Abstract
The development and outcome of inflammatory diseases are associated with genetic and lifestyle factors, which include chemical and nonchemical stressors. Persistent organic pollutants (POPs) are major groups of chemical stressors. For example, dioxin-like polychlorinated biphenyls (PCBs), per- and polyfluoroalkyl substances (PFASs), and polybrominated diphenyl ethers (PBDEs) are closely associated with the incidence of inflammatory diseases. The pathology of environmental chemical-mediated inflammatory diseases is complex and may involve disturbances in multiple organs, including the gut, liver, brain, vascular tissues, and immune systems. Recent studies suggested that diet-derived nutrients (e.g., phytochemicals, vitamins, unsaturated fatty acids, dietary fibers) could modulate environmental insults and affect disease development, progression, and outcome. In this article, mechanisms of environmental pollutant-induced inflammation and cardiometabolic diseases are reviewed, focusing on multi-organ interplays and highlighting recent advances in nutritional strategies to improve the outcome of cardiometabolic diseases associated with environmental exposures. In addition, advanced system biology approaches are discussed, which present unique opportunities to unveil the complex interactions among multiple organs and to fuel the development of precision intervention strategies in exposed individuals.
Keywords: persistent organic pollutants, PCB, PFAS, PBDE, intervention, toxicity, multi-organ
Graphical Abstract
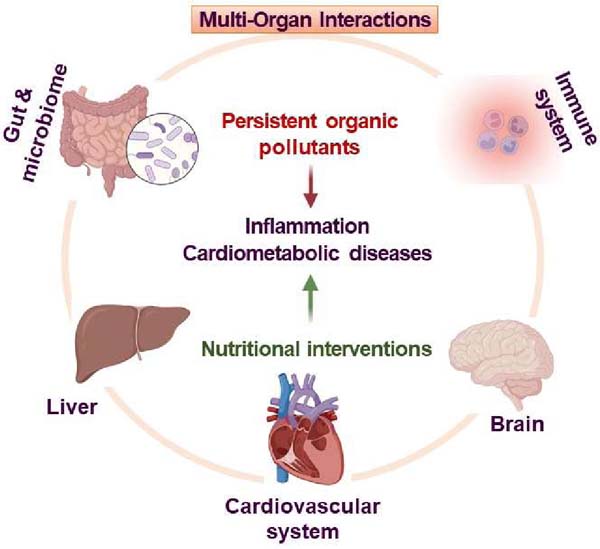
1. Introduction
Inflammation triggers the early phases of many disease processes, and the increased production of inflammatory cytokines is associated with a higher risk of developing multiple disorders. For example, it has been established that inflammatory processes play a crucial role in the development and complications of cardiometabolic diseases (CMD) 1, including cardiovascular disease (CVD), diabetes, and nonalcoholic fatty liver disease (NAFLD) 2, 3. POPs are the most investigated organic environmental contaminants. POPs disturb the ecological balance and threaten the health of all organisms. Multiple epidemiological studies have reported the associations between exposure to persistent organic pollutants (POPs) and air pollutants (a mixture of POPs with gaseous pollutants, such as nitrogen dioxide, ozone, and sulfur dioxide) and an increased risk of CMD 4–9. A common mechanism underlying most environmental pollutant-mediated disease risks is attributed to enhanced inflammation 10, 11. Recent reports have linked the pathology of CMD with the involvement of multiple organ systems, including liver, heart, and gut 12–15, and mounting evidence suggests that lipophilic and amphipathic POPs, such as dioxin-like polychlorinated biphenyls (PCBs) and per- and polyfluorinated substances (PFASs), promote CMD risks via gut microbiota dysbiosis and liver dysfunctions 15–19. Emerging evidence supports a significant impact of POPs on immune functions 20, gastrointestinal system 21, and pulmonary health 22. Organ interactions such as gut-lung axis 23, gut-liver axis 24, and gut-brain axis 25 play important roles in environmental pollution-associated inflammatory disease progression. These reports highlight the complex network of interactions between host organs and the gut/microbiome. In addition, organ crosstalk has been established not only between two organs but among multiple organ systems, such as the cardiovascular, gastrointestinal, nervous, endocrine, metabolic, and immune systems 26, 27. Thus, prevention or intervention strategies to counteract disease risks associated with exposure to environmental stressors should consider multiple organ interactions when aiming to reduce inflammation.
Consumption of healthy nutrients, i.e., foods rich in antioxidant and anti-inflammatory components, has been linked to CVD prevention for both healthy individuals and people at higher disease risk of CVD 28–30. Studies indicated that phytochemical interventions, specifically those involving polyphenols and polysaccharides (dietary fiber), can reduce or prevent the development of inflammatory events induced by exposure to environmental pollutants 31–35. This review highlights inflammatory disease mechanisms related to POPs (PCBs, PFASs, and PBDEs) exposure, and discusses the potential nutritional intervention approaches, with a focus on multi-organ systems interactions, thus, providing a scientific basis for the design of precision nutritional intervention strategies to prevent toxicity from environmental insults.
2. Search Strategy
We utilized the PubMed search to assess the topic-related literature. The specific methods are as follows: The PubMed database retrieval language is “persistent organic pollutant (Title/Abstract) AND inflammatory disease (Title/Abstract)”, “polychlorinated biphenyl (Title/Abstract) AND inflammatory disease (Title/Abstract)”, “PFAS (Title/Abstract) AND inflammatory disease (Title/Abstract)”, “intervention (Title/Abstract) OR nutrient (Title/Abstract) AND persistent organic pollutant (Title/Abstract)”, “intervention (Title/Abstract) OR nutrient (Title/Abstract) AND polychlorinated biphenyl (Title/Abstract)”, “intervention (Title/Abstract) OR nutrient (Title/Abstract) AND PFAS (Title/Abstract)”, and the publications date range from 2012 to 2023. We then filtered the literature based on the title, abstract, and full text of each paper, and a total number of 100 articles relevant to our topic were collected.
3. Persistent organic pollutants
3.1. Polychlorinated biphenyls (PCBs)
PCBs are POPs found in soil, air, and water. Fish and small organisms can absorb PCBs from the water and sediments in their habitat. PCBs accumulate in organisms through the food chain. Therefore, the major source of human exposure to PCBs is the dietary intake of contaminated food and water. In mammals, the liver is the most important tissue for the initial distribution of PCBs because it is highly perfused. Fatty tissue is the major storage compartment for PCBs, and adipose has the highest PCB tissue-to-blood partition coefficient due to the high lipophilicity of PCBs. For example, the human adipose/serum partition coefficient ranges from 50 to 370 36. The estimated half-lives of PCBs in different species range from 0.13 to 7.9 years depending on the degree of chlorination and the substitution pattern of the PCB congeners 37. According to the information compiled by the Agency for Toxic Substances and Disease Registry (ATSDR, https://wwwn.cdc.gov/TSP/MRLS/mrlsListing.aspx), the minimal toxic dose (minimal risk levels, MRL) in humans for orally and chronically exposed PCBs (Aroclor 1254) is 20 ng/kg/day using immune toxicity endpoints. Dioxin-like PCBs likely modulate CVD and related pathologies by inducing oxidative stress, cellular dysfunction, and chronic inflammation in key cell types associated with atherosclerosis 38. They have also been shown to initiate vascular endothelial cell dysfunction, induce key pro-atherogenic adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), increase monocyte attraction, and induce vascular permeability 38, 39. PCBs could damage organ systems in which they are known to bioaccumulate, such as the liver and adipose tissues 12, 40. At the transcriptional level, PCB126 activated the aryl hydrocarbon receptor (AhR) to produce an inflammatory response in preadipocytes 41. In a recent study, the exposure of insulin-resistant adipocytes to PCB126 in vitro led to impaired glucose uptake in myotubes, suggesting adipose-muscle communications 42. Animal experiments demonstrated that PCBs exposure led to inflammation in multiple organs, including the liver, adipose, gut, and vascular 40, 43, 44 (Figure 1).
Figure 1.
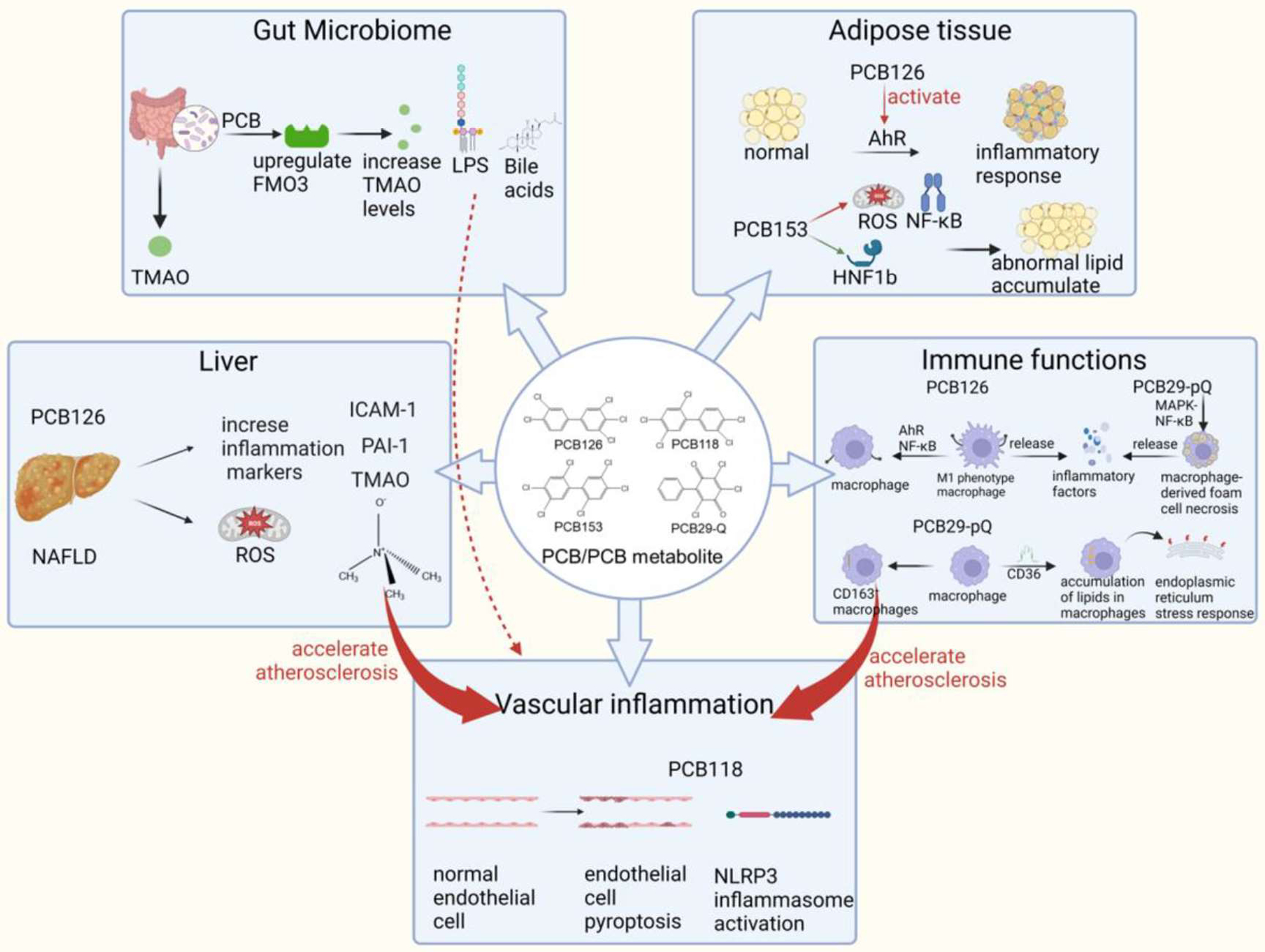
The involvement of multiple organ systems and interactions in PCB exposure induced toxicity (created with BioRender.com). AhR: aryl hydrocarbon receptor; FMO3: flavin-containing monooxygenase 3; HNF-1b: hepatocyte nuclear factor-1b; ICAM-1: intercellular cell adhesion molecule-1; LPS: lipopolysaccharide; MAPK: mitogen-activated protein kinase; NAFLD: nonalcoholic fatty liver disease; NF-κB: nuclear factor kappa-B; NLRP3: NOD-like receptor thermal protein domain associated protein 3; PAI-1: plasminogen activator inhibitor-1; PCB: polychlorinated biphenyl; ROS: reactive oxygen species; TMAO: trimethylamine oxide.
The liver is a major toxic organ for PCBs. It has been reported that PCBs altered normal hepatic nuclear receptor signaling and AhR function, disturbed hepatic metabolism, and promoted the production of inflammatory cytokines, all of these contribute to the development of NAFLD 45. The role of a compromised liver in defining dioxin-like PCB126 toxicity on the peripheral vasculature and associated inflammatory diseases has been studied 40, 43. In a mouse model of NAFLD, it was found that PCB126 exposure led to increased plasma inflammatory markers such as ICAM-1, plasminogen activator inhibitor-1 (PAI-1), and trimethylamine N-oxide (TMAO) 40. In addition, redox stress-related metabolites were elevated in NAFLD mice exposed to PCB126 43. The data suggested that a compromised liver altered PCB-mediated toxicity, resulting in increased systemic inflammation 40, 43, 46.
The interactions between the liver, gut, and vascular tissues contribute to the toxicity of PCBs. Certain metabolites (e.g., TMAO) formed by intimate crosstalk between gut microbiota and host liver enzymes could be causative mediators of pollutant-linked disease risks 47–49. TMAO was positively associated with dioxin-like pollutant exposure in the Anniston and Alabama cohorts (residents who live close to a former PCB production site) 50. An animal study revealed that PCB-induced upregulation of FMO3 led to increased circulating plasma levels of pro-atherogenic TMAO 51. TMAO was found to be associated with CMD by inducing foam cell formation, activating platelets, and promoting vascular inflammation 52. Other gut microbiota-derived metabolites, such as short-chain fatty acids (SCFAs) and bile acids, might also be involved in cardiovascular health and disease 53, especially after exposure to PCBs 54. These data suggested that environmental exposures could lead to changes in gut microbial metabolites, which act as mediators of CVD.
The immune system plays an important role in PCB-mediated inflammation. For example, PCB126 promoted macrophage inflammation and polarized monocytes to an M1-like phenotype through AhR and nuclear factor kappa-B (NF-κB) pathways (Figure 1). As a result, inflammatory factors such as tumor necrosis factor alpha (TNFα) and Interleukin-1 beta (IL-1β), and oxidative stress-sensitive markers such as heme oxygenase 1 (HMOX1) and NAD(P)H quinone dehydrogenase 1 (NQO1) were induced55. In addition to the parent compound, the PCB metabolites could be stronger inducers of inflammatory markers. As a possible biotransformation product of PCBs, PCB29-pQ might be involved in the MAPK-NF-κB inflammatory pathway by activating the RIPK1/3-MLKL pathway through a reactive oxygen species (ROS)-dependent mechanism, thus promoting macrophage-derived foam cell necrosis and ultimately accelerating the release of inflammatory cytokines to form the necrotic core of plaques 56. Furthermore, PCB29-pQ could promote macrophage/monocyte polarization to CD163+ macrophages, which would be a potential incentive to accelerate atherosclerosis through the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway 57. Additionally, PCB29-pQ promoted the accumulation of lipids in macrophages via upregulation of lipid transporter CD36, activation of the endoplasmic reticulum stress response, and accompanied apoptosis and necrosis 58. The mechanisms of other dioxin-like PCB (PCB118) and non-dioxin-like PCB (PCB153) induced inflammation are shown in Supplemental Table S1. Besides the direct effects of PCB on immune cells, the gut microbiota alterations may also contribute to changes in immune functions after PCB exposure. Environmental exposure to PCBs led to gut dysbiosis and increased gut permeability 59, which could introduce toxicants, bioactive endogenous metabolites, and pathogens into the systemic circulation, causing immune alternations in the liver and other extrahepatic organs.
MicroRNAs (miRNAs) are a class of small oligonucleotides that interact with messenger RNA. PCB-induced oxidative stress and production of pro-inflammatory cytokines could be regulated by miRNAs. For example, it was found that co-exposure to TCDD and PCB could increase the mRNA level of ICAM-1 which was regulated by miR-130a-3p 60. Aroclor 1260 increased miR-21, miR-31, miR-126, miR-221 and miR-222 expression levels 61. miR-21 was reported to increase fibrosis and cardiac hypertrophy 62, while miR-31, miR-126, miR-221, and miR-222 modulated inflammation 63. A significant increase in miR-192–5p was observed in PCB126 and/or Aroclor1260 exposed mouse livers 64. In addition, an epidemiology study revealed that circulating miR-192–5p was a hepatotoxicity biomarker from PCB exposures 65. miR-192–5p could promote the activation of M1 macrophages and increase the expression of iNOS, IL-6, and TNF-α via Rictor/Akt/FoxO1 signaling pathway 66, which might contribute to PCB-induced toxicity. It was reported that circulating miRNAs could be secreted from different organs, including the liver, adipose, muscle, and the cardiovascular system 67. Therefore, miRNAs might serve as messengers that facilitate communication between secreted cells/tissues with receptor cells/tissues, thereby potentially having important roles in organ crosstalk.
As multiple organs are involved in PCB toxicity, it is interesting to know the rankings of the effects on different organs. Oral ingestion of contaminated food and water is one of the major routes of PCB exposure. Therefore, the gut and microbiome represent the first defensive barrier towards PCBs. The liver is a critical organ for PCB metabolism, and the fat is the major storage compartment for PCBs, suggesting these organs are more likely to be exposed to higher concentrations of PCBs than other tissues. Increasing evidence suggests that the gut microbiome mediates liver functions and plays an important role in the progression of toxicant-induced liver diseases. In addition, the liver regulates the metabolism and innate immunity in other organs, therefore alterations in liver functions can also impact the metabolic signaling and immunity of other host organs 26. It was reported that PCBs induced adipose tissue dysfunction, including abnormal lipid accumulation 68, altered adipokine and cytokine secretion 40, 41, i.e., adiponectin, IL-6, MCP-1, and TNF-α, which in turn affected muscle metabolism 42. These effects could be consequences of adipocyte AhR activation69. Therefore, it seems plausible that exposure to PCBs may affect the gut microbiome function and structure, accompanied by liver and adipose dysfunction and then the effects could be extended to distal organs and the physiological health of the host. To provide a quantitative estimation of which organ is affected first and most seriously, the toxicokinetics needs to be taken into consideration.
3.2. Polyfluoro- and perfluoroalkyl substances (PFASs)
PFASs are widely used in industrial processes and consumer products. These compounds are found to be ubiquitous in environmental media because of their innate chemical stability. Widespread human exposure to PFASs via drinking water and contaminated food, along with their long biological half-lives have led to measurable PFASs in many populations worldwide. PFASs are highly efficiently absorbed in animals and humans 70. The vast majority of PFASs entering the body are absorbed in the gastrointestinal tract and then distributed to various tissues. A large percentage of PFASs is retained in serum, liver, and kidney, and some can also accumulate in the intestine 71. The estimated half-lives of PFAS range from 0.63 y (perfluoropolyether) to 18.5 y (6:2 chlorinated polyfluorinated ether sulfonate) in humans 72, 73.
The health effects of PFASs exposure have become a global concern. Emerging evidence suggested that exposure to PFASs was associated with inflammatory diseases, including metabolic dysfunctions (i.e., CVD, NAFLD, and chronic kidney diseases) and immune-related health conditions (i.e., allergic diseases, infection, and vaccine response) 74, 75. The liver is a well-established toxic target organ for many PFASs. With hepatocellular hypertrophy being used as the toxicology endpoint, it was found that the shapes of the dose-response curves were similar for short- and long-chain perfluoroalkyl carboxylic acids (PFCAs) and perfluorobutanesulfonic acid (PFBS) (slopes ranged from 12.0 to 18.0) 76. However, different dose-response curves were observed for perfluorooctane sulfonate (PFOS) and perfluorohexanesulfonic acid (PFHxS), with slope values being 4.1 and 4.6 respectively. The results suggested that liver toxicity among PFASs was different. According to ATSDR, the MRL of orally exposed PFASs (PFOA, PFOS, PFNA, and PFHxS) is 2–20 ng/kg/day in humans by using developmental and endocrine toxicity endpoints. Using data from the National Health and Nutrition Examination Survey (NHANES) 2005–2012, Omoike et al. found that increased exposure to PFASs resulted in increased serum concentrations of markers associated with chronic inflammation and oxidative stress, including lymphocyte count, serum iron, albumin, and bilirubin 77. Meneguzzi et al. reviewed epidemiological studies exploring the relationship between PFASs exposure and thromboembolic cardiovascular disease and found that changes in plasma membrane fluidity and calcium signaling were observed with PFASs exposure, along with platelet activation hypersensitivity 78. Animal and cell culture experiments demonstrated that PFASs exposure resulted in oxidative effects in cells and tissues, leading to inflammation in organs including the liver, gut, brain, and immune system (Figure 2).
Figure 2.
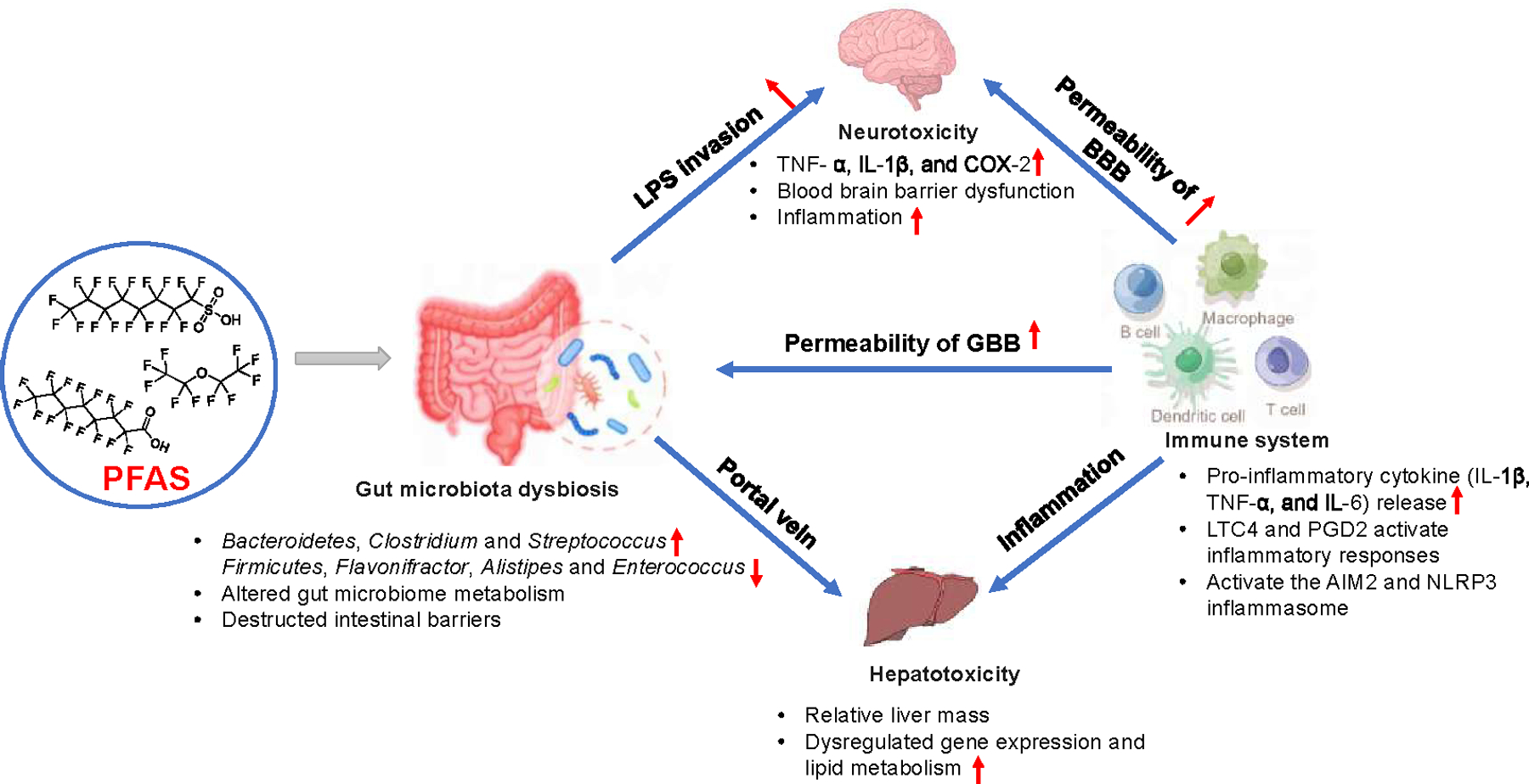
The involvement of multiple organ systems and interactions in PFAS exposure induced toxicity (created with Figdraw.com). BBB: blood-brain barrier; GBB: gut-blood barrier; LTC4: leukotriene C4; LPS: lipopolysaccharide; PFAS: polyfluoro- and perfluoroalkyl substances; PGD2: prostaglandin D2.
Gut-liver interactions play an important role in PFAS toxicity 19. Mouse studies suggested that PFOS disturbed liver metabolism at transcription and metabolome levels, which overlapped with increases in Bacteroidetes, Clostridium, and Streptococcus, and decreases in Firmicutes, Flavonifractor, Alistipes, and Enterococcus 17, 18, 35. Suppression of gut microbiota using antibiotics and supplementation of bacteria by fecal cell transplant (L. reuteri, E. faecalis, and Akk. muciniphila) attenuated the adverse liver effects induced by PFOS, suggesting that PFOS-induced liver injury is mediated through remodeling of the gut microbiota 17. Furthermore, the correlation between the gut microbiome and alterations in the hepatic PPAR pathway (lpl, slc27a2a, and Acox3) after PFAS exposure suggested a probable interaction between changes in the gut microbiota and hepatic lipid metabolism.79. Several new PFASs have also been shown to induce dysbiosis, which has been linked to a series of intestinal and liver diseases. For example, exposure to 6:2 chlorinated polyfluoroalkyl ether sulfonate 80, hexafluoropropylene oxide dimer acid (HFPO-DA) 81, and sodium ρ-perfluorous nonenoxybenzene sulfonate (OBS) 82 could cause inflammation in the gut, destruction of tight junction structure, reduced intestinal mucus secretion, and hepatic metabolism disorder.
PFAS exposure has been implicated in neurotoxicity in humans 83, and recent studies suggested that the gut-brain axis was an important link in PFAS-induced neurotoxicity 84, 85. For example, PFOA exposure led to cognitive deficits in mice and caused inflammation in the gut and brain by increasing lipopolysaccharide (LPS), TNFα, IL-1β, and cyclooxygenase-2, while fecal microbiota transplantation mitigated these symptoms 85. These effects could be related to PFOA-induced alterations in gut microbial composition and functions. The levels of Bifidobacterium-psudolongum and Bifidobacterium-bifidum, the gut bacteria that sustain mucosa thickness and barrier integrity, decreased after PFOA exposure in mice. In addition, acetic acid, propionic acid, and butyric acid, the SCFAs that suppress colonic inflammation by activating GPR43 and GPR109a were decreased. These effects led to impaired barrier integrity which resulted in higher LPS invasion into the systemic circulation and induced inflammation in the brain as demonstrated by the increased levels of LPS, TLR4, NF-κB, and IBA-1 in the brain. Similarly, perfluoroalkyl phosphonic acids (PFPiAs), significantly increased the abundance of Gram-negative bacteria in zebrafish 84, which led to increased levels of LPS and inflammation in the gut and brain. The LPS was delivered to the brain through the gut–brain axis, damaged the blood–brain barrier (BBB), and caused mitochondrial damage as well as apoptosis in the brain. This mechanism was verified by the fact that antibiotics reduced the LPS levels in the gut and brain, accompanied by reduced inflammatory responses.
Emerging evidence suggests that PFAS exposure in humans is associated with immune-related health conditions, including allergic diseases, infection, and vaccine response 75. Cardio-metabolic disorders are typically associated with increased inflammation. However, PFAS are already known to be immuno-suppressive, reflected by the inverse relationship between PFAS exposure levels and TNFα in humans 86. PFAS affects multiple aspects of the immune system, including modulation of nuclear receptors (e.g., NF-κB, PPARs), Ca2+-signaling, as well as modulation of oxidative stress 87. Moreover, PFAS exposures are associated with altered lipid levels by upregulating gene expressions in lipid metabolism, e.g., CD36 88, which might contribute to immune cell dysfunctions and cardio-metabolic disorders 89. It has become increasingly understood that immune cells could sense environmental signals and promote multi-organ interplays 90. PFOS and PFOA are the most commonly studied PFASs in relation to immunotoxicity. They altered the expression of proinflammatory cytokines (IL-1β, TNF-α, and IL-6) in macrophages 91, upregulated cytokine production (IL-4 and IL-13) in Th2 cells 92, and activated inflammatory responses in mast cells by increasing the production of inflammatory eicosanoids, leukotriene C4 (LTC4) and prostaglandin D2 (PGD2) 93. Inflammasome, a high-molecular-weight complex present in the cytosol of stimulated immune cells that mediates the activation of inflammatory caspases, was identified as the key factor regulating PFAS recognition in the cells and triggering cellular inflammatory responses in organs 94, 95. PFOA exposure increased NLRP3 inflammasome aggregation in mice liver, and autophagy was found to be associated with PFOA-induced NLRP3 activation, highlighting the mechanisms used by PFOA to trigger cellular inflammatory responses 95. PFOS could induce AIM2 inflammasome dependent IL-1β production in macrophages. This mechanism was further verified using Aim2-deficient mice, which were protected from PFOS-induced multiple tissue inflammation and damage, including liver, lung, and kidney, proving the important role of the immune system and AIM2 inflammasome in multi-organ toxicity after PFOS exposure 94. Mechanistically, this process was mediated by PFOS-induced mitochondrial dysfunction and mitochondrial DNA release (Supplemental Table S2). Together, these studies indicated that the immune cells could regulate multi-organ inflammation after sensing environmental insults. Nevertheless, we still need a better understanding of the toxicity mechanisms of environmental exposure underlying immune system-organ interactions.
3.3. Polybrominated diphenyl ethers (PBDEs)
Polybrominated diphenyl ethers (PBDEs) are widely used as flame-retardant additives in consumer and household products. PBDEs have become a global environmental organic pollutant due to the properties of persistence and bioaccumulation 96. Exposure to these chemicals has been associated with hepatotoxicity, developmental neurotoxicity, metabolic dysfunctions, and reproductive disorders 97, 98. According to ATSDR, the MRL for PBDE is 3 ng/kg/day for oral administration in humans using development and endocrine toxicities endpoints (https://wwwn.cdc.gov/TSP/MRLS/mrlsListing.aspx). BDE-209 is one of the most predominant PBDE 96. It was reported that BDE-209 enhances oxLDL-induced macrophage foam cell formation by increasing Toll-like receptor 4 (TLR4)-dependent lipid uptake in macrophages 99. The effect and potential molecular mechanism of BDE-209 adhesion between THP-1 monocytes and human aortic endothelial cells (HAECs) were investigated. It was found that BDE-209 potentiated ICAM-1 expression and increased adhesion by downregulating miR-141 100. BDE-209 induced oxidative stress and inflammation and ultimately led to endothelial dysfunction and cardiovascular injury in rats 101. In addition, PBDEs impacted host metabolism in an intestinal microbiome-dependent manner in mice, indicating that gut dysbiosis may contribute to PBDE-mediated metabolic disturbance 102 (Supplemental Table S3).
4. Nutritional intervention of environmental pollutant-mediated inflammatory diseases
Nutritional interventions have been considered as an effective strategy to reduce the risk of inflammatory diseases 103, 104. Flavonoids are a large group of phenolic compounds present in foods of plant origin and have a broad range of health-beneficial effects, including antioxidation and anti-inflammation105. Growing evidence suggests that flavonoids contribute to the prevention or reduction of the damage caused by certain environmental pollutants (Table 1–3). The gut microbiota plays an important role in overall host health, and disruptions in microbiota homeostasis by environmental exposures have been implicated in developing inflammatory diseases 106. Therefore, utilization of dietary interventions to mitigate the negative effects of environmental exposures on gut microbiota could be a feasible approach to reduce inflammatory disease risks associated with pollutant exposure. Dietary fibers are a diverse set of carbohydrate polymers. Numerous studies have reported that diets that are high in fiber play a protective role in the occurrence of inflammatory diseases. The mechanisms behind the protective effects of high-fiber diets included modulation of lipid metabolism 107, 108, alterations in the intestinal microbiome structure and metabolism 109, and immunomodulation effects 110. Growing evidence highlighted the importance of dietary fibers in influencing health maintenance and disease development after environmental exposures 34, 35, 111, indicating the great potential of using dietary fiber in the intervention of environmental diseases.
Table 1.
Mechanisms of nutritional intervention against PCB-mediated toxicity
| Environmental Chemicals (Dose, route, duration) | Nutrients (Dose, route, duration) | Subjects | Disease endpoint | Mechanisms | Reference |
|---|---|---|---|---|---|
| PCB126 (5 μmol/kg mouse, gavage, in weeks 10, 11, and 12) | Green tea extract (1%GTE-supplemented diet, oral, 12 weeks) | C57BL/6 mice | Liver injury. Reduce the expression of key markers of inflammation (MCP-1 and CCL3) | Upregulate a battery of antioxidant enzymes; upregulate genes transcriptionally controlled by AhR and Nrf2 proteins | 33 |
| PCB126 (1.5 mg/kg, gavage, twice a week for two weeks) | Inulin (250 mg/kg/day fiber, given drinking water containing inulin, throughout the PCB126 | male C57BL/6 J mice | Liver inflammation and fibrosis. Inulin treatment decreases the hepatic mRNA levels of TNFα, Ccl2, Ccl3, CoL1a1, and Sirius red staining | Affect the structure of gut microbiome. Inhibit PCB126-induced reduction of intestinal ZO-1 expression and reduce the release of inflammatory cytokines | 111 |
| PCB126 (1 μmol/kg, oral gavage, at weeks 2 and 4) | A high cholesterol diet with 8% inulin, 12 weeks | male Ldlr−/− mice | Atherosclerosis. Inulin decreases aortic root lesion area in Ldlr−/− mice | Production of protective metabolites, decrease in atherogenic lipoproteins and cholesterol | 34 |
| POPs (diet containing a mixture of POPs, including PCB, PBDE, dioxin, and DDT weeks 0 to 10) | Cranberry extract (200 mg/kg, from week 10 to week 16) | male C57BL/6J mice | Harmful effects of the weight loss process. Cranberry extract treatment leads to lower fasting glycemia and improved glucose tolerance | Improve glucose homeostasis, target the gut microbiota, and increase the relative abundance of Parvibacter | 125 |
| POPs (PCBs and organochlorine pesticides) | Vitamin C (1000 mg/day Vitamin C, oral, 2 months) | 15 healthy California women | Blood concentrations of POPs | Reduce body burdens of POPs | 113 |
Table 3.
Mechanisms of nutritional intervention against BDE-209 mediated toxicity
| Environmental Chemicals (Dose, route, duration) | Nutrients (Dose, route, duration) | Subjects | Disease endpoint | Mechanisms | Reference |
|---|---|---|---|---|---|
| BDE-209 (5 μmol/L, media, 12 h) | Luteolin (5, 50, 100 μmol/L, media, 24 h) | Caco-2 cells | Intestinal barrier damage. Luteolin increases the expression of tight junction proteins (ZO-1, occludin, and claudin-1) | Reduce the level of reactive oxygen species, inhibit the secretion of proinflammatory cytokines, inhibit the ERK and NF-κB/MLCK signaling pathways, and activate the Nrf2/ARE signaling pathways | 137 |
| BDE-47 (150 mg/kg/day, oral gavage, 12 w) | Troxerutin (150 mg/kg/day, oral gavage, 12 w) | male ICR mice | Liver index, serum ALT level, F4/80 (Kupffer cell marker) and IL-1β | Attenuate oxidative stress-mediated NAD+-depletion, restore SirT1 expression and activity | 138 |
In addition to the effects on toxicodynamics, nutritional intervention could affect the toxicokinetic processes of environmental pollutants. For example, antioxidant vitamins, such as vitamin C, can remove the free radicals inside or outside cells and keep the stability of the redox system in the body. It has been reported that vitamin C exerts hepatoprotective effects against chemical-induced liver injuries in mice 112. A pilot study reported that vitamin C intervention might lower the levels of POPs, including organochlorine pesticides and PCBs, in the blood of healthy women 113. This was proposed to be related to the accelerated hydroxylation metabolism of POPs by vitamin C, which could reduce the duration of the toxic effects of POPs in vivo. However, only 15 female subjects were included in this pilot study, and follow-up investigations should include more subjects to confirm the intervention effects of vitamin C. Fiber-rich food intake has been associated with lower environmental pollutant levels in humans, including PFAS 114, acrylamide115, and isopentanaldehyde 116. Although the mechanism of high dietary fiber intake on decreased pollutant exposure in humans remains unknown, it was postulated that dietary fiber reduced the absorption of pollutants and increased their excretion into feces. In an animal study, lower PFOS accumulation in livers was observed in mice fed with soluble fiber-supplemented diets compared with the control diet. This could be because soluble fibers can potentially reduce the reabsorption of PFOS by regulating apical sodium-dependent bile acid transporters, Na+ taurocholate cotransport polypeptides, and organic anion transporting polypeptide transporters, and thus increase PFOS excretion in feces 35.
Recently, there has been an increasing interest in probiotics intervention with high xenobiotics binding capacity 117. For example, lactic acid bacteria (LAB), including Lactobacillus plantarum 118 and Lactobacillus rhamnosus 119 have been discovered to accelerate the removal of heavy metal (cadmium 118, 119), POPs (phthalates 120), and bisphenol A 121. Additionally, a pilot study suggested that L. rhamnosus GR-1 supplemented yogurt protected against the increased mercury and arsenic levels in the blood of pregnant women 122. Furthermore, it was found that a probiotic mixture (Saccharomyces boulardii + Lactobacillus rhamnosus + Lactobacillus planarum LP 6595+ Lactobacillus planarum HEAL9) ameliorated systemic inflammation caused by phthalates and bisphenol A mixture in Wistar rats 123. Thus, probiotic cultures are expected to become important among the other protective agents, such as antioxidants, vitamins, and dietary fibers, to reduce environmental toxicity 117, 124 by affecting both toxicokinetic (accelerate excretion and reduce body burden) and toxicodynamic (decrease inflammation) processes induced by pollutants. A summary diagram of the mechanisms of nutritional intervention against environmental chemical-mediated diseases is shown in Figure 3.
Figure 3.
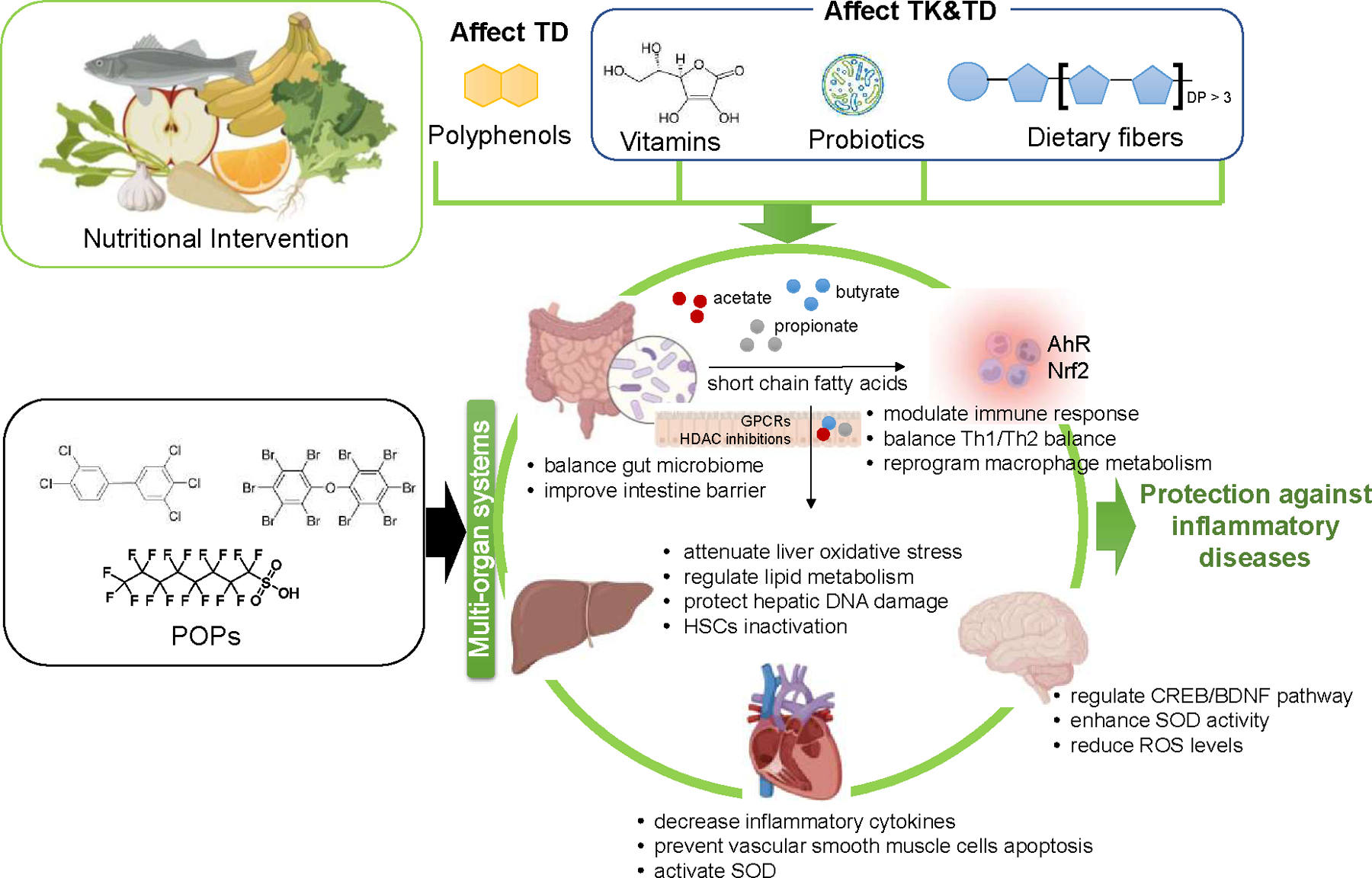
Mechanisms of nutritional intervention against environmental chemical-mediated inflammatory diseases via multi-organ interactions (created with BioRender.com). Polyphenols mainly affect the toxicodynamic processes of environmental pollutants (antioxidant and anti-inflammation). However, vitamin C, probiotics, and dietary fibers affect both toxicodynamic and toxicokinetic processes associated with exposure to POPs. AhR: aryl hydrocarbon receptor; BDNF: brain-derived neurotrophic factor; CREB: cAMP-response element binding; DP: degree of polymerization; GPCR: G protein-coupled receptor; HDAC: histone deacetylase; HSC: hepatic stellate cells; Nrf2: nuclear factor erythroid-related factor 2; POPs: persistent organic pollutants; ROS: reactive oxygen species; SOD: superoxide dismutase; TD: toxicodynamics; TK: toxicokinetics.
4.1. Nutritional intervention of PCB exposure-mediated diseases
Polyphenols could protect against the toxicity of dioxin-like PCBs due to their antioxidation properties. For example, PCB-exposed mice kept on a green tea-supplement diet exhibited an overall decrease in oxidative stress primarily due to the upregulation of a battery of antioxidant enzymes and upregulation of genes transcription controlled by AhR and Nrf2 proteins 33.
Since the primary route of exposure to dioxin-like PCBs is ingestion, and gut microbiome disturbance has been observed in animals exposed to PCBs, interventions are thus tailored to this observation. It was demonstrated that cranberry extract reduced the harmful effects of POPs (a mixture of PCB, PBDE, dioxin, and DDT and metabolites) exposure by targeting the gut microbiome and causing the increased abundance of Parvibacter, a genus involved in xenobiotic metabolism 125. The work with prebiotic fibers showed that consumption of the dietary fiber inulin could reduce dioxin-like PCB-mediated hepatotoxicity and gut dysbiosis in hyperlipidemic LDLR-deficient mice 34. In another study, it was found that inulin treatment ameliorated both inflammation and fibrosis in the liver of PCB126-exposed mice 111. Interestingly, Hoffman et. al found inulin consumption decreased PCB126-induced hepatic lipid accumulation 34; however, in the study reported by Su et.al, inulin treatment did not change the hepatic lipid accumulation in PCB126-exposed mice 111. This discrepancy might be related to the dose of inulin. In the first fiber intervention study, the mice were fed with an 8% inulin-supplemented diet (inulin is the sole dietary fiber source) for 12 weeks (approximately 20 g/kg/day), while in the second study, mice were treated with drinking water containing inulin (equivalent to 250 mg/kg/day). Laboratory mice are usually fed with a standardized chow diet that typically contains 5% of fiber. Therefore, the 8% fiber-supplemented diet reported by Hoffman et al. represented an approximate 60% increase in fiber contents compared with the standard chow. For humans, it was suggested that dietary fiber intake should be increased by about 50% compared to current intake 126, which is 16–24 g/day and 16.2 g/day in European countries and the US, respectively 127, 128. Therefore, the diets used in the animal study reported by Hoffman et. al represented the increase in dietary fiber intake recommended for humans. Nutritional intervention strategies against PCB-mediated toxicity are listed in Table 1.
4.2. Nutritional intervention of PFAS exposure-mediated diseases
The toxicity of PFASs could be attenuated by dietary fibers and polyphenols. It has been demonstrated that quercetin could ameliorate liver injury induced by PFOA exposure by reducing oxidative stress and inflammation 129. In addition, liver injury in mice treated with PFOS was reduced by naringin. The mechanisms were associated with the regulation of oxidation stress, inflammation, and apoptosis pathways 130. Steatosis and liver inflammation were observed in mice exposed to PFOS for 21 days, and concurrent administration of grape seed proanthocyanidin extract (GSPE) reduced PFOS-induced liver toxicity by normalizing lipid metabolism, oxidative stress, and inflammatory responses 131. Eke et al. reported that curcumin (diferuloylmethane), a natural compound present in the rhizome of the turmeric plant (Curcuma longa), protected against PFOS-induced genotoxic/apoptotic effects and the DNA damage in liver tissues of Wistar albino rats 132. In addition, Su et al. found that vitamin C protected against PFOS-induced liver damage in mice through suppressing inflammatory reactions and endoplasmic reticulum stress 133. Dietary interventions targeting the gut microbiome showed that soluble dietary fiber (inulin or pectin) fed mice were less susceptible to PFOS-induced liver metabolism disruption, hepatic lipid accumulation, and transcriptional changes 35. Nutritional intervention strategies against PFAS-mediated toxicity are presented in Table 2.
Table 2.
Mechanisms of nutritional intervention against PFAS-mediated toxicity
| Environmental Chemicals (Dose, route, duration) | Nutrients (Dose, route, duration) | Subjects | Disease endpoint | Mechanisms | Reference |
|---|---|---|---|---|---|
| PFOA (10 mg/kg/day, i.g., 14 d) | Quercetin (75 mg/kg/day, i.g., 14 d) | Male Kunming mice | Liver injury. AST, ALT, lactate dehydrogenase, and total bile acids | Reduce oxidative stress and inflammation | 129 |
| PFOS (10 mg/kg/day, i.g., 3 w) | Naringin (100 mg/kg/day, i.g., 3 w) | Male mice | Liver injury. Naringin supplementation led to the resumption of elevated serum hepatic enzyme activities in PFOS-exposed mice | Relate to the regulation of oxidation, inflammation, and apoptosis pathways | 130 |
| PFOS (10 mg/kg/day, i.g., 21 d) | Grape seed proanthocyanidin extract (150 mg/kg/day, i.g., 21 d) | Male Kunming Mice | Steatohepatitis. Grape seed proanthocyanidin extract restores decreased serum liver enzyme activity and histological abnormalities in PFOS-exposed mice | Regulate lipid metabolism, oxidative stress, and inflammatory responses | 131 |
| PFOS (3 ug/kg/day, by drinking water, 7 w) | soluble fibers (inulin or pectin) (8% insulin or pectin, diet, 7 w) | Male C57BL/6J mice | Inulin supplementation ameliorates PFOS-induced metabolic disturbance in liver | Affect microbe-liver metabolism and interactions | 35 |
| PFOS (10 mg/kg/day, i.g., 21 d) | Vitamin C (100/200 mg/kg/day, i.g., 21 d) | Male ICR mice | Liver steatosis. VC treatment decreases serum levels of ALT and AST | Suppress hepatocellular inflammatory reaction and ER stress | 133 |
| PFOS (0.6/1.25/ 2.5 mg/kg, gavage, 4 w) | Curcumin (80 mg/kg, gavage, 30 d at 48 h intervals) | Wistar rat | DNA damage in the liver. Curcumin supplementation reduces liver DNA damage | Decrease the expression levels of caspase 3 and 8 | 132 |
The dose of those phytochemicals (quercetin, naringin, proanthocyanidin, and curcumin) ranges from 75–150 mg/kg/day in animal intervention studies (Table 2). Although the average human daily intake of those phytochemicals from food is relatively low (e.g., daily intake of quercetin is estimated to be 10–100 mg), higher intakes can be achieved using selected nutraceuticals 134. After oral administration, glycosides (quercetin, naringin) are mostly hydrolyzed and absorbed from the intestines as deconjugated aglycones, which could be further metabolized in the liver. It has been demonstrated that the metabolism of glycosides was different between rodents and humans 135. Therefore, species differences in metabolism should be taken into consideration when translating rodent studies to human intervention strategies.
4.3. Nutritional intervention of PBDE exposure-mediated diseases
The toxicity of PBDE could be attenuated by polyphenols. Luteolin is a natural flavonoid compound in different fruits and vegetables 136. It was reported that in Caco2 cells exposed to BDE-209 (a major congener of PBDE), luteolin reduced the level of reactive oxygen species, inhibited the secretion of proinflammatory cytokines, and played a protective role in the intestinal barrier (increased tight junction proteins ZO-1, occludin, and claudin-1) 137. In another study, it was demonstrated that troxerutin, a trihydroxyethylated derivative of the natural bioflavonoid rutin, protected against BDE-47 induced liver inflammation by attenuating oxidative stress-mediated NAD+ depletion 138. These studies indicated that natural compounds had important protective effects and could potentially be used as dietary supplements against the toxicity of PBDEs (Table 3).
5. System biology approaches to explore multi-organ toxicity and precision intervention
Accumulating evidence suggests that the gut microbiome-tissue crosstalk is a major player in POPs-associated diseases. Dietary supplementation with prebiotics/probiotics appears to be a promising intervention for reducing the damage caused by pollutants and restoring the balanced structure of the gut microbial community. Further investigations on gut microbiome-host interactions are warranted by using system biology techniques such as metabolomics, metagenomics, metatranscriptomics, and stable isotope probing 139–142. Metabolomics enables holistic and systematic analyses of metabolites in a biological system. Integrative analysis of metabolomics, proteomics, and transcriptomics is a very appealing technology to investigate toxicity and precision nutritional effects that can provide an in-depth understanding of the etiology of functional dysbiosis and multi-organ interactions. Metagenomics and metatranscriptomics provide taxonomical and functional profiles of microbiomes 143, which in combined with metabolomic analysis could produce a more comprehensive picture of the etiology of dysbiosis and gut microbiome-tissue axis. As systems biology extends its application from bulk assays to more precise identifications of cells perturbed by toxicants, single cell techniques represent a valuable tool for understanding cell specific alterations in response to toxicant insults as well as the diversity and spatial changes within microorganism communities 144, 145, which would be anticipated to provide new insights on the mechanism of organ crosstalk at the dimension of intra- and inter-cellular responses. It should be noted that the effects of multi-organ toxicity and nutritional intervention may vary from person to person, depending on factors such as the baseline gut microbiome and intrinsic host factor. Therefore, precision/personalized intervention regimens should be considered.
The latest development of multiorgan-on-a-chip, a 3D engineered biological model implemented in microfluidic platforms, is a microphysiological system capable of modeling organ-level responses. Compared with the traditional single organ-on-a-chip, the multiorgan model allows investigations of cross-organ communications 146. For example, a liver/lung-on-a-chip was developed and applied to investigate the liver-lung interactions in the toxicity of aflatoxin B1 147. It was found that both tissues remained viable and functional for 28 days when cocultured in the chip, and detoxification of aflatoxin B1 by the liver protected against its lung toxicity. Multiorgan-on-a-chip technology offers new opportunities in system toxicity and precision nutrition studies while supporting the implementation of the 3Rs of animal research (replacement, reduction, and refinement).
6. Conclusions
The toxicity of POPs is linked to the multi-organ interplays involving the liver, gut, brain, vascular, and immune system, which lead to inflammation and cardiometabolic diseases. Consumption of food rich in anti-inflammatory phytochemicals, dietary fibers, and vitamins may be a feasible way to counteract POP toxicity by acting on multi-organ interactions and preventing inflammatory diseases. Future research should consider the investigation of lesser studied nutrient mixtures rather than single compounds. By integrating data from a single cell- to ecosystem-level processes, from single- to multiple-organ systems, together with omics analyses, a more comprehensive understanding of the multi-organ interactions can be generated, and meaningful insights into the mechanisms of toxicity will be provided, which will accelerate the development of precision intervention strategies.
Supplementary Material
Highlights.
Organ interactions play important roles in POPs-induced inflammatory diseases.
A complex network of interactions exists between host organs and gut/microbiome.
Nutritional intervention strategies should consider multiple organ interactions.
Acknowledgements
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD), SU-RCSI joint PhD programme, and the National Institute of Environmental Health Sciences, National Institutes of Health grant P42 ES007380. The content is solely the responsibility of the authors and does not necessarily represent the official views the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Aksentijevich M; Lateef SS; Anzenberg P; Dey AK; Mehta NN, Chronic inflammation, cardiometabolic diseases and effects of treatment: Psoriasis as a human model. Trends Cardiovasc Med 2020, 30, (8), 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattar N; Gill JMR; Alazawi W, Improving prevention strategies for cardiometabolic disease. Nat Med 2020, 26, (3), 320–325. [DOI] [PubMed] [Google Scholar]
- 3.Tahir UA; Gerszten RE, Omics and Cardiometabolic Disease Risk Prediction. Annu Rev Med 2020, 71, 163–175. [DOI] [PubMed] [Google Scholar]
- 4.Ward-Caviness CK, A review of gene-by-air pollution interactions for cardiovascular disease, risk factors, and biomarkers. Hum Genet 2019, 138, (6), 547–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warburton DER; Bredin SSD; Shellington EM; Cole C; de Faye A; Harris J; Kim DD; Abelsohn A, A Systematic Review of the Short-Term Health Effects of Air Pollution in Persons Living with Coronary Heart Disease. J Clin Med 2019, 8, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clementi EA; Talusan A; Vaidyanathan S; Veerappan A; Mikhail M; Ostrofsky D; Crowley G; Kim JS; Kwon S; Nolan A, Metabolic syndrome and air pollution: a narrative review of their cardiopulmonary effects. Toxics, 7, (1) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y; Tang R; Qiu H; Lai PC; Wong P; Thach TQ; Allen R; Brauer M; Tian L; Barratt B, Long term exposure to air pollution and mortality in an elderly cohort in Hong Kong. Environ Int 2018, 117, 99–106. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q; Gu X; Deng F; Mu L; Baccarelli AA; Guo X; Wu S, Ambient particulate air pollution and circulating C-reactive protein level: A systematic review and meta-analysis. Int J Hyg Environ Health 2019, 222, (5), 756–764. [DOI] [PubMed] [Google Scholar]
- 9.Lawal AO, Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol Lett 2017, 270, 88–95. [DOI] [PubMed] [Google Scholar]
- 10.Munzel T; Daiber A, Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid Redox Signal 2018, 28, (9), 735–740. [DOI] [PubMed] [Google Scholar]
- 11.Zhong S; Li L; Shen X; Li Q; Xu W; Wang X; Tao Y; Yin H, An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med 2019, 144, 266–278. [DOI] [PubMed] [Google Scholar]
- 12.Petriello MC; Brandon JA; Hoffman J; Wang C; Tripathi H; Abdel-Latif A; Ye X; Li X; Yang L; Lee E; Soman S; Barney J; Wahlang B; Hennig B; Morris AJ, Dioxin-like PCB 126 Increases Systemic Inflammation and Accelerates Atherosclerosis in Lean LDL Receptor-Deficient Mice. Toxicol Sci 2018, 162, (2), 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismaiel A; Dumitrascu DL, Cardiovascular Risk in Fatty Liver Disease: The Liver-Heart Axis-Literature Review. Front Med (Lausanne) 2019, 6, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J; Li H, The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front Pharmacol 2018, 9, 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng P; Wang C; Wahlang B; Sexton T; Morris AJ; Hennig B, Co-exposure to PCB126 and PFOS increases biomarkers associated with cardiovascular disease risk and liver injury in mice. Toxicol Appl Pharmacol 2020, 409, 115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai KP; Ng AH; Wan HT; Wong AY; Leung CC; Li R; Wong CK, Dietary Exposure to the Environmental Chemical, PFOS on the Diversity of Gut Microbiota, Associated With the Development of Metabolic Syndrome. Front Microbiol 2018, 9, 2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang L; Hong Y; Xiao P; Wang X; Zhang J; Liu E; Li H; Cai Z, The Role of Fecal Microbiota in Liver Toxicity Induced by Perfluorooctane Sulfonate in Male and Female Mice. Environ Health Perspect 2022, 130, (6), 67009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L; Rimal B; Nichols RG; Tian Y; Smith PB; Hatzakis E; Chang SC; Butenhoff JL; Peters JM; Patterson AD, Perfluorooctane sulfonate alters gut microbiota-host metabolic homeostasis in mice. Toxicology 2020, 431, 152365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G; Pan R; Liang X; Wu X; Wu Y; Zhang H; Zhao J; Chen W, Perfluorooctanoic acid-induced liver injury is potentially associated with gut microbiota dysbiosis. Chemosphere 2021, 266, 129004. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T; Hidaka T; Kumagai Y; Yamamoto M, Environmental pollutants and the immune response. Nat Immunol 2020, 21, (12), 1486–1495. [DOI] [PubMed] [Google Scholar]
- 21.Feng J; Cavallero S; Hsiai T; Li R, Impact of air pollution on intestinal redox lipidome and microbiome. Free Radic Biol Med 2020, 151, 99–110. [DOI] [PubMed] [Google Scholar]
- 22.Marzec JM; Nadadur SS, Inflammation resolution in environmental pulmonary health and morbidity. Toxicol Appl Pharmacol 2022, 449, 116070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford MS; Nordgren TM; McCole DF, Every breath you take: Impacts of environmental dust exposure on intestinal barrier function-from the gut-lung axis to COVID-19. Am J Physiol Gastrointest Liver Physiol 2021, 320, (4), G586–G600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Ciaula A, Baj J, Garruti G, Celano G, De Angelis M, Wang HH, Di Palo DM, Bonfrate L, Wang DQ, Portincasa P Liver steatosis, gut-liver Axis, microbiome and environmental factors. A never-ending bidirectional cross-talk. J. Clin. Med 2020. 9 (8), 2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S; Sharma P; Pal N; Kumawat M; Shubham S; Sarma DK; Tiwari RR; Kumar M; Nagpal R, Impact of Environmental Pollutants on Gut Microbiome and Mental Health via the Gut-Brain Axis. Microorganisms 2022, 10, (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand S; Mande SS, Host-microbiome interactions: Gut-Liver axis and its connection with other organs. NPJ Biofilms Microbiomes 2022, 8, (1), 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oishi Y; Manabe I, Organ System Crosstalk in Cardiometabolic Disease in the Age of Multimorbidity. Front Cardiovasc Med 2020, 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu E; Malik VS; Hu FB, Cardiovascular Disease Prevention by Diet Modification: JACC Health Promotion Series. J Am Coll Cardiol 2018, 72, (8), 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satija A; Hu FB, Plant-based diets and cardiovascular health. Trends Cardiovasc Med 2018, 28, (7), 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacks FM; Lichtenstein AH; Wu JHY; Appel LJ; Creager MA; Kris-Etherton PM; Miller M; Rimm EB; Rudel LL; Robinson JG; Stone NJ; Van Horn LV; American Heart, A., Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, (3), e1–e23. [DOI] [PubMed] [Google Scholar]
- 31.Tong H, Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta 2016, 1860, (12), 2891–8. [DOI] [PubMed] [Google Scholar]
- 32.Lim CC; Hayes RB; Ahn J; Shao Y; Silverman DT; Jones RR; Thurston GD, Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation 2019, 139, (15), 1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newsome BJ; Petriello MC; Han SG; Murphy MO; Eske KE; Sunkara M; Morris AJ; Hennig B, Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. J Nutr Biochem 2014, 25, (2), 126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman JB; Petriello MC; Morris AJ; Mottaleb MA; Sui Y; Zhou C; Deng P; Wang C; Hennig B, Prebiotic inulin consumption reduces dioxin-like PCB 126-mediated hepatotoxicity and gut dysbiosis in hyperlipidemic Ldlr deficient mice. Environ Pollut 2020, 261, 114183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng P; Durham J; Liu J; Zhang X; Wang C; Li D; Gwag T; Ma M; Hennig B, Metabolomic, Lipidomic, Transcriptomic, and Metagenomic Analyses in Mice Exposed to PFOS and Fed Soluble and Insoluble Dietary Fibers. Environ Health Perspect 2022, 130, (11), 117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haddad S; Poulin P; Krishnan K, Relative lipid content as the sole mechanistic determinant of the adipose tissue:blood partition coefficients of highly lipophilic organic chemicals. Chemosphere 2000, 40, (8), 839–43. [DOI] [PubMed] [Google Scholar]
- 37.Kania-Korwel I; Lehmler HJ, Toxicokinetics of chiral polychlorinated biphenyls across different species--a review. Environ Sci Pollut Res Int 2016, 23, (3), 2058–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkins JT; Petriello MC; Newsome BJ; Hennig B, Polychlorinated biphenyls and links to cardiovascular disease. Environ Sci Pollut Res Int 2016, 23, (3), 2160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petriello MC; Han SG; Newsome BJ; Hennig B, PCB 126 toxicity is modulated by cross-talk between caveolae and Nrf2 signaling. Toxicol Appl Pharmacol 2014, 277, (2), 192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahlang B; Barney J; Thompson B; Wang C; Hamad OM; Hoffman JB; Petriello MC; Morris AJ; Hennig B, Editor's Highlight: PCB126 Exposure Increases Risk for Peripheral Vascular Diseases in a Liver Injury Mouse Model. Toxicol Sci 2017, 160, (2), 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gourronc FA; Helm BK; Robertson LW; Chimenti MS; Joachim-Lehmler H; Ankrum JA; Klingelhutz AJ, Transcriptome sequencing of 3,3',4,4',5-Pentachlorobiphenyl (PCB126)-treated human preadipocytes demonstrates progressive changes in pathways associated with inflammation and diabetes. Toxicol In Vitro 2022, 83, 105396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caron A; Ahmed F; Peshdary V; Garneau L; Atlas E; Aguer C, Effects of PCB126 on Adipose-to-Muscle Communication in an in Vitro Model. Environ Health Perspect 2020, 128, (10), 107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng P; Barney J; Petriello MC; Morris AJ; Wahlang B; Hennig B, Hepatic metabolomics reveals that liver injury increases PCB 126-induced oxidative stress and metabolic dysfunction. Chemosphere 2019, 217, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim JJ; Li X; Lehmler HJ; Wang D; Gu H; Cui JY, Gut Microbiome Critically Impacts PCB-induced Changes in Metabolic Fingerprints and the Hepatic Transcriptome in Mice. Toxicol Sci 2020, 177, (1), 168–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahlang B; Hardesty JE; Jin J; Falkner KC; Cave MC, Polychlorinated Biphenyls and Nonalcoholic Fatty Liver Disease. Curr Opin Toxicol 2019, 14, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahlang B; Perkins JT; Petriello MC; Hoffman JB; Stromberg AJ; Hennig B, A compromised liver alters polychlorinated biphenyl-mediated toxicity. Toxicology 2017, 380, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petriello MC; Charnigo R; Sunkara M; Soman S; Pavuk M; Birnbaum L; Morris AJ; Hennig B, Relationship between serum trimethylamine N-oxide and exposure to dioxin-like pollutants. Environ Res 2018, 162, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang WH; Wang Z; Levison BS; Koeth RA; Britt EB; Fu X; Wu Y; Hazen SL, Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013, 368, (17), 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maksymiuk KM; Szudzik M; Gawrys-Kopczynska M; Onyszkiewicz M; Samborowska E; Mogilnicka I; Ufnal M, Trimethylamine, a gut bacteria metabolite and air pollutant, increases blood pressure and markers of kidney damage including proteinuria and KIM-1 in rats. J Transl Med 2022, 20, (1), 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavuk M; Olson JR; Wattigney WA; Dutton ND; Sjodin A; Shelton C; Turner WE; Bartell SM; Anniston Environmental Health Research, C., Predictors of serum polychlorinated biphenyl concentrations in Anniston residents. Sci Total Environ 2014, 496, 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petriello MC; Hoffman JB; Sunkara M; Wahlang B; Perkins JT; Morris AJ; Hennig B, Dioxin-like pollutants increase hepatic flavin containing monooxygenase (FMO3) expression to promote synthesis of the pro-atherogenic nutrient biomarker trimethylamine N-oxide from dietary precursors. J Nutr Biochem 2016, 33, 145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang S; Li X; Yang F; Zhao R; Pan X; Liang J; Tian L; Li X; Liu L; Xing Y; Wu M, Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front Pharmacol 2019, 10, 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z; Zhao Y, Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, (5), 416–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng SL; Li X; Lehmler HJ; Phillips B; Shen D; Cui JY, Gut Microbiota Modulates Interactions Between Polychlorinated Biphenyls and Bile Acid Homeostasis. Toxicol Sci 2018, 166, (2), 269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C; Petriello MC; Zhu B; Hennig B, PCB 126 induces monocyte/macrophage polarization and inflammation through AhR and NF-kappaB pathways. Toxicol Appl Pharmacol 2019, 367, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang B; Wang Y; Qin Q; Xia X; Liu Z; Song E; Song Y, Polychlorinated Biphenyl Quinone Promotes Macrophage-Derived Foam Cell Formation. Chem Res Toxicol 2019, 32, (12), 2422–2432. [DOI] [PubMed] [Google Scholar]
- 57.Liu J; Yang B; Wang Y; Wu Y; Fan B; Zhu S; Song E; Song Y, Polychlorinated biphenyl quinone promotes macrophage polarization to CD163(+) cells through Nrf2 signaling pathway. Environ Pollut 2020, 257, 113587. [DOI] [PubMed] [Google Scholar]
- 58.Yang B; Qin Q; Xu L; Lv X; Liu Z; Song E; Song Y, Polychlorinated Biphenyl Quinone Promotes Atherosclerosis through Lipid Accumulation and Endoplasmic Reticulum Stress via CD36. Chem Res Toxicol 2020, 33, (6), 1497–1507. [DOI] [PubMed] [Google Scholar]
- 59.Rude KM; Pusceddu MM; Keogh CE; Sladek JA; Rabasa G; Miller EN; Sethi S; Keil KP; Pessah IN; Lein PJ; Gareau MG, Developmental exposure to polychlorinated biphenyls (PCBs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environ Pollut 2019, 253, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shan Q; Qu F; Chen N, 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and Polychlorinated Biphenyl Coexposure Alters the Expression Profile of MicroRNAs in the Liver Associated with Atherosclerosis. Biomed Res Int 2020, 2020, 2652756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang B; Ye Z; Wang Y; Guo H; Lehmler HJ; Huang R; Song E; Song Y, Evaluation of Early Biomarkers of Atherosclerosis Associated with Polychlorinated Biphenyl Exposure: An in Vitro and in Vivo Study. Environ Health Perspect 2022, 130, (3), 37011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jenike AE; Halushka MK, miR-21: a non-specific biomarker of all maladies. Biomark Res 2021, 9, (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez-Pedrera C; Barbarroja N; Patino-Trives AM; Luque-Tevar M; Torres-Granados C; Aguirre-Zamorano MA; Collantes-Estevez E; Perez-Sanchez C, Role of microRNAs in the Development of Cardiovascular Disease in Systemic Autoimmune Disorders. Int J Mol Sci 2020, 21, (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petri BJ; Piell KM; Wahlang B; Head KZ; Andreeva K; Rouchka EC; Pan J; Rai SN; Cave MC; Klinge CM, Multiomics analysis of the impact of polychlorinated biphenyls on environmental liver disease in a mouse model. Environ Toxicol Pharmacol 2022, 94, 103928. [DOI] [PubMed] [Google Scholar]
- 65.Cave MC; Pinkston CM; Rai SN; Wahlang B; Pavuk M; Head KZ; Carswell GK; Nelson GM; Klinge CM; Bell DA; Birnbaum LS; Chorley BN, Circulating MicroRNAs, Polychlorinated Biphenyls, and Environmental Liver Disease in the Anniston Community Health Survey. Environ Health Perspect 2022, 130, (1), 17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu XL; Pan Q; Cao HX; Xin FZ; Zhao ZH; Yang RX; Zeng J; Zhou HP; Fan JG, Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192–5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, (2), 454–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji C; Guo X, The clinical potential of circulating microRNAs in obesity. Nat Rev Endocrinol 2019, 15, (12), 731–743. [DOI] [PubMed] [Google Scholar]
- 68.Wu H; Yu W; Meng F; Mi J; Peng J; Liu J; Zhang X; Hai C; Wang X, Polychlorinated biphenyls-153 induces metabolic dysfunction through activation of ROS/NF-kappaB signaling via downregulation of HNF1b. Redox Biol 2017, 12, 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker NA; Shoemaker R; English V; Larian N; Sunkara M; Morris AJ; Walker M; Yiannikouris F; Cassis LA, Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ Health Perspect 2015, 123, (10), 944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pizzurro DM; Seeley M; Kerper LE; Beck BD, Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regul Toxicol Pharmacol 2019, 106, 239–250. [DOI] [PubMed] [Google Scholar]
- 71.Perez F; Nadal M; Navarro-Ortega A; Fabrega F; Domingo JL; Barcelo D; Farre M, Accumulation of perfluoroalkyl substances in human tissues. Environ Int 2013, 59, 354–62. [DOI] [PubMed] [Google Scholar]
- 72.Xu Y; Fletcher T; Pineda D; Lindh CH; Nilsson C; Glynn A; Vogs C; Norstrom K; Lilja K; Jakobsson K; Li Y, Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ Health Perspect 2020, 128, (7), 77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi Y; Vestergren R; Xu L; Zhou Z; Li C; Liang Y; Cai Y, Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environ Sci Technol 2016, 50, (5), 2396–404. [DOI] [PubMed] [Google Scholar]
- 74.Fenton SE; Ducatman A; Boobis A; DeWitt JC; Lau C; Ng C; Smith JS; Roberts SM, Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem 2021, 40, (3), 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang ET; Adami HO; Boffetta P; Wedner HJ; Mandel JS, A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit Rev Toxicol 2016, 46, (4), 279–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goodrum PE; Anderson JK; Luz AL; Ansell GK, Application of a Framework for Grouping and Mixtures Toxicity Assessment of PFAS: A Closer Examination of Dose-Additivity Approaches. Toxicol Sci 2021, 179, (2), 262–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omoike OE; Pack RP; Mamudu HM; Liu Y; Strasser S; Zheng S; Okoro J; Wang L, Association between per and polyfluoroalkyl substances and markers of inflammation and oxidative stress. Environ Res 2021, 196, 110361. [DOI] [PubMed] [Google Scholar]
- 78.Meneguzzi A; Fava C; Castelli M; Minuz P, Exposure to Perfluoroalkyl Chemicals and Cardiovascular Disease: Experimental and Epidemiological Evidence. Front Endocrinol (Lausanne) 2021, 12, 706352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Q; Huang J; Liu S; Wang C; Jin Y; Lai H; Tu W, Aberrant hepatic lipid metabolism associated with gut microbiota dysbiosis triggers hepatotoxicity of novel PFOS alternatives in adult zebrafish. Environ Int 2022, 166, 107351. [DOI] [PubMed] [Google Scholar]
- 80.Pan Z; Yuan X; Tu W; Fu Z; Jin Y, Subchronic exposure of environmentally relevant concentrations of F-53B in mice resulted in gut barrier dysfunction and colonic inflammation in a sex-independent manner. Environ Pollut 2019, 253, 268–277. [DOI] [PubMed] [Google Scholar]
- 81.Xie X; Zhou J; Hu L; Shu R; Zhang M; Xiong Z; Wu F; Fu Z, Exposure to hexafluoropropylene oxide dimer acid (HFPO-DA) disturbs the gut barrier function and gut microbiota in mice. Environ Pollut 2021, 290, 117934. [DOI] [PubMed] [Google Scholar]
- 82.Wang C; Zhang Y; Deng M; Wang X; Tu W; Fu Z; Jin Y, Bioaccumulation in the gut and liver causes gut barrier dysfunction and hepatic metabolism disorder in mice after exposure to low doses of OBS. Environ Int 2019, 129, 279–290. [DOI] [PubMed] [Google Scholar]
- 83.Starnes HM; Rock KD; Jackson TW; Belcher SM, A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior. Front Toxicol 2022, 4, 881584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang T; Zhao S; Dong F; Jia Y; Chen X; Sun Y; Zhu L, Novel Insight into the Mechanisms of Neurotoxicity Induced by 6:6 PFPiA through Disturbing the Gut-Brain Axis. Environ Sci Technol 2023, 57, (2), 1028–1038. [DOI] [PubMed] [Google Scholar]
- 85.Shi L; Zheng J; Yan S; Li Y; Wang Y; Liu X; Xiao C, Exposure to Perfluorooctanoic Acid Induces Cognitive Deficits via Altering Gut Microbiota Composition, Impairing Intestinal Barrier Integrity, and Causing Inflammation in Gut and Brain. J Agric Food Chem 2020, 68, (47), 13916–13928. [DOI] [PubMed] [Google Scholar]
- 86.Bassler J; Ducatman A; Elliott M; Wen S; Wahlang B; Barnett J; Cave MC, Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut 2019, 247, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ehrlich V; Bil W; Vandebriel R; Granum B; Luijten M; Lindeman B; Grandjean P; Kaiser AM; Hauzenberger I; Hartmann C; Gundacker C; Uhl M, Consideration of pathways for immunotoxicity of per- and polyfluoroalkyl substances (PFAS). Environ Health 2023, 22, (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wan HT; Zhao YG; Wei X; Hui KY; Giesy JP; Wong CK, PFOS-induced hepatic steatosis, the mechanistic actions on beta-oxidation and lipid transport. Biochim Biophys Acta 2012, 1820, (7), 1092–101. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y; Zhang J; Cui W; Silverstein RL, CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J Exp Med 2022, 219, (6), e20211314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rankin LC; Artis D, Beyond Host Defense: Emerging Functions of the Immune System in Regulating Complex Tissue Physiology. Cell 2018, 173, (3), 554–567. [DOI] [PubMed] [Google Scholar]
- 91.Tian J; Hong Y; Li Z; Yang Z; Lei B; Liu J; Cai Z, Immunometabolism-modulation and immunotoxicity evaluation of perfluorooctanoic acid in macrophage. Ecotoxicol Environ Saf 2021, 215, 112128. [DOI] [PubMed] [Google Scholar]
- 92.Yang M; Li LY; Qin XD; Ye XY; Yu S; Bao Q; Sun L; Wang ZB; Bloom MS; Jalava P; Hu LW; Yu HY; Zeng XW; Yang BY; Dong GH; Li CW, Perfluorooctanesulfonate and perfluorooctanoate exacerbate airway inflammation in asthmatic mice and in vitro. Sci Total Environ 2021, 766, 142365. [DOI] [PubMed] [Google Scholar]
- 93.Park SJ; Sim KH; Shrestha P; Yang JH; Lee YJ, Perfluorooctane sulfonate and bisphenol A induce a similar level of mast cell activation via a common signaling pathway, Fyn-Lyn-Syk activation. Food Chem Toxicol 2021, 156, 112478. [DOI] [PubMed] [Google Scholar]
- 94.Wang LQ; Liu T; Yang S; Sun L; Zhao ZY; Li LY; She YC; Zheng YY; Ye XY; Bao Q; Dong GH; Li CW; Cui J, Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat Commun 2021, 12, (1), 2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weng Z; Xu C; Zhang X; Pang L; Xu J; Liu Q; Zhang L; Xu S; Gu A, Autophagy mediates perfluorooctanoic acid-induced lipid metabolism disorder and NLRP3 inflammasome activation in hepatocytes. Environ Pollut 2020, 267, 115655. [DOI] [PubMed] [Google Scholar]
- 96.Marques M; Nadal M; Domingo JL, Human exposure to polybrominated diphenyl ethers (PBDEs) through the diet: An update of the scientific literature. Food Chem Toxicol 2022, 167, 113322. [DOI] [PubMed] [Google Scholar]
- 97.Wu Z; He C; Han W; Song J; Li H; Zhang Y; Jing X; Wu W, Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ Res 2020, 187, 109531. [DOI] [PubMed] [Google Scholar]
- 98.Zhang T; Zhou X; Xu A; Tian Y; Wang Y; Zhang Y; Gu Q; Wang S; Wang Z, Toxicity of polybrominated diphenyl ethers (PBDEs) on rodent male reproductive system: A systematic review and meta-analysis of randomized control studies. Sci Total Environ 2020, 720, 137419. [DOI] [PubMed] [Google Scholar]
- 99.Zhi H; Wu JP; Lu LM; Li Y; Chen XY; Tao J; Mai BX, Decabromodiphenyl ether (BDE-209) enhances foam cell formation in human macrophages via augmenting Toll-like receptor 4-dependent lipid uptake. Food Chem Toxicol 2018, 121, 367–373. [DOI] [PubMed] [Google Scholar]
- 100.Zhi H; Wu JP; Lu LM; Zhang XM; Chen XY; Wu SK; Tao J; Mai BX, Decarbromodiphenyl ether (BDE-209) promotes monocyte-endothelial adhesion in cultured human aortic endothelial cells through upregulating intercellular adhesion molecule-1. Environ Res 2019, 169, 62–71. [DOI] [PubMed] [Google Scholar]
- 101.Jing L; Sun Y; Wang Y; Liang B; Chen T; Zheng D; Zhao X; Zhou X; Sun Z; Shi Z, Cardiovascular toxicity of decabrominated diphenyl ethers (BDE-209) and decabromodiphenyl ethane (DBDPE) in rats. Chemosphere 2019, 223, 675–685. [DOI] [PubMed] [Google Scholar]
- 102.Scoville DK; Li CY; Wang D; Dempsey JL; Raftery D; Mani S; Gu H; Cui JY, Polybrominated Diphenyl Ethers and Gut Microbiome Modulate Metabolic Syndrome-Related Aqueous Metabolites in Mice. Drug Metab Dispos 2019, 47, (8), 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peter S; Holguin F; Wood LG; Clougherty JE; Raederstorff D; Antal M; Weber P; Eggersdorfer M, Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients 2015, 7, (12), 10398–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hennig B; Petriello MC; Gamble MV; Surh YJ; Kresty LA; Frank N; Rangkadilok N; Ruchirawat M; Suk WA, The role of nutrition in influencing mechanisms involved in environmentally mediated diseases. Rev Environ Health 2018, 33, (1), 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dias MC, Pinto D, Silva AMS Plant flavonoids: chemical characteristics and biological activity. Molecules 2021, 26 (17), 5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tu P; Chi L; Bodnar W; Zhang Z; Gao B; Bian X; Stewart J; Fry R; Lu K, Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics 2020, 8, (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng P; Hoffman JB; Petriello MC; Wang CY; Li XS; Kraemer MP; Morris AJ; Hennig B, Dietary inulin decreases circulating ceramides by suppressing neutral sphingomyelinase expression and activity in mice. J Lipid Res 2020, 61, (1), 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soliman GA, Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cong J, Zhou P, Zhang R, 2022. Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients, 14, (9), 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuo SM, The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr 2013, 4, (1), 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su H; Liu J; Wu G; Long Z; Fan J; Xu Z; Liu J; Yu Z; Cao M; Liao N; Peng J; Yu W; Li W; Wu H; Wang X, Homeostasis of gut microbiota protects against polychlorinated biphenyl 126-induced metabolic dysfunction in liver of mice. Sci Total Environ 2020, 720, 137597. [DOI] [PubMed] [Google Scholar]
- 112.Wu W; Su M; Li T; Wu K; Wu X; Tang Z, Cantharidin-induced liver injuries in mice and the protective effect of vitamin C supplementation. Int Immunopharmacol 2015, 28, (1), 182–7. [DOI] [PubMed] [Google Scholar]
- 113.Guo W; Huen K; Park JS; Petreas M; Crispo Smith S; Block G; Holland N, Vitamin C intervention may lower the levels of persistent organic pollutants in blood of healthy women - A pilot study. Food Chem Toxicol 2016, 92, 197–204. [DOI] [PubMed] [Google Scholar]
- 114.Lin PD; Cardenas A; Hauser R; Gold DR; Kleinman KP; Hivert MF; Fleisch AF; Calafat AM; Sanchez-Guerra M; Osorio-Yanez C; Webster TF; Horton ES; Oken E, Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: Cross-sectional results from the Diabetes Prevention Program Trial. Environ Int 2020, 137, 105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gong H; Liao S, Inverse relationship between dietary fiber intake and environmental exposure to acrylamide. Environ Sci Pollut Res Int 2023, 30, (12), 35326–35333. [DOI] [PubMed] [Google Scholar]
- 116.Shi S; Zhu Q; Liao S; Zhu X; Tang X; Zhou Y, The association between dietary fiber intake and the concentrations of aldehydes in serum. Environ Sci Pollut Res Int 2022, 29, (17), 25790–25798. [DOI] [PubMed] [Google Scholar]
- 117.Srednicka P; Juszczuk-Kubiak E; Wojcicki M; Akimowicz M; Roszko ML, Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem Toxicol 2021, 153, 112306. [DOI] [PubMed] [Google Scholar]
- 118.Zhai Q; Wang G; Zhao J; Liu X; Narbad A; Chen YQ; Zhang H; Tian F; Chen W, Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl Environ Microbiol 2014, 80, (13), 4063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Daisley BA; Monachese M; Trinder M; Bisanz JE; Chmiel JA; Burton JP; Reid G, Immobilization of cadmium and lead by Lactobacillus rhamnosus GR-1 mitigates apical-to-basolateral heavy metal translocation in a Caco-2 model of the intestinal epithelium. Gut Microbes 2019, 10, (3), 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lili Z; Hongfei Z; Shoukat S; Xiaochen Z; Bolin Z, Screening lactic acid bacteria strains with ability to bind di-n-butyl phthalate via Turbiscan technique. Antonie Van Leeuwenhoek 2017, 110, (6), 759–769. [DOI] [PubMed] [Google Scholar]
- 121.Zhu Y.-t.; Yang C.-x.; Luo B-B; Zhou K; Liu S.-l., Efficiency of dairy strains of lactic acid bacteria to bind bisphenol A in phosphate buffer saline. Food Control 2017, 73, 1203–1209. [Google Scholar]
- 122.Bisanz JE; Enos MK; Mwanga JR; Changalucha J; Burton JP; Gloor GB; Reid G, Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 2014, 5, (5), e01580–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baralic K; Zivancevic K; Javorac D; Buha Djordjevic A; Andelkovic M; Jorgovanovic D; Antonijevic Miljakovic E; Curcic M; Bulat Z; Antonijevic B; Dukic-Cosic D, Multi-strain probiotic ameliorated toxic effects of phthalates and bisphenol A mixture in Wistar rats. Food Chem Toxicol 2020, 143, 111540. [DOI] [PubMed] [Google Scholar]
- 124.Feng P, Ye Z, Kakade A, Virk AK, Li X, Liu P, 2018. A review on gut remediation of selected environmental contaminants: possible roles of probiotics and gut microbiota. Nutrients 2018, 11 (1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi BS; Varin TV; St-Pierre P; Pilon G; Tremblay A; Marette A, A polyphenol-rich cranberry extract protects against endogenous exposure to persistent organic pollutants during weight loss in mice. Food Chem Toxicol 2020, 146, 111832. [DOI] [PubMed] [Google Scholar]
- 126.Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO, 2020. The health benefits of dietary fibre. Nutrients 2020, 12 (10), 3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stephen AM; Champ MM; Cloran SJ; Fleith M; van Lieshout L; Mejborn H; Burley VJ, Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev 2017, 30, (2), 149–190. [DOI] [PubMed] [Google Scholar]
- 128.Quagliani D; Felt-Gunderson P, Closing America's Fiber Intake Gap: Communication Strategies From a Food and Fiber Summit. Am J Lifestyle Med 2017, 11, (1), 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zou W; Liu W; Yang B; Wu L; Yang J; Zou T; Liu F; Xia L; Zhang D, Quercetin protects against perfluorooctanoic acid-induced liver injury by attenuating oxidative stress and inflammatory response in mice. Int Immunopharmacol 2015, 28, (1), 129–35. [DOI] [PubMed] [Google Scholar]
- 130.Lv Z; Wu W; Ge S; Jia R; Lin T; Yuan Y; Kuang H; Yang B; Wu L; Wei J; Zhang D, Naringin protects against perfluorooctane sulfonate-induced liver injury by modulating NRF2 and NF-kappaB in mice. Int Immunopharmacol 2018, 65, 140–147. [DOI] [PubMed] [Google Scholar]
- 131.Huang T; Zhang Y; Zhang W; Lin T; Chen L; Yang B; Wu L; Yang J; Zhang D, Attenuation of Perfluorooctane Sulfonate-Induced Steatohepatitis by Grape Seed Proanthocyanidin Extract in Mice. Biomed Res Int 2020, 2020, 8818160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eke D; Celik A; Yilmaz MB; Aras N; Kocaturk Sel S; Alptekin D, Apoptotic gene expression profiles and DNA damage levels in rat liver treated with perfluorooctane sulfonate and protective role of curcumin. Int J Biol Macromol 2017, 104, (Pt A), 515–520. [DOI] [PubMed] [Google Scholar]
- 133.Su M; Liang X; Xu X; Wu X; Yang B, Hepatoprotective benefits of vitamin C against perfluorooctane sulfonate-induced liver damage in mice through suppressing inflammatory reaction and ER stress. Environ Toxicol Pharmacol 2019, 65, 60–65. [DOI] [PubMed] [Google Scholar]
- 134.Bischoff SC, Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care 2008, 11, (6), 733–40. [DOI] [PubMed] [Google Scholar]
- 135.Bai Y; Peng W; Yang C; Zou W; Liu M; Wu H; Fan L; Li P; Zeng X; Su W, Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front Pharmacol 2020, 11, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Z; Zeng M; Wang Z; Qin F; Chen J; He Z, Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J Agric Food Chem 2021, 69, (5), 1441–1454. [DOI] [PubMed] [Google Scholar]
- 137.Yuan J; Che S; Ruan Z; Song L; Tang R; Zhang L, Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem Toxicol 2021, 150, 112098. [DOI] [PubMed] [Google Scholar]
- 138.Zhang ZF; Zhang YQ; Fan SH; Zhuang J; Zheng YL; Lu J; Wu DM; Shan Q; Hu B, Troxerutin protects against 2,2',4,4'-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD(+)-depletion. J Hazard Mater 2015, 283, 98–109. [DOI] [PubMed] [Google Scholar]
- 139.Wang WL; Xu SY; Ren ZG; Tao L; Jiang JW; Zheng SS, Application of metagenomics in the human gut microbiome. World J Gastroenterol 2015, 21, (3), 803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bashiardes S; Zilberman-Schapira G; Elinav E, Use of Metatranscriptomics in Microbiome Research. Bioinform Biol Insights 2016, 10, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deng P; Li X; Petriello MC; Wang C; Morris AJ; Hennig B, Application of metabolomics to characterize environmental pollutant toxicity and disease risks. Rev Environ Health 2019, 34, (3), 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Deng P; Valentino T; Flythe MD; Moseley HNB; Leachman JR; Morris AJ; Hennig B, Untargeted Stable Isotope Probing of the Gut Microbiota Metabolome Using (13)C-Labeled Dietary Fibers. J Proteome Res 2021, 20, (5), 2904–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aguiar-Pulido V; Huang W; Suarez-Ulloa V; Cickovski T; Mathee K; Narasimhan G, Metagenomics, Metatranscriptomics, and Metabolomics Approaches for Microbiome Analysis. Evol Bioinform Online 2016, 12, (Suppl 1), 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y; Huang WE; Cui L; Wagner M, Single cell stable isotope probing in microbiology using Raman microspectroscopy. Curr Opin Biotechnol 2016, 41, 34–42. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Q; Caudle WM; Pi J; Bhattacharya S; Andersen ME; Kaminski NE; Conolly RB, Embracing Systems Toxicology at Single-Cell Resolution. Curr Opin Toxicol 2019, 16, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Picollet-D'hahan N; Zuchowska A; Lemeunier I; Le Gac S, Multiorgan-on-a-Chip: A Systemic Approach To Model and Decipher Inter-Organ Communication. Trends Biotechnol 2021, 39, (8), 788–810. [DOI] [PubMed] [Google Scholar]
- 147.Bovard D; Sandoz A; Luettich K; Frentzel S; Iskandar A; Marescotti D; Trivedi K; Guedj E; Dutertre Q; Peitsch MC; Hoeng J, A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, (24), 3814–3829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


