Abstract
Despite decades of research and anti-tobacco messaging, nicotine addiction remains an important public health problem leading to hundreds of thousands of deaths each year. While fundamental studies have identified molecular, circuit-level and behavioral mechanisms important for nicotine reinforcement and withdrawal, recent studies have identified additional pathways that are important for both nicotine seeking and aversion. In particular, although dopaminergic mechanisms are necessary for nicotine-dependent reward and drug-seeking, novel glutamate and GABA signaling mechanisms in the mesolimbic system have been identified for their contributions to reward-related behaviors. An additional area of active investigation for nicotine addiction focuses on molecular mechanisms in the habenula-interpeduncular pathway driving nicotine aversion and withdrawal. Across all these domains, sex differences in the molecular basis of nicotine-induced behaviors have emerged that identify important new directions for future research. Recent studies reviewed here highlight additional pathways that could provide therapeutic targets for smoking cessation and problematic nicotine vaping.
Introduction
The dopamine (DA) hypothesis has dominated addiction research for many years and can explain a number of aspects of substance use, including behaviors related to initial reward, cue-reward learning, compulsive drug seeking and motivation to seek addictive substances [1,2]. Despite the central role of DA in behaviors underlying addiction, emerging evidence implicates a number of different neurotransmitters in the rewarding properties of drugs and the negative consequences experienced during drug withdrawal. Here we review a number of recent studies in the field of nicotine addiction identifying molecular- and circuit-level mechanisms underlying nicotine reward, aversion and withdrawal, particularly in canonical mesolimbic reward and habenular-interpeduncular aversive circuits. We also highlight the need for further investigation into sex differences in these mechanisms perpetuating nicotine addiction. These studies identify pathways that could be targets for development of more effective cessation aids for smoking and nicotine vaping.
Ventral Tegmental Area (VTA) circuits and nicotine reinforcement
Nicotine acts by binding to, activating, and/or desensitizing nicotinic acetylcholine receptors (nAChRs), ligand-gated ion channel pentamers composed of a combination of α2-α10 and β2-β4 subunits [3]. The various subunit compositions allow for the formation of functionally distinct receptors with different binding affinities and desensitization rates for nicotine [3]. The primary reinforcing properties of nicotine are mediated via high affinity nAChRs containing the β2 subunit (β2* nAChRs, where * represents additional subunits in the receptor), that can increase the activity of VTA DA neurons and release of DA from terminals in the nucleus accumbens (NAc) [4,5]. The β2 nicotinic subunit generally co-assembles with the α4 and/or α6 subunit, both of which are critical for primary nicotine reinforcement, as mice lacking the α4 or α6 subunit self-administer fewer infusions of nicotine into the VTA [6]. The expression of α6* nAChRs, often as a part of α4β2* nAChRs, pre-synaptically on VTA DA terminals in the NAc allows these receptors to regulate dopamine release in response to nicotine to mediate nicotine reward [7]. In addition to their role in primary nicotine reinforcement, α4β2* nAChRs may also contribute to the ability of nicotine to increase the reinforcing effects of other reinforcers (“reinforcement-enhancement”; see [8]). Varenicline, a drug used for smoking cessation, is a partial agonist of α4β2* nAChRs. While varenicline can mimic the reinforcement-enhancing effects of nicotine [9], it does not show primary reinforcing effects when administration of varenicline is dissociated from a visual cue that acts as a mild reinforcer [10]. These results suggest that the efficacy of varenicline as a smoking cessation aid could be due to reinforcement-enhancement, and these effects are at least partially mediated by α4β2* nAChRs. Furthermore, nicotine can also enhance the reinforcing effects of sweet flavors, which is relevant to the exponential growth in popularity of flavored nicotine vape liquids [11]. These studies highlight the importance of further investigation into the reinforcement-enhancing effects of nicotine to aid tobacco and e-cigarette users in successful cessation.
The β2* nAChRs in the VTA are critical for nicotine reinforcement. Mice lacking the β2 subunit (β2 KO mice) do not show nicotine-induced firing of VTA DA neurons and are unable to acquire self-administration of intravenous nicotine [12]. Further, selective re-expression of the β2* nAChRs in the VTA of β2 KO mice restores nicotine-induced DA release and nicotine self-administration [6,13,14]. These data are consistent with the idea that nAChR-dependent DA signaling is essential for nicotine reinforcement. However, re-expressing β2* nAChRs in VTA DA neurons alone is insufficient to rescue nicotine-induced burst firing of DA neurons or self-administration [15], suggesting that additional VTA neuronal populations are necessary for nicotine reinforcement.
Although most research on the VTA has focused on its dopaminergic functions, it is increasingly clear that the VTA is a heterogeneous structure composed of not only dopaminergic neurons, but GABAergic and glutamatergic neurons as well [16,17]. Notably, β2* nAChRs are expressed, to varying degrees, on all neurotransmitter-defined neuron subtypes in the VTA [18,19]. The involvement of glutamate and GABA signaling in nicotine reinforcement has been highlighted for some time [20]; however, more recent work has addressed the question of how nicotine’s effects on the various neuronal populations in the VTA work together in nicotine reinforcement. For example, although re-expression of the β2* nAChRs in VTA DA or GABA neurons alone is not sufficient to rescue nicotine self-administration, re-expression in both DA and GABA neurons in the VTA of β2 KO mice restores burst firing of DA neurons and results in rapid and steady acquisition of intra-VTA nicotine self-administration [15]. Interestingly, a similar re-expression study in β2 KO mice found that expression of β2* nAChRs on VTA GABA neurons alone is sufficient for nicotine reward [21]. An important distinction between these two studies is that Tolu et al. [15] used intra-VTA nicotine self-administration, while Grieder et al. [21] administered nicotine systemically in a conditioned place preference paradigm. The frequency of nicotine administration, amount of nicotine exposure, and kinetics of brain exposure differ between these two paradigms, potentially leading to different expression levels and desensitization states of nAChRs in the VTA, which could explain the contradictory findings. These two studies highlight the heterogeneity of the VTA, suggesting that further work is needed to fully elucidate how distinct populations of VTA DA and GABA neurons mediate nicotine reward and aversion.
Recent work has provided additional support for the importance of VTA GABA neurons in reward processing. VTA GABA neurons have been thought to act primarily as local inhibitory interneurons, regulating the activity of VTA DA neurons [22]. For example, VTA GABA neurons in the medial VTA (mVTA) make functional connections mostly with DA neurons in the lateral VTA (latVTA) [23]. Low doses of nicotine in this microcircuit inhibit the activity of mVTA GABA neurons that project to latVTA [23], although it is not clear whether this is due to desensitization of nAChRs or activation of other GABA neurons in the circuit. Nonetheless, this would result in a net disinhibition of latVTA DA neurons, suggesting that local VTA GABAergic signaling can contribute to nicotine reward. However, a subset of VTA GABA neurons make long-range, functional connections outside the VTA to regions such as the ventral pallidum (VP) that are also involved in reward processing [24,25]. Activity of these VTA-to-VP GABA neurons is not only stimulated consistently by unconditioned rewards, but also scales according to the value of the reward [25]. Optogenetic activation of these VTA-to-VP GABA neurons accelerates acquisition of a cue-reward contingency and sustains high responding for reward in a progressive ratio task [25]. Therefore, VTA GABA neurons may be able to mediate nicotine reward, reward-based learning and motivation.
VTA glutamate neurons are also receiving attention for their role in reward-related behaviors. VTA glutamate neurons make functional connections within the VTA as well as with other reward-related brain regions like the NAc [26]. A subset of VTA glutamate neurons express tyrosine hydroxylase (TH), the key enzyme in dopamine synthesis, and are capable of co-releasing glutamate and DA [27]. Conditional knock-out (cKO) of TH in VGLUT2-expressing neurons abolishes DA release only from these neurons capable of co-release [28]. Interestingly, these cKO mice lacking DA signaling from VTA glutamate fibers in NAc still sustain intracranial self-stimulation and behave like control littermates in a real-time place preference test [28]. Similarly, when DA synthesis and release is disrupted selectively in adult VTA neurons that express VGLUT2, mice can still acquire an operant task to stimulate VTA glutamate neurons independent of DA release [28]. Thus, glutamatergic signaling from VTA to NAc, independent of DA, is sufficient for positive reinforcement.
However, nicotine-mediated VTA glutamate signaling within the VTA is more heterogeneous. Nicotine equally potentiates and suppresses the EPSCs in latVTA neurons induced by targeted optogenetic stimulation (oEPSCs) of mVTA glutamatergic neurons, suggesting that nicotine can both strengthen and attenuate this VTA microcircuit [23]. Interestingly, nicotine-mediated suppression of oEPSCs may be explained by the co-release of GABA and glutamate from these mVTA neurons, as these neurons express both VGluT2 and Gad2 mRNA [23]. While the majority of neurons release either glutamate or GABA, neurons with the capability to co-release both neurotransmitters are present in the VTA [25,29]. A subset of VTA-to-VP GAD+ neurons can induce post-synaptic currents that are incompletely blocked by GABA or glutamate receptor antagonists alone, but are abolished by co-administration of GABA and glutamate antagonists together [25]. Similarly, mVTA neurons expressing both VGLUT2 and VGAT send axon terminals to the lateral habenula (LHb), where they form both asymmetric synapses that release glutamate and symmetric synapses that release GABA [29]. These VTA glutamate/GABA co-releasing neurons can signal both rewarding and aversive outcomes to the LHb [30]. Furthermore, the LHb sends projections to the rostromedial tegmental nucleus (RMTg), where nicotine binding to presynaptic α7 nAChRs expressed on these terminals induces glutamate release [31]. These studies suggest that VTA glutamate neurons can modulate nicotine reward through VTA DA neurons and projections to the NAc, but may also mediate aversive properties of nicotine through reciprocal connections to the LHb.
Circuits mediating nicotine aversion
While glutamate release from the VTA to the NAc is reinforcing, glutamate signaling from the habenula is implicated in aversive effects of nicotine. The habenular complex, particularly the medial habenula (MHb) projections to the interpeduncular nucleus (IPN), is an important circuit mediating nicotine aversion [32]. These IPN-projecting MHb neurons are mostly cholinergic and can co-release both acetylcholine and glutamate [33,34]. Loss of glutamatergic signaling from these MHb cholinergic neurons increases intravenous nicotine self-administration, suggesting that glutamate signaling in the MHb-to-IPN circuit can mediate the aversive properties of nicotine, inhibiting consumption [35].
Nicotine also binds directly to nAChRs in the MHb to drive an aversive stop signal for nicotine self-administration [36]. Mice lacking α5* nAChRs self-administer more nicotine at higher doses that are typically considered to be aversive; this is reversed when the α5 subunit is re-expressed in the MHb or IPN [36]. The β4* nAChRs are also involved in signaling nicotine aversion, as β4 KO mice also self-administer more nicotine at higher doses than wildtype (WT) controls, while over-expression of these receptors results in avoidance of the nicotine-reinforced arm [37]. Re-expression of β4* nAChRs specifically in the MHb restores a WT-like profile of nicotine self-administration [37].
In addition to the β4 and α5 subunits, the α3 nicotinic subunit is required to self-regulate nicotine intake. Knockdown of the α3 subunit in MHb increases nicotine self-administration, particularly at higher doses [38]. Furthermore, specific antagonism of α3β4* nAChRs with α-conotoxin AuIB significantly attenuates nicotine-induced currents in MHb neurons [38]. The α5/α3/β4* nAChRs in the IPN are also involved in limiting nicotine self-administration. For example, selective re-expression of β4* nAChRs in the IPN alone is also sufficient to revert the increased nicotine self-administration patterns seen in β4 KO mice [37]. Furthermore, infusion of α-conotoxin AuIB, the α3β4* nAChR antagonist, directly into the IPN increases nicotine self-administration [38]. Thus, nAChRs containing the α5, α3 and β4 subunits in the MHb and IPN are important for signaling nicotine aversion and limiting nicotine self-administration.
The IPN sends GABAergic projections to the laterodorsal tegmentum (LDTg), which in turn, sends cholinergic, GABAergic and glutamatergic projections to the VTA [39]. This circuit is another critical pathway underlying nicotine aversion. Optogenetic activation of the GABAergic IPN terminals in LDTg results in place avoidance, while inhibition of these neurons blocks nicotine conditioned place aversion [40]. High concentrations of nicotine applied on ex vivo slices increases the amplitude of optically-evoked inhibitory post-synaptic currents, whereas application of a β2* nAChR-specific antagonist dihydro-β-erythroidine hydrobromide (DhβE) partially reverses this increase [40]. Furthermore, GABAergic projections from the LDTg make direct inhibitory synaptic connections onto VTA DA neurons that project to the NAc lateral shell [39], the canonical reward prediction error-encoding population of VTA DA neurons [41]. Optogenetic inhibition of these GABAergic LDTg axon terminals in VTA also blocks nicotine conditioned place aversion. In addition, positive allosteric modulators of GABAB receptors inhibit nicotine self-administration and decrease cue-induced nicotine-seeking without affecting food-seeking behaviors [42]. Therefore, increased GABAergic tone within the IPN-LDTg-VTA circuit likely contributes to nicotine aversion.
Circuits mediating nicotine withdrawal
Nicotine aversion and withdrawal are both negative states induced by nicotine, but aversive properties of nicotine limit nicotine intake, while physical and affective symptoms of nicotine withdrawal often lead to relapse of nicotine seeking. Despite the opposing motivational forces on nicotine consumption, the molecular and circuit mechanisms mediating aversion and withdrawal overlap [43]. For example, activity of the MHb plays a critical role in nicotine withdrawal in both rodents and humans [44–46]. During nicotine withdrawal in overnight-abstinent smokers, nicotine but not placebo, decreases habenula responsivity to both negative and positive feedback, and higher session-specific habenula activity among smokers correlates with greater tobacco craving, indicating that activity in the structure could contribute to relapse to nicotine seeking [47]. In rodents, optogenetic inhibition of habenular cholinergic projections to the IPN alleviates affective symptoms of nicotine withdrawal, such as anxiety-like behaviors [48]. Furthermore, mice with reduced glutamate release from MHb terminals in the IPN show no withdrawal symptoms after cessation of chronic nicotine treatment [34]. Thus, the MHb is strongly implicated in mediating nicotine withdrawal.
The nAChR subtypes important for nicotine withdrawal partially coincide with those that mediate nicotine aversion, but also overlap with nAChRs important for nicotine reinforcement. The α5* and β4* nAChRs are highly expressed in MHb and IPN, and both β4 KO mice and α5 KO mice show milder somatic symptoms of withdrawal [49,50]. Activity of IPN GABA neurons is increased during nicotine withdrawal, whereas optogenetic silencing of IPN GABA neurons reduces somatic and affective signs of withdrawal [51]. IPN GABA neurons express α5* and α7* nAChRs, implicating these receptor subtypes in nicotine withdrawal [52,53]. In addition, α5* nAChRs are expressed on DA neurons in the VTA and their activity is modulated by nicotine consumption and withdrawal [54]. Indeed, chronic nicotine exposure in α5 KO mice results in abnormally elevated function of VTA DA neurons for many weeks throughout withdrawal [54]. Activity of VTA neurons is further implicated in nicotine withdrawal, as α4β2* nAChRs are upregulated in the VTA, striatum, habenula and IPN during withdrawal [55]. Furthermore, the high sensitivity isoform of α4β2 nAChRs (containing two α4 and three β2 subunits) also contributes to both the somatic and affective components of nicotine withdrawal [56]. Together, these studies highlight the role of multiple nAChR subtypes in the MHb, IPN and VTA in nicotine withdrawal.
Sex differences
Historically, many studies of nicotine reinforcement and withdrawal have used only male rodents; however, more recent studies using both sexes have shown that nicotine exposure and withdrawal can differentially alter gene expression of nAChR subunits in a sex-dependent manner. Although male and female rats show similar increases in expression of the genes encoding the β3 and β4 subunits in the IPN during nicotine withdrawal, only female rats have increased levels of α5 mRNA transcripts and only males have increased levels of α2 and α3 transcripts during withdrawal [57]. Furthermore, female rats display greater anxiety-like behavior than males in nicotine withdrawal, and this is associated with increased gene expression of α4, α5, and β2 nAChR subunits in the IPN in female animals [57]. In addition, GABA release in the IPN is closely associated with withdrawal severity, and female rats display elevated GABA release in the IPN during withdrawal compared to males [58]. Female mice also show stronger stress-induced reinstatement of conditioned place preference when nicotine is paired with an initially non-preferred side, while males show reinstatement when nicotine is paired with an initially preferred side, suggesting that the anxiolytic and reward-enhancing effects of nicotine may be sex-dependent [59]. Finally, at the molecular level, chronic nicotine and withdrawal significantly alter the expression of many more proteins in the VTA of female mice compared to male mice [60]. These results suggest that the negative consequences of nicotine withdrawal may be mediated by multiple nAChR subunits, and by different subtypes in females and males. These studies highlight the need for further investigation of sex differences in nicotine reinforcement and withdrawal.
Conclusions
Significant advances in understanding the heterogeneity of circuits involved in nicotine reward and aversion have been made over the last several years (Fig. 1). Most exciting has been an expanded appreciation for the role of GABA and glutamate signaling in regulation of behaviors that had been thought to depend primarily on DA signaling. The increasing body of work identifying important roles for non-DA projection neurons originating from the VTA has broadened the understanding of neuronal substrates mediating reward-related behaviors and provides additional therapeutic targets for interventions to treat nicotine addiction.
Figure 1. Overview of brain areas and nAChR subtypes involved in nicotine reward, aversion and withdrawal.
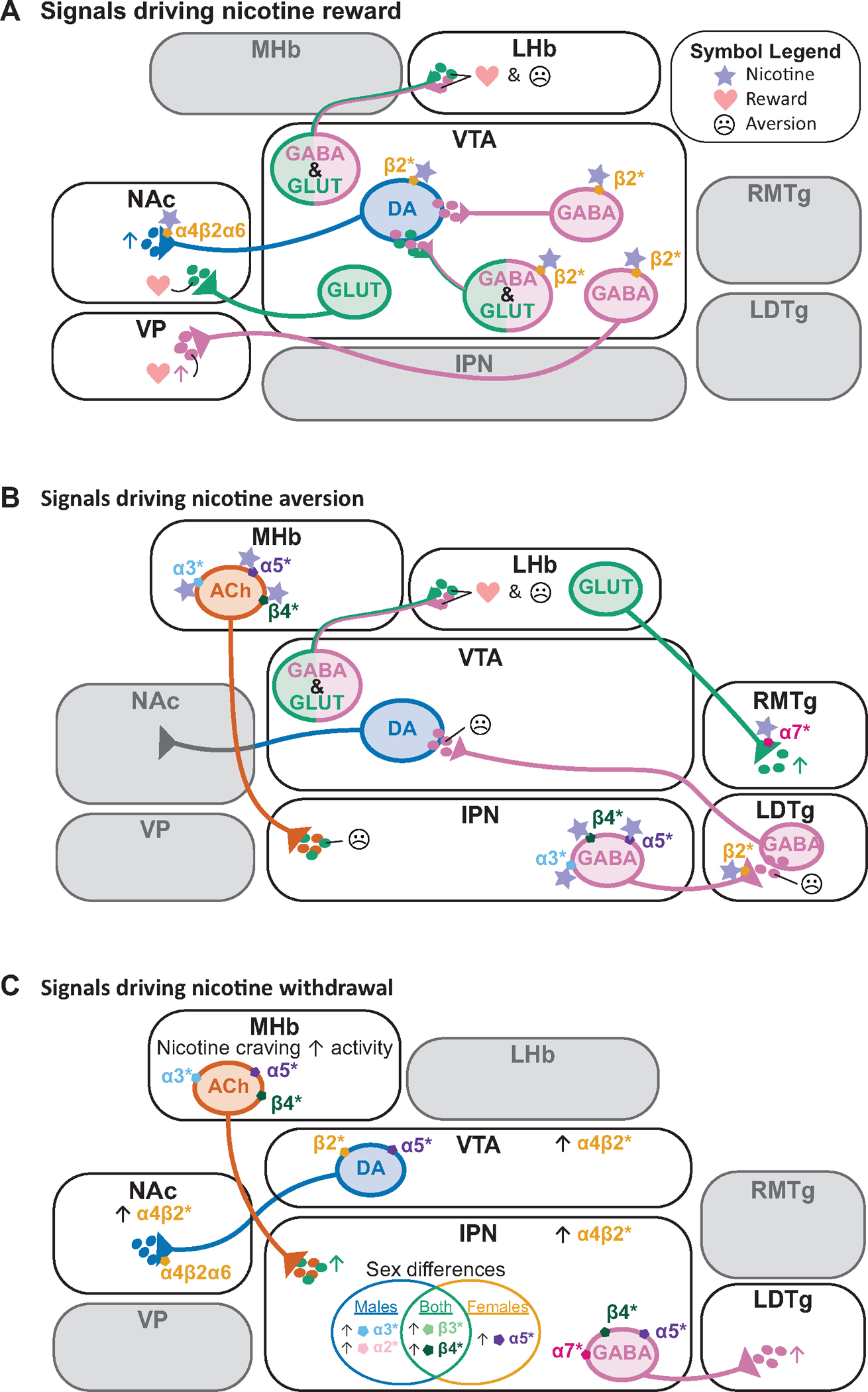
A) Brain areas known to be involved in nicotine reward include the VTA, NAc and VP. Activity in these brain regions can be regulated by α4β2* nAChRs, as well as α6* nAChRs. Specific projections from the VTA that mediate reward include glutamatergic neurons projecting to NAc, GABA neurons projecting to VP, and GABA/glutamate co-releasing neurons projecting to LHb.
B) Brain areas known to be involved in nicotine aversion include the VTA, habenula (MHb and LHb), IPN, LDTg, and RMTg. Nicotine aversion is mediated by α3*, α5*, β4*, β2*, and α7* nAChRs in these various brain regions.
C) Brain areas known to be involved in nicotine withdrawal include the VTA, NAc, MHb, IPN and LDTg. α4β2* nAChRs are upregulated in many of these regions during withdrawal. The α3*, α5*, β4*, and α7* nAChRs in these brain areas also mediate nicotine withdrawal. Sex-specific changes in expression of nicotinic subunit mRNAs in IPN during withdrawal are also summarized.
Similarly, it has been clear for some time that circuits involved in aversive behaviors are important for limiting intake of high doses of nicotine and for mediating the negative consequences of nicotine withdrawal, and more recent studies have confirmed the involvement of these pathways in human nicotine-related behaviors. The MHb-to-IPN pathway includes a high proportion of cholinergic neurons that signal directly through local and distal α5/α3/β4* nAChRs, but these circuits also release glutamate, and both ACh and glutamate signaling are involved in limiting intake of high-dose nicotine (Fig. 1B). These studies highlight the need to study additional neurotransmitter systems and circuits beyond the canonical mesolimbic DA system in initiation and development of nicotine reinforcement.
Recent research has also highlighted opportunities for further investigation into sex differences in molecular and behavioral responses to nicotine. Human studies have identified differences in development of nicotine dependence, the severity of nicotine withdrawal and difficulty in smoking cessation between men and women. Molecular studies have identified differential roles for particular nAChR subtypes in female and male rodents (Fig. 1C), and sex differences in protein expression profiles in the mesolimbic system at baseline and following nicotine intake. These studies suggest that including females and males in both preclinical and human studies of nicotine reward and reinforcement will be essential for a full understanding of the complexities of nAChR-mediated regulation of circuits and behaviors.
Finally, while smoking rates continue to decrease in many parts of the world, nicotine vaping has become more prevalent, especially among youth and those who have never smoked. Vaping has been shown to have harmful effects to both physical and mental health, with higher prevalence of depression and anxiety found in those who vape. As a result, studies of nAChR function, cholinergic signaling and nicotine-mediated behaviors continue to be relevant for understanding the consequences of human nicotine intake. Extending the current understanding of nAChR function in glutamate and GABA signaling in circuits such as the VTA and MHb-to-IPN to human subjects will be challenging, but could provide new avenues for understanding the consequences of nicotine on overall mental health.
Acknowledgements
This work was supported by National Institutes of Health grants DA14241 and DA050986. This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
Footnotes
Declaration of Interest Statement
The authors declare that they have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wise RA, Robble MA: Dopamine and Addiction. Annu Rev Psychol 2020, 71:79–106. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Michaelides M, Baler R: The Neuroscience of Drug Reward and Addiction. Physiol Rev 2019, 99:2115–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Changeux J-P: Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 2010, 11:389–401. [DOI] [PubMed] [Google Scholar]
- 4.Wittenberg RE, Wolfman SL, De Biasi M, Dani JA: Nicotinic acetylcholine receptors and nicotine addiction: A brief introduction. Neuropharmacology 2020, 177:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papke RL, Brunzell DH, De Biasi M: Cholinergic Receptors and Addiction. In Behavioral Pharmacology of the Cholinergic System. Edited by Shoaib M, Wallace TL. Springer International Publishing; 2020:123–151. [DOI] [PubMed] [Google Scholar]
- 6.Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W: Crucial Role of α4 and α6 Nicotinic Acetylcholine Receptor Subunits from Ventral Tegmental Area in Systemic Nicotine Self-Administration. J Neurosci 2008, 28:12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills L, Kenny PJ: Addiction-related neuroadaptations following chronic nicotine exposure. J Neurochem 2021, 157:1652–1673. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF: Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006, 184:353–366. [DOI] [PubMed] [Google Scholar]
- 9.Barrett ST, Geary TN, Steiner AN, Bevins RA: A behavioral economic analysis of the value-enhancing effects of nicotine and varenicline and the role of nicotinic acetylcholine receptors in male and female rats. Behav Pharmacol 2018, 29:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schassburger RL, Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Donny EC, Sved AF: Differentiating the primary reinforcing and reinforcement-enhancing effects of varenicline. Psychopharmacology (Berl) 2015, 232:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmatier MI, Smith AL, Odineal EM, Williams EA, Sheppard AB, Bradley CA: Nicotine Self-Administration With Tobacco Flavor Additives in Male Rats. Nicotine Tob Res Off J Soc Res Nicotine Tob 2020, 22:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP: Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998, 391:173–177. [DOI] [PubMed] [Google Scholar]
- 13.Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux J-P, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, et al. : Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 2005, 436:103–107. [DOI] [PubMed] [Google Scholar]
- 14.Orejarena MJ, Herrera-Solís A, Pons S, Maskos U, Maldonado R, Robledo P: Selective re-expression of β2 nicotinic acetylcholine receptor subunits in the ventral tegmental area of the mouse restores intravenous nicotine self-administration. Neuropharmacology 2012, 63:235–241. [DOI] [PubMed] [Google Scholar]
- 15.Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, Baudonnat M, Husson M, Besson M, Reperant C, et al. : Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry 2013, 18:382–393. [DOI] [PubMed] [Google Scholar]
- 16.Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA: Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 2008, 152:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi T, Qi J, Wang H-L, Zhang S, Morales M: Glutamatergic and Dopaminergic Neurons in the Mouse Ventral Tegmental Area. Eur J Neurosci 2015, 41:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP: Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci Off J Soc Neurosci 2001, 21:1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Y, Peng C, Arvin MC, Jin X-T, Kim VJ, Ramsey MD, Wang Y, Banala S, Wokosin DL, McIntosh JM, et al. : Nicotinic Cholinergic Receptors in VTA Glutamate Neurons Modulate Excitatory Transmission. Cell Rep 2018, 23:2236–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markou A: Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci 2008, 363:3159–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grieder TE, Besson M, Maal-Bared G, Pons S, Maskos U, van der Kooy D: β2* nAChRs on VTA dopamine and GABA neurons separately mediate nicotine aversion and reward. Proc Natl Acad Sci U S A 2019, 116:25968–25973. This study shows that β2* nAChRs on VTA GABA neurons are necessary and sufficient for the conditioned rewarding effects of nicotine. This study also found that conditioned place aversion from high doses of nicotine is disrupted in β2 KO mice, and rescued following β2* nAChR re-expression in all VTA neurons or only VTA DA neurons.
- 22.Mansvelder HD, Keath JR, McGehee DS: Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 2002, 33:905–919. [DOI] [PubMed] [Google Scholar]
- 23.Yan Y, Beckley NA, Kim VJ, Drenan RM: Differential Nicotinic Modulation of Glutamatergic and GABAergic VTA Microcircuits. eNeuro 2019, 6:ENEURO.0298–19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SR, Badurek S, DiLeone RJ, Nashmi R, Minichiello L, Picciotto MR: GABAergic and Glutamatergic Efferents of the Mouse Ventral Tegmental Area. J Comp Neurol 2014, 522:3308–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou W-L, Kim K, Ali F, Pittenger ST, Calarco CA, Mineur YS, Ramakrishnan C, Deisseroth K, Kwan AC, Picciotto MR: Activity of a direct VTA to ventral pallidum GABA pathway encodes unconditioned reward value and sustains motivation for reward. Sci Adv 2022, 8:eabm5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai J, Tong Q: Anatomy and Function of Ventral Tegmental Area Glutamate Neurons. Front Neural Circuits 2022, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi T, Wang H-L, Li X, Ng TH, Morales M: Mesocorticolimbic glutamatergic pathway. J Neurosci Off J Soc Neurosci 2011, 31:8476–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zell V, Steinkellner T, Hollon NG, Warlow SM, Souter E, Faget L, Hunker AC, Jin X, Zweifel LS, Hnasko TS: VTA Glutamate Neuron Activity Drives Positive Reinforcement Absent Dopamine Co-release. Neuron 2020, 107:864–873.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Root DH, Zhang S, Barker DJ, Miranda-Barrientos J, Liu B, Wang H-L, Morales M: Selective Brain Distribution and Distinctive Synaptic Architecture of Dual Glutamatergic-GABAergic Neurons. Cell Rep 2018, 23:3465–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Root DH, Barker DJ, Estrin DJ, Miranda-Barrientos JA, Liu B, Zhang S, Wang H-L, Vautier F, Ramakrishnan C, Kim YS, et al. : Distinct Signaling by Ventral Tegmental Area Glutamate, GABA, and Combinatorial Glutamate-GABA Neurons in Motivated Behavior. Cell Rep 2020, 32:108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castillo-Rolón D, Ramírez-Sánchez E, Arenas-López G, Garduño J, Hernández-González O, Mihailescu S, Hernández-López S: Nicotine Increases Spontaneous Glutamate Release in the Rostromedial Tegmental Nucleus. Front Neurosci 2021, 14:604583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowler CD, Kenny PJ: Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 2014, 76:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M: Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 2011, 69:445–452. [DOI] [PubMed] [Google Scholar]
- 34.Frahm S, Antolin-Fontes B, Görlich A, Zander J-F, Ahnert-Hilger G, Ibañez-Tallon I: An essential role of acetylcholine-glutamate synergy at habenular synapses in nicotine dependence. eLife 2015, 4:e11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Souter EA, Chen Y-C, Zell V, Lallai V, Steinkellner T, Conrad WS, Wisden W, Harris KD, Fowler CD, Hnasko TS: Disruption of VGLUT1 in Cholinergic Medial Habenula Projections Increases Nicotine Self-Administration. eNeuro 2022, 9:ENEURO.0481–21.2021. Using conditional knock-out mice lacking VGLUT1 in cholinergic MHb neurons, this study demonstrates that loss of glutamate co-release from MHb-to-IPN neurons increases nicotine self-administration. These data suggest that glutamate co-release from these projections limits nicotine self-administration and can potentially signal nicotine aversion.
- 36.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ: Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 2011, 471:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Husson M, Harrington L, Tochon L, Cho Y, Ibañez-Tallon I, Maskos U, David V: β4-Nicotinic Receptors Are Critically Involved in Reward-Related Behaviors and Self-Regulation of Nicotine Reinforcement. J Neurosci 2020, 40:3465–3477. This study characterizes nicotine-related behaviors in β4 KO mice. These mice show deficits in nicotine self-administration at low doses that is reversed at high nicotine doses typically considered to be aversive. Following broad over-expression of β4* nAChRs, mice avoid nicotine; however, viral re-expression of β4* nAChRs in the VTA potentiates nicotine reward, whereas re-expression in the MHb-to-IPN pathway attenuates self-administration of high nicotine doses.
- 38. Elayouby KS, Ishikawa M, Dukes AJ, Smith ACW, Lu Q, Fowler CD, Kenny PJ: α3* Nicotinic Acetylcholine Receptors in the Habenula-Interpeduncular Nucleus Circuit Regulate Nicotine Intake. J Neurosci 2021, 41:1779–1787. This study found that mice expressing low levels of α3* nAChRs self-administer more nicotine. This phenotype can be replicated with viral knock-down of α3* nAChRs in the MHb or IPN. Using electrophysiology and specific α3β4* and α3β2* nAChR antagonists, this study further suggests that α3β4* nAChRs mediate nicotine aversion that typically limits nicotine consumption.
- 39.Liu C, Tose AJ, Verharen JPH, Zhu Y, Tang LW, de Jong JW, Du JX, Beier KT, Lammel S: An inhibitory brainstem input to dopamine neurons encodes nicotine aversion. Neuron 2022, 110:3018–3035.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfman SL, Gill DF, Bogdanic F, Long K, Al-Hasani R, McCall JG, Bruchas MR, McGehee DS: Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nat Commun 2018, 9:2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, Lammel S: A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 2019, 101:133–151.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Sturchler E, Kaczanowska K, Cameron M, Finn MG, Griffin P, McDonald P, Markou A: KK-92A, a novel GABAB receptor positive allosteric modulator, attenuates nicotine self-administration and cue-induced nicotine seeking in rats. Psychopharmacology (Berl) 2017, 234:1633–1644. [DOI] [PubMed] [Google Scholar]
- 43.Antolin-Fontes B, Ables JL, Görlich A, Ibañez-Tallon I: The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology 2015, 96:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HW, Yang SH, Kim JY, Kim H: The Role of the Medial Habenula Cholinergic System in Addiction and Emotion-Associated Behaviors. Front Psychiatry 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paolini M, De Biasi M: Mechanistic insights into nicotine withdrawal. Biochem Pharmacol 2011, 82:996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jennings C, Gosnell S, Curtis KN, Kosten T, Salas R: Altered habenula resting state functional connectivity in deprived veteran tobacco smokers: A pilot study. Bull Menninger Clin 2020, 84:21–34. [DOI] [PubMed] [Google Scholar]
- 47.Flannery JS, Riedel MC, Poudel R, Laird AR, Ross TJ, Salmeron BJ, Stein EA, Sutherland MT: Habenular and striatal activity during performance feedback are differentially linked with state-like and trait-like aspects of tobacco use disorder. Sci Adv 2019, 5:eaax2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, Gao G, Rando OJ, Martin GE, George O, et al. : Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun 2015, 6:6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salas R, Pieri F, Biasi MD: Decreased Signs of Nicotine Withdrawal in Mice Null for the β4 Nicotinic Acetylcholine Receptor Subunit. J Neurosci 2004, 24:10035–10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salas R, Sturm R, Boulter J, De Biasi M: Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci Off J Soc Neurosci 2009, 29:3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klenowski PM, Zhao-Shea R, Freels TG, Molas S, Tapper AR: Dynamic activity of interpeduncular nucleus GABAergic neurons controls expression of nicotine withdrawal in male mice. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 2022, 47:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu Y-WA, Tempest L, Quina LA, Wei AD, Zeng H, Turner EE: Medial habenula output circuit mediated by α5 nicotinic receptor-expressing GABAergic neurons in the interpeduncular nucleus. J Neurosci Off J Soc Neurosci 2013, 33:18022–18035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin X-T, Drenan RM: Functional α7 nicotinic acetylcholine receptors in GABAergic neurons of the interpeduncular nucleus. Neuropharmacology 2022, 208:108987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang K, McLaughlin I, Shaw JK, Quijano-Cardé N, Dani JA, De Biasi M: CHRNA5 gene variation affects the response of VTA dopaminergic neurons during chronic nicotine exposure and withdrawal. Neuropharmacology 2023, 235:109547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varani AP, Pedrón VT, Aon AJ, Canero EM, Balerio GN: GABAB receptors blockage modulates somatic and aversive manifestations induced by nicotine withdrawal. Biomed Pharmacother Biomedecine Pharmacother 2021, 140:111786. [DOI] [PubMed] [Google Scholar]
- 56.Hamouda AK, Bautista MR, Akinola LS, Alkhlaif Y, Jackson A, Carper M, Toma WB, Garai S, Chen Y-C, Thakur GA, et al. : Potentiation of (α4)2(β2)3, but not (α4)3(β2)2, Nicotinic Acetylcholine Receptors Reduces Nicotine Self-Administration and Withdrawal Symptoms. Neuropharmacology 2021, 190:108568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Correa VL, Flores RJ, Carcoba LM, Arreguin MC, O’Dell LE: Sex differences in cholinergic systems in the interpeduncular nucleus following nicotine exposure and withdrawal. Neuropharmacology 2019, 158:107714. This study is one of few that has investigated sex differences in the effects of chronic nicotine and withdrawal. Female rats display greater anxiety-like behavior than males during withdrawal, and gene expression of specific nAChR subunits in the IPN is regulated by chronic nicotine and withdrawal in a sex-dependent manner.
- 58.Carcoba LM, Uribe KP, Ortegon S, Mendez IA, DeBiasi M, O’Dell LE: Amino acid systems in the interpeduncular nucleus are altered in a sex-dependent manner during nicotine withdrawal. J Neurosci Res 2022, 100:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee AM, Calarco CA, McKee SA, Mineur YS, Picciotto MR: Variability in nicotine conditioned place preference and stress-induced reinstatement in mice: Effects of sex, initial chamber preference, and guanfacine. Genes Brain Behav 2020, 19:e12601. This study examined sex differences in the mesolimbic dopamine pathway by analyzing the proteome in the VTA and NAc shell of male and female mice at baseline, after sub-chronic and chronic administration of nicotine and withdrawal. These data demonstrate that many more proteins are differentially expressed following chronic nicotine administration and withdrawal in the VTA of females than in that of males.
- 60.Lee AM, Mansuri MS, Wilson RS, Lam TT, Nairn AC, Picciotto MR: Sex Differences in the Ventral Tegmental Area and Nucleus Accumbens Proteome at Baseline and Following Nicotine Exposure. Front Mol Neurosci 2021, 14:657064. [DOI] [PMC free article] [PubMed] [Google Scholar]


