Abstract
Background:
Survivors of intracerebral hemorrhage (ICH) face an increased risk of ischemic cardiovascular events. Current ICH guidelines do not provide definitive recommendations regarding the use of antithrombotic and statin therapies. We therefore sought to study practice patterns and factors associated with the use of such medications after ICH.
Methods:
This was a cross-sectional study of patients with ICH in the Get With The Guidelines–Stroke registry, between 2011 and 2021. Patients transferred to another hospital, died during hospitalization, and those with missing information on discharge medications were excluded. The study exposure was the proportion of patients who were prescribed antithrombotic or statin medications. We first ascertained the proportion of patients prescribed antithrombotic and lipid lowering medications at discharge overall and across strata defined by pre-ICH use and history of prior ischemic vascular disease or atrial fibrillation. We then studied factors associated with discharge prescription of these medications after ICH, using multiple logistic regression.
Results:
In the final cohort, 50,416 (10.4%) of 486,586 ICH patients were prescribed antiplatelet medications, 173,322 (35.1%) of 493,491 statins, and 27,085 (5.4%) of 486,585 anticoagulation therapy at discharge. The proportion of patients with antiplatelet therapy were 16.6% with pre-ICH use, and 15.6% in those with prior ischemic vascular disease. Statins were prescribed in 41.1% and 43.7% among patients on prior lipid lowering therapy and prior ischemic vascular disease, respectively. Anticoagulation therapy was restarted in 11.1%. In logistic regression analysis, factors associated with higher use of antithrombotic or statin therapies after ICH were younger age, male sex, pre-ICH medication use, prior ischemic vascular disease, atrial fibrillation, lower admission NIHSS, longer length of stay, and favorable discharge outcome.
Conclusions:
Few ICH patients are prescribed antithrombotic or statin therapies at hospital discharge. Given the emerging association between ICH and future major cardiovascular events, trials examining the net benefit of antiplatelet and lipid lowering therapy after ICH are warranted.
Keywords: intracerebral hemorrhage, ischemic Stroke, myocardial Infarction, antithrombotic agents, antiplatelet drugs, anticoagulant drugs, statins
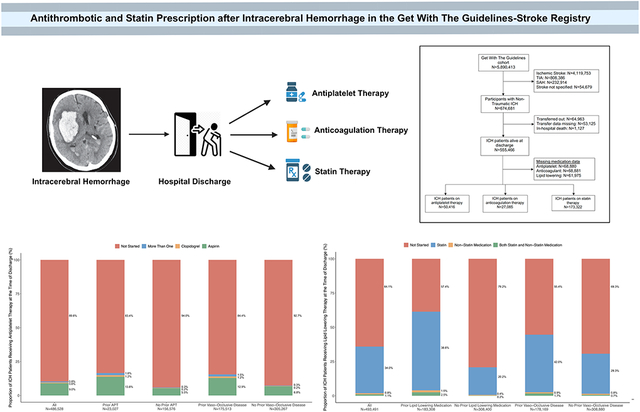
Graphical Abstract
Introduction
Non-traumatic intracerebral hemorrhage (ICH) shares risk factors with ischemic cardiovascular disease, particularly ischemic stroke and myocardial infarction.1 Recent data have shown that ICH is associated with a 2-3-fold heightened risk of major cardiovascular events including stroke, myocardial infarction, and vascular death.2-4 Major cardiovascular events, in turn, are a leading cause of poor outcome after ICH.5,6 Despite this increased cardiovascular risk among ICH survivors, there is uncertainty about the use of secondary prevention strategies such as antithrombotic and lipid lowering therapies, due to a potential increased risk of ICH recurrence.7 While emerging data from randomized trials suggest that resumption of antiplatelet medications after ICH may be safe8, whether initiation of oral anticoagulation among ICH survivors with atrial fibrillation is superior to avoiding it has been inconclusive.9,10 Furthermore, there is also a paucity of prospective, high quality data on the safety of statin medications after ICH.11 Consequently, current ICH guidelines do not provide definitive recommendations about the use of these medications, including the timing of initiation, potentially resulting in wide variation in the implementation of secondary cardiovascular prevention strategies after ICH.12 We therefore sought to leverage a large, heterogeneous, representative, nationwide hospital-level U.S. registry to study the proportion of ICH patients discharged on antithrombotic and lipid lowering medications. In this analysis, we also identified factors associated with implementation of these strategies at the time of discharge following hospitalization for an ICH.
Methods
Data Availability
The data used in this analysis are restricted per the terms of AHA-GWTG’s data use agreement and therefore cannot be shared directly with other investigators. However, interested researchers may submit a proposal to the American Heart Association-GWTG committee to formally request access to the dataset.
Design and Population
We performed a cross-sectional analysis of the GWTG-Stroke registry, an ongoing, national stroke registry and quality improvement initiative sponsored by the American Heart Association and American Stroke Association. Details of the GWTG-Stroke registry, including case ascertainment and data collection methodologies, have been described previously.13,14 Standardized data collection includes patient demographic characteristics, comorbidities, medication history prior to admission, diagnostic testing, brain imaging, in-hospital treatment, medications at discharge, and outcomes. The validity and reliability of data collection have been reported in prior research.15 Each participating hospital received either human research approval to enroll cases without individual patient consent under the common rule, or a waiver of authorization and exemption from subsequent review by their institutional review board (IRB). Advarra, the IRB for the American Heart Association, determined that this study is exempt from IRB oversight. Our analysis was also approved by the institutional review board of Weill Cornell Medicine. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies.16
Measurements
We included patients admitted with a diagnosis of a non-traumatic ICH in the GTWG-Stroke registry between January 2011 and December 2021. We excluded patients with a concurrent diagnosis of subarachnoid hemorrhage, subdural hemorrhage, or ischemic stroke to limit the erroneous inclusion of patients who had an ICH as a complication of therapeutic interventions. Patients who were transferred out to another hospital were excluded to prevent double counting of participants, similar to prior analyses.17,18 We also excluded patients who died during hospitalization, because our objective was to analyze discharge prescriptions of antiplatelet and lipid lowering medications. We identified patients with a medical history of ischemic vascular disease, defined as a composite of ischemic stroke, transient ischemic attack, myocardial infarction, peripheral artery disease, or carotid stenosis. In the GTWG-Stroke registry, carotid stenosis was defined as 50% or greater stenosis, or a history of carotid endarterectomy/stenting.
The primary outcome was the proportion of ICH patients who were prescribed antiplatelet and statin medications at the time of discharge from hospitalization for an ICH. Patients with missing data on these medications were excluded from the analyses. The secondary outcomes were factors associated with the initiation/resumption of these medications at the time of discharge in ICH patients.
Statistical Analysis
We used standard descriptive statistics with confidence intervals to report crude rates. To examine the use of antiplatelet therapy after ICH, we first used the full final analytical sample and subsequently stratified the sample based by prior antiplatelet medication use and presence of ischemic vascular disease. Similarly, we assessed the prescription of statin medications at the time of discharge from an ICH hospitalization in the final study population, and then among patients with prior lipid lowering therapy and ischemic vascular disease. Additionally, we also evaluated the proportion of patients with combined use of antiplatelet and statin medications at discharge after an ICH. Similarly, for anticoagulant medications, we used the full sample initially followed by stratification based on prior anticoagulant use, presence of atrial fibrillation with CHA2DS2-VASc >1, presence of AF with prior stroke or transient ischemic attack, and presence of venous thromboembolism. These results were plotted as bar graphs. We also studied the time trends of the use of these medications using logistic regression.
In secondary analyses, we studied factors associated with prescription of a specific secondary prevention therapy compared to those not getting the intervention. Pearson X2 tests were used for categorical variables, and Student t-tests and Wilcoxon Rank sum tests were used for continuous variables as appropriate, to evaluate intergroup differences. Using multiple logistic regression, we studied the association between demographic and clinical factors, and starting a specific secondary prevention therapy after ICH. To account for hospital-level clustering, we used robust estimator method. The covariates in the models evaluating the prescription of antiplatelet and statin medications were selected a priori and included, age, sex, race, prior use of the medication in question, history of ischemic vascular disease, congestive heart failure, atrial fibrillation, admission National Institutes of Health Stroke Scale (NIHSS), favorable functional outcome at discharge (modified Rankin Score [mRS] of 0-3, length of stay, and hospital factors (teaching status, academic institutions, location, and ICH case volume). The regression models examining the use of anticoagulant medications were prior history of venous thromboembolism. Collinear covariates, defined by a variance inflation factor >4, were subsequently identified and removed from the model.
Results
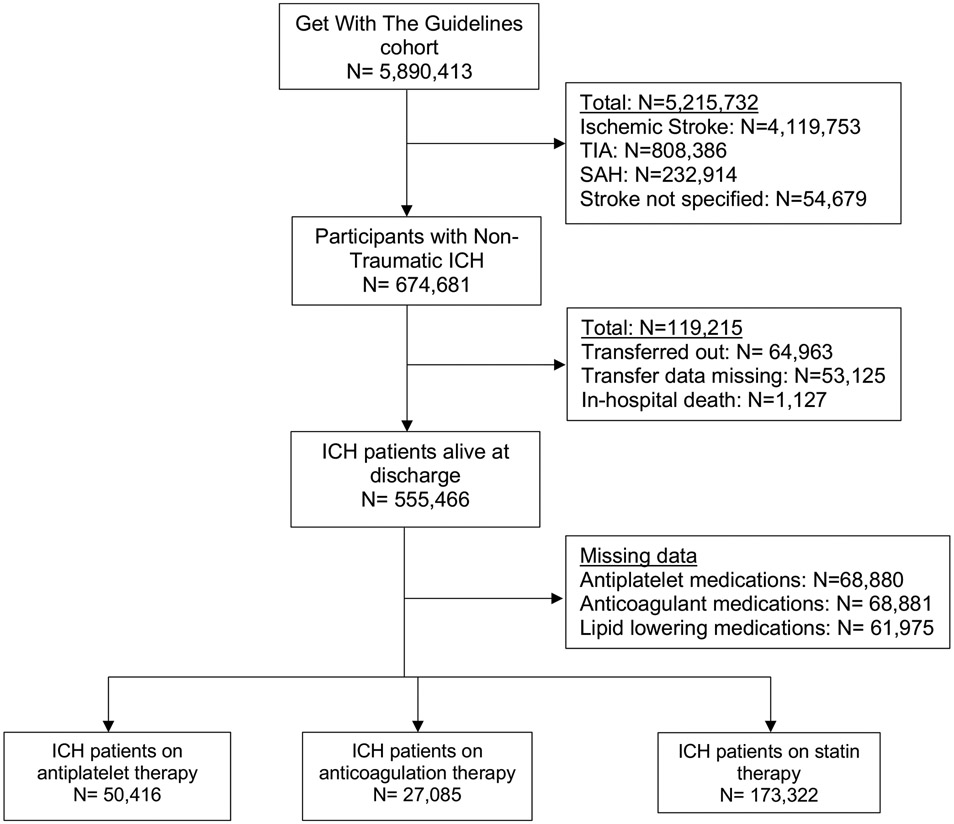
The GWTG-Stroke cohort comprised nearly 5.9 million patients admitted from April 2009 to December 2021, of whom 674,681 had an ICH (Figure 1). In the final analytical cohort of 555,466 patients, the mean age was 67.9 years (15.3) and 47.3% were female. Differences in the baseline characteristics of ICH patients with and without missing data are shown in Supplemental Table S1. The mean length of stay for ICH hospitalization was 8.5 (SD, 11.3) days. The time-trends in the prescription of antithrombotic and statin medications after ICH are shown in Supplemental Figures S1-S3.
Figure 1: Flowchart Showing Inclusion Criteria for Patients with an Intracerebral Hemorrhage in the Get With The Guidelines-Stroke Registry.
Abbreviations: ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage; TIA, transient ischemic attack.
Antiplatelet medications
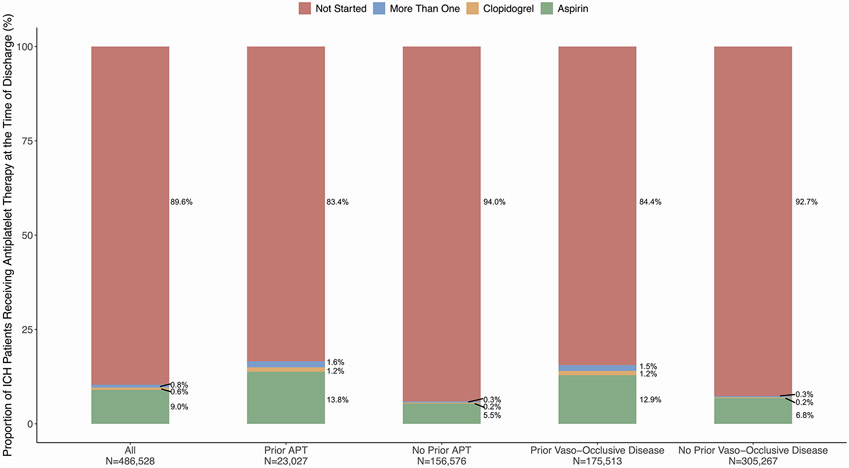
Among 486,586 patients with ICH, 50,416 (10.4%) were prescribed antiplatelet medications at discharge. Patients who were prescribed antiplatelet therapy after ICH were older, had lower ICH severity on admission, and had more vascular risk factors including hypertension, atrial fibrillation, carotid stenosis, coronary artery disease and prior stroke (Table 1). The proportion of patients with antiplatelet therapy were 16.6% with pre-ICH use, and 15.6% in those with prior ischemic vascular disease (Figure 2). Differences in baseline characteristics among patients on prior antithrombotic therapy versus those with a history of ischemic vascular disease are shown in Supplemental Table S2. In logistic regression analysis, factors associated with the use of antiplatelet therapy after ICH were younger age, male sex, prior antiplatelet medication use, prior ischemic vascular disease, atrial fibrillation, baseline NIHSS, longer length of stay, and favorable discharge mRS (Table 2).
Table 1:
Baseline Characteristics of Patients with ICH, Stratified by Medication Use at Discharge
| Variable | Antiplatelet Therapy N = 50,416 |
No Antiplatelet Therapy N = 436,170 |
Statin Medications N = 173,322 |
No Statin Medications N =320,169 |
Anticoagulant Therapy N = 27,085 |
No Anticoagulant Therapy N = 459,500 |
|---|---|---|---|---|---|---|
| Age, mean (SD), years | 71 (60, 80) | 69 (57, 80) | 70 (60, 79) | 69 (56, 81) | 66 (55, 77) | 70 (58, 80) |
| Female | 21,960 (44) | 208,500 (48) | 76,971 (44) | 156,642 (49) | 12,256 (45%) | 218,211 (48%) |
| Race | ||||||
| White | 31,599 (63) | 269,354 (62) | 105,694 (61) | 199,521 (62) | 15,883 (59%) | 285,059 (62%) |
| Black | 10,035 (20) | 77,769 (18) | 32,100 (19) | 56,836 (18) | 5,725 (21%) | 82,082 (18%) |
| Asian | 1,925 (3.8) | 22,243 (5.1) | 9,093 (5.3) | 15,579 (4.9) | 1,288 (4.8%) | 22,885 (5.0%) |
| Patients of other race | 2,608 (5.2) | 23,845 (5.5) | 8,759 (5.1) | 18,023 (5.6) | 1,711 (6.3%) | 24,741 (5.4%) |
| Hispanic ethnicity | 4,215 (8.4) | 42,459 (9.7) | 17,492 (10) | 29,906 (9.3) | 2,452 (9.1%) | 44,224 (9.6%) |
| NIHSS, admission | 4 (1, 12) | 9 (2, 20) | 4 (1, 11) | 13 (4, 23) | 8 (2, 17) | 8 (2, 19) |
| Atrial fibrillation | 11,209 (23) | 68,847 (16) | 31,728 (19) | 48,826 (15) | 8,076 (30%) | 71,967 (16%)# |
| Carotid Stenosis | 1,770 (3.6) | 6,634 (1.5) | 4,257 (2.5) | 4,275 (1.4) | 401 (1.5%) | 8,004 (1.8%) |
| Coronary artery disease | 14,891 (30) | 68,976 (16) | 39,280 (23) | 45,822 (14) | 5,173 (19%) | 78,684 (17%) |
| Diabetes mellitus | 18,728 (38%) | 107,848 (25) | 59,637 (35) | 69,557 (22) | 7,467 (28%) | 119,105 (26%) |
| Hypertension | 40,913 (82) | 312,691 (73) | 138,664 (81) | 220,105 (70) | 19,392 (72%) | 334,197 (74%) |
| Prosthetic heart valve | 1,070 (2.1) | 5,794 (1.3) | 2,716 (1.6) | 4,129 (1.3) | 1,571 (5.8%) | 5,293 (1.2%)# |
| Heart failure | 5,685 (11) | 29,782 (6.9) | 14,549 (8.5) | 21,337 (6.7) | 2,915 (11%) | 32,551 (7.2%) |
| Smoker | 7,390 (15) | 58,137 (13) | 23,087 (14) | 43,311 (14)# | 3,295 (12%) | 62,230 (14%) |
| Prior stroke/TIA | 17,698 (36) | 100,920 (23) | 52,158 (31) | 68,263 (22) | 6,873 (26%) | 111,733 (25%) |
| Length of hospital stay (median, IQR) | 6 (4,12) | 5 (3, 10) | 6 (4, 10) | 5 (2, 10) | 11 (6,21) | 5 (3, 9) |
| Favorable outcome at discharge (mRS 0-3) |
2,932 (44) | 16,530 (14)# | 9,996 (44) | 9,689 (9.3)# | 1,098 (32) | 18,364 (15)# |
| Rural hospital location | 1,479 (2.9) | 11,992 (2.8) | 4,641 (2.7) | 8,950 (2.8) | 438 (1.6) | 13,033 (2.9) |
| Academic hospital | 33,765 (75) | 269,135 (69) | 106,166 (69) | 201,030 (70) | 19,602 (81) | 283,318 (69) |
| Teaching hospital | 43,701 (88) | 366,892 (85) | 145,992 (85) | 270,135 (85) | 24,361 (91) | 386,236 (85) |
| Annual Volume of ICH Stroke Admissions | 72 (40, 119) | 66 (38, 111) | 65 (37, 110) | 67 (38, 115) | 83 (51, 128) | 65 (37, 111) |
Abbreviations: ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin Score; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; TIA, transient ischemic attack.
indicates a p value >0.05. All p values were <0.05 unless specified otherwise.
Figure 2: Bar Graphs Showing the Proportion of ICH Patients Receiving Antiplatelet Therapy at the Time of Discharge.
Abbreviations: APT, antiplatelet therapy; ICH, intracerebral hemorrhage.
Table 2:
Multiple Logistic Regression of Factors Associated with Antiplatelet and Statin Therapy at Discharge after ICH
| Covariate | Antiplatelet Therapy | Statin Therapy | Anticoagulation Therapy | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.02 (1.01-1.04) | 0.02 | 0.98 (0.97-0.99) | 0.003 | 0.99 (0.98-0.99) | <0.001 |
| Female | 0.93 (0.84-1.04) | 0.22 | 0.98 (0.93-1.04) | 0.52 | 1.06 (0.90-1.24) | 0.489 |
| Race | ||||||
| White | Reference | Reference | Reference | |||
| Black | 1.21 (0.96-1.51) | 0.10 | 1.45 (1.29-1.62) | <0.001 | 1.25 (0.97-1.62) | 0.088 |
| Asian | 0.83 (0.60-1.16) | 0.28 | 1.34 (1.14-1.57) | <0.001 | 1.17 (0.84-1.63) | 0.361 |
| Patients of other race | 1.02 (0.75-1.39) | 0.89 | 1.47 (1.26-1.70) | <0.001 | 0.89 (0.63-1.27) | 0.528 |
| Hispanic Ethnicity | 0.90 (0.69-1.18) | 0.45 | 1.37 (1.22-.55) | <0.001 | 1.05 (0.79-1.39) | 0.759 |
| Prior medication use# | 3.31 (2.62-4.19) | <0.001 | 6.20 (5.62-6.84) | <0.001 | 2.93 (2.05-4.19) | <0.001 |
| Vaso-occlusive history | 2.03 (1.67-2.46) | <0.001 | 1.17 (1.11-1.23) | <0.001 | 1.10 (0.89-1.36) | 0.358 |
| Atrial fibrillation | 1.45 (1.13-1.86) | 0.003 | 0.87 (0.82-0.94) | <0.001 | 2.10 (1.50-2.93) | <0.001 |
| Congestive heart failure | 1.20 (0.91-1.61) | 0.20 | 0.87 (0.80-0.94) | 0.001 | 1.16 (0.85-1.57) | 0.353 |
| Baseline NIHSS | 0.93 (0.92-0.94) | <0.001 | 0.90 (0.89-0.91) | <0.001 | 0.96 (0.96-0.97) | <0.001 |
| Length of Stay, per day | 1.02 (1.01-1.03) | <0.001 | 1.04 (1.02-1.04) | <0.001 | 1.03 (1.03-1.04) | <0.001 |
| Favorable outcome at discharge (mRS 0-3) | 1.62 (1.36-1.91) | <0.001 | 3.04 (2.67-3.47) | <0.001 | 1.41 (1.02-1.67) | 0.03 |
| Rural location of hospital | 1.24 (0.80-1.94) | 0.33 | 0.99 (0.75-1.31) | 0.97 | 0.25 (0.07-0.87) | 0.03 |
| Academic hospital | 1.19 (0.93-1.54) | 0.17 | 0.89 (0.73-1.08) | 0.25 | 1.31 (0.85-2.02) | 0.223 |
| Teaching hospital | 1.04 (0.79-1.40) | 0.75 | 1.11 (0.89-1.39) | 0.36 | 0.95 (0.57-1.61) | 0.860 |
| Annual ICH case volume | ||||||
| Quartile 2 | 0.96 (0.75-1.22) | 0.74 | 0.86 (0.71-1.04) | 0.71 | 1.40 (0.94-2.07) | 0.096 |
| Quartile 3 | 1.22 (0.89-1.68) | 0.21 | 1.10 (0.90-1.35) | 0.90 | 1.71 (1.12-2.61) | 0.013 |
| Quartile 4 | 1.16 (0.84-1.61) | 0.36 | 0.80 (0.63-1.03) | 0.09 | 1.46 (0.97-2.20) | 0.070 |
Abbreviations: CI, confidence interval; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale; OR, odds ratio.
Use of either antiplatelet, statin, or both prior to admission for ICH
Anticoagulant medications
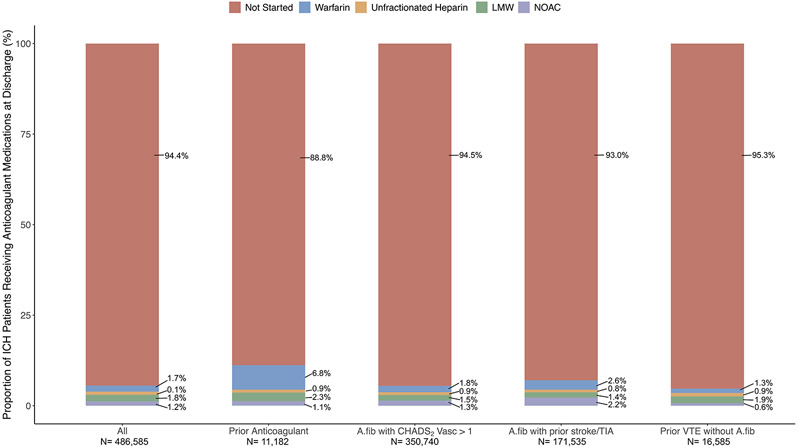
A total of 27,085 (5.6%) were prescribed anticoagulation therapy at ICH discharge. Prescription of anticoagulant therapy at discharge was more common in younger patients, and in those with more vascular risk factors including atrial fibrillation and prosthetic heart valves, with the exception of hypertension (Table 1). In pre-specified strata, 11.1% of ICH patients previously on anticoagulation therapy were restarted on the medication (Figure 3). In total, 6,779 ICH patients with atrial fibrillation were previously on anticoagulation medications at the time of ICH. Among them, 469 (7.0%) remained on anticoagulation, 627 (9.3%) switched to an antiplatelet medication, 187 (2.8%) received both medications, and 5,429 (81%) were discharged without antithrombotic therapy. In logistic regression analysis, factors associated with the use of antiplatelet therapy after ICH were age, prior anticoagulant medication use, presence of atrial fibrillation, prior venous thromboembolism, and favorable discharge mRS (Table 2).
Figure 3: Bar Graphs Showing the Proportion of ICH Patients Receiving Anticoagulation Therapy at the Time of Discharge.
Abbreviations: A.fib, atrial fibrillation; VTE, venous thromboembolism.
Lipid lowering medications
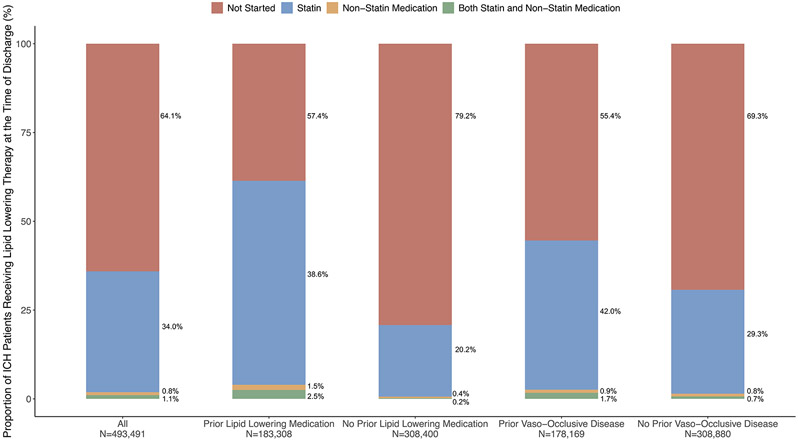
Of the 493,491 ICH patients who had data on lipid lowering medications at discharge, 173,322 (35.1%) were prescribed statin medications and 9,239 (1.9%) had non-statin medications. ICH patients who were prescribed statin medications were older, male, and had a higher prevalence of vascular risk factors such as hypertension, ischemic vascular disease, atrial fibrillation, and heart failure (Table 1). Statins were prescribed in 41.1% and 43.7% among patients on prior lipid lowering therapy and prior ischemic vascular disease, respectively (Figure 4). In logistic regression analysis, factors associated with the use of statin therapy after ICH were male sex, Black race, prior lipid lowering medication use, prior ischemic vascular disease, baseline NIHSS, longer length of stay, and favorable discharge mRS (Table 2).
Figure 4: Bar Graphs Showing the Proportion of ICH Patients Receiving Lipid Lowering Therapy at the Time of Discharge.
Abbreviations: ICH, intracerebral hemorrhage.
Discussion
In this large, clinical practice-based quality improvement registry of ICH survivors, we identified low rates of antithrombotic and statin prescription at the time of hospital discharge. Rates of prescription of antiplatelet, anticoagulation, and statin therapies were 10.4%, 5.6%, and 35.1%, respectively. While our analyses suggest that younger patients and those with lower admission NIHSS, mRS at discharge, a history of pre-morbid vascular events and antecedent prescription were more likely to receive antiplatelet or statin treatment, proportions of prescription in this high vascular risk population were low in all groups.
ICH is believed to disproportionately increase the risk of recurrent ICH, but emerging data have challenged this paradigm by demonstrating a heightened risk of future ischemic cardiovascular disease, including ischemic stroke and myocardial infarction.1 Although it is hypothesized that antithrombotic drug interruption in the acute phase of ICH and poor long-term risk factor control influence the risk of ischemic cardiovascular disease among ICH survivors19,20, the clinical factors and mechanisms underlying this risk are poorly understood. In this context, our real-world data from a large U.S. national registry highlight the low utilization of secondary stroke and cardiovascular prevention therapies after ICH, which likely play a role in the elevated ischemic cardiovascular disease risk. These findings assume importance particularly in patients with a deep ICH where the risk of major arterial ischemic events is higher than the risk of a recurrent ICH.21 While the risk of a recurrent ICH can be mitigated by optimal long-term blood pressure control22, antiplatelet and statin medications when used in conjunction for secondary cardiovascular prevention, can lower the risk of an arterial ischemic event by 30%.23,24 Therefore, antiplatelet and statin medications may have a role in reducing the long-term ischemic risk among survivors of a deep ICH.
Antiplatelets and statin medications are rarely utilized after ICH largely due to the perceived increased risk of ICH recurrence.11,19 The latest AHA guidelines state that it may be reasonable to resume antiplatelet and statin medications after ICH, but do not provide clarity on the timing of initiation of these medications after ICH.12 Although prior clinical trials targeting secondary stroke and cardiovascular disease prevention have shown an increased risk of ICH with antithrombotic therapy25,26, given the established benefit of these medications in reducing cardiovascular disease in patients with indications, there has been renewed interest in investigating the net benefit of antithrombotic medications after ICH. For instance, in the RESTART trial where ICH patients were randomized to stop or resume antiplatelet therapy, there was no relationship between antiplatelet therapy use and ICH recurrence.8 Despite these results, the use of antiplatelet therapy overall in our study was about 10%, and slightly higher at 17% among those previously on antiplatelet medications. Along similar lines, published literature provides conflicting data about the risk of ICH recurrence with statin use, particularly in lobar ICH.11,27 This uncertainty was reflective in a recent survey about antithrombotic treatment after ICH, where over 75% of stroke physicians in the United Kingdom were uncertain about antiplatelet therapy and an overwhelming 95% of the stroke clinicians expressed indecision over anticoagulant use after ICH.28 The low rates of antithrombotic medication prescription in our study also demonstrate the current clinical practice in the US, where these medications are withheld after an ICH with planned resumption in 2-6 weeks after discharge in many cases.29,30 Moreover, the current ICH clinical guidelines recommend delaying the initiation of anticoagulation for 7-8 weeks after ICH.12 Several randomized clinical trials are currently underway to specifically address the safety and efficacy of antithrombotic and statin medications after ICH, and will eventually provide concrete evidence.11,19
Our study has some important limitations. First, our study was subject to confounding by indication in that clinicians were more likely to start these medications in patients who had favorable ICH outcomes and were deemed to be low risk for ICH recurrence. Nevertheless, this limitation underscores the need for randomized trials examining the net benefit of antiplatelet and lipid lowering therapy after ICH. Second, while the Get with the Guidelines dataset collected some imaging data, the actual images were not available for further analysis. As a result, adjudication of ICH location (lobar vs. deep), or assessment of ICH imaging severity characteristics such as ICH volume, intraventricular hemorrhage volume, or hematoma expansion was not possible in this analysis. This precluded further study of the relationship between ICH location and statin use, particularly considering the safety of statin use being called into question in patients with lobar ICH and in those with cerebral amyloid angiopathy.11 Third, data on antithrombotic and lipid lowering medications were available at the time of ICH discharge, although it is likely that clinicians are inclined to starting these medications much later during outpatient follow up after ICH discharge, which may have accounted for low rates of medication use in our study. Fourth, data were obtained from hospitals participating in the GWTG-Stroke program and our results may, therefore, may not be generalizable to ICH patients treated in hospitals outside the registry or to patients in other countries. Lastly, the diagnosis codes for ICH are not 100% accurate which may have resulted in the inclusion of some patients who may have had an ICH as a complication of interventions. To explore this further, we identified the proportion of thrombolysis and thrombectomy interventions among ICH patients and found that none received thrombectomy, and about 0.1% of them received thrombolysis. In the absence of ischemic stroke code diagnoses, this likely represented thrombolysis administered for pulmonary embolism and unclogging of central venous catheters (including dialysis catheters).31,32 Despite these limitations, our results provide important insight into antithrombotic and lipid lowering medication use after ICH. Moreover, the GTWG-Stroke is the largest stroke registry that encompasses the majority of the tertiary hospitals, where ICH patients are likely to be treated, and is hence representative of the ICH treatment practices in the US.
Conclusions
Few survivors of ICH are prescribed secondary stroke prevention therapies, particularly antithrombotic and statin medications, at the time of discharge in the US. Given the emerging association between ICH and future ischemic stroke or myocardial infarction, studies examining the net benefit of antiplatelet and lipid lowering therapy, and timing of initiation of these medications after ICH are warranted.
Supplementary Material
Funding/Support:
This study is funded by the National Institutes of Health (NIH)/ National Institute of Neurological Disorders and Stroke (NINDS) through grants K23NS105948 (Murthy). The Get With The Guidelines®–Stroke (GWTG-Stroke) program is provided by the American Heart Association/American Stroke Association. GWTG-Stroke is sponsored, in part, by Novartis, Novo Nordisk, AstraZeneca, Bayer, Tylenol and Alexion, and AstraZeneca Rare Disease.
Non-Standard Abbreviations
- GWTG
Get With The Guidelines
- ICH
Intracerebral hemorrhage
- NIHSS
National Institutes of Health Stroke Scale
Footnotes
- Dr. Murthy has received grants from the NIH and reports personal fees for medicolegal consulting in stroke and neurological disorders.
- Dr. Ziai has received grants from the NIH, serves as the Associate Editor of Neurocritical Care, and reports personal fees from C. R. Bard DMC, outside the submitted work.
- Dr. Schwamm reported receiving personal fees from the Massachusetts Department of Public Health, Genentech, Penumbra, Diffusion Pharma, and Medtronic; grants from Medtronic and the National Institute of Neurological Disorders and Stroke outside the submitted work; and serving as volunteer chair of the American Heart Association/American Stroke Association Get With the Guidelines (GWTG)–Stroke ClinicalWork Group and as a consultant to Coverdell Grant.
- Dr. Fonarow reported receiving personal fees from AstraZeneca, Amgen, Bayer, Eli Lilly, Janssen, Merck, Novartis, and Pfizer outside the submitted work.
- Dr. Smith reported consulting for Eli Lilly, outside the submitted work.
- Dr. Bhatt discloses the following relationships - Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Angiowave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures, Hims; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda.
- Dr. Kamel is a co-PI for the ARCADIA trial (NIH/NINDS U01NS095869), which received in-kind study drug from the BMS-Pfizer Alliance for Eliquis® and ancillary study support from Roche Diagnostics; other funding from NIH (R01HL144541, R01NS123576, U01NS106513); Deputy Editor for JAMA Neurology; clinical trial steering/executive committees for Medtronic, Janssen, and Javelin Medical; endpoint adjudication committees for AstraZeneca, Novo Nordisk, and Boehringer Ingelheim; and household ownership interests in TETMedical, Spectrum Plastics Group, and Burke Porter Group.
- Dr. Sheth reported receiving grants from the National Institutes of Health, the American Heart Association, Bard, Hyperfine, and Biogen, He received consulting fees from Zoll and Sense for DSMB service and consulting fees from Astrocyte, CSL Behring and Rhaeos. He also holds equity in Alva outside the submitted work.
- No other disclosures were reported.
References
- 1.Li L, Murthy SB. Cardiovascular Events After Intracerebral Hemorrhage. Stroke. 2022;53:2131–2141. doi: 10.1161/STROKEAHA.122.036884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murthy SB, Zhang C, Diaz I, Levitan EB, Koton S, Bartz TM, DeRosa JT, Strobino K, Colantonio LD, Iadecola C, et al. Association Between Intracerebral Hemorrhage and Subsequent Arterial Ischemic Events in Participants From 4 Population-Based Cohort Studies. JAMA Neurol. 2021;78:809–816. doi: 10.1001/jamaneurol.2021.0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy SB, Diaz I, Wu X, Merkler AE, Iadecola C, Safford MM, Sheth KN, Navi BB, Kamel H. Risk of Arterial Ischemic Events After Intracerebral Hemorrhage. Stroke. 2020;51:137–142. doi: 10.1161/STROKEAHA.119.026207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaist D, Hald SM, Garcia Rodriguez LA, Clausen A, Moller S, Hallas J, Al-Shahi Salman R. Association of Prior Intracerebral Hemorrhage With Major Adverse Cardiovascular Events. JAMA Netw Open. 2022;5:e2234215. doi: 10.1001/jamanetworkopen.2022.34215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah VA, Thompson RE, Yenokyan G, Acosta JN, Avadhani R, Dlugash R, McBee N, Li Y, Hansen BM, Ullman N, et al. One-Year Outcome Trajectories and Factors Associated with Functional Recovery Among Survivors of Intracerebral and Intraventricular Hemorrhage With Initial Severe Disability. JAMA Neurol. 2022;79:856–868. doi: 10.1001/jamaneurol.2022.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parasram M, Parikh NS, Merkler AE, Falcone GJ, Sheth KN, Navi BB, Kamel H, Zhang C, Murthy SB. Risk of Mortality After an Arterial Ischemic Event Among Intracerebral Hemorrhage Survivors. Neurohospitalist. 2022;12:19–23. doi: 10.1177/19418744211026709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheth KN, Selim M. Focused Update on Vascular Risk and Secondary Prevention in Survivors of Intracerebral Hemorrhage. Stroke. 2022;53:2128–2130. doi: 10.1161/STROKEAHA.122.039819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaboration R. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open-label trial. Lancet. 2019;393:2613–2623. doi: 10.1016/S0140-6736(19)30840-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreuder F, van Nieuwenhuizen KM, Hofmeijer J, Vermeer SE, Kerkhoff H, Zock E, Luijckx GJ, Messchendorp GP, van Tuijl J, Bienfait HP, et al. Apixaban versus no anticoagulation after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation in the Netherlands (APACHE-AF): a randomised, open-label, phase 2 trial. Lancet Neurol. 2021;20:907–916. doi: 10.1016/S1474-4422(21)00298-2 [DOI] [PubMed] [Google Scholar]
- 10.So SC. Effects of oral anticoagulation for atrial fibrillation after spontaneous intracranial haemorrhage in the UK: a randomised, open-label, assessor-masked, pilot-phase, non-inferiority trial. Lancet Neurol. 2021;20:842–853. doi: 10.1016/S1474-4422(21)00264-7 [DOI] [PubMed] [Google Scholar]
- 11.Shoamanesh A, Selim M. Use of Lipid-Lowering Drugs After Intracerebral Hemorrhage. Stroke. 2022;53:2161–2170. doi: 10.1161/STROKEAHA.122.036889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, Hemphill JC 3rd, Johnson R, Keigher KM, Mack WJ, et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2022;53:e282–e361. doi: 10.1161/STR.0000000000000407 [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, Hernandez AF, Peterson ED, Schwamm LH, Committee GW-SS, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes. 2010;3:291–302. doi: 10.1161/CIRCOUTCOMES.109.921858 [DOI] [PubMed] [Google Scholar]
- 14.Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, Ellrodt G, Cannon CP, Liang L, Peterson E, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107–115. doi: 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 15.Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, et al. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J. 2012;163:392–398, 398 e391. doi: 10.1016/j.ahj.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 17.Xian Y, Zhang S, Inohara T, Grau-Sepulveda M, Matsouaka RA, Peterson ED, Piccini JP, Smith EE, Sheth KN, Bhatt DL, et al. Clinical Characteristics and Outcomes Associated With Oral Anticoagulant Use Among Patients Hospitalized With Intracerebral Hemorrhage. JAMA Netw Open. 2021;4:e2037438. doi: 10.1001/jamanetworkopen.2020.37438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xian Y, Xu H, Matsouaka R, Laskowitz DT, Maisch L, Hannah D, Smith EE, Fonarow GC, Bhatt DL, Schwamm LH, et al. Analysis of Prescriptions for Dual Antiplatelet Therapy After Acute Ischemic Stroke. JAMA Netw Open. 2022;5:e2224157. doi: 10.1001/jamanetworkopen.2022.24157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puy L, Forman R, Cordonnier C, Sheth KN. Protecting the Brain, From the Heart: Safely Mitigating the Consequences of Thrombosis in Intracerebral Hemorrhage Survivors With Atrial Fibrillation. Stroke. 2022;53:2152–2160. doi: 10.1161/STROKEAHA.122.036888 [DOI] [PubMed] [Google Scholar]
- 20.Mullen MT, Anderson CS. Review of Long-Term Blood Pressure Control After Intracerebral Hemorrhage: Challenges and Opportunities. Stroke. 2022;53:2142–2151. doi: 10.1161/STROKEAHA.121.036885 [DOI] [PubMed] [Google Scholar]
- 21.Casolla B, Moulin S, Kyheng M, Henon H, Labreuche J, Leys D, Bauters C, Cordonnier C. Five-Year Risk of Major Ischemic and Hemorrhagic Events After Intracerebral Hemorrhage. Stroke. 2019;50:1100–1107. doi: 10.1161/STROKEAHA.118.024449 [DOI] [PubMed] [Google Scholar]
- 22.Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, Rosand J. Association Between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. JAMA. 2015;314:904–912. doi: 10.1001/jama.2015.10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennekens CH, Sacks FM, Tonkin A, Jukema JW, Byington RP, Pitt B, Berry DA, Berry SM, Ford NF, Walker AJ, et al. Additive benefits of pravastatin and aspirin to decrease risks of cardiovascular disease: randomized and observational comparisons of secondary prevention trials and their meta-analyses. Arch Intern Med. 2004;164:40–44. doi: 10.1001/archinte.164.1.40 [DOI] [PubMed] [Google Scholar]
- 24.Kim BJ, Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, Wong LKS, Yu S, Hwang YH, Lee JS, et al. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurol. 2018;17:509–518. doi: 10.1016/S1474-4422(18)30128-5 [DOI] [PubMed] [Google Scholar]
- 25.Antithrombotic Trialists C, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang WY, Saver JL, Wu YL, Lin CJ, Lee M, Ovbiagele B. Frequency of Intracranial Hemorrhage With Low-Dose Aspirin in Individuals Without Symptomatic Cardiovascular Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76:906–914. doi: 10.1001/jamaneurol.2019.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endres M, Nolte CH, Scheitz JF. Statin Treatment in Patients With Intracerebral Hemorrhage. Stroke. 2018;49:240–246. doi: 10.1161/STROKEAHA.117.019322 [DOI] [PubMed] [Google Scholar]
- 28.Forfang E, Larsen KT, Salman RA, Bell SM, Wester P, Berge E, Wyller TB, Ronning OM. Antithrombotic treatment after intracerebral hemorrhage: Surveys among stroke physicians in Scandinavia and the United Kingdom. Health Sci Rep. 2023;6:e1059. doi: 10.1002/hsr2.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy SB, Biffi A, Falcone GJ, Sansing LH, Torres Lopez V, Navi BB, Roh DJ, Mandava P, Hanley DF, Ziai WC, et al. Antiplatelet Therapy After Spontaneous Intracerebral Hemorrhage and Functional Outcomes. Stroke. 2019;50:3057–3063. doi: 10.1161/STROKEAHA.119.025972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biffi A, Kuramatsu JB, Leasure A, Kamel H, Kourkoulis C, Schwab K, Ayres AM, Elm J, Gurol ME, Greenberg SM, et al. Oral Anticoagulation and Functional Outcome after Intracerebral Hemorrhage. Ann Neurol. 2017;82:755–765. doi: 10.1002/ana.25079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moradiya Y, Murthy SB, Newman-Toker DE, Hanley DF, Ziai WC. Intraventricular thrombolysis in intracerebral hemorrhage requiring ventriculostomy: a decade-long real-world experience. Stroke. 2014;45:2629–2635. doi: 10.1161/STROKEAHA.114.006067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murthy SB, Karanth S, Shah S, Shastri A, Rao CP, Bershad EM, Suarez JI. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke. 2013;44:3573–3576. doi: 10.1161/STROKEAHA.113.003058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this analysis are restricted per the terms of AHA-GWTG’s data use agreement and therefore cannot be shared directly with other investigators. However, interested researchers may submit a proposal to the American Heart Association-GWTG committee to formally request access to the dataset.