Abstract
Purpose:
Inflammatory bowel disease (IBD) has a rising global prevalence. However, the understanding of its impact on mortality remains inconsistent so we explored the association between IBD and all-cause and cause-specific mortality.
Methods:
This study included 502,369 participants from the UK Biobank, a large, population-based, prospective cohort study with mortality data through 2022. IBD was defined by baseline self-report or from primary care or hospital admission data. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and cause-specific mortality in multivariable Cox proportional hazards regression models.
Results:
A total of 5,799 (1.2%) participants had a history of IBD at baseline. After a median follow-up of 13.7 years, 44,499 deaths occurred. Having IBD was associated with an increased risk of death from all causes (HR=1.16, 95% CI=1.07–1.24) and cancer (HR=1.16, 95% CI=1.05–1.30), particularly colorectal cancer (CRC) (HR=1.56, 95% CI=1.17–2.09). We observed elevated breast cancer mortality rates for individuals with Crohn’s disease, and increased CRC mortality rates for individuals with ulcerative colitis. In stratified analyses of IBD and all-cause mortality, mortality risk differed by individuals’ duration of IBD, age at IBD diagnosis, body mass index (BMI) (PHeterogeneity=0.03) and smoking status (PHeterogeneity=0.01). Positive associations between IBD and all-cause mortality were detected in individuals diagnosed with IBD for 10 years or longer, those diagnosed before the age of 50, all BMI subgroups except obese individuals, and in never or current, but not former smokers.
Conclusions:
We found that having IBD was associated with increased risks of mortality from all causes, all cancers, and CRC. This underscores the importance of enhanced patient management strategies and targeted prevention efforts in individuals with IBD.
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, mortality, cancer mortality
Introduction
Inflammatory bowel disease (IBD) is a chronic and recurrent inflammatory disease of the gastrointestinal (GI) tract, comprised of two main subcategories: ulcerative colitis (UC) and Crohn’s disease (CD).1 With increasing rates in newly industrialized and westernized countries, IBD presents a growing public health challenge.2, 3 It affects greater than 0.3% of the global population, with the highest prevalence rates in Europe and North America.2 In the UK alone, approximately 640,000 individuals were estimated to have an IBD diagnosis in 2017, with predictions of an 11% increase by 2025.4 IBD often leads to acute complications and severe chronic conditions, posing life-threatening risks and potentially reducing life expectancy.5–7 Despite current treatment strategies providing temporary relief, their effectiveness in managing long-term symptoms is limited, and some treatments may lead to severe health conditions, including certain cancers.8, 9
Previous studies have reported inconsistent findings regarding the association between UC or CD and overall and cause-specific mortality.10–17 While most recent prospective cohort studies reported elevated mortality risks in both UC and CD patients, others found no significant differences in outcomes compared with individuals without IBD.11, 18–20 Such inconsistencies could be attributable to the limitations of each study, including small sample sizes, limited ability to control for confounders particularly for registry data, and population differences. Meanwhile, previous studies have linked IBD to increased mortality from GI disease, infection, respiratory conditions such as chronic obstructive pulmonary disease, and cardiovascular disease (CVD).21–23 Understanding the cause-specific mortality associated with IBD is essential for shaping future prevention and management efforts, including potential screening and surveillance strategies, thereby potentially improving outcomes for IBD patients.
Our study leveraged the substantial data available from the UK Biobank cohort, a large, population-based, prospective cohort study with long-term follow-up for mortality and extensive data on possible confounders and effect modifiers.24, 25 We aimed to evaluate the association of IBD with all-cause mortality and mortality from major causes of death, including CVD, respiratory disease, and cancer, and determine whether specific demographic, lifestyle, or other medical factors modify the association between IBD and all-cause mortality.
Materials and Methods
Study population
The UK Biobank recruited participants aged 40 to 69 years from 2006 to 2010 at 22 National Health Service (NHS) assessment centers across England, Scotland, and Wales in the UK. Detailed study design and data collection procedures have been described previously.24 In brief, about 9.2 million individuals who were registered in the NHS and resided within 40 km of the NHS assessment centers were invited to participate in the study. A total of 502,414 participants provided an electronic informed consent and had phenotypic and genotypic details collected at assessment visits using touchscreen questionnaires, physical and functional measures, and biological sample collections. The cohort tracks a wide range of health-related outcomes through linkage to participants’ primary care medical records, hospital admission records, cancer-screening data, and disease-specific registries. The NHS Northwest Multicenter Research Ethics Committee (MREC) reviewed and approved the UK Biobank’s study protocol and research activities. After excluding 45 participants who withdrew from the UK Biobank cohort, we included a total of 502,369 participants in the analytic cohort.
Exposure measurements
Participants with an IBD diagnosis prior to baseline based on self-report, primary care records, and/or hospital admission data (International Classification of Disease [ICD]-10 codes: K50 [CD] and K51 [UC]) were classified as having IBD. We considered participants without a history of IBD at baseline as the unexposed comparison group. Participants who self-reported an IBD diagnosis also provided the years since or age of their first diagnosis. Primary care records were available for approximately 230,000 UK Biobank participants via a linkage to NHS records since 1938 in England, 1948 in Wales, and 1939 in Scotland. A linkage to hospital admission records including diagnosis records was conducted for all cohort participants since 1997 in England, 1999 in Wales, and 1981 in Scotland. The diagnosis source of IBD was categorized as primary care only, hospital admission only, self-report only, and multiple sources for participants identified from more than one diagnosis source. We calculated the duration of IBD from the earliest diagnosis date of IBD until the recruitment date. For participants with multiple diagnosis records or from multiple diagnosis sources, we determined the diagnosis date of IBD using the earliest date reported for an IBD diagnosis.
Outcome measurements
The primary outcome of interest in this study was all-cause mortality. We conducted a secondary analysis to further investigate mortality from the major causes of death that were determined using the ICD-10 codes obtained through the linkage to national death registries.26 The causes of deaths (ICD-10) we investigated included all CVDs (I00-I79), ischemic heart disease (I20-I25), cerebrovascular disease (I60-I69); respiratory diseases (excluding cancer, J09-J18 and J40-J47); all cancers (C00-D48), breast cancer (C50), prostate cancer (C61), colorectal cancer (C18-C20, C26), and cancers of the respiratory system (including nose, nasal cavity and middle ear, larynx, lung and bronchus, pleura, trachea, mediastinum and other respiratory organs, C30–39). Mortality data were available through October 31, 2022 in England, through August 31, 2022 in Scotland, and through May 31, 2022 in Wales. We calculated person-years through the date of death, date of drop-out, or the end of the cohort follow-up, whichever occurred first.
Covariates
The baseline questionnaire collected information on demographic and lifestyle characteristics, comorbidities, family history of diseases, and other medical information. Standard measuring devices were used to collect and record anthropometric measurements such as body mass index (BMI) at baseline. We combined data on smoking status, lifetime smoking, previous/current smoking intensity, time since quitting for former smokers, type of tobacco smoked previously/currently to create a 25-level detailed smoking history and combined data on drinking status and amount consumed to create a 6-level variable for alcohol drinking as described previously.27 We also generated a simple 3-level smoking history variable (Supplemental Table 1). Since less than 8.5% of the population had missing values for any single covariate of interest, we used an indicator category for missing values for categorical variables, including race, BMI, overall health rating, smoking, alcohol drinking, and physical activity. The Townsend deprivation index (TDI) is an individual-level measure of material deprivation that is often associated with physical health and social well-being.28As it was a continuous variable, we replaced missing values with −1.3, the median score for the study population. We conducted a supplementary complete case analysis excluding 50,004 individuals with any missing data and found similar results. Therefore, all presented analyses included a missing category and simple imputation for the TDI to maximize our study sample size.
Statistical analysis
We computed mean (standard deviation [SD]) values for continuous variables and frequencies (proportions) for categorical variables to describe baseline characteristics within all participants and by IBD diagnosis status. We used multivariable Cox proportional hazards regression models to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between IBD, CD, and UC with all-cause and cause-specific mortality. We created multiple models which included confounders that we identified a priori according to the current literature and those statistically significantly associated with both the exposure and the outcome (P <0.05). Specifically, we adjusted for the assessment center; demographic information, including age, sex, race/ethnicity and TDI; lifestyle factors, including BMI, detailed smoking history, alcohol drinking, and physical activity; self-reported comorbidities, including diabetes, hypertension, asthma, and tuberculosis; and self-reported medication use, including aspirin, ibuprofen, and laxatives in Model 1. We included all confounders in Model 1 in addition to self-reported overall health rating in Model 2. This additional variable was included as it was the strongest predictor of mortality in the cohort. However, we acknowledge the potential for overadjustment if the overall health rating was part of the causal pathway. In the cause-specific models, we further adjusted for self-reported family history of relevant diseases for each model (e.g., family history of heart disease or stroke in the CVD mortality model, and family history of cancer in the cancer-specific mortality model). The proportional hazards assumption for the Cox regression models was evaluated by testing the interaction term between IBD and follow-up time for each mortality outcome with no violations of the proportional hazards assumption observed.
We stratified the all-cause mortality analysis by the following potential effect modifiers: duration of IBD prior to baseline, age at IBD diagnosis, age at baseline, sex, overall health rating, BMI, alcohol drinking history, smoking history, diabetes status, bowel cancer screening history, and self-reported major dietary changes in the last five years. We conducted a likelihood ratio test comparing the models with and without the interaction term for IBD and the stratifying variables and obtained the P values for heterogeneity across strata. Additionally, we investigated the associations of IBD with all-cause and selected cause-specific mortality by IBD diagnosis source to evaluate possible misclassification bias. We also conducted a sensitivity analysis using participants with available primary care data (N = 228,888) to provide further investigation into potential selection bias.
All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and R version 3.6.2, and P <0.05 was considered statistically significant.
Results
Of the 502,369 participants included in the study, 5,799 (1.2%) of the participants were identified to have a history of IBD at baseline with an average duration of 17.4 years since the IBD diagnosis. Of these participants with a history of IBD, 4,174 had UC and 2,115 had CD (3,684 UC only, 1,625 CD only, and 490 both UC and CD). The diagnosis source for IBD varied for participants with IBD: 363 were diagnosed from primary care data only, 774 were diagnosed from hospital admission data only, 501 were diagnosed from self-reported data only, and 4,161 were diagnosed from multiple sources (Supplemental Table 2). The mean age of the overall study population was 56.5 years at baseline, 52.5% were female, and 95.7% self-identified as white (Table 1). The baseline characteristics were generally similar between participants with and without IBD. However, participants with a history of IBD were more likely to report having poor health and being former smokers, and to have comorbidities such as diabetes, hypertension, asthma, and tuberculosis.
Table 1.
Baseline characteristics of UK Biobank participants by inflammatory bowel disease status at baseline assessmenta (n=502,369)
| IBD | |||||
|---|---|---|---|---|---|
| Characteristics | Total | Yes (N=5,789) | No (N=496,624) | UC (N=4,166) | CD (N=2,112) |
| Age at assessment, years, mean ± SD | 56.5 ± 8.1 | 57.3 ± 7.9 | 56.5 ± 8.1 | 57.6 ± 7.8 | 56.6 ± 8.0 |
| Townsend deprivation index, mean ± SD | −1.3 ± 3.1 | −1.3 ± 3.1 | −1.3 ± 3.1 | −1.31 ± 3.0 | −1.1 ± 3.2 |
| Sex, n (%) | |||||
| Male | 229,068 (45.6) | 2,752 (47.5) | 226,316 (45.6) | 2,050 (49.1) | 944 (44.6) |
| Female | 273,301 (54.0) | 3,047 (52.5) | 270,254 (54.4) | 2,124 (50.9) | 1,171 (55.4) |
| Race, n (%) | |||||
| Asian | 11,452 (2.3) | 125 (2.2) | 11,327 (2.3) | 98 (2.4) | 41 (1.9) |
| Black | 8,058 (1.6) | 38 (0.7) | 8,020 (1.6) | 26 (0.6) | 18 (0.9) |
| Mixed race | 2,953 (0.6) | 19 (0.3) | 2,934 (0.6) | 16 (0.4) | <5 (0.1) |
| Other races | 4,555 (0.9) | 34 (0.6) | 4,521 (0.9) | 28 (0.7) | 11 (0.5) |
| White | 472,573 (94.1) | 5,552 (95.7) | 467,021 (94.1) | 3,981 (95.4) | 2,032 (96.1) |
| Assessment center, n (%) | |||||
| England | 445,728 (88.7) | 5,042 (87.0) | 440,686 (88.8) | 3,625 (86.9) | 1,831 (86.6) |
| Scotland | 35,837 (7.1) | 488 (8.4) | 35,349 (7.1) | 359 (8.6) | 179 (8.5) |
| Wales | 20,804 (4.1) | 269 (4.6) | 20,535 (4.1) | 190 (4.6) | 105 (5.0) |
| BMI, kg/m 2 , n (%) | |||||
| <18.5 | 2,626 (0.5) | 45 (0.8) | 2,581 (0.5) | 22 (0.5) | 28 (1.3) |
| 18.5 to <25.0 | 162,352 (32.3) | 1,955 (33.7) | 160,397 (32.3) | 1,351 (32.5) | 750 (35.5) |
| 25.0 to <30.0 | 212,062 (42.2) | 2,485 (42.9) | 209,577 (42.2) | 1,808 (43.3) | 898 (42.5) |
| 30.0 to <35.0 | 87,534 (17.4) | 929 (16.0) | 86,605 (17.4) | 697 (16.7) | 297 (14.0) |
| ≥35.0 | 34,688 (6.9) | 344 (5.9) | 34,344 (6.9) | 257 (6.2) | 130 (6.2) |
| Overall health rating, n (%) | |||||
| Excellent | 81,833 (16.3) | 396 (6.8) | 81,437 (16.4) | 303 (7.3) | 107 (5.1) |
| Good | 288,948 (57.5) | 2,699 (46.5) | 286,249 (57.7) | 2,032 (48.7) | 855 (40.4) |
| Fair | 105,333 (21.0) | 1,961 (33.8) | 103,372 (20.8) | 1,357 (32.5) | 788 (37.3) |
| Poor | 22,768 (4.5) | 706 (12.2) | 22,062 (4.4) | 453 (10.9) | 349 (16.5) |
| Smoking, n (%) | |||||
| Never | 273,449 (54.4) | 2,727 (47.0) | 270,722 (54.5) | 2,015 (48.3) | 944 (44.6) |
| Former | 173,009 (34.4) | 2,482 (42.8) | 170,527 (34.3) | 1,836 (44.0) | 850 (40.2) |
| Current | 52,964 (10.5) | 562 (9.7) | 52,399 (10.6) | 302 (7.2) | 311 (14.7) |
| Alcohol drinking, n (%) | |||||
| Never | 22,379 (4.5) | 293 (5.1) | 22,086 (4.5) | 207 (5.0) | 127 (6.0) |
| Former | 18,093 (3.6) | 298 (5.1) | 17,795 (3.6) | 199 (4.8) | 119 (5.6) |
| Current, <3 drink/month | 113,825 (22.7) | 1,465 (25.3) | 112,360 (22.6) | 1001 (24.0) | 586 (27.7) |
| Current, <1 drink/day | 124,784 (24.8) | 1,383 (23.9) | 123,401 (24.9) | 998 (23.9) | 499 (23.6) |
| Current, 1 to 3 drink/day | 179,516 (35.7) | 1,920 (33.1) | 177,594 (35.8) | 1,426 (34.2) | 642 (30.4) |
| Current, >3 drink/day | 41,971 (8.4) | 420 (7.2) | 41,551 (8.4) | 327 (7.8) | 133 (6.3) |
| Physical activity, days/week, n (%) | |||||
| 0 | 52,690 (10.5) | 701 (12.1) | 51,989 (10.5) | 498 (11.9) | 266 (12.6) |
| 1–2 | 63,530 (12.7) | 756 (13.0) | 62,774 (12.6) | 506 (12.1) | 314 (14.9) |
| 3–4 | 81,860 (16.3) | 934 (16.1) | 80,926 (16.3) | 681 (16.3) | 326 (15.4) |
| >=5 | 261,669 (52.1) | 2,852 (49.2) | 258,817 (52.1) | 2,100 (50.3) | 996 (47.1) |
| Aspirin use, n (%) | 69,642 (13.9) | 818 (14.1) | 68,824 (13.9) | 620 (14.9) | 272 (12.9) |
| Ibuprofen use, n (%) | 73,279 (14.6) | 655 (11.3) | 72,624 (14.6) | 454 (10.9) | 246 (11.6) |
| Laxative use, n (%) | 14,888 (3.0) | 189 (3.3) | 14,699 (3.0) | 134 (3.2) | 69 (3.3) |
| Diabetes, n (%) | 21,719 (4.3) | 307 (5.3) | 21,412 (4.3) | 226 (5.4) | 109 (5.2) |
| Hypertension, n (%) | 130,956 (26.1) | 1,581 (27.3) | 129,375 (26.1) | 1,129 (27.1) | 594 (28.1) |
| Asthma, n (%) | 58,258 (11.6) | 802 (13.8) | 57,456 (11.6) | 561 (13.4) | 309 (14.6) |
| Tuberculosis, n (%) | 2,542 (0.5) | 42 (0.7) | 2,500 (0.5) | 28 (0.7) | 18 (0.9) |
NOTE: inflammatory bowel diseases (IBD); ulcerative colitis (CD); Crohn’s disease (CD); body mass index (BMI).
Numbers may not sum to n=502,369, and percentages may not sum to 100% owing to missing data.
Physical activity measured by number of days/week of >10 min of moderate or vigorous activity.
During a median follow-up of 13.7 years, 44,499 deaths occurred. Among those, 742, 503, and 317 deaths occurred in individuals with IBD, UC, and CD, respectively. After multivariable adjustment, we found that individuals with IBD had worse survival compared to those without IBD (Supplemental Figure 2). In Model 1, the adjusted HR for all-cause mortality was 1.33 (95% CI: 1.24–1.43). The HRs for all-cause mortality were 1.55 (95% CI: 1.39–1.73) for individuals with CD and 1.25 (95% CI: 1.14–1.36) for individuals with UC compared with those without the respective conditions (Table 2). After further adjusting for overall health rating, the association with all-cause mortality was attenuated for IBD (HR=1.16, 95% CI: 1.07–1.24), CD (HR=1.28, 95% CI: 1.15–1.43) and UC (HR=1.10, 95% CI: 1.01–1.20).
Table 2.
Association of inflammatory bowel disease, ulcerative colitis and Crohn’s disease with all-cause and cause-specific mortality (n=502,369)
| Cause of death | IBD | UC | CD |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| All-cause | |||
| No. of deaths for individuals with/without IBD | 742/43757 | 503/43996 | 317/44182 |
| Model 1 | 1.33 (1.24–1.43) | 1.25 (1.14–1.36) | 1.55 (1.39–1.73) |
| Model 2 | 1.16 (1.07–1.24) | 1.10 (1.01–1.20) | 1.28 (1.15–1.43) |
| Cancer | |||
| No. of deaths for individuals with/without IBD | 341/21266 | 233/21374 | 141/21466 |
| Model 11 | 1.28 (1.14–1.42) | 1.21 (1.06–1.37) | 1.45 (1.23–1.71) |
| Model 21 | 1.16 (1.05–1.30) | 1.11 (0.98–1.27) | 1.28 (1.08–1.51) |
| 26/1600 | 17/1609 | 13/1613 | |
| Model 13 | 1.42 (0.96–2.09) | 1.31 (0.81–2.11) | 1.88 (1.09–3.25) |
| Model 23 | 1.14 (0.77–1.68) | 1.09 (0.67–1.75) | 1.41 (0.82–2.44) |
| 25/1261 | 19/1267 | 7/1279 | |
| Model 15 | 1.58 (1.06–2.35) | 1.55 (0.99–2.44) | 1.40 (0.67–2.94) |
| Model 25 | 1.38 (0.93–2.06) | 1.37 (0.87–2.15) | 1.16 (0.55–2.44) |
| Colorectal cancer | |||
| No. of deaths for individuals with/without IBD | 47/2263 | 35/2275 | 15/2295 |
| Model 16 | 1.66 (1.24–2.21) | 1.66 (1.19–2.32) | 1.53 (0.92–2.54) |
| Model 26 | 1.56 (1.17–2.09) | 1.57 (1.13–2.20) | 1.40 (0.84–2.33) |
| Respiratory system cancer | |||
| No. of deaths for individuals with/without IBD | 49/3705 | 22/3732 | 30/3724 |
| Model 17 | 1.02 (0.77–1.35) | 0.70 (0.46–1.06) | 1.43 (0.99–2.04) |
| Model 27 | 0.93 (0.70–1.23) | 0.65 (0.43–0.98) | 1.27 (0.88–1.82) |
| Cardiovascular disease | |||
| No. of deaths for individuals with/without IBD | 131/8891 | 89/8933 | 53/8969 |
| Model 18 | 1.16 (0.98–1.38) | 1.08 (0.87–1.33) | 1.30 (0.99–1.70) |
| Model 28 | 1.00 (0.84–1.19) | 0.95 (0.77–1.17) | 1.07 (0.81–1.40) |
| Ischemic disease | |||
| No. of deaths for individuals with/without IBD | 57/4685 | 42/4700 | 20/4722 |
| Model 19 | 0.96 (0.74–1.24) | 0.96 (0.71–1.30) | 0.94 (0.61–1.46) |
| Model 29 | 0.83 (0.64–1.08) | 0.84 (0.62–1.14) | 0.78 (0.50–1.21) |
| Cerebrovascular disease | |||
| No. of deaths for individuals with/without IBD | 29/1924 | 17/1936 | 13/1940 |
| Model 110 | 1.17 (0.81–1.69) | 0.95 (0.59–1.54) | 1.44 (0.84–2.49) |
| Model 210 | 1.02 (0.70–1.47) | 0.84 (0.52–1.36) | 1.19 (0.69–2.05) |
| Respiratory disease | |||
| No. of deaths for individuals with/without IBD | 36/2159 | 23/2172 | 19/2176 |
| Model 111 | 1.20 (0.86–1.67) | 1.16 (0.77–1.75) | 1.43 (0.91–2.25) |
| Model 211 | 0.89 (0.64–1.23) | 0.89 (0.59–1.35) | 0.97 (0.62–1.53) |
NOTE: inflammatory bowel diseases (IBD); ulcerative colitis (UC); Crohn’s disease (CD; HR (hazard ratio); CI (confidence interval).
Model 1 adjusts for age, sex, center, race/ethnicity, Townsend deprivation index, body mass index, detailed smoking history, alcohol drinking, physical activity, diabetes, hypertension, asthma, tuberculosis, and use of aspirin, ibuprofen, or laxatives.
Model 2 includes all confounders from Model 1 with additional adjustment for overall health rating.
Also adjusted for family history of cancer.
In females only, N=273,301.
Also adjusted for family history of breast cancer.
In males only, N=229,068.
Also adjusted for family history of prostate cancer.
Also adjusted for family history of bowel cancer.
Also adjusted for family history of lung cancer.
Also adjusted for family history of heart disease or stroke.
Also adjusted for family history of heart disease.
Also adjusted for family history of stroke.
Also adjusted for family history of chronic bronchitis/emphysema.
For the cause-specific models, the risk of cancer-specific mortality in individuals with IBD was 1.28 (95% CI: 1.14–1.42) times the rate in individuals without IBD. Similarly, the cancer-specific mortality HR was 1.21 (95% CI: 1.06–1.37) for individuals with UC compared to those without, and 1.45 (95% CI: 1.23–1.71) for individuals with CD compared to those without. After further adjusting for overall health rating, the associations were attenuated but remained statistically significant in individuals with IBD (HR=1.16, 95% CI: 1.05–1.30) or CD (HR=1.28, 95% CI: 1.08–1.51). Meanwhile, we found strong associations with CRC-specific mortality for individuals with IBD and UC in Model 1; the associations for IBD and UC slightly dropped to 1.56 (95%: 1.17–2.09) and 1.57 (95% CI: 1.13–2.20), respectively, in the models with adjustment for overall health rating. However, the association between CD and CRC-specific mortality was weak and not statistically significant in either Model 1 or Model 2. In Model 1, we also found increased prostate cancer-specific mortality in participants with IBD (HR=1.58, 95% CI: 1.06–2.35) and increased breast cancer-specific mortality in participants with CD (HR=1.88, 95% CI: 1.09–3.25). These associations were attenuated and no longer statistically significant in Model 2 with additional adjustment for overall health rating. Additionally, we observed a decreased mortality risk for respiratory system cancer (HR=0.65, 95% CI: 0.43–0.98) in participants with CD in Model 2, although these findings were marginally statistically significant. We did not find associations of IBD with CVD, ischemic disease, cerebrovascular disease, or respiratory disease-specific mortality (Table 2).
In the stratified analyses, we found potential effect modification by BMI and smoking status (PHeterogeneity = 0.03, and 0.01, respectively) (Figure 1). Statistically significant, positive associations between IBD and all-cause mortality were observed among the normal weight (BMI=18.5 to <25.0 kg/m2, HR=1.23, 95% CI:1.07–1.40), and overweight (BMI=25.0 to <30.0 kg/m2, HR=1.15, 95% CI: 1.03–1.29), and Class 2 or 3 obese (BMI≥35.0 kg/m2, HR=1.22, 95% CI: 0.96–1.55) groups, although there was null association among those with a BMI ranged from 30.0 to less than 35.0 kg/m2 (HR=0.97, 95% CI: 0.81–1.16). The association between IBD and all-cause mortality was stronger in non-smokers (HR=1.28, 95% CI: 1.13–1.44) and current (HR=1.32, 95% CI: 1.11–1.58) smokers, compared to former smokers (HR=1.04, 95% CI: 0.93–1.16). The positive association between IBD and all-cause mortality was stronger in individuals who reported having good or excellent health (HR=1.32, 95% CI: 1.17–1.48) compared to individuals who reported fair or poor health (HR=1.05, 95% CI: 0.96–1.15), although the P for heterogeneity was marginally not statistically significant (PHeterogeneity= 0.07). Additionally, no significant association was observed in participants diagnosed with IBD for less than 10 years (HR=1.07, 95% CI: 0.95–1.21). In contrast, there was an increased all-cause mortality risk for those diagnosed with IBD for 10 years or more. There was also a positive association with all-cause mortality for participants diagnosed with IBD before the age of 50 (HR=1.24, 95% CI: 1.13–1.37), while this association became null for those diagnosed at age 50 or older (HR=1.05, 95% CI: 0.94–1.18). However, the associations of IBD and all-cause mortality did not appear to meaningfully differ by duration of IBD, sex, age at baseline, alcohol drinking, having a history of diabetes or bowel cancer screening, or having major dietary changes in the past five years.
Figure 1.
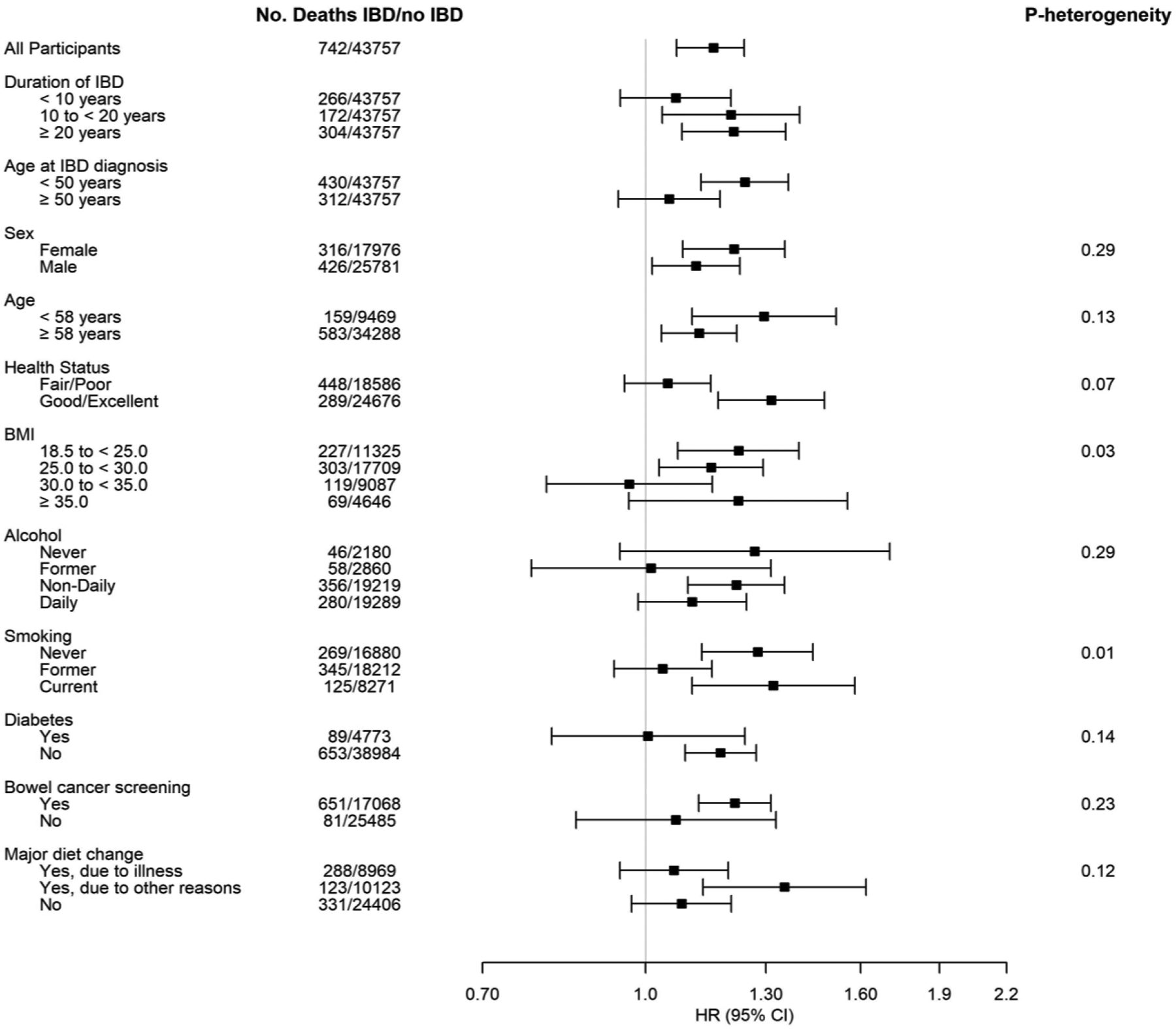
Associations between IBD and all-cause mortality* stratified by potential effect modifiers.
*Health status stratified analysis adjusted for age, sex, center, race/ethnicity, Townsend deprivation index, body mass index, detailed smoking history, alcohol drinking, physical activity, diabetes, hypertension, asthma, tuberculosis, and use of aspirin, ibuprofen or laxatives (Model 1); All other analyses additionally adjusted for overall health rating (Model 2).
When we categorized individuals by IBD diagnosis source, we found that IBD diagnosed via hospital admission records only and multiple sources were associated with increased all-cause (hospital admission: HR=1.29, 95% CI=1.09–1.54; multiple sources: HR=1.18, 95% CI=1.09–1.29) and cancer-specific mortality (hospital admission: HR=1.27, 95% CI=0.97–1.66; multiple sources: HR=1.21, 95% CI=1.07–1.37) (Supplemental Figure 2). However, IBD diagnosed via primary care records only were significantly associated with decreased all-cause (HR=0.57, 95% CI: 0.38–0.86). No associations were observed for IBD ascertained by self-report only with any of the mortality outcomes.
In the sensitivity analysis, which was restricted to participants with available primary care record data, we observed similar positive associations among individuals with IBD (HR=1.38, 95% CI=1.27–1.50), UC (HR=1.29, 95% CI=1.16–1.40) or CD (HR=1.59, 95% CI=1.39–1.80) in Model 1 (Supplemental Table 3). Notably, the association remained significant after additional adjustments were made for overall health rating.
Discussion
In this large cohort of 502,369 people living in the UK, having IBD was consistently associated with increased all-cause, all-cancer, and CRC-specific mortality. Within the subgroups of IBD, having CD or UC was also significantly associated with all-cause and all-cancer mortality. Individuals with UC had increased CRC-specific mortality compared with individuals without UC, and having CD possibly was associated with increased breast cancer-specific mortality. We observed effect modification for the association between IBD and all-cause mortality by duration of IBD, age at IBD diagnosis, BMI and smoking status where the strongest associations between IBD and all-cause mortality were among individuals who were diagnosed with IBD for over 10 years, were diagnosed at younger age, were not obese and either never or current smokers.
Our findings of the increased all-cause mortality among individuals with either UC or CD were similar to the most recent and largest meta-analysis.22 This previous meta-analysis incorporated 35 international cohort studies and found increased standardized mortality ratios (SMR) of 1.38 (95% CI: 1.23–1.55) and 1.19 (95% CI: 1.06–1.35), for the associations of CD and UC with all-cause mortality, respectively. More recent, large, prospective cohort studies in Sweden,29 Spain,30 and Canada31 have also shown similar, positive associations of UC or CD with all-cause mortality. However, a recent U.S. cohort study from Minnesota suggested that the overall mortality was not significantly higher among individuals with CD or UC compared with those without respective conditions.32 Prospective cohort studies conducted in Italy and South Korea both concluded that there was no association between UC and all-cause mortality while individuals with CD had an increased all-cause mortality.33, 34 Although the majority of previous studies found increased mortality for individuals with CD or UC, a large portion of these studies were conducted using registry data and thus were not able to control for a number of confounders not documented in registries such as alcohol consumption and detailed tobacco use. We found that, in particular, self-reported overall health rating had a large effect on the all-cause mortality associations, although it is not clear if health status is a confounder or on the causal pathway. Thus, we generated models with and without adjustment for self-reported overall health rating. In addition, our stratified analysis suggested that certain factors may modify the association between IBD and mortality which deserves further study.
In the cause-specific analyses, we observed a consistent association between IBD and cancer mortality. In the site-specific cancer mortality analyses, IBD was specifically associated with increased CRC mortality. Similar to our study, the previous Swedish cohort study suggested that individuals with IBD were at a 1.4 times increased risk of death from cancer29 and a recent study in the UK Biobank found that both UC and CD were associated with an increased risk of overall cancer incidence and cancer-specific mortality.35 However, the Canadian, Italian and South Korean cohorts found higher risks of cancer-specific mortality in individuals with CD than in the general population, but not individuals with UC.31, 33, 34 The differing results between studies could be related to insufficient power to detect a weaker association, geographic differences, differences in access to and utilization of cancer screening across countries, or other unknown factors. For CRC-specific mortality, we found that participants with UC had higher CRC-specific mortality. We also found that CD was not significantly associated with elevated CRC-specific mortality, although there were only nine individuals with CD who died from CRC. Some previous studies also found an association only for UC with CRC-specific mortality,36, 37 while other studies found positive associations for individuals with UC or CD.31 Mortality due to extraintestinal cancer (e.g., breast cancer, prostate cancer) in individuals with IBD has not been well studied, but a few studies reported a null association with breast cancer-specific death.31, 33, 38 In our study, we observed increased mortality for breast cancer among women with CD, but further studies are needed to confirm these findings. Although IBD has been recently described as a non-traditional risk factor of CVD incidence and mortality in a few studies,29, 39, 40 our finding of the null association between IBD and CVD mortality was in line with a recent meta-analysis.42 Similarly, we did not find associations between IBD and respiratory diseases, although recent research has suggested a potential positive association.41
Many biologically plausible mechanisms have linked IBD to cancer including: 1) chronic inflammation and the interaction between the GI tract microenvironment and immune response in IBD patients triggering carcinogenesis and tumor progression; and 2) prolonged exposures to treatments such as immunosuppressive drugs which can exhibit carcinogenic properties and promote intestinal and extraintestinal cancers.42, 43 For the IBD subtypes, emerging evidence suggests that individuals with CD may experience more severe outcomes, such as deficient innate immune response44 which may lead to increased mortality in this group, although we generally observed similar mortality associations within individuals with CD and UC. However, the impact of biologics and immunomodulators on mortality in individuals with IBD needs to be further tested, as current evidence had mixed findings.45–47 Given the absence of data on IBD treatment strategy in UK Biobank cohort, we were unable to consider how specific IBD treatments (e.g., TNF-α and opiates) were associated with mortality in the current study. However, future observational studies investigating the impact of treatment strategies on mortality in individuals with IBD may be subject to validity limitations due to confounding by indication. Therefore, the association between treatment strategy and mortality would be most rigorously studied in the context of Randomized Controlled Trials. Such studies would be invaluable in further illuminating the effect of different treatment strategies on IBD patient mortality and should be considered an area of future investigation.
The UK Biobank provided a unique opportunity to assess potential effect modification by multiple factors. The positive association differed by BMI status and smoking status and was generally stronger among participants who were not obese and were current or never smokers. Two recent reviews of the interaction of obesity and IBD concluded that current studies have conflicting results and therefore, the effects of obesity on IBD-related health outcomes are unknown.48, 49 Further investigation on effect modification of the association between IBD and mortality by BMI categories is needed. Similarly, the positive association between IBD and all-cause mortality appeared to be restricted to participants who either never smoked or currently smoked, but not among former smokers. Smoking cessation has numerous health benefits including reduced risk of heart disease, lung cancer, and chronic obstructive pulmonary disease.50, 51 It could be that former smokers may have adopted other health-promoting behaviors or maintain increased health vigilance, potentially mitigating IBD’s mortality risk. However, this finding requires independent validation and further study. Meanwhile, we found a null association between IBD and all-cause mortality among individuals reporting poor or fair health. Since poor or fair overall health is an indication of underlying physical or mental health conditions and is correlated to morbidity (e.g., CVD, cancer, and diabetes) in previous and current studies (correlation coefficient = 0.17–0.22; P<0.01),52, 53 it is possible that IBD does not have as significant of an impact on mortality among those who reported worse health. Moreover, the association between IBD and all-cause mortality was only detected in participants diagnosed with IBD before the age of 50 and those with IBD for greater than 10 years. This could suggest that early-onset or longer duration of the disease might exert a more considerable impact on overall health, thereby increasing mortality risk. However, this hypothesis warrants further scrutiny.
There is no existing sensitive serology for IBD, and the diagnosis of IBD largely relies on clinical, endoscopic and histopathologic classifications.54 Self-report, primary care records, and hospital in-patient records were used to identify individuals with IBD in our study. The positive associations between IBD and all-cause mortality and cancer-specific mortality were observed for individuals we identified through hospital in-patient records only or through multiple sources. An inverse association was observed for individuals identified through primary care records only, but this may represent rule-out procedures for IBD. Overall, individuals with an IBD diagnosis identified through multiple sources were less likely to be misclassified, which lends confidence to our finding of a positive association observed between IBD and all-cause and cancer-specific mortality. Additionally, in the sensitivity analysis restricted to participants with available primary care data, we observed similar results, further supporting the validity of our overall associations. The strengths of the study include its prospective design, large sample size, and extensive range of data for possible confounders that were collected in the UK Biobank cohort. Using this data, we were also able to evaluate possible effect modification by multiple demographic, health, and behavioral factors. This study also has limitations. First, it is possible that there was some misclassification in our exposure since IBD diagnosed via hospital admissions did not cover cases that occurred before 1981 and primary care records were only collected on a subset of participants. Nevertheless, the consistency in our findings, even after restricting participants to those with available primary care records, suggests the validity of our results. Second, we only included IBD diagnosed before baseline, which means that any individuals with incident diagnoses of IBD during the follow-up would be included in the referent group. However, as only a limited subset of participants provided self-report or primary care data during follow-up and hospital admission records would underrepresent any newly diagnosed IBD cases, we were unable to include new diagnoses of IBD after baseline. Third, we were likely underpowered for some of the cause-specific mortality analyses, so we may have been unable to detect associations with specific causes of death. Fourth, we did not evaluate the effect of IBD-related treatment on these associations, nor could control for disease severity in the analysis, so future studies should evaluate whether these treatments have an impact on mortality. Fifth, the study’s age range (40 to 69 years at recruitment) is not representative of all individuals with IBD, as many are diagnosed at a younger age, so individuals who died prior to age 40 would be excluded. Finally, the cohort is not demographically representative of the general population of the UK, as the majority (94.1%) of the participants were Caucasian.
In conclusion, in this large study within the UK Biobank cohort, individuals with IBD, UC, or CD had higher all-cause and cancer-specific mortality compared with individuals without IBD, UC, or CD. In addition, CRC- and potentially respiratory system cancer-specific mortality was increased in individuals with UC, and the presence of CD was possibly linked to increased breast cancer mortality. Additional studies in large cohorts with detailed confounder data are needed, particularly to further investigate effect modification by demographic, medical, and behavioral factors.
Supplementary Material
Acknowledgements
This research was conducted using the UK Biobank Resource under Application Number 52576. Copyright © (2023), NHS England. Data was used with the permission of the NHS England and UK Biobank. All rights reserved. This work uses data provided by patients and collected by the NHS as part of their care and support. This research used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation. This study was also supported by the Intramural Research Program of the National Cancer Institute in the National Institutes of Health. We want to thank all the UK Biobank management team for the assistance. We also want to thank NCI DCEG Fellows Editorial Board for assistance in reviewing and improving the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Considerations
The NHS Northwest Multicenter Research Ethics Committee (MREC) reviewed and approved the UK Biobank’s study protocol and research activities, and all participants signed written informed content. We followed the UKB data sharing policy and requested relevant data through the approved UK Biobank project (ID 52576).
Disclosures: No conflicts of interest to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Fangyu Li, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
Yesenia Ramirez, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
Yukiko Yano, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
Carrie R. Daniel, Department of Epidemiology, The University of Texas MD Anderson Cancer Center; 1155 Pressler St, Houston, TX, USA, 77030..
Shreela V. Sharma, Department of Epidemiology, Human Genetics and Environmental Sciences, School of Public Health, University of Texas Health Science Center at Houston; 1200 Pressler St, Houston, TX, USA, 77030..
Eric L. Brown, Department of Epidemiology, Human Genetics and Environmental Sciences, School of Public Health, University of Texas Health Science Center at Houston; 1200 Pressler St, Houston, TX, USA, 77030..
Ruosha Li, Department of Biostatistics and Data Science, School of Public Health, University of Texas Health Science Center at Houston; 1200 Pressler St, Houston, TX, USA, 77030..
Baharak Moshiree, Division of Gastroenterology, Hepatology, and Nutrition, Atrium Health, Wake Forest University, Charlotte, North Carolina; 1025 Morehead Medical Dr, Ste 300, Charlotte, NC, USA, 28204..
Erikka Loftfield, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
Qing Lan, Occupational and Environmental Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
Rashmi Sinha, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
Maki Inoue-Choi, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
Emily Vogtmann, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute; 9609 Medical Center Dr, Rockville, MD, USA, 20850..
References
- 1.Guan Q A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. doi: 10.1155/2019/7247238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. Dec 23 2017;390(10114):2769–2778. doi: 10.1016/s0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 3.Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology. 2020;5(1):17–30. doi: 10.1016/s2468-1253(19)30333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King D, Reulen RC, Thomas T, et al. Changing patterns in the epidemiology and outcomes of inflammatory bowel disease in the United Kingdom: 2000–2018. Alimentary Pharmacology & Therapeutics. 2020;51(10):922–934. doi: 10.1111/apt.15701 [DOI] [PubMed] [Google Scholar]
- 5.Marrero F, Qadeer MA, Lashner BA. Severe complications of inflammatory bowel disease. Med Clin North Am. May 2008;92(3):671–86, ix. doi: 10.1016/j.mcna.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 6.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. May 2009;6(5):297–305. doi: 10.1038/nrgastro.2009.44 [DOI] [PubMed] [Google Scholar]
- 7.Kuenzig ME, Manuel DG, Donelle J, Benchimol EI. Life expectancy and health-adjusted life expectancy in people with inflammatory bowel disease. Canadian Medical Association Journal. 2020;192(45):E1394–E1402. doi: 10.1503/cmaj.190976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. Apr-Jun 2019;12(2):113–122. doi: 10.25122/jml-2018-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav S, Singh S, Harmsen WS, Edakkanambeth Varayil J, Tremaine WJ, Loftus EV. Effect of Medications on Risk of Cancer in Patients With Inflammatory Bowel Diseases. Mayo Clinic Proceedings. 2015;90(6):738–746. doi: 10.1016/j.mayocp.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sewell JL, Yee HF. 13-year mortality trends among hospitalized patients with inflammatory bowel disease. BMC Gastroenterology. 2012;12(1):79. doi: 10.1186/1471-230x-12-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masala G Divergent patterns of total and cancer mortality in ulcerative colitis and Crohn’s disease patients: the Florence IBD study 1978–2001. Gut. 2004;53(9):1309–1313. doi: 10.1136/gut.2003.031476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Card T, Hubbard R, Logan RFA. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 2003;125(6):1583–1590. doi: 10.1053/j.gastro.2003.09.029 [DOI] [PubMed] [Google Scholar]
- 13.Uno H, Yao T, Matsui T, et al. Mortality and cause of death in Japanese patients with Crohn’s disease. Dis Colon Rectum. Oct 2003;46(10 Suppl):S15–21. doi: 10.1097/01.DCR.0000087485.03284.D3 [DOI] [PubMed] [Google Scholar]
- 14.Canavan C, Abrams KR, Hawthorne B, Mayberry JF. Long-term prognosis in Crohn’s disease: an epidemiological study of patients diagnosed more than 20 years ago in Cardiff. Alimentary Pharmacology & Therapeutics. 2007;25(1):59–65. doi: 10.1111/j.1365-2036.2006.03132.x [DOI] [PubMed] [Google Scholar]
- 15.Peneau A, Savoye G, Turck D, et al. Mortality and cancer in pediatric-onset inflammatory bowel disease: a population-based study. Am J Gastroenterol. Oct 2013;108(10):1647–53. doi: 10.1038/ajg.2013.242 [DOI] [PubMed] [Google Scholar]
- 16.Brunet E, Roig-Ramos C, Vela E, et al. Prevalence, incidence and mortality of inflammatory bowel disease in Catalonia. A population-based analysis. Ann Med. Nov 2018;50(7):613–619. doi: 10.1080/07853890.2018.1523550 [DOI] [PubMed] [Google Scholar]
- 17.Nocerino A, Feathers A, Ivanina E, Durbin L, Swaminath A. Mortality Risk of Inflammatory Bowel Disease: A Case-Control Study of New York State Death Records. Dig Dis Sci. Jun 2019;64(6):1604–1611. doi: 10.1007/s10620-018-5430-8 [DOI] [PubMed] [Google Scholar]
- 18.Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. May 2013;7(4):322–37. doi: 10.1016/j.crohns.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 19.Lin W-C, Weng M-T, Tung C-C, et al. Trends and risk factors of mortality analysis in patients with inflammatory bowel disease: a Taiwanese nationwide population-based study. Journal of Translational Medicine. 2019;17(1)doi: 10.1186/s12967-019-02164-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrokhyar F, Swarbrick ET, Grace RH, Hellier MD, Gent AE, Irvine EJ. Low mortality in ulcerative colitis and Crohn’s disease in three regional centers in England. Am J Gastroenterol. Feb 2001;96(2):501–7. doi: 10.1111/j.1572-0241.2001.03466.x [DOI] [PubMed] [Google Scholar]
- 21.Kassam Z, Belga S, Roifman I, et al. Inflammatory bowel disease cause-specific mortality: a primer for clinicians. Inflamm Bowel Dis. Dec 2014;20(12):2483–92. doi: 10.1097/mib.0000000000000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn’s disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis. Mar 2013;19(3):599–613. doi: 10.1097/MIB.0b013e31827f27ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jess T, Loftus EV Jr., Harmsen WS, et al. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940–2004. Gut. Sep 2006;55(9):1248–54. doi: 10.1136/gut.2005.079350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. Mar 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UK Biobank: Protocol for a large-scale prospective epidemiological resource. 2007;
- 26.UK Biobank Death Summary Report. https://biobank.ndph.ox.ac.uk/~bbdatan/DeathSummaryReport.html
- 27.Loftfield E, Cornelis MC, Caporaso N, Yu K, Sinha R, Freedman N. Association of Coffee Drinking With Mortality by Genetic Variation in Caffeine Metabolism: Findings From the UK Biobank. JAMA Intern Med. Aug 1 2018;178(8):1086–1097. doi: 10.1001/jamainternmed.2018.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend PPP, Beattie A. Health and deprivation: inequality and the north. London: Croom Helm. 1988; [Google Scholar]
- 29.Olen O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut. Mar 2020;69(3):453–461. doi: 10.1136/gutjnl-2018-317572 [DOI] [PubMed] [Google Scholar]
- 30.Brunet E, Roig-Ramos C, Vela E, et al. Prevalence, incidence and mortality of inflammatory bowel disease in Catalonia. A population-based analysis. Annals of Medicine. 2018;50(7):613–619. doi: 10.1080/07853890.2018.1523550 [DOI] [PubMed] [Google Scholar]
- 31.Bitton A, Vutcovici M, Sewitch M, Suissa S, Brassard P. Mortality Trends in Crohn’s Disease and Ulcerative Colitis: A Population-based Study in Quebec, Canada. Inflamm Bowel Dis. Feb 2016;22(2):416–23. doi: 10.1097/MIB.0000000000000608 [DOI] [PubMed] [Google Scholar]
- 32.Aniwan S, Harmsen WS, Tremaine WJ, Kane SV, Loftus EV Jr. Overall and Cause-Specific Mortality of Inflammatory Bowel Disease in Olmsted County, Minnesota, From 1970 Through 2016. Mayo Clin Proc. Oct 2018;93(10):1415–1422. doi: 10.1016/j.mayocp.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caini S, Bagnoli S, Palli D, et al. Total and cancer mortality in a cohort of ulcerative colitis and Crohn’s disease patients: The Florence inflammatory bowel disease study, 1978–2010. Dig Liver Dis. Oct 2016;48(10):1162–7. doi: 10.1016/j.dld.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: a nationwide population-based study. Inflamm Bowel Dis. Mar 2015;21(3):623–30. doi: 10.1097/MIB.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 35.Wu S, Xie S, Yuan C, et al. Inflammatory Bowel Disease and Long-term Risk of Cancer: A Prospective Cohort Study Among Half a Million Adults in UK Biobank. Inflammatory Bowel Diseases. 2022;doi: 10.1093/ibd/izac096 [DOI] [PubMed] [Google Scholar]
- 36.Olen O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. Jan 11 2020;395(10218):123–131. doi: 10.1016/S0140-6736(19)32545-0 [DOI] [PubMed] [Google Scholar]
- 37.Duricova D, Pedersen N, Elkjaer M, Gamborg M, Munkholm P, Jess T. Overall and cause-specific mortality in Crohn’s disease: a meta-analysis of population-based studies. Inflamm Bowel Dis. Feb 2010;16(2):347–53. doi: 10.1002/ibd.21007 [DOI] [PubMed] [Google Scholar]
- 38.Sogaard KK, Cronin-Fenton DP, Pedersen L, Sorensen HT, Lash TL. Survival in Danish patients with breast cancer and inflammatory bowel disease: a nationwide cohort study. Inflamm Bowel Dis. Apr 2008;14(4):519–25. doi: 10.1002/ibd.20341 [DOI] [PubMed] [Google Scholar]
- 39.Sun H-H, Tian F Inflammatory bowel disease and cardiovascular disease incidence and mortality: A meta-analysis. European Journal of Preventive Cardiology. 2018;25(15):1623–1631. doi: 10.1177/2047487318792952 [DOI] [PubMed] [Google Scholar]
- 40.Nevulis MG, Baker C, Lebovics E, Frishman WH. Overview of Link Between Inflammatory Bowel Disease and Cardiovascular Disease. Cardiol Rev. Nov/Dec 2018;26(6):287–293. doi: 10.1097/CRD.0000000000000214 [DOI] [PubMed] [Google Scholar]
- 41.Vutcovici M, Bitton A, Ernst P, Kezouh A, Suissa S, Brassard P. Inflammatory bowel disease and risk of mortality in COPD. European Respiratory Journal. 2016;47(5):1357–1364. doi: 10.1183/13993003.01945-2015 [DOI] [PubMed] [Google Scholar]
- 42.Greuter T, Vavricka S, König AO, Beaugerie L, Scharl M. Malignancies in Inflammatory Bowel Disease. Digestion. 2020;101 Suppl 1:136–145. doi: 10.1159/000509544 [DOI] [PubMed] [Google Scholar]
- 43.Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World Journal of Gastroenterology. 2016;22(20):4794. doi: 10.3748/wjg.v22.i20.4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sartor RB. Mechanisms of Disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nature Clinical Practice Gastroenterology & Hepatology. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528 [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious Infection and Mortality in Patients With Crohn’s Disease: More Than 5 Years of Follow-Up in the TREAT™ Registry. American Journal of Gastroenterology. 2012;107(9):1409–1422. doi: 10.1038/ajg.2012.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrinton LJ, Liu L, Chen L, et al. Association between anti-TNF-α therapy and all-cause mortality. Pharmacoepidemiology and Drug Safety. 2012;21(12):1311–1320. doi: 10.1002/pds.3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A Pooled Analysis of Infections, Malignancy, and Mortality in Infliximab- and Immunomodulator-Treated Adult Patients With Inflammatory Bowel Disease. American Journal of Gastroenterology. 2012;107(7):1051–1063. doi: 10.1038/ajg.2012.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson AM, Loftus EV. Impact of Obesity on the Management of Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). Jul 2020;16(7):350–359. [PMC free article] [PubMed] [Google Scholar]
- 49.Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World Journal of Gastroenterology. 2016;22(35):7868. doi: 10.3748/wjg.v22.i35.7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallucci G, Tartarone A, Lerose R, Lalinga AV, Capobianco AM. Cardiovascular risk of smoking and benefits of smoking cessation. Journal of Thoracic Disease. 2020;12(7):3866–3876. doi: 10.21037/jtd.2020.02.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. Feb 2000;161(2 Pt 1):381–90. doi: 10.1164/ajrccm.161.2.9901044 [DOI] [PubMed] [Google Scholar]
- 52.Jylha M What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. Aug 2009;69(3):307–16. doi: 10.1016/j.socscimed.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 53.Takahashi S, Tanno K, Yonekura Y, et al. Poor self-rated health predicts the incidence of functional disability in elderly community dwellers in Japan: a prospective cohort study. BMC Geriatrics. 2020;20(1)doi: 10.1186/s12877-020-01743-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.M’Koma AE. Inflammatory Bowel Disease: Clinical Diagnosis and Surgical Treatment-Overview. Medicina. 2022;58(5):567. doi: 10.3390/medicina58050567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


