Abstract
Magnetoencephalography (MEG) has proven valuable for presurgical language lateralization. Investigators have established that low-beta (13-23Hz) event-related desynchrony (ERD), a neuromagnetic signature for increased neuronal firing, maps to critical language centers for expressive language tasks in MEG. The distribution of low-beta ERD is relatively bilateral in early childhood, transitioning to left lateralized by adolescence or early adulthood. Recently, we showed that a complementary signal, low-beta event-related synchrony, thought to reflect neuronal inhibition, becomes increasingly right lateralized across development. Here, we introduce a hybrid laterality index for language derived from both low-beta ERD and ERS. We present findings from a large cohort of children performing verb generation in MEG, and show that inclusion of low-beta ERS provides relatively powerful estimation of language lateralization.
Introduction
Gross language representation is known to shift from bilateral to the predominantly left lateralized, from childhood through adolescence (e.g., Holland et al., 2001). However, in children experiencing early left hemisphere insult, the right hemisphere can partially or fully support gross language functions through adulthood (e.g., Helmstaedter et al., 1997; Kadis et al., 2009; Rasmussen & Milner, 1977). In children undergoing epilepsy surgery, it is important to lateralize language, prior to resection. Presurgical language lateralization (and localization) is particularly important when the seizure originates in the left hemisphere; in these cases, bilateral or right hemisphere language representation confers favorable outcomes.
In magnetoencephalography (MEG), low-beta (13-23Hz) event-related desynchrony (ERD) is thought to reflect increased neuronal firing and is a signature for expressive language (Ressel et al. 2008; Kadis et al., 2008). Low-beta ERD has been found to gradually lateralize from bilateral to the left hemisphere across normal development (Kadis et al., 2011; Sharma et al., 2021). Notably, the distribution of low-beta ERD for expressive language is concordant with increases of blood oxygen level dependent signal in language fMRI, lateralization from the intracarotid amobarbital procedure (IAP), and localization with direct electrocortical stimulation (Breier et al., 2000; Findlay et al., 2012; Foley et al., 2019; Hall et al., 2014; Herfurth et al., 2022; Pang et al., 2011).
In contrast to ERD, low-beta event-related synchrony (ERS) is thought to reflect cortical idling or inhibition (Neuper et al., 2006). During expressive language, low-beta ERS lateralizes from bilateral to right hemisphere across childhood (Sharma et al., 2021).
Clinicians may lack confidence in laterality estimations based on a small number of suprathreshold findings. The inclusion of low-beta ERD may result in improved power, and confidence in laterality assessments.
Here, we introduce a hybrid LI (LIhybrid) derived from both low-beta ERD and low-beta ERS in 80 participants, ages 4 to 18 years, performing verb generation in MEG. Importantly, LIhybrid is a relatively powerful for assessment of language lateralization, or hemispheric dominance, compared to established LIs derived from low-beta ERD, alone.
Method
Participants
Eighty-two typically developing children, ages 4.03 to 18.92 years, participated in this study. All participants were native English speakers, without history of neurological insult, speech or language impairment, or learning disability. Informed written consent from a parent and/or legal guardian was obtained for all participants 18 years of age; all participants under the age of 18 years assented to participate. Individuals older than 18 provided consent, directly. The study was approved by the Institutional Review Board (IRB) at Cincinnati Children’s Hospital Medical Center (data collection site), and the Research Ethics Board (REB) at the Hospital for Sick Children (analysis site).
Procedure
The experimental paradigm, preprocessing, and source localization procedures have been detailed previously (Sharma et al., 2021) and are described only briefly here. Participants listened to either concrete nouns (verb generation trials), or speech-shaped noise (control trials), in the MEG, and were instructed to think of an action word that corresponds to each target noun during the verb generation trials. Nouns consisted of commonly experienced items chosen from normative databases and standardized language assessments (see, Kadis et al., 2011). MEG data were acquired using a whole-head 275-channel CTF system (MEG International Services Ltd., Coquitlam, B.C., Canada) at a sampling rate of 1.2 kHz.
MRI
MEG head localization coils were replaced with multimodal radiographic markers, before acquiring structural MR images; fiducial marking facilitated accurate co-registration of MEG with individual anatomy. MRIs were acquired at 3.0T on a Philips Achieva or Ingenia Elition scanner (Philips Medical Systems, International). Whole-brain 3D T1-weighted images were acquired using an MDEFT sequence (flip angle = 90°, TE = 3.7 ms, TR = 8.1 ms, voxel size = 1.0×1.0×1.0 mm).
Data Analysis
Pre-processing Analysis of MEG
MEG data were processed using FieldTrip toolbox (Oostenveld et al., 2011) routines running in MATLAB version 2019b (Mathworks Inc., MA, USA). ICA was used to isolate and reject ocular and cardiac artifacts from the recordings. The data were bandpass filtered from 13-23Hz to isolate the signal of interest, then epoched for both verb generation and control trials at 700-1200 ms post-stimulus onset (Sharma et al., 2021; Yousoffzadeh et al., 2017). SQUID jump artifacts were automatically identified; an average of 1.68 ± 1.16 trials containing artifact were rejected across participants.
Head Modeling
Individual 3D T1-weighted images were segmented into brain, skull, and scalp compartments using SPM12 routines (FIL Methods Group, 2014). Realistic single-shell models were constructed from the segmentation (Nolte, 2003). A whole-brain template source model consisting of dipole positions placed at a regular 10mm interval inside the MNI152 brain was developed for this study; we have previously shown that language lateralization can be assessed within the presumed language network, and at the whole brain level (see, Sharma et al., 2021); however, for clinical applications, we prefer the whole-brain approach, requiring no assumptions about language network structure, which may be impacted by pathology.
Differential beamformer analyses with bootstrapping derived thresholds
Covariance matrices were computed from concatenated verb generation and control trials, for each participant. Low-beta (13-23Hz) source activity was estimated for each dipole location using a linearly constrained minimum variance beamformer (LCMV; Van Veen et al., 1997) with 1% regularization; source activity was projected along the orientation maximizing power, per location (via singular value decomposition). An independent samples t-contrast between verb generation and control trial oscillatory power was computed for each dipole location; significance was determined through Monte Carlo simulation, with 10,000 random permutations and an alpha of 0.05 (Maris & Oostenveld, 2007). Low-beta ERD and low-beta ERS statistical thresholding were carried out separately, for each participant. Suprathreshold t-values, reflecting significant ERD and ERS, were passed on for LI computation.
Laterality Index
Conventional low-beta ERD LIs (LIERD) were computed for each participant based on the total number of left (ERDL) versus right (ERDR) suprathreshold positions in the whole brain, using the following formula:
LIERD scores range from −1 to +1 for each participant; −1 represents completely right lateralized signal and +1 represents completely left lateralized signal. LI scores around 0 indicate bilateral contribution.
Left lateralized low-beta ERD and right lateralized low-beta ERS reflect a left lateralized language network (Sharma et al., 2021). As such, the hybrid LI (LIhybrid) is computed as:
Where ERSL and right ERSR reflect the number of left and right hemisphere dipole positions showing suprathreshold ERS. Again, scores range from −1 to +1, with −1 indicating right lateralization, and +1 indicating left lateralization.
Results
Two participants lacked suprathreshold low-beta ERD in frontal lobe; these participants were deemed unreliable, and were excluded from subsequent analyses (see, Sharma et al., 2021). LIERD was significantly positively correlated to age (r(78) = 0.40, p < .001). A significant positive correlation was also found between LIhybrid and age (r(78) = 0.50, p < .001). LIhybrid was not significantly different between females and males (t(79) = 0.25 p = .72). Importantly, the LIhybrid method considers more data than conventional LIERD (see Figure 1).
Figure 1.
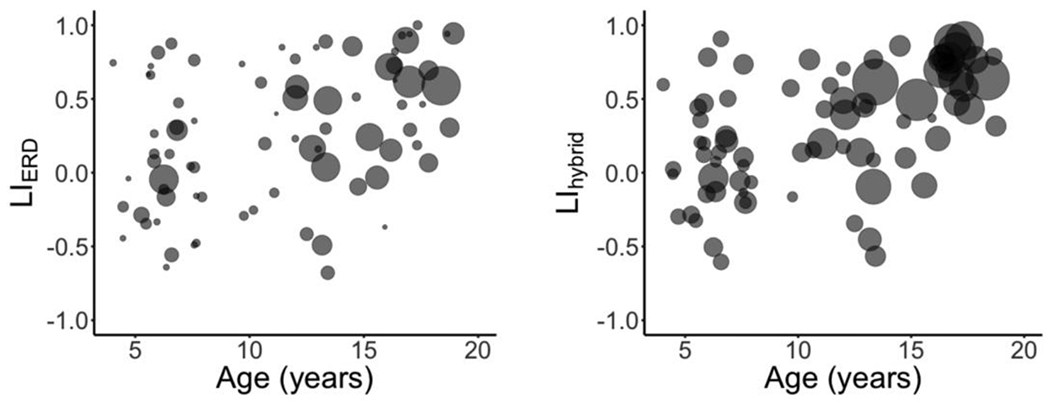
Scatterplots of age versus lateralization using the LIERD metric (left), and LIhyrbid (right). Participant age and LI scores are represented as the centroids of each circle; the size of each circle reflects the number of suprathreshold dipoles that contributed to LI calculation.
The ratio of dipoles showing suprathreshold low-beta ERD and low-beta ERS was not correlated to age. LIhybrid was concordant and positively correlated to LIERD (r = 0.88, p < .001). The number of suprathreshold low-beta ERD dipoles per participant was negatively correlated to the percentage of dipoles added with LIhybrid computation (r = −0.70, p < .001). This suggests that gains are largest for individuals with minimal suprathreshold low-beta ERD (see Figure 2).
Figure 2.
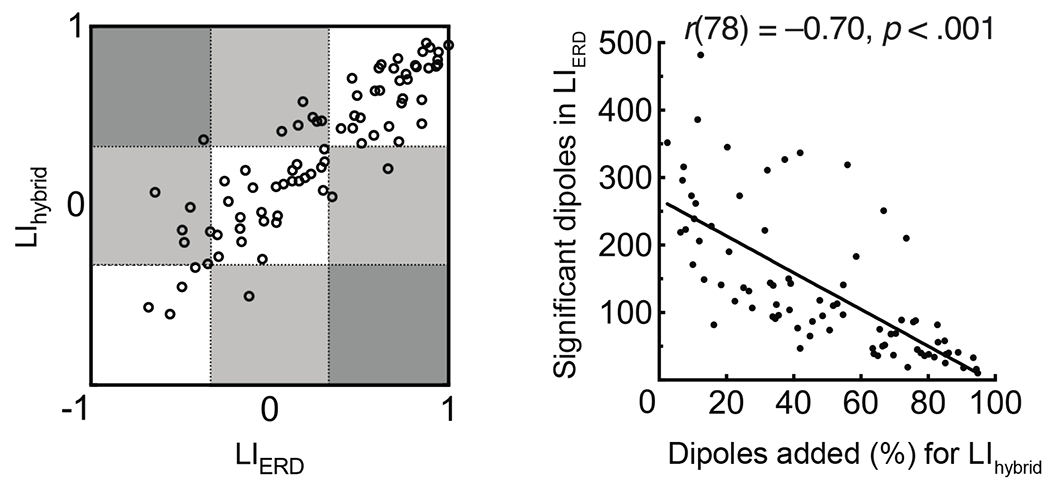
Bivariate plot (left) showing concordance between LIERD and LIhybrid. Dotted lines may be used to classify left (> 0.33), bilateral (≥ −0.33 and ≤ 0.33), and right (< −0.33) language lateralization. Scatterplot (right) shows number of suprathreshold low-beta ERD dipoles per participant is negatively correlated to the percentage of dipoles added for computation of LIhybrid.
Discussion
LIERD and LIhybrid were both positively correlated with age, consistent with the expected trajectory of language lateralization transitioning from bilateral to left, in childhood (Holland et al., 2007). Results suggests LIhybrid tracks maturation of language similarly to LIERD.
LIhybrid is computed from a greater number of data points than LIERD; laterality assessment for participants with the fewest suprathreshold low-beta ERD dipoles, enjoyed the largest gains. Because confidence may be low when few datapoints contribute to LI computation, LIhybrid may be preferable over conventional LIERD, particularly when a small number of dipoles showing suprathreshold low-beta ERD are detected.
Finally, we urge the community to evaluate the utility of LIhybrid, and caution against use until clinical validation has occurred with methods such as the intracarotid amobarbital procedure.
Conclusion
LIhybrid can be computed simply, and provides more power for assessing language lateralization in childhood by considering additional signal in computation. This may be is particularly important for cases where there are few positions showing suprathreshold low-beta ERD, which may be detrimental to clinician confidence when assessing language lateralization. With clinical validation, the metric, based on both low-beta ERD and ERS signals, may supplant conventional approaches that consider only the ERD signal.
Highlights.
Children and adolescents completed an expressive language task in MEG and language laterality was assessed.
Both increases and decreases in beta band power were used to generate a novel hybridized laterality index.
By considering multiple oscillatory signatures for language lateralization, the hybrid metric is more powerful than conventional approaches.
Acknowledgements
The study was supported, in part, by a grant provided the Research Institute of Cincinnati Children’s Hospital Medical Center [DSK] and by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health [R21NS106631, DSK]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. 2000. Lateralization of activity associated with language function using magnetoencephalography: a reliability study. Journal of Clinical Neurophysiology, 17, 503–510. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM, Lenhard A. Peabody Picture Vocabulary Test: PPVT 4 (Pearson Assessments, Bloomington, 2015). [Google Scholar]
- Findlay AM, Ambrose JB, Cahn-Weiner DA, Houde JF, Honma S, Hinkley LB, Berger MS, Nagarajan SS, Kirsch HE. 2012. Dynamics of hemispheric dominance for language assessed by magnetoencephalographic imaging. Annals of Neurology, 71, 668–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E, Cross JH, Thai NJ, Walsh AR, Bill P, Wood AG, Cerquiglini A, Seri S. 2019. MEG assessment of expressive language in children evaluated for epilepsy surgery. Brain Topography, 32, 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EL, Robson SE, Morris PG, Brookes MJ. 2014. The relationship between MEG and fMRI. Neuroimage, 102, 80–91. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Linke DB, Elger CE. 1997. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain and Cognition, 33(2), 135–150. [DOI] [PubMed] [Google Scholar]
- Herbst K. 2001. Differential effects of unilateral lesions on language production in children and adults. Brain and Language, 79(2), 223–265. [DOI] [PubMed] [Google Scholar]
- Herfurth K, Harpaz Y, Roesch J, Mueller N, Walther K, Kaltenhaeuser M, Pauli E, Goldstein A, Hamer H, Buchfelder M, Doerfler A, Prell J, Rampp S. 2022. Localization of beta power decrease as measure for lateralization in pre-surgical language mapping with magnetoencephalography, compared with functional magnetic resonance imaging and validated by Wada test. Frontiers in Human Neuroscience, 16, 996989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS. 2001. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage, 14(4), 837–843. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema J-M, Karunanayaka PR, Schmithorst VJ, Yuan WY, Plante E, Byars AW. 2007. Functional MRI of language lateralization during development in children. International Journal Audiology, 46(9), 533–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis DS, Kerr EN, Rutka JT, Snead OC 3rd, Weiss SK, Smith ML. 2009. Pathology type does not predict language lateralization in children with medically intractable epilepsy. Epilepsia, 50(6), 1498–1504. [DOI] [PubMed] [Google Scholar]
- Kadis DS, Pang EW, Mills T, Taylor MJ, McAndrews MP, Smith ML. 2011. Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. Journal of the International Neuropsychological Society, 17(5), 896–904 [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods, 164(1), 177–190. [DOI] [PubMed] [Google Scholar]
- Neuper C, Wörtz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. In Event-Related Dynamics of Brain Oscillations (eds. Neuper C & Klimesch W), 211–259 (Elsevier, Amsterdam, 2006). [DOI] [PubMed] [Google Scholar]
- Nolte G 2003. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Physics in Medicine & Biology, 48, 3637–3652. doi: 10.1088/0031-9155/48/22/002 [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG and invasive electrophysiological data. Computational Intelligence in Neuroscience, 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang EW, Wang F, Malone M, Kadis DS, Donner EJ. 2011. Localization of Broca’s area using verb generation tasks in the MEG: Validation against fMRI. Neuroscience Letters, 490(3), 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. 1977. The role of early left-brain injury in determining lateralization of cerebral speech functions. Annals of the New York Academy of Science, 299, 355–369. [DOI] [PubMed] [Google Scholar]
- Sharma VV, Vannest J, Greiner HM, Fujiwara H, Tenney JR, Williamson BJ, Kadis DS. 2021. Beta synchrony for expressive language lateralizes to right hemisphere in development. Scientific Reports, 11, 3949. doi: 10.1038/s41598-021-83373-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VV, Vannest J, Kadis DS. 2022. Asymmetric information flow in brain networks supporting expressive language in childhood. Human Brain Mapping, 1–8. doi: 10.1002/hbm26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. 1997. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Translational Biomedical Engineering, 44, 867–880. [DOI] [PubMed] [Google Scholar]
- Youssofzadeh V, Williamson BJ, Kadis DS. 2017. Mapping critical language sites in children performing verb generation: Whole-brain connectivity and graph theoretical analysis of MEG. Frontiers in Human Neuroscience, 11, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]


