Summary:
The importance of time is ever prevalent in our world and disruptions to the normal light/dark and sleep/wake cycle have now become the norm rather than the exception for a large part of it. All mood disorders, including seasonal affective disorder (SAD), major depressive disorder (MDD), and bipolar disorder (BD), are strongly associated with abnormal sleep and circadian rhythms in a variety of physiological processes. Environmental disruptions to normal sleep/wake patterns, light/dark changes and seasonal changes can precipitate episodes. Moreover, treatments that target the circadian system have proven to be therapeutic in certain cases. This review will summarize much of our current knowledge of how these disorders associate with specific circadian phenotypes, as well as the neuronal mechanisms that link the circadian clock with mood regulation. We also discuss what has been learned from therapies that target circadian rhythms and how we may use current knowledge to develop more individually designed treatments.
Keywords: depression, bipolar disorder, seasonal affective disorder, sleep, chronotherapy
In Brief
In Dollish et al., they review the clinical literature and provide mechanistic insights into how circadian rhythms contribute to mood disorders. They also describe chronotherapeutic treatments and provide hypotheses for testing in future studies of both mechanism and novel treatments.
Introduction:
Mood disorders including SAD, MDD, BD, all have abnormalities in sleep/wake patterns as a core symptom, and in fact this is one of the primary diagnostic criteria used clinically. Moreover, acute changes to the sleep/wake cycle can precipitate mood episodes.1 There are now hundreds of studies which find an association between having a late chronotype (i.e. preference for the evening over morning activities) and developing a mood disorder.2,3 For example, one study in the Netherlands of nearly 2000 people found a significant association between late chronotype and depression/anxiety (P = .004) even when adjusting for sociodemographic, somatic health, and sleep-related factors which was largely driven by those with depression.4 Furthermore, a very large study in the United Kingdom (433,268 adults) comparing definite evening type to definite morning types in a wide assessment of health related issues found that the associations were strongest for psychological disorders and late chronotype (OR 1.94, 95% CI 1.86–2.02, p = < 0.001) over other health issues, though interestingly having a late chronotype was associated overall with poorer health outcomes and even an increased risk for all causes of mortality.5
We also know that chronobiologic treatments aimed at changing circadian rhythms in particular ways can be highly beneficial in the treatment of these disorders.6,7 Due to the complex etiology of these diseases, however, it is difficult to say whether mood disorders are directly caused by circadian rhythm disruptions, or how much of the association between chronotype, circadian rhythms, sleep and mood disorders is due to desynchronization from the environment versus genetic or other abnormalities in the core molecular pacemaker.
Circadian clocks evolved to calculate and keep track of the amount of time that has passed for the daily rotation of the earth. Without this development, organisms would not be able to anticipate the daily sunrise, develop reliable wake and rest periods, or control metabolic demands in an efficient way.8,9 Humans share evolutionary conservation of this ancient time keeping mechanism10 and every organ, and in fact nearly every cell in the body, is tuned to this daily cycle. This allows the body to maximize energy production during active periods and conserve energy, as well as restore and repair cellular functions, during rest periods. The clock is also crucial in timing immune signaling to protect organisms from harm without creating internal immune problems.11 Thus, when this internal mechanism of time keeping is thrown off (which occurs frequently in our modern world), it can contribute to or even cause a plethora of physiological consequences, including diseases like sleep disorders, heart disease, metabolic disorders, cancer, neurodegenerative disease and psychiatric disorders.9,10,12,13
Circadian rhythms are centrally controlled in the brain by the suprachiasmatic nucleus (SCN) located at the base of the hypothalamus. The SCN core receives direct projections from the retina through cells which contain the photoreceptor, melanopsin, which allows the SCN to respond directly to light, the primary entrainer of circadian rhythms (Figure 1).14 An important aspect of circadian rhythms is that even in the absence of light they will continue to run with a period very close to 24 hrs and this is controlled largely by the shell of the SCN which receives and gives feedback to the core to regulate rhythms over the light/dark cycle.15 Without light or other entraining stimuli, rhythms will “free run” such that they drift ever so slightly every day leading in humans typically to later and later wake and sleep timing.16 Entrainment by light and other factors is critical for the proper functioning of all biological processes as it ensures rhythms are timed to the proper phase of the solar light/dark cycle as well as with each other. For example, when one travels to a new time zone, they typically experience some form of “jet lag” which is a condition in which the internal pacemaker in the SCN becomes out of synch with the environment and needs time to entrain to the new light/dark cycle. Jet lag is characterized by daytime fatigue, mood changes, headaches, inability to concentrate and digestive problems.17 Eastward travel produces a phase advance in rhythms while westward travel produces a phase delay, changing the timing of rhythmic peaks and troughs.18 It typically takes one day for every hour traveled to entrain to the new light/dark cycle, and the response to the phase advance of eastward travel is typically harder to adjust to compared to westward travel.17 In addition to entrained or non-entrained (e.g. free running) rhythms, a person’s chronotype or lifestyle choices can lead to either highly irregular sleep/wake patterns, or a phase advance or delay compared to normally entrained rhythms such that they wake up earlier or later than what is typical (Figure 2). In extreme cases, persistent advanced or delayed rhythms are classified as advanced or delayed sleep phase disorder in which people have a difficult time with school, work or social activities due to the rigid timing of their endogenous rhythms.19
Figure 1. The Retinohypothalamic circuit responds to light from the environment and uses it to entrain internal rhythms.

Light information is directly projected to the SCN through the retinal hypothalamic tract. Starting in the retina, it travels to ventral core of the SCN in the hypothalamus which communicates with the dorsal shell that houses the endogenous pacemaker. The bidirectional communication between the shell and core together entrain and synchronize peripheral clocks in the brain and body.
Figure 2. Circadian rhythm perturbations as depicted through activity patterns.

Daylight hours (yellow), darkness (blue), activity periods (black bars (1 line = 1 day) 1) Entrained rhythms align with the light/dark cycle, 2) delays are shifted with onset occurring later than observed entrained rhythms, 3) advances are shifted to occur earlier than usual, 4) fragmentation is a breakdown and disorganization of entrained rhythms, and 5)free running conditions are the absence of cues or the inability of rhythms to entrain to external cues.
While the SCN is the master pacemaker that conducts the orchestra of rhythms across the brain and body, a core molecular clock resides in nearly every cell. This core mechanism in mammals lies in a transcription-translation feedback loop between circadian genes and their products (Figure 3). In humans, the Circadian Locomotor Output Cycles Kaput (CLOCK) and Aryl hydrocarbon receptor nuclear translocator like protein (ARNTL also known as BMAL1) genes encode basic helix-loop-helix transcription factors which heterodimerize and bind to DNA E-boxes in a variety of genes.20,21 Among the many genes controlled by CLOCK:ARNTL, are the Period (PER) and Cryptochrome (CRY) genes. Once translated, PER and CRY proteins form a stable dimer which then can reenter the nucleus to inhibit their own transcription.20 In addition to this primary loop, there is a secondary stabilizing feedback loop involving the REV-ERBɑ and Retinoic acid-related orphan receptor alpha (RORɑ) proteins which regulate ARNTL expression to affect the timing of the primary loop. In addition, several other proteins contribute to the timing and speed of these molecular rhythms which lead to diurnal rhythms in gene expression of thousands of other clock-controlled genes whose identity can be vastly different depending on the cell type.22
Figure 3. Molecular machinery of the circadian clock.

Circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein 1 (BMAL1; a.k.a. ARNTL) heterodimerize and bind to Enhancer Box (E-box) elements to activate clock-controlled genes (CCGs) transcription, including the Period (Per) and Cryptochrome (Cry) genes. PER and CRY proteins dimerize and translocate back to the nucleus to inhibit CLOCK and BMAL1 activity, forming a negative loop, which cycles every 24 hours. In a secondary loop, CLOCK and BMAL1 proteins regulate the nuclear hormone receptors expression, Rev-erb and Ror, which inhibit or activate Bmal1 transcription, respectively.
This review will summarize current knowledge of the interwoven nature of circadian rhythms and mood disorders. In addition, we will explore possible circadian mechanisms and pathology of SAD, MDD, and BD, as well as current and experimental treatment options that add to the growing literature budding from interest in this field.
Seasonal Affective Disorder (SAD)
Although canonically called seasonal affective disorder (SAD) the current DSM-5 diagnosis is Major Depressive Disorder with a Seasonal Pattern and this is a common mood disorder. According to the American Psychiatric Association about 5% of adults in the United States experience SAD. Seasonal patterns of mood episodes are also experienced by approximately 25% of individuals with BD23. The diagnostic criteria for SAD can be similar to MDD24,25 and more closely overlaps with symptoms seen in atypical depression such as hyperphagia, hypersomnia, and fatigue/lethargy as opposed to symptoms of melancholic or typical depression.26 Though SAD is laregely recognized by the greater psychiatric community, one study by Traffanstedt et al. (2016)27 failed to find differences in the Patient Health Questionnaire-8 Depression Scale in a cross sectional survey of U.S. adults that varied with latitude or season, suggesting possibly that seaonal depression as a disorder may not be so clear cut, however this study is limited in that it is cross sectional self report, it only examines one point in time for each individual’s current symptoms, and combines a variety of depression-related symptoms together, some of which are not consistent with the greater litterature on seasonal depression.28 The feature that most distinguishes SAD from MDD is that depressive symptoms last roughly 40% of the year and symptom on and off set is timed to correspond with longer or shorter daylight hours indicating a seasonal change.24,25 Atypical depression also has diurnal features with symptoms sometimes worsening in the evening; however those with SAD often see a worsening of symptoms in the morning suggesting, along with other differences such as the response to timed light therapy, that although these diseases are similar they are indeed separate disorders.26,29,30 Typically, SAD occurs during the transition between fall to winter months where the rate of daylight change from long photoperiod to short photoperiods is the highest. However, SAD is not limited to the winter months, and also occurs during the summer months in about 10% of people with SAD and the symptoms of summer SAD often include insomnia and less appetite.31 The length and severity of symptom onset is individual and can be measured using the Seasonal Pattern Assessment Questionnaire (SPAQ) by looking at the Global Seasonality Score (GSS) which indicates the presense of SAD and its severity with anything over an 11 indicating moderate to severe SAD.25 Chronotype, gender, and age also play a role in developing or showing symptoms of SAD with a strong sex bias towards women who are 4x more likely to develop the disease. In a study by Höller et al they found that people under the age of 60 and those with evening chronotypes had a higher seasonality score on the SPAQ.32 Interestingly latitude (living in the north) weakly associates with SAD, however the rate of incidence of SAD generally increases as one moves further from the equator.33,34 A manuscript comparing two studies of environmental factors that could contribute to SAD by Young et. al. (1997)35 found in the first study which examined data from 5 different locations, that latitude and time of year contributed to SAD, but the second study examining data collected over 7 years from 1 location failed to find evidence of daily hours of sunshine or temperature that relate to SAD suggesting that photoperiod is related to the onset of SAD as opposed to total hours of sunlight. However, a study by Murray and Hay (1997)36 failed to find photoperiod specificity to SAD symptomology. However this study, although robust with 526 female participants from the Australian Twin Registry, only looked at a single location (Australia) and not locations across a broad latitude which is important in making this assessment.
Genetics seem to play a role in both developing SAD as well as seasonal symptom onset. Single nucleotide polymorphisms (SNPs) in the clock genes ARNTL, CLOCK, and PER3 have been shown to contribute to developing depression, anxiety and are particularly associated with seasonal depression.37 SNPs in CRY1 and CRY2, have also been linked to seasonal depression in a longitudinal study.38 In addition, Ho et. al. (2018) conducted a genome-wide association study of SAD and found that intronic variant rs139459337 in the zinc finger protein, ZBTB20, was the SNP most strongly associated with SAD, and individuals with this SNP had reduced RNA expression of ZBTB20 in the temporal cortex.39 ZBTB20 is important in the regulation of circadian activity output and of the 330 known human targets of this gene, a significant enrichment of these targets are found in SAD genetic association signals, further suggesting that ZBTB20 may be centrally important in seasonal mood regulation.39,40 However, it is important to note that most of these studies have been rather small candidate gene studies with the largest, aforementioned GWAS study consisting of only 1380 cases, so larger studies will need to be done to assess potential genetic associations.
Genetics also determine a person’s chronotype41 which can increase their susceptibility to developing SAD. A study looking at 1539 adolescents found that when evaluated with the Morning-Eveningness Questionnaire and the SPAQ, those with evening preference had significantly higher scores on the SPAQ than those with intermediate and morning chronotypes.42,43 Furthermore, a 3-year study evaluating 244 individuals found a positive association between lower mood and eveningness.44,45 This could be due to the fact that evening chronotypes often wake up later in the day compared to the rest of the population, which would reduce exposure to daytime light, especially in the winter months when individuals are more likely to develop SAD. However, chronotype does not entirely predict SAD and reduced exposure to light during the day does not track with all cases of SAD, since summer variations of SAD occur in a small subset of SAD suffers. Or another possibility is that evening types are already biologically out of phase from the environment, and have rhythms that are strongly set, making adaptation to seasonal changes challenging.
Potential mechanisms linking circadian rhythms and SAD
Although SAD is highly prevalent, its etiology is not fully understood. Animal studies point to an important role for hypothalamic and midbrain dopamine neuron populations in SAD. In a landmark study, adult rats exposed to a short-day photoperiod experienced what appears to be “neurotransmitter switching” in the hypothalamus in which somatostatin containing, GABAergic interneurons became dopamine neurons, and blockade or ablation of these new dopamine neurons increased anxiety and depression-like behavior.46 Long-day photoperiods produced the opposite effect. These results are intriguing in that they suggest that monoaminergic systems in the brain can be fundamentally altered simply by changing the photoperiod, and this can result in changes in mood-related behavior. Interestingly, a recent study by Jameson et al. (2023) also found that photoperiod length can modulate dopamine signaling in the nucleus accumbens (NAc) core and this is sex specific. Both dopamine release and uptake in the NAc were increased in female mice raised in a long photoperiod versus a short photoperiod however, there were no differences in male mice.47 This is particularly interesting given the higher prevalence of SAD in females compared to males.
Mice generally find light aversive as they are nocturnal, making some results such as those stated above difficult to compare to humans, so investigators have instead used the diurnal grass rat as model with greater face validity. Indeed, grass rats housed in 8:16 photoperiods mimicking winter develop an increase in depressive-like behavior.48 These studies also found that the neuropeptide orexin helps mediate light induced changes in mood and anxiety in these animals. As opposed to mice, in grass rats the number of hypothalamic dopamine neurons were reduced in a short photoperiod, and numbers of dopamine neurons can be altered with treatment with an orexin 1 receptor antagonist, suggesting that orexin is involved in mediating these changes in dopamine neurons. Another study by Costello et. al. (2023) found that administering light to diurnal grass rats under a protocol similar to that used in bright light therapy (BLT) induced higher wakefulness, increased nighttime sleep quality and improved rhythm entrainment, similar to what is observed in humans.49 Moreover, this treatment led to changes in prepro-orexin and orexin receptor expression which was brain region and sex specific. Taken together there is strong evidence that photoperiod changes directly impact dopamine signaling, potentially via orexin and that this contributes to seasonal mood changes. However, it is important to note that photoperiod induced changes in dopamine signaling are different in diurnal versus nocturnal species as well as brain region and sex specific.
Cortisol rhythms are widely known to be synchronized to the external light-dark cycle as seen by a nadir in the early evening and progressively increasing till it peaks just before waking along with a spike in serotonin before gradually tapering off again.50 Individuals with SAD have a cortisol awakening response (CAR) in the summer that does not differ from healthy populations, but during the winter months, where SAD patients report increased feelings of stress, depression and anxiety, the CAR is attenuated compared to healthy individuals.51,52 However, this finding does not necessarily indicate that SAD is a hypocortisolemic condition. In a systematic review of 13 papers, those that used heterogeneous methods and samples were unable to consistently replicate the attenuated CAR response in SAD patients.53 Thus further studies are needed to understand if perhaps there is a subgroup of subjects in which the winter CAR response is attenuated.
In long photoperiods, cells in the caudal SCN are desynchronized with rostral cells having a bi-modal pattern of activity with one activity peak at dawn and the other at dusk.54 Control of seasonal neural activity and behaviors also seems to be somewhat localized to the pars tuberalis (PT) a tubular sheath that wraps around the pituitary gland and may act in tandem with the SCN to produce seasonal timekeeping and entrainment signals and output in mammals.55 It might be that similar to the pineal gland which houses the “night clock” via melatonin secretion, that the pars tuberalis is the home of the “seasonal clock” with its own unique seasonal timekeeping system and zeitgebers. SCN plasticity occurs in response to adjustments in daylength. Long day photoperiods indicative of summer lead to an increase in the duration of neuronal activity periods at the population level in hamster SCN slice compared to short days.56 In addition to population activity, the phase distribution between specific types of SCN neurons is also important for seasonal entrainment.57 Short days result in a relatively synchronized firing pattern between these SCN subpopulations while long days result in the activation of these subpopulations at different phases of the day/night cycle.57 It is possible that individuals with SAD have an SCN that cannot properly adapt to these different seasonal light cues. It is also possible that people with SAD differ in the sensitivity to light. Light and photoperiod entrainment can be measured using a post-illumination pupil response (PIPR). In a study by Roecklein et. al. (2013) subjects with SAD or control subjects were exposed to either red or blue light in the fall/winter.58 They found that the SAD group had a reduced PIPR and lower overall change relative to controls, specifically to blue light. They propose that this reduced PIPR response may be linked to a specific melanopsin gene OPN4 variant, suggesting that this circadian photoreceptor is less sensitive to light input.
Importantly, while there is evidence as described above that mood-related responses to light are regulated via the pathway from the eye to the SCN and then other brain networks, work from Samar Hattar’s group has found that there are direct projections from the eye to the perihabenula region, and that depression-related responses to changes in the light/dark cycle in mice occur via this pathway which is completely independent of the SCN.59,60 Communication between the perihabenular region and the lateral habenula may modulate dopaminergic activity in response to photoperiod changes as described above, resulting in reduced dopaminergic transmission.61 Therefore, it is unclear how much of the mood related responses to light at night, or a shortened photoperiod are regulated by the SCN versus projections from the eye to the perihabenular region.
Melatonin in humans is released by the pineal gland in the evening to facilitate the onset of sleep. In multiple mammalian species however, melatonin also plays a much larger role in seasonal processes including breeding patterns, aggression and hibernation.62–64 Symptoms of SAD include increased fatigue, excessive sleeping, carbohydrate craving, and exaggerated seasonal changes in melatonin brought on by the reduction in day length may contribute, cause or be the result of these perturbances (Figure 4). Indeed, an overproduction of melatonin during the winter is phenotypical in some SAD patients.65,66 Individuals with SAD tend to also have strongly delayed timing of melatonin secretion, which is exacerbated in the winter, leading to more fatigue during the daytime.67 Thus, melatonin is likely to play a key role in many of the symptoms of SAD. However, evidence is increasingly pointing towards SAD being a system wide misalignment issue involving multiple neurotransmitters and modulators. The inability to entrain rhythms can be rectified through better understanding of how the SCN and other networks integrate daily and seasonal light information and disseminate that information to entrain biological rhythms.
Figure 4. Melatonin secretion duration changes as a function of seasonal night length.
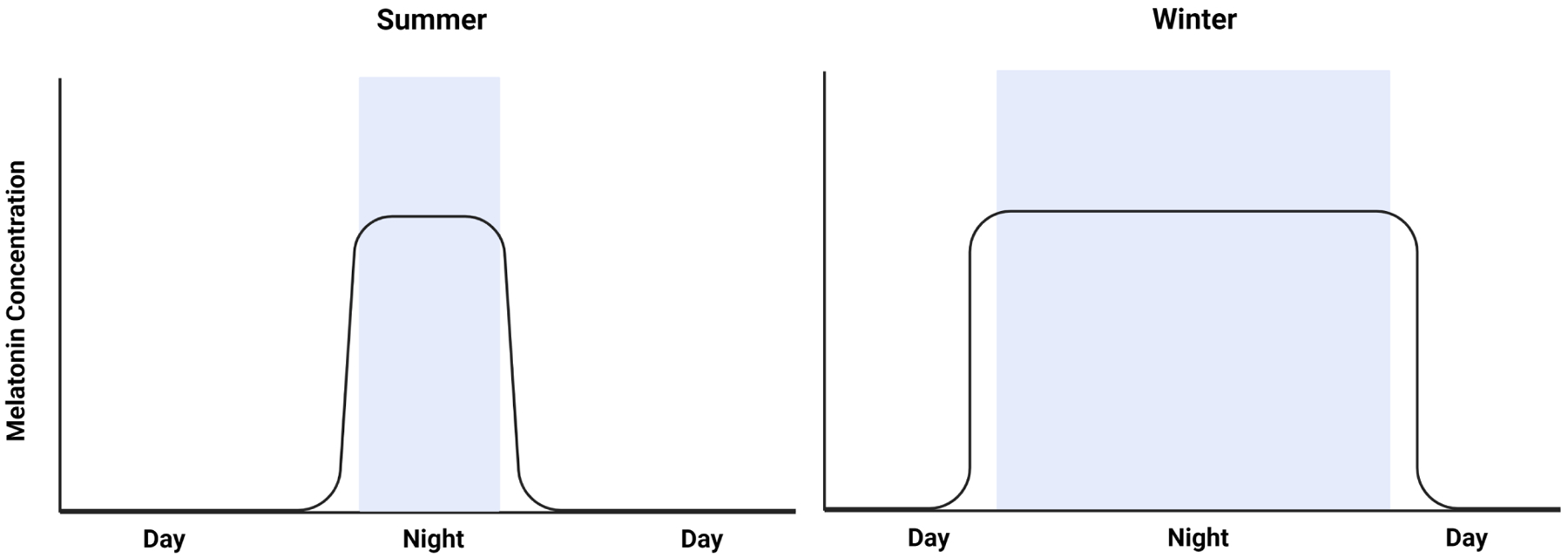
The summer melatonin secretion profile (Left) is shorter due to summers longer daylight hours suppressing melatonin secretion. In the winter (Right) melatonin secretion is longer because of the shorter daylight hours. The variation in melatonin secretion duration and amplitude changes are a factor of seasonal daylight presence allowing it to act as both a seasonal and night timekeeper.
Treatments for SAD
Bright light therapy (BLT), is the most widely used treatment for SAD. When exposed to bright broad-spectrum light (~10,000 lux) for 30 minutes, most patients with SAD report symptom amelioration or cessation with dedicated use and engagement with the therapy.6 Typically, BLT is prescribed in the early morning, as studies show that it is the most effective timing window as it can shift circadian rhythms forward to better align them with the environment and the majority of patients with SAD tend to be phase delayed.68,69 However, there are a smaller subset of patients who may experience SAD due to a phase advance, perhaps due to the earlier winter dusk. Differences in BLT timing and chronotype have not been observed70; however, differences in individual BLT timing exist and optimization of the prescribed time and “dose” of light is important.71 An important study from Lewy et al (2006) tested the phase shift hypothesis of SAD treatment through the administration of low-dose melatonin either in the morning or afternoon/evening to induce phase advances or delays.66 Their administration protocol varied over the course of the 4 years this study took place over, with daily melatonin totaling 0.225 mg (year 2) or 0.3 mg (years 1, 3, and 4). They found that there is an optimal “therapeutic window” for phase aligning patients based on the timing between their dim light melatonin onset and midpoint of sleep which is optimally 6 hrs. Treating people with appropriately timed melatonin (which was typically at night) significantly correlated with symptom improvement while inappropriately timed melatonin did not.66
Another way that BLT may be beneficial in SAD is that it decreases the amount of melatonin being produced. When exposed to light – specifically blue light – melatonin production is halted and is quickly eliminated from the body.72 For individuals that have either an increased production of melatonin during the day or a phase shift in melatonin release such that it is still present during the day, BLT will reduce melatonin levels. Selective serotonin reuptake inhibitors (SSRIs) have also been used in the treatment of SAD with promising results, however, it should be noted that further studies of the side-effect “cost-benefit” and replication of findings are still needed and noted in many of the studies.73–75 Further research is needed to determine if other treatments such as agomelatine, a melatonin receptor agonist and 5-HT2c receptor antagonist, has any efficacy in the treatment of SAD.
Major Depressive Disorder (MDD)
Major depressive disorder is a common and debilitating disorder affecting more than 300 million people worldwide.76 In the last several decades, prevalence of MDD has been steadily increasing.77 MDD is characterized by depressed mood, loss of interest in pleasurable activities as well as physiological and cognitive symptoms. Insomnia, or oversleeping, fatigue and decreased energy, and changes in appetite are common among patients with MDD. Either insomnia or oversleeping is one of the main diagnostic criteria for MDD, and more than 80% of patients with MDD report sleep disturbances.78 Mood fluctuates over the course of 24 hours in healthy individuals; however, diurnal variations in mood are altered in patients with MDD. Compared to healthy controls, depressed patients exhibit pronounced diurnal disturbances in positive and negative affect.79 Patients often report the worst symptoms early in the morning, with a subset of patients (about 20%) also experiencing an afternoon slump.80 Chronotype might also influence diurnal variations in mood, and MDD patients with an evening chronotype are more likely to experience worsening of their symptoms in the morning and overall increased rumination.81 Moreover, an evening chronotype is a strong risk factor for MDD and is associated with more frequent and severe depressive episodes3, greater risk for suicidality82, and reduced SSRI treatment efficacy.83 Increasing rates of depression worldwide can be linked to modernization of society, with its increased exposure to artificial light, shift work and air travel across multiple time zones.77,84 People living in areas with the highest levels of outdoor LAN show increased odds of depressive symptoms and suicidal behaviors.85
Shift work causes disturbances to circadian rhythms leading to a number of negative health consequences, including sleep disturbances, fatigue, cognitive impairments and increased risk of cardiovascular and metabolic disorders and certain types of cancers.86–88 A meta-analysis by Lee et al suggests that night shift work is also associated with the increased risk of developing depression.89 Moreover, compared to the general population, flight attendants have a higher prevalence of sleep disorders, fatigue, depression and anxiety.90 In rodents, exposure to jet lag paradigms worsens depressive-like behaviors, as evidenced in reduced sucrose intake, increased immobility time in the forced swim test, and reduced number of entries to the center zone in the open field test.91 There is also evidence that long-distance flights can trigger depressive episodes in people with a history of mental illness.92,93
A study of circadian gene expression in human postmortem brain found that when looking across a population of subjects who died at different times of day, rhythmic patterns of gene expression were much weaker (i.e. lower amplitude) in subjects with MDD and this was particularly true in cortico-limbic regions of the brain.94 This was due in part to shifts in timing and disrupted phase relationships between individual genes. Animal studies also indicate that circadian rhythms play a significant role in MDD. Knocking-down Bmal1 specifically in the SCN results in increased depression-like behavior in mice as evident by increased immobility times in the tail suspension test and increased escape latency times in the learned helplessness test.95 Furthermore, the severity of chronic stress-induced depression and anxiety-like phenotypes in mice correlates directly with a loss of amplitude of molecular rhythms in the SCN.95,96 Mice that are susceptible to developing a depression-like syndrome following chronic social defeat stress also have a loss of circadian rhythmicity in core body temperature while mice that are resilient to developing a depression-like phenotype following stress do not.97 Recently, a Bmal1 knock-out cynomolgus monkey was developed using CRISPR/Cas9 editing of monkey embryos.98 These monkeys had higher nocturnal locomotion and reduced sleep, which was exacerbated when they were housed in constant light. Moreover, these monkeys showed phenotypes consistent with anxiety and depression, including elevated blood cortisol. Transcriptome analysis in blood showed multiple changes in genes associated with inflammatory and stress responses including for example Toll-like receptor 4 (TLR4), which was also found in blood transcriptomes of subjects with MDD and in subjects with sleep deprivation.
Potential mechanisms linking circadian rhythms and MDD
Dysregulation of the hypothalamic-pituitary axis (HPA) is a common finding in MDD patients. HPA axis controls production and secretion of glucocorticoid hormone, a potent regulator of all major physiological systems. Glucocorticoids have a strong circadian rhythm in their expression. In healthy subjects, maximal cortisol secretion happens in the morning hours before awakening, and then gradually decreases during the day.99 In depressed individuals, however, lower levels of cortisol in the morning and higher evening levels of cortisol have been reported across studies.100 In addition, a phase advance of the morning cortisol in MDD patients compared to controls has also been reported.101 Furthermore, dysregulated cortisol levels may predispose individuals to subsequent depressive episodes.93 A number of studies suggest that crosstalk between the HPA axis and the immune system mediates behavioral responses to stress102,103, which is a common trigger in the development of depression. The immune system protects against injuries and infections, however a dysregulated immune system leads to chronic inflammation and contributes to the pathophysiology of psychiatric disorders, including depression.104–106 The circadian clock is directly involved in regulating the immune system, including production of cytokines11, phagocytosis107, anti-inflammatory responses108, as well as the expression of pattern recognition receptors.109 Disruption in circadian rhythms results in increased levels of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α).11 In turn, TNF-α has been shown to play an important role in regulating circadian rhythms primarily by interfering with E-box-mediated transcription and thus suppressing the expression of clock genes Per1 and Per2 both in vitro and in vivo.110,111 Chronic sleep deprivation which can result from disrupted circadian rhythms, leading to an increased inflammatory profile in mice and increased microglial activation and astrocytic phagocytosis.112 On the other hand, microglial dysfunction may also be a contributing factor in the pathophysiology of sleep disorders, potentially resulting in a vicious cycle of sleep and mood disruption.113
Similar to seasonal depression, MDD may also involve disruptions to monoamine signaling. Rats kept for 6 weeks in constant darkness such that they can’t entrain to light, develop a depressive like phenotype, as evidenced by increased immobility times in forced swim test, which is associated with impairment of the noradrenergic locus coeruleus system.114 In addition, these free running conditions lead to increased apoptosis of noradrenergic, dopaminergic and serotonergic neurons which can be partially rescued by desipramine treatment.115 Interestingly these circadian effects on monoaminergic cells were not associated with increased measures of stress, suggesting that this effect is independent of a general stress response. Circadian genes within monoaminergic-rich regions of the brain also play a role in mood regulation both with and without chronic stress. Chronic social defeat stress significantly reduces expression of Per1 and Per2 in the NAc, and consistent with this, knocking down Per1 and Per2 specifically in the NAc leads to increased anxiety-like behavior, as demonstrated by decreased number of entries into open arms in the elevated plus maze and decreased time spent in the center of an open field, in the absence of stress.97,116 Treatment with fluoxetine normalizes Per gene expression following stress and reverses social interaction deficit, suggesting Per1 and Per2 gene involvement in depression-like and anxiety-like behaviors. Intriguingly, knocking down Bmal1 in the NAc, leads to reduced susceptibility to helpless behavior in a learned helplessness paradigm.117 Furthermore, a knock-down of Bmal1 specifically in astrocytes in the NAc leads to increased exploratory drive (i.e. less anxiety-related behavior).118 Thus, more work needs to be done to understand the complex roles of individual circadian genes within these brain regions and specific cell types and how all of these various factors contribute to the development of MDD (Figure 5).
Figure 5. Mood Regulation by circadian rhythms and the environment.

There is a complex interplay between circadian rhythms, the environment, sleep and mood regulation. The circadian clock affects multiple brain regions and systems and certain mutations in the circadian genes might make an individual more vulnerable to mood disorders. Environmental factors such as seasonal changes, stress or night shift work can lead to sleep and circadian dysfunction and contribute to mood changes. DA, dopamine; 5-HT, serotonin; NE, norepinephrine; MEL, melatonin; SCN, suprachiasmatic nucleus; HPA, hypothalamus-pituitary-adrenal.
Treatments for MDD
Further evidence for the connection between circadian rhythms and depression comes from the fact that treatments directly targeting the circadian system (such as acute sleep deprivation, bright light therapy and sleep phase advance) can be successful in treating depression. Depressed patients typically exhibit sleep phase delay, with the sleep onset falling between 2 am – and 6 am.119,120 This pronounced phase delay might be due to either a biological or self-imposed misalignment between the internal biological clock and the sleep-wake cycle121 and treatments targeting the circadian system and/or cognitive behavioral therapy may help to phase advance and align circadian rhythms.122
One of the most effective chronotherapies used as a rapid acting treatment of depression is acute sleep deprivation, which has been shown to improve depressive symptoms in approximately 40% to 60% of patients123,124 and repetition of acute sleep deprivation treatment leads to increasingly better outcomes.124 However, the effects of an acute treatment are typically not long lasting and symptoms often return after a night of sleep. Sleep deprivation may exert its antidepressant action through the resetting of abnormalities in circadian gene expression.125 Interestingly, both sleep deprivation and ketamine, another rapid-acting treatment, result in common transcriptional changes in the anterior cingulate cortex, where a number of genes are downregulated, including Per2 and Npas4.125 Interestingly both the acute and lasting response to ketamine in people with MDD can be predicted based on their diurnal activity profiles. Prior to ketamine treatment, a phase advanced activity pattern and lower mesor (the rhythm-adjusted mean) distinguished subsequent responders from nonresponders.126 Moreover, on day 1, ketamine nonresponders had a blunted 24 hr amplitude relative to baseline while a higher amplitude following ketamine treatment on day 3 was associated with a persistent clinical response.127 These data suggest that ketamine is most effective in those with a particular circadian profile at baseline, and that it has a persistent anti-depressant response in those in which the drug induces higher amplitude rhythms.
As mentioned previously, similar to SAD many individuals with MDD are phase delayed and some patients with MDD have lower levels, or inappropriately timed melatonin, which is crucially important in maintaining appropriately timed sleep-wake rhythms.121 In clinical settings, 8 weeks of morning BLT alone or in combination with fluoxetine was well tolerated and efficacious in reducing depressive symptoms in patients with MDD as evidenced by score changes on the Montgomery-Asberg Depression Rating Scale.128 A potential mechanism for the antidepressive effects of BLT for MDD was recently proposed by Huang and colleagues (2019). They showed that a dedicated retina – ventral lateral geniculate nucleus and intergeniculate leaflet – (vLGN/IGL) lateral habenular (LHb) pathway regulates depressive-like behaviors. Specifically, activation of intrinsically photosensitive retinal ganglion cells (ipRGCs) projecting to vLGN/IGL, activation of vLGN/IGL neurons projecting to LHb, or inhibition of postsynaptic LHb neurons was sufficient to decrease depressive-like behaviors in rodents, tested through sucrose preference, forced swim and shuttle box tests.129
It is important to emphasize that while light exposure in the morning can aid in ameliorating depressive symptoms, exposure to light at night can produce negative health outcomes and associated with increased odds for depression.85,130 A recent study by An et al. (2020) helps explain why light exposure has different effects on mood during the day and night. The researchers discovered a circadian-gated neural pathway from intrinsically photosensitive retinal ganglion cells ipRGCs to the dorsal perihabenular nucleus (dpHb) to the nucleus accumbens (NAc), which preferentially relays light signals at night.131 In particular they, showed that subpopulation of dpHb neurons projecting to NAc were more excitable at night and preferentially responded to light at night and not daylight. Excitation of these dpHb neurons either optogenetically or chemogenetically led to reduced sucrose preference when administered at night but not during the day.
Studies conducted in both humans and rodents show that treatment with antidepressant medications also leads to a phase advancement of circadian rhythms.84,132 Fluoxetine, a selective serotonin reuptake inhibitor, can phase advance neuronal firing in the SCN.133 Treatment with citalopram, another SSRI, increases the sensitivity of the circadian system to light in MDD patients, which might explain why bright light therapy is more efficacious when administered together with SSRIs.93 Agomelatine, a high affinity agonist for melatonin MT1 and MT2 receptors and a 5HT2C antagonist, has proven to reduce depressive symptoms in MDD patients134 and has been shown to phase-shift circadian rhythms of body temperature and release of hormones and corrects the circadian rhythm disruptions caused by prenatal restraint stress in adult rats, suggesting that it’s mechanism of action as an antidepressant involves modulation of the circadian system.135,136
Insomnia is one of the common symptoms of MDD, and one of the non-medication approaches to treat insomnia is cognitive behavioral therapy for insomnia (CBT-I). Interestingly, CBT-I has been shown to be effective in reducing the risk for the onset of a depressive episode and in treating depression.137–139 Intriguingly, a randomized control trial evaluating CBT-I effectiveness in individuals with comorbid depression and insomnia found that CBT-I is particularly effective for individuals with an evening preference.140 The investigators propose that clinical care for patients with comorbid MDD and insomnia could be improved if circadian preferences of the patients are taken into account.140
MDD is very heterogeneous as a disorder and the key in future clinical studies will be to measure each individual’s circadian and sleep patterns as well as physiological rhythms such that appropriate chronotherapeutic treatments can be chosen which will produce the best response. A better mechanistic understanding of how chronic stress and other factors interact with the circadian system will also lead to the development of more targeted treatments.
Bipolar Disorder (BD)
Bipolar disorder is a complex disorder characterized by states of mania and depression and mixed states with both features. Between states individuals may experience a relatively stable period termed euthymia. An estimated 2.8% of individuals in the United States are diagnosed with BD and it tends to impact males and females fairly equally with some clinical differences in symptom presentation.141 Over the years, it has become apparent that in addition to the emotion and mood changes associated with these states, cardinal features of manic and depressive episodes involve changes in energy and activity.142 During mania, individuals are typically full of energy, taking on multiple tasks, rapid speech, and racing thoughts, despite very little sleep. During depressive episodes, individuals typically describe a lack of energy in which they are exhausted by routine tasks, behaviors, movements, speech, and thoughts can be slowed, and they typically oversleep. Sleep and circadian disturbances in BD have been found in multiple studies and indicate that individuals with BD have highly disrupted sleep/wake patterns which are significantly more desynchronized during episodes of mania or depression.143 Up to one-third of individuals with BD have a diagnosable circadian rhythm disorder with the most common being delayed-sleep phase disorder.144 Indeed, most individuals with BD also have an evening chronotype similar to individuals with MDD, SAD when compared to control populations. Moreover, a late chronotype predicts a greater number of depressive symptoms and less manic episodes in BD over a 5 year follow up.145 A number of studies have identified SNPs in genes that regulate circadian rhythms and have found nominally significant associations between various elements of the core circadian clock and BD. The most commonly found are SNPs in CLOCK, PER3, and ARNTL.146 ARNTL is of particular interest since it just falls short of genome wide significance in large GWAS studies of BD.147 In addition, when the circadian system is considered as an entire network as opposed to individual genes, there is a significant association with BD.148
Seasonal patterns of episodes occur in 15–25% of individuals with BD. In these individuals, manic episodes tend to occur during the spring while depressive episodes tend to occur in the fall/winter.149 Interestingly, a variant in the PER3 gene has been associated with seasonal patterns in BD.150 Along with seasonal changes, one of the most common triggers for manic or depressive episodes is changes to normal sleep patterns.151 Irregular and disrupted sleep/wake rhythms associate with and may predict first onset of BD, especially in high-risk individuals.152,153 Travel induced jet lag can be a significant trigger and interestingly a few studies have suggested that the direction of travel is important with eastward travel associated with increased manic episodes and westward travel associated with more depressive episodes.18 This is in line with studies that find that the genes that control circadian rhythms tend to run faster (i.e. with less than a 24 hour rhythm) in peripheral samples of subjects experiencing a manic episode, while these same genes run slower (greater than 24 hours) in those that are experiencing a depressive episode, with a return to a more conventional 24 hr period during euthymia.120 Interestingly, studies have also found that individuals with BD on average have a hypersensitivity to nocturnal light in that this light at night leads to a greater suppression of melatonin along with a delay in sleep, and some have suggested that this could be a trait marker of BD.143 Exposure to excessive caffeine, alcohol and drugs of abuse can also trigger episodes, as can exposure to stressful life events.154,155 Since these environmental factors can result in inappropriately timed, inefficient and shortened amounts of sleep, disruptions to the sleep/wake cycle could be the common thread that leads to the precipitation of episodes. These observations form the Social Zeitgeber Theory of BD, originally put forward by Ehlers, Frank and Kupfer in 1988, which postulates that “life events disturb social zeitgebers (“time givers”), which in turn disrupt biological rhythms, resulting in affective symptomatology in vulnerable individuals”.2
Animal studies also suggest a prominent role for circadian rhythms in BD. Mice with a mutation (a loss of exon 19; Δ19) in one of the central molecular clock genes, Clock, display a range of phenotypes that are highly reminiscent of mania.156 This includes hyperactivity, increased impulsivity and risk-taking behavior, increased reward value for multiple drugs and natural rewards, and decreased sleep time.157 Interestingly, these behavioral phenotypes are most pronounced during the inactive (i.e. light) phase of the mice, suggesting a shift between states during the active and inactive phase. Treatment with the mood-stabilizing medications, lithium and valproic acid, both reverse their behavioral phenotypes.157,158 Other mice with mutations in circadian genes share several phenotypes with the ClockΔ19 mice. For example, mice lacking Rev-erbα have a similar hyperactivity phenotype as do mice with a mutation in F-box and leucine rich repeat protein 13 (Fbxl3), a core circadian gene.159,160 Recently a mouse model was described that is termed the chronic unpredictable rhythm disturbances (CURD) model in which mice are subjected to a number of circadian, light and sleep disturbances.161 These manipulations lead to the development of a manic-like phenotype. Interestingly, this is in opposition to the effects of chronic unpredictable mild stress which leads to an overall depression like phenotype. The CURD mice showed behavioral and molecular improvements with both lithium and valproic acid. Thus, there are multiple lines of evidence from both human and animal studies suggesting that circadian rhythms are key to the pathophysiology of BD.2,18,120,143,149
Potential mechanisms linking circadian rhythms and BD
The molecular clock tightly controls cellular energy state, metabolism, protein translational processes, neurotransmission, and cellular growth, all processes implicated in BD.162 These rhythms are essential, as cells in the brain need to be highly metabolically active when the individual is awake, and then have a period of restoration in which they can rid the cell of misfolded proteins, free radicals and reactive oxygen species that have built up with activity. Core molecular clock proteins bind directly to metabolites that are produced with mitochondrial respiration, acting as a “redox sensor” informing the molecular clock in the nucleus of the metabolic state of the cell.163 In turn, the clock proteins directly regulate genes necessary for mitochondrial function and antioxidant function. Mitochondrial fusion and fission and the production of new mitochondria have a diurnal pattern and are dependent on having a functional molecular clock.164 Moreover, when the molecular clock is disrupted in animal models this leads to a large increase in reactive oxygen species in the brain, widespread gliosis and eventually neurodegeneration.165 A number of studies of BD have suggested that disruptions to mitochondrial function and oxidative stress are central to the disease166 thus the direct regulation of these processes by the circadian clock could be vitally important in disease development.
Multiple studies indicate that BD is associated with changes in dopaminergic transmission. Mania is generally associated with higher levels of dopamine while reuptake of dopamine may be greater in bipolar depression, though these studies need further replication.167 In particular, mania involves excessive goal-directed activity and impulsive decision-making driven by heightened reward seeking. These processes are known to be regulated by cortico-limbic dopaminergic systems. ClockΔ19 mice have increased midbrain dopaminergic activity, increased levels of TH and increased dopamine synthesis which is more pronounced during their inactive phase.163,168 The manic-like behavior of the ClockΔ19 mice can be recapitulated through chronic, optogenetic stimulation of VTA dopamine neurons in wild type mice168, but only during the mouse’s inactive phase, suggesting that it is inappropriate timing of increased dopamine that leads to mania and not necessarily just excess dopamine at any time of day. Blocking of TH during the light phase but not the dark phase reverses manic-like behavior.168 This is in line with the precipitation of mania in response to altered light/dark cycles, as this could lead to surges of dopamine at a time of day when it is not expected. Thus, the circadian system is directly regulating key cellular processes and dopaminergic transmission which could contribute to BD.
Another intriguing possibility lies in the concept of “metabolic jet lag”. BD is often associated with metabolic problems including obesity. Metabolic jet lag refers to “a state of shift in circadian patterns of energy homeostasis, effecting neuroendocrine, immune and adipose tissue function”.169 It is possible that erratic sleeping and eating patterns displayed by individuals with BD leads to highly disrupted rhythms in microbiome function, insulin signaling, expression of metabolic peptides like ghrelin and leptin, all of which are known to influence mood. Recent interest in the use of a ketogenic diet or time restricted feeding to control BD episodes may derive at least some of its effectiveness through stabilization of these systems though more work needs to be done to fully understand the mechanism and rates of efficacy for BD.170
Treatments for BD
Based on the Social Zeitgeber Theory, Interpersonal and Social Rhythms Therapy (IPSRT) was created as a treatment for BD.171 IPSRT is a manualized, evidence-based therapy that helps align and stabilize sleep/wake and social rhythms to help prevent the onset of episodes and reduce symptoms. In clinical studies, IPSRT produces therapeutic results that are more rapid and longer lasting than intensive clinical management in terms of occupational functioning in individuals with BD.172 Interestingly a recent study of SRT therapy on its own modified for telehealth delivery to adolescents and young adults with BD, support its role in reducing symptoms of BD, as well as suicide propensity.173
BD is commonly treated with mood-stabilizing medications that include lithium salts, anticonvulsant drugs such as valproic acid (VPA), and/or second-generation antipsychotic medications. Morning BLT commonly used to treat SAD and certain forms of MDD, has been used to treat bipolar depression, however, it can trigger a switch to mania.7 Studies find that bright light therapy given during the afternoon which will not lead to as great a phase advance in the circadian cycle, may be helpful for people with bipolar depression with less risk of triggering a manic episode.174 Importantly, all of these treatments can have a strong impact on circadian mechanisms. For example, lithium increases the amplitude of molecular rhythms, likely through inhibition of GSK3β, which is known to phosphorylate several members of the molecular clock, altering their stability and nuclear entry.175 This could act much like IPRST to keep sleep/wake patterns stable, preventing episodes from happening. In a multicenter prospective trial of lithium in 386 subjects with BD, individuals on stable lithium therapy had the least circadian disruptions while individuals with no prior lithium exposure had the most pronounced disruptions.176 Another well-known effect of lithium is that it lengthens the circadian period, increasing it to greater than 24 hrs.177 Lithium’s effects on IP3 signaling in addition to Gsk3β may underlie some of the period lengthening effects of lithium.178–180 Early studies found that lithium responders are those that have an endogenous period less than 24 hours, and this was also generally found during a manic state.181 In agreement with these studies, fibroblasts isolated from individuals with BD had muted circadian responses to lithium if they had a long period, and interestingly these individuals were not prescribed lithium by their physicians at the time of tissue collection, suggesting that they are not lithium responsive.182 In addition, iPSCs derived into neuronal precursor cells and glutamatergic neurons from lithium responders had a shorter circadian period, and lithium lengthened the period in these cells.183 Genetic variation in IP3 signaling may underlie some of these differences in responders and non-responders to lithium.180 Interestingly, VPA has a similar impact on rhythm amplitude in molecular rhythms in cell culture, but it can have the opposite effect of lithium on period, creating a shorter circadian cycle, and it’s tempting to speculate that measurement of circadian rhythms in BD may help predict treatment response to lithium or VPA.184 Multiple clinical trials have been performed testing the efficacy of melatonin or melatonin receptor agonists on BD and they have yielded mixed results. Two meta-analyses of these trials conclude that the receptor agonist, ramelteon might prevent relapse into depression in BD, and that the largest efficacy signal detected was for manic symptoms. Indeed, in the two trials assessing manic symptoms during acute mania, adjunctive melatonin had superior treatment effects versus placebo but there was substantial heterogeneity between studies and patient characteristics, and some studies found no significant differences in patient outcomes.185,186
Conclusions
In conclusion, it seems clear that circadian rhythms and the ability to adapt to photoperiod changes play a key role in mood regulation (Table 1). It is interesting that a consistently found risk factor across multiple studies for these diseases is a late chronotype. However, it is unclear if this association with psychiatric disease is due to the genetic factors that contribute to “eveningness” or a misalignment between internal rhythms and early wake times due to school or work.187 The recent COVID-19 pandemic and subsequent school and office closings provided a rare opportunity to examine chronotype on its own without environmental pressures for early wakening. Interestingly, a study of over 19,000 adults across 12 different countries found that evening-types had a large increase in sleep delay, particularly on workdays and an increase in sleep duration.188 However, they also had worse mental health, well-being, and quality of life self-report than other circadian types. These results held up with corrections for age, sex, socio-economic status or duration of confinement during the pandemic. These results suggest that even without the pressures for early wake times, evening types may be more at risk for depression or other mood episodes, particularly when faced with chronic stress. Other studies, however that have examined the impact of the delay of school start times, and flexible work schedules have suggested that environmental shifts to a later wake time have a beneficial effect on sleep and mood in evening chronotypes189,190 so it’s likely that both a biological vulnerability exists and its effects are exacerbated by social jet lag induced by early morning waking.
Table 1.
Sleep and circadian characteristics that associate with SAD, MDD and BD
| SAD | MDD | BD | |
|---|---|---|---|
| Sleep duration | Increased | Increased or decreased | Increased during depression and decreased during mania |
| Seasonal changes | Defined by seasonal symptoms which typically begin in fall/winter | More prominent in moderate depression with greatest risk in fall/winter. Stronger in women and little to no seasonal variation in psychotic depression | About 25% of cases show increased depression in fall/winter and mania in spring/summer |
| Chronotype | Strong association with evening chronotype | Evening types have both an increased risk for depression and increased symptom severity | Evening chronotype linked to depression more than mania |
| Circadian patterns | Mostly phase delayed with difficulty adapting to photoperiod change | Can be phase delayed, advanced, or low amplitude rhythms | Can be phase delayed or advanced, which can fluctuate with mood state. High intra daily variability and difficulty adapting to any change |
Another common feature across disorders is that an unstable sleep/wake or a changing light/dark cycle can precipitate episodes or make episodes worse. This suggests that many people with mood disorders may have an inflexible clock that is unable to properly adapt to change. This could be due to a deficiency in entrainment mechanisms in that there is perhaps less sensitivity to morning light, or the SCN is not able to properly respond to cues. Indeed, studies in animal models suggest that an SCN that is out of synch with the rest of the brain leads to worse behavioral outcomes than a total SCN lesion which can actually produce a protective effect against stress.95,191 It is also possible that particularly in the case of BD that the SCN is too easily thrown into a state of phase advance or phase delay and the intra-daily instability is what creates vulnerability for episodes. More work will need to be done to determine what types of mechanisms drive these episodes in the face of a change to sleep patterns or the environment.
There is also a growing body of evidence to suggest that stabilization and alignment of circadian rhythms is therapeutic for mood disorders. In the case of SAD, rhythms need to be better aligned with the environment which can typically be accomplished through BLT in the morning to shift rhythms forward, but some individuals may benefit from intervention with melatonin or other zeitgebers at a different time of day. For MDD phase advancing of rhythms may also be therapeutic for some individuals but for others, increasing rhythm amplitude through medications like ketamine may also be therapeutic. For BD, daily rhythm stabilization is key, but there is also evidence to suggest that rhythms might need to be delayed during mania and advanced during depression. In addition, the internal phase of each individual during a euthymic state should also be assessed to determine the appropriate chronotherapeutic treatment.192 Better circadian profiling of individuals and more thorough testing of chronotherapeutic treatments will ultimately guide appropriate personalized treatment options which can augment or replace existing therapies for mood regulation.
Acknowledgements:
Work from our group was funded by MH106460, NS127064, DA039865, DA046346, MH111601 to C.A.M. Figures employed the BioRender program (BioRender.com)
Footnotes
Declaration of interests: Dr. McClung is a member of the advisory boards for the Brain and Behavior Research Foundation, The BD2 Foundation, and Alkermes Pathway Awards for Bipolar Disorder and Schizophrenia. She is also on the chronobiology task force for the International Society for Bipolar Disorders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D, and Kupfer DJ (1998). Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: a preliminary investigation. Archives of general psychiatry 55, 702–707. [DOI] [PubMed] [Google Scholar]
- 2.Ehlers CL, Frank E, and Kupfer DJ (1988). Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Archives of general psychiatry 45, 948–952. [DOI] [PubMed] [Google Scholar]
- 3.Zou H, Zhou H, Yan R, Yao Z, and Lu Q (2022). Chronotype, circadian rhythm, and psychiatric disorders: Recent evidence and potential mechanisms. Front Neurosci 16, 811771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antypa N, Vogelzangs N, Meesters Y, Schoevers R, and Penninx BW (2016). Chronotype Associations with Depression and Anxiety Disorders in a Large Cohort Study. Depress Anxiety 33, 75–83. [DOI] [PubMed] [Google Scholar]
- 5.Knutson KL, and von Schantz M (2018). Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int 35, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirz-Justice A, and Terman M (2012). Chronotherapeutics (light and wake therapy) as a class of interventions for affective disorders. Handbook of clinical neurology / edited by Vinken PJ and Bruyn GW 106, 697–713. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy MJ, Gottlieb JF, Gonzalez R, McClung CA, Alloy LB, Cain S, Dulcis D, Etain B, Frey BN, Garbazza C, et al. (2022). Neurobiological and behavioral mechanisms of circadian rhythm disruption in bipolar disorder: A critical multi-disciplinary literature review and agenda for future research from the ISBD task force on chronobiology. Bipolar Disord 24, 232–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass J, and Takahashi JS (2010). Circadian integration of metabolism and energetics. Science 330, 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Ramsey KM, Marcheva B, and Bass J (2011). Circadian rhythms, sleep, and metabolism. The Journal of clinical investigation 121, 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan RW, and McClung CA (2019). Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci 20, 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logan RW, and Sarkar DK (2012). Circadian nature of immune function. Molecular and cellular endocrinology 349, 82–90. [DOI] [PubMed] [Google Scholar]
- 12.Yu EA, and Weaver DR (2011). Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging 3, 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belloir J, Makarem N, and Shechter A (2022). Sleep and Circadian Disturbance in Cardiovascular Risk. Curr Cardiol Rep 24, 2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reppert SM, and Weaver DR (2001). Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63, 647–676. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Billings HJ, and Lehman MN (2003). The suprachiasmatic nucleus: a clock of multiple components. J Biol Rhythms 18, 435–449. [DOI] [PubMed] [Google Scholar]
- 16.Sack RL, Brandes RW, Kendall AR, and Lewy AJ (2000). Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med 343, 1070–1077. [DOI] [PubMed] [Google Scholar]
- 17.Sack RL (2009). The pathophysiology of jet lag. Travel medicine and infectious disease 7, 102–110. [DOI] [PubMed] [Google Scholar]
- 18.Inder ML, Crowe MT, and Porter R (2016). Effect of transmeridian travel and jetlag on mood disorders: evidence and implications. Aust N Z J Psychiatry 50, 220–227. [DOI] [PubMed] [Google Scholar]
- 19.Dodson ER, and Zee PC (2010). Therapeutics for Circadian Rhythm Sleep Disorders. Sleep Med Clin 5, 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko CH, and Takahashi JS (2006). Molecular components of the mammalian circadian clock. Hum Mol Genet 15 Spec No 2, R271–277. [DOI] [PubMed] [Google Scholar]
- 21.Buhr ED, and Takahashi JS (2013). Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol, 3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patton AP, and Hastings MH (2023). The Mammalian Circadian Time-Keeping System. J Huntingtons Dis 12, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geoffroy PA, Bellivier F, Scott J, Boudebesse C, Lajnef M, Gard S, Kahn JP, Azorin JM, Henry C, Leboyer M, and Etain B (2013). Bipolar disorder with seasonal pattern: clinical characteristics and gender influences. Chronobiology international 30, 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnusson A, and Partonen T (2005). The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectr 10, 625–634; quiz 621–614. [DOI] [PubMed] [Google Scholar]
- 25.Melrose S (2015). Seasonal Affective Disorder: An Overview of Assessment and Treatment Approaches. Depress Res Treat 2015, 178564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh T, and Williams K (2006). Atypical depression. Psychiatry (Edgmont) 3, 33–39. [PMC free article] [PubMed] [Google Scholar]
- 27.Traffanstedt MK, Mehta S & LoBello SG (2016). Major depression with seasonal variation: Is it a valid construct? Clinical Psychological Science 4, 825–834. [Google Scholar]
- 28.Young MA (2017). Does seasonal affective disorder exist? A commentary on Traffanstedt, Mehta, and LoBello (2016). Clinical Psychological Science 5, 750–754. [Google Scholar]
- 29.Stewart JW, Quitkin FM, Terman M, and Terman JS (1990). Is seasonal affective disorder a variant of atypical depression? Differential response to light therapy. Psychiatry Res 33, 121–128. [DOI] [PubMed] [Google Scholar]
- 30.Graw P, Krauchi K, Wirz-Justice A, and Poldinger W (1991). Diurnal variation of symptoms in seasonal affective disorder. Psychiatry Res 37, 105–111. [DOI] [PubMed] [Google Scholar]
- 31.Rosen LN, Targum SD, Terman M, Bryant MJ, Hoffman H, Kasper SF, Hamovit JR, Docherty JP, Welch B, and Rosenthal NE (1990). Prevalence of seasonal affective disorder at four latitudes. Psychiatry Res 31, 131–144. [DOI] [PubMed] [Google Scholar]
- 32.Holler Y, Gudjonsdottir BE, Valgeirsdottir SK, and Heimisson GT (2021). The effect of age and chronotype on seasonality, sleep problems, and mood. Psychiatry Res 297, 113722. [DOI] [PubMed] [Google Scholar]
- 33.Bjorvatn B, Saxvig IW, Waage S, and Pallesen S (2021). Self-reported seasonality is strongly associated with chronotype and weakly associated with latitude. Chronobiol Int 38, 278–285. [DOI] [PubMed] [Google Scholar]
- 34.Mersch PP, Middendorp HM, Bouhuys AL, Beersma DG, and van den Hoofdakker RH (1999). Seasonal affective disorder and latitude: a review of the literature. J Affect Disord 53, 35–48. [DOI] [PubMed] [Google Scholar]
- 35.Young MA, Meaden PM, Fogg LF, Cherin EA, and Eastman CI (1997). Which environmental variables are related to the onset of seasonal affective disorder? J Abnorm Psychol 106, 554–562. [DOI] [PubMed] [Google Scholar]
- 36.Murray GW, and Hay DA (1997). Seasonal affective disorder in Australia: is photoperiod critical? Aust N Z J Psychiatry 31, 279–284. [DOI] [PubMed] [Google Scholar]
- 37.Jankowski KS, and Dmitrzak-Weglarz M (2017). ARNTL, CLOCK and PER3 polymorphisms - links with chronotype and affective dimensions. Chronobiol Int 34, 1105–1113. [DOI] [PubMed] [Google Scholar]
- 38.Kovanen L, Donner K, Kaunisto M, and Partonen T (2016). CRY1 and CRY2 genetic variants in seasonality: A longitudinal and cross-sectional study. Psychiatry Res 242, 101–110. [DOI] [PubMed] [Google Scholar]
- 39.Ho KWD, Han S, Nielsen JV, Jancic D, Hing B, Fiedorowicz J, Weissman MM, Levinson DF, and Potash JB (2018). Genome-wide association study of seasonal affective disorder. Transl Psychiatry 8, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu Z, Zhang H, Huang M, Shi G, Liu Z, Xie P, Li H, Wang W, Xu G, Zhang Y, et al. (2016). Loss of ZBTB20 impairs circadian output and leads to unimodal behavioral rhythms. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalmbach DA, Schneider LD, Cheung J, Bertrand SJ, Kariharan T, Pack AI, and Gehrman PR (2017). Genetic Basis of Chronotype in Humans: Insights From Three Landmark GWAS. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrer A, Costas J, Gratacos M, Martinez-Amoros E, Labad J, Soriano-Mas C, Palao D, Menchon JM, Crespo JM, Urretavizcaya M, and Soria V (2020). Clock gene polygenic risk score and seasonality in major depressive disorder and bipolar disorder. Genes Brain Behav 19, e12683. [DOI] [PubMed] [Google Scholar]
- 43.Tonetti L, Fabbri M, Martoni M, and Natale V (2012). Circadian type and mood seasonality in adolescents. Psychiatry Clin Neurosci 66, 157–159. [DOI] [PubMed] [Google Scholar]
- 44.Murray G, Allen NB, and Trinder J (2003). Seasonality and circadian phase delay: prospective evidence that winter lowering of mood is associated with a shift towards Eveningness. J Affect Disord 76, 15–22. [DOI] [PubMed] [Google Scholar]
- 45.Kivela L, Papadopoulos MR, and Antypa N (2018). Chronotype and Psychiatric Disorders. Curr Sleep Med Rep 4, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dulcis D, Jamshidi P, Leutgeb S, and Spitzer NC (2013). Neurotransmitter switching in the adult brain regulates behavior. Science 340, 449–453. [DOI] [PubMed] [Google Scholar]
- 47.Jameson AN, Siemann JK, Melchior J, Calipari ES, McMahon DG, and Grueter BA (2023). Photoperiod Impacts Nucleus Accumbens Dopamine Dynamics. eNeuro 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deats SP, Adidharma W, and Yan L (2015). Hypothalamic dopaminergic neurons in an animal model of seasonal affective disorder. Neurosci Lett 602, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costello A, Linning-Duffy K, Vandenbrook C, Donohue K, O’Hara BF, Kim A, Lonstein JS, and Yan L (2023). Effects of light therapy on sleep/wakefulness, daily rhythms, and the central orexin system in a diurnal rodent model of seasonal affective disorder. J Affect Disord 332, 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards S, Clow A, Evans P, and Hucklebridge F (2001). Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci 68, 2093–2103. [DOI] [PubMed] [Google Scholar]
- 51.Thorn L, Hucklebridge F, Evans P, and Clow A (2009). The cortisol awakening response, seasonality, stress and arousal: a study of trait and state influences. Psychoneuroendocrinology 34, 299–306. [DOI] [PubMed] [Google Scholar]
- 52.Thorn L, Evans P, Cannon A, Hucklebridge F, and Clow A (2011). Seasonal differences in the diurnal pattern of cortisol secretion in healthy participants and those with self-assessed seasonal affective disorder. Psychoneuroendocrinology 36, 816–823. [DOI] [PubMed] [Google Scholar]
- 53.Agustini B, Bocharova M, Walker AJ, Berk M, Young AH, and Juruena MF (2019). Has the sun set for seasonal affective disorder and HPA axis studies? A systematic review and future prospects. J Affect Disord 256, 584–593. [DOI] [PubMed] [Google Scholar]
- 54.Inagaki N, Honma S, Ono D, Tanahashi Y, and Honma K (2007). Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci U S A 104, 7664–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guilding C, Hughes AT, Brown TM, Namvar S, and Piggins HD (2009). A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mrugala M, Zlomanczuk P, Jagota A, and Schwartz WJ (2000). Rhythmic multiunit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod. Am J Physiol Regul Integr Comp Physiol 278, R987–994. [DOI] [PubMed] [Google Scholar]
- 57.VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, and Meijer JH (2007). Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol 17, 468–473. [DOI] [PubMed] [Google Scholar]
- 58.Roecklein K, Wong P, Ernecoff N, Miller M, Donofry S, Kamarck M, Wood-Vasey WM, and Franzen P (2013). The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res 210, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, et al. (2018). Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell 175, 71–84 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weil T, Daly KM, Yarur Castillo H, Thomsen MB, Wang H, Mercau ME, Hattar S, Tejeda H, and Fernandez DC (2022). Daily changes in light influence mood via inhibitory networks within the thalamic perihabenular nucleus. Sci Adv 8, eabn3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young CJ, Lyons D, and Piggins HD (2021). Circadian Influences on the Habenula and Their Potential Contribution to Neuropsychiatric Disorders. Front Behav Neurosci 15, 815700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Y, Zhao S, Zhang Y, and Zhang Q (2022). Melatonin Receptors: A Key Mediator in Animal Reproduction. Vet Sci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munley KM, Han Y, Lansing MX, and Demas GE (2022). Winter madness: Melatonin as a neuroendocrine regulator of seasonal aggression. J Exp Zool A Ecol Integr Physiol 337, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coomans CP, Ramkisoensing A, and Meijer JH (2015). The suprachiasmatic nuclei as a seasonal clock. Front Neuroendocrinol 37, 29–42. [DOI] [PubMed] [Google Scholar]
- 65.Danilenko KV, Putilov AA, Russkikh GS, Duffy LK, and Ebbesson SO (1994). Diurnal and seasonal variations of melatonin and serotonin in women with seasonal affective disorder. Arctic Med Res 53, 137–145. [PubMed] [Google Scholar]
- 66.Lewy AJ, Lefler BJ, Emens JS, and Bauer VK (2006). The circadian basis of winter depression. Proc Natl Acad Sci U S A 103, 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, Parry B, and Cardinali DP (2006). Melatonin in mood disorders. World J Biol Psychiatry 7, 138–151. [DOI] [PubMed] [Google Scholar]
- 68.Partonen T (1994). Effects of morning light treatment on subjective sleepiness and mood in winter depression. J Affect Disord 30, 47–56. [DOI] [PubMed] [Google Scholar]
- 69.Terman JS, Terman M, Lo ES, and Cooper TB (2001). Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry 58, 69–75. [DOI] [PubMed] [Google Scholar]
- 70.Knapen SE, Gordijn MC, and Meesters Y (2016). The relation between chronotype and treatment outcome with light therapy on a fixed time schedule. J Affect Disord 202, 87–90. [DOI] [PubMed] [Google Scholar]
- 71.Wirz-Justice A, and Terman AM (2022). CME: Light Therapy: Why, What, for Whom, How, and When (And a Postscript about Darkness). Praxis (Bern 1994) 110, 56–62. [DOI] [PubMed] [Google Scholar]
- 72.West KE, Jablonski MR, Warfield B, Cecil KS, James M, Ayers MA, Maida J, Bowen C, Sliney DH, Rollag MD, et al. (2011). Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol (1985) 110, 619–626. [DOI] [PubMed] [Google Scholar]
- 73.Cools O, Hebbrecht K, Coppens V, Roosens L, De Witte A, Morrens M, Neels H, and Sabbe B (2018). Pharmacotherapy and nutritional supplements for seasonal affective disorders: a systematic review. Expert Opin Pharmacother 19, 1221–1233. [DOI] [PubMed] [Google Scholar]
- 74.Gartlehner G, Nussbaumer B, Gaynes BN, Forneris CA, Morgan LC, Kaminski-Hartenthaler A, Greenblatt A, Wipplinger J, Lux LJ, Sonis JH, et al. (2015). Second-generation antidepressants for preventing seasonal affective disorder in adults. Cochrane Database Syst Rev, CD011268. [DOI] [PubMed] [Google Scholar]
- 75.Geoffroy PA, Schroder CM, Reynaud E, and Bourgin P (2019). Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Med Rev 48, 101213. [DOI] [PubMed] [Google Scholar]
- 76.Friedrich MJ (2017). Depression Is the Leading Cause of Disability Around the World. JAMA 317, 1517. [DOI] [PubMed] [Google Scholar]
- 77.Hidaka BH (2012). Depression as a disease of modernity: explanations for increasing prevalence. J Affect Disord 140, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy MJ, and Peterson MJ (2015). Sleep Disturbances in Depression. Sleep Med Clin 10, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peeters F, Berkhof J, Delespaul P, Rottenberg J, and Nicolson NA (2006). Diurnal mood variation in major depressive disorder. Emotion 6, 383–391. [DOI] [PubMed] [Google Scholar]
- 80.Wirz-Justice A (2008). Diurnal variation of depressive symptoms. Dialogues in clinical neuroscience 10, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antypa N, Verkuil B, Molendijk M, Schoevers R, Penninx B, and Van Der Does W (2017). Associations between chronotypes and psychological vulnerability factors of depression. Chronobiol Int 34, 1125–1135. [DOI] [PubMed] [Google Scholar]
- 82.Bahk YC, Han E, and Lee SH (2014). Biological rhythm differences and suicidal ideation in patients with major depressive disorder. J Affect Disord 168, 294–297. [DOI] [PubMed] [Google Scholar]
- 83.McGlashan EM, Drummond SPA, and Cain SW (2018). Evening types demonstrate reduced SSRI treatment efficacy. Chronobiol Int 35, 1175–1178. [DOI] [PubMed] [Google Scholar]
- 84.Walker WH 2nd, Walton JC, DeVries AC, and Nelson RJ (2020). Circadian rhythm disruption and mental health. Transl Psychiatry 10, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Min JY, and Min KB (2018). Outdoor light at night and the prevalence of depressive symptoms and suicidal behaviors: A cross-sectional study in a nationally representative sample of Korean adults. J Affect Disord 227, 199–205. [DOI] [PubMed] [Google Scholar]
- 86.Boivin DB, Boudreau P, and Kosmadopoulos A (2022). Disturbance of the Circadian System in Shift Work and Its Health Impact. J Biol Rhythms 37, 3–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brum MC, Filho FF, Schnorr CC, Bottega GB, and Rodrigues TC (2015). Shift work and its association with metabolic disorders. Diabetol Metab Syndr 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.group IMV (2019). Carcinogenicity of night shift work. Lancet Oncol 20, 1058–1059. [DOI] [PubMed] [Google Scholar]
- 89.Lee A, Myung SK, Cho JJ, Jung YJ, Yoon JL, and Kim MY (2017). Night Shift Work and Risk of Depression: Meta-analysis of Observational Studies. J Korean Med Sci 32, 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNeely E, Gale S, Tager I, Kincl L, Bradley J, Coull B, and Hecker S (2014). The self-reported health of U.S. flight attendants compared to the general population. Environ Health 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen Q, Wu J, Ni Y, Xie X, Yu C, Xiao Q, Zhou J, Wang X, and Fu Z (2019). Exposure to jet lag aggravates depression-like behaviors and age-related phenotypes in rats subject to chronic corticosterone. Acta Biochim Biophys Sin (Shanghai) 51, 834–844. [DOI] [PubMed] [Google Scholar]
- 92.Katz G, Knobler HY, Laibel Z, Strauss Z, and Durst R (2002). Time zone change and major psychiatric morbidity: the results of a 6-year study in Jerusalem. Compr Psychiatry 43, 37–40. [DOI] [PubMed] [Google Scholar]
- 93.Daut RA, and Fonken LK (2019). Circadian regulation of depression: A role for serotonin. Front Neuroendocrinol 54, 100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, et al. (2013). Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A 110, 9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, and Welsh DK (2016). Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice. Biol Psychiatry 80, 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, and McClung CA (2015). Chronic Stress Induces Brain Region-Specific Alterations of Molecular Rhythms that Correlate with Depression-like Behavior in Mice. Biol Psychiatry 78, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. [DOI] [PubMed] [Google Scholar]
- 98.Qiu P, Jiang J, Liu Z, Cai Y, Huang T, Wang Y, Liu Q, Nie Y, Liu F, Cheng J, et al. (2019). BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. Natl Sci Rev 6, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spencer RL, and Deak T (2017). A users guide to HPA axis research. Physiol Behav 178, 43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones SG, and Benca RM (2015). Circadian Disruption in Psychiatric Disorders. Sleep Med Clin 10, 481–493. [DOI] [PubMed] [Google Scholar]
- 101.Koenigsberg HW, Teicher MH, Mitropoulou V, Navalta C, New AS, Trestman R, and Siever LJ (2004). 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. J Psychiatr Res 38, 503–511. [DOI] [PubMed] [Google Scholar]
- 102.Iwata M, Ota KT, and Duman RS (2013). The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 31, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mileva GR, Rooke J, Ismail N, and Bielajew C (2017). Corticosterone and immune cytokine characterization following environmental manipulation in female WKY rats. Behav Brain Res 316, 197–204. [DOI] [PubMed] [Google Scholar]
- 104.Estes ML, and McAllister AK (2016). Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dantzer R (2018). Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol Rev 98, 477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rutsch A, Kantsjo JB, and Ronchi F (2020). The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol 11, 604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, A.G. N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, et al. (2013). Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, et al. (2014). An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 20, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palomino-Segura M, and Hidalgo A (2021). Circadian immune circuits. J Exp Med 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ertosun MG, Kocak G, and Ozes ON (2019). The regulation of circadian clock by tumor necrosis factor alpha. Cytokine Growth Factor Rev 46, 10–16. [DOI] [PubMed] [Google Scholar]
- 111.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, and Fontana A (2007). TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A 104, 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, and Cirelli C (2017). Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J Neurosci 37, 5263–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nadjar A, Wigren HM, and Tremblay ME (2017). Roles of Microglial Phagocytosis and Inflammatory Mediators in the Pathophysiology of Sleep Disorders. Front Cell Neurosci 11, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gonzalez MM, and Aston-Jones G (2006). Circadian regulation of arousal: role of the noradrenergic locus coeruleus system and light exposure. Sleep 29, 1327–1336. [DOI] [PubMed] [Google Scholar]
- 115.Gonzalez MM, and Aston-Jones G (2008). Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proceedings of the National Academy of Sciences of the United States of America 105, 4898–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, and McClung CA (2013). Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur J Neurosci 37, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Porcu A, Vaughan M, Nilsson A, Arimoto N, Lamia K, and Welsh DK (2020). Vulnerability to helpless behavior is regulated by the circadian clock component CRYPTOCHROME in the mouse nucleus accumbens. Proc Natl Acad Sci U S A 117, 13771–13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Becker-Krail DD, Ketchesin KD, Burns JN, Zong W, Hildebrand MA, DePoy LM, Vadnie CA, Tseng GC, Logan RW, Huang YH, and McClung CA (2022). Astrocyte Molecular Clock Function in the Nucleus Accumbens Is Important for Reward-Related Behavior. Biol Psychiatry 92, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glozier N, O’Dea B, McGorry PD, Pantelis C, Amminger GP, Hermens DF, Purcell R, Scott E, and Hickie IB (2014). Delayed sleep onset in depressed young people. BMC Psychiatry 14, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moon JH, Cho CH, Son GH, Geum D, Chung S, Kim H, Kang SG, Park YM, Yoon HK, Kim L, et al. (2016). Advanced Circadian Phase in Mania and Delayed Circadian Phase in Mixed Mania and Depression Returned to Normal after Treatment of Bipolar Disorder. EBioMedicine 11, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pandi-Perumal SR, Monti JM, Burman D, Karthikeyan R, BaHammam AS, Spence DW, Brown GM, and Narashimhan M (2020). Clarifying the role of sleep in depression: A narrative review. Psychiatry Res 291, 113239. [DOI] [PubMed] [Google Scholar]
- 122.Geoffroy PA, and Palagini L (2021). Biological rhythms and chronotherapeutics in depression. Prog Neuropsychopharmacol Biol Psychiatry 106, 110158. [DOI] [PubMed] [Google Scholar]
- 123.Wu JC, and Bunney WE (1990). The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. The American journal of psychiatry 147, 14–21. [DOI] [PubMed] [Google Scholar]
- 124.Benedetti F, and Colombo C (2011). Sleep deprivation in mood disorders. Neuropsychobiology 64, 141–151. [DOI] [PubMed] [Google Scholar]
- 125.Orozco-Solis R, Montellier E, Aguilar-Arnal L, Sato S, Vawter MP, Bunney BG, Bunney WE, and Sassone-Corsi P (2017). A Circadian Genomic Signature Common to Ketamine and Sleep Deprivation in the Anterior Cingulate Cortex. Biol Psychiatry 82, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duncan WC Jr., Slonena E, Hejazi NS, Brutsche N, Yu KC, Park L, Ballard ED, and Zarate CA Jr. (2017). Motor-Activity Markers of Circadian Timekeeping Are Related to Ketamine’s Rapid Antidepressant Properties. Biol Psychiatry 82, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duncan WC Jr., Slonena EE, Hejazi NS, Brutsche N, Park LT, Henter ID, Ballard ED, and Zarate CA Jr. (2018). Are 24-hour motor activity patterns associated with continued rapid response to ketamine? Neuropsychiatr Dis Treat 14, 2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, Morehouse R, Ramasubbu R, Yatham LN, and Tam EM (2016). Efficacy of Bright Light Treatment, Fluoxetine, and the Combination in Patients With Nonseasonal Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 73, 56–63. [DOI] [PubMed] [Google Scholar]
- 129.Huang L, Xi Y, Peng Y, Yang Y, Huang X, Fu Y, Tao Q, Xiao J, Yuan T, An K, et al. (2019). A Visual Circuit Related to Habenula Underlies the Antidepressive Effects of Light Therapy. Neuron 102, 128–142 e128. [DOI] [PubMed] [Google Scholar]
- 130.Zielinska-Dabkowska KM (2018). Make lighting healthier. Nature 553, 274–276. [DOI] [PubMed] [Google Scholar]
- 131.An K, Zhao H, Miao Y, Xu Q, Li YF, Ma YQ, Shi YM, Shen JW, Meng JJ, Yao YG, et al. (2020). A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat Neurosci 23, 869–880. [DOI] [PubMed] [Google Scholar]
- 132.Robillard R, Carpenter JS, Feilds KL, Hermens DF, White D, Naismith SL, Bartlett D, Whitwell B, Southan J, Scott EM, and Hickie IB (2018). Parallel Changes in Mood and Melatonin Rhythm Following an Adjunctive Multimodal Chronobiological Intervention With Agomelatine in People With Depression: A Proof of Concept Open Label Study. Front Psychiatry 9, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sprouse J, Braselton J, and Reynolds L (2006). Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biological psychiatry 60, 896–899. [DOI] [PubMed] [Google Scholar]
- 134.Srinivasan V, Zakaria R, Othman Z, Lauterbach EC, and Acuna-Castroviejo D (2012). Agomelatine in depressive disorders: its novel mechanisms of action. The Journal of neuropsychiatry and clinical neurosciences 24, 290–308. [DOI] [PubMed] [Google Scholar]
- 135.Mairesse J, Silletti V, Laloux C, Zuena AR, Giovine A, Consolazione M, van Camp G, Malagodi M, Gaetani S, Cianci S, et al. (2013). Chronic agomelatine treatment corrects the abnormalities in the circadian rhythm of motor activity and sleep/wake cycle induced by prenatal restraint stress in adult rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 16, 323–338. [DOI] [PubMed] [Google Scholar]
- 136.Leproult R, Van Onderbergen A, L’Hermite-Baleriaux M, Van Cauter E, and Copinschi G (2005). Phase-shifts of 24-h rhythms of hormonal release and body temperature following early evening administration of the melatonin agonist agomelatine in healthy older men. Clin Endocrinol (Oxf) 63, 298–304. [DOI] [PubMed] [Google Scholar]
- 137.Boland EM, Goldschmied JR, and Gehrman PR (2023). Does insomnia treatment prevent depression? Sleep 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mirchandaney R, Barete R, and Asarnow LD (2022). Moderators of Cognitive Behavioral Treatment for Insomnia on Depression and Anxiety Outcomes. Curr Psychiatry Rep 24, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carney CE, Edinger JD, Kuchibhatla M, Lachowski AM, Bogouslavsky O, Krystal AD, and Shapiro CM (2017). Cognitive Behavioral Insomnia Therapy for Those With Insomnia and Depression: A Randomized Controlled Clinical Trial. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Asarnow LD, Bei B, Krystal A, Buysse DJ, Thase ME, Edinger JD, and Manber R (2019). Circadian Preference as a Moderator of Depression Outcome Following Cognitive Behavioral Therapy for Insomnia Plus Antidepressant Medications: A Report From the TRIAD Study. J Clin Sleep Med 15, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Diflorio A, and Jones I (2010). Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry 22, 437–452. [DOI] [PubMed] [Google Scholar]
- 142.Kato T (2019). Current understanding of bipolar disorder: Toward integration of biological basis and treatment strategies. Psychiatry Clin Neurosci 73, 526–540. [DOI] [PubMed] [Google Scholar]
- 143.McCarthy MJ, Gottlieb JF, Gonzalez R, McClung CA, Alloy LB, Cain S, Dulcis D, Etain B, Frey BN, Garbazza C, et al. (2021). Neurobiological and behavioral mechanisms of circadian rhythm disruption in bipolar disorder: A critical multi-disciplinary literature review and agenda for future research from the ISBD task force on chronobiology. Bipolar Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Melo MCA, Abreu RLC, Linhares Neto VB, de Bruin PFC, and de Bruin VMS (2017). Chronotype and circadian rhythm in bipolar disorder: A systematic review. Sleep medicine reviews 34, 46–58. [DOI] [PubMed] [Google Scholar]
- 145.Vidafar P, Yocum AK, Han P, McInnis MG, and Burgess HJ (2021). Late chronotype predicts more depressive symptoms in bipolar disorder over a 5 year follow-up period. Int J Bipolar Disord 9, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gordovez FJA, and McMahon FJ (2020). The genetics of bipolar disorder. Mol Psychiatry 25, 544–559. [DOI] [PubMed] [Google Scholar]
- 147.Bipolar, D., Schizophrenia Working Group of the Psychiatric Genomics Consortium. Electronic address, d.r.v.e., Bipolar, D., and Schizophrenia Working Group of the Psychiatric Genomics, C. (2018). Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell 173, 1705–1715 e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McCarthy MJ, Nievergelt CM, Kelsoe JR, and Welsh DK (2012). A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PloS one 7, e32091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geoffroy PA, Bellivier F, Scott J, and Etain B (2014). Seasonality and bipolar disorder: a systematic review, from admission rates to seasonality of symptoms. J Affect Disord 168, 210–223. [DOI] [PubMed] [Google Scholar]
- 150.Aytac HM, Pehlivan M, Oyaci Y, and Pehlivan S (2022). PERIOD3 (PER3) VNTR Variant Associated with Seasonal Pattern and Family History in Bipolar Disorder. Psychiatr Danub 34, 695–699. [DOI] [PubMed] [Google Scholar]
- 151.Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT, Houck PR, and Kupfer DJ (2000). Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychological medicine 30, 1005–1016. [DOI] [PubMed] [Google Scholar]
- 152.Castro J, Zanini M, Goncalves Bda S, Coelho FM, Bressan R, Bittencourt L, Gadelha A, Brietzke E, and Tufik S (2015). Circadian rest-activity rhythm in individuals at risk for psychosis and bipolar disorder. Schizophr Res 168, 50–55. [DOI] [PubMed] [Google Scholar]
- 153.Melo MC, Garcia RF, Linhares Neto VB, Sa MB, de Mesquita LM, de Araujo CF, and de Bruin VM (2016). Sleep and circadian alterations in people at risk for bipolar disorder: A systematic review. J Psychiatr Res 83, 211–219. [DOI] [PubMed] [Google Scholar]
- 154.Rodrigues Cordeiro C, Corte-Real BR, Saraiva R, Frey BN, Kapczinski F, and de Azevedo Cardoso T (2023). Triggers for acute mood episodes in bipolar disorder: A systematic review. J Psychiatr Res 161, 237–260. [DOI] [PubMed] [Google Scholar]
- 155.Azorin JM, Perret LC, Fakra E, Tassy S, Simon N, Adida M, and Belzeaux R (2017). Alcohol use and bipolar disorders: Risk factors associated with their co-occurrence and sequence of onsets. Drug Alcohol Depend 179, 205–212. [DOI] [PubMed] [Google Scholar]
- 156.Kristensen M, Nierenberg AA, and Ostergaard SD (2018). Face and predictive validity of the ClockDelta19 mouse as an animal model for bipolar disorder: a systematic review. Mol Psychiatry 23, 70–80. [DOI] [PubMed] [Google Scholar]
- 157.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, et al. (2007). Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A 104, 6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Logan RW, Ozburn AR, Arey RN, Ketchesin KD, Winquist A, Crain A, Tobe BTD, Becker-Krail D, Jarpe MB, Xue X, et al. (2020). Valproate reverses mania-like behaviors in mice via preferential targeting of HDAC2. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, Kim KS, Dluzen DE, Lee I, Hwang O, et al. (2014). Impact of Circadian Nuclear Receptor REV-ERBalpha on Midbrain Dopamine Production and Mood Regulation. Cell 157, 858–868. [DOI] [PubMed] [Google Scholar]
- 160.Keers R, Pedroso I, Breen G, Aitchison KJ, Nolan PM, Cichon S, Nothen MM, Rietschel M, Schalkwyk LC, and Fernandes C (2012). Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PloS one 7, e38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li X, Chen B, Zhang D, Wang S, Feng Y, Wu X, Cui L, Ji M, Gong W, Verkhratsky A, et al. (2023). A novel murine model of mania. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ketchesin KD, Becker-Krail D, and McClung CA (2020). Mood-related central and peripheral clocks. Eur J Neurosci 51, 326–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Logan RW, Parekh PK, Kaplan GN, Becker-Krail DD, Williams WP 3rd, Yamaguchi S, Yoshino J, Shelton MA, Zhu X, Zhang H, et al. (2019). NAD+ cellular redox and SIRT1 regulate the diurnal rhythms of tyrosine hydroxylase and conditioned cocaine reward. Mol Psychiatry 24, 1668–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Goede P, Wefers J, Brombacher EC, Schrauwen P, and Kalsbeek A (2018). Circadian rhythms in mitochondrial respiration. J Mol Endocrinol 60, R115–R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Musiek ES, and Holtzman DM (2016). Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kato T, and Kato N (2000). Mitochondrial dysfunction in bipolar disorder. Bipolar disorders 2, 180–190. [DOI] [PubMed] [Google Scholar]
- 167.Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH, and Howes OD (2017). The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry 22, 666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, Arey RN, Enwright JF 3rd, Jacobsen JP, Kumar S, et al. (2015). Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koning E, McDonald A, Bambokian A, Gomes FA, Vorstman J, Berk M, Fabe J, McIntyre RS, Milev R, Mansur RB, and Brietzke E (2022). The concept of “metabolic jet lag” in the pathophysiology of bipolar disorder: implications for research and clinical care. CNS Spectr, 1–10. [DOI] [PubMed] [Google Scholar]
- 170.Dietch DM, Kerr-Gaffney J, Hockey M, Marx W, Ruusunen A, Young AH, Berk M, and Mondelli V (2023). Efficacy of low carbohydrate and ketogenic diets in treating mood and anxiety disorders: systematic review and implications for clinical practice. BJPsych Open 9, e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Frank E, Swartz HA, and Boland E (2007). Interpersonal and social rhythm therapy: an intervention addressing rhythm dysregulation in bipolar disorder. Dialogues in clinical neuroscience 9, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Frank E, Soreca I, Swartz HA, Fagiolini AM, Mallinger AG, Thase ME, Grochocinski VJ, Houck PR, and Kupfer DJ (2008). The role of interpersonal and social rhythm therapy in improving occupational functioning in patients with bipolar I disorder. The American journal of psychiatry 165, 1559–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sankar A, Panchal P, Goldman DA, Colic L, Villa LM, Kim JA, Lebowitz ER, Carrubba E, Lecza B, Silverman WK, et al. (2021). Telehealth Social Rhythm Therapy to Reduce Mood Symptoms and Suicide Risk Among Adolescents and Young Adults With Bipolar Disorder. Am J Psychother 74, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lam RW, Teng MY, Jung YE, Evans VC, Gottlieb JF, Chakrabarty T, Michalak EE, Murphy JK, Yatham LN, and Sit DK (2020). Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can J Psychiatry 65, 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moreira J, and Geoffroy PA (2016). Lithium and bipolar disorder: Impacts from molecular to behavioural circadian rhythms. Chronobiol Int 33, 351–373. [DOI] [PubMed] [Google Scholar]
- 176.Federoff M, McCarthy MJ, Anand A, Berrettini WH, Bertram H, Bhattacharjee A, Calkin CV, Conroy C, Coryell WH, D’Arcangelo N, et al. (2021). Correction of depression-associated circadian rhythm abnormalities is associated with lithium response in bipolar disorder. Bipolar Disord. [DOI] [PubMed] [Google Scholar]
- 177.Li J, Lu WQ, Beesley S, Loudon AS, and Meng QJ (2012). Lithium impacts on the amplitude and period of the molecular circadian clockwork. PloS one 7, e33292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kaladchibachi SA, Doble B, Anthopoulos N, Woodgett JR, and Manoukian AS (2007). Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lavoie J, Hebert M, and Beaulieu JM (2013). Glycogen synthase kinase-3beta haploinsufficiency lengthens the circadian locomotor activity period in mice. Behav Brain Res 253, 262–265. [DOI] [PubMed] [Google Scholar]
- 180.McCarthy MJ, Wei H, Nievergelt CM, Stautland A, Maihofer AX, Welsh DK, Shilling P, Alda M, Alliey-Rodriguez N, Anand A, et al. (2019). Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology 44, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kripke DF, Mullaney DJ, Atkinson M, and Wolf S (1978). Circadian rhythm disorders in manic-depressives. Biological psychiatry 13, 335–351. [PubMed] [Google Scholar]
- 182.Sanghani HR, Jagannath A, Humberstone T, Ebrahimjee F, Thomas JM, Churchill GC, Cipriani A, Attenburrow MJ, Perestenko OV, Cowley SA, et al. (2021). Patient fibroblast circadian rhythms predict lithium sensitivity in bipolar disorder. Mol Psychiatry 26, 5252–5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mishra HK, Ying NM, Luis A, Wei H, Nguyen M, Nakhla T, Vandenburgh S, Alda M, Berrettini WH, Brennand KJ, et al. (2021). Circadian rhythms in bipolar disorder patient-derived neurons predict lithium response: preliminary studies. Mol Psychiatry 26, 3383–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Johansson AS, Brask J, Owe-Larsson B, Hetta J, and Lundkvist GB (2011). Valproic acid phase shifts the rhythmic expression of Period2::Luciferase. Journal of biological rhythms 26, 541–551. [DOI] [PubMed] [Google Scholar]
- 185.McGowan NM, Kim DS, de Andres Crespo M, Bisdounis L, Kyle SD, and Saunders KEA (2022). Hypnotic and Melatonin/Melatonin-Receptor Agonist Treatment in Bipolar Disorder: A Systematic Review and Meta-Analysis. CNS Drugs 36, 345–363. [DOI] [PubMed] [Google Scholar]
- 186.Kishi T, Nomura I, Sakuma K, Kitajima T, Mishima K, and Iwata N (2019). Melatonin receptor agonists-ramelteon and melatonin-for bipolar disorder: a systematic review and meta-analysis of double-blind, randomized, placebo-controlled trials. Neuropsychiatr Dis Treat 15, 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Taillard J, Sagaspe P, Philip P, and Bioulac S (2021). Sleep timing, chronotype and social jetlag: Impact on cognitive abilities and psychiatric disorders. Biochem Pharmacol 191, 114438. 188. [DOI] [PubMed] [Google Scholar]
- 188.Merikanto I, Kortesoja L, Benedict C, Chung F, Cedernaes J, Espie CA, Morin CM, Dauvilliers Y, Partinen M, De Gennaro L, et al. (2022). Evening-types show highest increase of sleep and mental health problems during the COVID-19 pandemic-multinational study on 19 267 adults. Sleep 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Salfi F, D’Atri A, Amicucci G, Viselli L, Gorgoni M, Scarpelli S, Alfonsi V, and Ferrara M (2022). The fall of vulnerability to sleep disturbances in evening chronotypes when working from home and its implications for depression. Sci Rep 12, 12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dunster GP, de la Iglesia L, Ben-Hamo M, Nave C, Fleischer JG, Panda S, and de la Iglesia HO (2018). Sleepmore in Seattle: Later school start times are associated with more sleep and better performance in high school students. Sci Adv 4, eaau6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tataroglu O, Aksoy A, Yilmaz A, and Canbeyli R (2004). Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res 1001, 118–124. [DOI] [PubMed] [Google Scholar]
- 192.Gottlieb JF, Benedetti F, Geoffroy PA, Henriksen TEG, Lam RW, Murray G, Phelps J, Sit D, Swartz HA, Crowe M, et al. (2019). The chronotherapeutic treatment of bipolar disorders: A systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord 21, 741–773. [DOI] [PubMed] [Google Scholar]


