DEP are needed that measure chronic stress, anterior and posterior hippocampal volumes, and depressive symptoms over time to elucidate how effects of these variables on each other unfold over time.
In contrast to the results for PGS-DEP, PGS-EA was not associated with either anterior or posterior hippocampal volumes. Previous studies have distinguished between polygenic scores for psychiatric disorders and those for cognitive outcomes, such as educational attainment (Alemany et al., 2019; Kwong et al., 2021). Our results suggest that smaller anterior hippocampal volume may be more strongly associated with genetic factors associated with depression compared to genetic factors associated with educational attainment. Given that smaller hippocampal volumes have also been associated with other psychiatric disorders, future studies should examine whether these associations are specific to depression or similar for polygenic scores for other psychiatric disorders.
Parental History of Anxiety/Depression and PGS-DEP
In this sample of children and adolescents, 29% had a parent with a history of anxiety/depression, which is very similar to the percentage in the Adolescent Brain and Cognitive Development (ABCD) study sample (Pagliaccio et al., 2020). Having a parent with a history of anxiety/depression was associated with higher PGS-DEP in children and adolescents, consistent with previous work (Mars et al., 2022). The effect size was small, which aligns with previous research indicating that polygenic risk scores and family history measures represent related but different information (Agerbo et al., 2021; Loughnan et al., 2022; Lu et al., 2018). There was an indirect association between parental history of anxiety/depression and anterior hippocampal volume via PGS-DEP. Having a parent with a history of anxiety/depression was significantly associated with higher PGS-DEP, which was in turn associated with smaller anterior hippocampal volume. These findings suggest that one pathway through which family history of depression may lead to reductions in anterior hippocampal volume for children is through increases in genetic risk. Given that parental depression is also associated with more stressful rearing environments for children, future studies should disentangle how much the transmission of risk across generations, including at the neural level, is due to genetic and environmental influences (Goodman & Gotlib, 1999).
Parental history of anxiety/depression was not directly associated with anterior or posterior hippocampal volume in children and adolescents. While some previous studies have shown associations between parental history of depression and hippocampal volume in children or adolescents (Chen et al., 2010; Rao et al., 2010), others have not (Lupien et al., 2011; Mannie et al., 2014; Pagliaccio et al., 2020). These findings may be partially attributable to the type of family history assessment used. A more rigorous, clinical interview measure of parental history of depression may have yielded significant results.
Strengths and Limitations
Key strengths of this MRI study include the relatively large sample size coupled with the use of PGS-DEP derived from one of the largest recent GWAS of depression (Howard et al., 2019). Another major strength of this study stems from the careful implementation of a combined automated and manual segmentation approach to computing hippocampal subregional volumes (Decker et al., 2020). At the same time, several limitations of this study must be considered when interpreting the results. First, this study utilized a cross-sectional and correlational design. Second, analyses of PGS-DEP and PGS-EA included only participants of European ancestry, similar to many studies involving polygenic scores (Elliott et al., 2019; Kwong et al., 2021; von Stumm et al., 2020). Therefore, the findings from this study are not generalizable to populations beyond individuals of European ancestry. Third, data on depression symptoms were only collected for a small subsample, precluding analyses of the role of depression symptoms.
Conclusion
Findings from this study link higher polygenic risk for depression with reduced anterior but not posterior hippocampal volume in children and adolescents. Higher genetic risk for depression may lead to reductions in anterior hippocampal volume that contribute to the onset or worsening of depression. Parental history of depression may lead to smaller anterior hippocampal volumes in children in part by increasing children’s genetic risk for depression. These findings can be used to inform the design of more effective prevention and intervention strategies for depression. Anterior hippocampal volume could be investigated as a biomarker to match individuals with effective treatments. Early identification of children at risk for depression can be facilitated by increased knowledge of genetic and neural markers measurable prior to the onset of depression or early in the course of the disorder.
Supplementary Material
Highlights.
Polygenic risk scores and hippocampal volume measured in children and adolescents
Polygenic risk for depression associated with smaller anterior hippocampal volume
Polygenic risk for depression not associated with posterior hippocampal volume
Polygenic risk for depression associated with family history of anxiety/depression
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adnan A, Barnett A, Moayedi M, McCormick C, Cohn M, & McAndrews MP (2016). Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Structure and Function, 221(6), 2999–3012. 10.1007/s00429-015-1084-x [DOI] [PubMed] [Google Scholar]
- Agerbo E, Trabjerg BB, Børglum AD, Schork AJ, Vilhjálmsson BJ, Pedersen CB, Hakulinen C, Albiñana C, Hougaard DM, Grove J, McGrath JJ, Bybjerg-Grauholm J, Mors O, Plana-Ripoll O, Werge T, Wray NR, Mortensen PB, & Musliner KL (2021). Risk of Early-Onset Depression Associated With Polygenic Liability, Parental Psychiatric History, and Socioeconomic Status. JAMA Psychiatry, 78(4), 387–397. 10.1001/jamapsychiatry.2020.4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany S, Jansen PR, Muetzel RL, Marques N, El Marroun H, Jaddoe VWV, Polderman TJC, Tiemeier H, Posthuma D, & White T (2019). Common Polygenic Variations for Psychiatric Disorders and Cognition in Relation to Brain Morphology in the General Pediatric Population. Journal of the American Academy of Child and Adolescent Psychiatry, 58(6), 600–607. 10.1016/j.jaac.2018.09.443 [DOI] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, & Hen R (2018). Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature, 559(7712), Article 7712. 10.1038/s41586-018-0262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-Carter E, Wertz J, & Domingue BW (2021). Genetics and Child Development: Recent Advances and Their Implications for Developmental Research. Child Development Perspectives, 15(1), 57–64. 10.1111/cdep.12400 [DOI] [Google Scholar]
- Avenevoli S, Swendsen J, He J-P, Burstein M, & Merikangas K (2015). Major Depression in the National Comorbidity Survey- Adolescent Supplement: Prevalence, Correlates, and Treatment. Journal of the American Academy of Child and Adolescent Psychiatry, 54(1), 37–44.e2. 10.1016/j.jaac.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, & Rawlins JNP (2003). Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research, 139(1), 197–213. 10.1016/S0166-4328(02)00268-1 [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang W-N, Pothuizen HHJ, & Feldon J (2004). Regional dissociations within the hippocampus—Memory and anxiety. Neuroscience & Biobehavioral Reviews, 28(3), 273–283. 10.1016/j.neubiorev.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Belleau EL, Treadway MT, & Pizzagalli DA (2019). The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biological Psychiatry, 85(6), 443–453. 10.1016/j.biopsych.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. JSTOR. [Google Scholar]
- Blankenship SL, Redcay E, Dougherty LR, & Riggins T (2017). Development of hippocampal functional connectivity during childhood. Human Brain Mapping, 38(1), 182–201. 10.1002/hbm.23353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing EM, Beissner F, Schumann A, Brünner F, & Bär K-J (2016). A data-driven approach to mapping cortical and subcortical intrinsic functional connectivity along the longitudinal hippocampal axis. Human Brain Mapping, 37(2), 462–476. 10.1002/hbm.23042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botdorf M, Dunstan J, Sorcher L, Dougherty LR, & Riggins T (2022). Socioeconomic disadvantage and episodic memory ability in the ABCD sample: Contributions of hippocampal subregion and subfield volumes. Developmental Cognitive Neuroscience, 57, 101138. 10.1016/j.dcn.2022.101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, & Lee JJ (2015). Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience, 4, 7. 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Hamilton JP, & Gotlib IH (2010). Decreased Hippocampal Volume in Healthy Girls at Risk of Depression. Archives of General Psychiatry, 67(3), 270–276. 10.1001/archgenpsychiatry.2009.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Routledge. 10.4324/9780203771587 [DOI] [Google Scholar]
- Dalton MA, McCormick C, & Maguire EA (2019). Differences in functional connectivity along the anterior-posterior axis of human hippocampal subfields. NeuroImage, 192, 38–51. 10.1016/j.neuroimage.2019.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Yu Q, Flinn R, & Ofen N (2015). A reliable and valid method for manual demarcation of hippocampal head, body, and tail. International Journal of Developmental Neuroscience, 41, 115–122. 10.1016/j.ijdevneu.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Decker AL, Duncan K, Finn AS, & Mabbott DJ (2020). Children’s family income is associated with cognitive function and volume of anterior not posterior hippocampus. Nature Communications, 11(1), Article 1. 10.1038/s41467-020-17854-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedenhofen B, & Musch J (2015). cocor: A Comprehensive Solution for the Statistical Comparison of Correlations. PLOS ONE, 10(4), e0121945. 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott ML, Belsky DW, Anderson K, Corcoran DL, Ge T, Knodt A, Prinz JA, Sugden K, Williams B, Ireland D, Poulton R, Caspi A, Holmes A, Moffitt T, & Hariri AR (2019). A Polygenic Score for Higher Educational Attainment is Associated with Larger Brains. Cerebral Cortex (New York, NY), 29(8), 3496–3504. 10.1093/cercor/bhy219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euesden J, Lewis CM, & O’Reilly PF (2015). PRSice: Polygenic Risk Score software. Bioinformatics (Oxford, England), 31(9), 1466–1468. 10.1093/bioinformatics/btu848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Dong H-W (2010). Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron, 65(1), 7–19. 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fateh AA, Long Z, Duan X, Cui Q, Pang Y, Farooq MU, Nan X, Chen Y, Sheng W, Tang Q, & Chen H (2019). Hippocampal functional connectivity-based discrimination between bipolar and major depressive disorders. Psychiatry Research. Neuroimaging, 284, 53–60. 10.1016/j.pscychresns.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Flint J, & Kendler KS (2014). The Genetics of Major Depression. Neuron, 81(3), 484–503. 10.1016/j.neuron.2014.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, & O’Keane V (2013). How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of Disease, 52, 24–37. 10.1016/j.nbd.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Ge R, Torres I, Brown JJ, Gregory E, McLellan E, Downar JH, Blumberger DM, Daskalakis ZJ, Lam RW, & Vila-Rodriguez F (2019). Functional disconnectivity of the hippocampal network and neural correlates of memory impairment in treatment-resistant depression. Journal of Affective Disorders, 253, 248–256. 10.1016/j.jad.2019.04.096 [DOI] [PubMed] [Google Scholar]
- Goodman SH, & Gotlib IH (1999). Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review, 106(3), 458–490. 10.1037/0033-295X.106.3.458 [DOI] [PubMed] [Google Scholar]
- Grady CL (2020). Meta-analytic and functional connectivity evidence from functional magnetic resonance imaging for an anterior to posterior gradient of function along the hippocampal axis. Hippocampus, 30(5), 456–471. 10.1002/hipo.23164 [DOI] [PubMed] [Google Scholar]
- Guo T, Winterburn JL, Pipitone J, Duerden EG, Park MTM, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP, & Mallar Chakravarty M (2015). Automatic segmentation of the hippocampus for preterm neonates from early-in-life to term-equivalent age. NeuroImage : Clinical, 9, 176–193. 10.1016/j.nicl.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyger L, Regen F, Ramponi C, Marquis R, Mall J-F, Swierkosz-Lenart K, von Gunten A, Toni N, Kherif F, Heuser I, & Draganski B (2021). Gradient of electroconvulsive therapy’s antidepressant effects along the longitudinal hippocampal axis. Translational Psychiatry, 11(1), Article 1. 10.1038/s41398-021-01310-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsdottir T, Piechaczek C, Soares de Matos AP, Czamara D, Pehl V, Wagenbuechler P, Feldmann L, Quickenstedt-Reinhardt P, Allgaier A-K, Freisleder FJ, Greimel E, Kvist T, Lahti J, Räikkönen K, Rex-Haffner M, Arnarson EÖ, Craighead WE, Schulte-Körne G, & Binder EB (2019). Polygenic Risk: Predicting Depression Outcomes in Clinical and Epidemiological Cohorts of Youths. American Journal of Psychiatry, 176(8), 615–625. 10.1176/appi.ajp.2019.18091014 [DOI] [PubMed] [Google Scholar]
- Hawley DF, & Leasure JL (2012). Region-specific response of the hippocampus to chronic unpredictable stress. Hippocampus, 22(6), 1338–1349. 10.1002/hipo.20970 [DOI] [PubMed] [Google Scholar]
- Hawley DF, Morch K, Christie BR, & Leasure JL (2012). Differential Response of Hippocampal Subregions to Stress and Learning. PLOS ONE, 7(12), e53126. 10.1371/journal.pone.0053126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press. [Google Scholar]
- Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, Furukawa TA, Kessler RC, Kohrt BA, Maj M, McGorry P, Reynolds CF, Weissman MM, Chibanda D, Dowrick C, Howard LM, Hoven CW, Knapp M, Mayberg HS, … Wolpert M (2022). Time for united action on depression: A Lancet-World Psychiatric Association Commission. Lancet (London, England), 399(10328), 957–1022. 10.1016/S0140-6736(21)02141-3 [DOI] [PubMed] [Google Scholar]
- Herten A, Konrad K, Krinzinger H, Seitz J, & von Polier GG (2019). Accuracy and bias of automatic hippocampal segmentation in children and adolescents. Brain Structure & Function, 224(2), 795–810. 10.1007/s00429-018-1802-2 [DOI] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, Alloza C, Shen X, Barbu MC, Xu EY, Whalley HC, Marioni RE, Porteous DJ, Davies G, Deary IJ, … McIntosh AM (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience, 22(3), Article 3. 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Liu J, Liu Y, Wu X, Zhuang K, Chen Q, Yang W, Xie P, Qiu J, & Wei D (2021). Dysfunction of the anterior and intermediate hippocampal functional network in major depressive disorders across the adult lifespan. Biological Psychology, 165, 108192. 10.1016/j.biopsycho.2021.108192 [DOI] [PubMed] [Google Scholar]
- Hubachek S, Botdorf M, Riggins T, Leong H-C, Klein DN, & Dougherty LR (2021). Hippocampal subregion volume in high-risk offspring is associated with increases in depressive symptoms across the transition to adolescence. Journal of Affective Disorders, 281, 358–366. 10.1016/j.jad.2020.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, & Cascino GD (1989). Anterior temporal lobes and hippocampal formations: Normative volumetric measurements from MR images in young adults. Radiology, 172(2), 549–554. 10.1148/radiology.172.2.2748838 [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Hagler DJ Jr., Akshoomoff N, Bartsch H, Newman E, Thompson WK, Bloss CS, Murray SS, Schork N, Kennedy DN, Kuperman JM, McCabe C, Chung Y, Libiger O, Maddox M, Casey BJ, Chang L, Ernst TM, … Dale AM (2016). The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. NeuroImage, 124, Part B, 1149–1154. 10.1016/j.neuroimage.2015.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Zhou M, & Jhaveri DJ (2022). Dissecting the role of adult hippocampal neurogenesis towards resilience versus susceptibility to stress-related mood disorders. Npj Science of Learning, 7(1), Article 1. 10.1038/s41539-022-00133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, Leaver A, Woods RP, & Narr KL (2016). Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biological Psychiatry, 79(4), 282–292. 10.1016/j.biopsych.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D, Schwab SG, Mechawar N, & Matosin N (2021). How stress physically re-shapes the brain: Impact on brain cell shapes, numbers and connections in psychiatric disorders. Neuroscience and Biobehavioral Reviews, 124, 193–215. 10.1016/j.neubiorev.2021.01.025 [DOI] [PubMed] [Google Scholar]
- Kemp JVA, Bernier E, Lebel C, & Kopala-Sibley DC (2022). Associations Between Parental Mood and Anxiety Psychopathology and Offspring Brain Structure: A Scoping Review. Clinical Child and Family Psychology Review, 25(1), 222–247. 10.1007/s10567-022-00393-5 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Khundrakpam B, Vainik U, Gong J, Al-Sharif N, Bhutani N, Kiar G, Zeighami Y, Kirschner M, Luo C, Dagher A, & Evans A (2020). Neural correlates of polygenic risk score for autism spectrum disorders in general population. Brain Communications, 2(2). 10.1093/braincomms/fcaa092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach H-A, Murison R, Moser EI, & Moser M-B (2002). Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences, 99(16), 10825–10830. 10.1073/pnas.152112399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, & Maguire EA (2009). Tracking the Emergence of Conceptual Knowledge during Human Decision Making. Neuron, 63(6), 889–901. 10.1016/j.neuron.2009.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong ASF, Morris TT, Pearson RM, Timpson NJ, Rice F, Stergiakouli E, & Tilling K (2021). Polygenic risk for depression, anxiety and neuroticism are associated with the severity and rate of change in depressive symptoms across adolescence. Journal of Child Psychology and Psychiatry, 62(12), 1462–1474. 10.1111/jcpp.13422 [DOI] [PubMed] [Google Scholar]
- Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, Bowers P, Sidorenko J, Karlsson Linnér R, Fontana MA, Kundu T, Lee C, Li H, Li R, Royer R, Timshel PN, Walters RK, Willoughby EA, … Cesarini D (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50(8), Article 8. 10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levone BR, Cryan JF, & O’Leary OF (2015). Role of adult hippocampal neurogenesis in stress resilience. Neurobiology of Stress, 1, 147–155. 10.1016/j.ynstr.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levone BR, Moloney GM, Cryan JF, & O’Leary OF (2021). Specific sub-regions along the longitudinal axis of the hippocampus mediate antidepressant-like behavioral effects. Neurobiology of Stress, 14, 100331. 10.1016/j.ynstr.2021.100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M-N, Pantouw JG, Yang K-C, Hu L-Y, Liou Y-J, Lirng J-F, & Chou Y-H (2021). Sub-regional hippocampal volumes in first-episode drug-naïve major depression disorder. Neuroscience Letters, 763, 136178. 10.1016/j.neulet.2021.136178 [DOI] [PubMed] [Google Scholar]
- Loughnan RJ, Palmer CE, Makowski C, Thompson WK, Barch DM, Jernigan TL, Dale AM, & Fan CC (2022). Unique prediction of developmental psychopathology from genetic and familial risk. Journal of Child Psychology and Psychiatry, 63(12), 1631–1643. 10.1111/jcpp.13649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Pouget JG, Andreassen OA, Djurovic S, Esko T, Hultman CM, Metspalu A, Milani L, Werge T, & Sullivan PF (2018). Genetic risk scores and family history as predictors of schizophrenia in Nordic registers. Psychological Medicine, 48(7), 1201–1208. 10.1017/S0033291717002665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, & Séguin JR (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences, 108(34), 14324–14329. 10.1073/pnas.1105371108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, & Sheets V (2002). A comparison of methods to test mediation and other intervening variable effects. Psychological Methods, 7(1), 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, & Coupland NJ (2010). Structural changes in the hippocampus in major depressive disorder: Contributions of disease and treatment. Journal of Psychiatry and Neuroscience, 35(5), 337–343. 10.1503/jpn.100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie ZN, Filippini N, Williams C, Near J, Mackay CE, & Cowen PJ (2014). Structural and functional imaging of the hippocampus in young people at familial risk of depression. Psychological Medicine, 44(14), 2939–2948. 10.1017/S0033291714000580 [DOI] [PubMed] [Google Scholar]
- Maren S, & Holt WG (2004). Hippocampus and Pavlovian fear conditioning in rats: Muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience, 118(1), 97–110. 10.1037/0735-7044.118.1.97 [DOI] [PubMed] [Google Scholar]
- Mars N, Lindbohm JV, Della Briotta Parolo P, Widén E, Kaprio J, Palotie A, FinnGen, & Ripatti S. (2022). Systematic comparison of family history and polygenic risk across 24 common diseases. American Journal of Human Genetics, 109(12), 2152–2162. 10.1016/j.ajhg.2022.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, Kang HM, Fuchsberger C, Danecek P, Sharp K, Luo Y, Sidore C, Kwong A, Timpson N, Koskinen S, Vrieze S, Scott LJ, Zhang H, Mahajan A, … Haplotype Reference Consortium. (2016). A reference panel of 64,976 haplotypes for genotype imputation. Nature Genetics, 48(10), 1279–1283. 10.1038/ng.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology, 41(1), 3–23. 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Rosenthal R, & Rubin DB (1992). Comparing correlated correlation coefficients. Psychological Bulletin, 111, 172–175. 10.1037/0033-2909.111.1.172 [DOI] [Google Scholar]
- Merz EC, Strack J, Hurtado H, Vainik U, Thomas M, Evans A, & Khundrakpam B (2022). Educational attainment polygenic scores, socioeconomic factors, and cortical structure in children and adolescents. Human Brain Mapping, 43(16), 4886–4900. 10.1002/hbm.26034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BL, Thorp JG, Wu Y, Campos AI, Nyholt DR, Gordon SD, Whiteman DC, Olsen CM, Hickie IB, Martin NG, Medland SE, Wray NR, & Byrne EM (2021). Polygenic Risk Scores Derived From Varying Definitions of Depression and Risk of Depression. JAMA Psychiatry, 78(10), 1152–1160. 10.1001/jamapsychiatry.2021.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Hoscheidt S, & Ryan LR (2013). Spatial cognition and the hippocampus: The anterior-posterior axis. Journal of Cognitive Neuroscience, 25(1), 22–28. 10.1162/jocn_a_00313 [DOI] [PubMed] [Google Scholar]
- Nazarova A, Schmidt M, Cookey J, & Uher R (2022). Neural markers of familial risk for depression – A systematic review. Developmental Cognitive Neuroscience, 58, 101161. 10.1016/j.dcn.2022.101161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Thompson WK, Bartsch H, Hagler DJ, Chen C-H, Brown TT, Kuperman JM, McCabe C, Chung Y, Libiger O, Akshoomoff N, Bloss CS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Murray SS, … Jernigan TL (2015). Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Structure and Function, 221(6), 3013–3025. 10.1007/s00429-015-1085-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, & Cryan JF (2014). A ventral view on antidepressant action: Roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends in Pharmacological Sciences, 35(12), 675–687. 10.1016/j.tips.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Alqueza KL, Marsh R, & Auerbach RP (2020). Brain Volume Abnormalities in Youth at High Risk for Depression: Adolescent Brain and Cognitive Development Study. Journal of the American Academy of Child & Adolescent Psychiatry, 59(10), 1178–1188. 10.1016/j.jaac.2019.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine JG, Paul SE, Johnson E, Bogdan R, Kandala S, & Barch DM (2023). Polygenic Risk for Schizophrenia, Major Depression, and Post-traumatic Stress Disorder and Hippocampal Subregion Volumes in Middle Childhood. Behavior Genetics. 10.1007/s10519-023-10134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipitone J, Park MTM, Winterburn J, Lett TA, Lerch JP, Pruessner JC, Lepage M, Voineskos AN, & Chakravarty MM (2014). Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. NeuroImage, 101, 494–512. 10.1016/j.neuroimage.2014.04.054 [DOI] [PubMed] [Google Scholar]
- Pomponio R, Erus G, Habes M, Doshi J, Srinivasan D, Mamourian E, Bashyam V, Nasrallah IM, Satterthwaite TD, Fan Y, Launer LJ, Masters CL, Maruff P, Zhuo C, Völzke H, Johnson SC, Fripp J, Koutsouleris N, Wolf DH, … Davatzikos C (2020). Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. NeuroImage, 208, 116450. 10.1016/j.neuroimage.2019.116450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, & Nadel L (2013). Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. 10.1016/j.tics.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Poppenk J, & Moscovitch M (2011). A Hippocampal Marker of Recollection Memory Ability among Healthy Young Adults: Contributions of Posterior and Anterior Segments. Neuron, 72(6), 931–937. 10.1016/j.neuron.2011.10.014 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, & Reich D (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics, 38(8), 904–909. 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- Rao U, Chen L-A, Bidesi AS, Shad MU, Thomas MA, & Hammen CL (2010). Hippocampal Changes Associated with Early-Life Adversity and Vulnerability to Depression. Biological Psychiatry, 67(4), 357–364. 10.1016/j.biopsych.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus LM, Shen X, Gibson J, Wigmore E, Ligthart L, Adams MJ, Davies G, Cox SR, Hagenaars SP, Bastin ME, Deary IJ, Whalley HC, & McIntosh AM (2017). Association of polygenic risk for major psychiatric illness with subcortical volumes and white matter integrity in UK Biobank. Scientific Reports, 7(1), Article 1. 10.1038/srep42140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Riglin L, Thapar AK, Heron J, Anney R, O’Donovan MC, & Thapar A (2019). Characterizing Developmental Trajectories and the Role of Neuropsychiatric Genetic Risk Variants in Early-Onset Depression. JAMA Psychiatry, 76(3), 306–313. 10.1001/jamapsychiatry.2018.3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AB, Mumford JA, Naliboff BD, & Poldrack RA (2012). Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion (Washington, D.C), 12(1), 58–68. 10.1037/a0026517 [DOI] [PubMed] [Google Scholar]
- Schmaal L, Pozzi E, Ho C, van Velzen, Veer LS, Opel IM, Van Someren N, Han EJW, Aftanas LKM, Aleman L, Baune A, Berger BT, Blanken K, Capitão TF, Couvy-Duchesne L, Cullen B,R, Dannlowski K, Davey U, Erwin-Grabner, T C, … Veltman DJ. (2020). ENIGMA MDD: Seven years of global neuroimaging studies of major depression through worldwide data sharing. Translational Psychiatry, 10(1), Article 1. 10.1038/s41398-020-0842-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, Gauthier S, & Pruessner JC (2016). Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage, 129, 1–14. 10.1016/j.neuroimage.2016.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shengli C, Yingli Z, Zheng G, Shiwei L, Ziyun X, Han F, Yingwei Q, & Gangqiang H (2022). An aberrant hippocampal subregional network, rather than structure, characterizes major depressive disorder. Journal of Affective Disorders, 302, 123–130. 10.1016/j.jad.2022.01.087 [DOI] [PubMed] [Google Scholar]
- Sherif T, Rioux P, Rousseau M-E, Kassis N, Beck N, Adalat R, Das S, Glatard T, & Evans AC (2014). CBRAIN: A web-based, distributed computing platform for collaborative neuroimaging research. Frontiers in Neuroinformatics, 8, 54. 10.3389/fninf.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, & Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), Article 10. 10.1038/nrn3785 [DOI] [PubMed] [Google Scholar]
- Tang L, Pruitt PJ, Yu Q, Homayouni R, Daugherty AM, Damoiseaux JS, & Ofen N (2020). Differential Functional Connectivity in Anterior and Posterior Hippocampus Supporting the Development of Memory Formation. Frontiers in Human Neuroscience, 14. https://www.frontiersin.org/articles/10.3389/fnhum.2020.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti A, & Belzung C (2013). Neurogenesis along the septo-temporal axis of the hippocampus: Are depression and the action of antidepressants region-specific? Neuroscience, 252, 234–252. 10.1016/j.neuroscience.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Trivedi MA, & Coover GD (2004). Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiology of Learning and Memory, 81(3), 172–184. 10.1016/j.nlm.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, & Yurgelun-Todd DA (2000). Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biological Psychiatry, 47(12), 1087–1090. 10.1016/S0006-3223(99)00296-6 [DOI] [PubMed] [Google Scholar]
- von Stumm S, Smith-Woolley E, Ayorech Z, McMillan A, Rimfeld K, Dale PS, & Plomin R (2020). Predicting educational achievement from genomic measures and socioeconomic status. Developmental Science, 23(3), e12925. 10.1111/desc.12925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, DeWitt I, Goff D, Ditman T, & Heckers S (2005). Anterior and posterior hippocampal volumes in schizophrenia. Schizophrenia Research, 73(1), 103–112. 10.1016/j.schres.2004.05.018 [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Gameroff MJ, Warner V, Pilowsky D, Kohad RG, Verdeli H, Skipper J, & Talati A (2016). Offspring of Depressed Parents: 30 Years Later. American Journal of Psychiatry, 173(10), 1024–1032. 10.1176/appi.ajp.2016.15101327 [DOI] [PubMed] [Google Scholar]
- Willard SL, Friedman DP, Henkel CK, & Shively CA (2009). Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology, 34(10), 1469–1475. 10.1016/j.psyneuen.2009.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN, & Chakravarty MM (2013). A novel in vivo atlas of human hippocampal subfields using high-resolution 3T magnetic resonance imaging. NeuroImage, 74, 254–265. 10.1016/j.neuroimage.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, Bacanu S-A, Bækvad-Hansen M, Beekman AFT, Bigdeli TB, Binder EB, Blackwood DRH, Bryois J, Buttenschøn HN, Bybjerg-Grauholm J, … Sullivan PF (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics, 50(5), Article 5. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Gao Y, Feng R, Zhuo L, Bao W, Liang K, Qiu H, Cao L, Tang M, Li H, Zhang L, Huang G, & Huang X (2022). Aberrant intrinsic hippocampal and orbitofrontal connectivity in drug-naive adolescent patients with major depressive disorder. European Child & Adolescent Psychiatry. 10.1007/s00787-022-02086-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.