Abstract
Glutamate is the major excitatory neurotransmitter in the mammalian CNS. It is loaded into synaptic vesicles by a proton gradient-dependent uptake system and is released by exocytosis upon stimulation. Recently, two mammalian isoforms of a vesicular glutamate transporter, VGLUT1 and VGLUT2, have been identified, the expression of which enables quantal release of glutamate from glutamatergic neurons. Here, we report a novel isoform of a human vesicular glutamate transporter (hVGLUT3). The predicted amino acid sequence of hVGLUT3 shows 72% identity to both hVGLUT1 and hVGLUT2. hVGLUT3 functions as a vesicular glutamate transporter with similar properties to the other isoforms when it is heterologously expressed in a neuroendocrine cell line. Although mammalian VGLUT1 and VGLUT2 exhibit a complementary expression pattern covering all glutamatergic pathways in the CNS, expression of hVGLUT3 overlaps with them in some brain areas, suggesting molecular diversity that may account for physiological heterogeneity in glutamatergic synapses.
INTRODUCTION
Glutamate, a major excitatory neurotransmitter in the mammalian CNS, is transported into synaptic vesicles by a specific transport system driven by the proton electrochemical gradient generated by a V-type H+-ATPase (Masson et al., 1999; Gasnier, 2000). Glutamate uptake by synaptic vesicles is highly selective for l-glutamate, exhibits relatively low substrate affinity (∼mM range) and requires low concentration of chloride (2–4 mM) for full activity (Naito and Ueda, 1983, 1985).
Recently, a protein originally characterized as a brain-specific Na+-dependent inorganic phosphate transporter (BNPI) (Ni et al., 1994) has been shown to function as a vesicular glutamate transporter, and it is now termed VGLUT1 (Bellocchio et al., 2000; Takamori et al., 2000). The amino acid sequence of VGLUT1 shares weak homology to a rabbit Na+-dependent phosphate transporter, and in fact expression of this protein in Xenopus oocytes stimulated transport of inorganic phosphate across the plasma membrane (Ni et al., 1994). However, VGLUT1 is predominantly localized on synaptic vesicles, and its expression is confined to populations of glutamatergic neurons in the mammalian CNS (Bellocchio et al., 1998). Intracellular vesicles isolated from VGLUT1-expressing cell lines exhibit V-ATPase-dependent glutamate uptake activity with properties very similar to those observed in intact synaptic vesicles (Bellocchio et al., 2000; Takamori et al., 2000). In addition, expression of VGLUT1 in neuroendocrine cells and in GABAergic neurons causes quantal release of glutamate, suggesting that VGLUT1 expression is sufficient to define a glutamatergic phenotype in neurons (Takamori et al., 2000).
More recently, we and others found that DNPI (differentiation-associated BNPI) (Aihara et al., 2000), a protein highly homologous to VGLUT1, also functions as a vesicular glutamate transporter, and it has thus been renamed VGLUT2. Like VGLUT1, VGLUT2 is predominantly localized to synaptic vesicles (Fremeau et al., 2001; Takamori et al., 2001; Varoqui et al., 2002). Expression of VGLUT2 in neuroendocrine cells confers V-ATPase-dependent glutamate uptake with properties very similar to intact synaptic vesicles and to VGLUT1 (Bai et al., 2001; Fremeau et al., 2001; Hayashi et al., 2001; Herzog et al., 2001; Takamori et al., 2001; Varoqui et al., 2002). Furthermore, expression of VGLUT2 also causes glutamate release from GABAergic neurons (Takamori et al., 2001). Interestingly, the distribution of VGLUT2 in the CNS is largely complementary to that of VGLUT1 (Fremeau et al., 2001; Fujiyama et al., 2001; Herzog et al., 2001; Sakata-Haga et al., 2001; Kaneko et al., 2002; Varoqui et al., 2002). It is still unclear whether VGLUT1- and VGLUT2-expressing neurons exhibit physiological differences.
The distribution of VGLUT1 and VGLUT2 appears to include all glutamatergic pathways in the mammalian CNS. Nevertheless, we now report the identification of a cDNA in the Human Genome Database with high homology to VGLUT1 and VGLUT2. Expression of this new gene in neuroendocrine cells results in proton gradient-dependent glutamate uptake by intracellular vesicles, indicating that it represents a novel vesicular glutamate transporter, termed VGLUT3.
RESULTS AND DISCUSSION
Molecular cloning of hVGLUT3
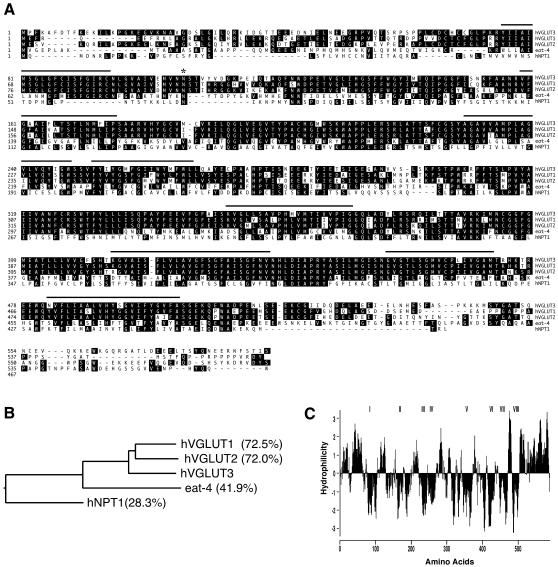
During searches for VGLUT homologues in the publicly available draft of the human genome, we have identified a gene locus on chromosome 12 that contains putative exons resembling VGLUT1 and VGLUT2. The analysis of exon/intron organizations of the novel gene predicts 12 exons that are dispersed over a 80 kb region. All of the donor–acceptor sites are ‘canonical’ GT/AG pairs (see Supplementary figure 1 available at EMBO reports Online). The resulting nucleotide sequence encoded by the gene has a start codon ATG (located on exon-1), which is preceded by Kozak sequences (Kozak, 1991), and is terminated by a stop codon TAA (located on exon-12). This information enabled us to isolate the entire cDNA clone encoding the novel gene from a human fetal brain cDNA library using a combination of PCR and 3′-RACE PCR amplification. The nucleotide and deduced amino acid sequences of the cDNA clone are shown in Supplementary figure 1. The predicted protein consists of 589 amino acids with a molecular mass of ∼65 kDa. Since the predicted amino acid sequence shares a high degree of identity with hVGLUT1 (72.5%) and hVGLUT2 (72.0%; Figure 1A and B), it was named hVGLUT3. A hydropathy plot (Kyte and Doolittle, 1982; Figure 1C) predicts eight membrane-spanning segments with a long C-terminal tail similar to hVGLUT1 and hVGLUT2. Construction of a phylogenetic tree based on the amino acid sequences of related proteins indicates that, in the Na+/Pi cotransporter family (a representative of hNPT-1; Chong et al., 1993), hVGLUT3 forms a small family together with hVGLUT1 and hVGLUT2. They are closely related to Caenorhabditis elegans EAT-4, originally suggested as an orthologue of mammalian VGLUT1 (Lee et al., 1999; Figure 1B).
Fig. 1. hVGLUT3 is highy homologous to hVGLUT1 and hVGLUT2. (A) The deduced amino acid sequence of human VGLUT3 is highly homologous to those of hVGLUT1 and hVGLUT2. Putative transmembrane segments predicted by Kyte–Doolittle hydropathy plot analysis are underlined (C). Black boxes indicate identical amino acid residues, and the asterisk shows a possible N-glycosylation site. (B) A phylogenic tree indicates the relationship between hVGLUT3 and other related proteins (hVGLUT1, hVGLUT2, C. elegans orthologue EAT-4, and hNPT1). The percentages indicate the percentage identity of each protein to hVGLUT3. (C) Hydropathy plot of hVGLUT3 indicates eight putative transmembrane segments.
Restricted distributions of hVGLUT3 transcript in brain
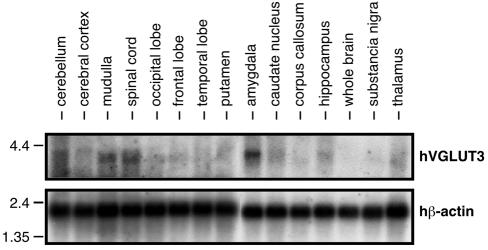
Although there is some overlap in the expression patterns of VGLUT1 and VGLUT2, in most brain regions the transcript of one isoform predominates, resulting in a largely complementary distribution (Aihara et al., 2000; Fremeau et al., 2001; Fujiyama et al., 2001; Herzog et al., 2001; Sakata-Haga et al., 2001; Kaneko et al., 2002; Varoqui et al., 2002). To gain insight into the distribution of hVGLUT3, we performed northern blot analysis using poly(A)+ RNA extracted from various regions of human brain. As shown in Figure 2, a transcript of ∼3.9 kb was detected in the amygdala, medulla and spinal cord. A weaker band was observed in the hippocampus, thalamus and cerebellum, whereas the transcript was not detectable in several other brain regions. Overall, these results indicate that expression of hVGLUT3 is restricted to specialized brain areas. Further characterization of its distribution will be dependent on the availability of immunoreagents crossreacting with non-human orthologues of VGLUT3.
Fig. 2. Expression of VGLUT3 transcripts in various regions of human brain tissue. Northern blotting shows the hybridization of 1 µg of poly(A)+ RNA extracted from each of the designated tissues with a 32P-labelled nick-translated hVGLUT3 fragment derived from the 3′-untranslated region. The upper panel shows hVGLUT3 mRNA (∼3.9 kb), the lower one hβ-actin mRNA that was used as an internal standard.
hVGLUT3 functions as proton gradient-dependent glutamate transporter
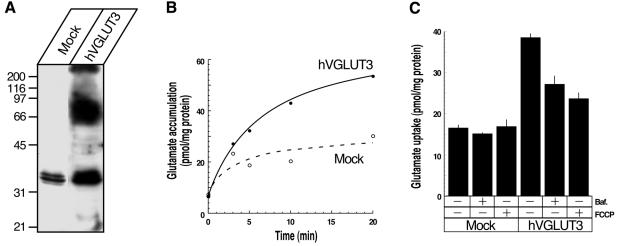
The structural similarity of hVGLUT3 with hVGLUT1 and hVGLUT2 suggests that VGLUT3 may also function as a vesicular glutamate transporter in brain. We therefore used heterologous expression of hVGLUT3 in BON cells (Parekh et al., 1994), a neuroendocrine cell line secreting serotonin, in order to determine whether hVGLUT3 expression results in proton gradient-dependent glutamate uptake by intracellular organelles. As shown previously, untransfected or mock-transfected BON cells exhibit scarce V-ATPase-dependent glutamate uptake activity and do not express detectable amounts of VGLUT1 and VGLUT2 (Takamori et al., 2000, 2001). BON cells were transfected with a bi-cistronic construct in order to express hVGLUT3 together with green fluorescence protein (GFP) for screening stable transfectants. The resulting clones were screened by green fluorescence under the microscope and further tested by western blotting for hVGLUT3 protein expression. One of the resulting clones (F4) was selected for further characterization. Antisera raised against the C-terminal region of hVGLUT3 recognized a distinct broad band with an apparent molecular weight of ∼70 kDa, whereas no such band was detectable in mock-transfected cells (Figure 3A). Light membrane fractions were prepared from both the F4 clone and a control clone (mock-transfected) and assayed for ATP-dependent uptake of 3H-labelled glutamate using standard assay conditions. As shown in Figure 3B, membrane vesicles isolated from F4 accumulated substantially higher (∼2-fold) amounts of glutamate than those isolated from mock cells (Figure 3B). Disruption of the proton gradient either by a proton ionophore, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 46 µM), or by a specific inhibitor for V-ATPase, bafilomycin A1 (100 nM), significantly reduced glutamate accumulation, indicating that it is driven by a proton electrochemical gradient, ΔµH+, generated by a V-ATPase (Figure 3C). Evans Blue at a concentration at 10 µM, which is known to block glutamate uptake into vesicles (Roseth et al., 1995), completely blocked FCCP-sensitive glutamate transport (data not shown). In contrast, inhibitors of other ATPases such as oligomycin B (5 µM), ouabain (2 mM) and vanadate (50 µM) (final concentrations) did not result in significant inhibition (data not shown), further documenting that glutamate transport activity in membranes derived from hVGLUT3-expressing cells depends on the proton electrochemical gradient of the V-ATPase.
Fig. 3. VGLUT3 functions as a vesicular glutamate transporter. (A) hVGLUT3 protein is expressed in hVGLUT3-transfected cells, but not in mock-transfected cells. Triton X-100 extracts from each cell line (50 µg/lane) were analysed by western blotting using hVGLUT3-specific antibody. A broad band with a molecular mass of ∼70 kDa was detected in hVGLUT3 cell extracts only. (B) Time course of glutamate accumulation in hVGLUT3-transfected (filled circles) and mock-transfected (open circles) BON cells. The membranes prepared from hVGLUT3-transfected cells accumulated ∼2-fold higher amounts of glutamate than those from mock-transfected cells. (C) Glutamate uptake by hVGLUT3 is driven by proton-electrochemical gradient as transport activity was reduced by the proton uncoupler, FCCP (46 µM), and the vacuolar H-ATPase inhibitor, bafilomycin A1 (100 nM). Error bars are the standard error of the mean (n = 3).
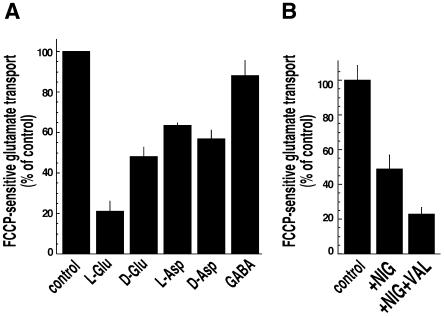
We then examined the substrate specificity of FCCP-sensitive glutamate uptake by hVGLUT3. Glutamate uptake by synaptic vesicles is selective for glutamate, and both VGLUT1- and VGLUT2-mediated transport exhibits the same properties (Bai et al., 2001; Fremeau et al., 2001; Hayashi et al., 2001; Herzog et al., 2001; Takamori et al., 2001; Varoqui et al., 2002). As expected, uptake of labelled glutamate was strongly reduced by l-glutamate, whereas similar concentrations of l-aspartate were less effective. Thus, like the other vesicular glutamate transporters, hVGLUT3 prefers glutamate over aspartate as a substrate. However, [3H]glutamate uptake was inhibited by 10 mM unlabelled d-aspartate and by d-glutamate to a similar extent (∼50%; Figure 4A), although we failed to detect significant uptake of [3H]d-aspartate by hVGLUT3 (data not shown).
Fig. 4. Characterization of glutamate transport by hVGLUT3. (A) Substrate specificity of glutamate transport by hVGLUT3. Glutamate uptake was measured in the absence (100%) and the presence of various amino acids (10 mM). l-glutamate inhibited [3H]glutamate incorporation as anticipated. Note that d-aspartate and d-glutamate inhibited glutamate uptake to a similar extent. (B) Transport by hVGLUT3 is dependent on both ΔΨ and ΔpH. Glutamate uptake was measured in the absence (100%) and presence of 5 µM nigericin and 20 µM valinomycin. In both (A) and (B), only FCCP-sensitive glutamate uptake was calculated by subtracting FCCP-insensitive glutamate accumulation. Error bars are the standard error of the mean (n = 6 from two independent experiments using different membrane preparations).
To further characterize the bioenergetics of glutamate uptake by hVGLUT3, the effects of the K+/H+ exchanger, nigericin, and the K+ ionophore, valinomycin, which selectively abolish ΔpH or ΔΨ of ΔµH+, respectively, were tested. In contrast to the vesicular uptake of GABA, acetylcholine and monoamines, glutamate uptake by synaptic vesicles depends on the electrical potential component ΔΨ to a greater extent than on the ΔpH component of the proton motive force ΔµH+, at least at low chloride concentrations (Naito and Ueda, 1985; Maycox et al., 1988). FCCP-sensitive glutamate transport by hVGLUT3 was reduced by ∼50% by 5 µM nigericin, suggesting that not only ΔΨ but also ΔpH is required for full activity of hVGLUT3, at least in our assay conditions (Figure 4B). Further addition of valinomycin (20 µM) reduced the remaining activity (∼20%; Figure 4B), indicating that glutamate transport by hVGLUT3 depends on both ΔΨ and ΔpH. Thus, it is possible that the transport mechanism of hVGLUT3 differs from that of the other two isoforms, although additional experiments are needed to clarify this issue.
Taken together, the data described above show that three isoforms of vesicular glutamate transporters are expressed in human brain. Furthermore, using database searches, we found a related expressed sequence tag clone (DDBJ/EMBL/GenBank accession number BB396899) that may represent the mouse orthologue of hVGLUT3, suggesting that expression of this third isoform is widespread among mammalian species. Previous immunohistochemical studies demonstrated that the expression patterns of VGLUT1 and VGLUT2 in the CNS complement each other, and these two transporters together might cover all glutamatergic pathways (Fremeau et al., 2001; Fujiyama et al., 2001; Herzog et al., 2001; Sakata-Haga et al., 2001; Kaneko et al., 2002; Varoqui et al., 2002). Furthermore, it has been speculated that the differential expression of VGLUT1 and VGLUT2 correlates with functional differences between VGLUT1- and VGLUT2-containing neurons. In particular, it has been proposed that VGLUT1 synapses may exhibit a lower release probability, and VGLUT2 synapses may display a high release probability (Fremeau et al., 2001; Varoqui et al., 2002). Although the precise distribution of VGLUT3 remains to be determined, our northern blot analysis suggests that VGLUT3 is co-expressed in some brain regions both with VGLUT1 (e.g. hippocampus and cerebellum) and with VGLUT2 (medulla and spinal cord). It remains to be established whether the transporters are co-expressed in the same neurons in these regions or whether they are confined to different neuronal subpopulations and, furthermore, whether VGLUT3 expression is correlated with differences in synaptic activity or in release properties of glutamatergic synapses.
METHODS
Homology search of the human genome draft for a hVGLUT homologue.
In order to search for VGLUT homologues, we used the publicly available draft of the human genome (‘Golden Path’, version available July 2001, http://genome.ucsc.edu/) and the protein sequence specified in the SwissProt SW:q9p2u7 database entry (corresponding to hVGLUT1, originally called hBNPI; Ni et al., 1996). In addition to the exons covering the hVGLUT1 and hVGLUT2 (hDNPI) that were identified on chromosomes 19 and 11, respectively, we have observed exons on chromosome 12 with high homology to hVGLUT1 and hVGLUT2. The analysis of the region covering these exons on chromosome 12 (CHR12–11654043, a genomic region from 12 170 to 94 170 bp) by the GeneWise program (http://www.sanger.ac.uk/Software/Wise2/) indicated that this novel gene (termed hVGLUT3) is composed of 12 exons.
Isolation of hVGLUT3 cDNA.
hVGLUT3 cDNA was amplified by PCR from a human fetal brain cDNA library in pCMV.SPORT2 (Invitrogen) using primers that were designed on the basis of the predicted exon-1 and exon-12 sequences from the human genome draft. PCRs were performed using the primers: sense, 5′-CCACTATGCCTTTTAAAGCATTTGATACC-3′; and anti-sense, 5′-AGATCTTCTCCCCTCCCAATATTTG-3′. In the design of sense primer, a Kosak consensus sequence (CCACT) was introduced upstream from ATG. The PCR-amplified fragment (1920 bp) was subcloned into pCR2.1 (Invitrogen). The nucleotide sequence of the inserts was determined by automated cycle sequencing (Applied Biosystems). The hVGLUT3 cDNA was excised from PCR2.1 with EcoRV and BglII and subcloned into pcDNA3.1(–) at the EcoRV–BamHI site. The 3′-untranslated region of hVGLUT3 was isolated by the 3′-RACE PCR technique using 3′-AmpliFINDER RACE kit (Clontech). The sequence data has been submitted to the DDBJ/EMBL/GenBank database under accession number AJ459241. For transfection experiments, an EcoRV–HindIII fragment of hVGLUT3 was subcloned simultaneously with a HindIII–NotI fragment of IRES–EGFP (a kind gift from J. Rettig, Homburg, Germany) into pcDNA3.1(+) at the EcoRV–NotI site.
Northern blot.
Multiple tissue northern blots from various human regions were purchased from Clontech. After pre-hybridization for 4 h at 42°C, the RNA blots were hybridized for 18 h with a 32P-labelled nick-translated PCR-derived cDNA probe from the 3′-untranslated region of hVGLUT3 (corresponding to nucleotides 1948–3645). A 32P-labelled nick-translated cDNA probe derived from human β-actin was used as an internal standard. The membranes were rinsed in 0.1× SSC and 0.2% SDS at 65°C for 1 h and exposed to X-ray film at –70°C with an intensifying screen for 4 days.
Generation of a hVGLUT3-specific antibody.
An antiserum was raised in rabbits using a bacterially expressed fusion protein as an antigen, which contained glutathione S-transferase (GST) and the amino acids 530–589 of hVGLUT3. The resulting serum was cleared from GST antibodies by absorption to a GST-coupled column. The flow-through fraction from the GST column was applied to an antigen-conjugated column to affinity purify hVGLUT3-specific antibodies.
Generation of BON stable transfectants.
BON cells (Parekh et al., 1994) were transfected with 20 µg of either hVGLUT3-IRES-EGFP-pcDNA3.1 or pEGFP (Clontech) by the calcium-phosphate method (Chen and Okayama, 1987). The transfected cells were then selected in the presence of 800 µg/ml G418 and the resulting clones were screened for EGFP fluorescence by fluorescence microscopy. hVGLUT3 expression in EGFP-positive clones was confirmed by western blotting using the hVGLUT3-specific antibody.
Glutamate uptake assay.
The cultured BON cells (hVGLUT3 cells and mock-transfected cells) were washed twice with ice-cold PBS and then harvested in 0.32 M sucrose and 4 mM HEPES–NaOH pH 7.4. The cells were homogenized, and cell nuclei and cell debris were cleared by centrifugation at 2000 g for 10 min. To remove large membranes, the supernatant was centrifuged further at 10 000 g for 10 min. The supernatant containing the light membrane fraction was sedimented by centrifugation at 200 000 g for 30 min in a TLA100.2 rotor. The resulting pellet was resuspended in standard uptake assay buffer (0.32 M sucrose, 4 mM KCl, 4 mM MgCl2, 10 mM HEPES–KOH pH 7.4). The glutamate uptake assay was carried out as described previously (Takamori et al., 2000).
Supplementary data.
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to M. Ebeling for his advice in bioinformatics. We thank C. Kratzeisen, M. Druminski and D. Diezmann for their skillful technical assistance. We also thank J. Rettig for the IRES–GFP construct. S.T. is a recipient of the JSPS Postdoctoral Fellowships for Research Abroad. This work was supported by the Leibniz Award of the DFG (to R.J.).
REFERENCES
- Aihara Y. et al. (2000) Molecular cloning of a novel brain-type Na+-dependent inorganic phosphate cotransporter. J. Neurochem., 74, 2622–2625. [DOI] [PubMed] [Google Scholar]
- Bai L., Xu, H., Collins, J.F. and Ghishan, F.K. (2001) Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J. Biol. Chem., 276, 36764–36769. [DOI] [PubMed] [Google Scholar]
- Bellocchio E.E., Hu, H., Pohorille, A., Chan, J., Pickel, V.M. and Edwards, R.H. (1998) The localization of the brain-specific inorganic phosphate trans- porter suggests a specific presynaptic role in glutamatergic transmission. J. Neurosci., 18, 8648–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio E.E., Reimer, R.J., Fremeau, R.T., Jr and Edwards, R.H. (2000) Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science, 289, 957–960. [DOI] [PubMed] [Google Scholar]
- Chen C. and Okayama, H. (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol., 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S.S., Kristjansson, K., Zoghbi, H.Y. and Hughes, M.R. (1993) Molecular cloning of the cDNA encoding a human renal sodium phosphate transport protein and its assignment to chromosome 6p21.3-p23. Genomics, 18, 355–359. [DOI] [PubMed] [Google Scholar]
- Fremeau R.T. Jr et al. (2001) The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron, 31, 247–260. [DOI] [PubMed] [Google Scholar]
- Fujiyama F., Furuta, T. and Kaneko, T. (2001) Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J. Comp. Neurol., 435, 379–387. [DOI] [PubMed] [Google Scholar]
- Gasnier B. (2000) The loading of neurotransmitters into synaptic vesicles. Biochimie, 82, 327–337. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Otsuka, M., Morimoto, R., Hirota, S., Yatsushiro, S., Takeda, J., Yamamoto, A. and Moriyama, Y. (2001) Differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. J. Biol. Chem., 276, 43400–43406. [DOI] [PubMed] [Google Scholar]
- Herzog E., Bellenchi, G.C., Gras, C., Bernard, V., Ravassard, P., Bedet, C., Gasnier, B., Giros, B. and El Mestikawy, S. (2001) The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci., 21, RC181, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Fujiyama, F. and Hioki, H. (2002) Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J. Comp. Neurol., 444, 39–62. [DOI] [PubMed] [Google Scholar]
- Kozak M. (1991) An analysis of vertebrate mRNA sequences: intimations of translational control. J. Cell Biol., 115, 887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. and Doolittle, R.F. (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol., 157, 105–132. [DOI] [PubMed] [Google Scholar]
- Lee R.Y., Sawin, E.R., Chalfie, M., Horvitz, H.R. and Avery, L. (1999) EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J. Neurosci., 19, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson J., Sagne, C., Hamon, M. and El Mestikawy, S. (1999) Neurotransmitter transporters in the central nervous system. Pharmacol. Rev., 51, 439–464. [PubMed] [Google Scholar]
- Maycox P.R., Deckwerth, T., Hell, J.W. and Jahn, R. (1988) Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J. Biol. Chem., 263, 15423–15428. [PubMed] [Google Scholar]
- Naito S. and Ueda, T. (1983) Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J. Biol. Chem., 258, 696–699. [PubMed] [Google Scholar]
- Naito S. and Ueda, T. (1985) Characterization of glutamate uptake into synaptic vesicles. J. Neurochem., 44, 99–109. [DOI] [PubMed] [Google Scholar]
- Ni B., Rosteck, P.R., Jr, Nadi, N.S. and Paul, S.M. (1994) Cloning and expression of a cDNA encoding a brain-specific Na+-dependent inorganic phosphate cotransporter. Proc. Natl Acad. Sci. USA, 91, 5607–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B., Yansheng, D., Wu, X., DeHoff, B.S., Rosteck, P.R., Jr and Paul, S.M. (1996) Molecular cloning, expression, and chromosomal localization of a human brain-specific Na+-dependent inorganic phosphate cotransporter. J. Neurochem., 66, 2227–2238. [DOI] [PubMed] [Google Scholar]
- Parekh D. et al. (1994) Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas, 9, 83–90. [DOI] [PubMed] [Google Scholar]
- Roseth S., Fykse, E.M. and Fonnum, F. (1995) Uptake of l-glutamate into rat brain synaptic vesicles: effect of inhibitors that bind specifically to the glutamate transporter. J. Neurochem., 65, 96–103. [DOI] [PubMed] [Google Scholar]
- Sakata-Haga H. et al. (2001) Differential localization and colocalization of two neuron-types of sodium-dependent inorganic phosphate cotrans- porters in rat forebrain. Brain Res., 902, 143–155. [DOI] [PubMed] [Google Scholar]
- Takamori S., Rhee, J.S., Rosenmund, C. and Jahn, R. (2000) Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature, 407, 189–194. [DOI] [PubMed] [Google Scholar]
- Takamori S., Rhee, J.S., Rosenmund, C. and Jahn, R. (2001) Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J. Neurosci., 21, RC182, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H., Schafer, M.K., Zhu, H., Weihe, E. and Erickson, J.D. (2002) Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J. Neurosci., 22, 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.